Abstract
Understanding the development process of male and female mosquitoes provides important basic information for sterile insect release programs and is important for improving other vector control strategies. However, little is known about the molecular mechanisms that distinguish male from female-specific developmental processes in this species. We used IlluminaRNA-seq to identify sex-specific genes during pupal and adult stages. One hundred forty-seven genes were expressed only in pupal males, 56 genes were expressed in adult males, and another 82 genes were commonly expressed in both male samples. In addition, 26 genes were expressed only in the pupal females, 163 genes were found in the adult females, and only one gene was expressed in both female samples. A further qRT-PCR validation of selected genes from the RNA-seq analysis confirmed upregulation of those genes in a sex-specific manner, including: fibrinogen and fibronectin, a zinc finger protein, phospholipase A(2), and a serine protein for female pupae; venom allergen 3, a perlecan, testis-specific serine/threonine-protein kinase 1, testis-specific serine/threonine-protein kinase 6, and cytochrome c-2 for male pupae; a salivary protein, D7 protein precursor, trypsin 7 precursor, D7 protein, and nanos for female adults; and tetraspanin F139, cytosol aminopeptidase, testis-specific serine/threonine-protein kinase 1, a testis-specific serine/threonine-protein kinase 6, and a C-type lectin for male adults. These findings provide insight into the development and physiology of Culex mosquitoes, which will help in the development of more effective control methods for these disease vectors.
Introduction
Mosquitoes are vectors for transmission of human diseases such as West Nile virus (WNV), Zika virus, chikungunya, yellow fever, malaria, and dengue, putting approximately 3 billion people worldwide at risk and causing millions of human deaths (Wilke et al., 2018). The economic impact of such disease outbreaks is also drastic. For instance, a 2012 West Nile Virus outbreak in Texas cost an estimated $47.6 million for vector control, medical costs, and reduced workforce productivity of affected people (Poh et al., 2019). Occurrences of these diseases in the United States are only rising and causing concern, such as the increased dispersal of WNV by the Culex mosquitoes in North America (Gould et al., 2017). Insecticide treatment, the common method for mosquito control today, is not only expensive but also eventually ineffective since mosquito populations develop insecticide resistance in approximately two years (Luz, et al., 2011). Therefore, an effective new strategy of disease vector control is urgently needed.
Recently, along with genetic modification strategies, sterile insect technique (SIT) has gained more attention as a mosquito control strategy. In essence, the SIT releases a mass of sterile males which reduce mosquito populations by mating with native females who then are unable to produce offspring, given that Culex females mate only once, both in the wild and in laboratory conditions (Cator et al., 2021, Kim et al., 2018). This method has successfully suppressed serious insect pest populations from certain regions, including those of the screwworm fly, the fruit fly, and the tsetse fly (Chung et al., 2018). However, effective use of the SIT in mosquito control is impeded by the lack of an adequate method for sorting male from female mosquitos. This problem can be solved through identification of sex-specific genes that could serve as targets to induce male feminization or female masculinization. Ultimately, this method of genetic manipulation or chemical mutagenesis can render sterile male mosquitoes. In addition, inadequate funding for SIT programs, which can be costly against large-scale populations, has, to date, prevented the success of SIT in suppressing mosquito vectors over extended periods of time. Yet this technique offers promise if new technologies that more effectively reduce the high mosquito fecundity are developed or if funding for these programs over longer periods of time will be allocated (Benedict 2021).
In the disease vector mosquito species Anopheles gambiae and Aedes aegypti, several male-specific (Y- or M-linked) gene sequences including Nix are hypothesized to be responsible for expression of masculine traits such as male antennae and genitals (Hall et al., 2015). After searching Illumina sequences from male genomic DNA across different mosquito genera, a homologous gene to the Nix gene could not be found in Culex pipiens mosquitoes (Hall et al., 2015). Yet, it has been shown that the key sex-specific transcription factor, the doublesex protein, shares the same molecular mechanism related to sex-specific developmental process in various insects (Price et al., 2015). Therefore, it is conceivable that downstream genes of the doublesex transcription factor will show similar expression patterns in a sex-specific manner. In this study, we identified genes showing significant differences in expression levels between males and females in pupal and adult developmental stages. Among these genes, nineteen functionally relevant genes were validated through qRT-PCR analysis.
Results
Raw Data Analysis
Illumina HiSeqX sequencing generates raw images utilizing sequencing control software for system control and base calling through an integrated primary analysis software called RTA (Real Time Analysis). The BCL (base calls) binary is converted into FASTQ utilizing Illumina package bcl2fastq. The FASTAQ statistics from each sample are summarized as follows: (1) female pupae, 48,698,276 reads have been produced, and total read bases are 4.9G bp and Q20 is 96.4%; (2) male pupae, 51,063,692 reads have been produced, and total read bases are 5.1G bp and Q20 is 96.9%; (3) female adults, 32,916,095 reads have been produced, and total read bases are 6.6G bp and Q20 is 96.9%; (4) male adults 54,062,230 reads have been produced, and total read bases are 5.4G bp and Q20 is 96.7%.
