Abstract
Objective
The effects of coffee consumption on serum uric acid (SUA) levels and gout risk are controversial. There have hitherto been no reports based on Mendelian randomization (MR) analysis of its effects that consider pleiotropy. Here, we evaluated the effects of coffee consumption across ancestry populations, taking pleiotropy into account.
Methods
We performed the first MR analyses for coffee consumption on SUA levels and gout, considering pleiotropy. We used the following summary statistics of genome‐wide association studies from a Japanese population: habitual coffee consumption (152,634 subjects), gout (3053 cases and 4554 controls), and SUA levels (121,745 subjects). In addition to fixed‐effect inverse variance weighted (IVW) meta‐analysis, we performed a robust evaluation of heterogeneity and removed several instruments for reasons of possible pleiotropy. Previous European datasets were also reevaluated while heterogeneity was considered.
Results
Habitual coffee consumption was significantly and inversely associated with gout (odds ratio [OR] = 0.29, 95% confidence interval [95% CI]: 0.16‐0.51, P = 1.9 × 10−5) in random‐effect IVW (P het = 5.5 × 10−19). Excluding pleiotropic instruments, the protective effect on gout was confirmed in fixed‐effect IVW analysis (OR = 0.75, 95% CI: 0.58‐0.97, P = 0.026) without heterogeneity (P het = 0.39). However, we observed no significance in the previous European datasets when heterogeneity was considered. Associations were not observed between coffee consumption and SUA levels in either ancestry in MR analyses that considered pleiotropy. Multivariable MR analysis showed that increased coffee consumption significantly reduced gout risk, even after adjusting for SUA levels (OR = 0.50, 95% CI: 0.31‐0.81, P = 0.0046).
Conclusion
With pleiotropy taken into account, our MR analyses revealed that coffee consumption can causally reduce gout risk, and that it may reduce gout risk independently of SUA levels.
INTRODUCTION
Gout is a common arthritis that results from hyperuricemia due to environmental and genetic factors. Previous observational studies have shown an inverse association between habitual coffee consumption and gout (1). In 2018, Larsson et al performed a Mendelian randomization (MR) analysis using genome‐wide association study (GWAS) data in Europeans to infer the causal effects of coffee consumption on gout, which showed that coffee consumption can reduce gout risk (2). However, as Lee pointed out in his corresponding letter (3) to the report by Larsson et al (2), pleiotropy should be considered in MR analyses, and other approaches, including the random effect inverse variance weighted (IVW) meta‐analysis, should be applied to adjust the causal effect and achieve a robust assessment. Hitherto there have been no reports on MR analysis that investigate the association between coffee consumption and gout susceptibility while taking pleiotropy into account. MR is also generally performed within a single ancestry, whereas validations within independent ancestries are necessary.
Here, to clarify the question of whether or not habitual coffee consumption is causally associated with serum uric acid (SUA) levels and gout risk, we performed the first MR analysis of the effect of coffee consumption on SUA levels and gout considering pleiotropy.
Furthermore, we reanalyzed the European datasets reported by Larsson et al (2), taking the heterogeneity into account. Considering the heterogeneity of genetic instruments is expected to reduce the pleiotropic effect, which is important to ensure that the MR analysis results are robust.
MATERIALS AND METHODS
This study was approved by the respective institutions' ethical committees (Osaka University and National Defense Medical College). All procedures were performed in accordance with the Declaration of Helsinki, with written informed consent obtained from each participant.
We obtained the Japanese GWAS summary statistics for habitual coffee consumption, defined as the number of days per week that a subject drank coffee (152,634 subjects) (4), gout (3053 cases and 4554 controls) (5), and SUA levels (121,745 subjects) (6), all of which were among the largest studies with non‐Europeans. As genetic instruments, we obtained the 11 lead single nucleotide polymorphisms (SNPs) at loci with a genome‐wide significance threshold (P < 5.0 × 10−8) in the Japanese GWAS dataset of coffee consumption (Supplementary Table S1). Among these SNPs, rs75544042 was not contained in the Japanese gout GWAS dataset, so we adopted rs141471965 as a proxy SNP, which has a linkage disequilibrium of r 2 = 1 in the 1000 Genomes Project phase 3v5 East Asian populations (1KGP p3v5 EAS). The forest plot describing MR effect size for individual SNPs (Figure 1) and the funnel plot (Supplementary Figure S1) showed an apparent outlier SNP (rs141471965 of ABCG2). We therefore excluded rs141471965 and performed MR analysis using 10 instruments. For the same reason, we excluded rs141471965 in the MR analysis between coffee consumption and SUA levels.
Figure 1.
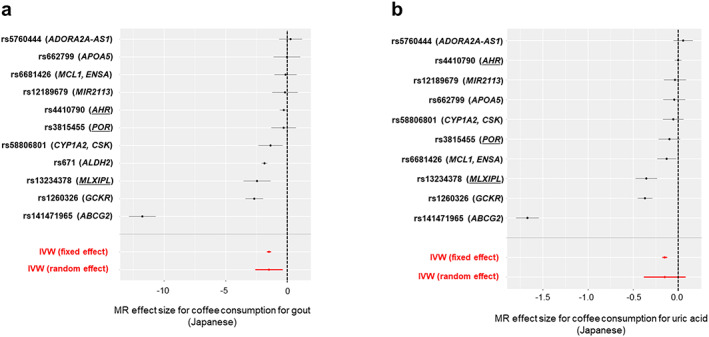
Forest plots describing MR effect size for individual SNPs in the Japanese population. Effect size estimates for coffee consumption on (a) gout or (b) uric acid levels are plotted with 95% confidence intervals for individual SNPs (black) and inverse variance‐weighted analysis (red). The vertical dotted line indicates an effect size estimate of 0. The individual SNPs and the mapped genes are labeled on the left. Coffee‐associated genes common to Europeans and Japanese are underlined. IVW, inverse variance weighted; MR, Mendelian randomization; SNP, single nucleotide polymorphism.
For the European analysis, we used summary statistics provided by the Coffee and Caffeine Genetics Consortium (CCGC) (7) as exposure and used Global Urate Genetics Consortium–provided GWAS summary statistics (8) as outcome. In the CCGC GWAS, coffee consumption was defined in terms of cups per day. We adopted five instruments, as did Larsson et al previously.
We used R statistical software with the TwoSampleMR package (9) for MR analysis. We began by performing an IVW analysis using multiple instruments. A P value of 0.05 by Cochran's Q test was used as the threshold for determining whether to prioritize the fixed effects model or the random effects model. We also performed weighted‐median, weighted‐mode, and MR‐Egger analysis to relax the instrumental variable assumption. The three methods adopt individually different strategies to avoid the impact of invalid instruments.
For a more conservative analysis with the Japanese dataset, we additionally excluded the three instruments suspected to have a pleiotropic effect on gout. Matoba et al reported that polymorphisms on ALDH have a genetic impact on alcohol drinking behavior as well as on coffee consumption (4). Polymorphisms of ALDH therefore may affect gout independently of coffee consumption. Furthermore, Hutton et al reported that the risk alleles of GCKR and MLXIPL affect gout independently of coffee intake (10). Based on these facts, we removed rs671 (ALDH2), rs1260326 (GCKR), and rs13234378 (MLXIPL) from 10 instruments and reanalyzed using the remaining seven instruments. As a complementary analysis, we applied Mendelian randomization pleiotropy residual sum and outlier (MR‐PRESSO) (11) to the original 11 lead SNPs to examine whether they had a statistically significant pleiotropic effect.
Finally, we performed a multivariable MR analysis (12) to estimate the association between coffee consumption and gout while adjusting for SUA levels. We performed a multivariable MR analysis in the Japanese population because we were able to access the full summary statistics of coffee consumption in only the Japanese cohort. In the multivariable MR analysis, we also excluded rs141471965 (ABCG2), and the three instruments were removed in the aforementioned conservative analysis.
RESULTS
Of the 11 lead SNPs from the Japanese GWAS dataset of coffee consumption, rs141471965 of ABCG2 was an apparent outlier in the forest plot (Figure 1) and funnel plot (Supplementary Figure S1). A random effect IVW analysis for gout was therefore performed using the remaining 10 instruments (P het = 5.5 × 10−19 in Cochran's Q test: Table 1, Figure 2) with an F statistic of 132, indicating that the instruments are sufficiently associated with coffee consumption to permit us to ignore weak instrument bias. Coffee consumption was significantly inversely associated with gout (odds ratio [OR] = 0.29, 95% confidence interval [95% CI]: 0.16‐0.51, P = 1.9 × 10−5).
Table 1.
Mendelian randomization analyses results inferring causality between coffee consumption and gout or SUA levels
| Exposure | Outcome | No. of instruments | Method | β | SE | OR [95% CI] | P | Heterogeneity | Pleiotropy | |
|---|---|---|---|---|---|---|---|---|---|---|
| Population | Cochran's Q P het | Intercept P | ||||||||
| Japanese | Coffee | Gout | 10 a | IVW (fixed‐effect) | −1.25 | 0.08 | 0.29 [0.24‐0.34] | 2.5 × 10−49 | 5.5 × 10−19 | — |
| IVW (random‐effect) | −1.25 | 0.29 | 0.29 [0.16‐0.51] | 1.9 × 10−5 | — | |||||
| MR‐Egger | −1.76 | 0.48 | 0.17 [0.07‐0.44] | 6.1 × 10−3 | 1.4 × 10−15 | 0.22 | ||||
| Weighted‐median | −1.40 | 0.33 | 0.25 [0.13‐0.47] | 2.7 × 10−5 | — | — | ||||
| Weighted‐mode | −1.85 | 0.19 | 0.16 [0.11‐0.23] | 3.8 × 10−6 | — | — | ||||
| Coffee | Gout | 7 b | IVW (fixed‐effect) | −0.29 | 0.13 | 0.75 [0.58‐0.97] | 0.026 | 0.39 | — | |
| IVW (random‐effect) | −0.29 | 0.13 | 0.75 [0.58‐0.97] | 0.030 | — | |||||
| MR‐Egger | −0.34 | 0.30 | 0.71 [0.39‐1.28] | 0.31 | 0.29 | 0.84 | ||||
| Weighted‐median | −0.27 | 0.16 | 0.77 [0.57‐1.04] | 0.086 | — | — | ||||
| Weighted‐mode | −0.28 | 0.16 | 0.76 [0.55‐1.04] | 0.14 | — | — | ||||
| Coffee | SUA levels | 9 a | IVW (fixed‐effect) | −0.080 | 0.014 | 0.92 [0.9‐0.95] | 9.6 × 10−9 | 2.0 × 10−16 | — | |
| IVW (random‐effect) | −0.080 | 0.047 | 0.92 [0.84‐1.01] | 0.090 | — | |||||
| MR‐Egger | 8.0 × 10−4 | 0.11 | 1.00 [0.81‐1.24] | 0.99 | 2.2 × 10−15 | 0.44 | ||||
| Weighted‐median | −0.022 | 0.020 | 0.98 [0.94‐1.02] | 0.26 | — | — | ||||
| Weighted‐mode | −0.011 | 0.017 | 0.99 [0.96‐1.02] | 0.55 | — | — | ||||
| European | Coffee | Gout | 5 | IVW (fixed‐effect) c | −0.58 | 0.21 | 0.56 [0.38‐0.84] | 5.3 × 10−3 | 0.027 | — |
| IVW (random‐effect) | −0.58 | 0.34 | 0.56 [0.29‐1.1] | 0.092 | — | |||||
| MR‐Egger | −0.29 | 0.83 | 0.75 [0.15‐3.8] | 0.75 | 0.016 | 0.72 | ||||
| Weighted‐median | −0.92 | 0.30 | 0.4 [0.22‐0.72] | 2.1 × 10−3 | — | — | ||||
| Weighted‐mode | −0.98 | 0.39 | 0.37 [0.17‐0.8] | 0.065 | — | — | ||||
| Coffee | SUA levels | 5 | IVW (fixed‐effect) | −0.15 | 0.03 | 0.86 [0.8‐0.92] | 7.9 × 10−6 | 6.8 × 10−8 | — | |
| IVW (random‐effect) | −0.15 | 0.11 | 0.86 [0.7‐1.06] | 0.15 | — | |||||
| MR‐Egger | −0.16 | 0.26 | 0.85 [0.51‐1.43] | 0.58 | 1.7 × 10−8 | 0.97 | ||||
| Weighted‐median | −0.03 | 0.05 | 0.97 [0.87‐1.07] | 0.51 | — | — | ||||
| Weighted‐mode | −0.10 | 0.06 | 0.91 [0.81‐1.01] | 0.16 | — | — |
Abbreviations: IVW, inverse variance weighted; MR, Mendelian randomization; OR, odds ratio; SUA, serum uric acid.
After exclusion of the outlier instruments (rs141471965 on ABCG2) in the forest plot.
After additional exclusion of the three instruments suspected to have pleiotropy effects.
IVW (fixed‐effect) analyses were previously performed by Larsson et al (2).
Figure 2.
