Abstract
Background:
A growing body of evidence has confirmed the association between fine particulate matter (PM2.5) and ocular diseases, but little is known on the effect of long-term PM2.5 exposure on glaucoma.
Methods:
A national cross-sectional study of the Rural Epidemiology for Glaucoma was conducted in 10 provinces of China, and 33,701 adults aged 40 years or more were included. A satellite-based model at 1-km resolution level was used to estimate PM2.5 concentrations which were assigned to each participant according to geocoded home addresses. Logistic regression model was performed to investigate associations of long-term PM2.5 exposure with glaucoma and its subtypes.
Results:
Estimated PM2.5 concentrations ranged from 28.0 to 96.4 μg/m3. For each 10 μg/m3 increment in PM2.5, the adjusted odds ratios (ORs) were 1.07 (95% CI: 1.00–1.15) and 1.14 (95% CI: 1.02–1.26) for glaucoma and primary angle-closure glaucoma (PACG), respectively. A positive but non-significant association (OR = 1.05, 95% CI: 0.92–1.18) was detected between long-term exposure to PM2.5 and odds of primary open-angle glaucoma. The middle aged residents and non-smokers were more sensitive to the adverse effects of PM2.5.
Conclusions:
Long-term PM2.5 exposure was associated with glaucoma and PACG in Chinese adults, which provided new insights on adverse ophthalmic effect of PM2.5.
Keywords: PM2.5, Eye, Satellite-based model
1. Introduction
Ambient fine particulate matter (PM2.5) has posed a serious threat to public health worldwide as the global fifth-ranking risk factor of mortality (Cohen et al., 2017). Long-term exposure to air pollution increased risks of respiratory diseases (Liu et al., 2021), cardiovascular disease (Liang et al., 2020a) and cancer (Hvidtfeldt et al., 2020). In addition, the eye is one of the few organs directly exposed to ambient pollutants (Lin et al., 2019), and biological evidence has illustrated that air pollution can induce intraocular inflammation, corneal cell apoptosis, and oxidative stress on eyes (Jung et al., 2018; Torricelli et al., 2011). Population-based studies have already observed associations between PM2.5 exposure and ocular diseases, such as conjunctivitis (Aik et al., 2020; Mimura et al., 2014), presbyopia (Lin et al., 2019), dry eye disease (Mo et al., 2019) and visual impairment in children (Yang et al., 2020).
Glaucoma is a multifactorial neurodegenerative disease leading to the loss of retinal ganglion cells and visual field defects (Okamoto et al., 2020). The Global Burden of Disease Study estimated that 3.6 million patients aged 50 years or older were blind due to glaucoma in 2020, which has ranked as the second leading cause of blindness worldwide (GBD 2019 Blindness and Vision Impairment Collaborators, 2021). Epidemiological studies have found some risk determinants of glaucoma such as elderly, women, as well as several modifiable factors including elevated intraocular pressure (IOP), diabetes and hypertension (Flammer et al., 2013; Jonas et al., 2017). To our best knowledge, three studies investigated the adverse effects of PM2.5 exposure on glaucoma (Chua et al., 2019; Grant et al., 2021; Sun et al., 2021). Chua et al. (2019) and Grant et al. (2021) explored the association between PM2.5 and glaucoma using data from the UK Biobank and Canadian Longitudinal Study on Aging, respectively, but their levels of PM2.5 concentrations were much lower than that in China. Another study in Taiwan, China found increased PM2.5 exposure was associated with primary open-angle glaucoma (POAG), one subtype of glaucoma, but it did not assess for total glaucoma (Sun et al., 2021). Although it is plausible biologically, human evidence on association of long-term PM2.5 exposure with glaucoma and each subtype remained scarce, especially in China and other countries with challenges of both aging population and PM2.5 pollution from rapid industrialization.
It is estimated that the global number of individuals with glaucoma will increase to 111.8 million by 2040 (Tham et al., 2014). Given air pollution as an ongoing challenge for global health, if evidence on glaucoma linked to high PM2.5 exposure was obtained, it would provide a modifiable risk factor of glaucoma and highlight novel health benefits potentially gained from reduction in air pollution. Based on an established spatiotemporal model at 1-km resolution level (Huang et al., 2019; Liang et al., 2020a), the study is aimed to combine satellite-based estimates of PM2.5 level with a nation-wide survey data to investigate associations between odds of glaucoma and long-term PM2.5 exposure. Furthermore, it will identify individuals susceptible to PM2.5 exposure and glaucoma among Chinese adults, underlying a wide concentration gradient of PM2.5 pollution.
2. Methods
2.1. Study population
The Rural Epidemiology for Glaucoma in China (REG-China) study is a nation-wide cross-sectional survey of glaucoma and related ocular diseases in rural Chinese populations, which is aimed to investigate the regional distributions and risk factors for epidemic of glaucoma. The details of study design were described elsewhere (Shan et al., 2021). Briefly, a multi-stage stratified cluster sampling procedure was used to enroll a nationally representative sample of study participants. First, ten provinces or municipalities were identified from Eastern China (Liaoning, Shandong and Jiangsu), Central China (Heilongjiang, Henan, Shaanxi and Shanxi) and Western China (Ningxia Hui Autonomous Region, Sichuan and Chongqing) (eFigure 1 in the Supplement). Second, a large city and a midsize city were selected from each province at random, and then two rural townships were randomly identified from each city. Last, one rural community was selected from each township at random. A total of 52,041 individuals aged 6 years or more were invited to participant in the project of REG-China, and 48,398 local residents completed the survey with a response rate of 93%, and 36,081 participants aged ≥40 years were involved in the study. Finally, 33,701 participants without missing exposure and health data were included in the further analysis (Fig. 1). The study protocol was approved by Institute Review Board of Tianjin Medical University, and written informed consents were obtained from participants before survey.
