Abstract
Objective
Comorbidities in rheumatoid arthritis (RA) can influence treatment selection, impact treatment persistency, and increase health care costs. This study assessed the magnitude of comorbidity burden via epidemiology (incidence and prevalence) and associated costs of select comorbidities in RA patients: anemia, malignancy, venous thromboembolism (VTE), major adverse cardiovascular events (MACE), and infections, stratified by history of disease‐modifying antirheumatic drug (DMARD) exposure.
Methods
From the IQVIA PharMetrics® Plus database, we selected adult patients with RA (2 or more RA diagnostic codes at least 30 days apart) at initiation of a new DMARD (DMARD‐naïve), after the first conventional synthetic DMARD (csDMARD) or after the first biologic DMARD (bDMARD). We assessed pre‐index prevalence (percentage) and on‐treatment incidence (per 100 patient‐years [P100PY]) of the aforementioned comorbidities. For patients with versus without incident conditions, we compared total all‐cause health care costs as unadjusted and adjusted for baseline characteristics and health care costs.
Results
Prior to initiating a new treatment, among DMARD‐naïve patients (N = 28,201), csDMARD switchers (N = 7,816), or bDMARD switchers (N = 4,656), the overall prevalence ranged from 14.1% to 16.2% (anemia), from 1.3% to 5.2% (malignancy, evaluated in csDMARD and bDMARD switchers), from 1.5% to 2.1% (VTE), from 1.8% to 2.9% (MACE), and from 66.6% to 76.1% (infections). Once on index treatment, overall incidence (P100PY) among the cohorts ranged from 6.9 to 8.9 (anemia), from 2.0 to 2.3 (malignancy), from 0.7 to 0.9 (VTE), from 1.6 to 2.0 (MACE), and from 77.4 to 87.7 (infections). The incident comorbidities (except herpes zoster) were associated with increased adjusted health care costs.
Conclusion
Anemia, malignancy, VTE, MACE, and infections affect patients with RA at all stages of their treatment journey and are associated with increased health care costs.
INTRODUCTION
American College of Rheumatology (ACR) 2021 guidelines on rheumatoid arthritis (RA) disease management recommend initiating treatment with a conventional synthetic disease‐modifying antirheumatic drug (csDMARD) and giving consideration to a patient's comorbidity history (1). After a failure (the development of side effects or the lack or loss of effectiveness) on the first csDMARD, the ACR recommends csDMARD combination therapy, a biologic DMARD (bDMARD), or a Janus kinase inhibitor (JAKi) (1). If the patient fails treatment on a bDMARD, guidelines recommend switching to another bDMARD or a JAKi (1).
The emergence of JAKi for the treatment of RA stimulated discussions around assessing risk of venous thromboembolisms (VTEs), anemia, and infections such as herpes zoster (HZ) (2, 3, 4, 5). Estimates for comorbidities frequently associated with DMARD therapies exist for malignancies (6, 7), VTE (deep‐vein thrombosis [DVT] or pulmonary embolism [PE]) (8, 9, 10), major adverse cardiovascular events (MACE) (11, 12), infections (2, 3, 4, 5, 13, 14), and anemia (15, 16, 17, 18). However, contemporary estimates are lacking by treatment history.
The presence of these comorbidities may lead to treatment discontinuation and increased health care costs. The treatment of acute VTE incurs substantial added direct medical costs (19). Cardiovascular disease, including acute myocardial infarction (MI) and ischemic stroke, is also associated with high direct costs (20). Patients with RA and concomitant anemia exhibit longer hospital stays, undergo more procedures, and incur higher hospitalization costs than patients without anemia (21). The presence of comorbidities can also lead to DMARD switching, which is associated with increased health care costs (22). Given the potential impact of these comorbidities on RA management and health care costs, understanding their burden in the real world can help optimize treatment decisions.
RA clinical trials often select DMARD‐naïve patients or patients who previously failed a csDMARD or bDMARD (23). Clinical trials are required to report the incidence of adverse events including anemia, malignancy, VTE, MACE, and infections. However, narrow inclusion and exclusion criteria in clinical trials are not representative of real‐world populations (23, 24), and retrospective observational studies can help expand the understanding of the complexity of patient comorbidities beyond the limits of clinical trial participation.
Reports of the real‐world incidence, prevalence, and economic burden of anemia, malignancy, VTE, MACE, and infections in patients with RA are outdated and ignore DMARD exposure history (6, 15, 25, 26, 27). This study aimed to investigate the baseline prevalence of, on‐treatment incidence of, and costs associated with the comorbidities—anemia, malignancy, VTE, MACE, and infections—in RA patients stratified by history of DMARD exposure and by initiated treatment.
METHODS
Data source
This retrospective cohort analysis used data from the IQVIA PharMetrics® Plus database, a fully adjudicated commercial medical and pharmacy (including Medicare Advantage) health insurance claims database, which contains data for approximately 40 million children and adults in the United States annually. In compliance with the Health Insurance Portability and Accountability Act, patient data were de‐identified; therefore, informed consent and institutional review board approval were unnecessary.
Patient population and study duration
We defined three study cohorts based on patient history of DMARD exposure. The DMARD‐naïve cohort comprised patients with RA newly initiating a DMARD treatment, defined as no evidence of DMARD therapy during the 1‐year pre‐index period. csDMARD switchers included patients who received a csDMARD (as monotherapy or combined with another csDMARD) as their first regimen and then switched to or added another DMARD (as monotherapy or combined with a csDMARD). bDMARD switchers included patients who received a first bDMARD and then switched to another targeted immunomodulator treatment regimen (with or without a concomitant csDMARD). In some instances, the cohorts were nested within each other; some DMARD‐naïve patients moved into the csDMARD switcher cohort and then moved into the bDMARD switcher cohort.
Between 1 January 2011 and 31 March 2017, we identified adult patients with RA, defined as two or more International Classification of Diseases, Ninth Revision–Clinical Modification (ICD‐9‐CM) codes (714.0, 714.1, 714.2, 714.4, 714.81) or ICD‐10‐CM codes (M05, M06) for RA occurring at least 30 days apart and at least 12 months prior to index date (Supplementary Figure 1). Exclusion criteria included evidence of pregnancy at any point in the study, evidence of autoimmune disease during the pre‐index period or index date, and (specific to the DMARD‐naïve cohort) pre‐index presence of malignancy. Supplementary Table 1 lists inclusion and exclusion criteria for each cohort.
Index date was defined as the date that patients initiated their first DMARD (for the DMARD‐naïve cohort), another DMARD therapy after the first csDMARD (csDMARD switchers), or another targeted therapy after the first bDMARD (bDMARD switchers). All patients had continuous health plan enrollment for 12 months prior to (pre‐index) and at least 12 months after index date (follow‐up). Follow‐up ended at disenrollment or the end of the study period, whichever occurred first.