Bioinformatics Analysis of RNA-seq reads
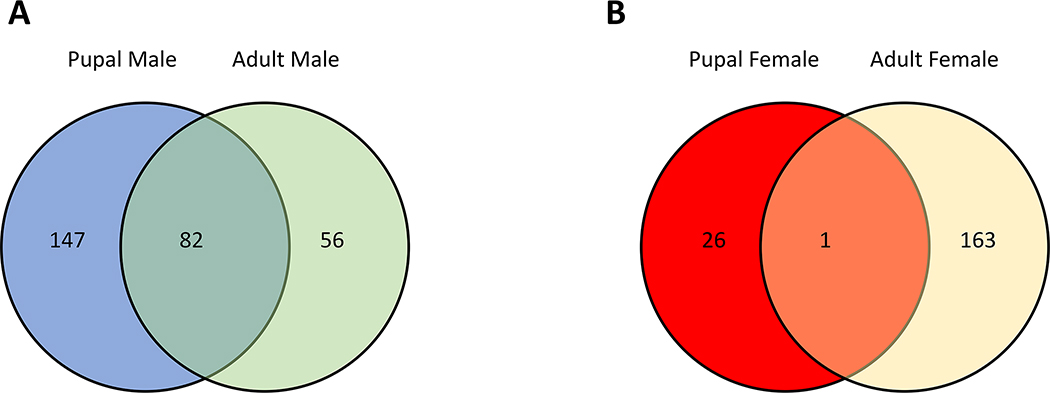
One hundred forty-seven genes were expressed only in pupal males, 56 genes were expressed in adult males, and another 82 genes were commonly expressed in both male samples. In addition, 26 genes were expressed only in the pupal females, 163 genes were found in the adult females, and only one gene was expressed in both female samples (Fig. 1 and Suppl. file 1, 2). Among these, in pupal stage, five male-specific genes and four female-specific genes were chosen for qRT-PCR validation (Table 1). Likewise, in adult stage, five female-specific genes and five male-specific genes were chosen for qRT-PCR validation (Table 2).
Figure 1.
Summary statistics of sex-specific genes from female and male Cx. pipiens (A) Venn diagram of common and unique male-specific genes of pupae and adult Cx. pipiens. (B) Venn diagram of common and unique female-specific genes of pupal and adult Cx. pipiens.
Table 1.
Sex-specific genes in pupal stage from RNA-seq and qRT-PCR analysis.
| Gene name | VectorBase accession number | Locus | Description | HTseq reads count in male pupae | HTseq reads count in female pupae |
|---|---|---|---|---|---|
|
| |||||
| venom | CPIJ004028 | supercont3.57:710052-711002 | Venom allergen 3 | 211 | 0 |
| perlecan | CPIJ004150 | supercont3.64:395817-396360 | Perlecan | 455 | 0 |
| kinase-6 | CPIJ007354 | supercont3.157:555143-556392 | Testis-specific serine/threonine-protein kinase 6 | 79 | 0 |
| cytochrome | CPIJ010388 | supercont3.273:384990-385317 | Cytochrome c-2 | 397 | 0 |
| kinase-1 | CPIJ017995 | supercont3.1194:69648-70580 | Testis-specific serine/threonine-protein kinase 1 | 87 | 0 |
| fibrinogen | CPIJ005843 | supercont3.115:850553-854405 | Fibrinogen and fibronectin | 0 | 68 |
| z-finger | CPIJ006424 | supercont3.128:457331-490530 | Zinc finger protein | 0 | 50 |
| phospholipase | CPIJ010429 | supercont3.267:151596-156038 | Phospholipase A(2) | 0 | 114 |
| serine protease | CPIJ017729 | supercont3.986:48829-54348 | Serine protease | 0 | 66 |
Table 2.
Sex-specific genes in adult stage from RNA-seq and qRT-PCR analysis.
| Gene Name | VectorBase accession number | Locus | Description | HTseq reads count in male adults | HTseq reads count in female adult |
|---|---|---|---|---|---|
|
| |||||
| tetraspanin | CPIJ002008 | supercont3.24:443502-444249 | Tetraspanin F139 | 89 | 0 |
| aminopeptidase | CPIJ003539 | supercont3.48:1146610-1148394 | Cytosol aminopeptidase | 4087 | 0 |
| kinase-6 | CPIJ007354 | supercont3.157:555143-556392 | Testis-specific serine/threonine-protein kinase 6 | 116 | 0 |
| kinase-1 | CPIJ012984 | supercont3.441:347896-348819 | Testis-specific serine/threonine-protein kinase 1 | 102 | 0 |
| c-lectin | CPIJ002079 | supercont3.21:295023-296521 | C-type lectin | 231 | 0 |
| salivary | CPIJ002046 | supercont3.24:1424410-1425153 | Salivary protein | 0 | 537 |
| d7 precursor | CPIJ014551 | supercont3.618:185252-186507 | D7 protein precursor | 0 | 19208 |
| trypsin-7 | CPIJ017964 | supercont3.1053:62790-63689 | Trypsin 7 precursor | 0 | 799 |
| d7 | CPIJ018735 | supercont3.1256:23045-45443 | D7 protein | 0 | 810 |
| nanos | CPIJ011551 | supercont3.340:412618-413450 | Nanos | 0 | 156 |
DAVID Analysis
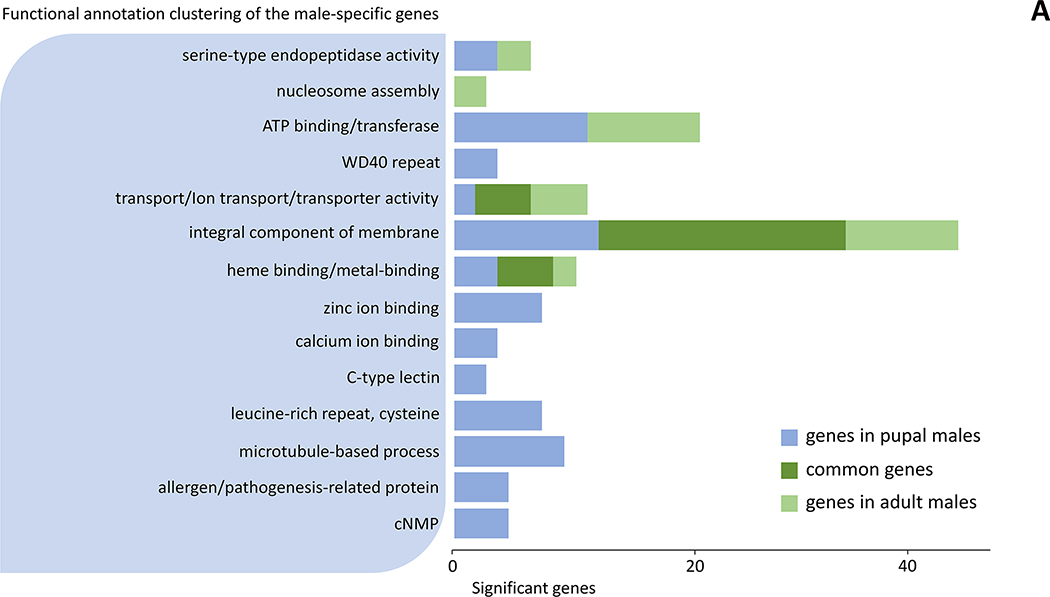
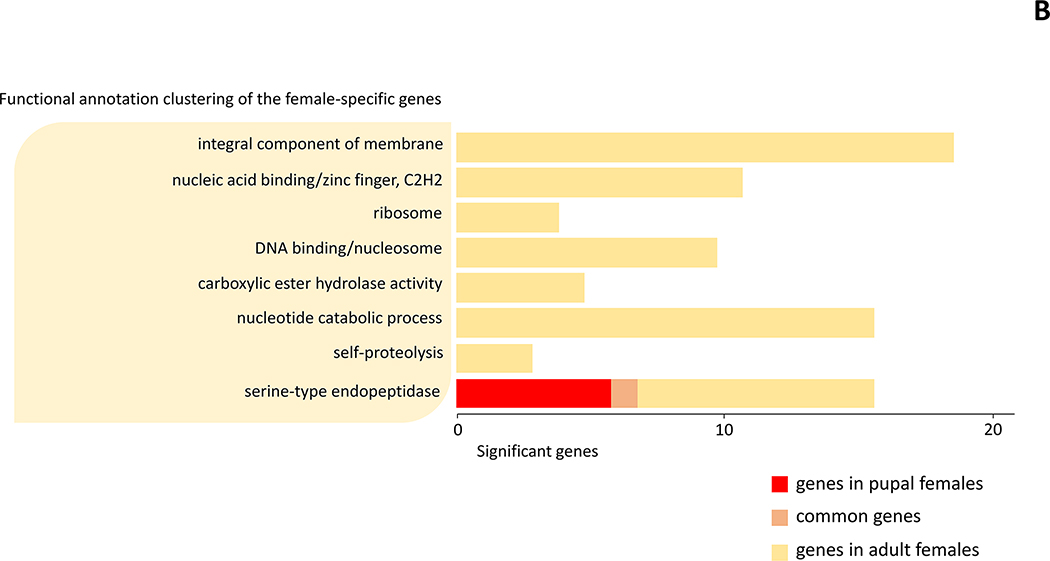
DAVID analysis was performed to gain further insight into the biological processes of the male-specific genes expressed only in pupal (147 genes) and adult (56 genes) stages, as well as in both stages (82 genes) (Fig. 1 and Suppl. file 3). Out of this total of 285 genes, fourteen functional annotation clusters were found and significantly enriched for the selected genes. Those clusters are: serine-type endopeptidase activity, nucleosome assembly, ATP binding/transferase, WD40 repeat, transport/ion transport, integral component of membrane, heme binding/metal-binding, zinc ion binding, calcium ion binding, C-type lectin, leucine-rich repeat, microtubule-based process, allergen/pathogenesis-related protein, cNMP (Fig. 2A and Suppl. file 3). In the same way, we analyzed how female-specific genes were differentiated into functional clusters in pupal (26 genes), adult (163 genes), or both stages (1 gene). Those clusters are: integral component of membrane, nucleic acid binding/zinc finger, ribosome, DNA binding/nucleosome, carboxylic ester hydrolase activity, nucleotide catabolic process, self-proteolysis, serine-type endopeptidase (Fig. 2B and Suppl. file 3)
Figure 2.
Functional annotation clustering for sex-specific genes in pupae and adults of Cx. pipiens. (A) Functional clusters of the male-specific genes (B) Functional clusters of the female-specific genes.
