Keywords: cardiopulmonary exercise testing, exercise capacity, long COVID, recovery
Abstract
A failure to fully recover following coronavirus disease 2019 (COVID-19) may have a profound impact on high-functioning populations ranging from frontline emergency services to professional or amateur/recreational athletes. The aim of the study is to describe the medium-term cardiopulmonary exercise profiles of individuals with “persistent symptoms” and individuals who feel “recovered” after hospitalization or mild-moderate community infection following COVID-19 to an age, sex, and job-role matched control group. A total of 113 participants underwent cardiopulmonary functional tests at a mean of 159 ± 7 days (∼5 mo) following acute illness; 27 hospitalized with persistent symptoms (hospitalized-symptomatic), 8 hospitalized and now recovered (hospitalized-recovered); 34 community managed with persistent symptoms (community-symptomatic); 18 community managed and now recovered (community-recovered); and 26 controls. Hospitalized groups had the least favorable body composition (body mass, body mass index, and waist circumference) compared with controls. Hospitalized-symptomatic and community-symptomatic individuals had a lower oxygen uptake (V̇o2) at peak exercise (hospitalized-symptomatic, 29.9 ± 5.0 mL/kg/min; community-symptomatic, 34.4 ± 7.2 mL/kg/min; vs. control 43.9 ± 3.1 mL/kg/min, both P < 0.001). Hospitalized-symptomatic individuals had a steeper V̇e/V̇co2 slope (lower ventilatory efficiency) (30.5 ± 5.3 vs. 25.5 ± 2.6, P = 0.003) versus. controls. Hospitalized-recovered had a significantly lower oxygen uptake at peak (32.6 ± 6.6 mL/kg/min vs. 43.9 ± 13.1 mL/kg/min, P = 0.015) compared with controls. No significant differences were reported between community-recovered individuals and controls in any cardiopulmonary parameter. In conclusion, medium-term findings suggest that community-recovered individuals did not differ in cardiopulmonary fitness from physically active healthy controls. This suggests their readiness to return to higher levels of physical activity. However, the hospitalized-recovered group and both groups with persistent symptoms had enduring functional limitations, warranting further monitoring, rehabilitation, and recovery.
NEW & NOTEWORTHY At 5 mo postinfection, community-treated individuals who feel recovered have comparable cardiopulmonary exercise profiles to the physically trained and active controls, suggesting a readiness to return to higher intensity/volumes of exercise. However, both symptomatic groups and the hospital-recovered group have persistent functional limitations when compared with active controls, supporting the requirement for ongoing monitoring, rehabilitation, and recovery.
INTRODUCTION
Approximately ∼80% of the >450 million global cases of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) are asymptomatic or mild. Most individuals recover within 2 to 4 wk (1, 2). However, between 2% and 12% of individuals report prolonged symptoms following their acute illness with coronavirus disease 2019 (COVID-19) (3, 4). Prolonged illness, lasting from several months up to 2 years, can cause exercise intolerance, breathlessness, fatigue, and palpitations (5–8). Functional limitations (9–11) and abnormalities on cross-sectional cardiothoracic imaging, including lung fibrosis and myocardial inflammation (12, 13) have been described, raising concerns regarding long-term impairment of cardiopulmonary function.
Cardiorespiratory fitness (CRF) can be compromised following COVID-19 infection (4, 14–16), with SARS-CoV-2 impacting the systems that most influence it; the cardiac, pulmonary, and skeletal muscle systems (4, 17–19). Cardiopulmonary exercise testing (CPET) provides the most accurate and standardized quantification of CRF and allows the identification of ventilatory inefficiency or other causes of exercise limitation (20, 21), therefore providing an ideal method of assessing for objective functional limitation and pathology, associated with persistent symptoms following SARS-CoV-2 (22, 23).
A failure to fully recover may have a profound impact on diverse high functioning populations such as emergency services (e.g., police, firefighters, paramedics), armed services, and professional or amateur/recreational athletes. All can be exposed to high volume/intensities of exercise as a core component of their occupational role or lifestyle choices, often under challenging, unpredictable environmental conditions. Any enduring pathology may therefore impair their ability to return to high levels of physical function, with many potentially becoming physically deconditioned, increasing the risk of injury as they return to work/exercise.(24)
The impact of the severity of the acute phase of SARS-CoV-2 on potential long-term sequelae remains unclear (25, 26), particularly regarding exercise response. Return to operational duty or strenuous activity advice for recovering patients will often be determined by residual symptoms and persisting effects such as fatigue, musculoskeletal discomfort, and dyspnea (27). It is unclear whether persistent symptoms or subjective markers of recovery are sufficient to determine an individual’s readiness to return to exercise or physically demanding work.
Using data from the Military COVID-19 Observational Outcome in a Viral Infectious Disease (M-COVID) study (developed to describe the effects of SARS-CoV-2 on the UK Armed Forces), our aim was to:
Characterize the medium-term cardiopulmonary function of individuals who feel recovered and those with persistent symptoms from two different acute disease severity groups (severe hospitalized and mild-moderate community managed) following COVID-19 illness.
Compare participants with “persistent symptoms” following hospitalization or community management to an age, sex, and job-role matched control group.
Compare participants who feel “recovered” following hospitalization or community management to an age, sex, and job-role matched control group.
METHODS
Study Design
Prospective observational cohort study. Ethical approval from the Ministry of Defense research ethic committee, July 2020 (1061/MODREC/20).
Setting
Investigations were performed as part of the UK Defence COVID-19 recovery service (DCRS) at Defence Medical Rehabilitation Centre (DMRC) Stanford Hall.
Participants
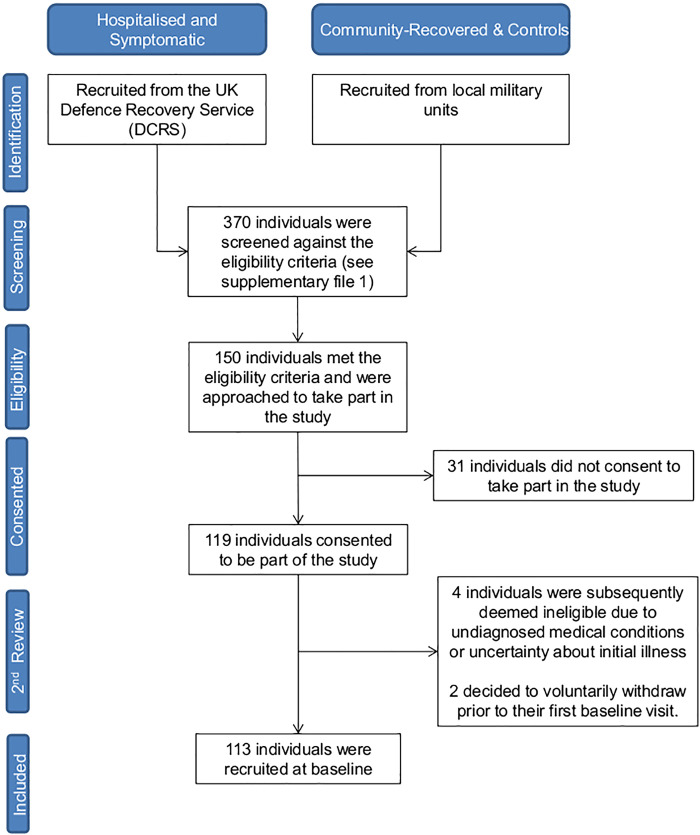
One hundred thirteen participants were categorized into one of five groups: severe initial disease requiring hospitalization with persistent symptoms (hospitalized-symptomatic, H-S, n = 27); severe initial disease requiring hospitalization and now recovered (hospitalized-recovered, H-R, n = 8); mild-moderate initial disease community managed with persistent symptoms (community-symptomatic, C-S, n = 34); mild-moderate initial disease community managed and now recovered (community-recovered, C-R, n = 18), and control (CON, n = 26). A detailed study eligibility criteria are provided in Supplemental Data S1 (https://doi.org/10.6084/m9.figshare.19704271). Exposed participants were recruited via a specially commissioned clinical pathway (28). Recovered and control participants were recruited from appropriate military units. Two senior consultants clinically adjudicated participant involvement based on positive SARS-CoV-2 antigen testing, history, blood tests, and imaging. Written informed consent was received by all participants before commencing data collection. Hospitalized and symptomatic participants were recruited consecutively from the DCRS, whereas community-recovered and controls were recruited from local military units (Fig. 1). Physical activity levels and behavior are not recorded in this study. However, to meet the occupational standards expected in the British Armed Forces, UK military personnel are required to pass an annual fitness test known as physical employment standards (PES). Personnel are assessed against components of muscle strength, power, and endurance to ensure a baseline level of operational readiness is achieved at all times (29). Expected levels of CRF are therefore supranormal in comparison to the general population at large.
Figure 1.
CONSORT flow diagram of recruitment process in this study.
Determining Recovery Status
Nonrecovery was defined as the continued presence of one or more post-COVID-19 symptoms at recruitment (see Supplemental Table S2: https://doi.org/10.6084/m9.figshare.19704325). Table 1 describes the most prevalent symptoms reported between each group.
Table 1.
Prevalence of primary symptoms across all groups
| Symptom | H-S | H-R | C-S | C-R | CON |
|---|---|---|---|---|---|
| Numbers | 27 | 8 | 34 | 18 | 26 |
| Any shortness of breath | 81% | 0% | 71% | 0% | 0% |
| Fatigue | 70% | 0% | 68% | 0% | 4% |
| Chest pain | 26% | 0% | 35% | 0% | 0% |
| Exercise intolerance | 26% | 0% | 35% | 0% | 0% |
| Joint pain | 33% | 0% | 15% | 0% | 0% |
| Loss of smell | 11% | 0% | 21% | 0% | 0% |
CON, control population; C-R, community-recovered; C-S, community-symptomatic; H-R, hospitalized-recovered; H-S, hospitalized-symptomatic.
Cardiopulmonary Functional Testing
Spirometry test.
Standing spirometry assessments (MicroMedical MicroLab 3500, CA) were performed to measure forced vital capacity (FVC) and forced expiratory volume in the first second of expiration (FEV1) (30).
Cardiopulmonary exercise testing.
CPET was conducted in a temperature-controlled exercise laboratory on an electromagnetically braked cycle ergometer (Lode Carnival, Lobe BV, Groningen, the Netherlands) using indirect calorimetry (Metalyzer 3B, Cortex Biophysik, Leipzig, Germany) with continuous 12-lead ECG monitoring (Custo Diagnostic software, Custo-Med, Ottoburn, Germany). Ventilation (V̇e), oxygen consumption (V̇o2), expired carbon dioxide (V̇co2), and HR were monitored continuously. Blood pressure (BP) and peripheral oxyhemoglobin saturations () were recorded every 2 min using an IPM 8 Mindray Patient Monitor (Mindray UK Ltd, Huntingdon, UK). A ramp protocol to volitional fatigue was used. A maximal test was defined by a respiratory exchange ratio (RER) of >1.1 and a plateau in V̇o2 over 30-s despite the increasing workload. The protocol started with a 2-min rest period, then 2 min of unloaded pedaling, followed by a progressive increase in workload based on a workload/min ramp to achieve 8 to 12 min of loaded exercise.
Data Management and Statistical Methods
Study data were collected and managed using REDCap (31).
Statistical analysis.
Data are presented as means ± SD. The normality of all variables was assessed using Shapiro–Wilk tests and inspection of the frequency histogram distributions and Q-Q plots. Results showed approximate normal distribution across the majority of variables. Parametric tests were applied throughout.
To measure for differences in demographics, and cardiopulmonary function between the five groups, a one-way analysis of variance (ANOVA) was performed on all continuous data and a χ2 test on ordinal and categorical data. An α threshold of 0.05 was taken to indicate significance. Post hoc tests were carried out for any results where significant between-group differences were identified following an ANOVA. Bonferroni corrections were applied to allow for multiple post hoc comparisons.
RESULTS
The mean number of days from acute illness to CPET is 159 ± 72 days with no differences reported between groups (H-S, 141 ± 58 days; H-R, 159 ± 79 days; C-S, 166 ± 63 days; C-R, 127 ± 60 days; F = 1.017, P = 0.391). Hospitalized-symptomatic individuals had 2.5 ± 1.3 and community-symptomatic 2.4 ± 1.3 symptoms (Table 1). No groups differed in age to controls; however, the hospitalized groups were the oldest, with hospitalized-recovered individuals older than both community groups (Table 2). Both hospitalized groups had the least favorable body composition values (Table 2). The hospitalized and community-symptomatic groups had higher body mass index (BMI) (H-S, 31 ± 4 kg/m2; H-R, 31 ± 4 kg/m2, C-S, 29 ± 4 kg/m2; C-R, 26 ± 2 kg/m2; CON, 25 ± 3 kg/m2) and waist circumference (H-S, 101 ± 13 cm; H-R, 101 ± 11 cm; C-S, 96 ± 13 cm; C-R, 85 ± 10 cm; CON 86 ± 7 cm) compared with community-recovered and controls. Body mass was also greater in the two hospitalized and community-symptomatic groups compared with controls. There were no differences in any body composition value between hospitalized-symptomatic, hospitalized-recovered and community-symptomatic, or between community-recovered and controls.
Table 2.
Participant information
| H-SA | H-RB | C-SC | C-RD | CONE | F Value | P Value | A vs. D | A vs. E | B vs. C | B vs. D | B vs. E | C vs D | C vs. E | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | 27 | 8 | 34 | 18 | 26 | |||||||||
| Sex | ||||||||||||||
| Male, n (%) | 23 (85%) | 8 (100%) | 28 (82%) | 15 (83%) | 22 (85%) | |||||||||
| Female, n (%) | 4 (15%) | 0 (0%) | 6 (18%) | 3 (17%) | 4 (15%) | |||||||||
| Age | 41 ± 8 | 48 ± 8 | 37 ± 10 | 34 ± 6 | 38 ± 8 | 4.437 | 0.002 | * | * | |||||
| Body composition | ||||||||||||||
| Height, cm | 176 ± 6 | 177 ± 9 | 179 ± 10 | 180 ± 8 | 176 ± 8 | 0.873 | 0.483 | |||||||
| Body mass, kg | 97 ± 14 | 96 ± 17 | 94 ± 19 | 83 ± 11 | 79 ± 8 | 7.493 | <0.001 | * | *** | * | *** | |||
| Body mass index, kg/m² | 31 ± 4 | 31 ± 4 | 29 ± 4 | 26 ± 2 | 25 ± 3 | 13.350 | <0.001 | *** | *** | ** | ** | ** | *** | |
| Waist circumference, cm | 101 ± 13 | 101 ± 11 | 96 ± 13 | 85 ± 10 | 86 ± 7 | 10.346 | <0.001 | *** | *** | * | ** | * | ** | |
| Hip circumference, cm | 106 ± 10 | 106 ± 9 | 103 ± 14 | 93 ± 12 | 94 ± 6 | 7.305 | <0.001 | ** | ** | * | * | |||
| Waist-to-hip ratio | 0.96 ± 0.11 | 0.96 ± 0.04 | 0.94 ± 0.12 | 0.92 ± 0.09 | 0.91 ± 0.07 | 0.946 | 0.440 | |||||||
| Service | ||||||||||||||
| Army, n, % | 22 (81%) | 4 (50%) | 16 (47%) | 10 (56%) | 14 (54%) | |||||||||
| Royal Navy, n, % | 2 (7%) | 0 (0%) | 8 (24%) | 4 (22%) | 0 (0%) | |||||||||
| RAF, n, % | 3 (11%) | 4 (50%) | 10 (29%) | 4 (22%) | 12 (46%) | |||||||||
| Rank | ||||||||||||||
| JNCO, n, % | 8 (30%) | 2 (25%) | 12 (35%) | 4 (22%) | 2 (8%) | |||||||||
| SNCO, n, % | 11 (41%) | 4 (50%) | 12 (35%) | 3 (17%) | 12 (46%) | |||||||||
| Officer, n, % | 8 (30% | 2 (25%) | 10 (30%) | 11 (61%) | 12 (46%) | |||||||||
| Role | ||||||||||||||
| GCC | 6 (22%) | 2 (25%) | 5 (15%) | 3 (17%) | 5 (19%) | |||||||||
| Non-GCC | 21 (78%) | 6 (75%) | 30 (85%) | 15 (83%) | 21 (81%) |
GCC roles typically demand a higher level of physical fitness to successfully deliver main duties and require personnel to pass higher physical employment standards (PES), examples of GCC roles include; infantry and armored corps. PES are less exacting for individuals employed in non-GCC roles, which encompass the remaining occupational roles within the British Armed Forces. There were no significant differences between C-R and CON for any parameter. CON, control; C-R, community-recovered; C-S, community-symptomatic; GCC, ground close combat roles; H-R, hospitalized-recovered; H-S, hospitalized-symptomatic; JNCO, junior noncommissioned officer; non-GCC, non-ground close combat roles; RAF, Royal Air Force; SNCO, senior noncommissioned officer. Level of significance: *P < 0.05, **P < 0.01, ***P < 0.001.
A detailed description of all variables captured during CPET can be found in Supplemental Table S3 (https://doi.org/10.6084/m9.figshare.19704367). The main results are presented in Table 3.
Table 3.
Cardiopulmonary function parameters
| Variable | H-SA | H-RB | C-SC | C-RD | CON E | F Score | P Value | A vs. D | A vs. E | B vs. D | B vs. E | C vs D | C vs. E |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPET | |||||||||||||
| V̇o2 at rest, mL/kg/min | 4.9 ± 1.0 | 4.8 ± 0.7 | 4.9 ± 1.0 | 5.5 ± 1.2 | 5.5 ± 1.8 | 1.930 | 0.111 | ||||||
| V̇o2 at VT1, mL/kg/min | 12.1 ± 1.7 | 12.9 ± 2.5 | 14.5 ± 3.9 | 17.2 ± 3.0 | 18.2 ± 5.6 | 10.995 | <0.001 | *** | *** | ** | ** | ||
| V̇o2 at peak (mL/kg/min) | 29.9 ± 5.0 | 32.6 ± 6.6 | 34.4 ± 7.2 | 44.3 ± 7.4 | 43.9 ± 13.1 | 13.448 | <0.001 | *** | *** | * | * | ** | *** |
| V̇o2 at VT1 (% peak predicted) | 43 ± 5 | 46 ± 10 | 47 ± 13 | 47 ± 7 | 56 ± 17 | 4.628 | 0.002 | ** | * | ||||
| V̇o2 at peak (% predicted) | 105 ± 13 | 116 ± 24 | 111 ± 19 | 122 ± 19 | 133 ± 25 | 7.701 | <0.001 | * | *** | ** | |||
| Work rate, W/kg, at VT1 | 0.70 ± 0.16 | 0.87 ± 0.18 | 0.92 ± 0.36 | 1.20 ± 0.29 | 1.38 ± 0.38 | 19.566 | <0.001 | *** | *** | ** | * | *** | |
| Work rate, W/kg, at peak | 2.40 ± 0.45 | 2.56 ± 0.55 | 2.77 ± 0.68 | 3.73 ± 0.67 | 3.89 ± 0.82 | 24.076 | <0.001 | *** | *** | ** | *** | *** | *** |
| Δ V̇o2, L/min/Δ work, W | 10.8 ± 1.0 | 11.3 ± 0.94 | 11.2 ± 2.2 | 11.2 ± 0.9 | 11.5 ± 0.7 | 1.855 | 0.124 | ||||||
| Lactate at rest, mmol/L | 1.2 ± 0.4 | 1.67 ± 0.5 | 1.3 ± 0.5 | 1.4 ± 0.4 | 1.2 ± 0.4 | 2.019 | 0.097 | ||||||
| Lactate at peak, mmol/L | 11.5 ± 2.3 | 14.0 ± 2.5 | 13.1 ± 2.3 | 14.1 ± 2.4 | 14.2 ± 1.5 | 6.119 | <0.001 | ** | *** | ||||
| O2 pulse at peak | 16.2 ± 4.0 | 18.4 ± 2.0 | 18.5 ± 4.7 | 21.1 ± 3.7 | 21.1 ± 4.7 | 5.785 | <0.001 | ** | *** | ||||
| O2 pulse (% predicted at peak) | 95 ± 21 | 104 ± 15 | 105 ± 20 | 119 ± 17 | 126 ± 22 | 9.377 | <0.001 | ** | *** | ** | |||
| RPE at peak (scored 6–20) | 18 ± 2 | 17 ± 2 | 17 ± 2 | 18 ± 2 | 17 ± 2 | 0.975 | 0.425 | ||||||
| SoB at peak (rated 0–10) | 7 ± 2 | 7 ± 3 | 6 ± 3 | 7 ± 2 | 7 ± 2 | 1.004 | 0.409 | ||||||
| Heart rate profile | |||||||||||||
| HR at rest, beats/min | 81 ± 11 | 82 ± 12 | 84 ± 13 | 77 ± 15 | 73 ± 8 | 3.566 | 0.009 | ** | |||||
| HR at peak, beats/min | 173 ± 15 | 169 ± 15 | 175 ± 16 | 178 ± 7 | 175 ± 8 | 0.792 | 0.533 | ||||||
| Ventilation | |||||||||||||
| V̇e/V̇co2 at rest | 30.7 ± 4.8 | 31.2 ± 5.2 | 30.5 ± 5.3 | 28.1 ± 2.0 | 28.0 ± 3.1 | 2.591 | 0.041 | ||||||
| V̇e/V̇co2 at VT1 | 28.1 ± 3.9 | 26.7 ± 4.6 | 26.7 ± 4.0 | 24.1 ± 1.7 | 24.3 ± 2.0 | 6.124 | <0.001 | ** | ** | ||||
| V̇e/V̇co2 at peak | 34.7 ± 5.6 | 33.1 ± 5.3 | 33.2 ± 4.0 | 30.5 ± 3.1 | 31.3 ± 3.3 | 3.525 | 0.010 | * | * | ||||
| V̇e/V̇co2 slope | 30.5 ± 5.3 | 26.5 ± 3.1 | 27.9 ± 5.3 | 24.1 ± 6.0 | 25.5 ± 2.6 | 6.012 | <0.001 | *** | ** | ||||
| OUES | 3.00 ± 0.57 | 3.16 ± 0.63 | 3.34 ± 0.96 | 4.88 ± 1.11 | 3.79 ± 0.93 | 4.135 | 0.004 | * | ** | ||||
| RER at peak | 1.18 ± 0.05 | 1.20 ± 0.05 | 1.18 ± 0.07 | 1.20 ± 0.06 | 1.22 ± 0.07 | 1.879 | 0.119 |
Values are means ± SD. The oxygen uptake efficiency slope (OUES) is a regression-derived parameter. It is the gradient of the slope (δy/δx) of the relationship between oxygen uptake (V̇o2) (y-axis) and log-transformed minute ventilation (V̇e) (x-axis) from 1 min after the onset of exercise until the second ventilatory threshold (VT2; also known as the respiratory compensation point). There were no significant differences between H-S vs. C-S, H-R vs. C-S, and C-R vs. CON for any CPET parameter. BF, breathing frequency; BP, blood pressure; CON, control population; CPET, cardiopulmonary exercise testing; C-R, community-recovered; C-S, community-symptomatic; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; H-R, hospitalized-recovered; HR, heart rate; HRR, heart rate recovery; H-S, hospitalized-symptomatic; OUES, oxygen uptake efficiency slope; RER, respiratory exchange ratio; RPE, rate of perceived exertion; SoB, shortness of breath; VT1, 1st ventilatory threshold. Level of significance: *P < 0.05, **P < 0.01, ***P < 0.001.
Cardiopulmonary Functional Status between Symptomatic Participants versus Healthy Controls
Post hoc analysis revealed no differences between symptomatic groups (hospitalized-symptomatic vs. community-symptomatic) in any CPET or resting spirometry parameter (Table 3).
Oxygen uptake.
Hospitalized-symptomatic and community-symptomatic individuals have a lower oxygen uptake (V̇o2) at VT1 (earlier anaerobic transition) (H-S, 12.1 ± 1.7 mL/kg/min; C-S, 14.5 ± 3.9 mL/kg/min; vs. CON, 18.2 ± 5.6 mL/kg/min, P < 0.001 and P = 0.002, respectively) and at peak exercise (H-S, 29.9 ± 5.0 mL/kg/min; C-S, 34.4 ± 7.2 mL/kg/min; vs. CON 43.9 ± 3.1 mL/kg/min, both P < 0.001). These symptomatic groups also had a reduced mean percent predicted V̇o2 at VT1 compared with controls (H-S, 43 ± 5%; C-S, 47 ± 13%; vs. CON, 56 ± 17%; P = 0.001 and P = 0.034, respectively) and at peak exercise compared with controls (H-S, 105 ± 13%, C-S, 111 ± 19%; vs. CON, 133 ± 25%, P < 0.001 and P = 0.001, respectively) (Fig. 2). Two participants from each symptomatic group recorded a predicted peak V̇o2 value <85%. This is the arbitrary clinical standard used to define functional limitation in a civilian population.
Figure 2.
Cardiopulmonary exercise performance. A: percent predicted V̇o2 at VT1 and peak. B: relative power output at VT1 and peak. C: V̇e/V̇co2 slope. Level of significance: *P < 0.05, **P < 0.01, ***P < 0.001. CON, control population; C-R, community-recovered; C-S, community-symptomatic; H-R, hospitalized-recovered; H-S, hospitalized-symptomatic.
Work rate.
Work rate at VT1 and peak were lower by 39% and 25%, respectively, for hospitalized-symptomatic compared with control (both P < 0.001). Work rate at VT1 and peak were also less in community-symptomatic compared with control by 22% and 16%, respectively (P = 0.013 and P = 0.010). The differences between symptomatic groups and controls in work rate produced at VT1 and peak exercise are, predictably, even greater when presented relative to their body mass (W/kg) [H-S, 49% and 38% (reduction in VT1 and peak values, respectively, vs. controls); C-S, 30% and 29% (reduction in VT1 and peak values, respectively, vs. controls), all P < 0.001], respectively (Fig. 2). No differences in the rate of perceived exertion (RPE) or respiratory exchange ratio (RER) at peak exercise were demonstrated between groups (P > 0.05).
Heart rate profile.
The only difference in HR profile measurements was a higher (11 beats/min) resting HR between community-symptomatic and control (84 ± 13 vs. 73 ± 8 beats/min, P = 0.007). No differences in HR profiles were identified between hospitalized-symptomatic and control (P > 0.05).
Blood pressure profile.
Hospitalized-symptomatic individuals had a higher mean resting diastolic BP compared with control (86 ± 6 vs. 79 ± 6 mmHg, P = 0.011). No other between-group differences in BP profiles were identified.
Ventilation and spirometry.
There were no differences between community-symptomatic individuals and controls in any ventilatory or spirometry parameter (P > 0.05) (Table 3). However, hospitalized-symptomatic individuals had lower % predicted FVC (96 ± 13 vs. 108 ± 10, P = 0.004), ventilatory efficiency (higher V̇e /V̇co2) at VT1 (28.1 ± 3.9 vs. 24.3 ± 2.0, P = 0.001), peak exercise (34.7 ± 5.6 vs. 31.3 ± 3.3, P = 0.045), and slope (30.5 ± 5.3 vs. 25.5 ± 2.6, P = 0.003) compared with controls (Fig. 2). Hospitalized-symptomatic individuals also had a lower oxygen uptake efficiency slope (OUES, 3.00 ± 0.57 vs. 3.79 ± 0.93, P = 0.005) than controls.
Cardiopulmonary Functional Status between Recovered Participants versus Healthy Controls
Post hoc analysis revealed no differences between community-recovered and control groups in any CPET or spirometry value (Table 3). However, there were differences between hospitalized-recovered and controls.
Oxygen uptake.
Hospitalized-recovered have a lower oxygen uptake (V̇o2) at VT1 (12.9 ± 2.5 vs. 18.2 ± 5.6 mL/kg/min, P = 0.007) and V̇o2 at peak exercise (32.6 ± 6.6 vs. 43.9 ± 13.1 mL/kg/min, P = 0.015) compared with controls (Table 3). There were no between-group differences for mean percent of predicted (% predicted) V̇o2 at VT1 (H-R, 46 ± 10%; C-R, 47 ± 7%; vs. CON, 56 ± 17%, P = 0.391 and P = 0.165, respectively) V̇o2 at peak (H-R, 116 ± 24%; C-R, 122 ± 19%; vs. CON, 133 ± 25%, P = 0.430, P = 0.971, respectively) (Fig. 2). One participant from the hospitalized-recovered group met the clinical definition of functional limitation, with a predicted peak V̇o2 value <85%.
Work rate.
Work rate at VT1 and peak were lower by 26% and 21%, respectively, in hospital-recovered versus controls, however, only differences in peak work rate were statistically different (P = 0.046). The difference between hospitalized-recovered and controls in work rate produced at VT1 and peak is increased when presented relative to their body mass (W/kg) (H-R, 37%, P = 0.001; and 34%, P < 0.001), reflecting the adverse body habitus in the hospitalized groups. There were no differences between community-recovered participants and controls in work rate (at VT1 and peak) and no differences in RER at the peak were demonstrated between groups (P > 0.05).
Heart rate, blood pressure, ventilation, and spirometry.
No differences were reported in HR profiles, BP, ventilation, or spirometry parameters between recovered groups and controls (P > 0.05).
Other Results of Interest
Post hoc analysis revealed no differences between hospitalized-symptomatic and hospitalized-recovered (P > 0.05), or hospitalized-recovered and community-symptomatic groups (P > 0.05), in any CPET variable. Within the community-managed groups, community-recovered individuals had a higher mean oxygen uptake (V̇o2) at peak (44.3 ± 7.4 vs. 34.4 ± 7.2 mL/kg/min, P = 0.001), work rate at peak (308 ± 60 vs. 255 ± 61 W, P = 0.014), relative work rate at VT1 and at peak (1.20 ± 0.29 vs. 0.92 ± 0.36 W/kg, P = 0.020; and 3.73 ± 0.67 vs. 2.77 ± 0.68, P < 0.001; 35% higher) compared with community-symptomatic.
DISCUSSION
This study compared the medium-term cardiopulmonary exercise response of individuals who feel “recovered” and individuals with “prolonged symptoms” following either hospitalized or community-managed COVID-19 illness with an age, sex, and job-role matched control group. The study found that hospitalized and symptomatic participants have lower levels of exercise capacity 5 mo following the onset of illness, yet community-recovered participants have “supranormal” values that are comparable to controls. The implications of these findings form the basis of the discussion.
Symptomatic Participants versus Controls
No differences in cardiopulmonary function were reported between the two symptomatic groups. This suggests that the severity of initial acute illness (severe hospitalized vs. mild-moderate community managed) is not a useful predictor of objective medium-term cardiopulmonary performance.
The main differences between the two symptomatic participant groups (hospitalized and community) and controls were in oxygen uptake (earlier anaerobic transition, lower peak V̇o2, and lower % predicted peak V̇o2) and work-rate profiles (lower absolute and relative work rates at VT1 and peak exercise). Given how the respiratory, cardiac and skeletal muscular systems can be negatively impacted by COVID-19, it is not surprising to see a diminished peak V̇o2 and work rate response in symptomatic individuals. Importantly, hospitalized-symptomatic individuals also had a higher (less efficient) V̇e / V̇co2 slope (30.5 ± 5.3 vs. 25.5 ± 2.6) compared with controls. There are two contrasting mechanisms that might explain this finding: 1) an elevated V̇e/V̇co2 slope (>30) may suggest a ventilation-perfusion (VQ) abnormality (32) or 2) inappropriate ventilation in excess of the body’s physiological gas-exchange requirements (hyperventilation). In our group, hospitalized-symptomatic individuals did not have a significant lowering of peak capillary PCO2 or elevated breathing frequency compared with controls at rest, VT1, or peak exercise. Furthermore, there were no differences in end-tidal CO2 at rest or peak. This would suggest that a VQ abnormality, rather than inappropriate hyperventilation resulting from a resetting of the peripheral chemosensitivity to CO2, explains the elevated V̇e/V̇co2 slope. VQ mismatch could be explained by pulmonary vasculopathy (33) and would be supported by mild reductions of DLCO, as described by other investigators (34–36). At 3 mo after discharge from hospitalization for COVID-19, hyperpolarized xenon (129Xe) MRI has identified abnormalities due to alveolar capillary diffusion limitation in patients with dyspnea. Such findings would be in keeping with VQ mismatch (37). However, this is at odds with the findings of Baratto et al. (38). In a cohort of 18 patients studied at the time of hospital discharge, they concluded that the elevation in V̇e/V̇co2 values was “mainly explained by increased chemosensitivity.” Lower oxygen uptakes (V̇o2) at peak exercise and higher V̇e/V̇co2 slopes have also previously been reported in individuals with severe (hospitalized) COVID-19 (10, 11, 14, 39). Our findings are largely consistent with Raman et al. (14) who found that % predicted peak V̇o2 was lower (80.5 ± 23.1% vs. 112.7 ± 27.0%) and the V̇e/V̇co2 slope higher [mean 33.4 (SD, 29.2–40.3) vs. 28.2 (26.7–30.0)] in individuals who were hospitalized with COVID-19 versus healthy controls, respectively. Unlike this study, our hospitalized participants (at ∼5 mo postillness) have a comparatively “normal” % predicted peak V̇o2 (H-S,105 ± 13%; and H-R 116 ± 24%) and more efficient V̇e/V̇co2 slopes (H-S, 30.5 ± 5.3; and H-R, 26.5 ± 3.1), likely related to military-specific physical fitness employment standards.
Despite “normal” values of % predicted V̇o2 at VT1 in the symptomatic groups, a reduced value compared with controls is clinically significant because this represents the upper limit of workloads during exercise that can be sustained over a prolonged period of time. Most activities of daily living do not require maximal effort; therefore, a widely used submaximal index of exercise capacity is the anaerobic, or first ventilatory, threshold (VT1) (32). In endurance sports, it is understood that the anaerobic threshold is a better indicator of endurance capacity than peak V̇o2 (40, 41). Anaerobic thresholds may provide a more sensitive indicator of changes in CRF status. The functional outcome for the hospitalized-symptomatic group is a lower power output that can be sustained for any significant period. This would result in a reduced capacity to perform other tasks and activities of daily living. When taking on the same tasks as would have been readily sustainable premorbidly, the effect would be early onset of fatigue. This is most likely to reflect impairment in whole body oxidative metabolism. Although the peak lactate in symptomatic hospitalized patients was slightly lower than in community-recovered patients and controls, the magnitude of this difference was much lower than the decrement in peak power (both absolute and, even more so, power controlled for bodyweight). In spite of the lower workloads achieved by symptomatic patients, the subjective perception of effort and breathlessness at peak exertion were the same. These contrasting differences may result in part from changes in the perception of effort. However, the relative decrease in “efficiency” of power/lactate would also be consistent with the impairment of oxidative pathways. Peripheral factors, including reduced oxygen extraction by peripheral muscles, have been reported as major determinants of exercise intolerance following COVID-19 (38, 42). Impaired mitochondrial function may occur during both presymptomatic and acute COVID-19 infection in skeletal muscle (43) and peripheral blood mononuclear cells (44). In theory, alterations in mitochondrial function may influence the metabolic state and redox balance of numerous organs and tissues, and subsequently could play an important role in post-COVID-19 syndromes (45, 46).
Exercise limiting factors can be related to abnormalities of ventilation, circulation, or oxygen use in peripheral tissues oxygen (11). Decreased exercise capacity can, of course, also result from deconditioning from a variety of factors. Inactivity due to persistent symptoms (high prevalence of marked fatigue and dyspnea reported in symptomatic groups) and immobilization during hospitalization may have contributed to deconditioning in the symptomatic groups. Mancini et al. (47) performed CPET on 41 patients with unexplained dyspnea 9 ± 3 mo following the onset of COVID illness (22% hospitalized, 78% community managed) and assessed this cohort against criteria to diagnose myalgia encephalomyelitis/chronic fatigue syndrome (ME/CFS). Interestingly, a high prevalence (46%) of patients with postacute sequelae SARS-CoV-2 infection (PASC) fulfilled the criteria for ME/CFS. Although this finding is consistent with outcomes reported following the SARS COVID-1 outbreak (48), Naeije and Caravita (49) in their study question whether symptoms of ME/CFS (which they describe as patient advocacy-derived entities) are appropriate comparators for CPET phenotyping of PASC or long-COVID, especially when 76% of the cohort had preexisting comorbidities. Instead, Naeije and Caravita (49) in their study suggest the circulatory and ventilatory limitations reported in a study by Mancini et al. (47) (59% of patients had a peak V̇o2 <80% of predicted; 88% had ventilatory abnormalities consistent with dysfunctional breathing, resting hypocapnia, and/or elevated V̇e/V̇co2 slope) are more consistent with the findings from an analysis that grouped 581 patients with long-COVID from 11 studies (50). This analysis found that following recovery from an acute inflammatory process and prolonged bed rest, CPET profiles appear consistent with deconditioning with a tendency for hyperventilation (50). The hospitalized-recovered group would also have been exposed to a period of immobilization and physical inactivity during their hospital admission. The effect of their severe acute illness and recovery status on exercise capacity is discussed next.
Recovered Participants Versus Controls
Hospitalized-recovered participants demonstrate an earlier anaerobic transition, lower values for V̇o2 at peak exercise, lower work rate at VT1 and peak exercise, and lower % predicted peak V̇o2 compared with controls. This suggests that participants who were hospitalized but now feel recovered following their illness might not present with circulatory or respiratory limitations but nevertheless remain functionally compromised/deconditioned compared with a physically active control population at ∼5 mo. Although lower-limb muscle strength and size were not quantified in this study, decreases in the amount of external loading and/or neural activation of a muscle will lead to a loss of muscle mass (51). Disuse of skeletal muscle (often associated with prolonged bed rest or physical inactivity, as experienced by the hospitalized and persistent symptom groups) can lead to relatively rapid and progressive skeletal muscle atrophy, shortening of muscle fibers, decreased oxidative capacity, and reduced muscle compliance (52). All can influence strength, power, and somatotype resulting in reduced exercise capacity and performance (53). It is also widely recognized that skeletal muscle atrophy can prolong the duration of rehabilitation, negatively impact the ability to perform daily tasks, and prevent optimal recovery (54). It is not possible, from our study, to comment on the extent to which the functional limitation and persistently reduced cardiopulmonary exercise parameters in the hospitalized recovered group are the result of deconditioning, or direct pathological impact of the virus on mitochondrial function. Ultimately, both of these mechanisms, or a combination, may explain the findings. From a pragmatic, clinical perspective, caution must still be applied regardless of recovery status in previously hospitalized individuals when making decisions on returning to full activity (i.e., unlimited exposure to physically active/high hazardous job roles). To address the multifaceted nature of post-COVID19 syndrome, longer periods of symptom-guided rehabilitation and recovery may be warranted in this population (55).
Community-recovered participants demonstrate no difference in any ventilatory variable, HR profile, or resting spirometry value compared with controls at ∼5 mo following acute illness (see Supplemental Table S3). Singh et al. (42) in their study previously reported reduced V̇o2 max with increased V̇e/V̇co2 slopes during CPET in “recovered individuals”—however, these participants were recruited from an unexplained exercise intolerance clinic and therefore would still be classified as symptomatic by our study’s definition. Our findings should reassure the majority of recovered individuals with mild-moderate disease, as well as the clinicians responsible for their care. They will also facilitate the dedication of resources to those who have a continuing rehabilitation requirement, which may include interventions to improve body composition.
Impact of Body Composition
Hospitalized and community-symptomatic groups have greater body mass and BMI values compared with controls, consistent with increased BMI as a risk factor for COVID-19 severity (3, 56, 57). Increased waist circumference values suggest that this increased body mass can be attributed to a larger amount of abdominal fat (increased visceral adipose tissue). Of note, high CRF is associated with lower abdominal adipose tissue and is independent of BMI (58). The impact of body composition on CRF is obvious when work rate at VT1 and peak exercise are presented relative to body mass: relative work rate (W/kg). Relative work rate values are comparable between community-recovered individuals and physically active controls suggesting a protective effect of favorable body composition values on derivatives of peak power output (work rate). In the groups with less favorable body composition values (H-S, H-R, and C-S), relative work rates at VT1 and peak exercise were 30%–49% and 29%–38% lower than controls, respectively.
Strengths and Limitations
This is, to our knowledge, the first study that has compared cardiopulmonary function, across the spectrum of acute COVID-19 severity and persistence of symptoms, with an age, sex, and job-role matched control group. A major strength of this study is the use of an appropriate control group with which to identify limitations objectively and quantify exercise response, especially in the assessment of long-term sequelae (59). A nonrecovered status was defined by the presence of symptoms at the time of recruitment (∼5 mo postillness). The most common symptoms presented (Table 1) are reflective of those reported in other studies, which support the generalizability of other findings (such as objective cardiorespiratory fitness), which have not been reported in other case-controlled cohort studies (57, 60–62).
These are cross-sectional data, which do not determine whether patients are improving or regressing with regard to their subjective symptoms and objective cardiopulmonary functional profiles. However, the use of a control group enables a comparison to be made to a physically active population. To determine if limitations resolve over time, additional follow-up testing is required. Although the sample size (n = 113) is modest, it remains comparatively large when combined with other cohort studies using physically active participants (63). The impact of COVID-19 on sportspeople and others in physically demanding occupations is underreported and remains a research priority (64). We would not expect trained military personnel to have the same CRF levels as the more sedentary referenced population (65), we therefore recognize that not all of the findings from this previously active military cohort can be extrapolated to the wider general population. The data provided reflect the medium-term cardiopulmonary fitness status of individuals in physically active job roles following acute COVID-19 illness. From an occupational re-employability perspective, it is important to recognize that persistent symptoms following COVID-19 infection are multifaceted and may also cause unfavorable long-term changes to neurocognitive function, mental health, and organ pathology (13). When considering the return of frontline emergency personnel to physically demanding high-hazard roles, further consideration of these additional side effects should be taken into account.
Conclusions
This study found that individuals with more severe acute disease, and/or persistent symptoms had reduced CRF compared with a “healthy” age, sex, and job-role matched control population. There were no differences between hospitalized-recovered and community-symptomatic participants, so a similar degree of caution must be applied to individuals who were hospitalized and those who remain persistently symptomatic when considering a returning to exercise. Return to work guidance for previously hospitalized patients (regardless of recovery status) and/or symptomatic individuals need not be a binary decision for employers who oversee the management of personnel in physically active/high hazardous job roles. Greater consideration of the physical nature of an individual’s job role and graded exposure to their primary duties are likely to support a safer, and earlier, return to work.
Reassuringly, this study found that community-treated individuals, who have recovered symptomatically, do not differ from controls in cardiorespiratory fitness. This is a significant finding for the vast majority of physically active individuals who recover quickly following COVID-19 infection. Given the potentially high numbers of individuals with post-COVID-19 syndrome, a pragmatic approach is appropriate. Our findings suggest that for individuals who will be exposed to high volume/intensity physical exercise, who experience persistent symptoms, or were hospitalized during acute illness (regardless of recovery), continued monitoring of cardiopulmonary function and appropriate rehabilitation and recovery is warranted beyond 5 mo.
DATA AVAILABILITY
Data relate to the serving population of the Ministry of Defense and thus are sensitive. Research teams requesting data are invited to contact the corresponding author and appropriate permissions will be sought for release.
SUPPLEMENTAL DATA
Supplemental Data S1: https://doi.org/10.6084/m9.figshare.19704271;
Supplemental Table S2: https://doi.org/10.6084/m9.figshare.19704325;
Supplemental Table S3: https://doi.org/10.6084/m9.figshare.19704367.
GRANTS
The study was supported by a grant from the Defense Medical Services Research Steering Group.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.L., O.O., A.N.B., R.B.-D., E.D.N., and D.A.H. conceived and designed research; P.L., O.O., R.B.-D., R.C., S.M., D.M., D.D., K.R.-S., C.W., and J.T.. performed experiments; A.H. analyzed data; P.L., O.O., R.B.-D., A.H., J.M., J.N., and D.A.H. interpreted results of experiments; A.H. prepared figures; P.L., O.O., A.H., and D.A.H. drafted manuscript; P.L., A.N.B., R.B.-D., E.D.N., and D.A.H. edited and revised manuscript; P.L., O.O., A.N.B., R.B.-D., A.H., R.C., S.M., D.M., D.D., K.R.-S., C.W., J.T., J.M., J.N., E.D.N., and D.A.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the participants who volunteered for the Military COVID-19 Observational Outcome in a Viral Infectious Disease (M-COVID) study for determination and positive engagement during the cardiopulmonary exercise test. The authors also acknowledge the tireless efforts of all the research support staff at the UK Defense Medical Rehabilitation Centre (DMRC), Stanford Hall for the role in ensuring this study could be delivered during challenging circumstances.
REFERENCES
- 1.John Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu/ [2022 Mar 3].
- 2.Moghimi N, Di Napoli M, Biller J, Siegler JE, Shekhar R, McCullough LD, Harkins MS, Hong E, Alaouieh DA, Mansueto G, Divani AA. The neurological manifestations of post-acute sequelae of SARS-CoV-2 infection. Curr Neurol Neurosci Rep 21: 1–17, 2021. doi: 10.1007/s11910-021-01130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayoubkhani D, Pawelek P. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 3 February 2022 (Online). Off Natl Stat 1–6, 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/3february2022 [2022 Mar 1]. [Google Scholar]
- 4.Evans RA, McAuley H, Harrison EM, Shikotra A, Singapuri A, Sereno M, et al.; PHOSP-COVID Collaborative Group. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med 9: 1275–1287, 2021. doi: 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 324: 603–605, 2020. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Sullivan O, Barker-Davies R, Thompson K, Bahadur S, Gough M, Lewis S, Martin M, Segalini A, Wallace G, Phillip R, Cranley M. Rehabilitation post-COVID-19: cross-sectional observations using the Stanford Hall remote assessment tool. BMJ Mil Health, 2021. doi: 10.1136/bmjmilitary-2021-001856. [DOI] [PubMed] [Google Scholar]
- 7.Mandal S, Barnett J, Brill SE, Brown JS, Denneny EK, Hare SS, Heightman M, Hillman TE, Jacob J, Jarvis HC, Lipman MCI, Naidu SB, Nair A, Porter JC, Tomlinson GS, Hurst JR, ARC Study Group. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 76: 396–398, 2021. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ladlow P, O’Sullivan O, Houston A, Barker-Davies R, May S, Mills D, Dewson D, Chamley R, Naylor J, Mulae J, Bennett AN, Nicol ES, Holdsworth DA. Dysautonomia following COVID-19 is not associated with subjective limitations or symptoms, but is associated with objective functional limitations. Heart Rhythm 19: 613–620, 2021. doi: 10.1016/j.hrthm.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brawner CA, Ehrman JK, Bole S, Kerrigan DJ, Parikh SS, Lewis BK, Gindi RM, Keteyian C, Abdul-Nour K, Keteyian SJ. Inverse relationship of maximal exercise capacity to hospitalization secondary to coronavirus disease 2019. Mayo Clinic Proc 96: 32–39, 2021. doi: 10.1016/j.mayocp.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinaldo RF, Mondoni M, Parazzini EM, Pitari F, Brambilla E, Luraschi S, Balbi M, Papa GFS, Sotgiu G, Guazzi M, Di Marco F, Centanni S. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur Respir J. 58: 2100870, 2021. doi: 10.1183/13993003.00870-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skjørten I, Ankerstjerne OAW, Trebinjac D, Brønstad E, Rasch-Halvorsen Ø, Einvik G, Lerum TV, Stavem K, Edvardsen A, Ingul CB. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur Respir J 58: 2100996, 2021. doi: 10.1183/13993003.00996-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 5: 1265–1273, 2020. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, Li Y, Cao Y, Gu J, Wu H, Shi H. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology 299: E177–E86, 2021. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raman B, Cassar MP, Tunnicliffe EM, Filippini N, Griffanti L, Alfaro-Almagro F, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 31: 100683, 2021. doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debeaumont D, Boujibar F, Ferrand-Devouge E, Artaud-Macari E, Tamion F, Gravier F-E, Smondack P, Cuvelier A, Muir J-F, Alexandre K, Bonnevie T. Cardiopulmonary exercise testing to assess persistent symptoms at 6 months in people with COVID-19 who survived hospitalization: a pilot study. Phys Ther 101: pzab099, 2021. doi: 10.1093/ptj/pzab099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohr A, Dannerbeck L, Lange TJ, Pfeifer M, Blaas S, Salzberger B, Hitzenbichler F, Koch M. Cardiopulmonary exercise pattern in patients with persistent dyspnoea after recovery from COVID-19. Multidiscip Respir Med 16: 732, 2021. doi: 10.4081/mrm.2021.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva RN, Goulart CDL, Oliveira MR, Tacao GY, Back GD, Severin R, Faghy MA, Arena R, Borghi-Silva A. Cardiorespiratory and skeletal muscle damage due to COVID-19: making the urgent case for rehabilitation. Expert Rev Respir Med 15: 1–14, 2021. doi: 10.1080/17476348.2021.1893169. [DOI] [PubMed] [Google Scholar]
- 18.Laveneziana P, Di Paolo M, Palange P. The clinical value of cardiopulmonary exercise testing in the modern era. Eur Respir Rev 30: 200187, 2021. doi: 10.1183/16000617.0187-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dennis A, Wamil M, Alberts J, Oben J, Cuthbertson DJ, Wootton D, Crooks M, Gabbay M, Brady M, Hishmeh L, Attree E, Heightman M, Banerjee R, Banerjee A. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open 11: e048391, 2021. doi: 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross R, Blair SN, Arena R, Church TS, Després J-P, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisløff U, Stroke Council. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation 134: e653–e99, 2016. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 21.Palange P, Ward SA, Carlsen K-H, Casaburi R, Gallagher CG, Gosselink R, O'Donnell DE, Puente-Maestu L, Schols AM, Singh S, Whipp BJ, ERS Task Force. Recommendations on the use of exercise testing in clinical practice. Eur Respir J 29: 185–209, 2007. doi: 10.1183/09031936.00046906. [DOI] [PubMed] [Google Scholar]
- 22.Arena R, Faghy MA. Cardiopulmonary exercise testing as a vital sign in patients recovering from COVID-19. Expert Rev Cardiovasc Ther 19: 877–880, 2021. doi: 10.1080/14779072.2021.1985466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Y, Chen R, Geng Q, Mo X, Zhan C, Jian W, Li S, Zheng J. Cardiopulmonary exercise testing might be helpful for interpretation of impaired pulmonary function in recovered COVID-19 patients. Eur Respir J 57: 2004265, 2021. doi: 10.1183/13993003.04265-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caterisano A, Decker D, Snyder B, Feigenbaum M, Glass R, House P, Sharp C, Waller M, Witherspoon Z. CSCCa and NSCA joint consensus guidelines for transition periods: safe return to training following inactivity. Strength Condition J 41: 1–23, 2019. doi: 10.1519/SSC.0000000000000477. [DOI] [Google Scholar]
- 25.Gaber T. Assessment and management of post‐COVID fatigue. Prog Neurol Psychiatry 25: 36–39, 2021. doi: 10.1002/pnp.698. [DOI] [Google Scholar]
- 26.Stormorken E, Jason LA, Kirkevold M. Factors impacting the illness trajectory of post-infectious fatigue syndrome: a qualitative study of adults’ experiences. BMC Public Health 17: 952, 2017. doi: 10.1186/s12889-017-4968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy FM, Sharma S. COVID-19, the heart and returning to physical exercise. Occup Med;70: 467–469, 2020. doi: 10.1093/occmed/kqaa154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Sullivan O, Barker-Davies R, Chamley R, Sellon E, Jenkins D, Burley R, Holden L, Nicol AM, Phillip R, Bennett AN, Nicol E, Holdsworth DA. Defence Medical Rehabilitation Centre (DMRC) COVID-19 recovery service. BMJ Mil Health e001681, 2021. doi: 10.1136/bmjmilitary-2020-001681. [DOI] [PubMed] [Google Scholar]
- 29.Ladlow P, Conway D, Hayhurst D, Suffield C, Cassidy R, Coppack R. Integration of strength training into UK Defence Rehabilitation practice: current trends and future challenges. BMJ Mil Health. 2020. doi: 10.1136/bmjmilitary-2020-001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, Kaminsky DA, McCarthy K, McCormack MC, Oropez CE, Rosenfeld M, Stanojevic S, Swanney MP, Thompson BR. Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med 200: e70–e88, 2019. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377–381, 2009. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV; Interdisciplinary Council on Quality of Care and Outcomes Research. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 122: 191–225, 2010. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 33.Weatherald J, Farina S, Bruno N, Laveneziana P. Cardiopulmonary exercise testing in pulmonary hypertension. Ann Am Thorac Soc 14: S84–S92, 2017. doi: 10.1513/AnnalsATS.201610-788FR. [DOI] [PubMed] [Google Scholar]
- 34.Smet J, Stylemans D, Hanon S, Ilsen B, Verbanck S, Vanderhelst E. Clinical status and lung function 10 weeks after severe SARS-CoV-2 infection. Respir Med 176: 106276, 2021. doi: 10.1016/j.rmed.2020.106276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, Lei C, Chen R, Zhong N, Li S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eu Respir J 55: 2001217, 2020. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y-M, Shang Y-M, Song W-B, Li Q-Q, Xie H, Xu Q-F, Jia J-L, Li L-M, Mao H-L, Zhou X-M, Luo H, Gao Y-F, Xu A-G. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 25: 100463, 2020. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grist JT, Chen M, Collier GJ, Raman B, Abueid G, McIntyre A, Matthews V, Fraser E, Ho L-P, Wild JM, Gleeson F. Hyperpolarized 129Xe MRI abnormalities in dyspneic patients 3 months after COVID-19 pneumonia: preliminary results. Radiology 301: E353–E360, 2021. doi: 10.1148/radiol.2021210033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baratto C, Caravita S, Faini A, Perego GB, Senni M, Badano LP, Parati G. Impact of COVID-19 on exercise pathophysiology: a combined cardiopulmonary and echocardiographic exercise study. J Appl Physiol 130: 1470–1478, 2021. doi: 10.1152/japplphysiol.00710.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aparisi Á, Ybarra-Falcón C, García-Gómez M, Tobar J, Iglesias-Echeverría C, Jaurrieta-Largo S, Ladrón R, Uribarri A, Catalá P, Hinojosa W, Marcos-Mangas M, Fernández-Prieto L, Sedano-Gutiérrez R, Cusacovich I, Andaluz-Ojeda D, de Vega-Sánchez B, Recio-Platero A, Sanz-Patiño E, Calvo D, Baladrón C, Carrasco-Moraleja M, Disdier-Vicente C, Amat-Santos IJ, San Román JA. Exercise ventilatory inefficiency in post-COVID-19 syndrome: insights from a prospective evaluation. JCM 10: 2591, 2021. doi: 10.3390/jcm10122591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen WK, Seals DR, Hurley BF, Ehsani AA, Hagberg JM. Lactate threshold and distance-running performance in young and older endurance athletes. J Appl Physiol (1985) 58: 1281–1284, 1985. doi: 10.1152/jappl.1985.58.4.1281. [DOI] [PubMed] [Google Scholar]
- 41.Bishop D, Jenkins DG, Mackinnon LT. The relationship between plasma lactate parameters, Wpeak and 1-h cycling performance in women. Med Sci Sports Exerc 30: 1270–1275, 1998. doi: 10.1097/00005768-199808000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Singh I, Joseph P, Heerdt PM, Cullinan M, Lutchmansingh DD, Gulati M, Possick JD, Systrom DM, Waxman AB. Persistent exertional intolerance after COVID-19: insights from invasive cardiopulmonary exercise testing. Chest. 161: 54–63, 2022. doi: 10.1016/j.chest.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trinity JD, Craig JC, Fermoyle CC, McKenzie AI, Lewis MT, Park SH, Rondina MT, Richardson RS. Impact of presymptomatic COVID-19 on vascular and skeletal muscle function: a case study. J Appl Physiol (1985) 130: 1961–1970, 2021. doi: 10.1152/japplphysiol.00236.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ajaz S, McPhail MJ, Singh KK, Mujib S, Trovato FM, Napoli S, Agarwal K. Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19. Am J Physiol Cell Physiol 320: C57–C65, 2021. doi: 10.1152/ajpcell.00426.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saleh J, Peyssonnaux C, Singh KK, Edeas M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion 54: 1–7, 2020. doi: 10.1016/j.mito.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh KK, Chaubey G, Chen JY, Suravajhala P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am J Physiol Cell Physiol 319: C258–C267, 2020. doi: 10.1152/ajpcell.00224.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mancini DM, Brunjes DL, Lala A, Trivieri MG, Contreras JP, Natelson BH. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. Heart Failure 9: 927–937, 2021. doi: 10.1016/j.jchf.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol 11: 37–37, 2011. doi: 10.1186/1471-2377-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naeije R, Caravita S. CPET for long COVID-19. JACC Heart Fail 10: 214–215, 2022. doi: 10.1016/j.jchf.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naeije R, Caravita S. Phenotyping long COVID. Eur Respir J 58: 2101763, 2021. doi: 10.1183/13993003.01763-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atherton PJ, Greenhaff PL, Phillips SM, Bodine SC, Adams CM, Lang CH. Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence. Am J Physiol Endocrinol Metab 311: E594–E604, 2016. doi: 10.1152/ajpendo.00257.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slysz J, Stultz J, Burr JF. The efficacy of blood flow restricted exercise: A systematic review & meta-analysis. J Sci Med Sport 19: 669–675, 2016. doi: 10.1016/j.jsams.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Herda AA, Herda TJ, Costa PB, Ryan ED, Stout JR, Cramer JT. Muscle performance, size, and safety responses after eight weeks of resistance training and protein supplementation: a randomized, double-blinded, placebo-controlled clinical trial. The J Strength Cond Res 27: 3091–3100, 2013. doi: 10.1519/JSC.0b013e31828c289f. [DOI] [PubMed] [Google Scholar]
- 54.Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med 34: 329–348, 2004. doi: 10.2165/00007256-200434050-00005. [DOI] [PubMed] [Google Scholar]
- 55.Barker-Davies RM, O'Sullivan O, Senaratne KPP, Baker P, Cranley M, Dharm-Datta S, Ellis H, Goodall D, Gough M, Lewis S, Norman J, Papadopoulou T, Roscoe D, Sherwood D, Turner P, Walker T, Mistlin A, Phillip R, Nicol AM, Bennett AN, Bahadur S. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med 54: 949–959, 2020. doi: 10.1136/bjsports-2020-102596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, Liu L, Shan H, Lei C-L, Hui DSC, Du B, Li L-J, Zeng G, Yuen K-Y, Chen R-C, Tang C-L, Wang T, Chen P-Y, Xiang J, Li S-Y, Wang J-L, Liang Z-J, Peng Y-X, Wei L, Liu Y, Hu Y-H, Peng P, Wang J-M, Liu J-Y, Chen Z; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maxwell E. Living with Covid 19. A dynamic review of the evidence around ongoing Covid 19 symptoms (often called Long Covid). NIHR Centre for Engagement and Dissemination. 2020.
- 58.Wong SL, Katzmarzyk P, Nichaman MZ, Church TS, Blair SN, Ross R. Cardiorespiratory fitness is associated with lower abdominal fat independent of body mass index. Med Sci Sports Exerc 36: 286–291, 2004. doi: 10.1249/01.MSS.0000113665.40775.35. [DOI] [PubMed] [Google Scholar]
- 59.Amin-Chowdhury Z, Ladhani SN. Causation or confounding: why controls are critical for characterizing long COVID. Nat Med 27: 1129–1130, 2021. doi: 10.1038/s41591-021-01402-w. [DOI] [PubMed] [Google Scholar]
- 60.Ladds E, Rushforth A, Wieringa S, Taylor S, Rayner C, Husain L, Greenhalgh T. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res 20: 1–13, 2020. doi: 10.1186/s12913-020-06001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaira LA, Hopkins C, Petrocelli M, Lechien JR, Chiesa-Estomba CM, Salzano G, Cucurullo M, Salzano FA, Saussez S, Boscolo-Rizzo P, Biglioli F, De Riu G. Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J Laryngol Otol 134: 703–709, 2020. doi: 10.1017/S0022215120001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goërtz YMJ, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FVC, Houben-Wilke S, Burtin C, Posthuma R, Franssen FME, van Loon N, Hajian B, Spies Y, Vijlbrief H, van ’t Hul AJ, Janssen DJA, Spruit MA. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res 6: 00542-2020, 2020. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Komici K, Bianco A, Perrotta F, Dello Iacono A, Bencivenga L, D'Agnano V, Rocca A, Bianco A, Rengo G, Guerra G. Clinical characteristics, exercise capacity and pulmonary function in post-COVID-19 competitive athletes. J Clin Med 10: 3053, 2021. doi: 10.3390/jcm10143053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Udelson JE, Curtis MA, Rowin EJ. Return to play for athletes after coronavirus disease 2019 infection—making high-stakes recommendations as data evolve. JAMA Cardiol 6: 136–138, 2021. doi: 10.1001/jamacardio.2020.5896. [DOI] [PubMed] [Google Scholar]
- 65.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ (Editors). Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications (4th ed.), Philadelphia, PA: Lippincott Williams & Wilkins, 2004, p. 612. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data S1: https://doi.org/10.6084/m9.figshare.19704271;
Supplemental Table S2: https://doi.org/10.6084/m9.figshare.19704325;
Supplemental Table S3: https://doi.org/10.6084/m9.figshare.19704367.
Data Availability Statement
Data relate to the serving population of the Ministry of Defense and thus are sensitive. Research teams requesting data are invited to contact the corresponding author and appropriate permissions will be sought for release.