Fig. 1.
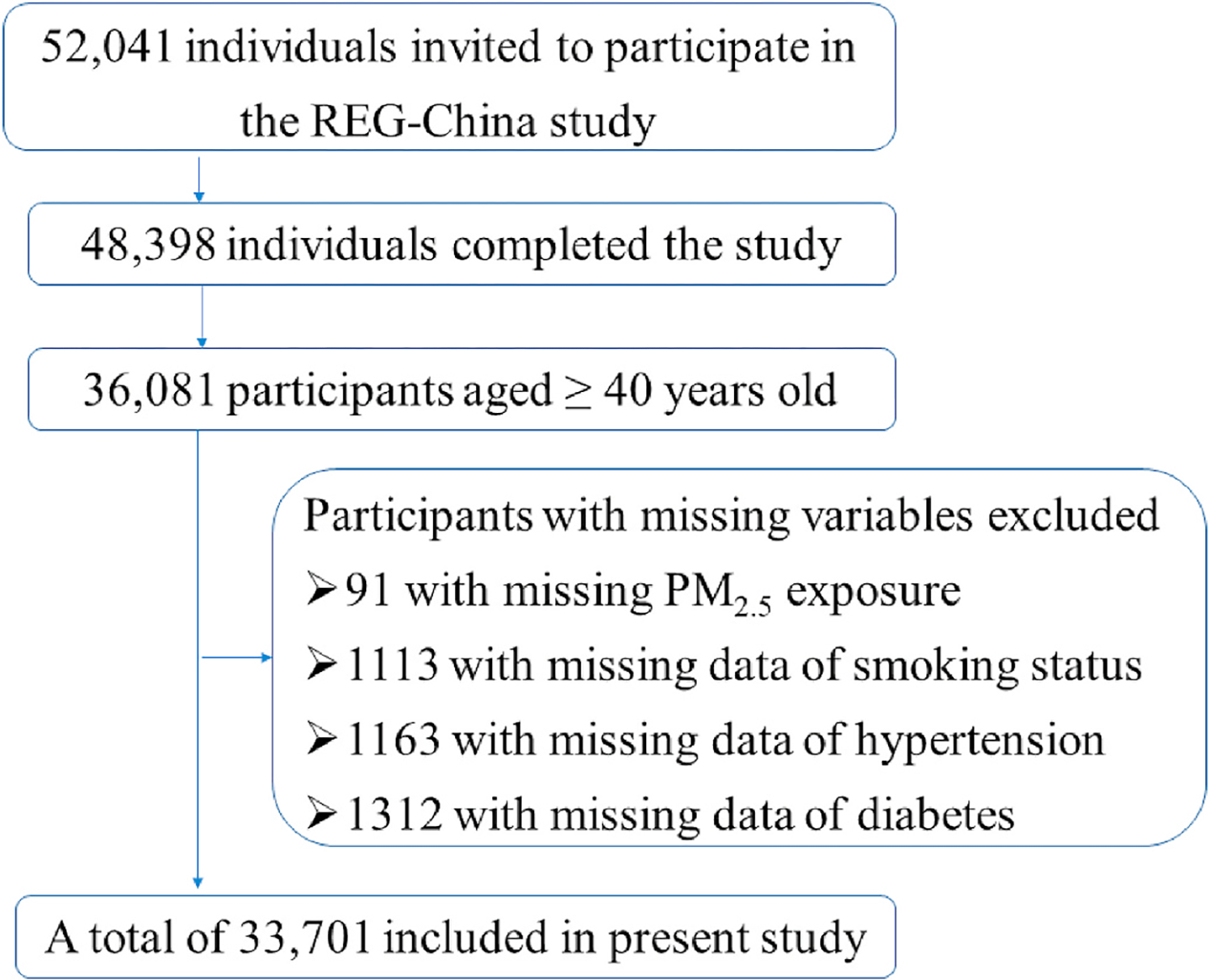
The flowchart for inclusion and exclusion of study participants.
2.2. Eye examinations and definition of outcome
Each participant underwent eye examinations, including measurements of visual acuity (VA), limbal anterior chamber depth (LACD), IOP and optic disk. A standard logarithmic VAE chart (Tianjin ZhengDa Medical Care Equipment Factory, Tianjin, China) was given at a distance of 5 m to measure the VA. LACD was assessed using results of slit-lamp microscopy. The IOP was measured with non-contact tonometry (NCT; Type-NIDEK NT-2000 and Type-NIDEK NT-510, NIDEK CO, LTD, Japan). Direct ophthalmoscopy and slit-lamp biomicroscopy with a 90D convex lens were done to obtain vertical cup-to-disc ratio (VCDR) without pupil dilation. Further examinations were tested among patients classified as suspect for glaucoma, including fundus photography, Goldmann applanation tonometry, visual field and gonioscopy. Details in eye examination were described in eAppendix 1 in the Supplement.
The diagnosis of glaucoma was made by ophthalmologists according to three levels of evidence, claimed by the International Society for Geographical and Epidemiological Ophthalmology (ISGEO) (eAppendix 2 in the Supplement) (Foster et al., 2002). Furthermore, cases of primary glaucoma were divided into primary angle-closure glaucoma (PACG) and POAG based on the gonioscopic finding of a narrow angle after excluding secondary factors.