Initiated treatment classes included csDMARD (hydroxychloroquine sulfate, leflunomide, methotrexate, or sulfasalazine), tumor necrosis factor‐α inhibitors (TNFi; adalimumab, certolizumab pegol, etanercept, golimumab, or infliximab), non‐TNFi bDMARD (abatacept, rituximab, sarilumab, and tocilizumab), and JAKi (tofacitinib citrate). Monotherapy was defined as initiation and continued use of a single DMARD for at least 30 days post‐index without overlap with another DMARD agent in the initial 30 days post‐index. Combination therapy was defined as at least 30 days of overlapping use of a DMARD agent and one or two additional csDMARDs, with the overlap starting within 30 days after the index date. No refill within 60 days after finishing a prescription's supplied days signified a treatment discontinuation, a designation that prioritized the supply status of the biologic or JAKi agent in combination scenarios.
Demographic and clinical characteristics
Patient baseline demographics (age and sex) and clinical characteristics (duration of RA, Deyo‐Charlson comorbidity index [DCCI] [28], and prevalence of comorbidities of interest) were reported for all cohorts.
Comorbidities of interest included anemia (excluding hereditary hemolytic anemias), malignancies (excluding nonmelanoma skin cancers), DVT, PE, VTE (DVT or PE or both), two‐ and four‐part nonfatal MACE (two‐part: nonfatal MI and nonfatal stroke; four‐part: nonfatal MI, nonfatal stroke [including hemorrhagic and ischemic stroke and transient ischemic attack], hospitalization for heart failure, and hospitalization for unstable angina), and infections (including serious infections, opportunistic infections, and HZ). Anemia was defined by ICD‐9‐CM or ICD‐10‐CM diagnosis codes or documented use of anemia medication (iron, vitamin B12, erythropoietin). MACE were defined by ICD‐9‐CM or ICD‐10‐CM diagnosis codes. Infections were defined by ICD‐9‐CM or ICD‐10‐CM diagnosis codes or documented use of antibiotics, antifungals, or antivirals. Serious infections were defined as inpatient admission or emergency department visit with an infection diagnosis (in any position) or outpatient visit with infection diagnosis and use of intravenous (IV) antibiotics, IV antifungals, or IV antivirals. Opportunistic infections and HZ were defined by ICD‐9‐CM or ICD‐10‐CM diagnosis codes. Unless otherwise specified, a single occurrence of an associated diagnostic code qualified as occurrence of a comorbidity.
Pre‐index prevalence referred to the proportion of patients with a specified comorbidity within the 12 months prior to the index date. On‐treatment incidence rates were assessed among patients without the comorbidity of interest in the 60 days prior to index date (for VTE, MACE, and infection) or in the 1‐year pre‐index period (for anemia and malignancy). The incidence rates (per 100 patient‐years [P100PY]) were calculated as the number of patients with new events divided by the total time (patient‐years) from index date until the earliest among the following: first event of interest, end of index treatment, end of follow‐up, or end of study.
Health care costs included the negotiated amount that a health plan had agreed to pay a provider. Total costs comprised all medical and pharmacy claims accrued over the duration of the index therapy. Medical costs denoted costs for all inpatient and outpatient medical services provided including diagnostics, office visits, and procedures, but did not include the costs of medications dispensed from a pharmacy.
Statistical analyses
Baseline demographic and clinical characteristics for each cohort (total and stratified by monotherapy and combination therapy) were summarized using descriptive statistics. Prevalence and incidence rates of comorbidities were reported for all patients and were stratified by monotherapy and combination therapy in each cohort.
t tests (continuous variables) and χ2 or Fisher's exact test (categorical variables) compared baseline demographics, clinical characteristics (including prevalence and incidence of comorbidities), and treatment patterns between the monotherapy and combination therapy groups. Within monotherapy and within combination regimens, an analysis of variance test compared prevalence among DMARD classes, and univariate Poisson regression models compared incidence rates among DMARD classes. For all analyses, a P value less than 0.05 was considered statistically significant.
Unadjusted mean total annualized per‐patient per‐year (PPPY) all‐cause health care costs in 2017 US dollars accumulated during the index treatment were compared between patients with and without incidence of each type of comorbidity. Generalized linear models with gamma distribution and log link function adjusting for index regimen and baseline characteristics (demographics, clinical history, and cost) estimated the impact of various baseline characteristics on PPPY costs associated with each incident comorbidity. The estimated adjusted total cost differences were calculated via the recycled prediction method (29). Analyses used SAS® version 9.4 (SAS Institute Inc.).
RESULTS
DMARD‐naïve cohort
Patient baseline demographics and clinical characteristics
Within the DMARD‐naïve cohort (N = 28,201; Supplementary Figure 2), the majority of patients were female, the mean age was 52.9 years (Table 1), and the mean DCCI was 1.2.
Table 1.
Patient baseline demographics and clinical characteristics
| All Patients | Index: Monotherapy | Index: Combination Therapy a | P Value b | |
|---|---|---|---|---|
| DMARD‐naïve | ||||
| N | 28,201 | 26,261 | 1,940 | |
| Age, mean (SD), years | 52.9 (10.9) | 53.0 (10.9) | 52.7 (10.6) | 0.3876 |
| Sex, female, n (%) | 20,597 (73.0) | 19,158 (73.0) | 1,439 (74.2) | 0.2415 |
| Duration of RA, mean (SD), years | 0.6 (1.0) | 0.6 (1.0) | 0.7 (1.1) | <0.0001 |
| DCCI, mean (SD) | 1.2 (1.1) | 1.2 (1.1) | 1.1 (1.0) | 0.2381 |
| csDMARD‐switchers | ||||
| N | 7,816 | 2,947 | 4,869 | |
| Age, mean (SD), years | 52.5 (10.4) | 52.5 (10.6) | 52.6 (10.2) | 0.7258 |
| Sex, female, n (%) | 5806 (74.3) | 2248 (76.3) | 3558 (73.1) | 0.0017 |
| Duration of RA, mean (SD), years | 1.0 (1.0) | 1.1 (1.1) | 0.9 (1.0) | <0.0001 |
| DCCI, mean (SD) | 1.5 (1.0) | 1.5 (1.0) | 1.4 (0.9) | 0.0906 |
| bDMARD‐switchers | ||||
| N | 4,656 | 2,149 | 2,507 | |
| Age, mean (SD), years | 52.4 (10.3) | 52.0 (10.7) | 52.8 (9.8) | 0.0041 |
| Sex, female, n (%) | 3,627 (77.9) | 1,678 (78.1) | 1,949 (77.7) | 0.7801 |
| Duration of RA, mean (SD), years | 1.8 (1.3) | 1.8 (1.3) | 1.8 (1.2) | 0.9634 |
| DCCI, mean (SD) | 1.6 (1.1) | 1.6 (1.1) | 1.6 (1.0) | 0.0778 |
Combination therapy is defined as the addition of an RA therapy to the index therapy within 30 days after the index date, with continuation of the index therapy.
P value for comparison of monotherapy and index combination therapy group was calculated using t test for continuous variables and χ2 or Fisher's exact test for categorical variables.
