Abstract
Light and water availability are likely to vary over the lifespan of closed-canopy forest trees, with understory trees experiencing greater limitations to growth by light and canopy trees greater limitation due to drought. As drought and shade have opposing effects on isotope discrimination (Δ13C), paired measurement of ring width and Δ13C can potentially be used to differentiate between water and light limitations on tree growth. We tested this approach for Cedrela trees from three tropical forests in Bolivia and Mexico that differ in rainfall and canopy structure. Using lifetime ring width and Δ13C data for trees of up to and over 200 years old, we assessed how controls on tree growth changed from understory to the canopy. Growth and Δ13C are mostly anti-correlated in the understory, but this anti-correlation disappeared or weakened when trees reached the canopy, especially at the wettest site. This indicates that understory growth variation is controlled by photosynthetic carbon assimilation due to variation in light levels. Once trees reached the canopy, inter-annual variation in growth and Δ13C at one of the dry sites showed positive correlations, indicating that inter-annual variation in growth is driven by variation in water stress affecting stomatal conductance. Paired analysis of ring widths and carbon isotopes provides significant insight in what environmental factors control growth over a tree’s life; strong light limitations for understory trees in closed-canopy moist forests switched to drought stress for (sub)canopy trees in dry forests. We show that combined isotope and ring width measurements can significantly improve our insights in tree functioning and be used to disentangle limitations due to shade from those due to drought.
Keywords: gap dynamics, growth release, suppression, tree rings, tropical forest
Introduction
Besides CO2, all trees require light and water for growth and survival. The relative supply of these resources is often inversely related over a tree’s life time. For example, small understory trees are often severely light-limited especially in tall closed-canopy tropical forests (Kira et al. 1969, Turner 2002, Poorter et al. 2005), while canopy trees face difficulties keeping their crowns well-watered (Ryan et al. 2006, McDowell and Allen 2015). The availability of these resources similarly varies between forests, with greater competition for light in wet forests and greater drought stress in dry forests. Disentangling the relative strengths of light versus water controls on tree photosynthesis, and how they impact growth throughout a tree’s life, is important for understanding forest dynamics and differences in tree functioning under different climatic and environmental conditions.
Tropical forest trees in the understory often receive only a fraction (1–2%) of the light levels of canopy trees (Chazdon et al. 1984, Montgomery and Chazdon 2001). This lack of light strongly limits the growth of tropical forest seedlings and saplings, which may remain supressed for several decades or longer and require growth releases for trees to get to the canopy (Clark and Clark 2001, Baker and Bunyavejchewin 2006, Brienen et al. 2010a). Once trees reach the canopy, competition for light with surrounding trees is reduced, and water demand increases due to an increase in irradiance and leaf internal to atmospheric vapor pressure deficit (VPD; Kira and Yoda 1989). At the same time, the longer pathlength from soil to tree crown will increase the resistance of water transport for tall trees (Ryan and Yoder 1997). Despite mechanisms to cope with increased resistance for water transport, such as greater investment in roots (Dawson 1996, Slot et al. 2012, Brum et al. 2019), water stress increases as trees increase in height (Koch et al. 2004, Ryan et al. 2006). These lifetime changes when growing from the understory to the upper canopy are known to affect tree’s leaf morphology and physiology (Rijkers et al. 2000, Cavaleri et al. 2010, McDowell et al. 2011, Steppe et al. 2011, Houter and Pons 2012), tree hydraulics (Olson et al. 2018) and patterns of tree growth (Clark and Clark 1992, 2001), reproduction (Thomas 2011) and mortality (Bennett et al. 2015, Johnson et al. 2018). However, there is still a lack of detailed insight into how these changes in light and water availability over a trees’ life may limit growth throughout tree ontogeny in different forest types, especially for tropical trees.
Tree ring studies have improved our understanding of controls on tropical tree growth. Climate growth analysis in tropical trees showed that growth can be limited by the amount of rainfall (Vlam et al. 2014, Granato-Souza et al. 2019) with stronger controls of rainfall in dry forests (Brienen et al. 2010b, López and Villalba 2011, Mendivelso et al. 2014). In forests with higher water availability and denser canopies, competition is more important than precipitation, especially when trees are small (Baker and Bunyavejchewin 2006, Brienen et al. 2010a). Temporal analysis of ring widths indicates that growth in moist forests is more strongly controlled by variation in light due to canopy gap dynamics, resulting in longer periods of suppressions and more growth releases compared with drier forests (Brienen et al. 2010a). These results indicate that the effects of light and water on growth are likely to vary between different life stages and forest types. However, a lack of historical records of light levels at the time of ring formation means that analysis of temporal variation in tree rings alone does not provide conclusive evidence of to what degree growth is controlled by variation in understory light levels or arises from other controls. One potentially useful tool to obtain insights in the effects of light and water availability on tree growth is the analysis of stable carbon isotopes in tree rings (δ13Ctr). Carbon isotopes provide information on the relative strengths of limits to carbon assimilation versus stomatal conductance on photosynthesis (Farquhar and Richards 1984, Barbour and Farquhar 2000, Aranda et al. 2007).
From δ13Ctr and historical records of δ13Cair, the plant-to-air isotope discrimination (Δ13Ctr) can be calculated as follows:
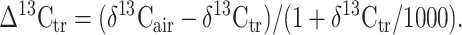 |
(1) |
Variation in Δ13Ctr arises from changes in the ratio of leaf intercellular to atmospheric [CO2], Ci/Ca during photosynthesis. The simplest model relating these two metrics is the linear or reduced model of Farquhar et al. (1982), where Δ13Ctr = a + (b − a) * (ci/ca), with a (4.4‰) referring to discrimination due to slower diffusion of 13CO2 compared with 12CO2 through the stomata, and b (27‰) to discrimination by the CO2-fixing enzyme Rubisco. According to this model, for a constant atmospheric CO2 level (Ca), a change in Δ13Ctr thus reflects changes in ci, which can result from a change in uptake of CO2 through assimilation (A) and/or a change in CO2 supply regulated by stomatal conductance (gs). For example, if photosynthesis rate (A) is limited by carboxylation as a result of light limitation (and not by CO2 supply), Ci and therefore Δ13Ctr increases (Carelli et al. 1999, Coste et al. 2010). Limits on photosynthesis due to low CO2 supply as a result of low stomatal conductance (gs) caused by, e.g., limitations in water supply, result in a decrease in ci and therefore Δ13Ctr (Farquhar et al. 1989). Combined analysis of growth (i.e., ring width) and Δ13Ctr thus may enable a determination of the degree to which growth is limited by light or water availability. Growth increases associated with lower Δ13Ctr (i.e., anti-correlations of Δ13Ctr and growth) indicate that growth is predominantly controlled by variation in light availability, while positive correlations between growth and Δ13Ctr indicate controls of stomatal conductance and water availability on growth (van der Sleen et al. 2014, Giuggiola et al. 2016). Some studies have previously used this approach to disentangle drought from light effects (Saurer et al. 1997, van der Sleen et al. 2014, Voelker et al. 2014, Giuggiola et al. 2016), but no study has used this to evaluate how these controls may change over a tree’s life.
Previous analysis of temporal growth patterns of trees toward the canopy identified longer periods of suppressions and more growth releases for trees growing at a moist tropical forest with high canopy compared with a more open, low stature dry forest (Brienen et al. 2010a). These differences are most likely driven by greater spatial and temporal variation in trees’ light environment, but growth rate analysis by itself does not allow drivers of variation to be distinguished. By combining tree ring width and δ13Ctr data, we here test whether this approach is indeed suitable to indicate light versus water limitation, and study whether limitations on growth change throughout a tree’s lifetime or vary between sites. The study comprises tropical Cedrela trees from three sites differing in annual rainfall, dry season length, stand density and canopy height (Figure 1, Table 1). These trees form distinct proven annual rings and reach ages of up to 300 years (Brienen et al. 2010a).
Figure 1.
Site locations (a) and allometric diameter–height relationships (b) for all sites. Note that data for the two locations in northern Bolivia were treated as one single site. Lines in (b) indicate the assumed tree height and diameter thresholds at which trees reach the lower forest canopy (see Table 1).
Table 1.
Characteristics of the forests and the Cedrela trees at the three study sites.
| Site | Forest type | Forest height | Soil type | Rainfall | Cedrela | |||
|---|---|---|---|---|---|---|---|---|
| Max tree height | Max diameter | Height lower canopy1 | Diameter lower canopy1 | |||||
| Northern Bolivia | Tropical semi-deciduous | 30–35 m | Xanthic ferrasols | 1750 mm | 46 m | 180 cm | ~17 m | ~30 cm |
| Yucatan | Tropical dry | ~15 m | Karstic | 1100 mm | 22 m | 52 cm | ~10 m | ~20 cm |
| Oaxaca | Tropical dry | ~10 m | Karstic | 900 mm | 13 m | 38.5 cm | ~7.5 m | ~15 cm |
1Defined as tree height corresponding to about two-thirds of the total canopy height.
Our hypothesis is that trees are generally more light limited in the understory and thus show negative correlations between growth and Δ13C when small, but that these correlations disappear when reaching the canopy. We further anticipate that the relative strength of light and water limitation varies between sites, with (i) a stronger light limitation of growth in the wetter sites resulting in anti-correlations between growth and Δ13Ctr, and (ii) a stronger water availability limitation on growth at the driest site resulting in positive correlations between growth and Δ13Ctr. We test these hypotheses by analyzing temporal co-variation in growth and Δ13Ctr over a tree’s life from understory to canopy stages and compare these patterns for the different sites. We further assess the relative effects of tree height, light and atmospheric CO2 on Δ13Ctr (cf., McDowell et al. 2011, Brienen et al. 2017, Vadeboncoeur et al. 2020), as these are fundamental to the interpretation of our results. For example, changes in light as trees grow to the canopy are accompanied by changes in tree height, as well as changes in atmospheric CO2 concentration due to CO2 emissions.
Materials and methods
Study sites and species
This study is based on data from four locations (Table 1, Figure 1). Two sampling locations are located in northern Bolivia in the department of Pando, one 50 km south of Cobija (Purissima, 11°24′S, 68°43′W) and one north of Riberalta (Selva Negra, 10°5′S, 66°18′W). These sample locations (cf., 350 km apart) are treated as one site as they share the same climate and vegetation. The annual precipitation is ~1750 mm with a distinct, dry season (<50 mm per month) of 3 months from June to August. The vegetation consists of semi-deciduous, moist tropical forests with a maximum canopy height of 30–35 m. The other two sites are in Mexico, in the state of Campeche on the Yucatan Peninsula (Ejido Pich, 19°03′N, 90°00′W), and in the state of Oaxaca on the Pacific slope of the Isthmus of Tehuantepec, close to Nizanda (16°39′N, 95°00′W). Both sites are much drier than those in northern Bolivia. The site in Yucatan receives ~1100 mm annual precipitation and has a 5-month long dry season (December–April), and the vegetation consists of tropical dry forest mostly on hilly terrain with karstic soils and very good drainage. Average canopy height at this site is ~15 m and the forest structure is open. The site in Oaxaca receives ~ 930 mm rainfall with a 7-month long dry season (November–May) and consists of tropical dry forest on steep slopes and karstic soils. Canopy height varies between 10 and 15 m and the forest structure is very open. All sites are old growth forests, although two of the sites (Selva Negra and Yucatan) have experienced selective logging in the past of less than one tree per hectare.
The sampled tree species are Cedrela odorata L. in Bolivia and Yucatan and Cedrela salvadorensis Standl. (Meliaceae) in Oaxaca. Both are deciduous species that lose their leaves during the dry season and form distinct annual rings, marked by terminal parenchyma bands often in conjunction with variation in vessel density and size (Worbes 1999, Baker et al. 2017). Cedrela odorata is a relatively light demanding canopy tree that can survive for relatively long periods at low growth and reach ages of over 300 years (Brienen et al. 2010a). In moist forest, the species reached heights of up to 45 m and diameters of 200 cm. Cedrela salvadorensis performs best as seedlings in intermediate light conditions (Guzmán-Q et al. 2016). The species reaches heights of up to 15 m, diameters of 50 cm and ages of ~120 years (Groenendijk 2010).
Sample collections and field measurements
Samples used in this study consisted of a mix of large stem disks, disk fragments and increment cores of trees. Samples were taken in 2001 (Bolivia, Purissima), 2007 (Yucatan and Oaxaca) and 2011 (Bolivia, Selva Negra) from trees ranging in size from 50-cm tall seedlings to canopy trees. At all three sites, tree ring chronologies were build using standard ring width cross-dating (Brienen and Zuidema 2005, Brienen et al. 2010a), complemented with oxygen isotope chronologies (Brienen et al. 2012, Baker et al. 2015). For the sites in Bolivia, we collected over 100 stem discs from large trees (>60 cm in diameter at breast height, DBH) and 55 small discs from seedlings and saplings, and increment cores (2–3 radii) from >150 trees. In Yucatan, we collected 10 discs from large trees and cores from 70 trees (2–3 radii), and in Oaxaca, we collected 6 discs and increment cores (2–3 radii) from 70 trees. For each tree, we measured its DBH, estimated tree height and assessed light availability using the modified Crown Illumination Index (CII) of Dawkins (Clark and Clark 1992) varying from 1 (no direct lateral or overhead light) to 5 (full overhead and lateral, direct light). The CII values of trees at the Bolivian and the Yucatan sites were estimated by R.B., and for trees at the Oaxaca site by both P.G. and R.B. Note that these estimates are only gross indications for differences in light levels between sites and that actual light levels for trees in the dry site are possibly higher in the same CII class compared with the moist site as crown structure in the dry site is more open. Tree heights were mostly estimated by eye by R.B. and P.G. and calibrated with reference to measurements from felled trees.
Ring width and isotope measurements
Samples were air-dried and sanded until rings were visible, and measured using a ring measurement device (Velmex or LINTAB). Rings width measurements from different radii, or cores were averaged and converted to diameter growth (see Brienen and Zuidema 2005). Carbon isotope ratios of the tree ring cellulose (δ13Ctr) were measured over the trees’ entire lifetimes for a subset of 28 large trees, and over the last 2–10 rings for 72 smaller trees to assess effects of tree size and crown illumination, totaling more than 3200 δ13Ctr measurements. For the site in Yucatan, only large trees were analyzed for δ13Ctr. The oldest ages of trees included in this analysis are 110 years for the Yucatan site, 118 years for the Oaxaca and 203 years for northern Bolivia.
Individual rings were cut using a scalpel and cellulose was extracted following the batch method of Wieloch et al. (2011). Cellulose was homogenized and then freeze-dried, and weighed in tin capsules. Isotope analysis was undertaken at the German Research Centre for Geosciences (GFZ, Postdam and Julich, Germany) for samples from Bolivia and Yucatan, at the National Environmental Isotope Facility (NEIF), British Geological Survey, for samples from Bolivia, Selva Negra and at the University of Leicester (UK) for samples from Oaxaca.
We calculated Δ13Ctr according to Eq. (1), and using atmospheric records of δ13Ca obtained from Antarctic ice cores (Francey et al. 1999), complemented with recent data from Mauna Loa from the NOAA ESRL Global Monitoring Laboratory (http://www.esrl.noaa.gov/gmd/ccgg/trends/full.html). We did not calculate internal leaf CO2 concentrations (ci) or derive intrinsic water-use efficiency (iWUE), as different equations have been used including models with uncertain terms for mesophyll conductance and photorespiration (Seibt et al. 2008, Schubert and Jahren 2015) causing unnecessary complexity and uncertainty in the interpretations of plant isotope discrimination for our purpose. We did however correct all our calculations of Δ13Ctr for lower δ13Ca above the soil due to respiration of depleted soil organic carbon (Buchmann et al. 1997). This was done using a previously developed relationship for the difference between below- and above- canopy δ13Ca and tree height using literature data for tropical forests (see Brienen et al. 2017). These adjustments were relatively small for trees taller than 3 m (84% of all data) (~0.1‰ decrease in Δ13Ctr), but larger (2–3.5‰) for a small portion of rings formed when trees were <1 m in height.
Analyzing lifetime change in Δ13C
To assess controls of lifetime change in Δ13C, we first related Δ13C to age, tree height, diameter growth and atmospheric CO2 (ca) for large trees with long trajectories containing more than 40 years of data. For each site, we selected the most parsimonious model to explain variation in Δ13C based on r-squared and AIC selection criteria. Separate analysis was performed using the Δ13C records of the last five rings, including a range of different tree sizes to additionally test for the influence of crown illumination on Δ13C.
We assessed if Δ13C differed between periods of suppressed versus high growth, and if Δ13C changed during growth releases. Suppressions were defined as periods of growth lower than a defined threshold over at least five consecutive years. Thresholds were calculated as the midpoint of mean growth at CII2 and CII3 (see Figure 3). Growth releases were defined as percent growth changes greater than 100% between two adjacent 10 year windows (see Brienen et al. 2010a).
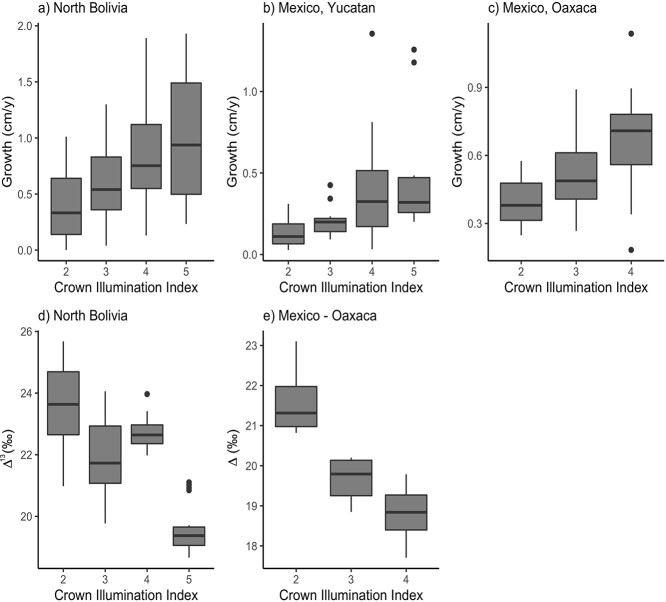
Figure 3.
Diameter growth rate and isotope discrimination (Δ13Ctr) in relation to crown illumination index (CII). The CII varies from 2 for sapling and seedlings without direct sunlight to 4 and 5 for trees with full direct sunlight from above or for emergent crowns. Both growth and isotope discrimination are calculated as the mean over the past five rings for extant trees. Boxes denote 25th, 50th and 75th percentile and whiskers extend to the largest or smallest value no more than 1.5 times the interquartile range. Letters indicate significant (P < 0.05) differences between CII classes using paired t-tests. In all three sites, CII explains greater amount of variation than diameter using ANOVA with tree diameter as covariable.
Growth–Δ13C relationships
To compare changes in growth–Δ13C relationships across life stages, we defined a size threshold to distinguish between understory and canopy trees. Canopy trees were defined as those taller than about two-thirds of the total local forest canopy. We chose this threshold as more than 80% of the trees above these heights had their crowns exposed to full overhead light (i.e., reached a CII index of 3b or higher). Thresholds corresponded to heights of 7.5, 10 and 17 m and diameters of 15, 20 and 30 cm for Oaxaca, Yucatan and Bolivia, respectively (Table 1, Figure 1).
Growth–Δ13C relationships were analyzed using a simple Pearson’s r correlation analysis on two types of data: (i) raw data and (ii) detrended (i.e., high-frequency) data from which we removed effects arising from age or ontogenetic effects and from canopy gap dynamics to enhance the underlying climate signal (Cook and Peters 1980). The detrended data were calculated for both ring width and Δ13C as the ratio of raw values to smoothing trends using a flexible spline with a rigidity of 15 years and a wavelength cut-off of 0.8. Examples of these splines are shown in Figure 4 and Figure S2, available as Supplementary data at Tree Physiology Online.
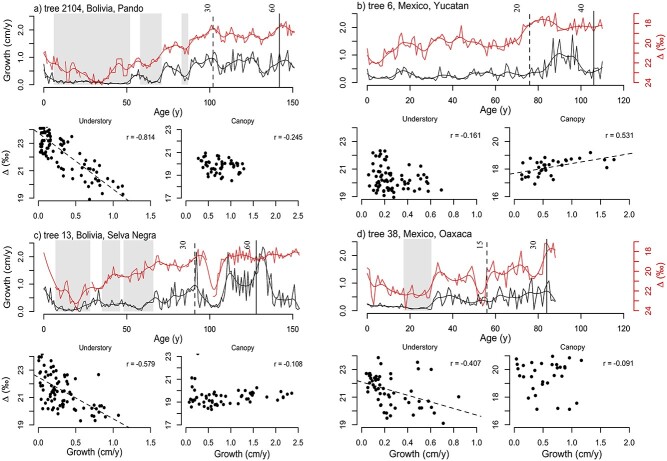
Figure 4.
Examples of trajectories for growth (black lines) and C-isotope discrimination (Δ13C, red lines in reversed axes) of individual trees, and scatterplots of growth versus Δ13C for understory and canopy growth phases. Note the reverse axis for Δ13C time series in red in the upper panels. Understory is defined as trees smaller than 30, 20 and 15 cm in diameter for Bolivia, Yucatan and Oaxaca, respectively, which is the approximate size at which trees reach the lower canopy (see Materials and methods). The age at which the individual trees reached these diameter thresholds for understory versus canopy growth phases or larger canopy sizes are indicated in the trajectory plots with vertical broken and continuous lines, respectively, with numbers referring to tree diameter in cm. The trend lines in the scatter plots indicate significant (P < 0.05) relationships between early growth rate and Δ13C, and Pearson correlation coefficients (r) are shown. Smooth curves for growth and Δ13C trajectories are smoothing splines (see Materials and methods). Periods of suppression (i.e., minimum of 5 years of growth below a site-specific threshold, see Materials and methods) are indicated by shaded areas.
Pearson correlation analyses were performed in the following three different ways:
(i) All-data correlations using and mixing all data from different trees and years. Correlations in this analysis are due to variation in growth and Δ13C within trees as well as between trees. This analysis was done for understory and canopy stages as well as for small size classes of 5-cm widths using raw and detrended data.
(ii) Within-tree correlations were calculated as the mean of correlations between growth and Δ13C within individual trees using both raw and detrended data. These correlations were calculated for understory and canopy stages, for trees with more than 40 years of data.
(iii) Between-tree correlations were calculated between mean growth and Δ13C of different trees for canopy and understory life-stages, as well as for small size classes of 5-cm widths.
These different types of analyses provide different insights: within-tree correlations highlight temporal co-variation between growth and Δ13C, while the between-tree correlations highlight variation between individuals due spatial differences in, e.g., soil conditions or light availability, and all-data correlations capture both effects.
Results
Lifetime changes in carbon isotope discrimination (Δ13C)
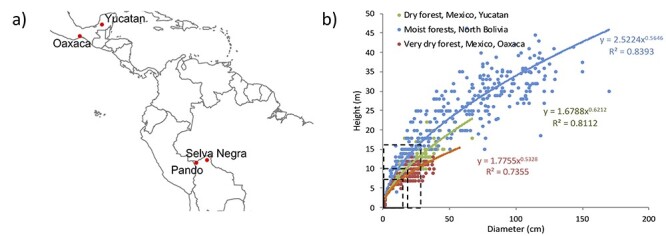
At all three sites, carbon isotope discrimination (Δ13C) decreased strongly over a tree’s lifetime from maxima of 24–25‰ in the understory to minima of 17–18‰ in the canopy stage (Figure 2). Tree height is the strongest predictor for lifetime changes in Δ13C in Bolivia, explaining more variation than age, growth or [CO2]. For the other two sites, height and [CO2] are equally strong predictors for changes in Δ13C (Table 2). The change in Δ13C with tree height was greatest in the driest site in Oaxaca (−0.41 ± 0.02‰ m−1), intermediate for Yucatan (−0.20 ± 0.01‰ m−1) and smallest for Bolivia (−0.15 ± 0.00‰ m−1). Within individual trees, we found a close relationship between lifetime changes in Δ13C and tree height, with tree height explaining 50–86% of the variation in Δ13C in most trees (Figure S1, available as Supplementary data at Tree Physiology Online). Variation in Δ13C between and within trees was generally greatest in the understory stage and was especially large in the wettest site in Bolivia (Figure 2).
Figure 2.
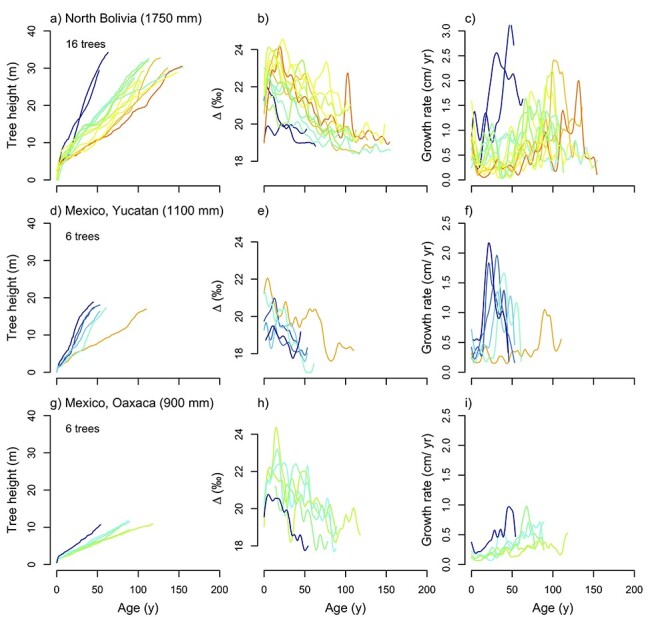
Relationships of tree height, C-isotope discrimination (Δ13C) and growth with tree age. The color scheme in figures corresponds to the trees’ average growth rates in understory, defined as trees smaller than 30, 20 and 15 cm in diameter for Bolivia, Yucatan and Oaxaca, respectively (see Materials and methods). Tree heights in left panels were estimated from tree diameter and site-specific mean diameter-height allometries (see Figure 1b). For clarity, curves for Δ13C and growth are smoothed using smoothing splines (see Materials and methods).
Table 2.
Individual effects of tree height, growth, crown illumination, age and [CO2] on Δ13C; values shown are the R2; significant relationships are shown in bold; note that all relationships here are negative.
| Site | Height | Diameter growth | Basal area growth | Crown illumination | Age | [CO2] | Most parsimonious model2 | R2 |
|---|---|---|---|---|---|---|---|---|
| Lifetime change in Δ13C | ||||||||
| Northern Bolivia | 0.59 | 0.18 | 0.26 | NA | 0.43 | 0.33 | Height, Diameter growth | 0.66 |
| Yucatan | 0.45 | 0.07 | 0.19 | NA | 0.20 | 0.46 | Height, Diameter growth | 0.48 |
| Oaxaca | 0.31 | 0.18 | 0.23 | NA | 0.25 | 0.38 | [CO2], Diameter growth | 0.41 |
| Last 5-years1 | ||||||||
| Northern Bolivia | 0.75 | 0.12 | 0.41 | 0.52 | 0.62 | − | Height, Crown illumination | 0.77 |
| Oaxaca | 0.55 | 0.15 | 0.41 | 0.81 | 0.41 | − | Crown illumination | 0.81 |
1No last 5-year data across diameter or light classes available for Yucatan.
2From a comparison of models with diameter growth and either Height, CO2 or Age. Due to strong collinearity, only one of these variables was included. The most parsimonious model was chosen based on highest R2 and lowest AIC.
Effects of light on growth and carbon isotope discrimination (Δ13C)
Variation in Δ13C of the five most recent rings of extant trees was most strongly related to tree height and light (i.e., CII) at the two sites for which we had data (Table 2, lower panel). An additional relationship between Δ13C and age arose mainly due to the correlation between age and tree height but disappeared in a multiple regression when accounting for their co-linearity. Trees with limited direct sunlight (i.e., low CII) had significantly lower growth and higher Δ13C (Figure 3). This effect of CII may partially arise due to the correlation between tree height and higher CIIs. Our analysis showed however that the effects of CII on growth only disappeared in Oaxaca after correcting for tree height, and the effects of CII on Δ13C remained significant at both sites. Note that full statistical separation of the strongly correlated effects of height and CII on Δ13C and growth is difficult.
Relationship between growth and carbon isotope discrimination (Δ13C)
Figure 4 shows some chosen examples of lifetime growth and Δ13C trajectories for individual trees. These examples as well as most trees (see Figure S2, available as Supplementary data at Tree Physiology Online, for all individual trajectories) illustrate strong temporal changes in growth especially for trees in the moist site in Bolivia going through prolonged periods of slow and fast growth when in the understory (Figure 4a and c). The shaded areas indicate periods of growth suppression. These periods of suppressed growth were generally associated with higher Δ13C (note the reverse axis for Δ13C). Differences in Δ13C between periods of suppressed and non-suppressed growth in the understory trees were significant for Bolivia (Δ13Csuppression = 22.6‰, Δ13Chigh_growth = 21.3‰, P < 0.001) and Oaxaca (Δ13Csuppression = 21.8‰, Δ13Chigh_growth = 20.7‰, P < 0.001), but not for Yucatan (Δ13Csuppression = 19.5‰, Δ13Chigh_growth 19.7‰, P = 0.6). Across all trees, we further found that growth releases (i.e., growth increases >100% between two subsequent 10-year periods) were associated with a 0.70‰ decrease in Δ13C (Welch two sample t-test, t = 7.4, P < 0.001).
The scatterplots in Figure 4 show the relationship between growth and Δ13C for each tree in both the understory (left panel) and canopy stages (right panel). The two examples for Bolivia clearly show strong negative relationships between Δ13C and growth when trees were in the understory (i.e., when <30 cm in diameter), but correlations disappeared after trees reached the canopy (>30 cm in diameter). The example for Oaxaca shows a similar pattern, while the Yucatan example shows no correlation in the understory stages and positive correlations between growth and Δ13C after reaching the canopy.
Growth–Δ13C relationships for understory versus canopy trees
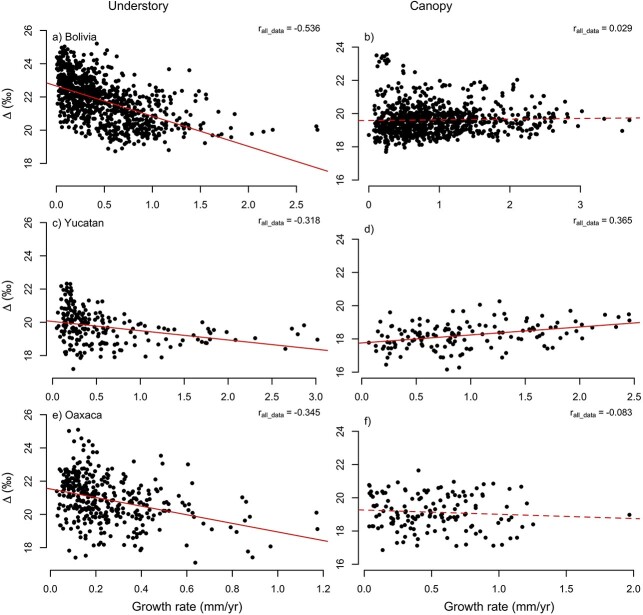
We first assessed changes in growth–Δ13C relationships between different life-stages by separating data into understory and canopy stages. This showed that during the understory stage, growth and Δ13C were negatively related, but there was no relation (Bolivia and Oaxaca), or a weakly positive relationship (Yucatán) at the canopy stages (Figure 5). This analysis mixes data from different trees and multiple years within trees, and relationships may thus arise from between-tree as well as within-tree co-variation between growth and Δ13C (e.g., examples in Figure 4). Further analyses showed that both factors cause anti-correlations in the understory stages in Bolivia with relatively strong mean within-tree correlations (r = −0.50) as well as between-tree correlations (r = −0.70, Table 3). In contrast, for the two other sites within-tree correlations in the understory were weaker (r = −0.18 and −0.35), but between-tree correlations are relatively strong (r = −0.59 and −0.57). At the canopy stages, most correlations disappeared apart from within-tree correlations in Yucatan, which change to positive correlations. We also found some strong negative between-tree correlations for canopy trees at the site of Oaxaca, but sample sizes were very low (Table 3).
Figure 5.
Relationship between discrimination and diameter growth using all data from all trees, separated between understory and canopy stages, per site. Correlation coefficients (r) are shown. Continuous lines indicate significant relationships (P < 0.05), and broken lines non-significant relationships (P > 0.05). See Figure S3, available as Supplementary data at Tree Physiology Online, for the same analysis using detrended data.
Table 3.
Correlation coefficients between growth and Δ13C for understory and canopy trees for the three sites; within tree correlations are calculated as the mean of individual tree correlations that have at least eight data points using raw and detrended data; correlations between trees consist of correlations between the mean growth and mean Δ13C in the understory and canopy life stages; understory is defined as trees smaller than 30, 20 and 15 cm in diameter for Bolivia, Yucatan and Oaxaca, respectively; values between brackets for the within-tree correlations give the standard error of the variation between trees in correlation coefficients. ns, *, P< 0.05.
| Mean correlation (r) within trees | Correlation (r) between trees | ||||||
|---|---|---|---|---|---|---|---|
| Raw data | Detrended data | Raw data | |||||
| Understory | Canopy | Understory | Canopy | Understory | Canopy | ||
| Bolivia | −0.50 (0.08) | −0.07 (0.08) | −0.11 (0.06) | −0.01 (0.06) | −0.70* | 0.09ns | |
| Yucatan | −0.18 (0.14) | 0.41 (0.09) | 0.06 (0.10) | 0.43 (0.07) | −0.59ns | 0.19ns | |
| Oaxaca | −0.35 (0.4) | 0.18 (0.13) | 0.00 (0.08) | 0.19 (0.10) | −0.57ns | −0.71ns | |
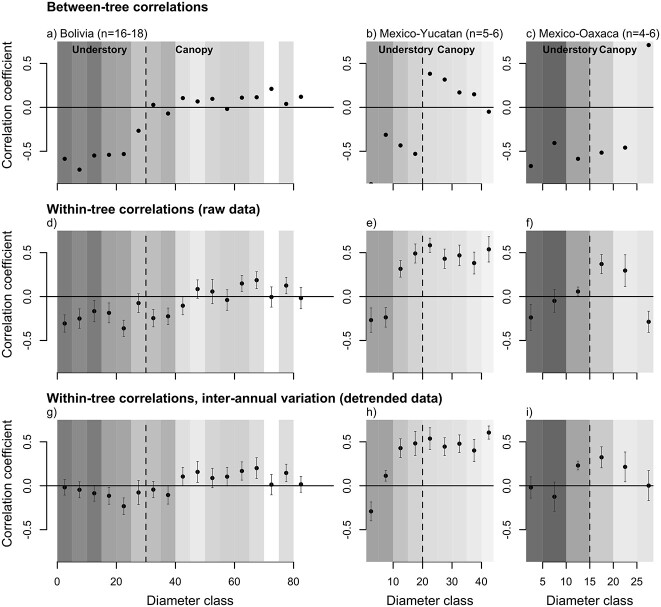
We further assessed changes in mean between-tree growth-Δ13Ctr relationships of trees across size classes (Figures 6 and Figures 7a-c). This analysis showed strong negative relationships between mean growth and Δ13C in the smallest size class (0–5 cm diameter) at all three sites. In Bolivia, the slope of the negative relationship weakened gradually and completely disappeared from ca 30 cm DBH onward, while in Yucatan, the relationship deteriorated immediately after the smallest size class. In Oaxaca, negative relationship remained apparent until 25 cm diameter.
Figure 6.
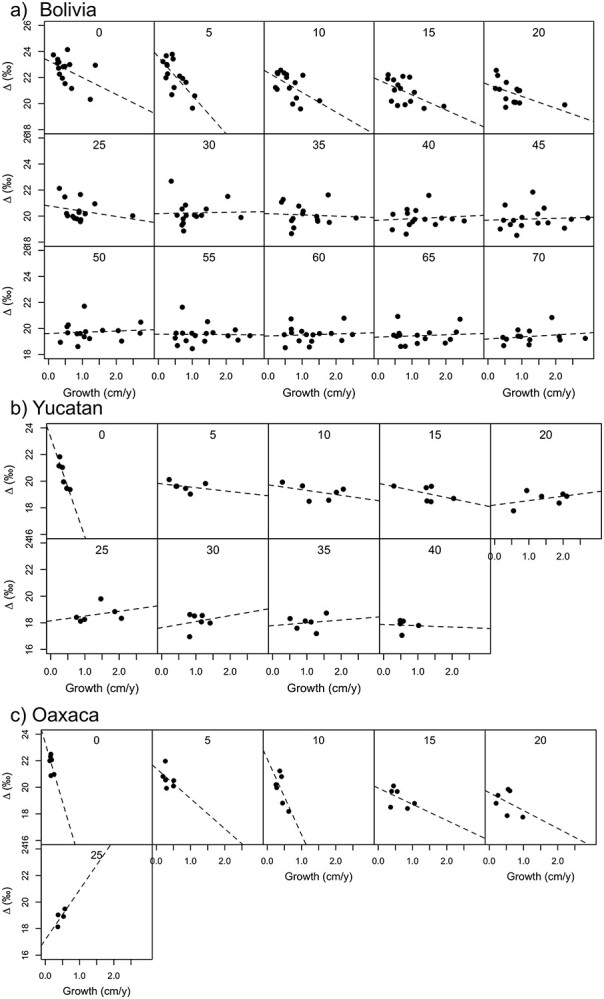
Relationship between mean diameter growth and Δ13C in diameter classes of 5-cm widths for Bolivia (a), Yucatan (b) and Oaxaca (c). Each point represents the mean of diameter growth and Δ13C for a different tree. Numbers at the tops of the graphs denote the lower boundary for each size class in cm.
Figure 7.
Correlation coefficients between growth and discrimination by diameter class for three sites between trees (cf., Figure 6) and within-trees using detrended data. Vertical broken lines indicate the approximate size at which trees reach the canopy for the three sites, respectively, at 30, 20 and 15 cm in diameter for Bolivia, Yucatan and Oaxaca. The gray scale of the background corresponds to the average crown exposure index, with darker colors representing lower light levels. Error bars in panels (d–i) indicate the standard error of the variation between trees in correlation coefficients (i.e., standard deviation/sqrt(n)). Sample sizes refer to the number of trees included in the correlations for each size class.
Effects of inter-annual variation in growth and Δ13C
To assess the effects of year-to-year variation in climate on growth and Δ13C, we used detrended data that removed variation due to ontogeny and gap dynamics. This analysis showed no or weak correlations between detrended growth and Δ13C in the understory stage for all three sites, but strong positive correlations at the canopy stage for the site of Yucatán (Table 3). At this site, positive relationships between detrended growth and Δ13C were observed from relatively small diameters onward (i.e., >10 cm DBH, Figure 7h). In Bolivia, positive relationships between detrended growth and Δ13C were only evident for trees larger than 50-cm DBH (Figure 7g). At the site of Oaxaca, we found positive relationships between detrended growth and Δ13C at intermediate sizes of 10–20 cm diameter (Figure 7i).
Discussion
Lifetime changes in Δ13C
Isotope discrimination (Δ13Ctr) decreased over tree’s lifetimes by 4–6‰ in all three sites. These decreases are not due to below-canopy profiles of δ13Cair or plant responses to changing atmospheric CO2 but are caused by height-related changes in microclimate (e.g., light, humidity and temperature) and in tree structure and functioning as trees grow through the canopy. Below-canopy 13C depletion due to soil respiration (cf., Buchmann et al. 1997) does not explain the observed Δ13C trends as all values of Δ13C were ‘adjusted’ for any impact from the respiration of soil carbon (see Materials and methods), plus the impact is estimated to be relatively small (2–3‰) and affects the first few rings only. The trends in Δ13C are also not due to long-term CO2 increases. Firstly, our results show that the effect of CO2 is weaker than height-related effects (Table 2). Secondly, the most common plant response to increasing CO2 is either to maintain a constant Δ13C (i.e., constant ci/ca ratio, Saurer et al. 2004, Franks et al. 2013, van der Sleen et al. 2015) or to increase Δ13C (Voelker et al. 2016, Keeling et al. 2017), while we find strong decreases in Δ13C over time (i.e., tree age).
The observed decreases in Δ13C and thus leaf intercellular CO2 (ci) with tree height are in line with temperate forest trees (e.g., mean of 6‰ in McDowell et al. 2011, 0–6‰ in Brienen et al. 2017, 1.2–4.5‰ in Klesse et al. 2018, 3.7–7.2‰ in Vadeboncoeur et al. 2020) and are thus due to greater increases in CO2 demand for assimilation relative to stomatal conductance (CO2 supply). As trees increase in height, stomatal conductance (gs) becomes more rate limiting for CO2 uptake, due to stomatal closure in response to increases in leaf temperature (Fauset et al. 2018) and thus VPD associated with higher irradiance (Lloyd and Farquhar 2008), as well as increases in hydraulic pathlength and resistance resulting in decreases in leaf water potentials (Koch et al. 2004, Ryan et al. 2006). The relative contributions of the limitation through hydraulics (i.e., water transport to the canopy) versus changes in canopy level irradiance and VPD in controlling these height-related changes in Δ13C are still debated (McDowell et al. 2011, Vadeboncoeur et al. 2020), but our results suggest a dominant influence of irradiance. Firstly, we find that the difference in discrimination between small understory and canopy trees (Figure 2b) is of similar magnitude to the difference between sun and shade trees (Figure 3). Secondly, the absolute magnitude of lifetime change in discrimination (from understory to canopy trees) does not vary between sites, despite the large difference in maximum tree height between sites, pointing perhaps toward a relative limited role of hydraulic limitation in C isotope discrimination. Thirdly, we find that once trees reached the canopy, Δ13C remained relatively constant or showed only slight decreases, especially at the Bolivian sites (Figure 2b, Figure S1, available as Supplementary data at Tree Physiology Online). Thus, light seems one of the most critical drivers for variation in Δ13C in line with previous studies (McDowell et al. 2011, Brienen et al. 2017, Klesse et al. 2018, Vadeboncoeur et al. 2020). Apart from direct effects of light on discrimination, leaf morphological changes, such as leaf thickness (Aranda et al. 2007), leaf nitrogen (Duursma and Marshall 2006) and leaf mesophyll conductance (Seibt et al. 2008), have also been shown to affect discrimination. Leaf morphological changes between sun and shade leaves in the two Cedrela species are large with, for example, an almost twofold variation in a specific leaf area (Poorter and Hayashida-Oliver 2000, Guzmán-Q et al. 2016). Regardless of the causes, lifetime trends in Δ13C from understory to canopy show increasing limitations of stomatal conductance on carbon assimilation, most likely driven by a combination of increased evaporative demand (irradiance) and restrictions to water supply (hydraulic limitations). The extent of the relative contributions from these drivers still requires further study.
Finally, despite large differences in mean annual rainfall (900–1750 mm), Δ13C does not vary strongly between sites and converges to a narrow range (~18–20‰) once trees reached the canopy, contrasting with general observations of increases in discrimination with rainfall (Schulze et al. 2006, Prentice et al. 2011, Givnish et al. 2014). This may be due to the relatively small differences in water availability during the rainy season, the actual growing period for this deciduous species. Alternatively, decreases in soil water potential across sites could be compensated for by decreasing maximum tree heights toward drier sites (Figure 1), or hydraulic adjustments such as the ratio of leaf area to water-conducting tissue could result in maintenance of similar leaf water potentials (Mencuccini and Grace 1995, McDowell et al. 2002, McDowell et al. 2011), and thus Δ13C, for canopy trees.
Co-variation of Δ13C and growth in the understory
Previous analysis of ring width data alone suggested that the observed growth rate variation in the moist forest in northern Bolivia was due to variation in light related to canopy gap dynamics (Brienen and Zuidema 2005). Here, we confirm this interpretation using the paired ring width–Δ13C approach. We find a strong match in temporal variation between Δ13C and growth in the understory within individual trees (Figure 4a and c, Table 3). Periods of low growth (i.e., suppressions) were associated with higher Δ13C, while growth increases (i.e., releases) led to decreases in Δ13C. Increases in Δ13C and ci during periods of low growth indicate that growth is limited by demand for CO2, and not by CO2 supply through stomatal conductance (which would result in decreases in ci). Theoretically, the negative covariation between growth and Δ13C could be due to variation in nutrients (Cernusak et al. 2013), but nutrients are unlikely to vary over time and cause temporal covariation of growth and Δ13C within trees. In all, these results provide strong evidence that growth in the understory is light limited, consistent with the observed effect of crown illumination on Δ13C and growth (Figure 3). However, we also find large variation in Δ13C at low growth rates (Figure 5a), indicating that growth of understory trees may not be exclusively limited by light and that water stress could play a role by reducing stomatal conductance and growth in some years.
Consistent with the temporal co-variation of growth and Δ13C within trees, we find that mean growth and Δ13C are also strongly negatively correlated between small trees in Bolivia (Figure 6a). Fast-growing, small trees had lower average Δ13C than slow-growing trees, resulting in strong negative relationships between growth and Δ13C in the smallest size classes. The negative slope of these relationships gradually weakened toward larger size classes, indicating diminishing controls of light on variation in growth and/or Δ13C with increasing tree size. The size at which this relationship disappeared in Bolivia is surprisingly close to the estimated size at which most trees reach (sub)canopy levels and receive full overhead light (~30 cm diameter).
At the two drier sites, we also observed negative correlations between growth and Δ13C in the understory, both within trees, as well as between trees (cf., Table 3, Figure 6). At the Yucatan site, the strength of the correlations declines rapidly as trees get bigger, showing that light is only a limiting factor to growth in the smallest trees (<5 cm). This can be explained by the canopy being lower and more open, with trees at this site showing faster canopy accession and significantly shorter periods of suppressed growth compared with the moist site (Brienen et al. 2010a). Patterns are different for the site of Oaxaca as negative relationships between growth and Δ13C continued even for large trees that had reached canopy positions with full sunlight (Figure 6c). These negative growth–Δ13C relationships for canopy trees are entirely due to differences in growth and Δ13C between trees (Figure 6c), as within-tree correlations are slightly positive (Table 3). As this site had a very open and low canopy structure, light was not expected to be a limiting growth factor for taller trees. Besides light, spatial variation in soil fertility could be driving variation in growth and Δ13C.
In all, paired growth–Δ13C showed that spatial and temporal variation in light limits growth at all three sites during the understory life phases. At all three sites, those fast-growing trees that reached the canopy early had the lowest mean Δ13C (cf., dark colored trajectories in Figure 2), indicating that light is the main control behind growth differences. Sites differed in the strength and the duration of light limits on growth, with the strongest limitations at the moist site with the highest and most dense canopy structure.
Interannual variation in growth and Δ13C
Detrended growth and Δ13C showed positive correlations at inter-annual scales in Yucatan once trees reached diameters >10 cm, and some weaker positive correlations for mid-sized and large trees in Bolivia and Oaxaca (Figure 7g–i). Positive correlations are indicative for water stress reducing growth in dry years through greater reductions in stomatal conductance, resulting in lower leaf intercellular CO2 (ci) and lower Δ13C, caused by either reduced soil water content or leaf water status controlled by VPD (Tardieu and Davies 1993, Harris et al. 2004). The differences in C. odorata between Yucatan and Bolivia are consistent with other studies showing strong positive relationships between growth and Δ13C at semi-arid sites (Andreu et al. 2008, Brienen et al. 2011, Leavitt et al. 2011, Voelker et al. 2014), and a weakening or even opposite directions of correlations at sites with greater water availability (Brooks et al. 1998, Young et al. 2010, Voelker et al. 2014).
The lack of correlations between inter-annual variation in growth and discrimination for trees at Oaxaca is surprising as it is the driest site and as inter-annual variation in growth by itself shows relatively good synchrony between trees (Groenendijk 2010). These results thus probably do not reflect insensitivity to climate but may be due to decoupling of leaf level isotope discrimination and growth as a result of post-photosynthetic fractionation processes and use of stored carbohydrate reserves (Gessler et al. 2014), or asynchronism between canopy photosynthesis and actual cambial activity and wood growth (Wagner et al. 2016).
The increase in strength of the association between growth and Δ13Ctr as trees get bigger in Yucatan (and to lesser degree in Bolivia) is consistent with observations (Mérian and Lebourgeois 2011, Bennett et al. 2015, Trouillier et al. 2019, McGregor et al. 2021) and theory (Ryan and Yoder 1997) of strengthening climate-growth correlations with increased tree size. Taller trees have a longer hydraulic pathlength, and larger evaporative demand, which increase trees’ water stress (McDowell and Allen 2015). Our results indicate that any potential increase in rooting depth and soil water uptake as trees grow bigger (Dawson 1996, Brum et al. 2019) is not sufficient to completely offset increased water demand for trees in the canopy of Cedrela, which is consistent with its shallow root system and reliance on water from the top soil (Kunert et al. 2010). A greater control of water availability on growth variation of canopy trees in Yucatan could be indicative of greater mortality risk under drought, as shown recently (DeSoto et al. 2020), and explain significantly shorter lifespan (Figure 2) and tree height (Figure 1) in this dry site compared with the wetter forest in Bolivia.
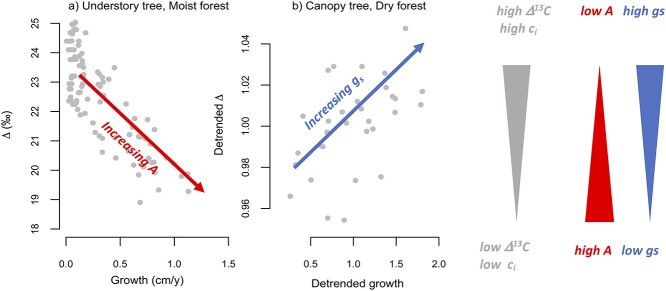
In summary, we observed a distinct change in drivers of growth rate variation from light limitation in the understory to drought limitations in the canopy. This is especially pronounced when comparing correlations between growth and discrimination for small trees in the moist site with large trees of the same species in the dry site (see Figure 8). Opposite directions of correlations indicate different fundamental controls on growth rates at different life stages and sites.
Figure 8.
Graphical summary of opposing growth–Δ13C correlations of Cedrela trees in understory (Bolivia) and canopy phases (Yucatan) indicating light (a) and water (b) limitations on growth. Increasing discrimination results from increase in ci/ca, and can be caused by increases in stomatal conductance (gs, relative to A) or decreases in assimilation rate (A, relative to gs). Increasing growth combined with decreasing discrimination (panel a) indicates a release of constraints of photosynthetic carboxylation rate on growth (i.e., greater A due to increase in light), whereas increasing growth with increase in discrimination indicates increasing stomatal conductance as the principal driver for increased growth (panel b).
Further implications for tree ring studies
We find an increase in the strength of the co-variation between growth and Δ13C as trees reach the canopy, especially in Yucatan. This may be due to either true changes in climate sensitivity of trees (i.e., magnitude of the response in growth or Δ13C per change in climate variable) or simply to reductions of other influencing factors as trees get bigger (e.g., light). Whatever the causes, these size- or age-related changes in variance caused by climate are common across many species (Carrer and Urbinati 2004, Mérian and Lebourgeois 2011, Trouillier et al. 2019). This may violate the stationarity principle for growth–climate relationships in dendroclimatic reconstructions (Wilmking et al. 2020) and affect assessment of (changes in) trees’ drought sensitivity over time (Anderegg et al. 2020) or in response to CO2 (Zuidema et al. 2020). This highlights that ring width variation in the juvenile phases is unlikely to provide a reliable climate proxy, especially for shade-tolerant species, and disentangling ontogenetic effects from climate or CO2-related effects requires great care.
Our results also have significant implications for the use of δ13Ctr to assess responses of iWUE of trees to climate or atmospheric CO2. Understory trajectories that are governed by light, as observed here, cannot be interpreted meaningfully in such a context. In our case, these understory phases extended to more than 100 years in at least one tree in the wettest site (cf., Figure 3a), which is significantly longer than the few decades of ‘juvenile’ phase suggested to be excluded (Gagen et al. 2008). This may vary however between species and sites; according to our criteria (see Materials and methods), the average age when reaching the canopy ranged for C. odorata from 37 years in Yucatan to 64 years in Bolivia. Similarly, shade-tolerant species may have much stronger and longer-lasting trends (Vadeboncoeur et al. 2020) compared with shade-intolerant tree species, which do not survive long in the shadow and may lack longer term trends (McCarroll et al. 2020). Even a lack of trends in isotope series (McCarroll et al. 2020) need to be interpreted cautiously, however, as insidious trends due to ontogeny can be hard to distinguish from climate or CO2 effects (Brienen et al. 2017). The danger of possible mis-interpretations of ontogenetic trends in Δ13C for trees’ responses to CO2 is real. For example, Adams et al. (2019) recently showed that increases in iWUE were two times larger in dry compared with wetter tropical forests, but these differences are of the same magnitude as observed in this study, which we show to arise due to differences in the canopy height and structure between forests (Figure 2). Results from Adams et al. (2019) may thus similarly be due to variation in tree height and leaf area index with water availability (Klein et al. 2015, Tao et al. 2016), rather than to different responses to CO2.
Conclusions
We showed that paired measurement of ring width and Δ13C can provide powerful insights in the causes of growth rate variation over trees’ lives. This approach could be highly effective for studying gap regeneration in closed canopy forests (Canham 1989, Clark and Clark 2001) and provide insights in the role of large-scale disturbances in tropical forest dynamics (Vlam et al. 2017) or the causes of tree death by allowing distinguishing light and drought effects on, e.g., pre-death growth declines (Cailleret et al. 2017, Gessler et al. 2018). We find significant shifts in controls on growth over a tree’s lifetime, from light availability when trees are small to drought when trees are tall, with light limitations playing a much more pronounced role in moist forests compared with dry forests. Two lines of evidence suggest increasing limitations of stomatal conductance on carbon gains as trees increase in height and reach full canopy positions. Firstly, at all three sites, we find monotonic decreases in discrimination with increasing tree height, and thus leaf internal CO2 concentration (ci), indicating that stomatal conductance increasingly becomes the bottleneck for carbon assimilation. Secondly, we find that interannual variation in growth and discrimination are increasingly positively correlated when reaching larger tree heights, especially at one of the drier sites. These results are consistent with hydraulic limitation theory, as described by Ryan et al. (2006), and indicate increasing limitations of stomatal conductance on growth for canopy trees. Trees are not limited by one single resource, but resource limitations may change dramatically over a tree’s lifetime. Analysis of ring widths and carbon isotope ratios, as applied here, provides a powerful tool to partition between environmental factors controlling growth over a tree’s life.
Supplementary Material
Acknowledgments
This work has been supported by the Dirección General de Asuntos del Personal Académico of UNAM (Mexico) and the National Environmental Research Council (UK) through an NERC Research Fellowship (grant NE/L0211160/1), NERC standard grant (NE/K01353X/1) and by NERC Isotope Geosciences Facilities grants (IP-1424-0514 and IP-1314-0512). P.G. acknowledges the Miquelfonds and Alberta Mennega Stichting for fieldwork support and current funding by the São Paulo Research Foundation (FAPESP grant 2018/01847-0). We thank Sarah Hunt for help with cellulose extraction and sample preparations.
Contributor Information
Roel Brienen, School of Geography, University of Leeds, Leeds LS2 9JT, UK.
Gerhard Helle, GFZ—German Research Centre for Geosciences, Section 4.3 Climate Dynamics and Landscape Evolution, 14473 Potsdam, Germany.
Thijs Pons, Plant Ecophysiology, Institute of Environmental Biology, Utrecht University, 3512 Utrecht, The Netherlands.
Arnoud Boom, School of Geography, University of Leicester, Leicester LE1 7RH, UK.
Manuel Gloor, School of Geography, University of Leeds, Leeds LS2 9JT, UK.
Peter Groenendijk, Department of Plant Biology, Institute of Biology, PO Box: 6109, University of Campinas, UNICAMP, Campinas 13083-970, Brazil; Ecology and Biodiversity, Institute of Environmental Biology, Utrecht University, 3584 Utrecht, The Netherlands.
Santiago Clerici, School of Geography, University of Leeds, Leeds LS2 9JT, UK.
Melanie Leng, National Environmental Isotope Facility, British Geological Survey, Nottingham NG12 5GG, UK.
Christopher Jones, School of Geography, University of Leeds, Leeds LS2 9JT, UK.
Data availability
All the data and codes used in this publication are available from the authors upon request.
Conflict of interest
None declared.
Authors’ contributions
R.B., T.P. and G.H. designed the research. R.B. and P.G. collected tree ring samples and performed tree ring measurements and dating. G.H., M.L., A.B., C.J., S.C. and R.B. extracted cellulose and measured isotopes. R.B. performed data analysis and wrote the first draft of the manuscript, and all co-authors contributed to revisions.
References
- Adams MA, Buckley TN, Turnbull TL (2019) Rainfall drives variation in rates of change in intrinsic water use efficiency of tropical forests. Nat Commun 10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg WR, Trugman AT, Badgley G, Konings AG, Shaw J (2020) Divergent forest sensitivity to repeated extreme droughts. Nat Clim Change 10:1091–1095. [Google Scholar]
- Andreu L, Planells O, Gutiérrez E, Helle G, Schleser GH (2008) Climatic significance of tree-ring width and δ13C in a Spanish pine forest network. Tellus B Chem Phys Meteorol 60:771–781. [Google Scholar]
- Aranda I, Pardos M, Puértolas J, Jiménez MD, Pardos JA (2007) Water-use efficiency in cork oak (Quercus suber) is modified by the interaction of water and light availabilities. Tree Physiol 27:671–677. [DOI] [PubMed] [Google Scholar]
- Baker JC, Hunt SF, Clerici SJ, et al. (2015) Oxygen isotopes in tree rings show good coherence between species and sites in Bolivia. Glob Planet Change 133:298–308. [Google Scholar]
- Baker JC, Santos GM, Gloor M, Brienen RJ (2017) Does Cedrela always form annual rings? Testing ring periodicity across South America using radiocarbon dating. Trees 31:1999–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PJ, Bunyavejchewin S (2006) Suppression, release and canopy recruitment in five tree species from a seasonal tropical forest in western Thailand. J Trop Ecol 22:521–529. [Google Scholar]
- Barbour MM, Farquhar GD (2000) Relative humidity- and ABA-induced variation in carbon and oxygen isotope ratios of cotton leaves. Plant Cell Environ 23:473–485. [Google Scholar]
- Bennett AC, McDowell NG, Allen CD, Anderson-Teixeira KJ (2015) Larger trees suffer most during drought in forests worldwide. Nat Plants 1:15139. [DOI] [PubMed] [Google Scholar]
- Brienen R, Gloor E, Clerici S,et al. (2017) Tree height strongly affects estimates of water-use efficiency responses to climate and CO2 using isotopes. Nat Commun 8:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brienen RJW, Zuidema PA (2005) Relating tree growth to rainfall in Bolivian rain forests: a test for six species using tree ring analysis. Oecologia 146:1–12. [DOI] [PubMed] [Google Scholar]
- Brienen RJW, Zuidema PA, Martinez-Ramos MM (2010a) Attaining the canopy in dry and moist tropical forests: strong differences in tree growth trajectories reflect variation in growing conditions. Oecologia 163:485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brienen RJW, Lebrija-Trejos E, Zuidema PA, MartÍnez-Ramos MM (2010b) Climate-growth analysis for a Mexican dry forest tree shows strong impact of sea surface temperatures and predicts future growth declines. Glob Chang Biol 16:2001–2012. [Google Scholar]
- Brienen RJW, Wanek W, Hietz P (2011) Stable carbon isotopes in tree rings indicate improved water use efficiency and drought responses of a tropical dry forest tree species. Trees (Berlin) 25:103–113. [Google Scholar]
- Brienen RJW, Helle G, Pons TL, Guyot J-L, Gloor M (2012) Oxygen isotopes in tree rings are a good proxy for Amazon precipitation and El Niño-southern oscillation variability. Proc Natl Acad Sci USA 109:16957–16962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JR, Flanagan LB, Ehleringer JR (1998) Responses of boreal conifers to climate fluctuations: indications from tree-ring widths and carbon isotope analyses. Can J For Res 28:524–533. [Google Scholar]
- Brum M, Vadeboncoeur MA, Ivanov V et al. (2019) Hydrological niche segregation defines forest structure and drought tolerance strategies in a seasonal Amazon forest. J Ecol 107:318–333. [Google Scholar]
- Buchmann N, Guehl JM, Barigah TS, Ehleringer JR (1997) Interseasonal comparison of CO2 concentrations, isotopic composition, and carbon dynamics in an Amazonian rainforest (French Guiana). Oecologia 110:120–131. [DOI] [PubMed] [Google Scholar]
- Cailleret M, Jansen S, Robert EM et al. (2017) A synthesis of radial growth patterns preceding tree mortality. Glob Chang Biol 23:1675–1690. [DOI] [PubMed] [Google Scholar]
- Canham CD (1989) Different responses to gaps among shade-tolerant tree species. Ecology 70:548–550. [Google Scholar]
- Carelli MLC, Fahl JI, Trivelin PCO, RB QV (1999) Carbon isotope discrimination and gas exchange in coffea species grown under different irradiance regimes. Revista Brasileira de Fisiologia Vegetal (Brasil). 11:63–68. [Google Scholar]
- Carrer M, Urbinati C (2004) Age-dependent tree-ring growth responses to climate in Larix decidua and Pinus cembra. Ecology 85:730–740. [Google Scholar]
- Cavaleri MA, Oberbauer SF, Clark DB, Clark DA, Ryan MG (2010) Height is more important than light in determining leaf morphology in a tropical forest. Ecology 91:1730–1739. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Ubierna N, Winter K, Holtum JA, Marshall JD, Farquhar GD (2013) Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytol 200:950–965. [DOI] [PubMed] [Google Scholar]
- Chazdon RL, Fetcher N, Medina E, Mooney HA, