Abstract
We tested the hypothesis that obesity influences the pharmacodynamics of volatile general anesthetics (VGAs) by comparing effects of anesthetic exposure on mortality from traumatic brain injury (TBI) in lean and obese Drosophila melanogaster. We induced TBI with a high-impact trauma device. Starvation-selection over multiple generations resulted in an obese phenotype (SS flies). Fed flies served as lean controls (FC flies). Adult (1–8-day-old) SS and FC flies were exposed to equianesthetic doses of isoflurane or sevoflurane either before or after TBI. The principal outcome was percent mortality 24 hours after injury, expressed as the Mortality Index at 24 hours (MI24). TBI resulted in a lower MI24 in FC than in SS flies [21 (2.35) and 57.8 (2.14), respectively n = 12, P = 0.0001]. Pre-exposure to isoflurane or sevoflurane preconditioned FC flies to TBI, reducing the risk of death to 0.53 (0.25 to 1.13) and 0.82 (0.43 to 1.58), respectively, but had no preconditioning effect in SS flies. Postexposure to isoflurane or sevoflurane increased the risk of death in SS flies, but only postexposure to isoflurane increased the risk in FC flies [1.39 (0.81 to 2.38)]. Thus, obesity affects the pharmacodynamics of VGAs, thwarting the preconditioning effect of isoflurane and sevoflurane in TBI.
SIGNIFICANCE STATEMENT
Inadvertent preconditioning in models of traumatic brain injury (TBI) is a recognized confounder. The findings in a fruit fly (Drosophila melanogaster) model of closed-head TBI indicate that anesthetic pharmacodynamics are profoundly affected by obesity. Specifically, obesity thwarts the brain-protective effect of anesthetic preconditioning. This finding is important for experimental studies of TBI and supports the versatility of the fruit fly as a model for the exploration of anesthetic pharmacodynamics in a wide parameter space.
Introduction
Preconditioning, i.e., the capacity of anesthetics to induce tolerance to injury when administered prior to ischemia, is a potentially valuable property of volatile general anesthetics (VGAs). Anesthetic preconditioning effectively protects the brain (Kitano et al., 2007) and the heart (Stadnicka et al., 2007) from ischemic damage. However, its effectiveness in protecting the myocardium is suppressed in obese rodents (Song et al., 2011) and in models of diabetes (Ge et al., 2018). Although the cause of this failure is not fully understood, it may be due to metabolic abnormalities linked to excessive accumulation of lipids in the heart (Nakanishi and Kato, 2014). This pathologic entity is termed "lipotoxic cardiomyopathy" or "fatty heart syndrome" (Szczepaniak et al., 2007). Rodents develop this syndrome in experimentally induced diabetes and obesity (Zhou et al., 2000). Whether these common comorbidities also influence anesthetic preconditioning of nervous tissue remains unknown as no analogous "lipotoxic" brain phenotype has been yet described.
Examining anesthetic interactions with experimental brain injury is notoriously complicated because nonanesthetized control groups are impossible, as experiments require exposure to anesthesia for technical (e.g., immobility for surgery) and/or animal welfare reasons. VGAs, however, profoundly influence almost all aspects of brain physiology (Statler et al., 2006b; Tétrault et al., 2008; Staib-Lasarzik et al., 2014; Semple et al., 2016). As a result, in both focal and diffuse brain damage, injury and intervention always occur on the background of a brain exposed to anesthetics, and inadvertent preconditioning may confound the interpretation of experiments and interventions. Further complicating matters, the extent and molecular mechanisms of preconditioning differ among anesthetic agents (Statler et al., 2006a). This long-standing problem has been explicitly recognized by many research groups and may contribute to the frequent failure in translating findings from mammalian models to human patients.
To address some of these limitations, we have tested the effect of VGAs on mortality in a traumatic brain injury (TBI) model implemented in the fruit fly (Drosophila melanogaster). This model reproduces key characteristics of TBI in mammals, including temporary incapacitation (concussion), ataxia, death, neurodegeneration, and shortened lifespan (Katzenberger et al., 2013; Putnam et al., 2019; Saikumar et al., 2020), while also faithfully mimicking the behavioral effects of VGAs (Olufs et al., 2018). Crucially, as flies pose no animal welfare concerns, control experiments without exposure to anesthetics are feasible. We have found that exposure of a standard laboratory fly line (w1118) to the VGAs isoflurane and sevoflurane prior to TBI effectively preconditioned the brain, as indicated by suppression of 24 hour mortality (Fischer et al., 2018). By contrast, exposure to isoflurane after TBI increased mortality. To test the hypothesis that obesity modulates anesthetic pharmacodynamics, we inflicted TBI in a fly model of obesity acquired by starvation selection (see Methods for details), which mimics many of the phenotypic characteristics of obesity in mammals, including increased weight and triglyceride storage as well as behavioral, anatomic, and metabolic abnormalities (Reynolds, 2013; Masek et al., 2014; Hardy et al., 2015). We found that obesity thwarts anesthetic preconditioning by isoflurane and sevoflurane in TBI. These data indicate that preconditioning with VGAs in fruit flies is responsive to biologic variables and reproduces the effect of obesity on anesthetic pharmacodynamics in mammalian ischemia. Although this information complicates the design of experiments that require the use of anesthetics, it can be instrumentalized to improve our understanding and treatment of TBI.
Materials and Methods
The experiments adhere to applicable ARRIVE (Animal Research: Reporting of In Vivo Experiments) reporting guidelines (preclinical animal research). Approval from the Institutional Animal Care and Use Committee has been waived.