Abbreviations: bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; DCCI, Deyo‐Charlson comorbidity index; DMARD, disease‐modifying antirheumatic drug; RA, rheumatoid arthritis; SD, standard deviation.
Pre‐index prevalence and on‐treatment incidence
Anemia, malignancies, DVT, PE, MACE, and infections were prevalent in patients with RA in the 12 months prior to initiating their first DMARD, with the exception of zero baseline prevalence of VTE and two‐ and four‐part MACE in patients who initiated JAKi combined with csDMARD (Figure 1, Supplementary Figure 3, and Supplementary Tables 2‐4). After initiating index treatment, all examined comorbidities occurred in the DMARD‐naïve cohort, with a P100PY incidence of 8.9 for anemia, 2.3 for malignancy, 0.8 for VTE (0.7 for DVT, 0.3 for PE), 1.6 for two‐part MACE, 1.9 for four‐part MACE, and 77.4 for infections (Figure 2, Supplementary Figure 4, and Supplementary Tables 5‐7).
Figure 1.
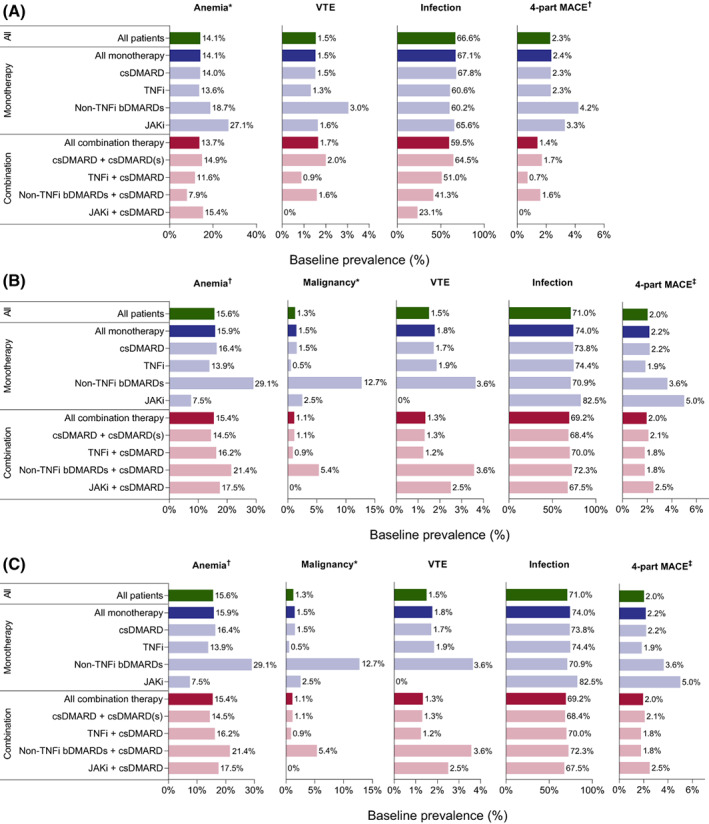
Pre‐index prevalence of select comorbidities by index treatment in DMARD‐naïve patients (A), csDMARD switchers (B), and bDMARD switchers (C). *Malignancy excludes nonmelanoma skin cancers, and presence of malignancy was an exclusion criterion for the DMARD‐naïve cohort. †Anemia excludes hereditary hemolytic anemia. ‡Four‐part MACE includes nonfatal MI, nonfatal stroke, hospitalization for heart failure, and hospitalization for unstable angina. bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; DMARD, disease‐modifying antirheumatic drug; JAKi, Janus kinase inhibitor; MACE, major adverse cardiovascular events; MI, myocardial infarction; TNFi, tumor necrosis factor‐α inhibitor; VTE, venous thromboembolism.
Figure 2.
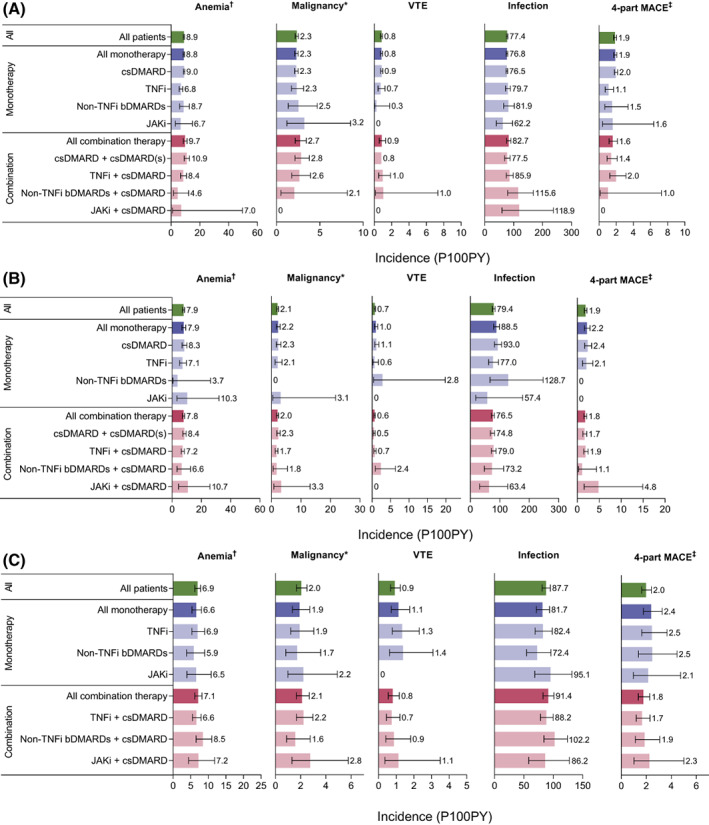
Incidence (95% confidence interval) while on index treatment of select comorbidities by index treatment in DMARD‐naïve patients (A), csDMARD switchers (B), and bDMARD switchers (C). *Malignancy excludes nonmelanoma skin cancers. †Anemia excludes hereditary hemolytic anemia. ‡Four‐part MACE includes nonfatal MI, nonfatal stroke, hospitalization for heart failure, and hospitalization for unstable angina. bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; DMARD, disease‐modifying antirheumatic drug; JAKi, Janus kinase inhibitor; MACE, major adverse cardiovascular events; MI, myocardial infarction; P100PY, per 100 patient‐years, TNFi, tumor necrosis factor‐α inhibitor; VTE, venous thromboembolism.
Health care costs
Total mean unadjusted health care costs were higher for patients with the examined comorbidities than for patients without them (Supplementary Table 8). After adjusting for baseline differences, total health care costs for patients with comorbidities were higher than costs for patients without the comorbidity, for all examined comorbidities. For patients with DVT, PE, malignancy, or serious infection, costs were more than 2 times higher than costs for patients without each of those comorbidities (Table 2). The effects of factors other than comorbidities on adjusted health care costs are summarized in Supplementary Tables 9‐11.