Validated Sex-Specific Genes
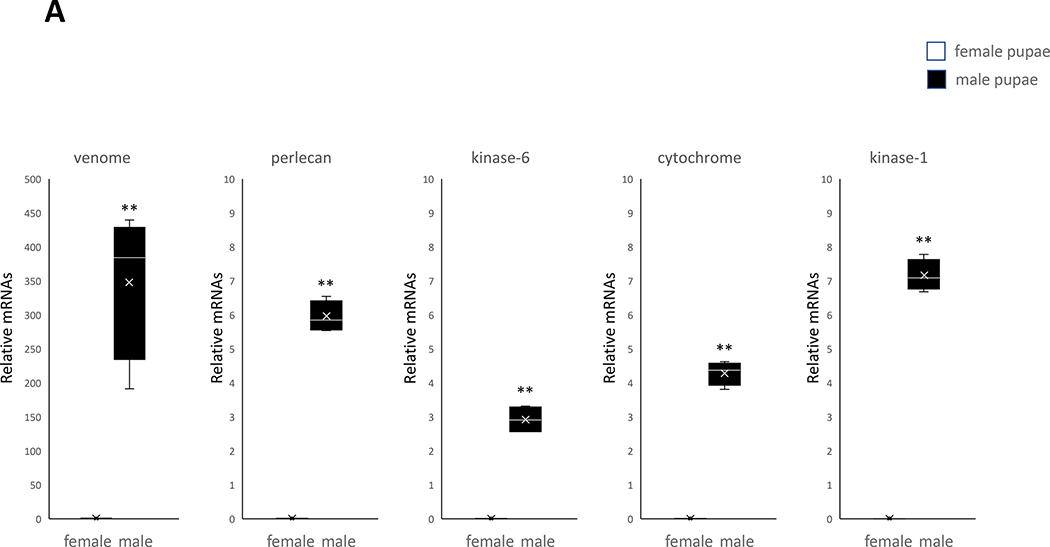
Measurement of gene expression based on Ct values from qRT-PCR shows a significant difference between female pupae (Fig. 3A) and male pupae (Fig. 3B) genes (P< 0.05). A significant difference is also observed between female adults (Fig. 4A) and male adults (Fig. 4B) genes (P< 0.05). After the first RNA-seq experiment, we focused on genes that are expressed in only one sex. Therefore, only the genes expressed in pupae and/or adult stages were selected to confirm that the genes were sex-specifically expressed. Validated sex-specific genes include: fibrinogen and fibronectin, a zinc finger protein, phospholipase A(2), and a serine protein for female pupae; venom allergen 3, a perlecan, testis-specific serine/threonine-protein kinase 1, testis-specific serine/threonine-protein kinase 6, and cytochrome c-2 for male pupae; a salivary protein, D7 protein precursor, trypsin 7 precursor, D7 protein, and nanos for female adults; and tetraspanin F139, cytosol aminopeptidase, testis-specific serine/threonine-protein kinase 1, testis-specific serine/threonine-protein kinase 6, and a C-type lectin for male adults. These genes were expressed in only one sex, and expression in the other sex was measured to be less than the minimum basal expression level from qRT-PCR experiments, which is consistent with the results of RNA-seq analysis (Tables 1 and 2).
Figure 3.
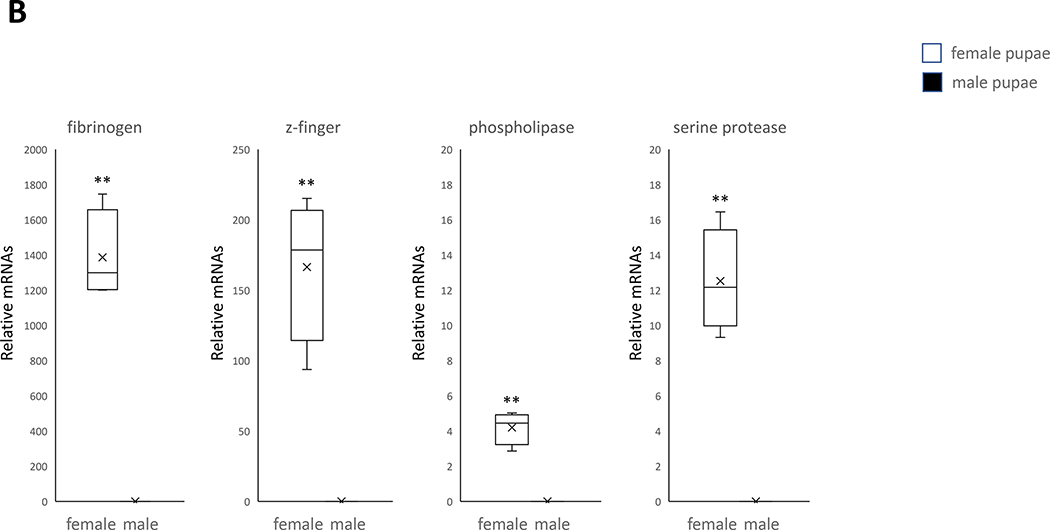
Female-specific expression of genes in pupae and adults of the mosquito Cx. pipiens (A) Five genes expressed only in male pupae. (B) Four genes expressed only in female pupae. A student’s t test was performed on the expression levels of male and female replicates for each gene. (*, P< 0.05), (**, P< 0.01). Error bars represent the 95% confidence interval of the average relative expression, n = 4 groups (each group contains 10 individuals). The box indicates the 25th and 75th percentiles and the whiskers caps represent the maximum and minimum values. A center line across the boxes indicates the median. The x in the box represents the mean.
Figure 4.