2.3. Long-term PM2.5 exposure assessment
An established satellite-based spatiotemporal model was used to estimate PM2.5 concentrations at 1-km spatial resolution, and detailed procedures have been described elsewhere (Liang et al., 2020b; Xiao et al., 2018). Briefly, the aerosol optical depth (AOD) product retrieved by the Multi-Angle Implementation of Atmospheric Correction (MAIAC) algorithm was derived from the US National Aeronautics and Space Administration (NASA) Moderate Resolution Imaging Spectrometer (MODIS) satellite. A machine learning algorithm was used to link AOD with other predictors of meteorology, road network, land cover index and air pollution emissions to estimate PM2.5 concentrations. The cross validation showed a highly agreement between predicted historical PM2.5 concentrations with available ground monitoring data at the annual level (prediction R2 = 0.80). Afterwards, the estimated PM2.5 concentrations were assigned to individual home address that has been encoded as longitude and latitude data. The annual PM2.5 concentrations from 2000 to 2016 were available, and the 17-year mean value was calculated as long-term PM2.5 exposure level used in the association analyses.
2.4. Covariates collection
Each participant was required to answer a standardized questionnaire to obtain information on sociodemographic characteristic, medical history and lifestyle factors. Participants who smoked at least one cigarette daily over 1 year were defined as smokers. Physician-diagnosed hypertension and/or diabetes was self-reported by participant. Data of disposable income per capita was obtained from National Bureau of Statistics (http://www.stats.gov.cn/).
2.5. Statistical analysis
Baseline characteristics of participants were displayed for continuous variables using means with corresponding standard deviations and for categorical variables using percentages. Logistic regression models were used to explore the potential association of long-term PM2.5 exposure with glaucoma and the subtypes. We reported odds ratios (ORs) with corresponding 95% confidence intervals (95% CIs) for each 10 μg/m3 increment in PM2.5 concentration, after multivariable adjustments for sex, age, region (Eastern China, Central China vs Western China), disposable income per capita (RMB Yuan), smoking (yes vs no), hypertension (yes vs no), IOP (mmHg) and lowering-IOP treatment (yes vs no). ORs (95% CI) for glaucoma, PACG, and POAG were also estimated according to quarters of exposure to PM2.5 with a trend test. In addition, the relationship between IOP levels and PM2.5 concentrations was also tested by generalized linear regression model with covariates above except IOP.
We further did subgroup analyses stratified by sex, age, smoking and hypertension to assess the potential modification effect of covariates on the association between long-term PM2.5 exposure and odds of glaucoma as well as its subtypes. Furthermore, because the treatment of cataract could potentially affect the progression of glaucoma (Sun et al., 2017), sensitivity analyses by excluding residents with cataract were performed to test the robustness of our results.
All statistical analyses were done using IBM SPSS Statistics for Windows (version 24.0, IBM Corp, USA) and R software (version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria). The two-side P < 0.05 was considered as statistical significance.
3. Results
3.1. Descriptive results
The demographic characteristics of all participants are shown in Table 1 and summarized by each region in eTable 1 in the Supplement. Overall, the mean age of all participants was 62.3 years and 60.7% were female. Approximately 24.7% of participants were smokers. The screening study identified a total of 713 participants suffered from glaucoma, of which 328 cases were PACG and 247 cases were POAG. There were 343 patients, less than 50% among diagnosed glaucoma cases, having received treatment of lowering IOP. In addition, general characteristics of the included participants and the excluded individuals due to missing data are described in eTable 2 in the Supplement.
Table 1.
Baseline characteristics of the study participants.
| Total | Male | Female | |
|---|---|---|---|
|
| |||
| Participants, N | 33701 | 13249 | 20452 |
| Age, years | 62.3 ± 11.3 | 62.6 ± 11.4 | 62.0 ± 11.2 |
| Smoking, N (%) | 8332 (24.7) | 6218 (46.9) | 2114 (10.3) |
| Hypertension, N (%) | 8499 (25.2) | 3114 (23.5) | 5385 (26.3) |
| IOP, mmHg | 14.4 ± 2.9 | 14.4 ± 2.9 | 14.4 ± 2.8 |
| Lowering-IOP treatment, N (%) | 343 (1.0) | 110 (0.8) | 233 (1.1) |
| Glaucoma, N (%) | 713 (2.1) | 283 (2.1) | 430 (2.1) |
| PACG, N (%) | 328 (1.0) | 118 (0.9) | 210 (1.0) |
| POAG, N (%) | 247 (0.7) | 115 (0.9) | 132 (0.6) |
IOP: intraocular pressure; PACG: primary angle-closure glaucoma; POAG, primary open-angle glaucoma.
3.2. Associations between long-term PM2.5 exposure and glaucoma
The 17-year average concentration of ambient PM2.5 that the study participants were exposed to was 62.4 μg/m3, ranging from 28.0 μg/m3 to 96.4 μg/m3. All participants were exposed to higher PM2.5 than the World Health Organization recommended criteria (5 μg/m3). In the regression analyses after multi-variable adjustment (Table 2), there were significant associations of glaucoma and PACG with each 10 μg/m3 increment in PM2.5. For example, in the Model 3 with full adjustment of age, sex, region, disposable income per capita, smoking, hypertension, IOP and lowering-IOP treatment, the odds of glaucoma increased with an OR of 1.07 (95% CI: 1.00–1.15) per 10 μg/m3 increment in PM2.5. For the major subtypes of glaucoma, each 10 μg/m3 increment in PM2.5 increased odds of PACG by 14% (95% CI: 2%–26%), and a positive but non-significant association was found between PM2.5 exposure and POAG.
Table 2.
Odds ratios (95% CI) for glaucoma associated with each 10 μg/m3 increase in PM2.5.
| Odds ratios (95% CI) | |||
|---|---|---|---|
|
|
|
||
| Glaucoma | PACG | POAG | |
|
| |||
| Model 1 | 1.00 (0.95, 1.06) | 1.11 (1.03, 1.19) | 0.92 (0.84, 1.00) |
| Model 2 | 1.06 (1.00, 1.14) | 1.10 (1.00, 1.20) | 1.06 (0.94, 1.20) |
| Model 3 | 1.07 (1.00, 1.15) | 1.14 (1.02, 1.26) | 1.05 (0.92, 1.18) |
PACG, primary angle-closure glaucoma; POAG, primary open-angle glaucoma.
Model 1, adjusted for sex and age.
Model 2, Model 1 + adjusted for region, disposable income per capita, smoking and hypertension.
Model 3, Model 2 + adjusted for IOP and lowering-IOP treatment.
In addition, the effects of quartile exposures were also examined for each glaucomatous outcome with 3 quartile cutoff points of 51.4 μg/m3, 59.8 μg/m3, and 72.0 μg/m3 in eTable 3 in the supplement. Compared with the first exposure quartile, ORs of glaucoma associated with the second to fourth exposure quartiles of PM2.5 were 0.96 (95% CI: 0.74–1.26), 1.70 (95% CI: 1.27–2.26), and 1.48 (95% CI: 1.10–2.01), respectively, underlying a significant trend test (P = 0.025). A similar trend was only found for PACG but not for POAG (eTable 3 in the Supplement).
The relationship between long-term PM2.5 exposure and IOP was also tested in a linear regression, but the effect estimation was rather mild with an increase of 0.14 mmHg per 10 μg/m3 increment in PM2.5, which inferred that glaucoma linked to long-term PM2.5 exposure might not be mediated through the pathway of IOP elevation (eTable 4 in the Supplement).
3.3. Subgroup and sensitivity analyses
The results of stratified analyses described in Fig. 2 have indicated the effect modification of age and smoking status on the PM2.5-glaucoma association. For a 10 μg/m3 increase of PM2.5, the estimated effects on odds of glaucoma were 1.25 (95% CI: 1.12–1.39) and 0.94 (95% CI: 0.85–1.04) for individuals aged 40–65 years and those aged over 65 years, respectively, which suggested the middle-aged adults would be more susceptible to long-term exposure to fine particulate pollutants than the elderly (P for interaction = 0.001). Furthermore, compared with smokers, the effects of PM2.5 on glaucoma were strengthened among non-smokers with a significant interaction term tested by PM2.5 exposure and smoking status (P for interaction = 0.008). Similarly, we found the association between long-term PM2.5 exposure and PACG could be modified by both age and smoking status (eFigure 2 in the Supplement).
Fig. 2.
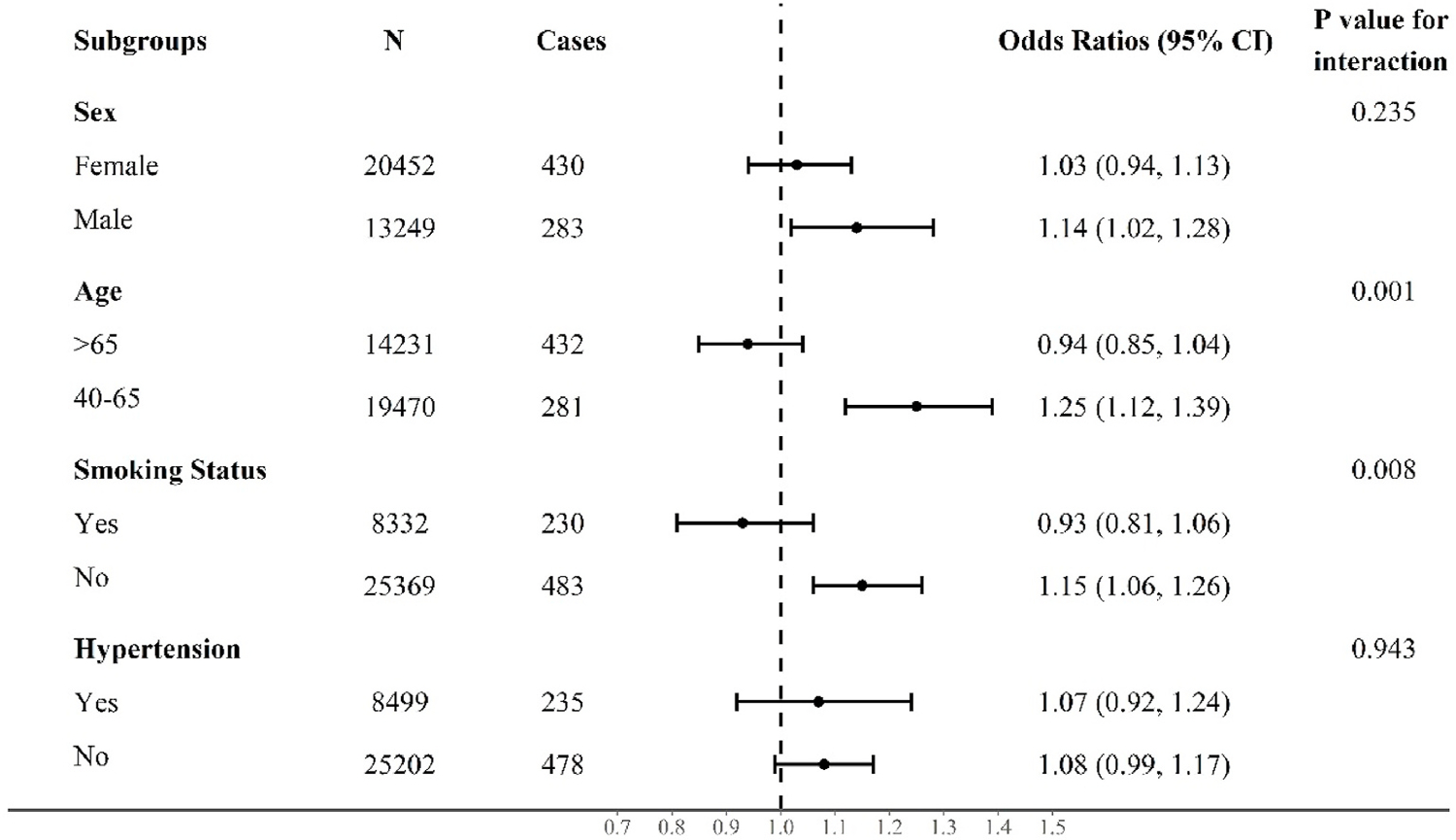
Odds ratios (95% CI) for associations of glaucoma with each 10 μg/m3 increase of PM2.5 by subgroups. Results from subgroup analyses stratified by sex, age, smoking, and hypertension after multivariable adjustments using the Model 3.
