Important Compound Classes
Title
Compounds and Compositions as Sppl2a Inhibitors
Patent Publication Number
WO 2022/058902 A1
URL:https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022058902
Publication Date
March 24, 2022
Priority Application
US 63/079,604
Priority Date
September 17, 2020
Inventors
Brandl, T.; Ehrhardt, C.; Epple, R.; Markert, C.; Rigollier, P.; Velcicky, J.
Assignee Company
Novartis AG, Switzerland
Disease Area
Autoimmune diseases and lymphomas
Biological Target
Sppl2a
Summary
The protein signal peptide peptidase-like protease 2a (Sppl2a) appears to play a role in innate and adaptive immunity by cleaving different transmembrane-anchored proteins and thereby affecting the function of a variety of immune cells. Sppl2a was initially described as the protease cleaving the membrane-spanning portion of TNF-α and thereby controlling the release of IL-12 from dendritic cells. Inhibition of this protease may be relevant for the repression of detrimental, uncontrolled immune responses, e.g., pathological conditions where autoantibodies might be critical to autoimmune diseases. Sppl2a inhibition might influence the proliferation of B-cell lymphomas.
The present application describes a series of novel diazepinone compounds as Sppl2a inhibitors for the treatment of autoimmune diseases and lymphomas. Further, the application discloses compounds, their preparation, use, and pharmaceutical composition, and treatment.
Definitions
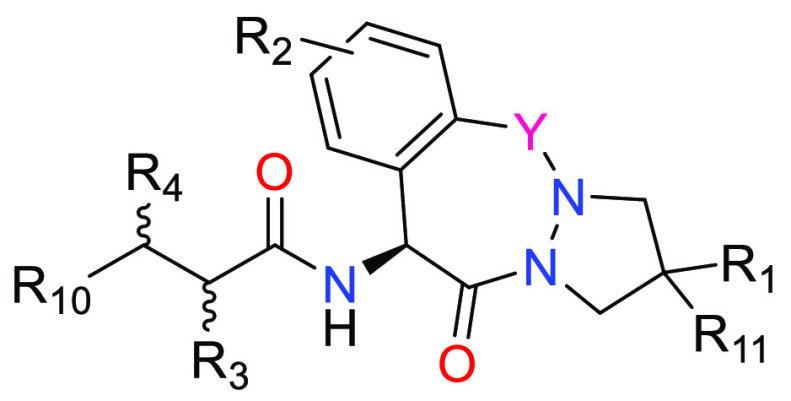
Y = CH2 or C=O;
R1 = H, C1–C6 alkyl or halogen; R2 = H or halogen;
R3 = H, C1–C6 alkyl, C1–C6 haloalkyl, C3–C6 cycloalkyl, C1–C6 alkyl-phenyl, or C1–C6 alkyl substituted with C1–C6 alkoxy;
R4 = H, C1–C6 alkyl, or C1–C6alkyl-phenyl;
R10 = -NHC(=O)R5, -C(=O)NHR5, or 9- or 10-membered bicyclic heteroaryl having 1–4 heteroatoms as ring members selected from N, O, and S, wherein the bicyclic heteroaryl is unsubstituted or the bicyclic heteroaryl is substituted with one or more R6; and
R11 = H, C1–C6 alkyl, or halogen.
Key Structures
Biological Assay
The Sppl2a TL assay was performed. The compounds described in this application were tested for their ability to inhibit Sppl2a. The Sppl2a IC50 (μM) are shown in the table below.
Biological Data
The table below shows representative compounds tested for Sppl2a
inhibition and the biological data obtained from testing
representative examples.
Claims
Total claims: 19
Compound claims: 13
Pharmaceutical composition claims: 1
Method of treatment claims: 4
Combination claims: 1
The author declares no competing financial interest.
Recent Review Articles. References
- Mentrup T.; Schroder B. Signal peptide peptidase-like 2 proteases: Regulatory switches or proteasome of the membrane?. Biochim. Biophys. Acta, Mol. Cell Res. 2022, 1869, 119163. 10.1016/j.bbamcr.2021.119163. [DOI] [PubMed] [Google Scholar]
- Yucel S. S.; Lemberg M. K. Signal Peptide Peptidase-Type Proteases: Versatile Regulators with Functions Ranging from Limited Proteolysis to Protein Degradation. J. Mol. Biol. 2020, 432, 5063. 10.1016/j.jmb.2020.05.014. [DOI] [PubMed] [Google Scholar]
- Marijt K. A.; van Hall T. To TAP or not to TAP: alternative peptides for immunotherapy of cancer. Curr. Opin. Immunol. 2020, 64, 15. 10.1016/j.coi.2019.12.004. [DOI] [PubMed] [Google Scholar]
- Pagano S.; Coniglio M.; Valenti C.; Federici M. I.; Lombardo G.; Cianetti S.; Marinucci L. Biological effects of Cannabidiol on normal human healthy cell populations: Systematic review of the literature. Biomed. Pharmacother. 2020, 132, 110728. 10.1016/j.biopha.2020.110728. [DOI] [PubMed] [Google Scholar]
- Nutt S. L.; Chopin M. Transcriptional Networks Driving Dendritic Cell Differentiation and Function. Immunity 2020, 52, 942. 10.1016/j.immuni.2020.05.005. [DOI] [PubMed] [Google Scholar]
- Beard H. A.; Barniol-Xicota M.; Yang J.; Verhelst S. H. L Discovery of Cellular Roles of Intramembrane Proteases. ACS Chem. Biol. 2019, 14, 2372. 10.1021/acschembio.9b00404. [DOI] [PubMed] [Google Scholar]