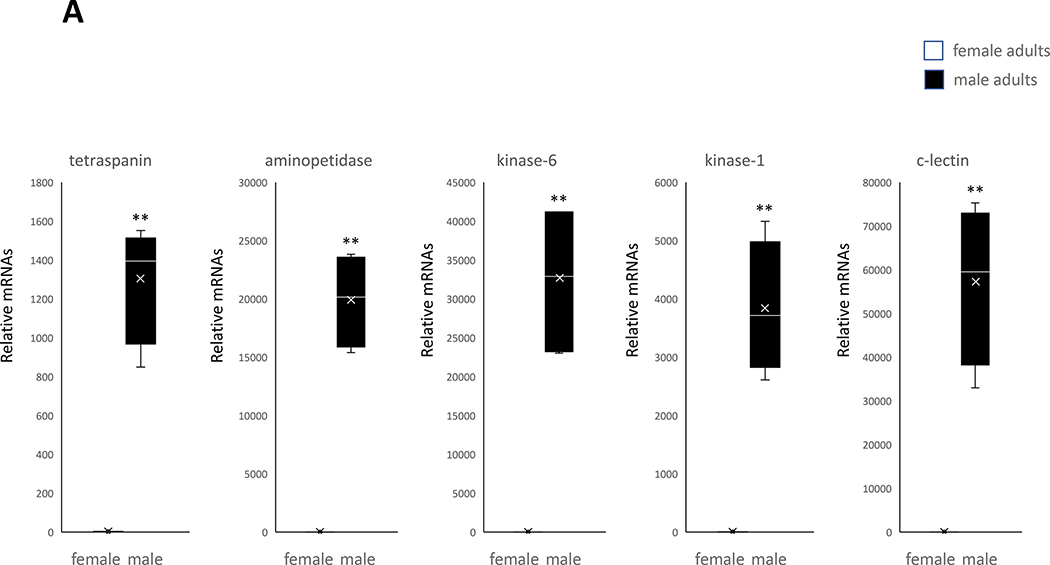
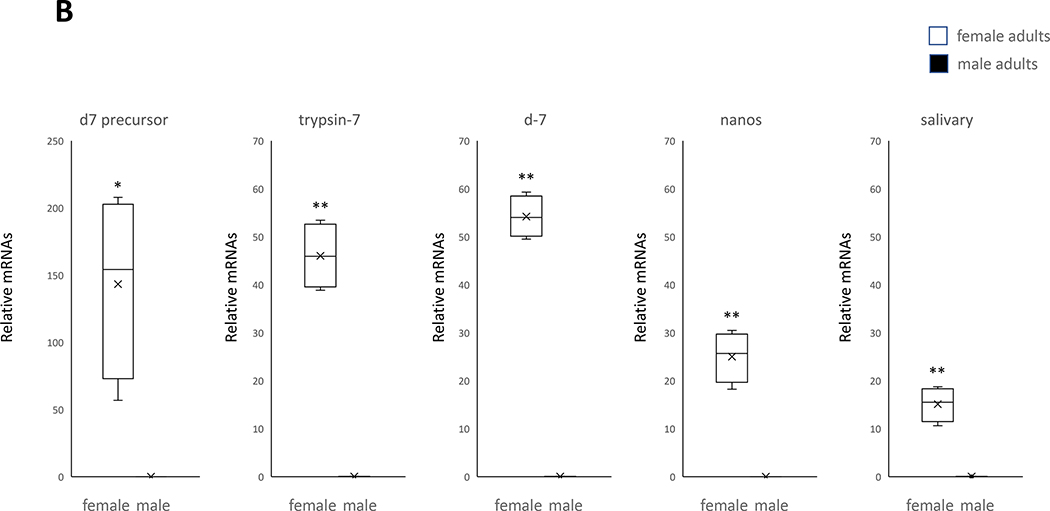
Male-specific expression of genes in pupae and adults of the mosquito Cx. pipiens (A) Five genes expressed only in male adults. (B) Five genes expressed only in female adults. A student’s t test was performed on the expression levels of male and female replicates for each gene. (*, P< 0.05), (**, P< 0.01). Error bars represent the 95% confidence interval of the average relative expression, n = 4 groups (each group contains 10 individuals). The box indicates the 25th and 75th percentiles and the whiskers caps represent the maximum and minimum values. A center line across the boxes indicates the median. The x in the box represents the mean.
Discussion
DAVID Analysis of Functional Annotation Clustering
Through functional annotation clustering, three functional clusters were found in both males and females: serine-type endopeptidase activity, nucleosome, and integral component of membrane. Genes belonging to these clusters are related to the regulation of gene expression, intracellular signals, and tissue composition, and this is expected to be linked to the development of sex-specific organs such as testes and ovaries, and other sex-specific cells. Comparing the differences between the functional clusters between the sexes, there were only 8 significant clusters in females, whereas 14 clusters in males (Fig. 1, 2). Furthermore, a total of 82 commonly expressed genes were observed in males, whereas one gene was commonly expressed in pupae and adults among the genes specific to females (Fig. 1, 2). This is probably because cells or organs that develop from pupa to early adult in males use the same signals and developmental mechanisms, but in females, this process may have different developmental mechanisms. The sex-specific functions and roles of candidate genes in this regard are discussed below.
The genes involved in microtubule-based process have been associated with mosquito sperm composition (Degner et al., 2019), which suggests the possibility that the tubulin and associate proteins are involved in the spermatogenesis process and play an important function in late male pupae and early male adults. The genes encoding allergen are also present, including venom allergen 5, in male pupae, which are most likely associated with insecticide resistance (Lv et al., 2015). The four genes encoding calcium ion binding proteins in male pupae are also consistent with the findings that calcium-dependent regulation is necessary for normal sperm motility (Degner & Harrington, 2016). The serine-type endopeptidase gene expression found in the male pupae may be associated with various functions in mosquitoes, including survival and development into adults (Park & Kwak, 2020). Other found gene clusters include ATP binding proteins and transporters in male pupae and the ABC transporter family previously found to be expressed in all stages of some mosquito species (Lu et al., 2016).
In adult males, the most notable functional cluster is the integral component of membrane. Most genes in this cluster are also expressed in the male pupae. It is likely that commonly expressed genes share functions associated with successive male-specific development (Fig. 2). In addition, ATP binding proteins such as testis-specific protein kinases found in male Ae. albopictus (Gamez et al., 2020) also had highly expressed (10 genes) in male adults of the mosquito Cx. quinquefasciatus, but their exact function in the male adults remains to be further investigated.
In the female pupal stage, only 7 genes encoding in serine-type endopeptidase were classified as a significant cluster (Fig. 2). Even in the female adults, many genes were classified into the same serine-related cluster. However, in the adult stage, these genes are different from those expressed in the pupa stage in that most of them contain a trypsin domain. Therefore, they may show functional differences even if they are classified into the same cluster. The genes expressed in the pupal stage might be related to the metamorphosis process. Furthermore, adult females display high expression of serine-type endopeptidase activity, in particular that of trypsin (10 genes). The function of trypsins in anautogenous mosquitoes, such as Cx. pipiens and Cx. quinquefasciatus females, is in digestion of the blood meal into substances needed for vitellogenesis (Borovsky et al., 2018). Expression of ribosomal subunits (4 genes) was also prominent in the female adults. This is consistent with the finding that decreased ribosomal subunit synthesis likely results in decreased vitellogenin protein synthesis which, in turn, leads to decreased ovary growth and fecundity in females (Wang et al., 2017).
There are certain limitations to the RNA-seq. and DAVID analysis. This is because there is a possibility that this sex-specific expression is only induced by differences in the size of sex-specific organs or cells that exist between males and females. Even taking these limitations into account, the pupal and early adult stages, which are the developmental periods chosen in this study, are the distinct developmental stages in which sex-specific organs and cells such as testis and ovaries are developed. Therefore, the expression of genes proportional to size cannot rule out the possibility that these genes’ activities will play an important role in functional maturation in sex-specific manner.
qRT-PCR Validation of Sex-Specific Genes
A zinc finger protein gene was found to be upregulated specifically in female pupae which, likewise, is consistent with previous findings that zinc finger proteins regulate ecdysone signaling involved in female oogenesis in An. gambiae (Swevers 2019). In female Ae. Aegypti the specific zinc finger gene AaKr h1 was found to be associated with egg production, and its knockout to lead to a reduction in fecundity (Ojani et al., 2018) Additionally, serine protease gene was found to be upregulated in female pupae which is also consistent with previous knowledge that serine proteases in females aid in blood digestion and help An. gambiae mosquitoes process products, such as substrates, pathogens, and inhibitors (Mancini et al., 2011) inserted into their bodies from males during the mating process (Dias-Lopes et al., 2015). Another gene expressed in female pupae, responsible for fibrinogen and fibronectin, was determined to be involved in female mosquito functions as well (Fig. 3). For instance, the fibrinogen-related protein 1 (FREP1) was found to be immediately expressed after a blood meal (Zhang et al., 2015) and the fibronectin gene FN3D1 was found to be connected to female An. arabiensis longevity (Debalke et al., 2020).
As revealed through qRT-PCR analysis, all four genes coding for proteins involved in the blood feeding (including D7s, a trypsin, and a salivary protein) were upregulated specifically in female adults (Fig. 3). While, as described above, these genes are associated with blood feeding, further research is needed to determine what effect each specific gene has on this process. Yet the association of the female genes mentioned above with reproduction or the female-specific blood feeding behavior makes them potentially good target genes for SIT application on female Cx. pipiens.
The testis-specific protein kinases associated with male mosquitoes were indeed found to be expressed in male pupae and adults by qRT-PCR (Fig. 4). The cytosol aminopeptidase protein expression found in male adults was previously determined to be an Ae. aegypti sperm composition protein (Degner et al., 2019) while the cytochrome protein expressed in male pupae (cytochrome c-2) was, likewise, found to be expressed in Ae. aegypti testes (Gamez et al., 2020). The association with sperm and testes of the male-specific genes mentioned above makes them potentially good target genes for SIT application on male Cx. pipiens. Other genes found to be expressed in males, thereby showing a consistency with other studies include tetraspanin F139 (Boes et al., 2014) in adults and venom allergen 3 in pupae (Lv et al., 2015). A C-type lectin expressed in male adults in this study, however, was previously found to be associated with females (Adelman & Myles, 2018). The fact that C-type lectins are not yet explored in Cx. pipiens leads to the speculation that this particular species might have male-specific C-type lectins, but more research is needed to investigate this question.
The results of this study provide basic information on molecular mechanisms during the mosquito’s sex determination and development process. Since only adult female mosquitoes feed on blood from host animals, the sex-specific characteristics of mosquitoes are being studied in depth as an important means of controlling mosquito-borne diseases by controlling sex ratio through genetic or physiological approaches and by releasing sterile males to endemic regions. In the future, studies are needed to show how each of these genes plays a role in development, physiology, and behavior in male or female mosquitoes. Because the genes selected in this study are validated to be sex-specific and are associated with functions and organs needed for mosquito reproduction, they are likely to be useful targets in the application of SIT against Cx. pipiens. In particular, genes associated with blood feeding or egg production in females and the testes organ in males can be chosen as targets if further studies indicate that knockout of these genes will, in fact, produce the expected sterility effect.
Experimental Procedures
Mosquito Rearing
Experiments utilized an anautogenous colony of Cx. pipiens L. established in September 2000, from larvae collected in Columbus, Ohio. Larvae are reared in de-chlorinated tap water and fed a diet of ground fish food (TetraMin). Adults are maintained on honey sponges and kept in large screened cages. All stages are reared at 25°C, 75% r.h., with a 12L:12D daily light cycle. To produce eggs, female adults feed on chicken blood using an artificial feeder (Hemotek® membrane feeding system) through parafilm.
Total RNA Extraction
Total RNA was extracted from four sample sets including male pupae, female pupae, male adults, and female adults. To separate the male and female pupae, individual pupae were sorted based on the pouch shape, length, and width as described in previous studies (Harbach et al., 1984). Briefly, sex-specific morphological differences were identified: male pupae exhibited narrow, pointed, and elongated pouches, while female displayed round, stunted and broad pouches. In the adult stage, based on morphological characteristics, males and females were separated right after adult eclosion. Each biological replicate was collected from three batches of 10 mosquitoes using TRIzol (Life Technologies, Carlsbad, CA). Total RNA purity was tested using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). Biological replicates were pooled for library preparation and sequencing.
Library Preparation and Sequencing
The libraries were built using TruSeq RNA Library Prep Kit v2 (Illumina, San Diego, CA, USA) according to the manufacturer protocol. Briefly, samples were purified twice using poly-T oligo-attached magnetic beads, before fragmentation and priming for cDNA synthesis. cDNA was synthesized using reverse transcriptase and random primers adapted into double stranded (ds) cDNA, which was then removed with Ampure XP beads (Beckman Coulter, Pasadena, CA). ds cDNA was end repaired, converting any resulting overhangs into blunt ends, before adapter adenylation of the 3’ end for pair-ended ligation. Next, paired-adapters were ligated to ds cDNA, which was selectively amplified by PCR. After quality control, bridge amplification was performed on a flow cell, which was loaded into an Illumina HiSeqX platform. A single molecular array was synthesized with reverse termination, resulting in unique clusters of nucleotides strands which were loaded for extension and imaging. Resulting clusters were extended one base at a time with nucleotides containing reversible fluorophores, resulting in clusters that gave a single, unified signal for each base.
Data Analysis
The Illumina adapters used during the library construction were removed from the reads using Trimmomatic (Bolger et al., 2014). In order to reduce the impact of lower quality reads on the alignment, all reads were trimmed to 60 bp using the FASTX Toolkit v-0.0.13 (Hannon, G.J., 2010) resulting in a Phred-Quality-Score greater than 30. Reads were aligned to Cx. quinquefasciatus genome (CpipJ2.5) using HISAT2 v2.20 (Kim et al., 2015). The output SAM files were converted to BAM using SAMtools v1.10 (Li et al, 2009). Htseq-count reads were created using htseq-count (Anders et al., 2015). Finally, in order to find genes specific to males, a gene list was prepared by filtering and sorting among the RNA-seq data of males and females. Among them, a high stringency level was applied to select only genes expressed in males, and only those with “10” or more htseq-count reads in males and those with of “0” reads value in females were selected. Female-specific genes were selected in the opposite way.
Then, a DAVID (Database for Annotation, Visualization and Integrated Discovery) analysis, v 6.8, was performed on male and female specific genes of both pupal and adult stages, grouping the genes into clusters based on functional annotations (Huang et al., 2009). The functional clusters were assigned using UniProt (https://www.uniprot.org/).
RNA Extraction and cDNA Synthesis
Total RNAs for qRT-PCR gene expression validation were extracted separately, with TRIzol reagent (Life Technologies, Carlsbad, CA), for males vs. females of pupal and adult stages. Each sample was centrifuged at 12,500 rpms for 10 minutes in 1 mL of TRIzol and 200 μL of phenol:chloroform:isoamyl alcohol, and then purified with 75% ethanol. A NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE) was used to measure purified RNA quality which was accepted if higher than 1.8 at the A260/A280 nm ratio. Each extraction contained approximately 30 mosquitoes of 3–7 days after adult eclosion. cDNA was consequently made from the purified RNA samples. A similar RNA extraction and cDNA synthesis were separately performed on approximately 30 male or female pupae.
qRT-PCR Validation of RNA Sequencing Data
Each cDNA sample was used in qRT-PCR validation of the nineteen candidate genes using an iQ5 real-time PCR detection system (Bio-Rad, Hercules, CA). 50ng RNA was reverse transcribed and amplified via superscript III RNase H-reverse transcriptase (Invitrogen, Carlsbad, CA), per the manufacturer’s protocol, and compared to ribosomal protein L19 (RpL19), an endogenous housekeeping gene, as an internal control. Transcript divergence from the qRT-PCR results was evaluated for statistical significance using the Student’s t-test. Candidate genes and primer information are reported in Tables 1 and 2; and Supplementary Tables 1 and 2.
Supplementary Material
Acknowledgements
This work was supported in part by the National Institutes of Health under grant number R15AI139861 and by the National Science Foundation under grant number IOS-1944214.
Abbreviations:
- RNA-seq
RNA-sequencing
- qRT-PCR
quantitative real-time PCR
- Cx. pipiens
Culex pipiens
- GO
gene ontology
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the Genbank database (accession no., PRJNA695583).