In addition, results of sensitivity analyses after excluding the 3928 participants with cataract showed that the estimated effects of PM2.5 on glaucoma and its subtypes were comparable to those in the main analyses (Table 3), although the ORs for associations of long-term exposure to PM2.5 with PACG changed slightly higher.
Table 3.
Sensitivity analyses on associations of glaucoma and the subtypes with each 10 μg/m3 increase in PM2.5 by excluding participants with cataract.
| Odds ratios (95% CI) |
|||||
|---|---|---|---|---|---|
| Glaucoma | PACG | POAG | |||
|
| |||||
| Model 1 | 1.01 (0.95, 1.07) | 1.14 (1.04, 1.24) | 0.90 (0.81, 1.00) | ||
| Model 2 | 1.06 (0.98, 1.15) | 1.12 (1.01, 1.25) | 1.07 (0.93, 1.24) | ||
| Model 3 | 1.10 (1.01, 1.20) | 1.25 (1.10, 1.41) | 1.06 (0.91, 1.22) | ||
PACG, primary angle-closure glaucoma; POAG, primary open-angle glaucoma.
Model 1, adjusted for sex and age.
Model 2, Model 1 + adjusted for region, disposable income per capita, smoking and hypertension.
Model 3, Model 2 + adjusted for IOP and lowering-IOP treatment.
4. Discussion
Based on multi-center population-based data in rural China covering a wide range of ambient PM2.5 concentrations, our findings first identified that long-term PM2.5 exposure was associated with increased odds of glaucoma in high pollution settings, and indicated that PACG seemed more sensitive to ambient PM2.5 exposure rather than POAG in Chinese adults, which provided new evidence on adverse effect of PM2.5 on ophthalmic damage. It was also observed that people aged 40–65 years or non-smokers might be susceptible populations to long-term PM2.5 exposure contributing to risk of glaucoma. Although further validations from independent studies are needed, the current findings suggest the long-term exposure to fine particulate pollutant could be an important modifiable risk factor for epidemic of glaucoma beyond some wellknown risk factors such as aging and elevated IOP, at least for some susceptible individuals living in high pollution environment.
To the best of our knowledge, limited studies have investigated the effect of long-term PM2.5 exposure on glaucoma and its subtypes worldwide. Loss of retinal ganglion cells is one of the common features of all glaucoma subtypes (Jonas et al., 2017). Thinner retinal nerve fiber layer and decreased retinal thickness were observed in residents exposed to higher PM2.5 pollution (Chua et al., 2020, 2021). Chua et al. identified a link between PM2.5 and self-reported glaucoma using a land use regression model for exposure assessment in the UK Biobank participants, with an OR of 1.06 (95% CI: 1.01–1.12) per IQR (1.07 μg/m3) increment in PM2.5 (Chua et al., 2019). However, the annual average level of PM2.5 exposure in the UK biobank data was approximately 9.9 μg/m3 with the lower and upper quartile of 9.38 μg/m3 and 10.45 μg/m3, which was much lower than the average level of PM2.5 as well as its interquartile range in this study. Similarly, increased PM2.5 (per IQR) was also associated with glaucoma (OR = 1.14; 95% CI, 1.01–1.29) in Canadian Longitudinal Study on Aging (Grant et al., 2021), underlying the lower mean PM2.5 concentration (6.5 μg/m3). The aforementioned studies did not investigate the subtypes of glaucoma linked to PM2.5 exposure (Chua et al., 2019, 2020, 2021; Grant et al., 2021). Recently, a nested case-control study in Taiwan, China, based on Longitudinal Health Insurance Database, found that increased PM2.5 exposure was associated with POAG among elder participants aged over 65 years (Sun et al., 2021). We only obtain a positive but non-significant association between PM2.5 and POAG in the study. Several potential explanations for the inconsistent results might include differences in study designs, methods for assessment of exposure, and participants’ demographic characteristics.
The current study was a multi-center survey conducted in 10 provinces of the mainland China for epidemic of glaucoma among 33,701 participants aged over 40 years. It had broader distributions in regions and age groups of study participants, compared with the study in Taiwan province. Another strength in the study is the use of established spatiotemporal model at 1-km resolution level to improve the measurement quality of ambient PM2.5 exposure at individual level (Liang et al., 2020b). Additionally, the proportion of PACG among glaucoma patients was 46.0% in the study, which was close to reports derived from regional surveys in China (Song et al., 2011; Zhong et al., 2012). Using the nation-wide data, it is the first time to identify a significant association of long-term PM2.5 exposure with PACG among adults in high pollution regions. In future, independent studies with prospective study design and more accumulated cases of glaucoma will be warranted to further investigate whether differences would exist in the adverse effect of PM2.5 exposure on PACG and POAG.
