Abstract
With the widespread use of volatile anesthetic agents in the prolonged sedation for COVID-19 pneumonia and ARDS, there is an urgent need to investigate the effects and treatments of lengthy low-concentration inhaled anesthetics exposure on cognitive function in adults. Previous studies showed that general anesthetics dose- and exposure length-dependently induced neuroinflammatory response and cognitive decline in neonatal and aging animals. The anti-diabetes drug metformin has anti-neuroinflammation effects by modulating microglial polarization and inhibiting astrocyte activation. In this study, we demonstrated that the inhalation of 1.3% isoflurane (a sub-minimal alveolar concentration, sub-MAC) for 6 h impaired recognition of novel objects from Day 1 to Day3 in adult mice. Prolonged sub-MAC isoflurane exposure also triggered typically reactive microglia and A1-like astrocytes in the hippocampus of adult mice on Day 3 after anesthesia. In addition, prolonged isoflurane inhalation switched microglia into a proinflammatory M1 phenotype characterized by elevated CD68 and iNOS as well as decreased arginase-1 and IL-10. Metformin pretreatment before anesthesia enhanced cognitive performance in the novel object test. The positive cellular modifications promoted by metformin pretreatment included the inhibition of reactive microglia and A1-like astrocytes and the polarization of microglia into M2 phenotype in the hippocampus of adult mice. In conclusion, prolonged sub-MAC isoflurane exposure triggered significant hippocampal neuroinflammation and cognitive decline in adult mice which can be alleviated by metformin pretreatment via inhibiting reactive microglia and A1-like astrocytes and promoting microglia polarization toward anti-inflammatory phenotype in the hippocampus.
Keywords: Cognitive impairment, Isoflurane, Neuroinflammation, Astrocyte, Microglia, Metformin
1. Introduction
Increasing experimental and clinical observations suggest that general anesthetics exposure, particularly prolonged or repeated exposure at the extremes of age, induces long-lasting cognitive decline [1], [2], [3], [4]. However, the role of lengthy general anesthetics exposure in adults remains largely unknown. With the widespread use of volatile anesthetic agents in the prolonged sedation for COVID-19 pneumonia and acute respiratory distress syndrome [5], there is an urgent need to investigate the effects of lengthy low-concentration inhaled anesthetics exposure on cognitive function in adults. Aberrant neuroinflammation was one of the primary mechanisms implicated in general anesthetic-induced cognitive decline in aging and neonatal rodents [6], [7], [8], [9]. Our previous study did find long-term 1.3% isoflurane (a sub-minimal alveolar concentration, sub-MAC) inhalation induced cognitive deficits in adult mice [10], [11]. However, whether neuroinflammation is involved in prolonged sub-MAC isoflurane-induced cognitive decline in adults remains to be elucidated.
Microglial cells and astrocytes are critical regulators of inflammatory responses in the brain. Microglia cells are resident immune cells in the brain which monitor and respond to “invaders” to maintain brain homeostasis. The anti-inflammatory state microglia (M2 phenotype) convert to a pro-inflammatory activated state (M1 phenotype) when the brain is under varying forms of insult [12]. M1 microglia cells release pro-inflammatory cytokines such as complement component 1 subcomponent q, interleukin-1α, tumor necrosis factor-α, and interleukin-18 which can induce neurotoxic A1-like astrocyte reactivity and further aggravate neuroinflammation [13], [14], [15]. While the A1-like astrocytes secreted abundant complement 3 (C3) which in turn interacted with the microglial C3 receptor to regulate microglial activation and phagocytosis [16], [17]. Previous evidence has shown that M1 microglia cells activation and A1-like astrocyte reactivation played a key role in general anesthetic-induced cognitive decline in aging and neonatal rodents [6], [7], [8], [18], [19]. In vitro study also found that prolonged sevoflurane exposure enhanced M1 polarization of microglial cell line BV-2 cells and inhibited M2 polarization of primary microglia and BV-2 cells [19], [20]. Preventing microglia polarization into the M1 phenotype and attenuating A1-like astrocyte reactivation may mitigate anesthetics-induced neuroinflammation and cognitive decline.
Metformin is a widely used first-line antidiabetic drug for type 2 diabetes. Besides the glucose-lowering effect, growing evidence suggested antidiabetic agents have anti-inflammatory effects [21]. Metformin can inhibit microglia cells activation and modulate the polarization of microglial cells exerting therapeutic effects for neuroinflammatory diseases [22], [23], [24], [25]. In vitro studies also suggested that metformin directly inhibited the activation of microglia cells and decreased the release of pro-inflammatory cytokines [22], [24], [26]. Furthermore, increasing evidence supports the anti-inflammatory effects of metformin by reducing the astrocyte activity in neurodegenerative diseases, normal aging, and diabetes [27], [28], [29]. As a target of metformin, adenosine-monophosphate-activated protein kinase (AMPK) also modulates the activation and polarization of microglia and astrocytes [28], [30], [31], [32]. These results hint that metformin may reduce glial activation and exert therapeutic effects for lengthy anesthetic-induced cognitive decline. Zhu and our recent studies did confirm that metformin had beneficial for inhalational anesthetic-induced cognitive impairment [10], [33]. However, whether metformin attenuated the prolonged sub-MAC isoflurane-induced cognitive decline in adult mice via the modulation of glial activation remains to be investigated. We hypothesis that metformin alleviated lengthy sub-MAC isoflurane exposure induced cognitive decline by preventing microglia polarization into the M1 phenotype and inhibiting reactive microglia and A1-like astrocytes in the hippocampus of adult mice.
2. Materials and methods
2.1. Animals and treatment
Adult male C57BL/6 mice (8 weeks old, Model Animal Research Center of Nanjing University) were housed in temperature-controlled facilities at 22–25 °C under a 12-hour light/dark cycle (lights on between 08:00 and 20:00), with free access to food and water. The animal care and study protocols were approved by the Laboratory Animal Ethics Committee of Drum Tower Hospital. After two weeks of acclimatization, mice were randomly divided into 4 groups receiving vehicle injection (Ctrl), isoflurane anesthesia plus vehicle injection (Anes), metformin injection (Met), or metformin pretreatment plus isoflurane anesthesia (Met + Anes). Mice in Anes and Met + Anes groups were exposed to isoflurane anesthesia in a chamber prefilled with 4% isoflurane (Lunan Better Pharmaceutical Co.) in 100% oxygen and then maintained with 1.3% isoflurane in 100% oxygen flowing at 2.5 L/min for 6 h. The respiratory activities of mice were monitored during anesthesia. To prevent hypothermia, mice were placed on heating pads to maintain body temperature during anesthesia and lasted until recovery. Mice in Met and Met + Anes groups were injected intraperitoneally with metformin (Sigma-Aldrich, D150959) 50 mg/kg body weight one hour before anesthesia.