References
- Adelman ZN, & Myles KM (2018). The C-type lectin domain gene family in Aedes aegypti and their role in arbovirus infection. Viruses, 10(7), 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, & Huber W (2015). HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics, 31(2), 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict MQ (2021). Sterile insect technique: lessons from the past. Journal of Medical Entomology. [DOI] [PubMed] [Google Scholar]
- Boes KE, Ribeiro JM, Wong A, Harrington LC, Wolfner MF, & Sirot LK (2014). Identification and characterization of seminal fluid proteins in the Asian tiger mosquito, Aedes albopictus. PLoS Negl Trop Dis, 8(6), e2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, & Usadel B (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics, 30(15), 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovsky D, Hancock RG, Rougé P, Powell CA, & Shatters RG Jr (2018). Juvenile hormone affects the splicing of Culex quinquefasciatus early trypsin messenger RNA. Archives of insect biochemistry and physiology, 99(3), e21506. [DOI] [PubMed] [Google Scholar]
- Cator LJ, Wyer CA, & Harrington LC (2021). Mosquito sexual selection and reproductive control programs. Trends in Parasitology, 37(4), 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HN, Rodriguez SD, Gonzales KK, Vulcan J, Cordova JJ, Mitra S, … & Attardo GM (2018). Toward implementation of mosquito sterile insect technique: the effect of storage conditions on survival of male Aedes aegypti mosquitoes (Diptera: Culicidae) during transport. Journal of insect science, 18(6), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debalke S, Habtewold T, Christophides GK, & Duchateau L (2020). Stability of the effect of silencing fibronectin type III domain-protein 1 (FN3D1) gene on Anopheles arabiensis reared under different breeding site conditions. Parasites & Vectors, 13, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degner EC, Ahmed-Braimah YH, Borziak K, Wolfner MF, Harrington LC, & Dorus S (2019). Proteins, transcripts, and genetic architecture of seminal fluid and sperm in the mosquito Aedes aegypti. Molecular & Cellular Proteomics, 18(Supplement 1), S6–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degner EC, & Harrington LC (2016). A mosquito sperm’s journey from male ejaculate to egg: Mechanisms, molecules, and methods for exploration. Molecular reproduction and development, 83(10), 897–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Lopes G, Borges-Veloso A, Saboia-Vahia L, Domont GB, Britto C, Cuervo P, & De Jesus JB (2015). Expression of active trypsin-like serine peptidases in the midgut of sugar-feeding female Anopheles aquasalis. Parasites & Vectors, 8(1), 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamez S, Antoshechkin I, Mendez-Sanchez SC, & Akbari OS (2020). The developmental transcriptome of aedes albopictus, a major worldwide human disease vector. G3: Genes|Genomes|Genetics, 10(3), 1051–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Pettersson J, Higgs S, Charrel R, & De Lamballerie X (2017). Emerging arboviruses: Why today?. One Health, 4, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AB, Basu S, Jiang X, Qi Y, Timoshevskiy VA, Biedler JK, … & Sharakhov IV (2015). A male-determining factor in the mosquito Aedes aegypti. Science, 348(6240), 1268–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon GJ (2010) FASTX-Toolkit. http://hannonlab.cshl.edu/fastx_toolkit/
- Harbach RE, Harbach RE, Harrison BA, & Gad AM (1984). Culex (Culex) Molestus Forskål (Diptera: Culicidae): neotype designation, description, variation, and taxonomic status. Proceedings of the Entomological Society of Washington, 86, 521–542. [Google Scholar]
- Huang DW, Sherman BT, & Lempicki RA (2009). Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic acids research, 37(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhn J, Marinotti O, Calvo E, & James AA (2008). Gene structure and expression of nanos (nos) and oskar (osk) orthologues of the vector mosquito, Culex quinquefasciatus. Insect molecular biology, 17(5), 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B and Salzberg SL (2015). HISAT: a fast spliced aligner with low memory requirements. Nature methods, 12(4), 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Trocke S, & Sim C (2018). Comparative studies of stenogamous behaviour in the mosquito Culex pipiens complex. Medical and veterinary entomology, 32(4), 427–435. [DOI] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, … & Durbin R (2009). The sequence alignment/map format and SAMtools. Bioinformatics, 25(16), 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Xu Y, & Cui F (2016). Phylogenetic analysis of the ATP-binding cassette transporter family in three mosquito species. Pesticide biochemistry and physiology, 132, 118–124. [DOI] [PubMed] [Google Scholar]
- Luz PM, Vanni T, Medlock J, Paltiel AD, & Galvani AP (2011). Dengue vector control strategies in an urban setting: an economic modelling assessment. The Lancet, 377(9778), 1673–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y, Lei Z, Hong S, Wang W, Zhang D, Zhou D, … & Zhu C (2015). Venom allergen 5 is associated with deltamethrin resistance in Culex pipiens pallens (Diptera: Culicidae). Journal of medical entomology, 52(4), 672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini E, Tammaro F, Baldini F, Via A, Raimondo D, George P, Audisio P, Sharakhov IV, Tramontano A, Catteruccia F, & Torre A. della. (2011). Molecular evolution of a gene cluster of serine proteases expressed in the Anopheles gambiae female reproductive tract. BMC evolutionary biology, 11(1), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojani R, Fu X, Ahmed T, Liu P, & Zhu J (2018). Krüppel homologue 1 acts as a repressor and an activator in the transcriptional response to juvenile hormone in adult mosquitoes. Insect molecular biology, 27(2), 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, & Kwak IS (2020). Cadmium-induced developmental alteration and upregulation of serine-type endopeptidase transcripts in wild freshwater populations of Chironomus plumosus. Ecotoxicology and Environmental Safety, 192, 110240. [DOI] [PubMed] [Google Scholar]
- Poh KC, Chaves LF, Reyna-Nava M, Roberts CM, Fredregill C, Bueno R Jr, … & Hamer GL (2019). The influence of weather and weather variability on mosquito abundance and infection with West Nile virus in Harris County, Texas, USA. Science of the Total Environment, 675, 260–272. [DOI] [PubMed] [Google Scholar]
- Price DC, Egizi A, & Fonseca DM (2015). The ubiquity and ancestry of insect doublesex. Scientific reports, 5, 13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swevers L (2019). An update on ecdysone signaling during insect oogenesis. Current opinion in insect science, 31, 8–13. [DOI] [PubMed] [Google Scholar]
- Wang JL, Saha TT, Zhang Y, Zhang C, & Raikhel AS (2017). Juvenile hormone and its receptor methoprene-tolerant promote ribosomal biogenesis and vitellogenesis in the Aedes aegypti mosquito. Journal of Biological Chemistry, 292(24), 10306–10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke AB, Beier JC, & Benelli G (2018). Transgenic Mosquitoes–Fact or Fiction?. Trends in parasitology, 34(6), 456–465. [DOI] [PubMed] [Google Scholar]
- Zhang G, Niu G, Franca CM, Dong Y, Wang X, Butler NS, … & Li J (2015). Anopheles midgut FREP1 mediates Plasmodium invasion. Journal of Biological Chemistry, 290(27), 16490–16501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.