In addition, a wide gradient of PM2.5 concentration in the multiple study centers strengthened the power to obtain a robust result. The range of PM2.5 exposure among participants was 28.0 μg/m3 to 96.4 μg/m3 with the mean level of 62.4 μg/m3, which was much higher than the recommended level of 5 μg/m3 from World Health Organization Air Quality Guideline. In 2017, the annual average of global PM2.5 concentration was 46 μg/m3, and population-weighted PM2.5 ranged from 7 μg/m3 in the United States to 91 μg/m3 in India among the 10 largest countries by population (Turner et al., 2020). The PM2.5 concentrations in the study covered much of global high-pollution levels, which was beneficial to capture the exposure-response relationship within a broader exposure gradient of PM2.5.
In the subgroup analyses, we identified that middle-aged adults and non-smokers had stronger effects of PM2.5 exposure on both glaucoma and PACG, after interaction terms of PM2.5 and several potential risk factors were tested. As a usually asymptomatic and chronic disease, glaucoma was associated with aging and IOP elevation (Jonas et al., 2017; Quigley 2011). However, after control multiple risk factors including age and IOP, we obtained significant association of PM2.5 with glaucoma and PACG among people aged 40–65 years, and it was inferred that glaucomatous optic nerve damage could be sensitive to PM2.5 exposure at the middle age or even earlier. In addition, for the heterogeneous results stratified by smoking status, previous studies also proved a stronger effect of PM2.5 on other deleterious outcomes (e.g., atherosclerosis and cardiovascular disease) in non-smokers (Künzli et al., 2005; Liang et al., 2020a). It was recorded that PM2.5 and cigarette smoking may share similar pathogenic pathways of increased oxidative stress and inflammation, and additional exposure to PM2.5 among smokers may not further enhance damages through competitive biopathways (Künzli et al., 2005).
It has not been illustrated very clearly in biological mechanisms for glaucoma associated with long-term PM2.5 exposure. The increase of IOP was associated with occluded aqueous humor outflow due to crowded ocular anatomy or angle closure (Nongpiur et al., 2011). Since elevated IOP can interfere with optic nerve axon transport, it was considered as one of major modifiable risk factors of glaucoma (Jonas et al., 2017; Maddineni et al., 2020). However, a significant but weak association between PM2.5 and IOP was detected in our study (eTable 4 in the Supplement), as is supported by similar findings in the UK biobank population (Chua et al., 2019). It suggested that other biological mechanisms beyond the pathway of IOP elevated might be involved in the high odds of glaucoma linked to long-term PM2.5 exposure, considering the PM2.5-glaucoma association obtained from multivariable regression after adjusting for IOP level and treatment of IOP. It has been demonstrated that PM2.5 exposure was related to increased levels of pro-inflammatory factors and oxidative stress (Münzel et al., 2018; Xia et al., 2019). And some epidemiological studies observed that inflammatory cytokines and oxidative stress markers were elevated in aqueous humor and serum of patients with glaucoma (Chua et al., 2012; Huang et al., 2014; Li et al., 2020; Takai et al., 2012; Takayanagi et al., 2020). Additional evidence showed that trabecular meshwork dysfunction may be mediated by oxidative stress and inflammation (Baudouin et al., 2020), which could lead to the obstruction of the circulation of aqueous humor. It was also recorded that reactive oxygen species and oxidative mitochondrial DNA may lead to apoptosis of retinal ganglion cells (Baudouin et al., 2020). Moreover, PM2.5-related endothelial dysfunction could lead to imbalance of endothelium-derived contracting (e.g., endothelin) and relaxing (e.g., nitric oxide) factors (Calderón-Garcidueñas et al., 2015; Wauters et al., 2013; Xia et al., 2019), which might affect the circulation of ophthalmic blood and aqueous humor (Cavet et al., 2014; Haefliger et al., 2001). Although the evidence above provides some perspectives in inflammation, oxidative stress, and endothelial dysfunction, more researches with careful design is needed to explore specific biomechanisms linking the risk of glaucoma and long-term exposure to PM2.5.
Several limitations must be addressed to interpret the study findings with caveats in mind. First, the health data derived from an epidemiological observational design made it impossible to establish causality between PM2.5 exposure and glaucoma. Second, the relatively small sample size of POAG limited the statistical power in testing the association between POAG and PM2.5 exposure, although the direction of effect estimation supported that long-term exposure to PM2.5 would increase odds of POAG in the study. Epidemiological data showed that risk of developing PACG had a greater prevalence in Asian populations than those of European and African descent (He et al., 2006). More studies considering both genetic and environment factors will be warranted to illustrate whether biological differences would exist in harmful effects of PM2.5 on PACG and POAG in diverse populations. Third, information about indoor air pollution and time-activity patterns were not collected in the survey, which might result in bias of health effect estimation. Forth, we did not consider the co-exposure effects of mixed pollutants such as ozone and nitrogen oxides since reliable gaseous exposure data at 1-km resolution was unavailable in the study. Fifth, the current data on smoke (yes or no) cannot be further classified into never, former, or current smoke due to the limitation of questionnaire. However, an additional analysis for association between PM2.5 exposure and glaucoma showed ORs of 1.07 (95% CI: 1.00–1.15) for glaucoma, 1.13 (95% CI: 1.02–1.26) for PACG, and 1.04 (95% CI: 0.92–1.18) for POAG, after excluding the smoke status from the covariates in Model 3. The results similar to the main analysis suggested that potential misclassification of smoke status would less bias the association estimation in the study. Last, 2380 participants were excluded in the study due to missing information in PM2.5 and several covariates (Fig. 1). Although the excluded people had a lower percentage of women and were slightly younger compared with the people included, the prevalence of glaucoma and average level of IOP showed similar between the included and excluded, which suggested estimations of PM2.5-glaucoma association might not be affected substantially by the excluded people due to missing data.