Fly Husbandry
Unless otherwise indicated, experiments were conducted on flies generously provided by Dr. Allen Gibbs (School of Life Sciences, University of Nevada, Las Vegas, NV). The original founding populations for these flies were D. melanogaster collected from Terhune Orchards, Princeton, NJ in 1999 and maintained as outbred stocks at 25°C on cornmeal medium. One population underwent starvation-selection (SS population) over multiple generations by subjecting sequential generations of flies to severe starvation on 1% agar until only 15%–20% of the original population survived. Surviving flies were then placed on food to lay eggs. The next generation of adults was selected for starvation resistance in the same manner. The obese phenotype that developed in the SS population was characterized by increased lipid storage of nearly two times the amount of total lipids as the unselected control population, including a 30% increase in weight and high fat stores along with high whole body triglyceride levels (Reynolds, 2013). SS flies also had a depressed metabolic rate, low activity levels, dilated cardiomyopathy, and excess sleep (Reynolds, 2013; Masek et al., 2014; Hardy et al., 2015). The control population was cultured under the same conditions as the SS population but was provided ad libitum food and water and is referred to as the FC (for fed control) population. We received one subpopulation each of SS and FC flies that had undergone at least 120 generations of selection. Once in our laboratory, all flies were maintained on cornmeal-molasses food at 25°C and used at 1–8 days post eclosion. As evolutionary pressure of starvation-selection is removed, SS flies gradually start losing the obese phenotype. Therefore, experiments reported in Figs. 1, 3, and 4 were performed on generations 2–4. For Figs. 2 and 5, later generations of SS flies were also used. Fly lines 2P9, D. virilis, and D. funebris were generously provided by Bob Kreber and Dr. Barry Ganetzky (Department of Genetics, College of Agricultural and Life Sciences, University of Wisconsin-Madison, Madison, WI). The w1118 line is a standard line maintained in our laboratory. All experiments were conducted using mixed sex samples except fly mass that was determined using males. Fly mass was determined by averaging the results of 12 replicates of 30 flies for each line using an analytical balance with 0.1 mg accuracy (Mettler Toledo XSE104, Columbus, OH).
Fig. 1.
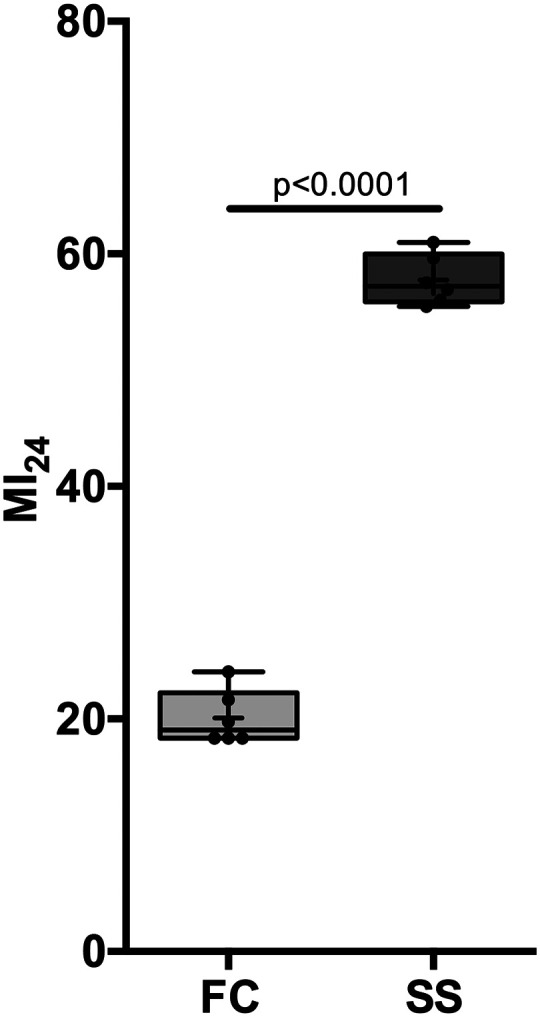
Obesity is associated with increased mortality. The Mortality Index at 24 hours after TBI (MI24) was determined in 1–8-day-old lean FC (fed control) and obese SS (starvation-selected) flies. The relative risk of death was 2.85 (CI [1.86 to 4.37], n = 6 per group). + indicates the mean, the horizontal line indicates the median, the box indicates 25th to 75th percentile, the whiskers extend to minimal and maximal values, and dots indicate individual replicates.
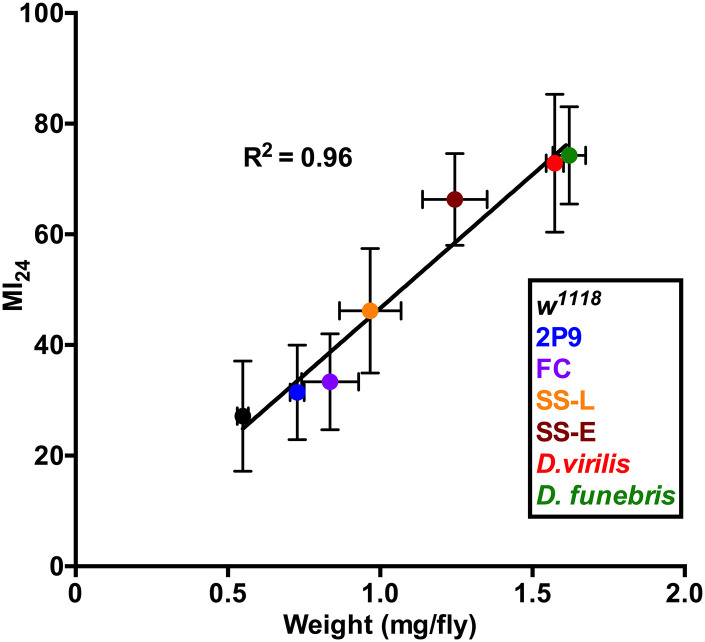
Fig. 2.
Early mortality (MI24) is correlated with weight for six fruit flies lines. Weight (independent variable) and the MI24 were determined for four lines of D. melanogaster (w1118, FC, SS, 2P9) and for D. funebris and D. virilis. The data were fitted with a simple linear regression. Tested SS flies were pooled into two groups determined by the time dots of their reproduction cycles in the laboratory: early (2nd–10th generation, SS-E) and late (up to generation 20, SS-L). For the determination of MI24, n = 14 except for SS-E where n = 24. The MI24 for the tested lines positively correlated with weight (R2= 0.96).
TBI
TBI was induced using a high-impact trauma device as described previously (Katzenberger et al., 2013). On the day prior to an experiment, eight vials containing 20 mixed sex flies were incubated at 25°C with cornmeal-molasses food. On the day of the experiment, flies were rapidly transferred into empty vials. TBI was induced with four strikes from the high-impact trauma device with the spring deflected to 90 degrees and 5 minutes between strikes. Anesthetics were administered either before or after TBI. After injury and anesthesia, flies were transferred to vials with cornmeal-molasses food and incubated at 25°C.
The primary outcome was mortality expressed as the Mortality Index determined 24 hours after TBI (MI24). We define the MI24 as the percentage of flies that are dead at 24 hours after TBI minus the percentage of matching uninjured flies that died within the same 24 hour period. Because mortality after TBI does not differ between male and female flies, we performed all experiments on mixed sex groups. Unless otherwise indicated, at least six independent replicates were performed for each experimental condition.
Anesthesia
We used a custom-built Serial Anesthesia Array to simultaneously expose up to eight samples of 20 flies each to precise doses of VGAs in air, as described previously (Fischer et al., 2018; Olufs et al., 2018). VGAs were administered through the Serial Anesthesia Array using a Datex-Ohmeda Aestiva/5 anesthesia machine equipped with commercial agent-specific vaporizers (Datex-Ohmeda Inc., Madison, WI). Compressed gas cylinders (Airgas USA, LLC., Radnor, PA) containing air (21% O2/79% N2) provided the carrier gas. To test the effect of obesity on anesthetic pharmacodynamics, we exposed the flies to anesthetics either immediately before or after inflicting TBI, mimicking pre- and postconditioning, respectively. We used either 2% isoflurane or 3.5% sevoflurane for both exposure protocols. These anesthetic concentrations are behaviorally equivalent and do not affect median and maximum lifespans (Olufs et al., 2018). The dose of anesthetic administered is reported as concentration (%) multiplied by duration (hours), e.g., 2% isoflurane for 2 hours equals 4%h. All flies resumed movement within less than 1 hour after discontinuing isoflurane or sevoflurane (i.e., no flies died immediately after TBI with or without anesthetic exposure, indicating that the doses were safe). A typical assay simultaneously tested two control conditions (i–ii) and two experimental condition (iii, iv): (i) no treatment, (ii) anesthesia alone, (iii) TBI alone, and (iv) TBI and anesthesia. All experiments were conducted under normobaric conditions. Mortality under control conditions (i and ii) was less than 1%.
Statistical Analysis
This study was exploratory with respect to examining the obese phenotype. The sample sizes were based on our experience with previous experiments testing the effect of anesthetics on the MI24. Data are presented as mean (± standard deviation), number of biologic replicates (n), and [95% confidence interval]. Each replicate included 20 individuals except for the determination of weight. To test for significance between treated and untreated FC and SS flies, we used the unpaired two-sample student’s t test and ANOVA with Bonferroni’s multiple comparison test. To compare the MI24 between FC and SS flies subjected to the same treatment, we used the independent two-sample t test. We quantified the effect of the VGAs on the MI24 using relative risk of death, calculated as the relative risk (Altman, 1991). Data underlying the calculations is plotted in box [interquartile range (IQR) of P25 to P75, i.e., 25th to 75th percentile] and whiskers to maximum and minimum values with ‘+’ = mean and ‘horizontal line’ = median. Dots indicate individual replicates. We used Graphpad© Prism for all statistical calculations. We used Hedge’s g to calculate effect size for comparing different sample sizes and four benchmarks to accommodate the range of our data (0 no effect; 0.2 small effect, cannot be discerned with the naked eye; 0.5 medium effect; and 0.8 large effect, can be seen with the naked eye) (https://www.statisticshowto.com/hedges-g). A negative value indicates an increase and a positive value indicates a decrease in the MI24.
Results
SS Flies Are Heavier and at Increased Risk of Mortality after TBI
Under our culture conditions, SS flies exhibited an easily recognizable obese phenotype weighing 40%–50% more than FC flies (1.25 versus 0.84 mg/fly), in agreement with previously reported data (Reynolds, 2013). To investigate whether the obesity phenotype alters the risk of early mortality after TBI, we determined the MI24 of FC and SS flies after four strikes with the high-impact trauma device (Fig. 1). The MI24 of FC flies was 20.1 (±2.35) n = 6, confidence interval (CI) [17.6 to 22.5], close to that previously reported for w1118 flies of the same age (Fischer et al., 2018). By contrast, the MI24 of SS flies was 57.8 (±2.14), n = 6, CI [55.5 to 60.0], which is in the top decile of MI24 values reported for inbred and outbred collections (Katzenberger et al., 2015) (Fig. 1). In summary, changes associated with obesity induced by experimental evolution increased the relative risk of TBI-induced early death 2.85-fold CI [1.86 to 4.37] (P < 0.0001).
Early Mortality Is Positively Correlated with Fly Weight
To examine whether the high MI24 of SS flies was attributable to their increased weight, we examined male flies from four other fly lines whose weights varied over a 3-fold range from 0.5 to 1.7 mg/fly, bracketing the weight of FC and SS flies (Fig. 2). In addition to FC and SS flies, we included two Drosophila melanogaster lines with the following weights: w1118 0.55 (±0.02) mg/fly, and 2P9 0.73 (±0.02) mg/fly as well as lines of Drosophila virilis 1.57 (±0.03) mg/fly and Drosophila funebris 1.62 (±0.05) mg/fly. w1118 is a standard laboratory line and 2P9 is an uncharacterized P-element insertion line. FC flies weighed 0.84 (±0.09) mg/fly. SS flies from the 2nd to 10th, i.e., early (E) generations maintained an obese phenotype (GenE) and weighed 1.25 (±0.1) mg/fly. The late (L) generation SS flies that were losing the obese phenotype (SS GenL) weighed 0.97 (±0.1) mg/fly by generation 20. All lines were tested at 1–8 days old. We found that fly weight was highly correlated with the MI24 (R2= 0.96) (Fig. 2). This might be expected because both the force imparted on the flies and the energy they were subjected to should be proportional to their mass [i.e., force = mass × acceleration (F = ma) and energy = 1/2 mass × velocity of impact squared (E = 1/2 mv2)]. The close, but not perfect, correlation between the weight of a fly and the MI24 leaves room for other factors to affect the MI24 (e.g., genetic background). We previously found that when tested at 0–7 days old, inbred fly lines from the Drosophila Genetic Reference Panel, whose males vary in weight from 0.58 to 0.87 mg/fly (Unckless et al., 2015), had MI24 values that varied from 8 to 58 (Katzenberger et al., 2015), which exceeds the expected variability based exclusively on weight of 23 to 37 predicted by the data in Fig. 2. These data indicate that the weight plays a major role in increasing TBI-induced mortality of SS over FC flies, but it remains possible that increased mortality of SS flies results from severe secondary injuries due to cellular and molecular effects associated with obesity.
Anesthetic Pretreatment Does Not Precondition SS Flies
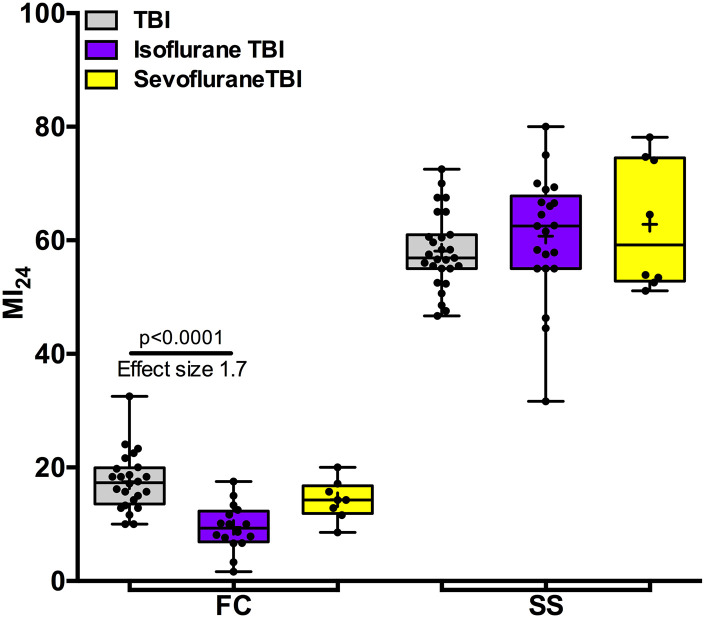
To test the hypothesis that obesity influences anesthetic pharmacodynamics, we assayed the effect of anesthetic exposure prior to TBI. In FC flies, pretreatment with equianesthetic doses of isoflurane (4%h) or sevoflurane (7%h) reduced the MI24 from 17.5 (5.1, n = 24) to 9.4 (4.1, n = 16) and to 14.3 (3.5, n = 8) for isoflurane and sevoflurane (P < 0.0001 and 0.18), respectively (Fig. 3). Thus, exposure to isoflurane preconditioned flies to TBI, whereas sevoflurane trended toward this phenotype. Preconditioning resulted in a reduction of the relative risk of death to 0.53 [CI 0.25 to 1.13] and 0.82 [CI 0.43 to 1.58] for isoflurane and sevoflurane, respectively. By contrast, pre-exposure of SS flies with the same doses of isoflurane or sevoflurane did not precondition to TBI. The MI24 without pre-exposure was 58.1 (±6.6, n = 27) and with pre-exposure was 60.7 (±10.9, n = 21) and 62.8 (±11.4, n = 8) for isoflurane and sevoflurane, respectively. We conclude that although the effectiveness of preconditioning in FC flies is comparable to the previously reported protective effects of these agents in w1118 flies (Fischer et al., 2018; Schiffman et al., 2020), the obese phenotype generated by starvation-selection is associated with changes that thwart molecular mechanisms underlying anesthetic preconditioning.
Fig. 3.
Obese flies are resistant to the preconditioning effect of pre-exposure to VGAs. Exposure of FC flies (fed control, i.e., lean flies) to the isoflurane prior to TBI (left) reduced the MI24. The effect was large for isoflurane (Hedge’s g 1.7; P < 0.0001, ANOVA with Bonferroni’s test) and medium (Hedge’s g 0.7; (P = 0.18, ANOVA with Bonferroni’s test) for sevoflurane. Exposure of SS flies (starvation-selected, i.e., obese flies) to either VGA did not appreciably affect the MI24 (right). + indicates the mean, the horizontal line indicates the median, the box indicates 25th to 75th percentile, whiskers extend to the minimal and maximal values, and dots indicate individual replicates.
Anesthesia after TBI Selectively Increases the MI24
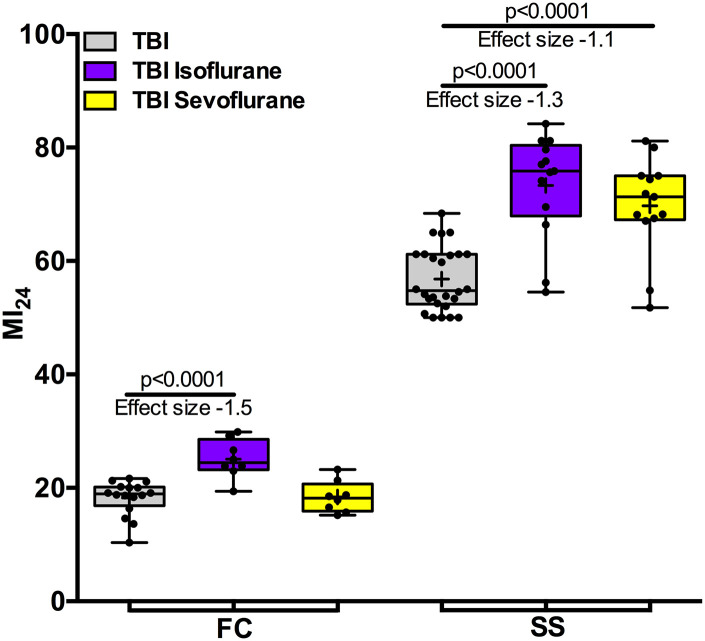
In contrast to the unambiguous effectiveness of preconditioning, the results from exposure to VGAs after ischemia (i.e., postconditioning) are mixed (Lucchinetti et al., 2005; Li and Zuo, 2011). We tested the effect of exposure to VGAs after TBI using the same doses of isoflurane and sevoflurane as used for pre-exposure. Exposure of FC flies to isoflurane after TBI increased the MI24 from 18.2 (±3.1, n = 16) to 25.1 (±6.5, n = 8), also increasing the risk ratio for death to 1.38 [CI 0.81 to 2.38), whereas the MI24 was not appreciably affected by sevoflurane (18.4 ± 4.3, n = 8). (Fig. 4). These results replicate our findings in w1118 flies, in that postexposure with isoflurane but not sevoflurane revealed a toxic potential of VGAs when administered after TBI (Fischer et al., 2018; Schiffman et al., 2020). The outcomes differed somewhat in SS flies where exposure to both isoflurane and sevoflurane increased the MI24 from 56.8 (±8.1, n = 26) to 73.3 (±18.4, n = 13) and to 69.7(±17.3, n = 13), respectively. Postconditioning hence increased the risk of death to 1.28 [CI 1.04 to 1.58] and 1.23 [CI 0.99 to 1.52] for isoflurane and sevoflurane, respectively. These results in SS flies resemble the increase in MI24 from post-treatment with both agents reported for old w1118 flies (Schiffman et al., 2020). We conclude that metabolic changes associated with obesity lower the threshold for VGA toxicity and reveal a toxic potential for sevoflurane in the context of an injured brain.
Fig. 4.
Exposure to VGAs after TBI increases mortality in obese flies. Exposure of FC flies (fed control, i.e., lean flies) to isoflurane but not to sevoflurane after TBI increased the MI24 (left). By contrast, exposure of SS flies (starvation-selected, i.e., obese flies) to both isoflurane and sevoflurane increased the MI24 (right). Hedge’s g effect size of isoflurane in FC and SS flies was large (-1.5 and -1.3, respectively, P < 0.0001 for both comparisons, ANOVA with Bonferroni’s multiple comparisons test). The effect of sevoflurane in SS flies was large (Hedge’s g −1.1, P < 0.0001), whereas there was no effect in FC flies (Hedge’s g 0). + indicates the mean, the horizontal line indicates the median, the box indicates 25th to 75th percentile, whiskers extend to the minimal and maximal values, and dots indicate individual replicates.
Pre-Exposure and Postexposure Phenotypes Normalize after Many Generations in the Absence of Starvation-Selection
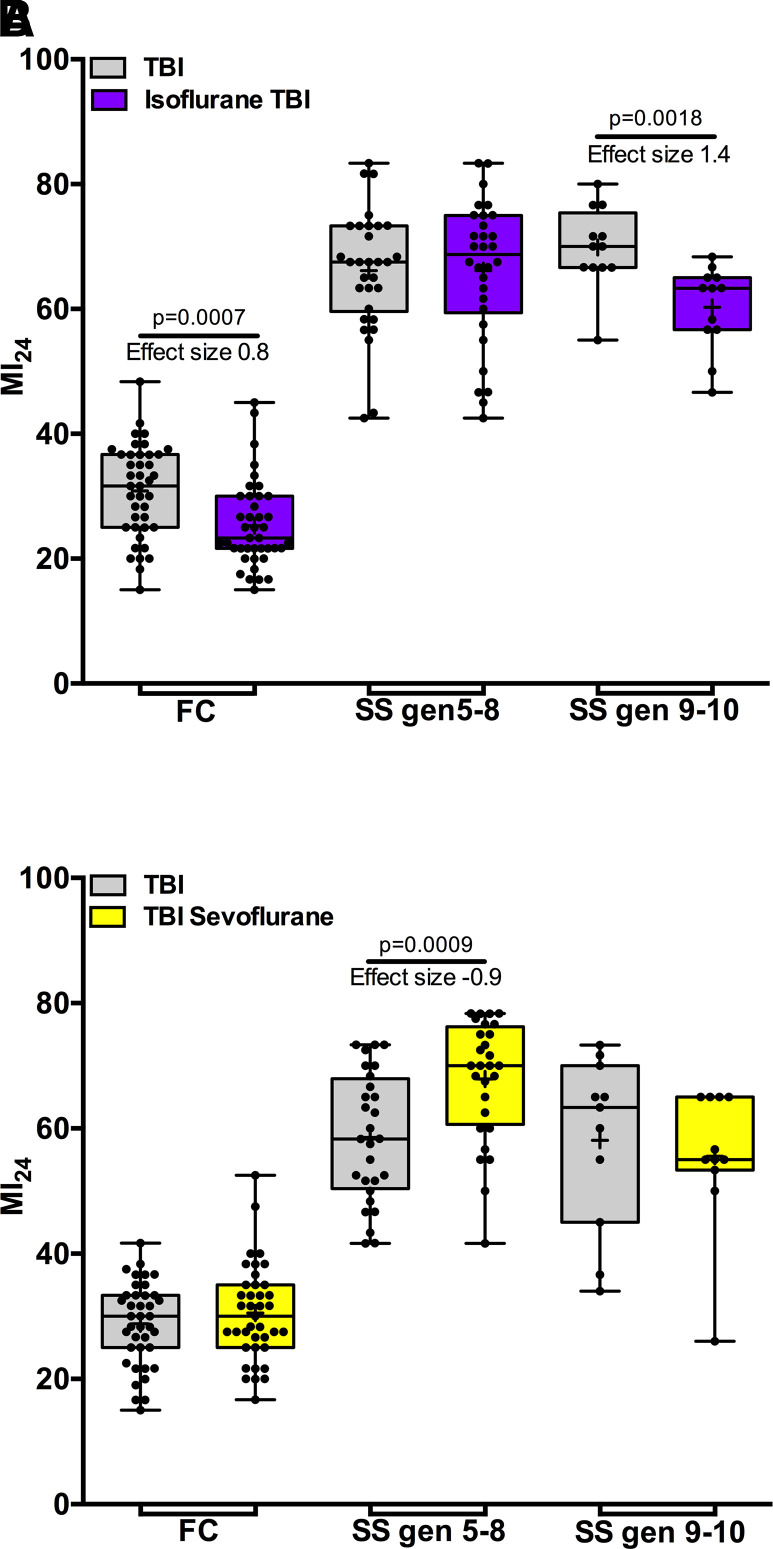
Figs. 3 and 4 show that early generation (≤ 5th generation) SS flies are distinct from FC flies in their resistance to preconditioning of TBI by isoflurane and sevoflurane and toxicity from postexposure to sevoflurane. To test whether these distinct phenotypes persist after SS flies lose the obese phenotype, we examined flies up to the 10th generation. The MI24 declined proportionally to the loss of weight (Fig. 2, SS-E and SS-L) but remained higher than that of FC flies (Fig. 5). After the eighth generation, pre-exposure to isoflurane suppressed the MI24 (Fig. 5A, SS gen 9–10). Concomitantly, postexposure to sevoflurane lost its toxic effect (Fig. 5B, SS gen 9–10). We conclude that pathways mediating the molecular mechanisms of the effects of VGAs on survival after TBI recover after prolonged absence of starvation-selection.
Fig. 5.
Anesthetic pharmacodynamics gradually normalize after termination of starvation-selection. (A) SS flies became susceptible to isoflurane preconditioning after the 8th generation. (B) Postexposure toxicity of sevoflurane disappears in SS flies after the 8th generation. Gray bars indicate the MI24 without anesthetic exposure, purple bars indicate preconditioning with 15 minutes of 2% isoflurane, and yellow bars indicate postexposure to 15 minutes of 3.5% sevoflurane. SS gen 5–8 and SS gen 9–10 indicate the number of generations that the flies reproduced on cornmeal-molasses food. FC are fed control, i.e., lean flies. Note: the MI24 of SS flies remains higher than that of FC flies. + indicates the mean, the horizontal line indicates the median, the box indicates 25th to 75th percentile, whiskers extend to the minimal and maximal values, and dots indicate individual replicates.
Discussion
The principal finding of this work is that obesity resulting from starvation-selection interferes with preconditioning of a TBI outcome by VGAs. To reach this conclusion, we combined two fly models (TBI and obesity) that reproduce many features of their counterparts in higher animals, and we took advantage of the fact that key pharmacokinetic and pharmacodynamic properties of VGAs (Fischer et al., 2018; Olufs et al., 2018) are also evolutionarily conserved.
Preconditioning by VGAs in mammals is well documented but its mechanisms are not fully understood. Diverse injurious stimuli can precondition the brain but all of them have exceedingly narrow therapeutic indices rapidly resulting in injury when a certain, largely ill-defined threshold is exceeded (Stenzel-Poore et al., 2004; Gidday, 2006; Obrenovitch, 2008; Yokobori et al., 2013). VGAs are exceptional in that preconditioning is induced rapidly, but even sustained exposure will not injure the adult "healthy" brain. Therefore, the mechanism underlying anesthetic preconditioning must differ qualitatively and/or quantitatively from other preconditioning stressors that have to be administered either very briefly (e.g., hypoxia and oxidative stress) or for prolonged periods of time [e.g., hyperthermia (Shohami et al., 1994; Su et al., 2009)].
The principal, not mutually exclusive, mechanisms of anesthetic preconditioning under investigation are: (i) signaling from an early increase of intracellular Ca2+ (Gray et al., 2005; Weber, 2012), (ii) triggering of proteostatic responses (e.g., the unfolded protein response) (Baker et al., 2011; McClintick et al., 2011), (iii) isoflurane-induced mitochondrial reactive oxygen species-mediated signaling cascades leading to ischemic tolerance (Hirata et al., 2011) by modulation of the AMPK (AMP-activated protein kinase) signaling pathway (Song et al., 2011), (iv) inducible nitric oxide synthase, implicated in both ischemic and anesthetic preconditioning (Kapinya et al., 2002) with possibly different sources of NO in the heart versus the brain (Kapinya et al., 2002; Amour et al., 2009), (v) modulation of the immune-inflammatory system, possibly via VGA-induced modulation of the transcription factor NF-kappaB (Zhang et al., 2013), and (vi) modulation of the mitochondrial inner membrane permeability transition pore (mPTP) (Sedlic et al., 2010). The mPTP serves as a rescue pathway for excessive mitochondrial Ca2+ accumulation and its opening is a critical, irreversible step committing a cell to apoptosis. The state of the mPTP is controlled by numerous upstream and downstream targets and even its exact molecular composition is under debate (Baines and Gutiérrez-Aguilar, 2018), but delays in its opening have been suggested with various types of preconditioning (Pravdic et al., 2009).
Obesity modulates some of these pathways. For example, the failure of preconditioning was attributed to interference with sevoflurane-induced phosphorylation of AMPK and activation of eNOS (endothelial nitric oxide synthase) in the myocardium of obese rats (Song et al., 2011) and with misregulation of microRNA 21 and NOS by isoflurane in the hearts of diabetic mice (Ge et al., 2018). The degree to which similar processes play a role in the brain remains to be investigated, and experiments presented in this paper are a first step.
The use of Drosophila as a model for clinical conditions is only possible because of extensive evolutionary conservation. For example, over 70% of human disease-causing genes have orthologs in the fly (Reiter et al., 2001) and, as basic cellular processes are conserved between flies and humans, both share secondary molecular and cellular events triggered by injury (Chow and Reiter, 2017). For example, oxidative stress is a major molecular driver of obesity-related complications (Furukawa et al., 2004) and plays similar role in obesity models in the fruit fly (Trindade de Paula et al., 2016).
Our previous work has shown that pretreatment with VGAs effectively protected flies from death due to TBI (Fischer et al., 2018; Schiffman et al., 2020), indicating that some molecular mechanisms by which anesthetics precondition are operational in flies. Here we expand on these findings by showing that, in agreement with data from the rodent myocardium (Song et al., 2011; Ge et al., 2018), obesity interferes with isoflurane preconditioning in brain injury. We cannot make a statement regarding sevoflurane in this context because the reduction in MI24 by sevoflurane preconditioning, despite a moderate effect size, did not reach the threshold for statistical significance (P = 0.18). These findings are particularly relevant for experimental studies of TBI. For example, in TBI induced in rodents either by fluid percussion (Wu et al., 2003) or controlled-cortical impact (Hoane et al., 2011), diet-induced obesity resulted in worsened outcomes. Both research groups attributed their findings to the effect of diet and/or obesity on biochemical alterations such as brain BDNF (brain derived neurotrophic factor) levels. It is notable though that all animals were exposed to general anesthetics around the time of injury. Therefore, although a role for BDNF is possible, the alternative explanation of differential preconditioning between experimental groups by anesthetics cannot be excluded, illustrating the value of unconventional approaches using invertebrate models to complex, multifactorial pathologies like TBI.
The high MI24 and the lack of preconditioning in SS flies resemble the phenotypes of aged laboratory flies (Schiffman et al., 2020). Aging increases vulnerability to TBI in humans (Maas et al., 2008) and flies (Katzenberger et al., 2013) and also reduces the effectiveness of preconditioning in the human myocardium (Mio et al., 2008). Because starvation-selection does not shorten lifespan (Archer et al., 2003), FC and SS flies were injured at the same point in their lifespan. One explanation for our findings may be that obesity associated changes result in a premature aging-like phenotype revealed under stress caused by TBI.
In summary, although neither TBI nor obesity in flies equals their human counterparts, flies are a useful tool to inform research in higher animals by exploring parameters that would be difficult, expensive, or simply unethical to examine in higher animals.
Acknowledgments
We thank Dr. Allen Gibbs (School of Life Sciences, University of Nevada, Las Vegas, NV) for generously providing the flies.
Abbreviations
- CI
confidence interval
- FC
fed control (lean control fly line from A. Gibbs laboratory – UNLV)
- MI24
Mortality Index at 24 hours
- SS
starvation-selected (obese phenotype fly line from A. Gibbs laboratory – UNLV)
- TBI
traumatic brain injury
- VGA
volatile general anesthetic
Authorship Contributions
Participated in research design: Wassarman, Perouansky.
Conducted experiments: Fischer, Schiffman.
Performed data analysis: Johnson-Schlitz, Olufs, Scharenbrock.
Wrote or contributed to the writing of the manuscript: Olufs, Wassarman, Perouansky.
Footnotes
The project was supported by a seed grant from the R&D fund of the Department of Anesthesiology, University of Wisconsin-Madison, by the Clinical and Translational Science Award (CTSA) program, through National Institutes of Health National Center for Advancing Translational Sciences (NCATS) [Grant UL1TR002373], and by the NIH National Institute of Neurological Disorders and Stroke [Grant RF1-NS114359]. The content is solely the responsibility of the authors and does not necessarily represent the official views of National Institutes of Health.
Primary laboratory of origin: Perouansky Laboratory (Misha Perouansky) (Department of Anesthesiology, University of Wisconsin-Madison, Madison, WI).
An earlier version of this paper appears in Preprints under the doi:10.20944/preprints202110.0339.v1.
No author has an actual or perceived conflict of interest with the contents of this article.
References
- Altman DG (1991) Practical Statistics for Medical Research, Chapman and Hall/CRC, London. [Google Scholar]
- Amour J, Brzezinska AK, Weihrauch D, Billstrom AR, Zielonka J, Krolikowski JG, Bienengraeber MW, Warltier DC, Pratt PF Jr, Kersten JR (2009) Role of heat shock protein 90 and endothelial nitric oxide synthase during early anesthetic and ischemic preconditioning. Anesthesiology 110:317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer MA, Phelan JP, Beckman KA, Rose MR (2003) Breakdown in correlations during laboratory evolution. II. Selection on stress resistance in Drosophila populations. Evolution 57:536–543. [DOI] [PubMed] [Google Scholar]
- Baines CP, Gutiérrez-Aguilar M (2018) The still uncertain identity of the channel-forming unit(s) of the mitochondrial permeability transition pore. Cell Calcium 73:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MR, Benton SK, Theisen CS, McClintick CA, Fibuch EE, Seidler NW (2011) Isoflurane’s effect on protein conformation as a proposed mechanism for preconditioning. Biochem Res Int 2011:739712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CY, Reiter LT (2017) Etiology of human genetic disease on the fly. Trends Genet 33:391–398. [DOI] [PubMed] [Google Scholar]
- Fischer JA, Olufs ZPG, Katzenberger RJ, Wassarman DA, Perouansky M (2018) Anesthetics influence mortality in a Drosophila model of blunt trauma with traumatic brain injury. Anesth Analg 126:1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114:1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge ZD, Li Y, Qiao S, Bai X, Warltier DC, Kersten JR, Bosnjak ZJ, Liang M (2018) Failure of isoflurane cardiac preconditioning in obese type 2 diabetic mice involves aberrant regulation of microRNA-21, endothelial nitric-oxide synthase, and mitochondrial complex I. Anesthesiology 128:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidday JM (2006) Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci 7:437–448. [DOI] [PubMed] [Google Scholar]
- Gray JJ, Bickler PE, Fahlman CS, Zhan X, Schuyler JA (2005) Isoflurane neuroprotection in hypoxic hippocampal slice cultures involves increases in intracellular Ca2+ and mitogen-activated protein kinases. Anesthesiology 102:606–615. [DOI] [PubMed] [Google Scholar]
- Hardy CM, Birse RT, Wolf MJ, Yu L, Bodmer R, Gibbs AG (2015) Obesity-associated cardiac dysfunction in starvation-selected Drosophila melanogaster. Am J Physiol Regul Integr Comp Physiol 309:R658–R667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata N, Shim YH, Pravdic D, Lohr NL, Pratt PF Jr, Weihrauch D, Kersten JR, Warltier DC, Bosnjak ZJ, Bienengraeber M (2011) Isoflurane differentially modulates mitochondrial reactive oxygen species production via forward versus reverse electron transport flow: implications for preconditioning. Anesthesiology 115:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoane MR, Swan AA, Heck SE (2011) The effects of a high-fat sucrose diet on functional outcome following cortical contusion injury in the rat. Behav Brain Res 223:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapinya KJ, Löwl D, Fütterer C, Maurer M, Waschke KF, Isaev NK, Dirnagl U (2002) Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO synthase dependent. Stroke 33:1889–1898. [DOI] [PubMed] [Google Scholar]
- Katzenberger RJ, Chtarbanova S, Rimkus SA, Fischer JA, Kaur G, Seppala JM, Swanson LC, Zajac JE, Ganetzky B, Wassarman DA (2015) Death following traumatic brain injury in Drosophila is associated with intestinal barrier dysfunction. eLife 4:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenberger RJ, Loewen CA, Wassarman DR, Petersen AJ, Ganetzky B, Wassarman DA (2013) A Drosophila model of closed head traumatic brain injury. Proc Natl Acad Sci USA 110:E4152–E4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano H, Kirsch JR, Hurn PD, Murphy SJ (2007) Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Flow Metab 27:1108–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z (2011) Isoflurane postconditioning induces neuroprotection via Akt activation and attenuation of increased mitochondrial membrane permeability. Neuroscience 199:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti E, da Silva R, Pasch T, Schaub MC, Zaugg M (2005) Anaesthetic preconditioning but not postconditioning prevents early activation of the deleterious cardiac remodelling programme: evidence of opposing genomic responses in cardioprotection by pre- and postconditioning. Br J Anaesth 95:140–152. [DOI] [PubMed] [Google Scholar]
- Maas AI, Stocchetti N, Bullock R (2008) Moderate and severe traumatic brain injury in adults. Lancet Neurol 7:728–741. [DOI] [PubMed] [Google Scholar]
- Masek P, Reynolds LA, Bollinger WL, Moody C, Mehta A, Murakami K, Yoshizawa M, Gibbs AG, Keene AC (2014) Altered regulation of sleep and feeding contributes to starvation resistance in Drosophila melanogaster. J Exp Biol 217:3122–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintick CA, Theisen CS, Ferns JE, Fibuch EE, Seidler NW (2011) Isoflurane preconditioning involves upregulation of molecular chaperone genes. Biochem Biophys Res Commun 411:387–392. [DOI] [PubMed] [Google Scholar]
- Mio Y, Bienengraeber MW, Marinovic J, Gutterman DD, Rakic M, Bosnjak ZJ, Stadnicka A (2008) Age-related attenuation of isoflurane preconditioning in human atrial cardiomyocytes: roles for mitochondrial respiration and sarcolemmal adenosine triphosphate-sensitive potassium channel activity. Anesthesiology 108:612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T, Kato S (2014) Impact of diabetes mellitus on myocardial lipid deposition: an autopsy study. Pathol Res Pract 210:1018–1025. [DOI] [PubMed] [Google Scholar]
- Obrenovitch TP (2008) Molecular physiology of preconditioning-induced brain tolerance to ischemia. Physiol Rev 88:211–247. [DOI] [PubMed] [Google Scholar]
- Olufs ZPG, Loewen CA, Ganetzky B, Wassarman DA, Perouansky M (2018) Genetic variability affects absolute and relative potencies and kinetics of the anesthetics isoflurane and sevoflurane in Drosophila melanogaster. Sci Rep 8:2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravdic D, Sedlic F, Mio Y, Vladic N, Bienengraeber M, Bosnjak ZJ (2009) Anesthetic-induced preconditioning delays opening of mitochondrial permeability transition pore via protein Kinase C-epsilon-mediated pathway. Anesthesiology 111:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam LJ, Willes AM, Kalata BE, Disher ND, Brusich DJ (2019) Expansion of a fly TBI model to four levels of injury severity reveals synergistic effects of repetitive injury for moderate injury conditions. Fly (Austin) 13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter LT, Potocki L, Chien S, Gribskov M, Bier E (2001) A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res 11:1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LA (2013) The Effects of Starvation Selection on Drosophila melanogaster Life History and Development. Doctoral dissertation, University of Nevada, Las Vegas, NV. [Google Scholar]
- Saikumar J, Byrns CN, Hemphill M, Meaney DF, Bonini NM (2020) Dynamic neural and glial responses of a head-specific model for traumatic brain injury in Drosophila. Proc Natl Acad Sci 117:17269–17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman HJ, Olufs ZPG, Lasarev MR, Wassarman DA, Perouansky M (2020) Ageing and genetic background influence anaesthetic effects in a D. melanogaster model of blunt trauma with brain injury†. Br J Anaesth 125:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlic F, Sepac A, Pravdic D, Camara AK, Bienengraeber M, Brzezinska AK, Wakatsuki T, Bosnjak ZJ (2010) Mitochondrial depolarization underlies delay in permeability transition by preconditioning with isoflurane: roles of ROS and Ca2+. Am J Physiol Cell Physiol 299:C506–C515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Sadjadi R, Carlson J, Chen Y, Xu D, Ferriero DM, Noble-Haeusslein LJ (2016) Long-term anesthetic-dependent hypoactivity after repetitive mild traumatic brain injuries in adolescent mice. Dev Neurosci 38:220–238. [DOI] [PubMed] [Google Scholar]
- Shohami E, Novikov M, Horowitz M (1994) Long term exposure to heat reduces edema formation after closed head injury in the rat. Acta Neurochir Suppl (Wien) 60:443–445. [DOI] [PubMed] [Google Scholar]
- Song T, Lv LY, Xu J, Tian ZY, Cui WY, Wang QS, Qu G, Shi XM (2011) Diet-induced obesity suppresses sevoflurane preconditioning against myocardial ischemia-reperfusion injury: role of AMP-activated protein kinase pathway. Exp Biol Med (Maywood) 236:1427–1436. [DOI] [PubMed] [Google Scholar]
- Stadnicka A, Marinovic J, Ljubkovic M, Bienengraeber MW, Bosnjak ZJ (2007) Volatile anesthetic-induced cardiac preconditioning. J Anesth 21:212–219. [DOI] [PubMed] [Google Scholar]
- Staib-Lasarzik I, Kriege O, Timaru-Kast R, Pieter D, Werner C, Engelhard K, Thal SC (2014) Anesthesia for euthanasia influences mRNA expression in healthy mice and after traumatic brain injury. J Neurotrauma 31:1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statler KD, Alexander H, Vagni V, Dixon CE, Clark RS, Jenkins L, Kochanek PM (2006a) Comparison of seven anesthetic agents on outcome after experimental traumatic brain injury in adult, male rats. J Neurotrauma 23:97–108. [DOI] [PubMed] [Google Scholar]
- Statler KD, Alexander H, Vagni V, Holubkov R, Dixon CE, Clark RS, Jenkins L, Kochanek PM (2006b) Isoflurane exerts neuroprotective actions at or near the time of severe traumatic brain injury. Brain Res 1076:216–224. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Simon RP (2004) Genomics of preconditioning. Stroke 35(11, Suppl 1)2683–2686. [DOI] [PubMed] [Google Scholar]
- Su Z, Han D, Sun B, Qiu J, Li Y, Li M, Zhang T, Yang Z (2009) Heat stress preconditioning improves cognitive outcome after diffuse axonal injury in rats. J Neurotrauma 26:1695–1706. [DOI] [PubMed] [Google Scholar]
- Szczepaniak LS, Victor RG, Orci L, Unger RH (2007) Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res 101:759–767. [DOI] [PubMed] [Google Scholar]
- Tétrault S, Chever O, Sik A, Amzica F (2008) Opening of the blood-brain barrier during isoflurane anaesthesia. Eur J Neurosci 28:1330–1341. [DOI] [PubMed] [Google Scholar]
- Trindade de Paula MPoetini Silva MRMachado Araujo SCardoso Bortolotto VBarreto Meichtry LZemolin APWallau GLJesse CRFranco JLPosser T, et al. (2016) High-fat diet induces oxidative stress and MPK2 and HSP83 gene expression in drosophila melanogaster. Oxid Med Cell Longev 2016:4018157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unckless RL, Rottschaefer SM, Lazzaro BP (2015) A genome-wide association study for nutritional indices in Drosophila. G3 (Bethesda) 5:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JT (2012) Altered calcium signaling following traumatic brain injury. Front Pharmacol 3:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Molteni R, Ying Z, Gomez-Pinilla F (2003) A saturated-fat diet aggravates the outcome of traumatic brain injury on hippocampal plasticity and cognitive function by reducing brain-derived neurotrophic factor. Neuroscience 119:365–375. [DOI] [PubMed] [Google Scholar]
- Yokobori S, Mazzeo AT, Hosein K, Gajavelli S, Dietrich WD, Bullock MR (2013) Preconditioning for traumatic brain injury. Transl Stroke Res 4:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang J, Yang L, Dong Y, Zhang Y, Xie Z (2013) Isoflurane and sevoflurane increase interleukin-6 levels through the nuclear factor-kappa B pathway in neuroglioma cells. Br J Anaesth 110 (Suppl 1):i82–i91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH (2000) Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA 97:1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]