Keywords: collagen, macrophage, muscle hypertrophy, resistance training, scRNA-seq
Abstract
In older individuals, hypertrophy from progressive resistance training (PRT) is compromised in approximately one-third of participants in exercise trials. The objective of this study was to establish novel relationships between baseline muscle features and/or their PRT-induced change in vastus lateralis muscle biopsies with hypertrophy outcomes. Multiple linear regression analyses adjusted for sex were performed on phenotypic data from older adults (n = 48 participants, 70.8 ± 4.5 yr) completing 14 wk of PRT. Results show that baseline muscle size associates with growth regardless of hypertrophy outcome measure [fiber cross-sectional area (fCSA), β = −0.76, Adj. P < 0.01; thigh muscle area by computed tomography (CT), β = −0.75, Adj. P < 0.01; dual-energy X-ray absorptiometry (DXA) thigh lean mass, β = −0.47, Adj. P < 0.05]. Furthermore, loosely packed collagen organization (CO, β = −0.44, Adj. P < 0.05) and abundance of CD11b+/CD206− immune cells (β = −0.36, Adj. P = 0.10) were negatively associated with whole muscle hypertrophy, with a significant sex interaction on the latter. In addition, a composite hypertrophy score generated using all three measures reinforces significant fiber level findings that changes in myonuclei (MN) (β = 0.67, Adj. P < 0.01), changes in immune cells (β = 0.48, Adj. P < 0.05; both CD11b+/CD206+and CD11b+/CD206− cells), and capillary density (β = 0.56, Adj. P < 0.01) are significantly associated with growth. Exploratory single-cell RNA-sequencing of CD11b+ cells in muscle in response to resistance exercise showed that macrophages have a mixed phenotype. Collagen associations with macrophages may be an important aspect in muscle response heterogeneity. Detailed histological phenotyping of muscle combined with multiple measures of growth response to resistance training in older persons identify potential new mechanisms underlying response heterogeneity and possible sex differences.
NEW & NOTEWORTHY Extensive analyses of muscle features associated with muscle size and resistance training response in older persons, including sex differences, and evaluation of multiple measures of hypertrophy are discussed. Collagen organization and CD11b-expressing immune cells offer potential new targets to augment growth response in older individuals. A hypertrophy composite score reveals that changes in immune cells, myonuclei, and capillary density are critically important for overall muscle growth while sc-RNAseq reveals evidence for macrophage heterogeneity.
INTRODUCTION
Skeletal muscle mass or size and strength are significant determinants of all-cause mortality and overall metabolic health among older adults (1, 2). Skeletal muscle is a highly adaptable tissue in response to environmental stimuli through a series of tightly regulated yet complex processes involving multiple cell types. However, aged muscle plasticity diminishes and becomes highly unpredictable, as evidenced by large variations in hypertrophic and functional responses to exercise stimuli (3–5). In some individuals, resistance exercise may fail to forestall or even exacerbate the natural age-related losses in skeletal muscle mass and strength, termed sarcopenia, commonly experienced by older individuals, leading to their being categorized as “poor” or “non” responders, leading to reduced health span (6, 7). Nevertheless, progressive resistance training (PRT) is the most widely prescribed and successful way to increase muscle mass and strength during aging (5, 8). As such, identifying factors associated with muscle responses to PRT in older individuals is crucial for increasing the effectiveness of PRT, and for developing new strategies to preserve and increase muscle mass, particularly in those exhibiting poor responses.
Molecular and cellular changes in muscle observed with aging have been proposed to contribute to exercise response heterogeneity. Anabolic resistance, or reduced sensitivity to the anabolic effects of nutrition and exercise, is one proposed mechanism (9, 10). Molecular characteristics of muscle related to inflammation, cell signaling, RNA processing, and extracellular matrix (ECM) organization before and their changes in response to acute exercise or PRT have been reported by our groups and others (6, 11–14). Our research groups have also demonstrated the importance of cellular muscle features such as inflammatory cells, fibrogenic cells, endothelial cells, and stem cells or satellite cells (SCs) in the context of exercise. The Bamman group has performed several analyses of differential outcomes to exercise between young and old volunteers using k-means clustering statistical techniques to separate by group the hypertrophic response ranging from poor to extremely positive. Supplemented by molecular analyses, findings support that the abundance of pretraining satellite cells was higher in the extreme responders compared with the other groups and that extreme responders also had the greatest increase in satellite cells following PRT (15). This would suggest that higher satellite cell content before starting resistance exercise training may contribute to greater myonuclear accretion and explain differences in myofiber hypertrophy between old and young men and women (13). The Peterson group has reported changes in inflammatory cells in human skeletal muscle in response to exercise. Findings suggest that aging alters macrophage function both at rest and in response to resistance exercise (14). Furthermore, an increase in cells expressing the pan-macrophage marker CD11b+ and the cell surface marker characteristic of an M2-like phenotype (CD206+) was associated with endurance exercise adaptations and fiber growth (16). Additional exercise-responsive components that are dysregulated in aging muscle continue to be discovered (17, 18), including circadian rhythm disruption, impairments in epigenetic factors such as microRNAs and DNA methylation, suppressed protein translation, and ribosome biogenesis (3, 19–24).
Our groups recently completed the two-site MASTERS (Metformin to Augment Strength Training Effective Response in Seniors) exercise trial in older adults, one of the largest trials to date, showing that ∼30% of participants on placebo did not show a hypertrophic response to PRT (25). Our initial report showed that when compared with placebo, the diabetic drug metformin, inhibited gains in whole muscle mass, size, and density but did not affect vastus lateralis fiber hypertrophy, as metformin did not affect satellite cells or macrophage abundance following training (25). It did, however, blunt fiber-type switching, which may be related to metformin’s effect on genes related to lipid metabolism and mitochondrial function (26); ultimately leading to altered fiber type and muscle lipid composition influencing strength gain (27). Using Weighted Gene Correlation Network Analysis (WGCNA) of RNA sequencing data, networks of genes (modules) whose baseline expression was predictive of the hypertrophic response to PRT in our older placebo adults in the MASTERS trial were identified (28). Although this previous investigation focused predominantly on practical indices of whole muscle hypertrophy, numerous underlying muscle-specific adaptations may be responsible for driving response to PRT. For instance, macrophage abundance increases following PRT, correlated to changes in muscle fiber size, capillary density, satellite cell abundance, and myonuclei (MN) within the MASTERS cohort (29).
Whether baseline muscle features are associated with hypertrophy outcomes in older adults is currently unknown, but this could provide key insight for personalized exercise medicine. Presently, we leveraged extensive phenotyping of muscle histological features in this population to gain a more mechanistic understanding of response heterogeneity to PRT in older adults. Collectively, these data, along with our previous investigations into the skeletal muscle transcriptome, emphasize the multifaceted nature of the hypertrophic response. Mechanistic understanding of muscle hypertrophy is compounded further by various measures used to quantify the hypertrophic response to exercise, for example, fiber cross-sectional area (fCSA) from biopsy samples versus muscle area by computed tomography (CT) versus thigh muscle mass by dual-energy X-ray absorptiometry (DXA) are often used, making comparison across studies, and even across measures, problematic. The MASTERS trial in which multiple measures of muscle size and mass, as well as an extensive characterization of muscle molecular and cellular features, enables this follow-up investigation on potential baseline muscle features associated with response heterogeneity to PRT in MASTERS participants (see graphical abstract). We also aimed to identify muscle feature dynamics associated with the hypertrophic response to provide a focused physiological framework for optimized skeletal muscle hypertrophy.
MATERIALS AND METHODS
Ethics Approval
This study was approved by the University of Kentucky institutional review board (IRB 14-0330) and the University of Alabama at Birmingham institutional review board (IRB F140722001) before any participants enrolled. Data and safety monitoring were provided by the UK CCTS DSMB on a quarterly basis.
Participants
Data were obtained from participants as part of the parent MASTERS clinical trial (NCT02308228) completed at the University of Kentucky and the University of Alabama at Birmingham, originally designed to determine whether metformin could be repurposed to enhance muscle hypertrophy in older individuals, aged ≥ 65 yr. Ninety-four participants, randomized to receive metformin (n = 46) or identical placebo (n = 48), in conjunction with 14 wk of PRT (centered on optimizing muscle mass and strength gains) completed the intervention. All participation was on a voluntary basis, and each participant was required to sign an approved IRB consent form and HIPAA (Health Insurance Portability and Accountability Act) authorization before any procedures took place. Detailed information and methodology of the clinical trial (30), primary outcomes (25), effects of metformin on gene expression (26), and muscle quality (27) have been previously published. Data reported here are from those on placebo only for which measures were obtained before and after the intervention to correlate heterogeneous hypertrophic response with muscle cellular and molecular properties across individuals.
Measures of Muscle Size
Computed tomography imaging (primary outcome).
Single-slice cross-sectional midthigh images of both legs, defined as the midpoint between the inguinal crease and superior border of the patella, were obtained. National Institutes of Health (NIH) ImageJ was used to quantify total muscle cross-sectional area using previously described step-by-step analysis methods (31). For this analysis, all computed tomography (CT) muscle areas were calculated from a density range of −29 to 150 Hounsfield units (HU) and dominant thigh was defined as the thigh with the higher muscle mass determined by dual-energy X-ray absorptiometry (DXA). Four CT scans were excluded due to probable positioning errors, resulting in n = 44 individuals with complete data sets.
DXA imaging.
For all participants (n = 48), total body DXA scans were utilized for whole and regional body composition including bilateral thigh muscle mass assessment using a Lunar Prodigy (UAB) and Lunar iDXA (UK). Custom analyses were performed to determine femur length and right and left thigh muscle and fat mass. Femur length was measured from the center of the junction of the femoral head (at the femoral neck) and the acetabulum to the center of the bottom of the medial condyle. Right and left thigh muscle and fat mass were calculated by subtracting the lower leg from the respective right or left leg total leg mass by creating a custom region of interest (ROI) through the center of the knee joint between the tibial plateau and the femoral condyles and encapsulating the lower leg past the toes. Thigh muscle mass was then normalized to femur length to account for different leg lengths.
Mean fiber cross-sectional area and fiber-type frequency.
Muscle biopsies were obtained from the vastus lateralis after administration of local anesthetic (1% lidocaine premixed with bicarbonate) using a 5-mm Bergstrom needle with a 60-mL syringe applied suction. Fiber-type-specific cross-sectional area (fCSA) was quantified on fresh 7-μm cryosections using an antibody recognizing laminin [antibody rabbit (1:100) (L9393, Sigma Aldrich, St. Louis, MO] to delineate muscle fiber borders, followed by a 90-min incubation with antibodies applied in combination for 90 min against myosin heavy chain (MyHC) isoforms; types 1 (BA.D5, IgG2b AF647), 2a (SC.71, IgG1 AF488) and 2ax (6H1, IgM AF555) from Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA (25). Digital images were captured of the entire cross-section (mean: 834 ± 366 fibers baseline vs. mean: 942 ± 526 post, P = 0.29) and mean fCSA was quantified using our validated automated algorithm (32). To ensure that only the highest quality data were analyzed, fCSA was not determined for samples that were freeze damaged, folded, or consisted of less than 300 fibers by visual inspection by the research team. This resulted in n = 38 at baseline and n = 32 following PRT. Of these, all participants had available data from both CT muscle area and DXA normalized bilateral thigh mass.
Associations between measurements of muscle size.
To provide an internal validation of the different methods to quantify the hypertrophic response, measures of whole and fiber level muscle size were correlated from all individuals who had all three baseline measures. As expected, at baseline, CT thigh muscle area was strongly and positively associated with bilateral thigh mass normalized to femur length (r = 0.90, P < 0.0001). Mean fCSA was significantly and positively associated with both CT (r = 0.53, P < 0.001) and DXA (r = 0.48, P < 0.001) measures of whole muscle size with CT muscle area providing slightly stronger associations (Supplemental Fig. S1, A and B; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.18450905). Slightly better relationships were apparent in posttraining comparisons of mean fCSA and CT thigh muscle area (r = 0.65, P < 0.0002) and between fCSA and DXA bilateral thigh mass normalized to femur length (r = 0.65, P < 0.0001) (Supplemental Fig. S1, C and D). Therefore, CT muscle area was used as the primary measure of whole muscle growth based on baseline relationships.