5. Conclusions
In this large-scale population-based study, long-term PM2.5 exposure was associated with higher odds of glaucoma across a wider range of PM2.5 concentrations, which added to novel evidence for the PM2.5-induced adverse health effects. The findings may provide pivotal reference for estimation of glaucoma burdens attributable to long-term PM2.5 pollution, and support insights on improvement of air quality being helpful in prevention of glaucoma especially in highly polluted regions.
Supplementary Material
Acknowledgement
This work was supported by the National Natural Science Foundation of China (grant numbers, 82020108007, 81830026), Beijing-Tianjin-Hebei Special Project (grant number, 19JCZDJC64300(Z)), and National Key Research and Development Program of China (grant numbers, 2016YFC0206503, 2017YFC0211605). The work of Y. Liu was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health (Award #1R01ES032140). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of competing interest
Authors declare no actual or potential competing financial interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijheh.2021.113858.
References
- Aik J, Chua R, Jamali N, Chee E, 2020. The burden of acute conjunctivitis attributable to ambient particulate matter pollution in Singapore and its exacerbation during South-East Asian haze episodes. Sci. Total Environ 740, 140129. [DOI] [PubMed] [Google Scholar]
- Baudouin C, Kolko M, Melik-Parsadaniantz S, Messmer EM, 2020. Inflammation in Glaucoma: from the back to the front of the eye, and beyond. Prog. Retin. Eye Res 100916. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Franco-Lira M, D’Angiulli A, et al. , 2015. Mexico City normal weight children exposed to high concentrations of ambient PM2.5 show high blood leptin and endothelin-1, vitamin D deficiency, and food reward hormone dysregulation versus low pollution controls. Relevance for obesity and Alzheimer disease. Environ. Res 140, 579–592. [DOI] [PubMed] [Google Scholar]
- Cavet ME, Vittitow JL, Impagnatiello F, Ongini E, Bastia E, 2014. Nitric oxide (NO): an emerging target for the treatment of glaucoma. Invest. Ophthalmol. Vis. Sci 55, 5005–5015. [DOI] [PubMed] [Google Scholar]
- Chua J, Vania M, Cheung CM, et al. , 2012. Expression profile of inflammatory cytokines in aqueous from glaucomatous eyes. Mol. Vis 18, 431–438. [PMC free article] [PubMed] [Google Scholar]
- Chua SYL, Khawaja AP, Dick AD, et al. , 2020. Ambient air pollution associations with retinal morphology in the UK Biobank. Invest. Ophthalmol. Vis. Sci 61, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua SYL, Khawaja AP, Morgan J, et al. , 2019. The relationship between ambient atmospheric fine particulate matter (PM2.5) and glaucoma in a large community cohort. Invest. Ophthalmol. Vis. Sci 60, 4915–4923. [DOI] [PubMed] [Google Scholar]
- Chua SYL, Warwick A, Peto T, et al. , 2021. Association of ambient air pollution with age-related macular degeneration and retinal thickness in UK Biobank. Br. J. Ophthalmol 10.1136/bjophthalmol-2020-316218. [DOI] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, et al. , 2017. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389, 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flammer J, Konieczka K, Bruno RM, Virdis A, Flammer AJ, Taddei S, 2013. The eye and the heart. Eur. Heart J 34, 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster PJ, Buhrmann R, Quigley HA, Johnson GJ, 2002. The definition and classification of glaucoma in prevalence surveys. Br. J. Ophthalmol 86, 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2019 Blindness and Vision Impairment Collaborators, 2021. Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. Lancet Glob. Health 9, e130–e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A, Leung G, Aubin MJ, Kergoat MJ, Li G, Freeman EE, 2021. Fine particulate matter and age-related eye disease: the canadian longitudinal study on aging. Invest. Ophthalmol. Vis. Sci 62, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefliger IO, Flammer J, Bény JL, Lüscher TF, 2001. Endothelium-dependent vasoactive modulation in the ophthalmic circulation. Prog. Retin. Eye Res 20, 209–225. [DOI] [PubMed] [Google Scholar]
- He M, Foster PJ, Ge J, et al. , 2006. Prevalence and clinical characteristics of glaucoma in adult Chinese: a population-based study in Liwan District, Guangzhou. Invest. Ophthalmol. Vis. Sci 47, 2782–2788. [DOI] [PubMed] [Google Scholar]
- Huang K, Liang F, Yang X, et al. , 2019. Long term exposure to ambient fine particulate matter and incidence of stroke: prospective cohort study from the China-PAR project. BMJ 367, l6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Chen S, Gao X, et al. , 2014. Inflammation-related cytokines of aqueous humor in acute primary angle-closure eyes. Invest. Ophthalmol. Vis. Sci 55, 1088–1094. [DOI] [PubMed] [Google Scholar]
- Hvidtfeldt UA, Severi G, Andersen ZJ, et al. , 2020. Long-term low-level ambient air pollution exposure and risk of lung cancer - a pooled analysis of 7 European cohorts. Environ. Int 146, 106249. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S, 2017. Glaucoma. Lancet 390, 2183–2193. [DOI] [PubMed] [Google Scholar]
- Jung SJ, Mehta JS, Tong L, 2018. Effects of environment pollution on the ocular surface. Ocul. Surf 16, 198–205. [DOI] [PubMed] [Google Scholar]
- Künzli N, Jerrett M, Mack WJ, et al. , 2005. Ambient air pollution and atherosclerosis in Los Angeles. Environ. Health Perspect 113, 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Shao M, Li Y, et al. , 2020. Relationship between oxidative stress biomarkers and visual field progression in patients with primary angle closure glaucoma. Oxid. Med. Cell. Longev 2020, 2701539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Liu F, Huang K, et al. , 2020a. Long-term exposure to fine particulate matter and cardiovascular disease in China. J. Am. Coll. Cardiol 75, 707–717. [DOI] [PubMed] [Google Scholar]
- Liang F, Xiao Q, Huang K, et al. , 2020b. The 17-y spatiotemporal trend of PM(2.5) and its mortality burden in China. Proc. Natl. Acad. Sci. U. S. A 117, 25601–25608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Guo Y, Ruan Z, et al. , 2019. Ambient PM2.5 and O3 and their combined effects on prevalence of presbyopia among the elderly: a cross-sectional study in six low-and middle-income countries. Sci. Total Environ 655, 168–173. [DOI] [PubMed] [Google Scholar]
- Liu S, Jørgensen JT, Ljungman P, et al. , 2021. Long-term exposure to low-level air pollution and incidence of asthma: the ELAPSE project. Eur. Respir. J 57, 2003099. [DOI] [PubMed] [Google Scholar]
- Maddineni P, Kasetti RB, Patel PD, et al. , 2020. CNS axonal degeneration and transport deficits at the optic nerve head precede structural and functional loss of retinal ganglion cells in a mouse model of glaucoma. Mol. Neurodegener 15, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura T, Ichinose T, Yamagami S, et al. , 2014. Airborne particulate matter (PM2.5) and the prevalence of allergic conjunctivitis in Japan. Sci. Total Environ 487, 493–499. [DOI] [PubMed] [Google Scholar]
- Mo Z, Fu Q, Lyu D, et al. , 2019. Impacts of air pollution on dry eye disease among residents in Hangzhou, China: a case-crossover study. Environ. Pollut 246, 183–189. [DOI] [PubMed] [Google Scholar]
- Münzel T, Gori T, Al-Kindi S, et al. , 2018. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur. Heart J 39, 3543–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nongpiur ME, He M, Amerasinghe N, et al. , 2011. Lens vault, thickness, and position in Chinese subjects with angle closure. Ophthalmology 118, 474–479. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Akagi T, Suda K, et al. , 2020. Longitudinal changes in superficial microvasculature in glaucomatous retinal nerve fiber layer defects after disc hemorrhage. Sci. Rep 10, 22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, 2011. Glaucoma. Lancet 377, 1367–1377. [DOI] [PubMed] [Google Scholar]
- Shan A, Chen X, Yang X, et al. , 2021. Association between long-term exposure to fine particulate matter and diabetic retinopathy among diabetic patients: a national cross-sectional study in China. Environ. Int 154, 106568. [DOI] [PubMed] [Google Scholar]
- Song W, Shan L, Cheng F, et al. , 2011. Prevalence of glaucoma in a rural northern China adult population: a population-based survey in kailu county, inner Mongolia. Ophthalmology 118, 1982–1988. [DOI] [PubMed] [Google Scholar]
- Sun HY, Luo CW, Chiang YW, et al. , 2021. Association between PM2.5 exposure level and primary open-angle glaucoma in Taiwanese adults: a nested case-control study. Int. J. Environ. Res. Publ. Health 18, 1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Dai Y, Chen Y, et al. , 2017. Primary angle closure glaucoma: what we know and what we don’t know. Prog. Retin. Eye Res 57, 26–45. [DOI] [PubMed] [Google Scholar]
- Takai Y, Tanito M, Ohira A, 2012. Multiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma, exfoliation glaucoma, and cataract. Invest. Ophthalmol. Vis. Sci 53, 241–247. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Takai Y, Kaidzu S, Tanito M, 2020. Evaluation of redox profiles of the serum and aqueous humor in patients with primary open-angle glaucoma and exfoliation glaucoma. Antioxidants 9, 1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY, 2014. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121, 2081–2090. [DOI] [PubMed] [Google Scholar]
- Torricelli AA, Novaes P, Matsuda M, Alves MR, Monteiro ML, 2011. Ocular surface adverse effects of ambient levels of air pollution. Arq. Bras. Oftalmol 74, 377–381. [DOI] [PubMed] [Google Scholar]
- Turner MC, Andersen ZJ, Baccarelli A, et al. , 2020. Outdoor air pollution and cancer: an overview of the current evidence and public health recommendations. CA A Cancer J. Clin 10.3322/caac.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauters A, Dreyfuss C, Pochet S, et al. , 2013. Acute exposure to diesel exhaust impairs nitric oxide-mediated endothelial vasomotor function by increasing endothelial oxidative stress. Hypertension 62, 352–358. [DOI] [PubMed] [Google Scholar]
- Xia B, Zhou Y, Zhu Q, et al. , 2019. Personal exposure to PM2.5 constituents associated with gestational blood pressure and endothelial dysfunction. Environ. Pollut 250, 346–356. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Chang HH, Geng G, Liu Y, 2018. An ensemble machine-learning model to predict historical PM2.5 concentrations in China from satellite data. Environ. Sci. Technol 52, 13260–13269. [DOI] [PubMed] [Google Scholar]
- Yang BY, Guo Y, Zou Z, et al. , 2020. Exposure to ambient air pollution and visual impairment in children: a nationwide cross-sectional study in China. J. Hazard Mater 407, 124750. [DOI] [PubMed] [Google Scholar]
- Zhong H, Li J, Li C, et al. , 2012. The prevalence of glaucoma in adult rural Chinese populations of the Bai nationality in Dali: the Yunnan Minority Eye Study. Invest. Ophthalmol. Vis. Sci 53, 3221–3225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