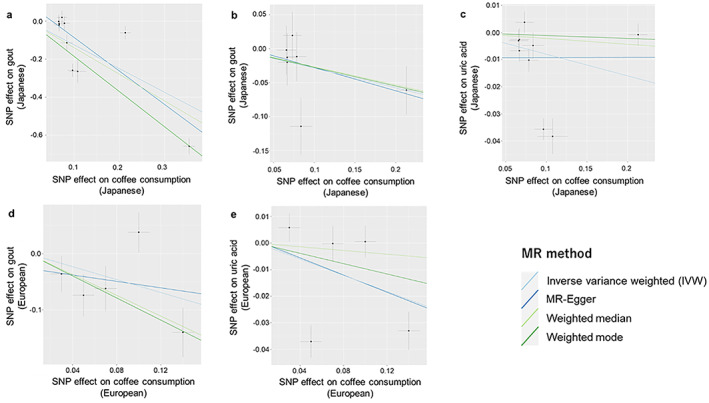
Scatter plots describing the association between coffee consumption (exposure) and gout or SUA levels (outcome). Dots represent the coffee‐associated SNPs plotted along with effect size estimates on coffee consumption (x‐axis) and gout or uric acid levels (y‐axis) with 95% confidence intervals (a‐c) in Japanese and (d and e) in Europeans. The line slopes indicate the effect size estimates in four MR analyses using multiple SNPs (ie, inverse variance weighted, MR‐Egger, weighted‐median, and weighted‐mode). Each method is indicated by differently colored lines. Prior to each of the analyses, we removed the outlier instrument (rs141471965 of ABCG2) in panels a and c and removed the three instruments suspected to have pleiotropic effects on gout in panel b. MR, Mendelian randomization; SNP, single nucleotide polymorphism; SUA, serum uric acid.
The previous European MR study used fixed‐effect IVW analysis despite the significant heterogeneity seen (P het = 0.027), and we did not obtain a significant result in random effect IVW analysis (OR = 0.56, 95% CI: 0.29‐1.1, P = 0.092).
With the Japanese dataset, we also applied weighted‐median, weighted‐mode, and MR‐Egger analysis to eliminate the impact of latent invalid instruments. We confirmed a significant inverse association between coffee consumption and gout in all the methods (P = 2.7 × 10−5 in weighted‐median, P = 3.8 × 10−6 in weighted‐mode, and P = 6.1 × 10−3 in MR‐Egger). The intercept analysis in MR‐Egger did not indicate any significant directional pleiotropy (P = 0.22). Similar results were obtained with all 11 instrumental variables except for MR‐Egger analysis (Supplementary Table S2).
As a conservative analysis, we excluded three more instruments (rs671 of ALDH2, rs1260326 of GCKR, and rs13234378 of MLXIPL) suspected to have a pleiotropic effect on gout and reanalyzed using the remained seven instruments. With this framework, no heterogeneity was supported (P het = 0.39), and we observed a consistent result of coffee consumption being inversely associated with gout in fixed‐effect IVW analysis (OR = 0.75, 95% CI: 0.58‐0.97, P = 0.026). As another strategy to test for pleiotropy, we applied MR‐PRESSO to the 11 lead SNPs to evaluate whether or not they were outliers. MR‐PRESSO revealed rs141471965 (ABCG2) and rs4410790 (AHR) to be statistical outliers. The MR analysis that removed them also supported our result, in which coffee consumption was negatively associated with gout (OR = 0.21, 95% CI: 0.12‐0.35, P = 8.8 × 10−9) as random effect IVW analysis (P het = 1.4 × 10−10).
MR analysis between coffee consumption and SUA levels in both ancestries showed no significant inverse causal association except for fixed‐effect IVW analysis, which did not take pleiotropy into account, and from which conclusions should not be drawn because of significant heterogeneity (P het = 2.0 × 10−16 in Japanese and P het = 6.8 × 10−8 in Europeans).
Furthermore, our multivariable MR analysis confirmed the independent effect of coffee consumption on gout after adjustment for SUA levels (OR = 0.50, 95% CI: 0.31‐0.81, P = 0.0046). These findings suggest that coffee consumption reduces gout risk independently of SUA levels. Coffee consumption thus appears to have protective effects on the progression from asymptomatic hyperuricemia to gout.
