Abstract
Background
Although current treatments for Post-Traumatic Stress Disorder (PTSD) in war veterans are effective, unfortunately 30–50% still do not benefit from these treatments. Trauma-focused therapies, eg exposure therapy, are primarily based on extinction processes in which the endocannabinoid system (ECS) plays a significant role. Therefore, it can be hypothesized that poor treatment response on trauma-focused therapy due to extinction deficits may be associated with a poorly functioning ECS. The present study examined whether the endocannabinoids anandamide (AEA) and 2-arachidonylglycerol (2-AG) are associated with post-treatment symptom reduction.
Methods
Blood plasma levels of AEA and 2-AG were determined in war veterans with a PTSD diagnosis (n = 54) and combat controls (n = 26) before and after a 6–8 month interval. During this period veterans with PTSD received trauma-focused therapy (eg cognitive behavioral therapy with exposure or eye-movement desensitization and reprocessing). Clinical symptoms were assessed before and after therapy with the Clinician Administered PTSD Scale (CAPS), State-Trait Anxiety Inventory (STAI) and Mood and Anxiety Symptom Questionnaire (MASQ).
Results
Regression analysis demonstrated that pretreatment endocannabinoid levels were not predictive of PTSD symptom reduction. Additionally, baseline endocannabinoid levels did not differ between either PTSD and combat controls or between combat controls, treatment responders, and non-responders. Only cortisol levels significantly decreased over time from pre- to posttreatment (p = .041). Endocannabinoid levels were significantly lower in individuals who reported cannabis use during their lifetime, independent of PTSD diagnosis. Furthermore, correlation analysis revealed that pretreatment 2-AG levels in PTSD were positively correlated with anxious arousal (r = .354, p = .015) and negatively with avoidance symptoms (r = -.271, p = .048). Both posttreatment AEA and 2-AG were positively correlated with trait anxiety (AEA r = .459, p = .003; 2-AG r = .423, p = .006), anxious arousal (AEA r = .351, p = .024; 2-AG r = .311, p = .048) and general distress depression symptoms (AEA r = .414, p = .007; 2-AG r = .374, p = .016).
Conclusion
Since endocannabinoids are mainly generated ‘on demand’, future work could benefit by investigating endocannabinoid circulation under both baseline and stressful conditions. In line with previous research cannabis use was associated with lower endocannabinoid levels. The correlation analysis between pre- and posttreatment endocannabinoid levels and pre- and posttreatment clinical symptomatology were exploratory analysis and should be replicated in future research.
Keywords: PTSD, endocannabinoid, anandamide, 2-AG, treatment outcome, cannabis use, anxious arousal, avoidance, trait anxiety, depression
Introduction
During military deployment exposure to traumatic situations can lead to the development of Post-Traumatic Stress Disorder (PTSD). PTSD is characterized by intrusive re-experiencing, avoiding reminders of the traumatic event, hyperarousal and alterations in mood and cognition. 1 European prevalence rates demonstrated that approximately 9% of soldiers develop PTSD symptomatology six months after returning from military deployment.2,3 Although there is a large number of effective treatments for PTSD 30–50% of patients does not adequately benefit from these therapies.4–6 Neurobiological mechanisms that are relevant to the etiology of PTSD are linked to the endocannabinoid system (ECS) and are suggested as a novel target for the development of pharmacological treatments besides current PTSD treatments.7,8
The ECS is a lipid signaling system that plays an important role in the regulation of stress, depressive and anxiety like behavior. 9 It also acts as a feedback loop to inhibit responses of the HPA-axis to stressors.10,11 The ECS consists of the CB1 and CB2 receptor which belong to the class of G protein-coupled receptors. The endogenous ligands that activate the CB1 and CB2 receptors are anandamide (AEA) and 2-arachidonylglycerol (2-AG). 12 AEA and 2-AG, are generated ‘on demand’, eg in reaction to stressful situations, and act in a retrograde manner to regulate neurotransmitter release, primarily through inhibition of GABAergic and glutamatergic neurotransmission.13–16 CB1 receptors are particularly of interest in relation to PTSD and anxiety disorders because they are expressed in most limbic structures in the brain, including the prefrontal cortex, amygdala and hippocampus, which are areas commonly reported as involved in PTSD and fear extinction learning. 17 CB2 receptors are located primarily in peripheral and immune tissues. 18
Preclinical research has demonstrated a crucial role for the ECS in the extinction of aversive memories. 19 Blocking or genetically deleting the CB1 receptor resulted in a failure to extinguish fear.19–21 On the other hand, augmenting endocannabinoid signaling by CB1 agonists or the pharmacological blockade of the enzyme FAAH, that breaks down the endocannabinoid AEA, enhances extinction learning.22,23 First line treatments for PTSD and anxiety disorders are often based on these (fear) extinction learning mechanisms, eg (imaginary) exposure therapy. 24 During treatment patients are repeatedly exposed to the feared stimuli without experiencing actual threat in order to achieve extinction of this fear response. Interestingly, PTSD patients often show an inability to inhibit the intense fear reaction to stimuli that reminds them of their trauma.17,25 PTSD and anxiety disorders are therefore often described as a disorder of learning and memory because of these deficits in extinction and recall of the extinction memory.17,25 Non-response to current treatments is possibly due through deficits in these fear extinction processes. Together these findings suggest that poor response to treatment due to an extinction deficit may be associated with a poorly functioning ECS.