Table 2.
Adjusted per‐patient per‐year total health care costs in patients with and without DVT, PE, malignancy, anemia, infection, or MACE while on index therapy
| Comorbidity of Interest | Cost With Comorbidity | Cost Without Comorbidity | Cost Ratio (95% CI) | P Value |
|---|---|---|---|---|
| DMARD‐naïve | ||||
| Anemia, mean PPPY | $30,364 | $15,956 | 1.90 (1.82‐1.99) | <0.0001 |
| Malignancy a , mean PPPY | $47,908 | $17,821 | 2.69 (2.49‐2.90) | <0.0001 |
| DVT, mean PPPY | $36,958 | $18,128 | 2.04 (1.77‐2.35) | <0.0001 |
| PE, mean PPPY | $46,991 | $18,180 | 2.58 (2.06‐3.24) | <0.0001 |
| Infection, mean PPPY | $19,634 | $13,594 | 1.44 (1.41‐1.48) | <0.0001 |
| Serious infection, mean PPPY | $37,714 | $15,932 | 2.37 (2.28‐2.46) | <0.0001 |
| Opportunistic infection, mean PPPY | $25,706 | $17,994 | 1.43 (1.35‐1.51) | <0.0001 |
| HZ, mean PPPY | $22,533 | $18,389 | 1.23 (1.12‐1.34) | <0.0001 |
| Two‐part MACE b , mean PPPY | $54,885 | $17,592 | 3.12 (2.87‐3.40) | <0.0001 |
| Four‐part MACE c , mean PPPY | $61,811 | $17,256 | 3.58 (3.32‐3.87) | <0.0001 |
| csDMARD‐switchers | ||||
| Anemia, mean PPPY | $50,097 | $26,959 | 1.86 (1.74‐1.99) | <0.0001 |
| Malignancy a , mean PPPY | $81,779 | $28,565 | 2.86 (2.55‐3.22) | <0.0001 |
| DVT, mean PPPY | $60,430 | $28,927 | 2.09 (1.68‐2.60) | <0.0001 |
| PE, mean PPPY | $77,942 | $29,000 | 2.69 (1.94‐3.73) | <0.0001 |
| Infections, mean PPPY | $32,431 | $22,774 | 1.42 (1.36‐1.49) | <0.0001 |
| Serious infection, mean PPPY | $52,935 | $26,723 | 1.98 (1.88‐2.09) | <0.0001 |
| Opportunistic infection, mean PPPY | $39,239 | $28,947 | 1.36 (1.25‐1.47) | <0.0001 |
| HZ, mean PPPY | $30,638 | $29,515 | 1.04 (0.92‐1.17) | 0.5429 |
| Two‐part MACE b , mean PPPY | $59,545 | $28,850 | 2.06 (1.83‐2.32) | <0.0001 |
| Four‐part MACE c , mean PPPY | $60,270 | $28,741 | 2.10 (1.87‐2.35) | <0.0001 |
| bDMARD‐switchers | ||||
| Anemia, mean PPPY | $64,944 | $51,920 | 1.25 (1.19‐1.32) | <0.0001 |
| Malignancy a , mean PPPY | $78,968 | $52,877 | 1.49 (1.37‐1.63) | <0.0001 |
| DVT, mean PPPY | $67,624 | $53,464 | 1.26 (1.09‐1.47) | 0.0025 |
| PE, mean PPPY | $92,635 | $53,435 | 1.73 (1.38‐2.17) | <0.0001 |
| Infections, mean PPPY | $53,658 | $49,163 | 1.09 (1.06‐1.13) | <0.0001 |
| Serious infection, mean PPPY | $66,967 | $51,402 | 1.30 (1.25‐1.35) | <0.0001 |
| Opportunistic infection, mean PPPY | $58,926 | $53,424 | 1.10 (1.04‐1.16) | 0.0004 |
| HZ, mean PPPY | $56,183 | $53,737 | 1.05 (0.96‐1.13) | 0.2876 |
| Two‐part MACE b , mean PPPY | $79,109 | $53,018 | 1.49 (1.37‐1.63) | <0.0001 |
| Four‐part MACE c , mean PPPY | $86,347 | $52,673 | 1.64 (1.51‐1.78) | <0.0001 |
Malignancy excludes nonmelanoma skin cancer.
Two‐part MACE includes nonfatal MI and nonfatal stroke.
Four‐part MACE includes nonfatal MI, nonfatal stroke, hospitalization for heart failure, and hospitalization for unstable angina.
Costs are calculated for the duration of index treatment in 2017 US dollars.
Abbreviations: bDMARD, biologic DMARD; CI, confidence interval; csDMARD, conventional synthetic DMARD; DMARD, disease‐modifying antirheumatic drug; DVT, deep vein thrombosis; HZ, herpes zoster; MACE, major adverse cardiovascular events; MI, myocardial infarction; PE, pulmonary embolism; PPPY, per‐patient per‐year.
csDMARD switchers
Patient baseline demographic and clinical characteristics
csDMARD switchers (N = 7,816; Supplementary Figure 2) were predominately female, had a mean (SD) age of 52.5 years (Table 1), and had a mean DCCI of 1.5.
Pre‐index prevalence and on‐treatment incidence
All the examined comorbidities of special interest manifested in the csDMARD switcher cohort in the 12 months prior to switching to their next treatment (Figure 1, Supplementary Figure 3, and Supplementary Tables 2‐4). New cases of all comorbidities presented while on index treatment (Figure 2, Supplementary Figure 4, and Supplementary Tables 5‐7), with overall on‐treatment P100PY incidence rates of 7.9 for anemia, 2.1 for malignancy, 0.6 for DVT, 0.3 for PE, 0.7 for VTE, 1.7 for two‐part MACE, 1.9 for four‐part MACE, and 79.4 for infections.
Health care costs
In csDMARD switchers, mean unadjusted and adjusted total health care costs for patients with malignancy, anemia, DVT, PE, MACE, serious infection, or opportunistic infection were higher than for those without these conditions (Table 2 and Supplementary Table 10). Adjusted costs increased by more than 2‐fold for patients with DVT, PE, and malignancy compared with patients unaffected by these comorbidities (Table 2). Other factors were also associated with change in adjusted health care costs and are summarized in Supplementary Tables 9‐11.
bDMARD switchers
Patient baseline demographics and clinical characteristics
Among bDMARD switchers (N = 4,656; Supplementary Figure 2), most patients were female, the mean age was 52.4 years (Table 1), and the mean DCCI was 1.6.
Pre‐index prevalence and on‐treatment incidence
During the 12 months pre‐index, each examined comorbidity was prevalent in bDMARD switchers (Figure 1, Supplementary Figure 3, and Supplementary Tables 2‐4). While on index treatment, patients experienced new cases of comorbidities, with incidence rates P100PY of 6.9 for anemia, 2.0 for malignancy, 0.7 for DVT, 0.3 for PE, 0.9 for VTE (for cohorts receiving JAKi, whether as monotherapy or combination, the incidence of VTE was 0), 1.7 for two‐part MACE, 2.0 for four‐part MACE, and 87.7 for infections (Figure 2, Supplementary Figure 4, and Supplementary Tables 5‐7).
Health care costs
Mean total PPPY unadjusted and adjusted health care costs for bDMARD switchers with anemia, malignancy, DVT, PE, MACE, any infections, and serious and opportunistic infection subtypes were higher than for those without these conditions (Table 2 and Supplementary Table 11). The highest increase in adjusted costs was for patients with PE, whose costs increased by a factor of 1.73 compared with patients not diagnosed with this comorbidity. The effects of additional factors on adjusted health care costs are summarized in Supplementary Tables 9‐11.
DISCUSSION
This study explored comorbidities frequently associated with RA therapies and their associated prevalence, incidence, and costs at different stages of the treatment journey and by initiated therapy. Patients with RA were affected by anemia, malignancy (except for DMARD‐naïve patients, for whom baseline malignancy was an exclusion criterion), VTE, MACE, and infections in the 12 months before initiating their first DMARD treatment and before switching from their first csDMARD or bDMARD to their next treatment. While on index treatment, all cohorts developed incident cases of these comorbidities, which varied by the type of initiated treatment.
These comorbidities were associated with economic burden. In most cases, their incident occurrences were associated with increased crude and adjusted health care costs. The increase in unadjusted total costs primarily involved medical costs, likely resulting from the need for provider visits and additional monitoring. The adjusted analyses showed that incident occurrence of the comorbidities examined were associated with increased cost (except for HZ), with PE, malignancies, and MACE associated with the most substantial increases in cost. Incidentally, the cost models showed differences in total health care costs by initiated DMARD regimens.
Our analysis revealed a pre‐index prevalence of VTE ranging from 0% to 3.7% and an on‐treatment incidence rate ranging from 0.7 to 0.9 P100PY. When examining patients prior to initiating a JAKi, pre‐index VTE prevalence ranged from 0% to 3.7%. On‐treatment incidence was 0 for all JAKi regimens for all cohorts except for JAKi plus csDMARD in bDMARD switchers, who had an incidence of 1.1 P100PY. In comparison, a cohort study in a UK primary care medical record database reported the prevalence of VTE in RA as 4.2% (incidence 0.7 P100PY) in patients not receiving a DMARD and 4.7% (0.8 P100PY) in patients on DMARD therapy (30), whereas a US commercial claims study reported incidence rates of 0.3 to 0.6 P100PY (10). Our calculated rates of VTE in JAKi monotherapy were unexpectedly low in the context of our overall findings and the black box warning issued by the US Food and Drug Administration for the JAKi tofacitinib, baricitinib, and upadacitinib. The warning states the risk of thrombosis—including DVT, PE, and arterial thrombosis—and recommends that the drugs be used with caution in patients with risk factors for thrombosis (such as prior history of thrombosis or use of estrogen‐based therapies). However, our findings could be due to the small number of patients on JAKi identified in the study selection.
Patients with RA may be at an increased risk for malignancy, potentially due to a link between chronic inflammation and malignant transformation or the suppression of anticancer immunosurveillance by the RA treatment regimen (6, 31). In the current study, we observed overall malignancy incidence of 2.0 to 2.3 P100PY. The incidence of malignancy in published reports is somewhat lower than our estimates. For instance, an analysis of data from a large‐scale US longitudinal observational study found an overall incidence of all malignancies of 1.3 P100PY (32). A UK study in bDMARD‐naïve patients who initiated a csDMARD treatment regimen reported overall malignancy incidence of 1.37 P100PY (33), whereas a cohort study using administrative databases from two US states and one Canadian province found incidence rates ranging from less than 0.1 to 1.4 P100PY for patients on a csDMARD and from 0.1 to 1.1 P100PY for patients on a bDMARD (34).
Anemia commonly affects patients with RA (15), and associated changes in hemoglobin levels may result in additional monitoring, further diagnostic workups, and modifications to treatment regimen. Within our study, the pre‐index prevalence of anemia ranged from 7.5% to 29.1%, and on‐treatment incidence ranged from 4.6 to 10.7 P100PY. In comparison, other real‐world studies have reported a prevalence of anemia in patients with RA at approximately 7% to 23% (35, 36, 37). Importantly, we observed that the prevalence of anemia was consistent across all patient cohorts, suggesting a constant need for management of this comorbidity with any RA treatment.
Patients with RA experience frequent infections. The real‐world analysis presented here demonstrates an overall incidence of 77.4, 79.4, and 87.7 in DMARD‐naïve patients, csDMARD switchers, and bDMARD switchers, respectively, whereas the incidence rate of serious infection was 8.9, 9.5, and 12.1 P100PY for DMARD‐naïve patients, csDMARD switchers, and bDMARD switchers, respectively (Supplementary Table 6). A population‐based study of 609 patients with RA in Rochester, MN, found an incidence rate of infections of 19.6 P100PY, whereas a systematic review of 106 published clinical trials in 42,330 patients with RA (19.8% csDMARD‐naïve, 68.9% csDMARD‐experienced, and 11.3% TNFi‐experienced) found an incidence rate for serious infections of 2 to 5.5 P100PY (13, 38). The higher incidence reported in this study than previously published results further underscores the need for appropriate management of this comorbidity.
The ACR guidelines encourage the use of a csDMARD combination regimen over the use of bDMARD or targeted synthetic DMARD for the treatment of patients with RA with previous serious infections (1). This study showed a prevalence for serious infection of approximately 10% to 15% during the pre‐index period for patients in all three cohorts receiving index TNFi treatment, suggesting that guidelines are not always followed.
In a clinical study, patients who began treatment with the JAKi tofacitinib 2 to 3 weeks after receiving a live HZ vaccine had a similar immune response to the vaccine as patients who received placebo (5). Of note, a non‐live adjuvanted varicella‐zoster virus glycoprotein E recombinant subunit vaccine received US Food and Drug Administration approval on 20 October 2017 and could potentially prove beneficial to patients with RA on an immunosuppressive treatment regimen (39, 40). Despite the high risk of infection and guideline recommendations, vaccination rates for HZ are reported to range from 1% to 21% (41, 42). In Medicare claims, incidence of HZ in bDMARD‐experienced patients who began a new bDMARD regimen ranged from 1.6 to 2.5 P100PY (42). The type of bDMARD was not associated with a significant change in HZ incidence, whereas HZ vaccination prior to new bDMARD initiation was associated with lower HZ incidence than no vaccination (42). These estimates of vaccination rates and HZ incidence—along with the incidence of HZ in the current study (ranging from 1.5 to 2.2)—suggest that, overall, there is an unmet need for HZ prevention in the RA population, both in terms of appropriate preventive options and their execution in clinical practice.
Patients with RA have an elevated risk of MACE compared with the general population (11, 12). Better control of RA disease activity over time is associated with reduced MACE risk (43), though traditional risk factors such as hypertension and diabetes are more prevalent in patients with RA than in the general population (26). In this study, the most inclusive composite MACE outcome, four‐part MACE, had a pre‐index prevalence ranging from 2.0% to 2.9% and on‐treatment incidence rate of 0.6 to 0.7 P100PY. Our study also identified differences in MACE incidence among DMARD classes, which a previous study did not (44). In the literature, estimates of MACE incidence in patients with RA vary, perhaps due, in part, to differing definitions of composite outcomes. One retrospective cohort study estimated MACE incidence in patients with RA to be 2.67 per 1,000 patient‐years (25), and another estimated cardiovascular event incidence to be 7.8 per 1,000 patient‐years (43). Analysis of records from a data repository found incidence of MACE in patients with RA to be 9.6 per 1,000 patient‐years (26).
The results of this study should be interpreted in the context of its limitations. A potential limitation of the study is generalizability: because sample selection focused on patients with RA who used DMARDs, it may not reflect the epidemiology of RA patients who have not initiated DMARDs. This study did not quantify the added comorbidity burden in RA relative to such burdens in the general population, nor those associated with normal aging. Within the JAKi class, we examined only tofacitinib because baricitinib and upadacitinib became available in the United States after the predetermined study period. Some conditions, such as anemia, may be underreported if hemoglobin levels were not tested or only over‐the‐counter therapies were used in managing the condition. Patients could have self‐managed upper respiratory infections without interacting with health care services. Claims‐based analyses do not account for the full range of clinical characteristics that may affect the occurrence and economic impact of these comorbidities in RA. For example, laboratory values or severity of comorbidities were not available to indicate the level of management needed, such as the stage of malignancy, localization of infection, or whether anemia affected oxygen saturation levels. Unobserved factors and interactions among the comorbidities may have affected cost estimates but were not captured by the models. For instance, malignancy may increase the risk of anemia, infection, and VTE, with an increase in associated costs. Future research can examine how specific health care resources contribute to cost differences. Current study strengths include the large sample size representative of the US insured population and the inclusion of different patient cohorts defined by treatment histories consistent with clinical trial programs.
In conclusion, because anemia, malignancies, DVT, PE, MACE, and infections can affect patients at each step of the treatment journey, their prevalence, incidence, and associated cost should be factored into the selection of the most optimal RA treatment to alleviate clinical and cost burdens.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Drs. Antonova and Chang had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Dore, Antonova, Chang, Genovese.
Acquisition of data
Burudpakdee, Gorritz.
Analysis and interpretation of data
Antonova, Burudpakdee, Chang, Gorritz, Genovese.
Supporting information
Supplementary Figure 1. Study cohort diagram
bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; DMARD, disease‐modifying antirheumatic drug; ICD, International Classification of Diseases; RA, rheumatoid arthritis; TIM, targeted immunomodulator.
Supplementary Figure 2. Patient disposition to index treatment regimen by cohort. A) DMARD‐naive patients, B) csDMARD switchers, and C) bDMARD switchers
bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; DMARD, disease‐modifying antirheumatic drug; JAKi, Janus kinase inhibitor; RA, rheumatoid arthritis; TNFi, tumor necrosis factor‐α inhibitor.
Supplemental Figure 3. Pre‐index prevalence of select comorbidities by index treatment in A) DMARD‐naïve patients, B) csDMARD switchers, and C) bDMARD switchers
‡2‐part MACE includes non‐fatal MI and non‐fatal stroke.
bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; DMARD, disease‐modifying antirheumatic drug; DVT, deep vein thrombosis; JAKi, Janus kinase inhibitor; MACE, major adverse cardiovascular event; MI, myocardial infarction; PE, pulmonary embolism; TNFi, tumor necrosis factor‐α inhibitor.
Supplemental Figure 4. Incidence while on index treatment of select comorbidities by index treatment in A) DMARD‐naïve patients, B) csDMARD switchers, and C) bDMARD switchers
‡ 2‐part MACE includes non‐fatal MI and non‐fatal stroke.
bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; DMARD, disease‐modifying antirheumatic drug; JAKi, Janus kinase inhibitor; MACE, major adverse cardiovascular event; MI, myocardial infarction; TNFi, tumor necrosis factor‐α inhibitor; VTE, venous thromboembolism
Supplemental Table 1. Full cohort inclusion and exclusion criteria
Supplementary Table 2. Pre‐index prevalence of select non‐MACE comorbidities by index treatment in DMARD‐naïve, csDMARD switchers, and bDMARD switchers
Supplementary Table 3. Pre‐index prevalence of select non‐MACE comorbidities by index treatment in DMARD‐naïve, csDMARD switchers, and bDMARD switchers
Supplementary Table 4. Pre‐index prevalence of 4‐part MACE and MACE subcomponent comorbidities by index treatment in DMARD‐naïve, csDMARD switchers, and bDMARD switchers
Supplementary Table 5. Incidence (per 100 patient‐years) while on index treatment of select non‐MACE comorbidities by index treatment in DMARD‐naïve, csDMARD switchers, and bDMARD switchers
Supplementary Table 6. Incidence (per 100 patient‐years) while on index treatment of select non‐MACE comorbidities by index treatment in DMARD‐naïve, csDMARD switchers, and bDMARD switchers
Supplementary Table 7. Incidence (per 100 patient‐years) while on index treatment of MACE and MACE subcomponent comorbidities by index treatment in DMARD‐naïve, csDMARD switchers, and bDMARD switchers
Supplementary Table 8. Unadjusted per‐patient per‐year healthcare costs during index treatment in DMARD‐naïve patients with and without DVT, PE, malignancy, anemia, infection, or MACE
Supplementary Table 9. Models for the association of an incident comorbidity of interest and mean total healthcare costs: covariate estimates
Supplementary Table 10. Factors affecting adjusted healthcare costs for subtypes of infection
Supplementary Table 11. Factors affecting adjusted healthcare costs for MACE
Supplementary Table 12. Unadjusted per‐patient per‐year healthcare costs during index treatment in csDMARD switcher patients with and without DVT, PE, malignancy, anemia, infection, or MACE
Supplementary Table 13. Unadjusted per‐patient per‐year healthcare costs during index treatment in bDMARD switcher patients with and without DVT, PE, malignancy, anemia, infection, or MACE
Disclosure Form
All analyses were conducted by IQVIA, Plymouth Meeting, PA, and were funded by Gilead Sciences, Inc., Foster City, CA. Medical writing support was provided by Jennifer Meyering, RN, MS, CMPP, of AlphaScientia, LLC, San Francisco, CA, and was funded by Gilead Sciences, Inc., Foster City, CA.
Robin K. Dore has received grants from and provided consulting services to AbbVie, Amgen, Biogen, Bristol‐Myers Squibb, Eli Lilly and Co., Gilead Sciences, Inc., GlaxoSmithKline, Myriad, Novartis, Pfizer, Radius, Regeneron, Sanofi, and UCB. Jenya N. Antonova is an employee of Gilead Sciences, Inc. Lawrence Chang is an employee of Gilead Sciences, Inc. Chakkarin Burudpakdee was an employee of IQVIA at the time of the study. Magdaliz Gorritz is an employee of IQVIA. Mark C. Genovese is an employee of Gilead Sciences, Inc., and has received honoraria or consulting fees from AbbVie, Amgen, BeiGene, Eli Lilly and Co., Genentech, Inc., Gilead Sciences, Inc., R‐Pharm, Sanofi‐Genzyme, and SetPoint.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr211376&file=acr211376‐sup‐0006‐Disclosureform.pdf.
REFERENCES
- 1. Fraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K, et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2021;73:924–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harigai M. Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology (Oxford, England) 2019;58(Supplement_1):i34–i42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taylor PC, Weinblatt ME, Burmester GR, Rooney TP, Witt S, Walls CD, et al. Cardiovascular safety during treatment with baricitinib in rheumatoid arthritis. Arthritis Rheum 2019;71:1042–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamaoka K. Benefit and risk of tofacitinib in the treatment of rheumatoid arthritis: a focus on herpes zoster. Drug Saf 2016;39:823–40. [DOI] [PubMed] [Google Scholar]
- 5. Winthrop KL, Wouters AG, Choy EH, Soma K, Hodge JA, Nduaka CI, et al. The safety and immunogenicity of live zoster vaccination in patients with rheumatoid arthritis before starting tofacitinib: a randomized phase ii trial. Arthritis Rheumatol 2017;69:1969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta‐analysis. Arthritis Res Ther 2015;17:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilton KM, Matteson EL. Malignancy incidence, management, and prevention in patients with rheumatoid arthritis. Rheumatol Ther 2017;4:333–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim SC, Schneeweiss S, Liu J, Solomon DH. Risk of venous thromboembolism in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65:1600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ungprasert P, Srivali N, Spanuchart I, Thongprayoon C, Knight EL. Risk of venous thromboembolism in patients with rheumatoid arthritis: a systematic review and meta‐analysis. Clin Rheumatol 2014;33:297–304. [DOI] [PubMed] [Google Scholar]
- 10. Kim SC, Solomon DH, Liu J, Franklin JM, Glynn RJ, Schneeweiss S. Risk of venous thromboembolism in patients with rheumatoid arthritis: initiating disease‐modifying antirheumatic drugs. Am J Med 2015;128:539. e7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schieir O, Tosevski C, Glazier RH, Hogg‐Johnson S, Badley EM. Incident myocardial infarction associated with major types of arthritis in the general population: a systematic review and meta‐analysis. Ann Rheum Dis 2017;76:1396–404. [DOI] [PubMed] [Google Scholar]
- 12. Ogdie A, Yu Y, Haynes K, Love TJ, Maliha S, Jiang Y, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population‐based cohort study. Ann Rheum Dis 2015;74:326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population‐based study. Arthritis Rheum 2002;46:2287–93. [DOI] [PubMed] [Google Scholar]
- 14. Ibrahim A, Ahmed M, Conway R, Carey JJ. Risk of infection with methotrexate therapy in inflammatory diseases: a systematic review and meta‐analysis. J Clin Med 2018;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson A, Yu HT, Goodnough LT, Nissenson AR. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med 2004;116 Suppl 7A:50s–7s. [DOI] [PubMed] [Google Scholar]
- 16. Alanazi AS. Sulfasalazine‐induced pancytopenia indicative of bone marrow suppression: a case report. J Med Cases 2014;5:289–91. [Google Scholar]
- 17. Wüsthof M, Smirnova A, Bacher U, Kröger N, Zander AR, Schuch G, et al. Severe aplastic anaemia following leflunomide therapy. Rheumatology (Oxford) 2009;49:1016–7. [DOI] [PubMed] [Google Scholar]
- 18. Qu C, Lu Y, Liu W. Severe bone marrow suppression accompanying pulmonary infection and hemorrhage of the digestive tract associated with leflunomide and low‐dose methotrexate combination therapy. J Pharmacol Pharmacother 2017;8:35–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grosse SD, Nelson RE, Nyarko KA, Richardson LC, Raskob GE. The economic burden of incident venous thromboembolism in the United States: a review of estimated attributable healthcare costs. Thromb Res 2016;137:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nicholson G, Gandra SR, Halbert RJ, Richhariya A, Nordyke RJ. Patient‐level costs of major cardiovascular conditions: a review of the international literature. Clinicoecon Outcomes Res 2016;8:495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zlateva G, Diazaraque R, Viala‐Danten M, Niculescu L. Burden of anemia in patients with osteoarthritis and rheumatoid arthritis in French secondary care. BMC Geriatr 2010;10:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shahabi A, Shafrin J, Zhao L, Green S, Curtice T, Marshall A, et al. The economic burden of switching targeted disease‐modifying anti‐rheumatic drugs among rheumatoid arthritis patients. J Med Econ 2019;22:350–8. [DOI] [PubMed] [Google Scholar]
- 23. Vashisht P, Sayles H, Cannella AC, Mikuls TR, Michaud K. Generalizability of patients with rheumatoid arthritis in biologic agent clinical trials. Arthritis Care Res (Hoboken) 2016;68:1478–88. [DOI] [PubMed] [Google Scholar]
- 24. Kilcher G, Hummel N, Didden EM, Egger M, Reichenbach S, GetReal Work Package 4. Rheumatoid arthritis patients treated in trial and real world settings: comparison of randomized trials with registries. Rheumatology (Oxford) 2018;57:354–69. [DOI] [PubMed] [Google Scholar]
- 25. Lauper K, Courvoisier DS, Chevallier P, Finckh A, Gabay C. Incidence and prevalence of major adverse cardiovascular events in rheumatoid arthritis, psoriatic arthritis, and axial spondyloarthritis. Arthritis Care Res (Hoboken) 2018;70:1756–63. [DOI] [PubMed] [Google Scholar]
- 26. Cooksey R, Brophy S, Kennedy J, Gutierrez FF, Pickles T, Davies R, et al. Cardiovascular risk factors predicting cardiac events are different in patients with rheumatoid arthritis, psoriatic arthritis, and psoriasis. Semin Arthritis Rheum 2018;48:367–73. [DOI] [PubMed] [Google Scholar]
- 27. Ni Mhuircheartaigh OM, Matteson EL, Green AB, Crowson CS. Trends in serious infections in rheumatoid arthritis. J Rheumatol 2013;40:611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- 29. Li z, Mahendra G. Using “Recycled Predictions” for Computing Marginal Effects. SAS Global Forum; 2010. [Google Scholar]
- 30. Ogdie A, Kay McGill N, Shin DB, Takeshita J, Jon Love T, Noe MH, et al. Risk of venous thromboembolism in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a general population‐based cohort study. European Heart J 2018;39:3608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smitten AL, Simon TA, Hochberg MC, Suissa S. A meta‐analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Res Ther 2008;10:R45‐R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheumatol 2007;56:2886–95. [DOI] [PubMed] [Google Scholar]
- 33. Mercer LK, Davies R, Galloway JB, Low A, Lunt M, Dixon WG, et al. Risk of cancer in patients receiving non‐biologic disease‐modifying therapy for rheumatoid arthritis compared with the UK general population. Rheumatology (Oxford) 2013;52:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Setoguchi S, Solomon DH, Weinblatt ME, Katz JN, Avorn J, Glynn RJ, et al. Tumor necrosis factor alpha antagonist use and cancer in patients with rheumatoid arthritis. Arthritis Rheum 2006;54:2757–64. [DOI] [PubMed] [Google Scholar]
- 35. Shafrin J, Ganguli A, Gonzalez YS, Shim JJ, Seabury SA. Geographic variation in the quality and cost of care for patients with rheumatoid arthritis. J Manag Care Spec Pharm 2016;22:1472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ershler WB, Chen K, Reyes EB, Dubois R. Economic burden of patients with anemia in selected diseases. Value Health 2005;8:629–38. [DOI] [PubMed] [Google Scholar]
- 37. Paul SK, Montvida O, Best JH, Gale S, Pethoe‐Schramm A, Sarsour K. Effectiveness of biologic and non‐biologic antirheumatic drugs on anaemia markers in 153,788 patients with rheumatoid arthritis: new evidence from real‐world data. Semin Arthritis Rheum 2018;47:478–84. [DOI] [PubMed] [Google Scholar]
- 38. Singh JA, Cameron C, Noorbaloochi S, Cullis T, Tucker M, Christensen R, et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta‐analysis. Lancet 2015;386:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Strezova A, Godeaux O, Aggarwal N, Leroux‐Roels G, Lopez‐Fauqued M, Van Damme P, et al. A randomized lot‐to‐lot immunogenicity consistency study of the candidate zoster vaccine HZ/su. Vaccine 2017;35:6700–6. [DOI] [PubMed] [Google Scholar]
- 40. Brosio F, Masetti G, Matteo G, Stefanati A, Gabutti G. A novel nonlive, adjuvanted herpes zoster subunit vaccine: a report on the emerging clinical data and safety profile. Infect Drug Resist 2018;11:1401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sandler DS, Ruderman EM, Brown T, Lee JY, Mixon A, Liss DT, et al. Understanding vaccination rates and attitudes among patients with rheumatoid arthritis. Am J Manag Care 2016;22:161–7. [PubMed] [Google Scholar]
- 42. Yun H, Xie F, Delzell E, Chen L, Levitan EB, Lewis JD, et al. Risks of herpes zoster in patients with rheumatoid arthritis according to biologic disease‐modifying therapy. Arthritis Care Res (Hoboken) 2015;67:731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Solomon DH, Reed GW, Kremer JM, Curtis JR, Farkouh ME, Harrold LR, et al. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol 2015;67:1449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sparks JA, Lesperance T, Accortt NA, Solomon DH. Subsequent cardiovascular events among patients with rheumatoid arthritis, psoriatic arthritis, or psoriasis: patterns of disease‐modifying antirheumatic drug treatment. Arthritis Care Res (Hoboken) 2019;71:512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Study cohort diagram
bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; DMARD, disease‐modifying antirheumatic drug; ICD, International Classification of Diseases; RA, rheumatoid arthritis; TIM, targeted immunomodulator.
Supplementary Figure 2. Patient disposition to index treatment regimen by cohort. A) DMARD‐naive patients, B) csDMARD switchers, and C) bDMARD switchers
bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; DMARD, disease‐modifying antirheumatic drug; JAKi, Janus kinase inhibitor; RA, rheumatoid arthritis; TNFi, tumor necrosis factor‐α inhibitor.
Supplemental Figure 3. Pre‐index prevalence of select comorbidities by index treatment in A) DMARD‐naïve patients, B) csDMARD switchers, and C) bDMARD switchers
‡2‐part MACE includes non‐fatal MI and non‐fatal stroke.
bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; DMARD, disease‐modifying antirheumatic drug; DVT, deep vein thrombosis; JAKi, Janus kinase inhibitor; MACE, major adverse cardiovascular event; MI, myocardial infarction; PE, pulmonary embolism; TNFi, tumor necrosis factor‐α inhibitor.
Supplemental Figure 4. Incidence while on index treatment of select comorbidities by index treatment in A) DMARD‐naïve patients, B) csDMARD switchers, and C) bDMARD switchers
‡ 2‐part MACE includes non‐fatal MI and non‐fatal stroke.
bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; DMARD, disease‐modifying antirheumatic drug; JAKi, Janus kinase inhibitor; MACE, major adverse cardiovascular event; MI, myocardial infarction; TNFi, tumor necrosis factor‐α inhibitor; VTE, venous thromboembolism
Supplemental Table 1. Full cohort inclusion and exclusion criteria
Supplementary Table 2. Pre‐index prevalence of select non‐MACE comorbidities by index treatment in DMARD‐naïve, csDMARD switchers, and bDMARD switchers
Supplementary Table 3. Pre‐index prevalence of select non‐MACE comorbidities by index treatment in DMARD‐naïve, csDMARD switchers, and bDMARD switchers
Supplementary Table 4. Pre‐index prevalence of 4‐part MACE and MACE subcomponent comorbidities by index treatment in DMARD‐naïve, csDMARD switchers, and bDMARD switchers
Supplementary Table 5. Incidence (per 100 patient‐years) while on index treatment of select non‐MACE comorbidities by index treatment in DMARD‐naïve, csDMARD switchers, and bDMARD switchers
Supplementary Table 6. Incidence (per 100 patient‐years) while on index treatment of select non‐MACE comorbidities by index treatment in DMARD‐naïve, csDMARD switchers, and bDMARD switchers
Supplementary Table 7. Incidence (per 100 patient‐years) while on index treatment of MACE and MACE subcomponent comorbidities by index treatment in DMARD‐naïve, csDMARD switchers, and bDMARD switchers
Supplementary Table 8. Unadjusted per‐patient per‐year healthcare costs during index treatment in DMARD‐naïve patients with and without DVT, PE, malignancy, anemia, infection, or MACE
Supplementary Table 9. Models for the association of an incident comorbidity of interest and mean total healthcare costs: covariate estimates
Supplementary Table 10. Factors affecting adjusted healthcare costs for subtypes of infection
Supplementary Table 11. Factors affecting adjusted healthcare costs for MACE
Supplementary Table 12. Unadjusted per‐patient per‐year healthcare costs during index treatment in csDMARD switcher patients with and without DVT, PE, malignancy, anemia, infection, or MACE
Supplementary Table 13. Unadjusted per‐patient per‐year healthcare costs during index treatment in bDMARD switcher patients with and without DVT, PE, malignancy, anemia, infection, or MACE
Disclosure Form