DISCUSSION
In this study, we performed the first MR analysis for estimating the impact of coffee consumption on SUA levels and gout risk, taking pleiotropy into account. Our findings from the Japanese population are the first to reveal that coffee consumption reduces gout risk, as revealed by MR analyses, whereas our reanalyses of previous datasets from Europeans did not show significant associations between coffee consumption and gout risk. By considering pleiotropy, the present results are more robust than those in the report by Larsson et al (2). Our robust MR analyses showed significant results from Japanese datasets. The outlier variant of rs141471965 in the 11 genetic instruments is a proxy (r 2 = 1 in 1KGP p3v5 EAS) for p.Gln141Lys (rs2231142), the ABCG2 missense SNP. The rs2231142 variant has been reported to alter the function of the urate transporter, resulting in increased SUA levels and gout risk in various populations, including Japanese (13, 14). This fact explains why rs141471965 has an obvious pleiotropic effect on gout risk not mediated by coffee consumption. A conservative analysis without three more instruments worked well because heterogeneity was not supported by the Cochran's Q test in the final analysis.
The present study suggests the effect of coffee consumption on gout risk to be independent of SUA levels. The previous European MR analysis reported that coffee consumption significantly reduced SUA levels (2), but when heterogeneity was taken into account, the significance was no longer supported. In a systematic review and meta‐analysis, Zhang et al reported coffee consumption to significantly reduce the gout risk but not reduce SUA levels (1). A recent observational study has also shown there to be no significant relationship between coffee consumption and SUA levels (15). Nevertheless, the relationship between coffee consumption and SUA levels is still unclear; the present results may suggest that coffee consumption reduces gout risk independently of uric acid, possibly acting against inflammation and/or innate immunity (16).
One of the limitations of this study is that in the Japanese MR analysis, we used as exposure the number of days per week that a subject drank coffee, because it was the only available data collected by questionnaire. The loci identified in the Japanese and European coffee consumption GWAS were mostly concordant (Supplementary Table S1), and we conclude that the genetic effects of each study reflect the same biological pathway. However, because data collected in different study designs can be a source of disjoint effects on outcome, a further study needs to be conducted to elucidate the mechanism by which coffee consumption protects against the development of gout.
In summary, by MR analyses using large‐scale GWAS results across ancestry populations and by considering the heterogeneity and pleiotropy of instruments, our study indicates that coffee consumption can causally reduce gout risk. Although the impact on SUA levels is still ambiguous, obvious protective effects of habitual coffee consumption on gout risk were observed. We consider that coffee consumption may reduce gout risk independently of SUA levels. Our study suggests the presence of biological pathways involved in gout pathogenesis other than SUA levels and should contribute to the development of preventive medicine against gout.
AUTHOR CONTRIBUTORS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Drs. Okada and Matsuo had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Shirai, Okada, Matsuo.
Acquisition of data
Nakayama, Kawamura, Okada, Matsuo.
Analysis and interpretation of data
Shirai, Nakayama, Kawamura, Toyoda, Nakatochi, Shimizu, Shinomiya, Okada, Matsuo.
Supporting information
Disclosure Form
Supplementary Table 1 Genetic instruments we used in the Mendelian randomization analysis in Japanese populations
Supplementary Table 2: Mendelian randomization analysis results using all instruments obtained from the Japanese coffee GWAS statistics
Supplementary Figure 1: Funnel plots describing the MR effect size for individual SNPs in relation to its precision in the Japanese population
ACKNOWLEDGMENTS
We would like to thank all the participants for their generous involvement in this study. We also thank K. Morichika, M. Miyazawa, and M. Seki (National Defense Medical College) for technical assistance.
Appendix A.
Members of the Japan Gout Genomics Consortium (Japan Gout) are as follows: Kimiyoshi Ichida, Tappei Takada, Hirofumi Nakaoka, Ken Yamamoto, Tony R. Merriman, Takahiro Nakamura, the Japan Multi‐Institutional Collaborative Cohort (J‐MICC) Study Group (principal investigator: Kenji Wakai), Toru Shimizu, Hiroshi Ooyama, Keiko Ooyama, Mitsuo Nagase, Yuji Hidaka, Satoko Suzuki, Satoko Iwasawa, Hiroshi Nakashima, Yutaka Sakurai, and Masashi Tsunoda.