Previous studies have demonstrated that alterations in circulating endocannabinoids levels were indeed associated with PTSD. In individuals who were exposed to the world trade center attacks, circulating 2-AG plasma levels measured 4–6 years after the event were reduced among those who had developed PTSD in comparison to those who did not. 26 Although no differences in plasma AEA levels were found, within the PTSD group AEA levels exhibited a negative relationship with the degree of intrusive symptoms experienced. Another study reported that PTSD was associated with reduced AEA levels accompanied by an upregulation of CB1 receptors within the amygdala-hippocampal-cortico-striatal neural circuit, compared with a trauma and healthy control group. 27 This might indicate a compensatory upregulation of CB1 receptors in PTSD due to low receptor occupancy by AEA. However, opposite or null effects were also reported on differences in AEA and 2-AG levels between PTSD patients and controls.14,28
So far, studies have mainly focused on endocannabinoid levels in individuals with PTSD, and hardly on the predictive value of these levels on treatment outcome. Since preclinical studies suggests that extinction processes depend on endocannabinoid signaling, higher pretreatment endocannabinoid levels might be associated with better treatment outcome in PTSD. Insight into the predictive value of endocannabinoid levels on treatment outcome can give more insight into the potential role of augmenting this system (eg with cannabidiol or FAAH inhibitors), and hence inform the development of novel pharmacotherapy aimed at the ECS in the treatment of PTSD and anxiety related disorders.
In the current study blood plasma concentration were analyzed from war veterans with and without PTSD diagnosis. Veterans with a PTSD diagnosis who received trauma-focused therapy were assessed before and after 6–8 months of treatment (treatment as usual). This treatment consisted of cognitive behavioral therapy with exposure (tfCBT) and/or eye-movement desensitization and reprocessing (EMDR). The combat control group did not receive treatment during this interval. The primary aim of this study was to examine if pretreatment endocannabinoid levels in individuals with PTSD can predict treatment outcome. Additionally, we examined whether baseline endocannabinoid levels (AEA and 2-AG) differed between PTSD and combat controls to replicate finding from previous studies. We hypothesized that PTSD patients demonstrate lower baseline endocannabinoid levels compared to controls, and that higher pretreatment endocannabinoid levels are associated with larger PTSD symptom reduction within the patient sample. Our secondary aim was to explore if pre- and posttreatment endocannabinoid levels are associated with specific pre- and post-treatment PTSD symptom clusters (re-experiencing, avoidance and hyperarousal), state and trait anxiety, and anxiety and mood symptoms (depressive symptoms, anxious symptoms, mixed symptoms, anxious arousal and anhedonic depression).
Method
Participants
Subjects that were included in the study were part of a larger study on the neurobiological mechanism underlying the recovery of PTSD. 29 Individuals diagnosed with PTSD (n = 57) were recruited from one of the four outpatient clinics of the Military Mental Healthcare, Ministry of Defence, The Netherlands. Veterans without a current psychiatric illness (combat controls; n = 29) were recruited through advertisements. Subjects were included in the study if they were active duty military or veterans who in the past participated in a military deployment for a duration of at least 4 months and who were aged between 18 and 60 years at time of the study. Subjects were excluded in case of substance abuse and/or substance dependency during the study. All measurements were assessed twice within a 6–8 month interval. In between the two assessments PTSD patients received trauma-focused therapy (treatment as usual) consisting of cognitive behavioral therapy with exposure (tfCBT) and/or eye-movement desensitization and reprocessing (EMDR). The study was approved by the Medical Ethics Committee of the University Medical Center Utrecht (09/314) and conducted in accordance with the Declaration of Helsinki. All participants provided verbal and written informed consent before screening and participation in the study.
Clinical Evaluation
PTSD symptom severity was assessed using the Clinician Administered PTSD Scale for DSM-IV (CAPS-IV) which was administered by a trained researcher. 30 Participants with a PTSD diagnosis were included when the total score on the CAPS was 45 or above. 31 Controls were included if they had a total CAPS score of ≤15 and no current psychiatric disorder. 30 In addition, the Structural Clinical interview for DSM-IV axis I disorders (SCID-I) 32 was conducted to access comorbid psychiatric disorders. Additionally, in the SCID-I interview cannabis use was probed: ‘Did you ever used cannabis during your lifetime.’ The interview asked people to describe the period of the heaviest substance use (age, date and duration). This period could then be divided in “substance never used”, “used substance less than 10 times a month” or “used substance at least 10 times a month or substance dependency.” However, since the description of the period of use was not clearly documented in the interviews we chose to score this question on cannabis use during someone's lifetime as “yes” or “no”.
Questionnaires
Questionnaires were assessed both pre- and posttreatment. State and trait anxiety were assessed with the State-Trait Anxiety Inventory (STAI-DY). 33 The Early Trauma Inventory (ETI) was used to assess traumatic experiences during childhood. 34 Lastly, The Mood and Anxiety Symptom Questionnaire (MASQ) was used to measure depressive, anxious, and mixed symptoms symptomatology. 35 The questionnaire consists of three scales, one scale that measures general distress (depressive symptoms, anxious symptoms and mixed symptoms), one scale that measures anxiety symptoms (anxious arousal) and one scale that measures depression symptoms (anhedonic depression). Additionally, data was collected about medication use (use during the time of the study visits) and cigarette use (average per week).
Endocannabinoid and Cortisol Measurements
Blood samples were taken both pre- and posttreatment between 08.00 and 11.00 AM All blood samples were collected between 2010–2013. After collection of the blood samples, they were immediately centrifuged to extract plasma and frozen at − 80 °C. In 2019 AEA and 2-AG levels were determined. Anandamide levels were determined using the AEA ELISA (abx258779, Abbexa Ltd, Cambridge, UK). The lower limit of detection was 3 ng/mL. Intra-assay variation at 60 ng/mL was 3.2%. Inter-assay variation at 60 ng/mL was 8.8%. Arachidonoylglycerol levels were determined using the 2-AG ELISA (abx258337, Abbexa Ltd, Cambridge, UK). The lower limit of detection was 4 ng/mL. Intra-assay variation at 95 ng/mL was 8.4%. Inter-assay variation at 95 ng/mL was 11.0%. Additionally, we determined cortisol levels because of the role of the ECS in mediating the actions of glucocorticoids. 10 Cortisol was measured in one batch using an electrochemiluminescence immunoassay on the Modular E411 (Cortisol II, Roche Diagnostics GmbH, D-68298 Mannheim, Germany). The lower limit of detection was 2 nmol/L and intra assay variation was <2.4% at 100–900 nmol/L respectively.
Statistical Analysis
Pre- and post-plasma levels of AEA, 2-AG and cortisol levels were first compared between individuals with PTSD and combat controls, using a mixed model analysis of variance (mixed ANOVA) for AEA, 2-AG and cortisol separately. In this analysis the factor Group (PTSD and combat controls) was the between-subjects factor and Time (pre- and post-plasma concentrations) the within-subject factor. Subsequently the PTSD subjects were divided into treatment responders and non-responders. Based on previous studies treatment responders were conceptualized to have a reduction in CAPS scores of ≥30% posttreatment.36,37 To investigate differences in pre- and post-plasma concentration between combat controls, treatment responders, and non-responders we used a mixed ANOVA with a three level between-subject factor Group. The aforementioned analysis was also performed including age, comorbidity (depression and anxiety), medication use (SSRIs and Benzodiazepines), childhood trauma, cigarette use, units of alcohol the day before blood sampling, and cannabis as separate covariates to the model. It was not possible to analyze type of treatment (tfCBT and EMDR) since most participants had received a combination of these treatments. Linear regression analysis was used to test whether pretreatment endocannabinoids levels (AEA and 2-AG) and cortisol in the PTSD group could predict symptom reduction. Symptom reduction was conceptualized as percentage reduction in symptoms from pre- to posttreatment. Lastly, bivariate correlations were performed to explore the relationship between pretreatment plasma levels and pretreatment CAPS symptom clusters (re-experiencing, avoidance, hyperarousal); state and trait anxiety; and the MASQ subscales (anxious arousal, anhedonic depression, depressive symptoms, anxious symptoms and mixed symptoms). The aforementioned bivariate correlation analyses were also performed to explore this relationship at posttreatment levels and clinical symptoms. The p-values from the bivariate correlations were corrected for multiple testing by using the Benjamini-Hochberg procedure. 38 All analyses were carried out with IBM SPSS Statistics (version 25) and p < 0.05 was considered statistically significant.
Results
Demographic and Clinical Characteristics
Clinical characteristics of the included sample (N = 80) are displayed in Table 1. Groups did not differ on any of these clinical characteristics, except, as expected, on state and trait anxiety, comorbid disorders (depression and anxiety), medication use (SSRIs and Benzodiazepines), CAPS and MASQ scores. Five participants were removed because of plasma levels that were far above the expected range. The only female participant was excluded because of known gender differences with regard to ECS activity. 39
Table 1.
Demographic and Clinical Characteristics of PTSD Patient and Combat Controls (N = 80).
| Combat Control (n = 26) | PTSD (n = 54) | Test statistic | p-value | |
|---|---|---|---|---|
| mean (sd) or n (%) | mean (sd) or n (%) | |||
| Age (in years) | 36.65 (9.61) | 36.33 (10.02) | F = .018 | .892 |
| Education Level (ISCED) | 5.31 (1.81) | 5.26 (1.43) | F = .014 | .908 |
| Number of missions | 2.42 (1.45) | 2.65 (2.95) | F = .141 | .708 |
| Early traumatic experiences | 3.13 (3.01) | 5.23 (4.73) | F = 3.777 | .056 |
| State anxiety | 30.70 (6.92) | 54.15 (9.52) | F = 110.638 | <.001 * |
| Trait anxiety | 31.78 (4.77) | 52.26 (7.97) | F = 128.588 | <.001 * |
| Substance use 1 | ||||
| Cannabis use (ever during life) | 12 (63.2%) | 27 (50.9%) | X2 = .840 | .359 |
| Cigarettes (average per week) | 4.56 (14.24) | 4.73 (6.94) | F = .005 | .946 |
| Comorbid disorders (number) 1 | ||||
| Depression current | 0 (0%) | 30 (55.6%) | X2 = 23.111 | <.001 * |
| Anxiety disorder current | 0 (0%) | 18 (33.3%) | X2 = 11.183 | .001 * |
| Alcohol dependence | 0 (0%) | 2 (4.1%) | X2 = 1.049 | .306 |
| Medication 1 | ||||
| SSRI | 0 (0%) | 15 (28.3%) | X2 = 9.083 | .003 * |
| BENZO's | 0 (0%) | 10 (18.9%) | X2 = 5.617 | .018 * |
| PTSD symptoms | ||||
| Re-experiencing (CAPS B) | 0.62 (1.20) | 23.56 (5.05) | F = 519.951 | <.001 * |
| Avoiding (CAPS C) | 1.04 (2.31) | 23.44 (9.55) | F = 138.434 | <.001 * |
| Hyperarousal (CAPS D) | 3.08 (3.14) | 24.63 (4.65) | F = 457.323 | <.001 * |
| Total (CAPS TOTAL) | 4.73 (4.81) | 71.63 (12.89) | F = 653.021 | <.001 * |
| MASQ | ||||
| Anhedonic Depression | 45.87 (8.95) | 76.23 (12.17) | F = 112.963 | <.001 * |
| Anxious Arousal | 21.61 (8.46) | 38.11 (12.52) | F = 32.560 | <.001 * |
| General Distress Depression | 16.65 (8.12) | 29.94 (9.15) | F = 34.950 | <.001 * |
| General Distress Anxiety | 14.65 (4.91) | 29.00 (7.67) | F = 66.820 | <.001 * |
| General Distress Mixed | 22.91 (8.76) | 45.53 (9.17) | F = 96.636 | <.001 * |
ISCED = International Standard Classification of Education.
MASQ = Mood & Anxiety Symptom Questionnaire.
CAPS = Clinician Administered PTSD Scale.
Because of missing data values and percentages will not always equal the total sample size.
Significant with a p < .05.