2.2. New object recognition test
The novel object recognition (NOR) test was used to assess the learning and memory function of mice and was conducted based on our previous protocol [11]. It involved three phases: the adaptive phase (3 days), the training phase, and the testing phase. During the adaptive phase, every mouse was placed in an empty box with a black-walled arena and white bottom (26 cm × 26 cm × 40 cm) to be adaptive to the environment for 10 min twice a day. One day after the adaptive session, two identical objects were placed in the box, and each mouse was trained to explore them in the box for 10 min. Two hours later, a novel object with different shapes and colors replaced one of the familiar objects in the box, and each mouse was permitted to explore it for another 10 min. To wipe the odor cues, the objects and box were thoroughly cleaned with 75% alcohol after each test. Touching, sniffing, or licking the object was identified as the exploratory behaviors. The movements of mice were recorded by an overhead camera and the exploratory behaviors were estimated by a blinded observer. The discrimination index = (novel object exploration time − familiar object exploration time)/total exploration time × 100% was used to analyze the cognitive level.
2.3. Immunofluorescence
Mice were deeply anesthetized with isoflurane and perfused intracardially with saline followed by paraformaldehyde fixation. Fixed brains were incubated in 30% sucrose in PBS at 4 °C for 48 h. Free-floating sections of the hippocampus (20 μm) were cut and blocked with 5% bovine serum albumin in PBS. The slices were then incubated with chicken polyclonal antiserum against GFAP (1:200, NBP-1-05198, NOVUS), rat monoclonal antiserum against C3 (1:50, NB200-540, NOVUS), rabbit polyclonal antiserum against IBA1 (1:500, 019-19741, Wako), or rat monoclonal antiserum against CD68 (1:50, MCA1957, BioRad) overnight at 4 °C. Secondary antibodies, Alexa Fluor 647 goat anti-chicken IgY, Alexa Fluor 594 goat anti- rabbit IgG, and Alexa Fluor 488 goat anti-rat IgG (Abcam, USA) were used at 1:1000 dilution. The slices were mounted in DAPI solution to label nuclei. Images were photographed using a Leica Dmi8 microscope with associated LAS-X software. The ImageJ (National Institutes of Health, USA) software is used to quantitate the fluorescence analysis.
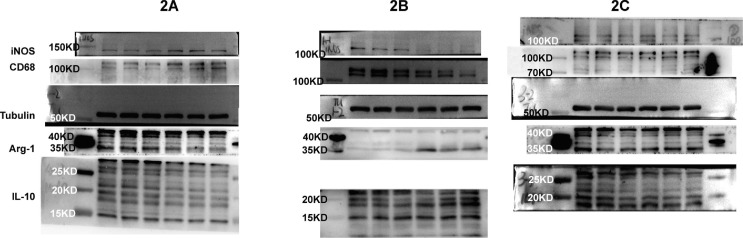
2.4. Western blotting
The expression levels of hippocampal CD68, inducible nitric oxide synthase (iNOS), interleukin-10 (IL-10), and arginase 1 (Arg1) proteins were assayed by western blotting. Hippocampal tissues were washed with ice-cold PBS and then lysed in RIPA buffer containing proteinase and phosphatase inhibitors (Sigma, USA). The samples were centrifuged at 12,000 rpm for 20 min at 4℃. A BSA protein assay was performed to determine protein concentration. Thirty microgram proteins were loaded per lane and separated by electrophoresis in 8% SDS-PAGE gels (KayGen Biotech, Co., Ltd), and were transferred onto polyvinylidene difluoride membranes (PVDF; Bio-Rad Laboratories, USA). Blots were blocked with 5% nonfat milk at normal temperature for 2 h and then were incubated with the following primary antibodies overnight at 4 °C: anti-CD68 (1:1000, 28058-1-AP, Proteintech), anti-iNOS (1:1000, 13120S, CST), anti-IL-10 (1:1000, ab9969, Abcam), and anti-Arg (1:1000, DF3791, Affinity). Tubulin (1:1000, AT819, Beyotime) was used as a loading control. Antibodies were diluted in 5% bovine serum albumin (BSA; Gentihold). The membranes were washed three times with TBST, incubated for 2 h with HRP-conjugated secondary antibodies. The Immunolabelled proteins were visualized using the chemiluminescence kit (ECL; Pierce, Illinois, USA). Band intensities of protein were quantified with ImageJ software (National Institutes of Health, USA).
2.5. Statistical analysis
All data were expressed as mean ± standard deviation (SD). Statistical analysis was carried out using SPSS 25.0 software (IBM Corporation, Armonk, NY). Results from cognitive behavioral tests and immunofluorescence analysis were analyzed by a one-way ANOVA test, followed by Bonferroni multiple comparison test. A two-tailed Student’s t test was used to analyze statistical differences between two groups for Western blotting results. For all analyses, values of *P < 0.05, or **P < 0.01 were denoted statistically significant.
3. Results
3.1. Metformin alleviated lengthy isoflurane exposure induced cognitive decline
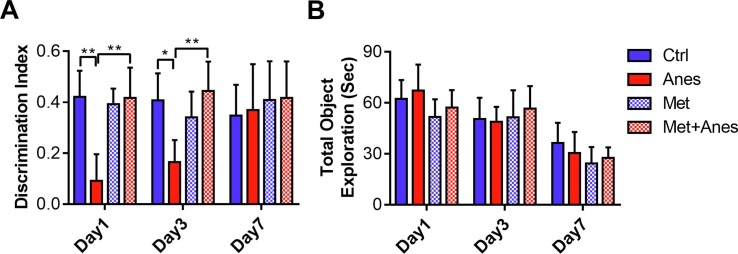
Our previous study confirmed that metformin attenuated hippocampus-dependent contextual fear memory impairment [10]. Hippocampus is also essential for novel object recognition in rodents [34]. We further investigated the effects of metformin on novel object recognition in mice treated with long-term isoflurane anesthesia. Lengthy isoflurane anesthesia significantly decreased the ability of the mice to distinguish the novel object from the familiar one. As shown in Fig. 1 A, the discrimination index of mice in the Anes group was lower than those of mice in the Ctrl group on Day 1 (0.09 ± 0.11 vs. 0.42 ± 0.10, P < 0.001) and Day 3 (0.16 ± 0.09 vs. 0.41 ± 0.11, P = 0.001) after anesthesia. Pretreatment with metformin (50 mg/kg) reversed the discrimination index decline induced by long-term isoflurane anesthesia on Day 1 (0.09 ± 0.11 vs. 0.42 ± 0.12, P < 0.001) and Day 3 (0.16 ± 0.09 vs. 0.44 ± 0.12, P < 0.001). However, the discrimination index was similar between the Met and Ctrl groups on Day 1 (0.42 ± 0.10 vs. 0.39 ± 0.06, P > 0.05) and Day 3 (0.41 ± 0.11 vs. 0.34 ± 0.10, P > 0.05) after anesthesia. In addition, both isoflurane and metformin had no impacts on the total amount of objection exploration time (Fig. 1B, all the P > 0.05). These results hinted that pretreatment with metformin could alleviate cognitive decline induced by lengthy isoflurane anesthesia in adult mice.