Muscle composite score.
It has been demonstrated and shown earlier that the measure used to quantify hypertrophy can have varying outcomes depending on the level of measurement (macroscopic vs. microscopic) (32). Thus, reasons to create a muscle composite score were threefold: 1) to minimize comparative discrepancies at the whole muscle and fiber levels, 2) to account for technical and measurement error between assessments, and 3) to provide proper directionality of the resulting correlations, as large or small values can skew results in a single measure. Our muscle composite score was calculated as the average z-score of the percent change measure for fCSA, CT thigh muscle area, and DXA bilateral thigh mass normalized to femur length.
Intrinsic Muscle Features Quantified via Immunohistochemistry
Hematoxylin and eosin (H&E) staining was performed to assess the quality of muscle samples for use in downstream analyses. For all analyses, digital images were captured of the entire cross-section, including two to three serial cross-sections for each sample. For each sample, areas containing longitudinal fibers, edge effects, bubbles, wrinkles, or folds were excluded from analyses. Samples found to have significant freeze artifact preventing quantification of fCSA or other muscle features, or less than 300 cross-sectional fibers, were excluded.
Macrophage profiling.
Resident macrophage number and polarization state were determined by our previously detailed methods (33). Pan monocyte/macrophage antibody against CD11b, together with 4′,6-diamidino-2-phenylindole (DAPI) staining, were used to quantify total macrophages in 7-µm cryosections. CD206 combined with CD11b immunohistochemistry (IHC) was used as a preliminary indicator of macrophage inflammatory state (16, 25), recognizing that this is an oversimplication due to heterogeneity within this cell population (34). Within the CD11b+ population, the number of CD206+ (M2-like) or CD206− immune cells were then expressed per total number of fibers. QA/QC (quality assurance/quality control) assessment of staining resulted in n = 37 pre-PRT samples and n = 32 matched pre-post pairs.
Satellite cells and myonuclei abundance.
Muscle stem cell (satellite cell) abundance per fiber was quantified with the Pax7 monoclonal antibody (DSHB) with GtαMs biotin secondary antibody, as reported previously (25). These analyses were combined with counting total myofiber nuclei (DAPI-stained nuclei residing within the dystrophin-labeled sarcolemma) using the automated MyoVision program (35) to monitor myonuclear accretion from satellite cell fusion that accompanies hypertrophic growth of myofibers. QA/QC assessment of staining resulted in n = 32 and n = 29 pre-PRT samples and n = 32 and n = 29 matched pre-post pairs for satellite cells and myonuclei, respectively.
Exploratory Muscle Features via Histochemistry
As part of a separate exploratory analysis, fibrous collagen content and organization and capillary content were quantified in a subset of participants that had gene expression data and/or had available remaining tissue (n = 33). This resulted in a slightly different sample of participants for these analyses as only 27 of the 33 also had fCSA data meeting fiber count quality control.
Collagen content.
Sirius Red (SR) staining and polarized light microscopy with imaging software (NIH ImageJ) were used to quantify total and varying thickness/architecture of collagen I and III within the ECM. Cryosections (7 µm) were fixed with 4% paraformaldehyde (PFA) and incubated in 0.1% SR for 2 h [Electron Microscopy Sciences (EMS), Hatfield, PA; 26357-02], as described previously (36). Total SR+ area under visible light was quantified as a percent of total area. Hue components of resulting whole section images under polarized light were obtained and the number of red (densely packed collagen) and green (loosely packed collagen) pixels were calculated using a thresholding technique and quantified per area. QA/QC assessment of staining resulted in n = 33 pre-PRT samples and n = 33 matched pre-post pairs.
Capillary density.
Capillary abundance per fiber was determined using fiber border anti-laminin-delineated fiber borders in combination with a mixture of two lectins: biotinylated Ulex Europaeus UEA-1 (1:50; Sigma, L4889) and biotinylated Griffonia (Bandeiraea) simplicifolia Lectin I GSL I, BSL I (1:50; Vector Laboratories, Burlingame, CA, RL-1102) for 1 h, as described in detail previously (36, 37). All images were captured using a Zeiss AxioImager M1 upright microscope at ×20 magnification and analyzed with Zen Lite software’s automated counting wizard validated against hand counts (Carl Zeiss AG, Oberkochen, Germany). QA/QC assessment of staining resulted in n = 33 pre-PRT samples and n = 33 matched pre-post pairs.
Gene Expression
RNA sequencing.
RNA sequencing (RNA-Seq) was performed on pre- (n = 31) and post-PRT (n = 22) vastus lateralis biopsies from a subset of participants (26). Approximately 35 mg of muscle biopsy was homogenized in Qiazol (Qiagen, Valencia, CA) and total RNA was isolated using miRNeasy Mini Kits (Qiagen). RNA content and integrity were determined with a 2100 Bioanalyzer (Agilent, Santa Clara, CA). A minimum RNA Integrity Number (RIN) of 6.5 was set for all samples. Library preparation and sequencing were performed at Novogene Corporation (Chula Vista, CA) on an Illumina HiSeq 4000 system, using paired-end 150 bp (PE150) dual indexing. Quality control, filtering and trimming, and alignment of raw sequence reads and normalization were performed as previously described in detail (26). Weighted Gene Correlation Network Analysis (WGCNA) was performed as described and resulted in 14 distinct gene networks (modules, each assigned an arbitrary color) (28).
Single-cell RNA gene expression from isolated CD11b+ cells.
To leverage molecular connections with our cellular associations, a secondary exploratory study was performed with one male aged 87 yr (classified as a “responder” in our MASTERS trial based on gains in thigh muscle size) (25), who performed an acute resistance exercise bout consisting of three sets of eight with a fourth set to failure on four different exercises; squat, lat pulldown, leg press, and leg extension following a detraining period from the completion of the original training. Muscle was then procured and immediately processed 24 h after exercise in a separate IRB-approved protocol. To isolate mononuclear cells from fresh human muscle, we used a modified version of the fluorescent-activated cell sorting (FACS) protocol from the Rando laboratory (38). Muscle was dissociated in collagenase (Thermo Fisher, Waltham, MA) followed by collagenase and dispase (Thermo Fisher), and the cell suspension was filtered through a 40-µm strainer. The suspension was incubated with the PE-conjugated CD11b antibody (BioLegend, San Diego, CA) and cleared of debris, dead cells, and doublets via FACS. The pellet was washed and re-suspended in PBS with 0.04% bovine serum albumin (BSA; Millipore Sigma) to minimize ambient RNA and cellular aggregation, in accordance with 10X Genomics recommendations (10X Genomics, Pleasanton, CA). Cell concentration was determined via hemocytometer before being loaded on the 10X Chromium chip. After cells were loaded into the 10X Chromium Controller, the Single Cell 3′ reagent kit was applied. Libraries were prepared using version 3.0 chemistry and were sequenced on the Illumina NovaSeq 6000 system. The Cell Ranger Single Cell Software Suite was used to perform sample demultiplexing, barcode processing, and single cell 3′ gene counting. The cDNA insert was aligned to an appropriate reference genome using STAR and hg 38 transcriptome (release 100). Partek Flow was used for all downstream analysis from Filtered_Barcode_Matrix.h5 files. For the postacute resistance exercise bout sample, 81.9% of reads were within cells, the estimated number of cells was 3,919, with a median genes per cell of 2,248. Filter criteria included expressed genes (Min: 400, Max: 6,000) and mitochondrial reads percent (Min: 0%, Max: 15.00%). Additional filtering was performed that excluded features where values were <1.0 in at least 99.9% of the samples yielding a total of 12,059 genes for downstream analysis. Samples were normalized using counts per million and log transformation. Principal component analysis (PCA) was used before Uniform Manifold Approximation and Projection (UMAP) for visual based clustering. All statistical comparisons were conducted in Partek. Gene Set Enrichment Analyses (GSEA) from differentially expressed genes (DEGs) among the resulting macrophage clusters, determined by unbiased graph-based clustering, were then performed using g:profiler (39) and enrichment maps (with genes significantly enriched using a false discovery rate < 0.01) were visualized in Cytoscape version 3.8.2 (40) using published step-by-step procedures (41) to determine major biological themes. scRNA-seq data can be found at ArrayExpress accession number E-MTAB-10988.
Statistical analysis.
Forty-eight older persons randomized to the placebo group in the MASTERS parent trial are included in the current analyses. Data are described as means ± SD and the median for continuous variables and frequencies and percentages for categorical variables with resulting n’s based on muscle feature were analyzed and quality of the tissue. Specific muscle features may differ as outliers in the data were removed using quantile range outliers calculated as values Q × the interquartile range past the lower and upper quartiles, which resulted in two individuals being removed for % SR, or because of technical error, as indicated earlier or in each Figure legend. In addition, sensitivity checks were done for individuals appearing to drive correlational analyses. Pairwise Pearson correlation coefficients were used for all univariate associations between muscle phenotype and muscle size (fCSA, CT thigh muscle area, and composite muscle score). Multiple linear regression was applied adjusting for sex and variable by sex to inspect for sex interactions for all standardized betas along with 95% confidence intervals. Paired t tests were used to examine changes in muscle features following PRT. For gene expression analysis, statistics were generated similarly using multiple linear regression adjusting for sex against individual module eigengene values and muscle phenotype to identify which modules, if any, were related to specific muscle features. For all RNA-Seq analysis, Bonferroni correction was applied by adjusting the α-level by the number of resulting modules (14) found to relate to muscle hypertrophy. This resulted in a significance level set at P ≤ 0.004 (P = 0.05/14) after multiple comparisons. Statistical analyses were performed using JMP Pro version 14.0 (Cary, NC) with a significance level set at P < 0.05. Trending variables, defined as a P value between 0.051 and 0.12, have been noted in Supplemental materials, tables, and figures for quick determination of closely associated variables.
RESULTS
Participant Characteristics
Baseline demographics for participants in the MASTERS trial (25) randomized to placebo are presented in Table 1. This cohort was 98% Caucasian, 60% female, with a mean age of 70.8 ± 4.5 yr. Participants may have been recreationally active but were not performing resistance exercise before entry into the parent study. All were relatively healthy, postmenopausal, with very few (n = 3) taking estrogen replacement therapy (n = 5), classified as obese [mean body mass index (BMI) 25.6 ± 3.0 kg/m2] or sarcopenic (6%) according to Studenski et al. (42), calculated as the total mineral-free lean mass of the arms and legs adjusted to BMI. Muscle lipid content within this cohort and changes in some muscle features following PRT have been reported previously (25, 27). Table 2 shows all muscle phenotypic features before and after PRT quantified in the MASTERS cohort used in the current correlational analyses. Supplemental Table S1 shows the muscle phenotypic features by sex as we had a higher percentage of females completing PRT in our placebo cohort.
Table 1.
Participant characteristics
| Baseline Demographics | Mean, SD/Median or Frequency, % |
|||
|---|---|---|---|---|
| Female, n = 29 | Male, n = 19 | All, n = 48 | Range Min-Max | |
| Age, yr | 69.6 (3.3)/68.8* | 72.7 (5.4)/73.1 | 70.8 (4.5)/69.5 | 64.4–82.8 |
| Non-Hispanic Caucasian | 28 (96.6%) | 19 (100%) | 47 (98%) | |
| Asian | 1 (3.4%) | 0 (0%) | 1 (2%) | |
| African American | 0 (0.0%) | 0 (0%) | 0 (0%) | |
| Obese | 3 (10.3%) | 2 (10.5%) | 5 (10%) | |
| Sarcopenic | 0 (0%) | 3 (15.8%) | 3 (6%) | |
| BMI, kg/m2 | 25.1 (3.3)/25.7 | 26.5 (2.3)/26.8 | 25.6 (3.0)/26.0 | 18.5–30.3 |
| DXA total body fat, % | 39.3 (6.6)/40.6* | 29.3 (4.3)/27.2 | 35.4 (7.6)/35.5 | 24.3–50.9 |
Baseline demographics by sex of n = 48 MASTERS participants randomized to placebo. BMI, body mass index; DXA, dual-energy X-ray absorptiometry; MASTERS, Metformin to Augment Strength Training Effective Response in Seniors; SD, standard deviation.
Significant difference found between males and females.
Table 2.
Muscle phenotypic features before and after PRT
| Baseline |
Percent Change after PRT |
|||||
|---|---|---|---|---|---|---|
| Cellular Muscle Features | Mean, SD/Median | Range Min-Max | n (Female) | Mean (SD)/Median | Range Min-Max | n (Female) |
| Mean fCSA | 3,951.5 (1,548.6)/3,592.8 | 2,079.1–8,471.9 | 38 (23) | 13.1 (30.1)/10.3$ | −38.4 to −91.6 | 32 (19) |
| T1 fCSA | 4,564.2 (1,610.0)/4,196.2 | 2,302.9–8,337.1 | 38 (23) | 8.6 (30.8)/2.6 | −39.7 to −85.1 | 32 (19) |
| T2 fCSA | 3,512.7 (1,758.4)/2,873.7 | 1,541.0–8,977.5 | 38 (23) | 19.6 (30.8)/14.0* | −28.4 to –100.9 | 32 (19) |
| T1 FF,% | 44.3 (17.0)/42.7 | 5.8–90.8 | 38 (23) | −10.1 (25.0)/−14.2* | −60.4 to −58.9 | 32 (19) |
| T2a FF,% | 26.6 (13.2)/26.6 | 1.0–59.7 | 38 (23) | 71.3 (82.2)/51.2* | −36.4 to –292.2 | 32 (19) |
| T2a/x FF,% | 27.6 (19.1)/26.7 | 0.0–91.3 | 38 (23) | −12.0 (76.4)/−25.2* | −97.1 to –315.1 | 32 (19) |
| CD11b+/CD206− cells/fiber | 0.04 (0.03)/0.03 | 0.01–0.14 | 37 (20) | 92.4 (140.4)/34.5 | −77.5 to –438.8 | 32 (19) |
| CD11b+/CD206+ macs/fiber | 0.2 (0.1)/0.1 | 0.05–0.75 | 37 (20) | 64.2 (84.1)/39.3* | −60.3 to –331.0 | 32 (19) |
| % CD11b+/CD206− cells/total | 17.2 (7.3)/17.2 | 7.0–38.8 | 37 (20) | 13.9 (57.6)/−4.6* | −66.1 to –163.8 | 32 (19) |
| % CD11b+/CD206+ macs/total | 82.8 (7.3)/82.8 | 61.2–93.0 | 37 (20) | 0.8 (9.3)/2.1 | −16.7 to −23.3 | 32 (19) |
| Total CD11b+ cells/fiber | 20.8 (14.4)/17.8 | 5.6–89.2 | 37 (20) | 63.6 (82.1)/48.2* | −63.0 to –309.9 | 32 (19) |
| T1 SC/fiber | 0.09 (0.04)/0.08 | 0.05–0.18 | 32 (20) | 17.5 (47.1)/14.5 | −57.0 to –97.2 | 32 (20) |
| T2 SC/fiber | 0.06 (0.03)/0.05 | 0.01–0.14 | 32 (20) | 34.1 (77.0)/13.2$ | −77.8 to –237.8 | 32 (20) |
| Mean SC/fiber | 0.08 (0.03)/0.08 | 0.03–0.13 | 32 (20) | 25.8 (55.7)/19.7$ | −64.9 to –154.6 | 32 (20) |
| All MN/fiber | 1.9 (0.7)/1.8 | 0.8–3.9 | 30 (17) | 6.0 (31.1)/5.2 | −43.4 to –92.2 | 30 (17) |
| T1 MN/fiber | 2.1 (0.8)/2.0 | 0.8–3.9 | 29 (16) | 4.7 (34.8)/−1.1 | −49.0 to –82.9 | 29 (16) |
| T2 MN/fiber | 1.8 (0.8)/1.6 | 0.8–4.0 | 29 (16) | 1.4 (31.2)/−6.7 | −50.2 to –94.0 | 29 (16) |
| Total collagen content, % SR+ | 6.9 (2.6)/6.7 | 2.3–13.7 | 31 (16) | 1.9 (61.4)/−9.7 | −72.7 to –262.7 | 31 (16) |
| Loose CO, Green per area | 318.7 (47.7)/303.9 | 139.4–382.3 | 33 (17) | −1.2 (13.5)/−2.0 | −19.4 to –58.4 | 33 (17) |
| Dense CO, Red per area | 508.3 (91.1)/508.7 | 205.0–835.9 | 33 (17) | 0.3 (17.4)/−1.7 | −38.6 to –62.1 | 33 (17) |
| Capillaries/Fiber | 1.2 (0.4)/1.2 | 0.6–2.1 | 33 (17) | 16.5 (30.3)/14.0* | −44.0 to –80.4 | 33 (17) |
| DXA bilateral thigh muscle mass normalized, kg/cm | 232.0 (43.7)/221.3 | 160.7–329.2 | 48 (29) | 3.8 (5.8)/5.0 | −10.4 to –16.5 | 48 (29) |
| CT thigh muscle (−29 to 150 HU) area, cm2 | 111.2 (25.1)/105.1 | 69.4–159.6 | 48 (29) | 5.7 (6.2)/5.1 | −14.9 to –23.3 | 44 (28) |
CO, collagen organization; CT, computed tomography; DXA, dual-energy X-ray absorptiometry; fCSA, fiber cross-sectional area; FF, fiber frequency; macs, macrophages; MN, myonuclei; PRT, progressive resistance training; SC, satellite cells; SD, standard deviation; SR, Sirius Red; T, type.
Significant pre/postdifference; $trend (P value = 0.051–0.12).
Influence of Sex and PRT on Intrinsic Muscle Features
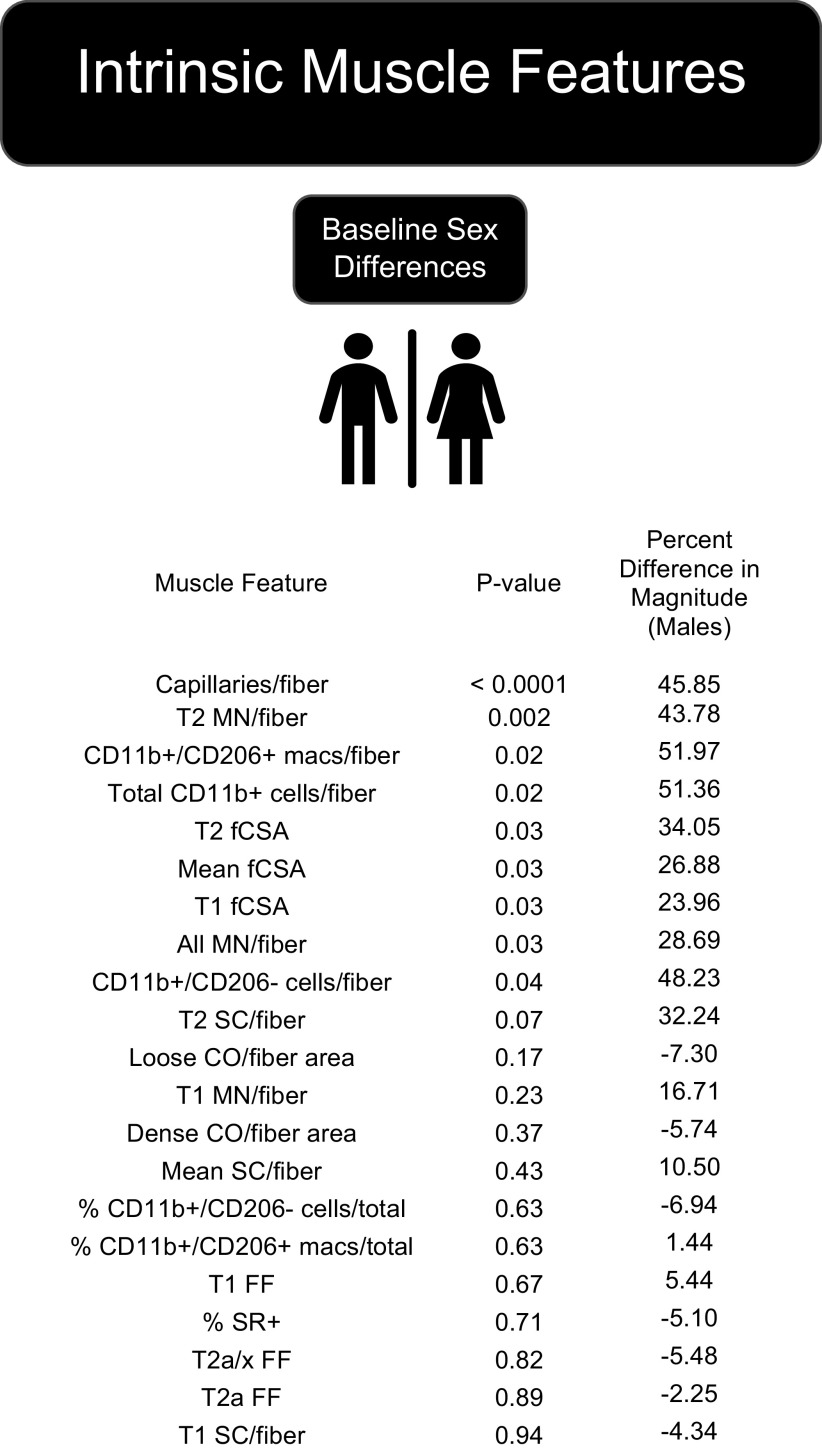
Figure 1 shows baseline sex differences of analyzed muscle features, and Table 2 shows muscle features that were significantly modified by PRT among placebo participants. At baseline, significant differences between males and females were found for capillaries per fiber, immune cells per fiber, myonuclei (MN) per fiber, and mean fiber cross-sectional area (fCSA) with males having greater mean values. Significant sex differences were not found for fiber frequencies, satellite cells per fiber, nor collagen measures at baseline. PRT had the most significant effect on immune cells, both CD11b+/CD206− and CD11b+/CD206+populations, ffiber-typeswitching, Type 2 fCSA, and capillaries per fiber, whereas satellite cells (overall and Type 2) per fiber were not significantly increased (P = 0.08). Both males and females responded similarly to PRT with no significant effect of sex found for any variable analyzed.
Figure 1.
Sex differences in muscle features at baseline sorted by P value showed that all significantly different (P ≤ 0.05) and trending (P = 0.051–0.12) muscle features were higher in males at baseline. CO, collagen organization by polarized light imaging of Sirius Red (SR)-stained sections; fCSA, fiber cross-sectional area; FF, fiber frequency; macs, macrophages; MN, myonuclei; SC, satellite cells; T, type. Refer to Table 2 for number of samples per assay.
Baseline muscle size is negatively correlated to changes in muscle size following PRT regardless of outcome measure. Baseline muscle size was consistently associated with hypertrophy outcomes regardless of the measurement method used (Table 3, Supplemental Fig. S2). Our sex-adjusted model shows that baseline thigh muscle area by CT (β = −0.75, P < 0.01) and thigh muscle mass by DXA (β = −0.47, P < 0.05) significantly negatively associated with whole muscle growth. Similar to whole muscle, baseline mean fCSA, with Type 1 fiber size being more significant (Table 3), was negatively associated with average fiber growth (β = −0.76, P < 0.001) revealing, as reported in young individuals, that smaller muscle size before beginning a PRT program is associated with greater growth in older individuals. These results underscore the usefulness of a composite score since the three measures of muscle growth move in the same direction.
Table 3.
Baseline muscle size predicts muscle growth regardless of primary outcome measure
| Baseline Variable | Postvariable | Correlation, r | n | Lower 95% CI | Upper 95% CI | P Value | Sex Adjusted β | AdjustedP Value |
|---|---|---|---|---|---|---|---|---|
| CT muscle area, cm2 | % Change in CT muscle area | −0.18 | 44 | −0.45 | 0.13 | 0.25 | −0.75 | 0.002 |
| Bilateral thigh muscle mass normalized, kg/cm | % Change in bilateral thigh muscle mass normalized | −0.26 | 48 | −0.51 | 0.02 | 0.07 | −0.47 | 0.04 |
| Mean fCSA, µm2 | % Change in mean fCSA | −0.42 | 32 | −0.67 | −0.09 | 0.02 | −0.76 | 0.003 |
| Type 1 fCSA, µm2 | % Change in Type 1 fCSA | −0.51 | 32 | −0.73 | −0.20 | 0.003 | −0.73 | 0.001 |
| Type 2 fCSA, µm2 | % Change in Type 2 fCSA | −0.27 | 32 | −0.56 | 0.09 | 0.14 | −0.43 | 0.12 |
CI, confidence interval; CT, computed tomography; fCSA, fiber cross-sectional area.
Baseline Cellular Muscle Features, but Not Change in the Features, Are Associated with Thigh Muscle Hypertrophy
Figure 2 shows additional baseline muscle features associated with changes in thigh muscle area following PRT. In addition to baseline muscle size, polarized light imaging of Sirus Red (SR)-stained sections showed a higher proportion of loosely organized collagen (green) was significantly correlated with whole muscle hypertrophy (Fig. 2A, β = −0.44, P < 0.05, representative image shown in Fig. 2D), whereas total fibrous collagen content detected by visible light imaging of SR staining was not correlated (representative image shown in Fig. 2E). Dense collagen organization (CO) by polarized light imaging (Fig. 2B, β = −0.32, P = 0.12) and CD11b+/CD206− immune cell abundance (Fig. 2C, β = −0.36, P = 0.10) were also trending to be negatively associated with whole muscle growth determined by CT. Interestingly, a differential sex response for baseline CD11b+/CD206− immune cell abundance was found showing that CD11b+/CD206− cells may be preferentially associated with lower muscle growth in females. All raw data, univariate and multiple linear regression model associations, are shown in Supplemental Table S2. Changes in the cellular features measured were not significantly correlated to change in our primary outcome; thigh muscle area measured by CT (Supplemental Table S3).
Figure 2.
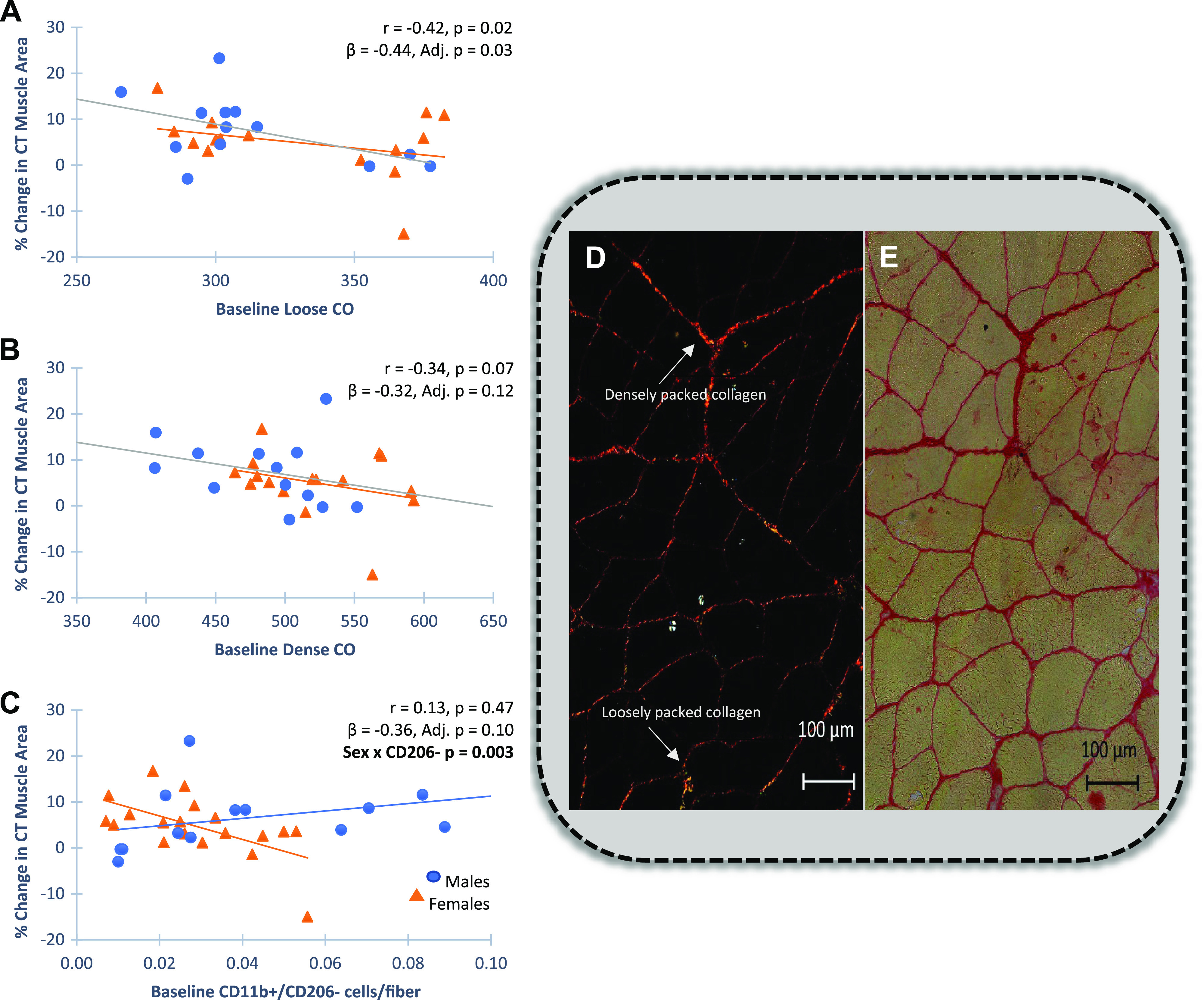
Baseline muscle features associated with change in thigh muscle area quantified by computed tomography (CT). Pearson correlations for univariant comparisons and multiple linear regression adjusting for sex (standardized betas) and variable by sex to identify significant sex interactions are shown. A: quantification of polarized light imaging of Sirius Red (SR)-stained sections with baseline green fluorescence, an indicator of loosely packed collagen, negatively associated with percent change in muscle area (n = 33). B: quantification of polarized light imaging of Sirius Red-stained sections. Baseline red fluorescence, an indicator of more densely packed collagen, trended to be negatively associated with increased muscle area (n = 33). C: CD11b+/CD206− immune cell abundance trended to be negatively associated with growth in females, with a significant sex by cell interaction (n = 37). Males are designated as blue circles and females are designated as orange triangles. Confidence intervals for all standardized βs are provided in Supplemental Table S1. D and E: representative images of Sirius Red (SR) staining of fibrous collagen in a baseline muscle section are shown. D: polarized light imaging shows hue components that reflect collagen organization. The number of red (densely packed collagen) and green (loosely packed collagen) pixels were quantified using a thresholding technique and expressed per area in ImageJ. E: visible light imaging of total fibrous collagen was quantified and expressed as percentage of total area.
To attempt to understand how baseline collagen organization and CD11b+/CD206− immune cells may be negatively associated with growth potential, muscle features were correlated to expression of basal gene networks generated in our group’s previous publication (28). This was done by examining the relationship between the module eigengene, the first principal component within a group of highly correlated genes designated with an arbitrary color (e.g., red, magenta), to baseline measures of collagen abundance and organization, and CD11b+/CD206− immune cell abundance. The Cyan module eigengene appeared positively associated (unadjusted P values) with both baseline collagen abundance and collagen organization whereas the Magenta and Purple modules were negatively associated with baseline collagen organization. On the other hand, baseline CD11b+/CD206− immune cell abundance trended negatively with the Blue module which also included a significant sex interaction, but positively with the Red module (Supplemental Table S4). After Bonferroni correction, nothing remained significant, but it is worth noting that the Magenta and Red modules have predictive value in CT muscle area outcomes with Gene Set Enrichment Analyses (GSEA) revealing connections to biological processes such as virus defense (Red module) and immune system processes (Magenta module) (28).
Changes in Intrinsic Muscle Features in Response to PRT, but Not Baseline Muscle Features, Associate with Muscle Fiber Growth
No cellular features of muscle at baseline (apart from fiber size presented earlier) were associated with increased fCSA following PRT. Even those features associated with increased thigh muscle area measured by CT were not associated at the fiber level (Supplemental Table S5); however, percent change in several features was associated with percent change in fCSA. After adjusting for sex, the percent change in capillaries (Fig. 3A, β = 0.56, P < 0.01), myonuclei (Fig. 3B, β = 0.67, P < 0.01), and CD11b+/CD206+ macrophages (Fig. 3C, β = 0.45, P < 0.05) were significantly associated with percent change in fiber growth, with a sex interaction approaching significance for the change in CD11b+/CD206+ macrophages. The change in CD11b+/CD206+ macrophages in response to PRT has been reported to be positively associated with changes in fCSA (29), but this new analysis has now been adjusted for sex. Unexpectedly, the change in CD11b+/CD206− immune cells was also significantly associated with change in fCSA (Fig. 3D, β = 0.52, P < 0.05). Although satellite cells tended to increase with PRT, the change in satellite cell abundance was not associated with fiber growth; satellite cell abundance was not associated with myonuclei at baseline (β = 0.21, P = 0.24) nor were the changes in satellite cells and myonuclei following PRT correlated (β = 0.26, P = 0.32) with no effect of sex.
Figure 3.
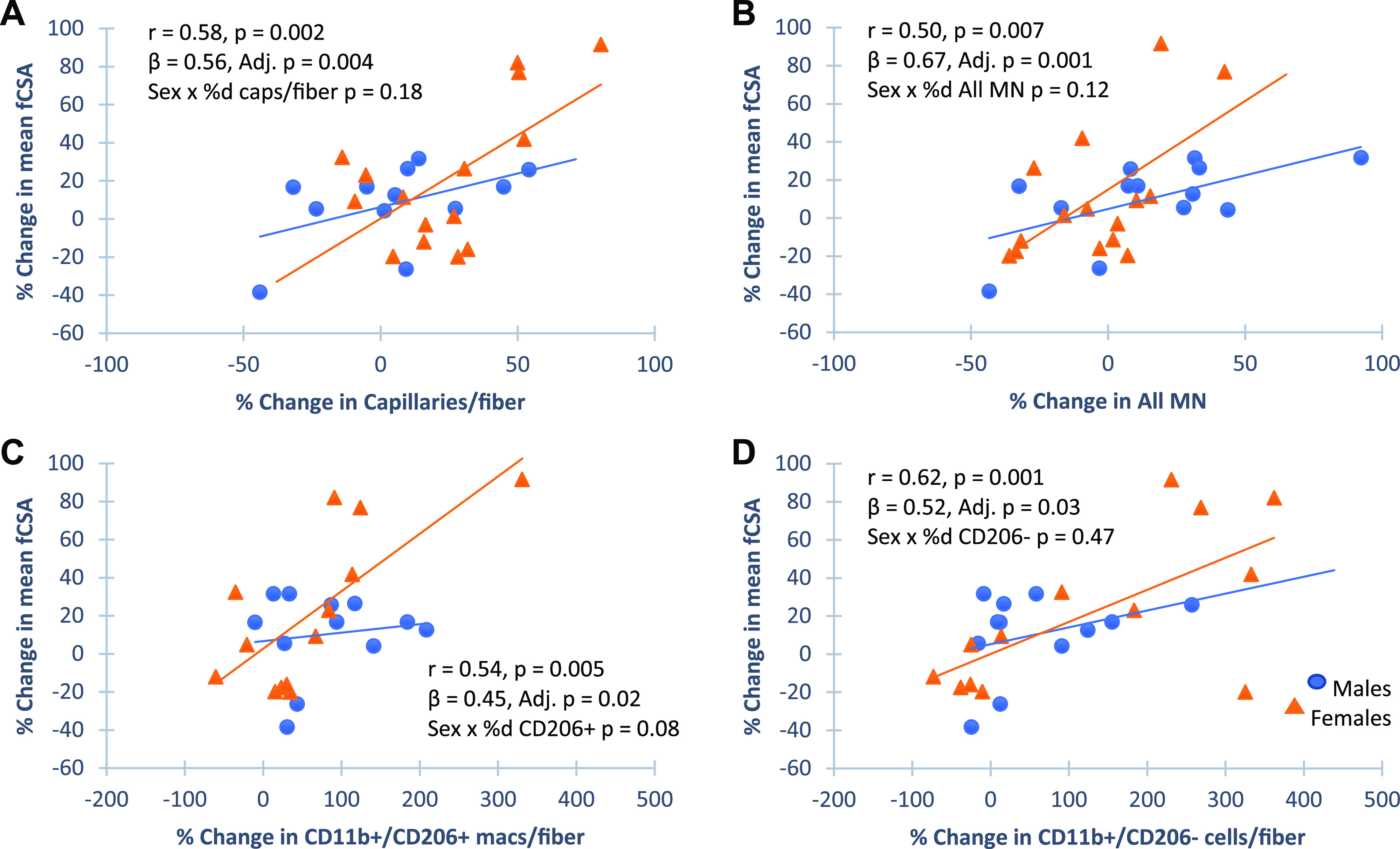
Changes in muscle features associated with change in muscle fiber cross-sectional area (fCSA). Pearson correlations for univariant comparisons and multiple linear regression adjusting for sex (standardized betas) and variable by sex to identify sex interactions are shown. Scatter plots show percent change in fCSA was positively correlated to change in capillary density (A, n = 32), myonuclei (MN; B) (n = 30), CD11b+CD206+ (C, n = 32), and CD11b+CD206− (D, n = 32) cell abundance. There was a trend for the change in CD11b+CD206+ cell abundance to be preferential in females. Males are designated as blue circles and females are designated as orange triangles. macs, Macrophages.
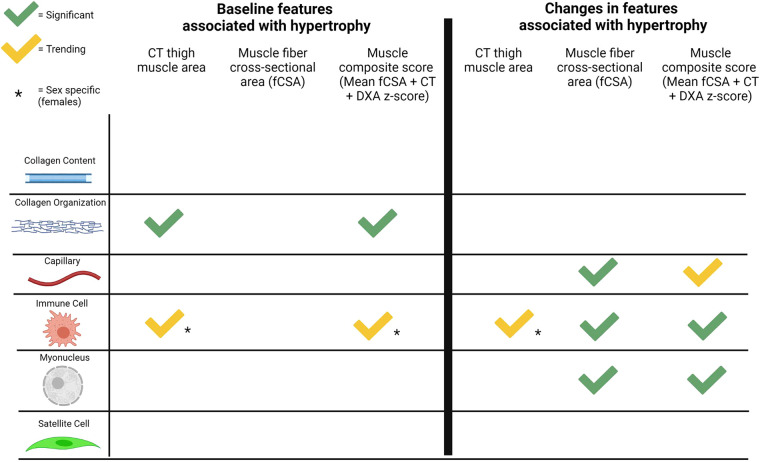
Using a muscle hypertrophy composite score aligns whole muscle and cellular myofiber percent change models. As shown in Supplemental Fig. S1, agreement among the three measures of muscle size (CT thigh muscle area, muscle mass by DXA, and mean fCSA from biopsies) pre- and post-PRT was very high. When assessing the degree of hypertrophy (% change) across methods, similar to our previous primary outcomes analyses (25), relationships became weaker within these same individuals, with the change in CT muscle area (r = 0.42, P = 0.03) still being superior to the change in bilateral thigh mass normalized by DXA (r = 0.28, P = 0.12) (Supplemental Fig. S3). This suggests that while agreement between measures is high at a single timepoint, every method has limits when assessing changes over time (32). Thus, the combined average z-score of the three muscle hypertrophy measures was used to identify potential muscle features that did not correlate using a single hypertrophy measure. Using a muscle composite score did not provide additional predictive value as baseline muscle features were not found to correlate with the composite score. However, change in overall immune cell response (β = 0.38, P = 0.05) and increases in myonuclei (β = 0.39, P < 0.05) were significantly correlated with the percent change in the muscle composite score. Percent change in capillaries per fiber was not significantly associated with change in the composite score (P = 0.15) making capillaries only significant at the fiber level. A summary showing correlations of the different hypertrophy measures to baseline muscle features and changes in those features is provided in Fig. 4.
Figure 4.
Summary of muscle features associated with whole muscle and/or muscle fiber hypertrophy. Created with BioRender.com with permission. CT, computed tomography; DXA, dual-energy X-ray absorptiometry.
Associations between Change in Macrophage Abundance in Response to PRT and Baseline Collagen Content
As stated earlier, PRT had a significant effect on both CD11b+/CD206+ and CD11b+/CD206− cell abundance. Our recent report showed that there was no correlation between change in collagen content and change in macrophage abundance in response to PRT (29). However, as reported here (see Fig. 2), collagen density and specific immune cell populations in females were associated with overall thigh muscle growth following PRT, leading us to narrow our focus on relationships between baseline collagen and change in macrophage profile to provide additional insight into muscle growth heterogeneity. There was a significant negative association between baseline collagen abundance and changes in CD11b+/CD206− immune cell content in response to PRT (Fig. 5A), with no relationship between baseline collagen content and change in CD11b+/CD206+ macrophages (Fig. 5B), suggesting that infiltrating cells may be preferentially affected by fibrous collagen content. In contrast to total fibrous collagen abundance, no significant correlation between baseline collagen organization using polarized light imaging and change in macrophage abundance in response to PRT was apparent (Fig. 5, C and D).
Figure 5.
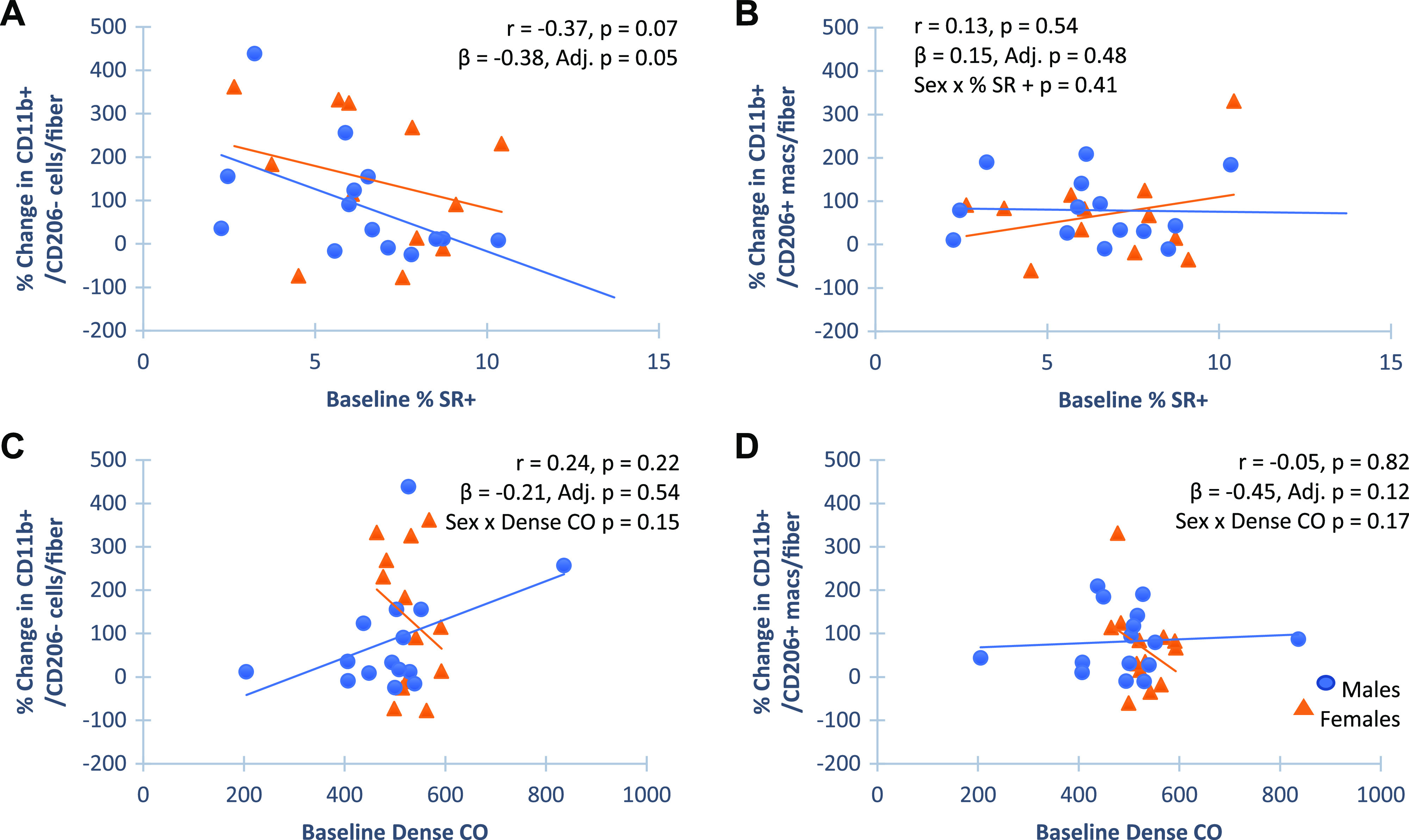
Associations of baseline collagen measures and change in immune cell abundance with progressive resistance training (PRT). Pearson correlations for univariant comparisons and multiple linear regression adjusting for sex (standardized betas) and variable by sex to identify sex interactions are shown. Scatterplots show baseline fibrous collagen content, quantified by area stained with Sirius Red (SR+) was negatively associated with change in CD11b+/CD206− (A, n = 31), but not CD11b+/CD206+ (B, n = 31) cell abundance. Polarized light imaging of Sirius Red-stained sections showed that baseline collagen density does not associate with either CD11b+/CD206− (C, n = 32), or CD11b+/CD206+ (D, n = 32) cell abundance. Collagen interactions with CD11b+/CD206+ cells in females only may contribute to sex differential response in whole muscle hypertrophy. Males are designated as blue circles and females are designated as orange triangles. CO, collagen organization.
Gene Expression in Macrophage Subclusters Following Resistance Exercise Reveals a Mixed Phenotype with Potential for Influencing Collagen Homeostasis
Increased abundance of both CD11b+/CD206+ and CD11b+/CD206− immune cells following PRT was associated with increased fCSA, warranting further investigation into CD11b+ cell heterogeneity. We hypothesized that the macrophage response to an acute bout of resistance exercise may provide insight into their potential contribution to the long-term hypertrophic response, as overall adaptation to training results from the cumulative effects of each acute exercise bout. A male participant who responded well in the MASTERS trial (∼10% gain in muscle size) returned to the laboratory and performed a lower-body focused exercise bout consisting of squats, leg press, and leg extensions to fatigue, a regimen similar to the regimen used in the MASTERS trial. A muscle biopsy was obtained 24 h postexercise, dissociated and cells sorted using the CD11b antibody followed by single-cell RNA-sequencing (scRNA-seq). From unbiased graph-based clustering, the majority of sorted cells were classified as macrophages and clustered into seven subclusters (Fig. 6A). The smallest cluster (cluster 7) displayed genes related to skeletal muscle and thus, was likely contaminated with ambient RNA leaving a total of six macrophage clusters. A gene atlas was developed to display known macrophage-associated markers within the entire gene set, cluster-specific genes within the top 100 differentially expressed genes (DEGs), genes shared by two clusters, and significantly enriched biological pathways from GSEA (Fig. 6B). Clusters 1 + 5, clusters 3 + 6, and clusters 2 + 4 were found to be the most similar to each other based on their shared genes and inflammatory profiles. Interestingly, at this early ttime point(24 h), two macrophage clusters (clusters 1 + 5) expressed the highest level of the mannose receptor C type 1 (MRC1) gene which encodes CD206, although each cluster had varying expression levels. Cluster 1 expressed LYVE1, a resident macrophage marker, while both of these clusters also expressed CD163 and CD209, suggesting they are M2-like. The MRC1 expressing clusters (1 + 5) also expressed genes with known roles in skeletal muscle growth and remodeling (IGF1, TGFB1, VEGFB). Clusters 3 + 6 expressed CCL2/CCR2, markers of infiltrating macrophages, but also expressed TIMD4, a resident marker, suggesting these clusters of macrophages had a mixed phenotype. Cluster 3 expressed both IL1A and IL1B and the IL1 receptor genes (IL1R1 and IL1R2), suggesting a more inflammatory phenotype than cluster 6. Cluster 2 also expressed IL1B, but also anti-inflammatory genes (IL10, PPARG, TNFAIP3, CD52, and VEGFA). Cluster 4 appeared to be the most inflammatory, expressing genes, including IL1B, CXCL2, CXCL10, and IFNG. Using GSEA for further examination of clusters 2, 4, and 6 revealed that some of the highest P value ranked DEGs following resistance exercise in clusters 2 and 4 were related to ECM breakdown (MMP19, ADAM9) whereas clusters 3 and 6 expressed genes involved with macrophage polarization to M2 and ECM remodeling (LGALS1, LGALS2, LGALS3, and TIMP1). Supplemental Table S6 shows DEGs of the six separately identified macrophage subclusters.
Figure 6.
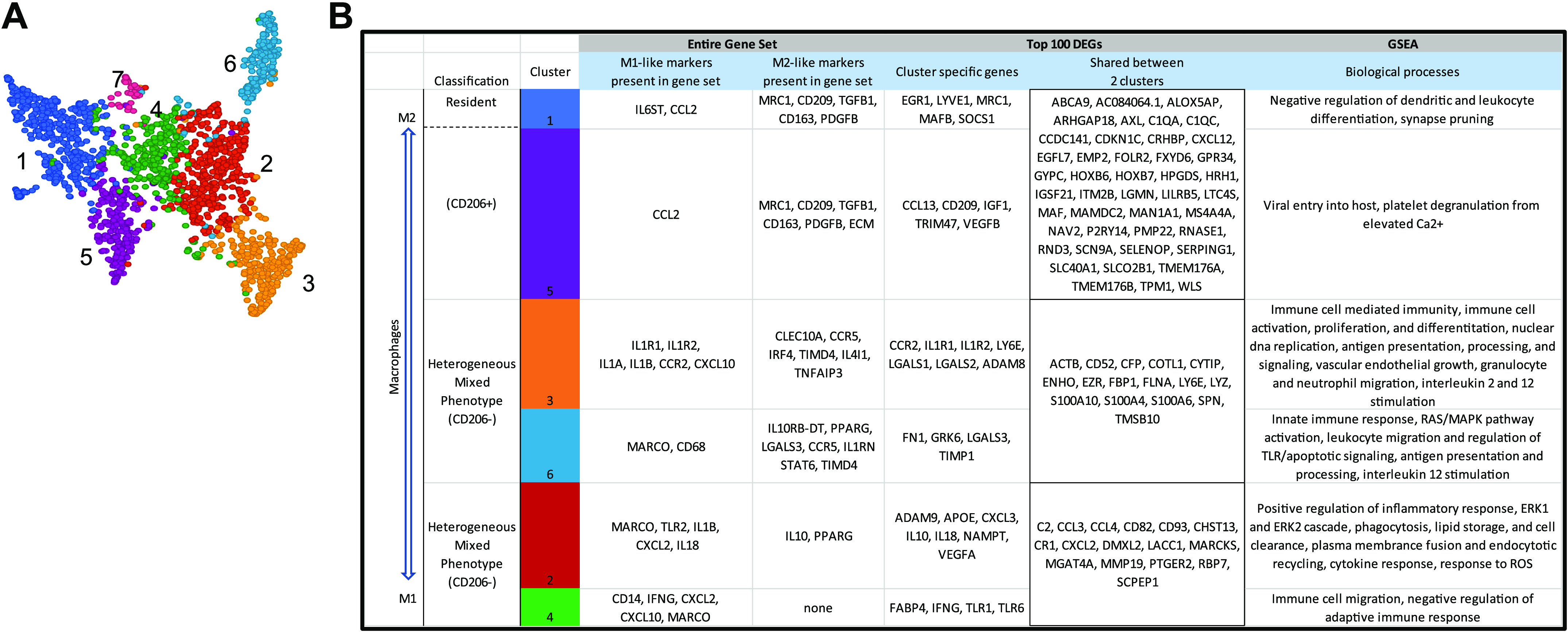
Single-cell transcriptome atlas of macrophages 24-h postresistance exercise (n = 1). Single-cell RNA sequencing of CD11b+ cells and unbiased graph-based clustering showed 6 macrophage clusters (A). B: from the top 100 differentially expressed genes (DEGs) in each cluster, genes characteristic of macrophage phenotype and/or functions are shown. Supplemental Table S6 shows DEGs of the six separately identified macrophage subclusters.
DISCUSSION
Muscle adaptation to resistance exercise relies on a complicated network of interconnected molecular mechanisms and changes in intrinsic cellular muscle features. The objective of this study was to investigate baseline intrinsic muscle molecular and cellular features that are associated with muscle adaptation to resistance exercise, as well as identify those features that may be most modifiable in accordance with hypertrophy in response to PRT in older individuals. Using an integrative approach assessing both anatomical whole muscle and histological fiber level dynamics, we report novel findings that along with muscle size, collagen organization, and CD11b+/CD206− immune cell abundance before exercise are associated with poor muscle growth in an older population, although the latter appears to be specific to females. Changes in several muscle features, including changes in capillary density, myonuclear number, and the abundance of both CD11b+/CD206+ and CD11b+/CD206− cells were positively associated only with fiber growth, even though greater variability was seen in fCSA compared with whole muscle growth measured by CT. This suggests an integrated indicator of muscle hypertrophic response to PRT may be useful, particularly as these histological features are associated with other critical muscle adaptations to exercise that confer increased resilience in aging muscle (e.g., metabolic flexibility, immune resistance). Creating a muscle composite score using all three measures of the muscle hypertrophic response (CT, fCSA, and DXA) showed that change in macrophage abundance was most significantly associated with the composite muscle score, increasing confidence in the significance of macrophages to the muscle adaptive response to PRT.
Muscle undergoes phenotypic changes during aging that may contribute to sarcopenia, defined as loss of muscle mass and strength (43), and may contribute to impaired response to resistance training. In advanced age, Type 2 fibers (the most responsive fiber type to resistance training) may be lost due to denervation or undergo a shift toward a more Type 1 fiber phenotype (44). This could account for the fact that fiber type distribution was not found to be associated with the hypertrophic response in our older group, either at the whole muscle or fiber level, as previously reported in young individuals (45). Surprisingly, no association of baseline satellite cell number or baseline myonuclear number was found to be associated with growth response, contrary to a previous report in a combined group of both young and older individuals (15). This disconnect may be due to the different populations studied, and the different methodologies used to quantify satellite cells and myonuclei. However, preclinical work by our laboratory has suggested that substantial muscle hypertrophy occurs in a genetic model of satellite cell depletion (46), suggesting that other factors predominate in muscle mass regulation in older individuals.
Baseline Contributors to Muscle Response Heterogeneity
We explored further possible influences of collagen content and organization on hypertrophic heterogeneity in older individuals due to a recent report demonstrating age-related declines in skeletal muscle collagen proteostasis (12). Previous work in this field has shown aged skeletal muscle exhibits increased stiffness due to increased ECM content leading to functional impairment (47–49). Our results show for the first time that ECM organization (loosely vs. densely packed collagen content), but not total collagen content before exercise significantly influences the muscle hypertrophic response to PRT in older individuals. Studies using mouse models typically find that collagen quantity is a poor predictor of muscle stiffness, whereas collagen organization is associated with muscle fibrosis or increased ECM content (50). Our data are consistent with findings from these studies, reporting that limb muscles of older mice showed higher proportions of loosely packed collagen than young mice, which we propose negatively impacts ability to adapt in response to muscle loading. Collagen disorganization may negatively affect lateral transmission of contractile force which could lead to less benefit from a resistance training program (51). In addition, collagen density may influence cytokine expression from proinflammatory macrophages that may be unfavorable for optimized muscle growth (52). We also found that lower collagen content at baseline was moderately associated with a greater increase in CD11b+/CD206+ macrophage abundance in response to PRT, further highlighting the importance of the ECM in muscle adaptability. This may be important as resistance training has been shown to blunt collagen accumulation (53), but it may have no effect on collagen already present in older individuals (54). These findings are consistent with the fact that ECM structural and remodeling genes were the most enriched in response to PRT in the MASTERS cohort (26).
In addition to collagen organization, CD11b+/CD206− cells at baseline were also negatively associated with changes in muscle area measured by CT, specifically in females. This finding suggests that these macrophages possess a more inflammatory profile (i.e., are more M1-like) and may be detrimental to the muscle adaptive response. The negative relationship between CD11b+/CD206− immune cells and muscle growth at baseline, only apparent in females, may also be involved in the higher susceptibility of females to disuse atrophy compared with males (55). There was no relationship between CD11b+/CD206+ macrophage abundance at baseline and the growth response to PRT in our older cohort. This is despite the fact that it has been shown that aged individuals typically have skewed macrophage profiles with higher prevalence of anti-inflammatory, M2-like macrophages at rest compared with young adults, as well as reduced inflammatory macrophages (56). A hypothetical model of macrophage involvement in aged skeletal has been proposed (57) such that M2 subtypes are increased during aging as a compensatory or adaptive response to increased fat content within the muscle, as they have found that in mice, M2-like macrophages colocalize with deposits of intermuscular adipose tissue (IMAT) and that slightly increased collagen deposits may also be responsible for M2 subtype polarization (56). Macrophage subtypes may also be influenced by adiposity as we found significant total body fat differences between females and males in this cohort at baseline. Limited evidence has shown that overall expression levels of macrophage genes (CD68, CD11b, CD206, CD16, CD40, and CD163) are lower in individuals with obesity (58). Thus, the various macrophage populations present in resting aged muscle are likely distinct from those responding to resistance training.
Important Changes during Resistance Training-Associated Growth
After training, increased myonuclei, capillary density, and both CD11b+/CD206+ and CD11b+/CD206− macrophages were positively and significantly associated with increased muscle fiber growth whereas only myonuclei and immune cell populations were significant with the muscle composite score. That myonuclei per fiber increased as fibers growth was expected, although the lack of correlation of growth to change in satellite cell number was unexpected, as satellite cell-mediated myonuclear accrual has been previously shown to be associated with myofiber growth (13, 15). However, in cluster analysis, only extreme responders seem to experience a significant change in the satellite cell pool (15). Consistent with our findings of lack of association of satellite cell abundance with hypertrophy, preclinical models show that satellite cell fusion cannot drive muscle hypertrophy in old mice (59). However, satellite cells may regulate other aspects of the muscle microenvironment that indirectly relate to the muscle growth response (60, 61). It is also possible that altered ECM and a thicker basal lamina may restrict satellite cell activation following resistance exercise (54). Our findings on capillary density are consistent with the work of two groups showing positive associations between capillarization and changes in fCSA (62–64). Our data support that PRT-induced changes in capillaries per fiber, especially in Type 2 fibers, were highly associated with growth. Although larger muscle fibers require increased oxygen and nutrient delivery, we hypothesize that circulating monocytes may be more efficiently recruited to muscle tissue by increased capillarization, contributing to muscle macrophage populations. In support of this hypothesis, our group recently reported that changes in capillary density in this cohort were strongly associated with changes in macrophages following PRT (29). That CD11b+/CD206+ macrophages are associated with growth is consistent with the reparative properties of M2 macrophages (65). It is also consistent with our previous work in middle-aged individuals demonstrating that the abundance of CD11b+/CD206+ macrophages is correlated with increases in muscle fiber size following cycle training (16). In that study, expression of several genes including those encoding growth factors and ECM remodeling proteins were correlated to CD11b+/CD206+ macrophage abundance following training which provides potential mechanisms facilitating growth. It has been shown that polarized M2-like macrophages play an important role in the process of tissue remodeling through the mannose receptor-mediated pathway (MRC1) for collagen uptake and degradation, allowing for proper collagen homeostasis (66).
The correlation between change in CD11b+/CD206− macrophage abundance and fiber growth is somewhat unexpected given the finding that at baseline, CD11b+/CD206− macrophages were negatively associated with growth. As mentioned earlier, this finding supports the idea that CD11b+/CD206− cells before and following PRT are distinct with a stimulus such as exercise providing beneficial changes. Although classically referred to as pro- (M1; CD11b+/CD206−) versus anti-inflammatory (M2, CD11b+/CD206+), it has recently been demonstrated that macrophages in human skeletal muscle are of a mixed phenotype, expressing both typical M1 and M2 markers (33). This is consistent with the findings of Jensen et al. (67) suggesting that neither pure pro- nor anti-inflammatory macrophages are present in muscle from older individuals after an acute bout of resistance exercise.
Increase in both CD11b+/CD206+ and CD11b+/CD206− cells were the most significant muscle features associated with fiber growth, as well as with the muscle hypertrophy composite score. In an attempt to explore macrophage heterogeneity in response to resistance exercise, scRNA-seq of CD11b+ cells in an older male was utilized after a single bout of resistance exercise, as overall adaptation to training likely results from the cumulative effects of each acute exercise bout. Focus was put on an early immune response (24 h) since at baseline, abundance of CD11b+/CD206− cells was negatively associated with hypertrophy, whereas an increase in CD11b+/CD206− cells post-PRT was positively associated with hypertrophy. Furthermore, the goal was to capture both potential changes in inflammatory macrophages and expansion of the M2-like CD206+ cells following a bout of resistance exercise. In an exploratory study using muscle from an 87-yr-old man who participated in the MASTERS trial and unbiased graph-based clustering, six distinct macrophage clusters present in muscle 24 h after the exercise bout were identified, each of which expressed unique inflammatory and anti-inflammatory gene markers and genes related to tissue remodeling. Three clusters (clusters 2 + 3 + 6) had very mixed phenotypes displaying both inflammatory and reparative/anti-inflammatory properties, whereas the remaining three clusters appeared more defined as M2- (clusters 1 + 5) or M1-like (cluster 4) with distinct anti-inflammatory versus inflammatory signatures. In addition, cross communication between resident and infiltrating macrophages through a possible CCL2 (MCP-1)/CCR2 mechanism and the potential for macrophage involvement in managing collagen homeostasis and ECM remodeling seems likely as some of the most highly expressed genes following resistance exercise encoded matrixmetalloproteinases and galectins (68). Thus, macrophage contribution to muscle adaptation to resistance exercise training is clearly complex and comprehensive analyses of macrophage heterogeneity related to exercise response heterogeneity are required.
Conclusions
This study suggests new cellular markers to identify those who may not experience muscle growth in response to resistance training, and potential new targets to increase training response in older individuals that may be useful in personalizing exercise regimens to individuals most at risk for sarcopenia. We show that collagen content and organization and immune cell content, potentially facilitated by change in capillary density, influence overall muscle growth. For the first time, we show immune cell populations are heterogeneous in muscle expressing a range of both inflammatory and reparative genes, and genes involved in ECM organization and remodeling. More research to follow up on these findings is required to define the importance of different macrophage populations at different timepoints in response to exercise. Muscle features, predictive and associated with change in function, independent of muscle growth, should also be examined to attempt to identify mechanisms underlying the discordance between muscle size and strength gains following resistance training.
DATA AVAILABILITY
The datasets used and/or analyzed during the current study are available at https://doi.org/10.6084/m9.figshare.18450905.
SUPPLEMENTAL DATA
Supplemental Tables S1–S6 and Figs. S1–S3: https://doi.org/10.6084/m9.figshare.18450905.
GRANTS
The study was funded by the National Institutes of Health (NIH) National Institute on Aging Grant AG046920 and supported by the NIH Clinical and Translational Science Awards (CTSA) (UL1TR001998) at the University of Kentucky and the NIH CTSA (UL1TR000165) at the University of Alabama at Birmingham. This study was also supported by an award from the Glenn Foundation for Medical Research.
DISCLAIMERS
The NIH had no role in the design of the study, collection, analysis, or interpretation of data, or in writing the manuscript.
DISCLOSURES
The first author, D.E.L., is a research coordinator at the University of Kentucky College of Health Sciences, and an exercise physiologist in the UK CCTS Functional Assessment and Body Composition Core Lab. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
M.M.B., P.A.K., and C.A.P. conceived and designed research; D.E.L., B.D.P., K.M.L., C.M.D., K.K., and S.C.T. performed experiments; D.E.L. and B.D.P. analyzed data; D.E.L., B.D.P., K.M.L., M.M.B., P.A.K., and C.A.P. interpreted results of experiments; D.E.L. prepared figures; D.E.L. drafted manuscript; D.E.L., B.D.P., K.M.L., C.M.D., K.K., M.M.B., P.A.K., and C.A.P. edited and revised manuscript; D.E.L., B.D.P., K.M.L., C.M.D., K.K., S.C.T., M.M.B., P.A.K., and C.A.P. approved final version of manuscript.
ENDNOTE
At the request of the authors, readers are herein alerted to the fact that additional materials related to this manuscript may be found at https://doi.org/10.6084/m9.figshare.18450905. These materials are not a part of this manuscript and have not undergone peer review by the American Physiological Society (APS). APS and the journal editors take no responsibility for these materials, for the website address, or for any links to or from it.
ACKNOWLEDGMENTS
The authors thank each of valuable research participants for time, effort, and dedication given during the study. We thank Tara Bennett PA-C and Dr. Sam Windham for performing muscle biopsies and Dr. Janna Jackson for performing immunohistochemistry. We also thank Dr. Ameya Kulkarni and Dr. Nir Barzilai of the Albert Einstein School of Medicine Nathan Shock Center for assistance with RNA sequencing data. Additional thanks goes to Dr. R. Grace Walton and Alex Simmons for technical expertise in the analysis of capillaries and collagen.
REFERENCES
- 1.Li R, Xia J, Zhang XI, Gathirua-Mwangi WG, Guo J, Li Y, McKenzie S, Song Y. Associations of muscle mass and strength with all-cause mortality among US older adults. Med Sci Sports Exerc 50: 458–467, 2018. doi: 10.1249/MSS.0000000000001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mesinovic J, McMillan LB, Shore-Lorenti C, De Courten B, Ebeling PR, Scott D. Metabolic syndrome and its associations with components of sarcopenia in overweight and obese older adults. J Clin Med 8: 145, 2019. doi: 10.3390/jcm8020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camera DM. Anabolic heterogeneity following resistance training: a role for circadian rhythm? Front Physiol 9: 569–569, 2018. doi: 10.3389/fphys.2018.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chmelo EA, Crotts CI, Newman JC, Brinkley TE, Lyles MF, Leng X, Marsh AP, Nicklas BJ. Heterogeneity of physical function responses to exercise training in older adults. J Am Geriatr Soc 63: 462–469, 2015. doi: 10.1111/jgs.13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med 34: 329–348, 2004. doi: 10.2165/00007256-200434050-00005. [DOI] [PubMed] [Google Scholar]
- 6.Bamman MM, Petrella JK, Jeong-Su K, Mayhew DL, Cross JM. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol 102: 2232–2239, 2007. doi: 10.1152/japplphysiol.00024.2007. [DOI] [PubMed] [Google Scholar]
- 7.Thalacker-Mercer A, Stec M, Cui X, Cross J, Windham S, Bamman M. Cluster analysis reveals differential transcript profiles associated with resistance training-induced human skeletal muscle hypertrophy. Physiol Genomics 45: 499–507, 2013. doi: 10.1152/physiolgenomics.00167.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Kotob R, Ponzano M, Chaput J-P, Janssen I, Kho ME, Poitras VJ, Ross R, Ross-White A, Saunders TJ, Giangregorio LM. Resistance training and health in adults: an overview of systematic reviews. Appl Physiol Nutr Metab 45: S165–S179, 2020. doi: 10.1139/apnm-2020-0245. [DOI] [PubMed] [Google Scholar]
- 9.Churchward-Venne TA, Breen L, Phillips SM. Alterations in human muscle protein metabolism with aging: Protein and exercise as countermeasures to offset sarcopenia. Biofactors (Oxford, England) 40: 199–205, 2014. doi: 10.1002/biof.1138. [DOI] [PubMed] [Google Scholar]
- 10.Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, Carter RE, Lanza IR, Nair KS. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab 25: 581–592, 2017. doi: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennis RA, Przybyla B, Gurley C, Kortebein PM, Simpson P, Sullivan DH, Peterson CA. Aging alters gene expression of growth and remodeling factors in human skeletal muscle both at rest and in response to acute resistance exercise. Physiol Genomics 32: 393–400, 2008. doi: 10.1152/physiolgenomics.00191.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbott CB, Lawrence MM, Kobak KA, Lopes EBP, Peelor FF III, Donald EJ, Van Remmen H, Griffin TM, Miller BF. A novel stable isotope approach demonstrates surprising degree of age-related decline in skeletal muscle collagen proteostasis. Function (Oxf) 2: zqab028, 2021. doi: 10.1093/function/zqab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrella JK, Kim J-S, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab 291: E937–E946, 2006. doi: 10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- 14.Przybyla B, Gurley C, Harvey JF, Bearden E, Kortebein P, Evans WJ, Sullivan DH, Peterson CA, Dennis RA. Aging alters macrophage properties in human skeletal muscle both at rest and in response to acute resistance exercise. Exp Gerontol 41: 320–327, 2006. doi: 10.1016/j.exger.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Petrella JK, Jeong-Su K, Mayhew DL, Cross JM, Marcas MB. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol 104: 1736–1742, 2008. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- 16.Walton RG, Kosmac K, Mula J, Fry CS, Peck BD, Groshong JS, Finlin BS, Zhu B, Kern PA, Peterson CA. Human skeletal muscle macrophages increase following cycle training and are associated with adaptations that may facilitate growth. Sci Rep 9: 969, 2019. doi: 10.1038/s41598-018-37187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moretti I, Ciciliot S, Dyar KA, Abraham R, Murgia M, Agatea L, Akimoto T, Bicciato S, Forcato M, Pierre P, Uhlenhaut NH, Rigby PWJ, Carvajal JJ, Blaauw B, Calabria E, Schiaffino S. MRF4 negatively regulates adult skeletal muscle growth by repressing MEF2 activity. Nat Commun 7: 12397–12397, 2016. doi: 10.1038/ncomms12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neppl RL, Wu C-L, Walsh K. lncRNA Chronos is an aging-induced inhibitor of muscle hypertrophy. J Cell Biol 216: 3497–3507, 2017. doi: 10.1083/jcb.201612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown DM, Goljanek-Whysall K. microRNAs: modulators of the underlying pathophysiology of sarcopenia? Ageing Res Rev 24: 263–273, 2015. doi: 10.1016/j.arr.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 20.McCormick R, Goljanek-Whysall K. MicroRNA dysregulation in aging and pathologies of the skeletal muscle. Int Rev Cell Mol Biol 334: 265–308, 2017. doi: 10.1016/bs.ircmb.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Ogasawara R, Akimoto T, Umeno T, Sawada S, Hamaoka T, Fujita S. MicroRNA expression profiling in skeletal muscle reveals different regulatory patterns in high and low responders to resistance training. Physiol Genomics 48: 320–324, 2016. doi: 10.1152/physiolgenomics.00124.2015. [DOI] [PubMed] [Google Scholar]
- 22.Rivas DA, Lessard SJ, Rice NP, Lustgarten MS, So K, Goodyear LJ, Parnell LD, Fielding RA. Diminished skeletal muscle microRNA expression with aging is associated with attenuated muscle plasticity and inhibition of IGF-1 signaling. FASEB J 28: 4133–4147, 2014. doi: 10.1096/fj.14-254490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC, Bamman MM. Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. Am J Physiol Endocrinol Metab 310: E652–E661, 2016. doi: 10.1152/ajpendo.00486.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figueiredo VC, Wen Y, Alkner B, Fernandez-Gonzalo R, Norrbom J, Vechetti IJ, Valentino T, Mobley CB, Zentner GE, Peterson CA, McCarthy JJ, Murach KA, Walden F. Genetic and epigenetic regulation of skeletal muscle ribosome biogenesis with exercise. J Physiol 599: 3363–3384, 2021. doi: 10.1113/JP281244. [DOI] [PubMed] [Google Scholar]
- 25.Walton RG, Dungan CM, Long DE, Tuggle SC, Kosmac K, Peck BD, Bush HM, Villasante Tezanos AG, McGwin G, Windham ST, Ovalle F, Bamman MM, Kern PA, Peterson CA. Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: A randomized, double-blind, placebo-controlled, multicenter trial: The MASTERS trial. Aging Cell 18: e13039, 2019. doi: 10.1111/acel.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulkarni AS, Peck BD, Walton RG, Kern PA, Mar JC, Windham ST, Bamman MM, Barzilai N, Peterson CA. Metformin alters skeletal muscle transcriptome adaptations to resistance training in older adults. Aging (Albany NY) 12: 19852–19866, 2020. doi: 10.18632/aging.104096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long DE, Peck BD, Tuggle SC, Villasante Tezanos AG, Windham ST, Bamman MM, Kern PA, Peterson CA, Walton RG. Associations of muscle lipid content with physical function and resistance training outcomes in older adults: altered responses with metformin. GeroScience 43: 629–644, 2021. doi: 10.1007/s11357-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavin KM, Bell MB, McAdam JS, Peck BD, Walton RG, Windham ST, Tuggle SC, Long DE, Kern PA, Peterson CA, Bamman MM. Muscle transcriptional networks linked to resistance exercise training hypertrophic response heterogeneity. Physiol Genomics 53: 206–221, 2021. doi: 10.1152/physiolgenomics.00154.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peck BD. A muscle cell-macrophage axis involving matrix metalloproteinase 14 facilitates extracellular matrix remodeling with mechanical loading. FASEB J 36: e22155, 2022. doi: 10.1096/fj.202100182RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long DE, Peck BD, Martz JL, Tuggle SC, Bush HM, McGwin G, Kern PA, Bamman MM, Peterson CA. Metformin to augment strength training effective response in seniors (MASTERS): study protocol for a randomized controlled trial. Trials 18: 192, 2017. doi: 10.1186/s13063-017-1932-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long DE, Villasante Tezanos AG, Wise JN, Kern PA, Bamman MM, Peterson CA, Dennis RA. A guide for using NIH Image J for single slice cross-sectional area and composition analysis of the thigh from computed tomography. PLoS One 14: e0211629, 2019. doi: 10.1371/journal.pone.0211629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haun CT, Vann CG, Roberts BM, Vigotsky AD, Schoenfeld BJ, Roberts MD. A critical evaluation of the biological construct skeletal muscle hypertrophy: size matters but so does the measurement. Front Physiol 10: 247, 2019. doi: 10.3389/fphys.2019.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosmac K, Peck BD, Walton RG, Mula J, Kern PA, Bamman MM, Dennis RA, Jacobs CA, Lattermann C, Johnson DL, Peterson CA. Immunohistochemical identification of human skeletal muscle macrophages. Bio Protoc 8: e2883, 2018. doi: 10.21769/BioProtoc.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege J-L, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41: 14–20, 2014. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan W, Murach KA, Vechetti IJ Jr, Fry CS, Vickery C, Peterson CA, McCarthy JJ, Campbell KS. MyoVision: software for automated high-content analysis of skeletal muscle immunohistochemistry. J Appl Physiol (1985) 124: 40–51, 2018. doi: 10.1152/japplphysiol.00762.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosmac K, Gonzalez-Freire M, McDermott MM, White SH, Walton RG, Sufit RL, Lu T, Lingyu L, Kibbe MR, Criqui MH, Guralnik JM, Polonsky TS, Leeuwenburgh C, Ferrucci L, Peterson CA, Tian L, Li L, S Polonsky T. Correlations of calf muscle macrophage content with muscle properties and walking performance in peripheral artery disease. J Am Heart Assoc 9: 1–15, 2020. doi: 10.1161/JAHA.118.015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirkeby S, Mandel U, Vedtofte P. Identification of capillaries in sections from skeletal muscle by use of lectins and monoclonal antibodies reacting with histo-blood group ABH antigens. Glycoconj J 10: 181–188, 1993. [Erratum in Glycoconj J 10: 466, 1993]. doi: 10.1007/BF00737716. [DOI] [PubMed] [Google Scholar]
- 38.Liu L, Cheung TH, Charville GW, Rando TA. Isolation of skeletal muscle stem cells by fluorescence-activated cell sorting. Nat Protoc 10: 1612–1624, 2015. doi: 10.1038/nprot.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, Vilo J. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res 47: W191–W198, 2019. doi: 10.1093/nar/gkz369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504, 2003. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reimand J, Isserlin R, Voisin V, Kucera M, Tannus-Lopes C, Rostamianfar A, Wadi L, Meyer M, Wong J, Xu C, Merico D, Bader GD. Pathway enrichment analysis and visualization of omics data using g: Profiler, GSEA, Cytoscape and EnrichmentMap. Nat Protoc 14: 482–517, 2019. doi: 10.1038/s41596-018-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam T-TL, Vassileva MT. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J GERONTOL SER A BIOL SCI MED SCI 69: 547–558, 2014. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siparsky PN, Kirkendall DT, Garrett WE. Muscle changes in aging: understanding sarcopenia. Sports Health 6: 36–40, 2014. doi: 10.1177/1941738113502296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelly NA, Hammond KG, Bickel CS, Windham ST, Tuggle SC, Bamman MM. Effects of aging and Parkinson's disease on motor unit remodeling: influence of resistance exercise training. J Appl Physiol (1985) 124: 888–898, 2018. doi: 10.1152/japplphysiol.00563.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haun CT, Vann CG, Mobley CB, Osburn SC, Mumford PW, Roberson PA, Romero MA, Fox CD, Parry HA, Kavazis AN, Moon JR, Young KC, Roberts MD. Pre-training skeletal muscle fiber size and predominant fiber type best predict hypertrophic responses to 6 weeks of resistance training in previously trained young men. Front Physiol 10: 297, 2019. doi: 10.3389/fphys.2019.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lacraz G, Rouleau A-J, Couture V, Söllrald T, Drouin G, Veillette N, Grandbois M, Grenier G. Increased stiffness in aged skeletal muscle impairs muscle progenitor cell proliferative activity. PloS one 10: e0136217, 2015. [Erratum in PLoS One 11: e0167661, 2016]. doi: 10.1371/journal.pone.0136217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pavan P, Monti E, Bondí M, Fan C, Stecco C, Narici M, Reggiani C, Marcucci L. Alterations of extracellular matrix mechanical properties contribute to age-related functional impairment of human skeletal muscles. Int J Mol Sci 21: 3992–3992, 2020. doi: 10.3390/ijms21113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood LK, Kayupov E, Gumucio JP, Mendias CL, Claflin DR, Brooks SV. Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. J Appl Physiol (1985) 117: 363–369, 2014. doi: 10.1152/japplphysiol.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith LR, Barton ER. Collagen content does not alter the passive mechanical properties of fibrotic skeletal muscle in mdx mice. Am J Physiol Cell Physiol 306: C889–C898, 2014. doi: 10.1152/ajpcell.00383.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brashear SE, Wohlgemuth RP, Gonzalez G, Smith LR. Passive stiffness of fibrotic skeletal muscle in mdx mice relates to collagen architecture. J Physiol 599: 943–962, 2021. doi: 10.1113/JP280656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sapudom J, Mohamed WKE, Garcia-Sabaté A, Alatoom A, Karaman S, Mahtani N, Teo JC. Collagen fibril density modulates macrophage activation and cellular functions during tissue repair. Bioengineering (Basel) 7: 33, 2020. doi: 10.3390/bioengineering7020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guzzoni V, Ribeiro MBT, Lopes GN, de Cássia Marqueti R, de Andrade RV, Selistre-de-Araujo HS, Durigan JLQ. Effect of resistance training on extracellular matrix adaptations in skeletal muscle of older rats. Front Physiol 9: 374, 2018. doi: 10.3389/fphys.2018.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nederveen JP, Joanisse S, Thomas ACQ, Snijders T, Manta K, Bell KE, Phillips SM, Kumbhare D, Parise G. Age-related changes to the satellite cell niche are associated with reduced activation following exercise. FASEB J 34: 8975–8989, 2020. doi: 10.1096/fj.201900787R. [DOI] [PubMed] [Google Scholar]
- 55.Rosa-Caldwell M, Greene NP. Mitochondrial contributions to disuse atrophy: let’s talk about sex. Biol Sex Differ 10: 43, 2019. doi: 10.1186/s13293-019-0257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cui C-Y, Driscoll RK, Piao Y, Chia CW, Gorospe M, Ferrucci L. Skewed macrophage polarization in aging skeletal muscle. Aging Cell 18: e13032, 2019. doi: 10.1111/acel.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cui C-Y, Ferrucci L. Macrophages in skeletal muscle aging. Aging (Albany NY) 12: 3–4, 2020. doi: 10.18632/aging.102740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu D, Morales FE, IglayReger HB, Treutelaar MK, Rothberg AE, Hubal MJ, Nadler EP, Robidoux J, Barakat H, Horowitz JF, Hoffman EP, Burant CF, Gordon PM. Expression of macrophage genes within skeletal muscle correlates inversely with adiposity and insulin resistance in humans. Appl Physiol Nutr Metab 43: 187–193, 2018. doi: 10.1139/apnm-2017-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JD, Fry CS, Mula J, Kirby TJ, Jackson JR, Fujun L, Lin Y, Dupont-Versteegden EE, McCarthy JJ, Peterson CA, Liu F, Yang L. Aged muscle demonstrates fiber-type adaptations in response to mechanical overload, in the absence of myofiber hypertrophy, independent of satellite cell abundance. J Gerontol A Biol Sci Med Sci 71: 461–467, 2016. doi: 10.1093/gerona/glv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28: 1654–1665, 2014. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murach KA, Fry CS, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Fusion and beyond: Satellite cell contributions to loading-induced skeletal muscle adaptation. FASEB J 35: e21893, 2021. doi: 10.1096/fj.202101096R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moro T, Brightwell CR, Phalen DE, McKenna CF, Lane SJ, Porter C, Volpi E, Rasmussen BB, Fry CS. Low skeletal muscle capillarization limits muscle adaptation to resistance exercise training in older adults. Exp Gerontol 127: 110723, 2019. doi: 10.1016/j.exger.2019.110723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snijders T, Nederveen JP, Joanisse S, Leenders M, Verdijk LB, van Loon LJC, Parise G. Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J Cachexia Sarcopenia Muscle 8: 267–276, 2017. doi: 10.1002/jcsm.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verdijk LB, Snijders TIM, Holloway TM, Van Kranenburg J, Van Loon LJC. Resistance training increases skeletal muscle capillarization in healthy older men. Med Sci Sports Exerc 48: 2157–2164, 2016. doi: 10.1249/MSS.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 65.Gehlert S, Jacko D. The role of the immune system in response to muscle damage./Die Rolle des Immunsystems bei Muskelverletzungen. Dtsch Z Sportmed 70: 242–248, 2019. doi: 10.5960/dzsm.2019.390. [DOI] [Google Scholar]
- 66.Madsen DH, Leonard D, Masedunskas A, Moyer A, Jürgensen HJ, Peters DE, Amornphimoltham P, Selvaraj A, Yamada SS, Brenner DA, Burgdorf S, Engelholm LH, Behrendt N, Holmbeck K, Weigert R, Bugge TH. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J Cell Biol 202: 951–966, 2013. doi: 10.1083/jcb.201301081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jensen SM, Bechshøft CJL, Heisterberg MF, Schjerling P, Andersen JL, Kjaer M, Mackey AL. Macrophage subpopulations and the acute inflammatory response of elderly human skeletal muscle to physiological resistance exercise. Front Physiol 11: 811, 2020. doi: 10.3389/fphys.2020.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slack RJ, Mills R, Mackinnon AC. The therapeutic potential of galectin-3 inhibition in fibrotic disease. Int J Biochem Cell Biol 130: 105881, 2021. doi: 10.1016/j.biocel.2020.105881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1–S6 and Figs. S1–S3: https://doi.org/10.6084/m9.figshare.18450905.
Data Availability Statement
The datasets used and/or analyzed during the current study are available at https://doi.org/10.6084/m9.figshare.18450905.
At the request of the authors, readers are herein alerted to the fact that additional materials related to this manuscript may be found at https://doi.org/10.6084/m9.figshare.18450905. These materials are not a part of this manuscript and have not undergone peer review by the American Physiological Society (APS). APS and the journal editors take no responsibility for these materials, for the website address, or for any links to or from it.