Endocannabinoid and Cortisol Levels in PTSD and Combat Controls
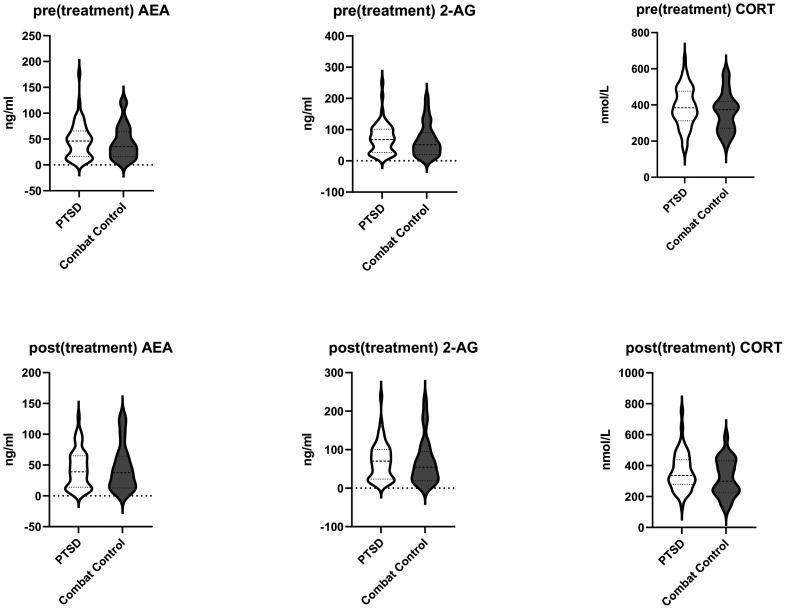
The mixed ANOVA for differences in AEA levels (see Table 2 and Figure 1 for data) demonstrated no effect of Group, F(1,71) = .004, p = .947, Time F(1,71) = .025, p = .874, or Time × Group interaction, F(1,71) = 1.388, p = .243. For 2-AG levels no main or interaction effects were found either (Group F(1,71) = .085, p = .771; Time F(1,71) = .789, p = .377; Time × Group F(1,71) = 1.021, p = .316). A main effect of time for cortisol was observed, F(1,71) = 4.313, p = .041, partial η2 = .06, reflecting that cortisol levels decreased over time. Finally, there was no main effect of group F(1,71) = 1.668, p = .201 or Time × Group interaction F(1,71) = .274, p = .603 for cortisol levels. The mixed ANOVA for differences in AEA levels and 2-AG levels was then tested with the addition of several covariates tested independently of each other. Out of these age, comorbidity (depression and anxiety), medication (SSRIs and Benzodiazepines), early childhood experience, units of alcohol the day before blood sampling, and cigarettes per week were tested (see supplementary data Table S1), but these covariates did not change the tested model, so none of these were retained in further analyses.
Table 2.
Pre and Post Endocannabinoid and Cortisol Levels for PTSD and Combat Controls.
| PTSD (n = 54) | Combat Controls (n = 26) | ||||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| AEA (ng/ml) | Pretreatment | 45.81 | 35.10 | 43.27 | 33.08 |
| Posttreatment | 44.08 | 31.01 | 45.53 | 36.76 | |
| 2-AG (ng/ml) | Pretreatment | 70.61 | 50.35 | 64.75 | 51.60 |
| Posttreatment | 70.35 | 48.00 | 68.88 | 58.64 | |
| Cortisol (nmol/L) | Pretreatment | 381.63 | 111.61 | 355.38 | 108.09 |
| Posttreatment | 361.27 | 121.02 | 321.29 | 119.26 |
Figure 1.
Pre- and post(treatment) endocannabinoid and cortisol levels for PTSD and combat controls.
Cannabis use Associated with Post AEA, and Pre and Post 2-AG Levels
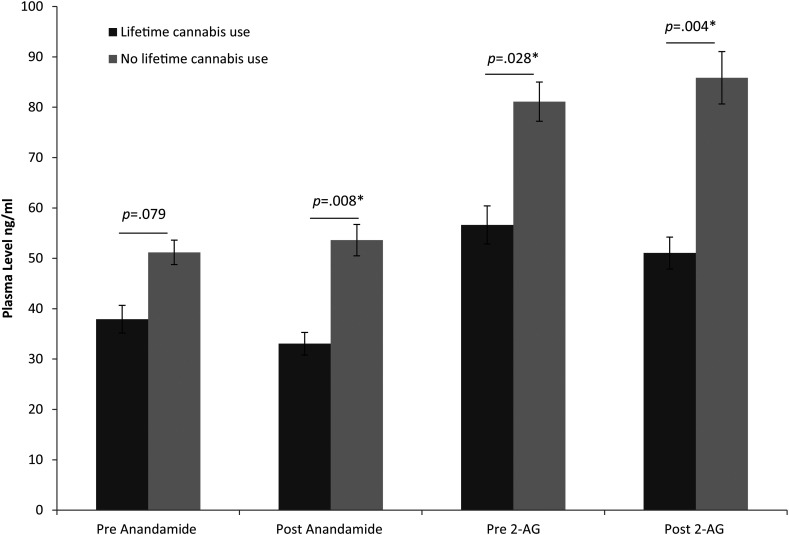
Adding Cannabis use as a covariate to the previous tested model resulted in a significant effect of Cannabis use (yes or no) on both AEA (F(1,62) = 4.780, p = .033, partial η2 = .07) and 2-AG levels (F(1,62) = 6.559, p = .013, partial η2 = .09). Furthermore, there was a significant Time × Cannabis use effect for both AEA (F(1,62) = 4.649, p = .035, partial η2 = .07) and 2-AG (F(1,62) = 4.879, p = .031, partial η2 = .07). Simple effect analysis revealed that pre(treatment) AEA did not differ between the Cannabis use groups, F(1,71) = 3.184, p = .79, contrary to post(treatment) AEA levels (F(1,64) = 7.541, p = .008, partial η2 = .11). Pre(treatment) 2-AG levels (F(1,71) = 5.033, p = .028, partial η2 = .07; and post(treatment) 2-AG levels F(1,64) = 8.841, p = .004, partial η2 = .12) also differed between the Cannabis use groups, in which lifetime cannabis users demonstrated lower levels in comparison to individuals who never used cannabis during their life, see also Figure 2. This suggests that cannabis use was associated with lower post AEA, and lower pre and post 2-AG levels independent of PTSD diagnosis.
Figure 2.
Post-Hoc test on mean difference and SEM on pre and post AEA and 2-AG levels between individuals who reported to have used cannabis during their lifetime and non-cannabis users, independent of PTSD diagnosis. *Significant with a p < .05.
No Differences in Endocannabinoid Levels Between Combat Controls, Treatment Responders and Treatment Non-Responders
The groups were divided into combat controls, treatment responders and non-responders to investigate differences in endocannabinoid levels between these three groups. Clinical characteristics of the differentiation are displayed in the supplementary data Table S2. The mixed ANOVA analysis demonstrated the same pattern of effects as the analysis with the 2 groups differentiation (combat controls and PTSD). For mean scores and standard deviations see supplementary data Table S3 and for the covariate analysis see supplementary data Table S4.
Pretreatment Endocannabinoid Levels are Not Predictive of Symptom Reduction
AEA, 2-AG and cortisol levels were added separately into a regression model to investigate their predictive value on treatment success. Because cannabis use has an influence (of effect) on endocannabinoid levels we also added this variable into our regression model. Regression analysis demonstrated that neither pretreatment AEA (F(2,45) = 1.222, p = .304), 2-AG (F(2,45) = .986, p = .381) nor cortisol (F(2,45) = .035, p = .965) were able to predict percentage symptom reduction in PTSD symptomatology as measured with the CAPS.
Pretreatment 2-AG Levels are Associated with Anxious Arousal and Avoidance, and Posttreatment AEA and 2-AG Levels are Associated with Trait Anxiety, General Distress Depression and Anxious Arousal
Correlations between endocannabinoid levels (AEA and 2-AG), cortisol and clinical symptoms (CAPS subscales, STAI and MASQ) were only investigated in the PTSD group (see supplementary data Table S5, S6 and S7), because of predominantly low scores for the combat controls on these questionnaires. The correlations were tested for both pre- and post-treatment levels and pre- and post-treatment clinical symptoms. Only the associations that survived the Benjamini-Hochberg procedure are reported, for the other correlations see supplementary data Table S5, S6 and S7.
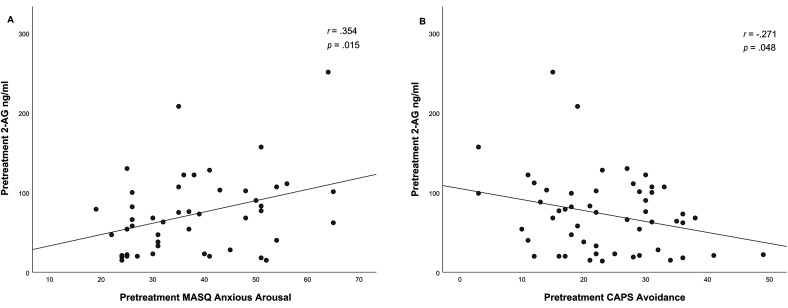
For the analysis on pre-treatment 2-AG and pre-treatment clinical symptoms the association between pretreatment 2-AG and pretreatment MASQ anxious arousal symptoms (r(45) = .354, p = .015. and pretreatment 2-AG and pretreatment CAPS avoidance symptoms (r(52) = -.271, p = .048) were significant, see Figure 3. This suggests that elevated 2-AG levels in PTSD are associated with higher anxious arousal and with less avoidance symptoms.
Figure 3.
Correlation between pretreatment 2-AG levels and the pretreatment MASQ anxious arousal subscale (A) and correlations between pretreatment 2-AG and pretreatment CAPS avoidance symptoms (B) in PTSD patients.
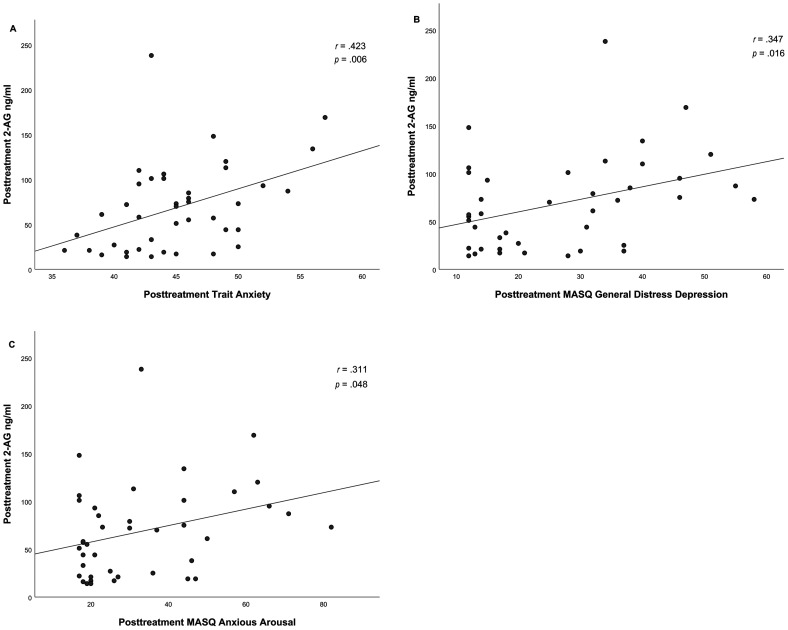
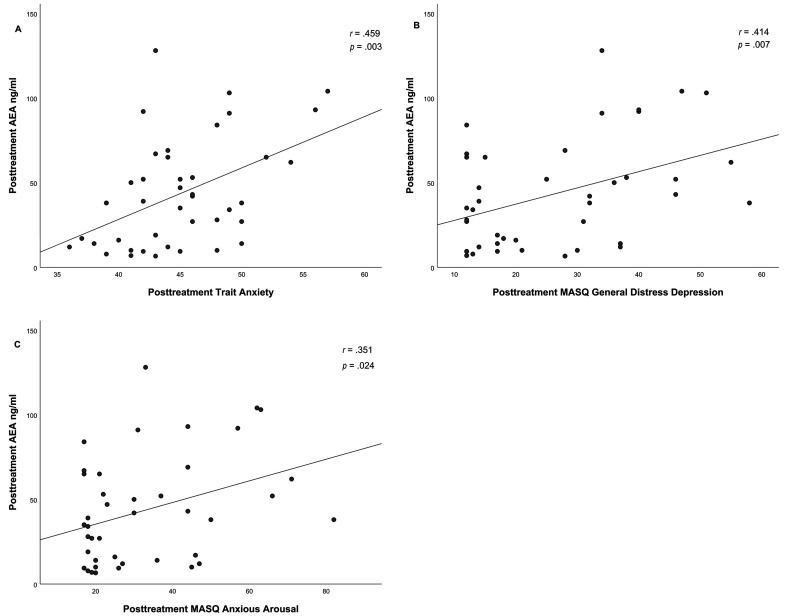
Additionally posttreatment 2-AG levels demonstrated positive correlations with posttreatment trait anxiety (r(41) = .423, p = .006), general distress depression (r(41) = .374, p = .016) and anxious arousal (r(41) = .311, p = .048), see Figure 4.
Figure 4.
Correlation between posttreatment 2-AG levels and posttreatment trait anxiety (A), posttreatment MASQ general distress depression (B) and posttreatment MASQ anxious arousal (C) PTSD patients.
The same pattern was demonstrated between posttreatment AEA levels and posttreatment trait anxiety (r(41) = .459, p = .003), general distress depression (r(41) = .414, p = .007) and anxious arousal (r(41) = .351, p = .024), see Figure 5.
Figure 5.
Correlation between posttreatment AEA levels and posttreatment trait anxiety (A), posttreatment MASQ general distress depression (B) and posttreatment MASQ anxious arousal (C) PTSD patients.
Discussion
Our study indicated that baseline endocannabinoid levels (AEA and 2-AG) did not differ between PTSD and combat controls nor between combat controls, treatment responders, and non-responders. Pretreatment endocannabinoid levels were also not predictive of treatment induced PTSD symptom reduction. However, our findings indicated that endocannabinoid levels were reduced in individuals who reported to have used cannabis during their life, independent of PTSD diagnosis. Lastly, pretreatment 2-AG levels in PTSD were observed to be associated with pretreatment anxious arousal and avoidance symptoms. Additionally, both posttreatment AEA and 2-AG levels were associated with posttreatment trait anxiety, general distress depression and anxious arousal.
Contrary to our expectations no baseline differences in endocannabinoid levels were found between combat controls and PTSD patients. Additionally, endocannabinoids remained stable over time, eg no differences between pre- and post-endocannabinoid levels. To date, most studies reported contradictory findings, namely either a reduction or an increase, or no differences in baseline endocannabinoid levels in PTSD patients compared to controls.26–28 Preclinical and clinical studies suggest that endocannabinoids are recruited in the brain during stress, which supports to terminate the stress response.10,16 Chronic elevation of these endocannabinoid levels might lead to a downregulation of endocannabinoid signaling. This could explain the reduction in endocannabinoid levels reported in some studies. On the other hand, it may not be the case that such chronic stress and corresponding endocannabinoid reactivity leads to ECS downregulation, which could explain studies that reported an increase in endocannabinoid levels in PTSD. 28
Our findings, however, correspond with the studies that reported no baseline differences in endocannabinoid levels between PTSD and controls.14,40 In addition one of these studies reported that in contrast to healthy controls, psychosocial stress did not induce an increase in 2-AG levels is PTSD. 14 This suggests an alternative explanation in which PTSD may be associated with an unresponsiveness of the ECS in reaction to stressful situations, also supported by data from healthy individuals.15,16 An increase in endocannabinoid levels during stress has been observed in individuals who successfully adapt to stressful situations, in contrast to those who displayed a maladaptive stress response.15,16 A possible cause that we found no differences in endocannabinoid levels is that they were measured at baseline instead of during a stress induction. More research is needed to gain further insight into which factors play a role in the elevation or reduction in baseline endocannabinoid levels and the reactivity of the ECS under different forms of stress in PTSD patients.
Given the important role that the ECS and specifically AEA has during extinction learning,41,42 we hypothesized that lower endocannabinoid levels prior to trauma-focused therapy could possibly predict reduced treatment effect, ie, less treatment induced reduction of symptoms. However, our study did not demonstrate a relationship between endocannabinoid levels and treatment success, possibly because our study has measured endocannabinoid levels during baseline conditions. Fear reduction during extinction depends on first activating this fear, and with that potentially also the ECS. This may explain why baseline endocannabinoid levels that we compared between combat controls, treatment responders and non-responders in our study were not representative of the level of ECS activation in patients during treatment. By measuring endocannabinoid levels during both baseline conditions and during fear extinction, future studies can elucidate possible difference in reactivity of the system during fear extinction.
With post-hoc analysis we observed that reduced endocannabinoid levels were associated with people who used cannabis occasionally during their life, independent of PTSD diagnosis. It is known that chronic cannabis use is associated with CB1 receptor desensitization and down-regulation of endocannabinoid signaling. 43 Reduction of endocannabinoid levels in chronic cannabis users have been explained by the impact of externally administered cannabinoids that cause the ECS to adapt. In our study, we observed that also more occasional use of cannabis is associated with lower endocannabinoid levels. In our study individuals with a diagnosis of cannabis use disorder or active users at the time were excluded, which makes it less likely that adaptation of the ECS could explain the findings. An alternative explanation could be that the difference between user and non-user already existed before starting to use cannabis. Future studies need to elucidate the association between occasional use of cannabis and the ECS.
The exploratory correlation analysis between clinical symptoms and endocannabinoid levels indicated two moderate associations with pretreatment 2-AG levels. Pretreatment endocannabinoid levels in the PTSD group were positively associated with pretreatment anxious arousal subscale of the MASQ, and negatively correlated with the pretreatment avoidance subscale of the CAPS. When focusing on posttreatment levels, six moderate associations were revealed, namely three with posttreatment 2-AG levels and three with posttreatment AEA. Both 2-AG and AEA were positively associated with trait anxiety, general distress depression and anxious arousal as measured with the MASQ. Anxious arousal is characterized by somatic symptoms (eg sweating, racing heart and muscle tension) and exaggerated physiological responses to stressful events.44,45 A study 46 indicated that in patients with panic disorder 2-AG correlated with different measures of panic and anxiety (bodily sensations and agoraphobic cognitions), which is in line with our findings. This relationship has also been confirmed by an animal study demonstrating a crucial role for 2-AG and not AEA in panic symptoms. 47 The negative association between 2-AG and avoidance behavior is contrary to a previous study that reported a positive correlation between 2-AG and avoidance behavior in PTSD patients. 26 The positive correlation with general depression symptoms is also contrary to previous studies.48,49 Since these aforementioned findings are a result of exploratory analysis, interpretation must be with caution and future research is needed to elucidate the association between AEA, 2-AG and clinical symptomatology.
Our study has a couple of limitations that must be addressed. Firstly, endocannabinoid levels were assessed on single time points which makes the interpretation difficult because of known impact of circadian rhythm on endocannabinoid levels. 50 Although it must be noted that all blood draws were taken place between 08:00–11:00 and differences in endocannabinoid levels were previously found based on a single time point.26–28 Furthermore, our study indicated that overall, endocannabinoid levels remained stable over time. Multiple time point and additional reactivity measures on the ECS could contribute to our understanding of the ECS. Secondly, in our study plasma levels were determined with the enzyme-linked immunosorbent assay method (ELISA). Most of the previous studies made use of chemical ionization liquid chromatography/mass spectrometry (LC-APCI-MS) to quantify AEA and 2-AG levels. Although they are both reliable and valid methods in determining AEA and 2-AG levels, ranges for these two approaches differ which can make it difficult to compare results between these methods. Thirdly, information about frequency and duration of cannabis use from the SCID-I interview was not documented. Therefore, this study only allowed the distinction between whether or not cannabis had been used before, and no detailed information on frequency and duration of cannabis use was available. Future studies in which the frequency and duration of cannabis use is archived more precisely may shed more light on how this is related to AEA and 2-AG levels. Lastly, our study consists of a male population and findings may not be generalized. For example, women generally show a higher CB1 receptor availability and AEA levels already under basal conditions, which is also confirmed in animal research. 27 Additionally, our sample consisted only of male participants which can also explain why we did not replicate the results from earlier studies which consisted of 50–80% females.14,26–28
Conclusion
Our study did not find indications that pretreatment endocannabinoid levels are associated with either PTSD or treatment outcome in PTSD patients. Furthermore, our findings confirm earlier findings that cannabis use is associated with reduced endocannabinoid levels. Lastly, pretreatment 2-AG in PTSD was associated with pretreatment anxious arousal and avoidance symptoms. Furthermore both posttreatment 2-AG and AEA was associated with posttreatment trait anxiety, general distress depression and anxious arousal. However, further research is needed to obtain more insights in these relations. Since endocannabinoids are mainly generated ‘on demand’ future work will benefit from investigating endocannabinoid circulation under both rest and stressful conditions. This will lead to a better understanding about how the ECS (dys)functions under stressful conditions and during extinction therapy sessions.
Supplemental Material
Supplemental material, sj-docx-1-css-10.1177_24705470221107290 for The Role of the Endocannabinoids 2-AG and Anandamide in Clinical Symptoms and Treatment Outcome in Veterans with PTSD by N.A. Leen, A.D. de Weijer, S.J.H. van Rooij, M. Kennis, J.M.P. Baas and E. Geuze in Chronic Stress
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ministerie van Defensie,
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
ORCID iDs: N.A. Leen https://orcid.org/0000-0002-8605-0863
Trial Registration: Not applicable, because this article does not contain any clinical trials.
Supplemental material: Supplemental material for this article is available online.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Eekhout I, Reijnen A, Vermetten E, et al. Post-traumatic stress symptoms 5 years after military deployment to Afghanistan: an observational cohort study. Lancet. 2016;3(1):58–64. [DOI] [PubMed] [Google Scholar]
- 3.Reijnen A, Rademaker AR, Vermetten E, et al. Prevalence of mental health symptoms in Dutch military personnel returning from deployment to Afghanistan: a 2-year longitudinal analysis. Eur. Psychiatry. 2015;30(2):341–346. [DOI] [PubMed] [Google Scholar]
- 4.Bradley R, Greene J, Russ E, et al. A multidimensional meta-analysis of psychotherapy for PTSD. Am. J. Psychiatry. 2005;162(2):214–227. [DOI] [PubMed] [Google Scholar]
- 5.Watts BV, Schnurr PP, Mayo L, et al. Meta-Analysis of the efficacy of treatments for posttraumatic stress disorder. J. Clin. Psychiatry. 2013;74(6):e541–e550. [DOI] [PubMed] [Google Scholar]
- 6.Stein DJ, Ipser JC, Seedat S, Sager C, et al. Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2006;(1): CD002795. DOI: 10.1002/14651858.CD002795.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neumeister A, Seidel J, Ragen BJ, et al. Translational evidence for a role of endocannabinoids in the etiology and treatment of posttraumatic stress disorder. Psychoneuroendocrinology. 2015;51:577–584. 10.1016/j.psyneuen.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill MN, Campolongo P, Yehuda R, et al. Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology. 2018;43(1):80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill MN, Patel S. Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses. Biol. Mood Anxiety Disord. 2013;3(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(5):791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience 2012 Mar 1;204:5–16. doi: 10.1016/j.neuroscience.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002 Aug;68–69:619–631. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- 13.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 14.Crombie KM, Leitzelar BN, Brellenthin AG, et al. Loss of exercise- and stress-induced increases in circulating 2-arachidonoylglycerol concentrations in adults with chronic PTSD. Biol Psychol. 2019 Jul;145:1–7. doi: 10.1016/j.biopsycho.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Strewe C, Feuerecker M, Nichiporuk I, et al. Effects of parabolic flight and spaceflight on the endocannabinoid system in humans. Reviews in the Neuroscience. 2012;23(5–6):673–680. 10.1515/revneuro-2012-0057 [DOI] [PubMed] [Google Scholar]
- 16.Choukèr A, Kaufmann I, Kreth S, et al. Motion sickness, stress and the endocannabinoid system. PLoS One. 2010;5(5):e10752. doi: 10.1371/journal.pone.0010752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VanElzakker MB, Dahlgren MK, Davis FC, et al. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem. 2014 Sep;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berdushev EV. Cannabinoid receptors and the regulation of immune response. Chem Phys Lipids. 2000;108(1–2):169–190. [DOI] [PubMed] [Google Scholar]
- 19.Marsicano G, Wotjak CT, Azad SC, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418(6897):530–534. [DOI] [PubMed] [Google Scholar]
- 20.Varvel SA, Num EA, Lichtman AH. Disruption of CB1 receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology. 2005;179(4):863–872. [DOI] [PubMed] [Google Scholar]
- 21.Niyuhire F, Varvel SA, Thorpe AJ, et al. The disruptive effects of the CB1 receptor antagonist rimonabant on extinction learning in mice are task-specific. Psychopharmacology. 2007;191(2):223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur. Neuropsychopharmacol. 2008;18(12):849–859. [DOI] [PubMed] [Google Scholar]
- 23.Gunduz-Cinar O, MacPherson K, Cinar R, et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry. 2013;18(7):813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNally RJ. Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clin. Psychol. Rev. 2007;27(6):750–759. [DOI] [PubMed] [Google Scholar]
- 25.Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry. 2009;66(12):1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill MN, Bierer LM, Makotkine I, et al. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the world trade center attacks. Psychoneuroendocrinology. 2013;38(12):2952–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumeister A, Normandin MD, Pietrzak RH, et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol. Psychiatry. 2013;18(9):1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauer D, Schelling G, Gola H, et al. Plasma concentrations of endocannabinoids and related primary fatty acid amides in patients with post-traumatic stress disorder. PLOS ONE. 2013;8(5):e62741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Rooij SJH, Kennis M, Sjouwerman R, et al. Smaller hippocampal volume as a vulnerability factor for the persistence of post-traumatic stress disorder. Psychol. Med. 2015;45(13):2737–2746. [DOI] [PubMed] [Google Scholar]
- 30.Blake D, Weathers F, Nagy L, et al. Clinican rating scale for assessing current and lifetime PTSD: the CAPS-1. Behav Ther. 1990;13(1):187–188. [Google Scholar]
- 31.Weathers F, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the clinician-administered posttraumatic stress disorder scale. Psychol Assess. 1999;11(2):124–133. [Google Scholar]
- 32.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders. SCID-I/P 1997.
- 33.Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists; 1983. [Google Scholar]
- 34.Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the early trauma inventory. Depress Anxiety. 2000;12(1):1–12. [DOI] [PubMed] [Google Scholar]
- 35.Clark LA, Watson D. A tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J. Abnorm. Psychol. 1991;100(3):316–336. [DOI] [PubMed] [Google Scholar]
- 36.Brady K, Pearlstein T, Asnis GM, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283(14):1837–1844. [DOI] [PubMed] [Google Scholar]
- 37.Davidson JT, Rothbaum BO, van der Kolk BA, et al. Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry. 2001;58(5):485–492. [DOI] [PubMed] [Google Scholar]
- 38.Benjamini Y, Hochberg J. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B. 1995;57(1):289–300. [Google Scholar]
- 39.Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res. 2009;203(2):264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaefer C, Enning F, Mueller JK, et al. Fatty acid ethanolamide levels are altered in borderline personality and complex posttraumatic stress disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2014;264(5):459–463. [DOI] [PubMed] [Google Scholar]
- 41.Mayo LM, Asratian A, Lindé J, et al. Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase: a randomized, controlled experimental medicine trial. Biol. Psychiatry. 2020;87(6):538–547. [DOI] [PubMed] [Google Scholar]
- 42.Spohrs J, Ulrich M, Grön G, et al.. Fear extinction learning and anandamide: An fMRI study in healthy humans. Transl Psychiatry. 2021 Mar 15;11(1):161. doi: 10.1038/s41398-020-01177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.González A, Cebeira M, Fernández-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol. Biochem. Behav. 2005 ;8(2):300–318. [DOI] [PubMed] [Google Scholar]
- 44.Nitschke JB, Heller W, Palmieri PA, et al. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36(5):628–637. [PubMed] [Google Scholar]
- 45.Finn AN, Sawyer CR, Behnke RR. A model of anxious arousal for public speaking. Commun. Educ. 2009;58(3):417–432. [Google Scholar]
- 46.Petrowski K, Kirschbaum C, Gao W, et al. Blood endocannabinoid levels in patients with panic disorder. Psychoneuroendocrinology. 2020 Dec;122:104905. doi: 10.1016/j.psyneuen.2020.104905. [DOI] [PubMed] [Google Scholar]
- 47.Viana TG, Bastos JR, Costa RB, et al. Hypothalamic endocannabinoid signalling modulates aversive responses related to panic attacks. Neuropharmacology. 2019 Apr;148:284–290. doi: 10.1016/j.neuropharm.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 48.Hill MN, Miller GE, Ho WSV, et al. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41(2):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill MN, Miller GE, Carrier EJ, et al. Circulating endocannabinoids and N-acylethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009;34(8):1257–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanlon EC. Impact of circadian rhythmicity and sleep restriction on circulating endocannabinoid (eCB) N-arachidonoylethanolamine (anandamide). Psychoneuroendocrinology. 2020 Jan;111:104471. doi: 10.1016/j.psyneuen.2019.104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-css-10.1177_24705470221107290 for The Role of the Endocannabinoids 2-AG and Anandamide in Clinical Symptoms and Treatment Outcome in Veterans with PTSD by N.A. Leen, A.D. de Weijer, S.J.H. van Rooij, M. Kennis, J.M.P. Baas and E. Geuze in Chronic Stress