Fig. 1.
Metformin alleviated lengthy isoflurane exposure induced cognitive decline. The discrimination index (A) and total object exploration time (B) by adult mice treated with 6 h 100% oxygen inhalation (Ctrl), 6 h 1.3% isoflurane inhalation (Anes), metformin (Met, 50 mg/kg) before oxygen inhalation, or metformin before isoflurane inhalation (Met + Anes) (n = 7 to 8). Data are expressed as mean ± SD and analyzed with one-way ANOVA test followed by Bonferroni multiple comparison test.
3.2. Metformin prevented long-term isoflurane exposure induced microglia activation in the hippocampus
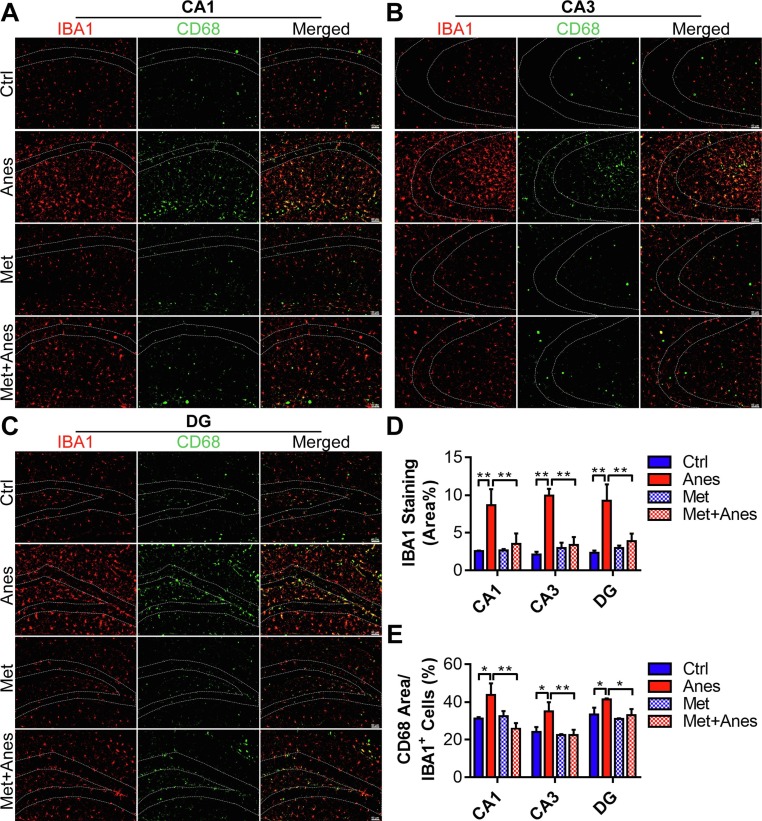
Immunofluorescence staining was used to examine the changes of hippocampal microglia among groups. Consistent with behavior changes, the microglial cells displayed ameboid shapes with larger soma in the CA1, CA3, and dentate gyrus (DG) of the hippocampus (Fig. 2 A-C). The expression level of ionised calcium-binding adaptor molecule-1 (IBA1) was also remarkably upregulated after isoflurane exposure (CA1: 2.55 ± 0.09% vs. 8.65 ± 2.14%, P = 0.002; CA3: 2.09 ± 0.40% vs. 9.93 ± 0.90%, P < 0.001; DG: 2.34 ± 0.30% vs. 9.24 ± 2.19%, P = 0.001) (Fig. 2A-D). Moreover, double immunofluorescence staining showed that the ratio of CD68 positive area in microglia also increased significantly after isoflurane anesthesia (CA1: 31.21 ± 0.79% vs. 43.76 ± 6.12%, P = 0.019; CA3: 24.13 ± 2.62% vs. 35.09 ± 4.81%, P < 0.014; DG: 33.38 ± 3.61% vs. 41.46 ± 0.41%, P = 0.022) (Fig. 2A-C, E). Metformin treatment prevented the morphological changes of hippocampal microglia induced by isoflurane (Fig. 2A-C). The expression level of IBA1 (CA1: 8.65 ± 2.14% vs. 3.53 ± 1.39%, P = 0.007; CA3: 9.93 ± 0.90% vs. 3.38 ± 1.06%, P < 0.001; DG: 9.24 ± 2.19% vs. 3.87 ± 1.03%, P = 0.004) (Fig. 2A-D) and the proportion of CD68 positive area in microglia (CA1: 43.76 ± 6.12% vs. 25.82 ± 2.98%, P = 0.002; CA3: 35.09 ± 4.81% vs. 22.51 ± 2.76%, P < 0.006; DG: 41.46 ± 0.41% vs. 33.03 ± 3.25%, P = 0.017) (Fig. 2A-C, E) also decreased when pretreatment with metformin. However, metformin alone had no effects on the morphological changes of hippocampal microglia and the expression levels of IBA1 and CD68 (Fig. 2). These data indicate that hippocampal microglia cells were activated by long-term isoflurane inhalation in mice, and prevented by metformin administration.
Fig. 2.