This study was supported by grants from the Japan Society for the Promotion of Science (JSPS) Kakenhi (20K21834, 19H01021, 20H00566, 21KK0173, 20K23152, 19K19434, 17H04128, 19K22786, 221S0001, 221S0002, 16H06277 [CoBiA], and 16H06279 [PAGS]), AMED (JP20km0405211, JP20ek0109413, JP20ek0410075, JP20gm4010006, and JP20km0405217), the Ministry of Defense, Gout and Uric Acid Foundation of Japan, the Takeda Science Foundation, and the Bioinformatics Initiative of Osaka University Graduate School of Medicine, Osaka University.
Members of the Japan Gout Genomics Consortium are shown in Appendix A.
Drs. Shirai, Nakayama, and Kawamura contributed equally to this work.
No potential conflicts of interest relevant to this article were reported.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr2.11425&file=acr211425-sup-0001-Disclosureform.pdf.
Contributor Information
Yukinori Okada, Email: yokada@sg.med.osaka-u.ac.jp.
Hirotaka Matsuo, Email: hmatsuo.ndmc@gmail.com.
REFERENCES
- 1. Zhang Y, Yang T, Zeng C, Wei J, Li H, Xiong Y‐L, et al. Is coffee consumption associated with a lower risk of hyperuricaemia or gout? A systematic review and meta‐analysis. BMJ Open 2016;6:e009809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Larsson SC, Carlström M. Coffee consumption and gout: a Mendelian randomisation study. Ann Rheum Dis 2018;77:1544–6. [DOI] [PubMed] [Google Scholar]
- 3. Lee YH. Coffee consumption and gout: a Mendelian randomisation study. Ann Rheum Dis 2019;78:e130. [DOI] [PubMed] [Google Scholar]
- 4. Matoba N, Akiyama M, Ishigaki K, Kanai M, Takahashi A, Momozawa Y, et al. GWAS of 165,084 Japanese individuals identified nine loci associated with dietary habits. Nat Hum Behav 2020;4:308–16. [DOI] [PubMed] [Google Scholar]
- 5. Nakayama A, Nakatochi M, Kawamura Y, Yamamoto K, Nakoaka H, Shimizu S, et al. Subtype‐specific gout susceptibility loci and enrichment of selection pressure on ABCG2 and ALDH2 identified by subtype genome‐wide meta‐analyses of clinically defined gout patients. Ann Rheum Dis 2020;79:657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakatochi M, Kanai M, Nakayama A, Hishida A, Kawamura Y, Ichihara S, et al. Genome‐wide meta‐analysis identifies multiple novel loci associated with serum uric acid levels in Japanese individuals. Commun Biol 2019;2:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cornelis MC, Byrne EM, Esko T, Nalls MA, Ganna A, Paynter N, et al. Genome‐wide meta‐analysis identifies six novel loci associated with habitual coffee consumption. Mol Psychiatry 2015;20:647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Köttgen A, Albrecht E, Teumer A, Vitart V, Krumsiek J, Hundertmark C, et al. Genome‐wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet 2013;45:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR‐Base platform supports systematic causal inference across the human phenome. Elife 2018;7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hutton J, Fatima T, Major TJ, Topless R, Stamp LK, Merriman TR, et al. Mediation analysis to understand genetic relationships between habitual coffee intake and gout. Arthritis Res Ther 2018;20:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in casual relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 2018;50:693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol 2015;181:251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsuo H, Takada T, Ichida K, Nakamura T, Nakayama A, Ikebuchi Y, et al. Common defects of ABCG2, a high‐capacity urate exporter, cause gout: a function‐based genetic analysis in a Japanese population. Sci Transl Med 2009;1:5ra11. [DOI] [PubMed] [Google Scholar]
- 14. Woodward OM, Köttgen A, Coresh J, Boerwinkle E, Guggino WB, Köttgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A. 2009;106:10338–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jung JH, Seok H, Choi SJ, Kim C, Bang CH, Song GG. Relationship between coffee consumption and serum uric acid level in the general Korean population: A nationwide cross‐sectional study. Int J Rheum Dis 2020;23:420–7. [DOI] [PubMed] [Google Scholar]
- 16. Paiva C, Beserra B, Reis C, Dorea JG, Da Costa T, Amato AA. Consumption of coffee or caffeine and serum concentration of inflammatory markers: a systematic review. Crit Rev Food Sci Nutr 2019;59:652–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Supplementary Table 1 Genetic instruments we used in the Mendelian randomization analysis in Japanese populations
Supplementary Table 2: Mendelian randomization analysis results using all instruments obtained from the Japanese coffee GWAS statistics
Supplementary Figure 1: Funnel plots describing the MR effect size for individual SNPs in relation to its precision in the Japanese population