Metformin prevented long-term isoflurane exposure induced microglia activation in the hippocampus. (A, B and C) Representative immunofluorescent images of the IBA1 positive microglia (red) expressing CD68 (green) in the hippocampal CA1 (A), CA3 (B), and DG (C) regions of adult mice receiving vehicle (Ctrl), isoflurane (Anes), metformin (Met) or metformin and isoflurane (Met + Anes). Scale bar = 50 μm. (D, E) The quantification of the percentage of IBA1 positive area in the total area of the image (D) and CD68 positive area in IBA1 + microglia (E) in each hippocampal region, (n = 3 mice per group). Data are expressed as mean ± SD and analyzed with one-way ANOVA test followed by Bonferroni multiple comparison test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Metformin promoted microglia cells polarization from M1 phenotype to the M2 phenotype in the hippocampus after long-term isoflurane anesthesia
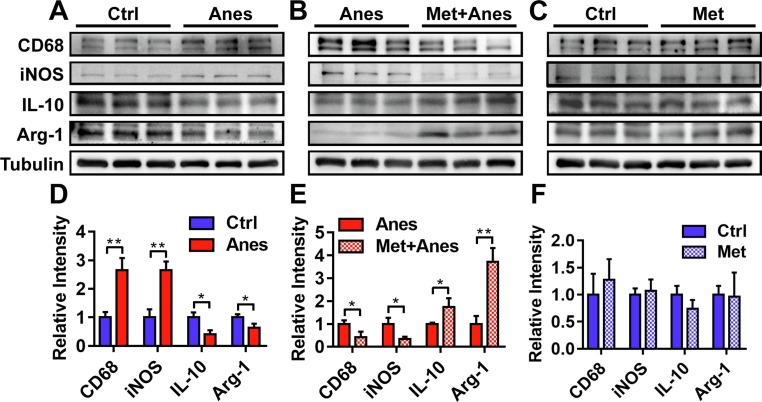
In vivo and in vitro studies had found that sevoflurane anesthesia induced microglial M1 activation and prevented microglial M2 polarization which contributed to cognitive decline in mice [8], [19], [20]. Consist with sevoflurane, western blotting analysis showed that the M1 markers (iNOS and CD68) of microglial cells were remarkably increased on Day 3 after long-term isoflurane anesthesia in the hippocampus of adult mice (iNOS: 1.00 ± 0.28 vs. 2.65 ± 0.30, P = 0.002; CD68: 1.00 ± 0.19 vs. 2.66 ± 0.42, P = 0.003) (Fig. 3 A, D). These increases were prevented by pretreatment with metformin (iNOS: 1.00 ± 0.27 vs. 0.34 ± 0.10, P = 0.016; CD68: 1.00 ± 0.16 vs. 0.42 ± 0.24, P = 0.025) (Fig. 3B, E). In contrast, the expression of hippocampal microglial M2 markers (Arg-1 and IL-10) was downregulated on Day 3 after long-term isoflurane anesthesia (Arg-1: 1.00 ± 0.11 vs. 0.64 ± 0.14, P = 0.025; IL-10: 1.00 ± 0.17 vs. 0.41 ± 0.15, P = 0.01) (Fig. 3A, D), and these downregulations were attenuated by the administration of metformin (Arg-1: 1.00 ± 0.35 vs. 3.72 ± 0.60, P = 0.002; IL-10: 1.00 ± 0.05 vs. 1.74 ± 0.39, P = 0.03) (Fig. 3B, E). While metformin alone had no effects on the expression levels of microglial M1 and M2 markers (Fig. 3C, F, all the P > 0.05). These results indicated that pretreatment with metformin promoted microglia cells polarization from M1 phenotype to the M2 phenotype in the hippocampus after long-term isoflurane anesthesia.
Fig. 3.
Metformin promoted microglia cells polarization from M1 phenotype to the M2 phenotype in the hippocampus of adult mice after lengthy isoflurane exposure. Representative western blots (above panels) and densitometric analysis (below panels, n = 3 mice per group) showing the effects of lengthy isoflurane inhalation (A, D), metformin pretreatment plus isoflurane inhalation (B, E), and metformin treatment (C, F) on expression levels of M1 microglia markers (CD68, iNOS) and M2 microglia markers (IL-10, Arg-1) in the hippocampus of adult mice. Tubulin was used as an internal control. Data are expressed as mean ± SD and compared by two-tailed Student’s t test.
3.4. Metformin inhibited A1-like astrocyte activation in the hippocampus of adult mice after long-term isoflurane inhalation.
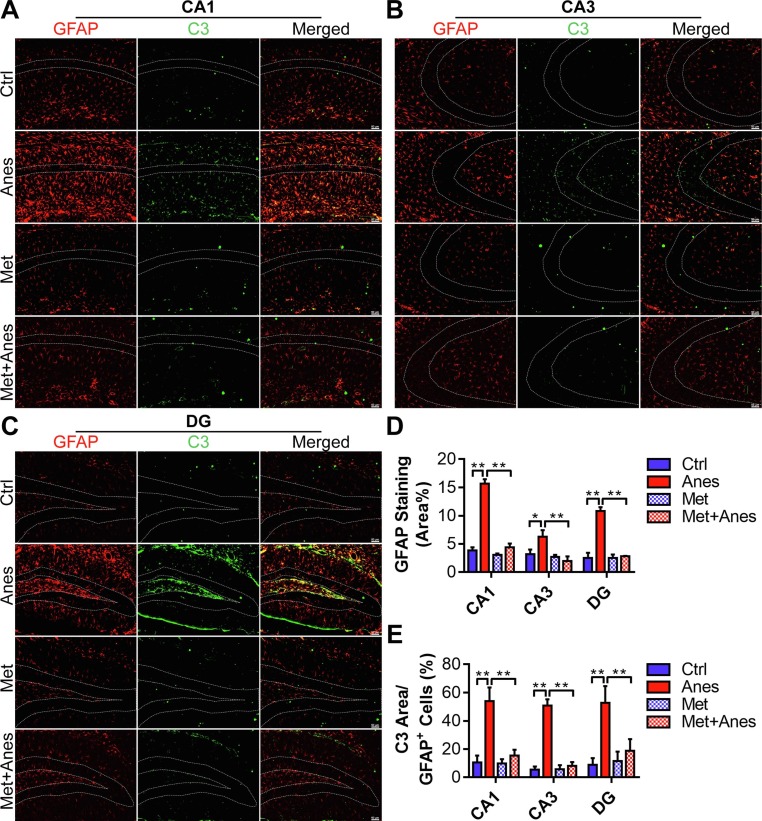
We examined the morphology changes of astrocytes by glial fibrillary acidic protein (GFAP) staining in the hippocampus. Reactive hypertrophic astrocytes with a pronounced overlap of thickened processes were increased throughout the hippocampus on day 3 after anesthesia (Fig. 4 A-C). Quantified results revealed that the GFAP positive area in the hippocampus of isoflurane-anesthetized mice was also upregulated, in comparison to mice in the Ctrl group (CA1: 3.84 ± 0.57% vs. 15.68 ± 0.76%, P < 0.001; CA3: 3.20 ± 0.81% vs. 6.27 ± 1.15%, P = 0.012; DG: 2.54 ± 0.91% vs. 10.82 ± 0.68%, P < 0.001) (Fig. 4D). Similar to microglia, the treatment with metformin decreased the size of astrocytes and partially restored their morphological changes (Fig. 4A-C). GFAP positive area in the Met + Anes group occupied a reduced area of the hippocampus compared with mice in the Anes group (CA1: 15.68 ± 0.76% vs. 4.41 ± 0.67%, P < 0.001; CA3: 6.27 ± 1.15% vs. 1.99 ± 0.82%, P = 0.001; DG: 10.82 ± 0.68% vs. 2.82 ± 0.07%, P < 0.001) (Fig. 4D). Moreover, metformin pretreatment also partially reversed the upregulation of the C3 (A1 reactive astrocytes marker) positive area in the astrocytes induced by isoflurane (CA1: 53.96 ± 9.61% vs. 15.30 ± 4.29%, P < 0.001; CA3: 50.75 ± 4.46% vs. 8.17 ± 2.67%, P < 0.001; DG: 52.67 ± 11.88% vs. 18.85 ± 8.15%, P = 0.006) (Fig. 4E). However, metformin alone had no effects on the morphologic changes and the expression levels of GFAP and C3 (Fig. 4, all the P > 0.05).
Fig. 4.
Metformin inhibited A1-like astrocyte activation in the hippocampus of adult mice after long-term isoflurane inhalation. (A, B and C) Representative immunofluorescent images of the GFAP positive astrocyte (red) expressing C3 (green) in the hippocampal CA1 (A), CA3 (B), and DG (C) regions of adult mice receiving vehicle (Ctrl), isoflurane (Anes), metformin (Met) or metformin and isoflurane (Met + Anes). Scale bar = 50 μm. (D, E) The quantification of the percentage of GFAP positive area in the total area of the image (D) and C3 positive area in GFAP + astrocyte (E) in each hippocampal region, (n = 3 mice per group). Data are expressed as mean ± SD and analyzed with one-way ANOVA test followed by Bonferroni multiple comparison test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
The brain of neonatal and aging animals is more vulnerable and easier to induce long-lasting neuroinflammation and cognitive decline [4]. Hence, though clinical evidence suggested that cognitive dysfunction is prevalent in all ages after major surgery [35], most studies about general anesthetic-induced cognitive decline have been conducted in aged or neonatal rodents [6], [7], [8], [18], [19]. Only a fraction of studies has been carried out in adult rodents to investigate the role of short-term general anesthetic exposure on neuroinflammation and cognitive performance [9], [36], [37], [38]. Besides the studies from our research group, sparse studies have been conducted to explore the effects of lengthy inhaled anesthetics exposure on cognitive performance [39]. However, with the pandemic of COVID-19 and the shortage of intravenous sedative drugs, inhaled anesthetics have been widely used for prolonged sedation of ventilated critically ill adult patients [5]. There is an urgent need to explore the effects and treatments of long-term inhaled anesthetics sedation on cognitive function in adults. The study demonstrated that 6 h sub-MAC isoflurane inhalation caused a significant hippocampal-dependent cognitive decline in adult mice. Consistent with cognitive impairment, typically activated microglia and astrocytes as well as upregulated microglial M1 markers (CD68 and iNOS) and A1-like astrocyte marker C3 were presented in the hippocampus of adult mice on Day3 after anesthesia. Whereas the markers of M2 microglia were decreased after isoflurane exposure. Metformin pretreatment leads to better cognitive performance in isoflurane-anesthetized adult mice. The positive cellular modifications facilitated by metformin pretreatment included shifting microglial polarization toward the M2 phenotype and reducing M1 phenotype microglia as well as A1-like astrocyte activation in the hippocampus. The promotion of activated microglia polarization toward the M2 phenotype and inhibition of overactivated M1 microglia are effective treatment strategies for neuroinflammation-related cognitive decline. Such as curcumin [40] and resveratrol [41] which had potencies to promote microglial polarization from the M1 to the M2 phenotype were proved to have therapeutic effects on cognitive impairment induced by neuroinflammation [42], [43]. In addition, pretreatment of curcumin and resveratrol also prevented inhalational anesthetic-induced cognitive decline by attenuating neuroinflammation in mice [8], [44].
Recently, Lai et al. found that 6 h isoflurane anesthesia had no impact on neuroinflammatory response and cognitive performance in young adult mice [39]. However, they used a high concentration of isoflurane (2%) which is unsuitable for prolonged sedation. In addition, a previous study suggested lower concentration isoflurane inhalation is more likely to induce a cognitive decline in adult mice [45]. Hence, we chose a sub-MAC concentration (1.3%) in our present study (the MAC of adult mice is 1.46% [46]). Furthermore, Wang and his colleagues found that 2 h exposure of 1.5% isoflurane caused hippocampal inflammation and cognitive decline in aged mice but not in young adult mice [9]. These results hinted that sub-MAC isoflurane inhalation induced neuroinflammation and cognitive decline also in a dose- and exposure length-dependent manner in adult mice. In clinical, the duration of isoflurane sedation for COVID-19 pneumonia and acute respiratory distress syndrome patients was more than 100 h [47], [48], [49]. The length of use in mice might not exactly mimic the time used in humans in consideration of their length of life respectively. In this study, 6 h of isoflurane exposure was used based on previous research in a cognitive impairment mouse model induced by lengthy isoflurane inhalation [7], [50], [51]. Our previous study also has proved that 6 h isoflurane exposure could induce a significant cognitive decline in adult mice [10], [11], [52]. In addition, different to 2 h isoflurane exposure which can’t elicit the activation of BV-2 cells or primary microglial cells [9], 6 h isoflurane or sevoflurane treatment can directly increase the expression level and transcription activity of nuclear factor kappa-B as well as the production of pro-inflammatory cytokines in H4 human neuroglioma cells, mouse primary microglia, and BV2 cells [7], [51], [53]. All in all, prolonged sub-MAC isoflurane inhalation can induce remarkable neuroinflammation and cognitive decline by promoting microglia and astrocyte activation in adults and should be fully considered for the use of inhaled anesthetics in prolonged sedation.
Our previous study found that synaptic plasticity impairment and tau hyperphosphorylation were the main mechanisms for length sub-MAC exposure induced cognitive decline in adult mice [10]. The imbalanced M1/M2 activation of microglia and several reactive A1-like astrocytes play an important role in inhibiting synaptic plasticity and triggering neurodegeneration [54], [55], [56], [57]. Therefore, we examined the activation and polarization of microglia and astrocyte in the hippocampus of adult mice after isoflurane inhalation. Consistent with sevoflurane exposure in neonatal and aging rodents [8], [19], prolonged sub-MAC isoflurane inhalation induced microglial M1 activation and inhibited microglial M2 polarization. Meanwhile, typically activated A1-like astrocytes surged in the hippocampus of adult mice after lengthy sub-MAC isoflurane exposure. As mentioned above, metformin exerts anti-inflammation effects by modulating the activation and polarization of microglia and astrocyte in neuroinflammatory diseases. Our results shown that metformin pretreatment shifted microglial polarization toward the M2 phenotype and reduced M1 phenotype microglia as well as A1-like astrocyte activation in the hippocampus of prolonged sub-MAC isoflurane-anesthetized adult mice. In line with the alleviation of neuroinflammation, we have in a recent study showed that metformin also mitigated the inhibition of synaptic plasticity and tau hyperphosphorylation induced by lengthy sub-MAC isoflurane exposure [10]. These results suggested that promoted microglia polarization into M2 phenotype and inhibited the activation of M1 microglia and A1-like neurotoxic astrocyte may be one of the mechanisms for metformin alleviated lengthy sub-MAC isoflurane-induced synaptic plasticity impairment and tau hyperphosphorylation.
The present study shows remarkable activated hippocampal M1 microglia and A1-like astrocytes contributing to lengthy sub-MAC isoflurane exposure-induced cognitive decline. The shortage is that we merely investigated the effects of prolonged sub-MAC isoflurane inhalation in healthy adult mice, but not seriously infected adults who required lengthy isoflurane sedation. However, in vitro studies had found that the exposure of primary microglia or BV2 cells to 0.4%-2% isoflurane for 6 h caused notable proinflammatory responses [7], [51], [53]. In addition, even 2 h isoflurane exposure can promote the release of proinflammatory cytokines in lipopolysaccharide-activated BV-2 cells or primary microglial cells [9]. These results suggested lengthy isoflurane exposure can directly activate microglia and aggravate the pro-inflammatory reactivity of activated microglia. Even so, the role of prolonged inhaled anesthetics sedation on neuroinflammation and cognitive performance in seriously infected adults deserved further study.
5. Conclusions
Prolonged sub-MAC isoflurane exposure triggered significant hippocampal neuroinflammation and cognitive decline in adult mice which can be alleviated by metformin pretreatment via inhibiting reactive M1 microglia and A1-like astrocytes and promoting microglia polarization toward M2 phenotype in the hippocampus.
6. Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation
7. Authors’ contributions
Gu X and Xia T conceived the original idea and provided the financial support for the study. Liu S, Xu J, Xie W and Fang X performed the animal experiments. Peng L generated and analyzed data as well as wrote the manuscript with input from all authors. All authors read and approved the final manuscript.
CRediT authorship contribution statement
Liangyu Peng: Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Shuai Liu: Project administration, Data curation, Formal analysis, Investigation, Methodology, Software. Jiyan Xu: Project administration, Data curation, Formal analysis, Investigation, Methodology, Software. Wenjia Xie: Project administration, Data curation, Formal analysis, Investigation, Methodology, Software. Xin Fang: Project administration, Data curation, Formal analysis, Investigation, Methodology, Software. Tianjiao Xia: Conceptualization, Funding acquisition, Resources. Xiaoping Gu: Conceptualization, Funding acquisition, Resources.
Funding
This study was supported by the National Natural Science Foundation of China [81730033, 81701371]; the Natural Science Foundation of Jiangsu Province of China [BK20170129]; and the Key Talents of 13th Five-Year Plan for Strengthening Health of Jiangsu Province [ZDRCA2016069].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2022.108903.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- 1.Cottrell J.E., Hartung J. Anesthesia and cognitive outcome in elderly patients: a narrative viewpoint. J. Neurosurg. Anesthesiol. 2020;32(1):9–17. doi: 10.1097/ANA.0000000000000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ing C., Hegarty M.K., Perkins J.W., Whitehouse A.J.O., DiMaggio C.J., Sun M., Andrews H., Li G., Sun L.S., von Ungern-Sternberg B.S. Duration of general anaesthetic exposure in early childhood and long-term language and cognitive ability. Br. J. Anaesth. 2017;119(3):532–540. doi: 10.1093/bja/aew413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maloney S.E., Yuede C.M., Creeley C.E., Williams S.L., Huffman J.N., Taylor G.T., Noguchi K.N., Wozniak D.F. Repeated neonatal isoflurane exposures in the mouse induce apoptotic degenerative changes in the brain and relatively mild long-term behavioral deficits. Sci. Rep. 2019;9(1):2779. doi: 10.1038/s41598-019-39174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vutskits L., Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat. Rev. Neurosci. 2016;17(11):705–717. doi: 10.1038/nrn.2016.128. [DOI] [PubMed] [Google Scholar]
- 5.Jerath A., Ferguson N.D., Cuthbertson B. Inhalational volatile-based sedation for COVID-19 pneumonia and ARDS. Intensive. Care. Med. 2020;46(8):1563–1566. doi: 10.1007/s00134-020-06154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li D., Chen M., Meng T., Fei J. Hippocampal microglial activation triggers a neurotoxic-specific astrocyte response and mediates etomidate-induced long-term synaptic inhibition. J. Neuroinflammation. 2020;17(1):109. doi: 10.1186/s12974-020-01799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang T., Xu S., Shen Y., Xu Y., Li Y. Genistein Attenuates Isoflurane-Induced Neuroinflammation by Inhibiting TLR4-Mediated Microglial-Polarization in vivo and in vitro. J. Inflamm. Res. 2021;14:2587–2600. doi: 10.2147/JIR.S304336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang X., Wang X., Fang G., Zhao Y., Yan J., Zhou Z., Sun R., Luo A., Li S. Resveratrol ameliorates sevoflurane-induced cognitive impairment by activating the SIRT1/NF-κB pathway in neonatal mice. J. Nutr. Biochem. 2021;90 doi: 10.1016/j.jnutbio.2020.108579. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z., Meng S., Cao L., Chen Y., Zuo Z., Peng S. Critical role of NLRP3-caspase-1 pathway in age-dependent isoflurane-induced microglial inflammatory response and cognitive impairment. J. Neuroinflammation. 2018;15(1):109. doi: 10.1186/s12974-018-1137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng L., Fang X., Xu F., Liu S., Qian Y., Gong X., Zhao X., Ma Z., Xia T., Gu X. Amelioration of Hippocampal Insulin Resistance Reduces Tau Hyperphosphorylation and Cognitive Decline Induced by Isoflurane in Mice. Front. Aging. Neurosci. 2021;13:301. doi: 10.3389/fnagi.2021.686506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Y., Li X., Gong X., Zhao X., Ma Z., Xia T., Gu X. Green tea polyphenols improve isoflurane-induced cognitive impairment via modulating oxidative stress. J. Nutr. Biochem. 2019;73 doi: 10.1016/j.jnutbio.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Salter M.W., Stevens B. Microglia emerge as central players in brain disease. Nat. Med. 2017;23(9):1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 13.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Münch A.E., Chung W.-S., Peterson T.C., Wilton D.K., Frouin A., Napier B.A., Panicker N., Kumar M., Buckwalter M.S., Rowitch D.H., Dawson V.L., Dawson T.M., Stevens B., Barres B.A. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou B., Zhang Y., Liang P., He Y., Peng B., Liu W., Han S., Yin J., He X. Inhibition of the NLRP3-inflammasome prevents cognitive deficits in experimental autoimmune encephalomyelitis mice via the alteration of astrocyte phenotype. Cell. Death. Dis. 2020;11(5):377. doi: 10.1038/s41419-020-2565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.L.E. Clarke, S.A. Liddelow, C. Chakraborty, A.E. Münch, M. Heiman, B.A. Barres, Normal aging induces A1-like astrocyte reactivity, Proceedings of the National Academy of Sciences of the United States of America 115(8) (2018) E1896-E1905. [DOI] [PMC free article] [PubMed]
- 16.Lian H., Litvinchuk A., Chiang A., Aithmitti N., Jankowsky J., Zheng H. Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer's Disease. J. Neurosci. Off. J. Soc. Neurosci. 2016;36(2):577–589. doi: 10.1523/JNEUROSCI.2117-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei Y., Chen T., Bosco D.B., Xie M., Zheng J., Dheer A., Ying Y., Wu Q., Lennon V.A., Wu L.-J. The complement C3–C3aR pathway mediates microglia-astrocyte interaction following status epilepticus. Glia. 2021;69(5):1155–1169. doi: 10.1002/glia.23955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H.-L., Liu H., Xue Z.-G., Liao Q.-W., Fang H. Minocycline attenuates post-operative cognitive impairment in aged mice by inhibiting microglia activation. J. Cell. Mol. Med. 2016;20(9):1632–1639. doi: 10.1111/jcmm.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H., Chu H., Jiang Q., Wang C., Tian Y. Irf6 participates in sevoflurane-induced perioperative neurocognitive disorder via modulating M2, but not M1 polarization of microglia. Brain. Res. Bull. 2021;177:1–11. doi: 10.1016/j.brainresbull.2021.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Pei Z., Wang S., Li Q. Sevoflurane suppresses microglial M2 polarization. Neurosci. Lett. 2017;655:160–165. doi: 10.1016/j.neulet.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Scheen A.J., Esser N., Paquot N. Antidiabetic agents: Potential anti-inflammatory activity beyond glucose control. Diabetes Metabol. 2015;41(3):183–194. doi: 10.1016/j.diabet.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Pan Y., Sun X., Jiang L., Hu L., Kong H., Han Y., Qian C., Song C., Qian Y., Liu W. Metformin reduces morphine tolerance by inhibiting microglial-mediated neuroinflammation. J. Neuroinflammation. 2016;13(1):294. doi: 10.1186/s12974-016-0754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inyang K.E., Szabo-Pardi T., Wentworth E., McDougal T.A., Dussor G., Burton M.D., Price T.J. The antidiabetic drug metformin prevents and reverses neuropathic pain and spinal cord microglial activation in male but not female mice. Pharmacol. Res. 2019;139:1–16. doi: 10.1016/j.phrs.2018.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin Q., Cheng J., Liu Y., Wu J., Wang X., Wei S., Zhou X., Qin Z., Jia J., Zhen X. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain. Behav. Immun. 2014;40:131–142. doi: 10.1016/j.bbi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Kodali M., Attaluri S., Madhu L.N., Shuai B., Upadhya R., Gonzalez J.J., Rao X., Shetty A.K. Metformin treatment in late middle age improves cognitive function with alleviation of microglial activation and enhancement of autophagy in the hippocampus. Aging. Cell. 2021;20(2):e13277. doi: 10.1111/acel.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Łabuzek K., Liber S., Gabryel B., Okopień B. Metformin has adenosine-monophosphate activated protein kinase (AMPK)-independent effects on LPS-stimulated rat primary microglial cultures. Pharmacological. Rep. PR. 2010;62(5):827–848. doi: 10.1016/s1734-1140(10)70343-1. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira W., Nunes A., França M., Santos L., Lós D., Rocha S., Barbosa K., Rodrigues G., Peixoto C. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain. Res. 2016;1644:149–160. doi: 10.1016/j.brainres.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Wang G., Cui W., Chen S., Shao Z., Li Y., Wang W., Mao L., Li J., Mei X. Metformin alleviates high glucose-induced ER stress and inflammation by inhibiting the interaction between caveolin1 and AMPKα in rat astrocytes. Biochem. Biophys. Res. Commun. 2021;534:908–913. doi: 10.1016/j.bbrc.2020.10.075. [DOI] [PubMed] [Google Scholar]
- 29.Ryu Y., Go J., Park H., Choi Y., Seo Y., Choi J., Rhee M., Lee T., Lee C., Kim K. Metformin regulates astrocyte reactivity in Parkinson's disease and normal aging. Neuropharmacology. 2020;175 doi: 10.1016/j.neuropharm.2020.108173. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y., Xu Y., Wang Y., Wang Y., He L., Jiang Z., Huang Z., Liao H., Li J., Saavedra J.M., Zhang L., Pang T. Telmisartan prevention of LPS-induced microglia activation involves M2 microglia polarization via CaMKKβ-dependent AMPK activation. Brain. Behav. Immun. 2015;50:298–313. doi: 10.1016/j.bbi.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Zhou X., Cao Y., Ao G., Hu L., Liu H., Wu J., Wang X., Jin M., Zheng S., Zhen X., Alkayed N.J., Jia J., Cheng J. CaMKKβ-dependent activation of AMP-activated protein kinase is critical to suppressive effects of hydrogen sulfide on neuroinflammation. Antioxid. Redox. Signal. 2014;21(12):1741–1758. doi: 10.1089/ars.2013.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Astakhova A., Chistyakov D., Thomas D., Geisslinger G., Brüne B., Sergeeva M., Namgaladze D. Inhibitors of Oxidative Phosphorylation Modulate Astrocyte Inflammatory Responses through AMPK-Dependent Ptgs2 mRNA Stabilization. Cells. 2019;8(10):1185. doi: 10.3390/cells8101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jinpiao Z., Zongze Z., Qiuyue Y., Peng F., Qi Z., Yanlin W., Chang C. Metformin attenuates sevoflurane-induced neurocognitive impairment through AMPK-ULK1-dependent autophagy in aged mice. Brain. Res. Bull. 2020;157:18–25. doi: 10.1016/j.brainresbull.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Cohen S., Munchow A., Rios L., Zhang G., Asgeirsdóttir H., Stackman R. The rodent hippocampus is essential for nonspatial object memory. Curr. Biol. :. CB. 2013;23(17):1685–1690. doi: 10.1016/j.cub.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monk T., Weldon B., Garvan C., Dede D., van der Aa M., Heilman K., Gravenstein J. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 36.Wu X., Lu Y., Dong Y., Zhang G., Zhang Y., Xu Z., Culley D.J., Crosby G., Marcantonio E.R., Tanzi R.E., Xie Z. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-α, IL-6, and IL-1β. Neurobiol. Aging. 2012;33(7):1364–1378. doi: 10.1016/j.neurobiolaging.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.L. Cao, L. Li, D. Lin, Z. Zuo, Isoflurane induces learning impairment that is mediated by interleukin 1β in rodents, PloS one 7(12) (2012) e51431. [DOI] [PMC free article] [PubMed]
- 38.D. Lin, Z. Zuo, Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats, Neuropharmacology 61(8) (2011) 1354–1359. [DOI] [PMC free article] [PubMed]
- 39.Lai Z., Min J., Li J., Shan W., Yu W., Zuo Z. Surgery trauma severity but not Anesthesia length contributes to postoperative cognitive dysfunction in mice. J. Alzheimer's Dis. JAD. 2021;80(1):245–257. doi: 10.3233/JAD-201232. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J., Zheng Y., Luo Y., Du Y., Zhang X., Fu J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/ TLR4/ NF-κB pathways in BV2 cells. Mol. Immunol. 2019;116:29–37. doi: 10.1016/j.molimm.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 41.Yang X., Xu S., Qian Y., Xiao Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain. Behav. Immun. 2017;64:162–172. doi: 10.1016/j.bbi.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Sorrenti V., Contarini G., Sut S., Dall'Acqua S., Confortin F., Pagetta A., Giusti P., Zusso M. Curcumin Prevents Acute Neuroinflammation and Long-Term Memory Impairment Induced by Systemic Lipopolysaccharide in Mice. Front. Pharmacol. 2018;9:183. doi: 10.3389/fphar.2018.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abraham J., Johnson R.W. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation. Res. 2009;12(6):445–453. doi: 10.1089/rej.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji M.-H., Qiu L.-L., Yang J.-J., Zhang H., Sun X.-R., Zhu S.-H., Li W.-Y., Yang J.-J. Pre-administration of curcumin prevents neonatal sevoflurane exposure-induced neurobehavioral abnormalities in mice. Neurotoxicology. 2015;46:155–164. doi: 10.1016/j.neuro.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Valentim A.M., Di Giminiani P., Ribeiro P.O., Rodrigues P., Olsson I.A.S., Antunes L.M. Lower isoflurane concentration affects spatial learning and neurodegeneration in adult mice compared with higher concentrations. Anesthesiology. 2010;113(5):1099–1108. doi: 10.1097/ALN.0b013e3181f79c7c. [DOI] [PubMed] [Google Scholar]
- 46.Sonner J.M., Gong D., Eger E.I. Naturally occurring variability in anesthetic potency among inbred mouse strains. Anesth. Analg. 2000;91(3):720–726. doi: 10.1097/00000539-200009000-00042. [DOI] [PubMed] [Google Scholar]
- 47.Hanidziar D., Baldyga K., Ji C.S., Lu J., Zheng H., Wiener-Kronish J., Xie Z. Standard Sedation and Sedation With Isoflurane in Mechanically Ventilated Patients With Coronavirus Disease 2019. Crit. Care. Explor. 2021;3(3):e0370. doi: 10.1097/CCE.0000000000000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flinspach A.N., Zacharowski K., Ioanna D., Adam E.H. Volatile Isoflurane in Critically Ill Coronavirus Disease 2019 Patients-A Case Series and Systematic Review. Crit. Care. Explor. 2020;2(10):e0256. doi: 10.1097/CCE.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kermad A., Speltz J., Danziger G., Mertke T., Bals R., Volk T., Lepper P.M., Meiser A. Comparison of isoflurane and propofol sedation in critically ill COVID-19 patients-a retrospective chart review. J. Anesthesia. 2021;35(5):625–632. doi: 10.1007/s00540-021-02960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y., Xu Z., Wang H., Dong Y., Shi H.N., Culley D.J., Crosby G., Marcantonio E.R., Tanzi R.E., Xie Z. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann. Neurol. 2012;71(5):687–698. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu P., Gao Q., Guan L., Sheng W., Hu Y., Gao T., Jiang J., Xu Y., Qiao H., Xue X., Liu S., Li T. Atorvastatin attenuates isoflurane-induced activation of ROS-p38MAPK/ATF2 pathway, neuronal degeneration, and cognitive impairment of the aged mice. Front. Aging Neurosci. 2020;12 doi: 10.3389/fnagi.2020.620946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia T., Cui Y., Chu S., Song J., Qian Y., Ma Z., Gu X. Melatonin pretreatment prevents isoflurane-induced cognitive dysfunction by modulating sleep-wake rhythm in mice. Brain Res. 2016;1634:12–20. doi: 10.1016/j.brainres.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L., Zhang J., Yang L., Dong Y., Zhang Y., Xie Z. Isoflurane and sevoflurane increase interleukin-6 levels through the nuclear factor-kappa B pathway in neuroglioma cells. Br. J. Anaesth. 2013;110(Suppl 1):i82–i91. doi: 10.1093/bja/aet115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhaskar K., Konerth M., Kokiko-Cochran O.N., Cardona A., Ransohoff R.M., Lamb B.T. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68(1):19–31. doi: 10.1016/j.neuron.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang T., Zhang Y.-D., Chen Q., Gao Q., Zhu X.-C., Zhou J.-S., Shi J.-Q., Lu H., Tan L., Yu J.-T. TREM2 modifies microglial phenotype and provides neuroprotection in P301S tau transgenic mice. Neuropharmacology. 2016;105:196–206. doi: 10.1016/j.neuropharm.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 56.Chun H., Im H., Kang Y.J., Kim Y., Shin J.H., Won W., Lim J., Ju Y., Park Y.M., Kim S., Lee S.E., Lee J., Woo J., Hwang Y., Cho H., Jo S., Park J.-H., Kim D., Kim D.Y., Seo J.-S., Gwag B.J., Kim Y.S., Park K.D., Kaang B.-K., Cho H., Ryu H., Lee C.J. Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer's disease via HO production. Nat. Neurosci. 2020;23(12):1555–1566. doi: 10.1038/s41593-020-00735-y. [DOI] [PubMed] [Google Scholar]
- 57.York E.M., Zhang J., Choi H.B., MacVicar B.A. Neuroinflammatory inhibition of synaptic long-term potentiation requires immunometabolic reprogramming of microglia. Glia. 2021;69(3):567–578. doi: 10.1002/glia.23913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation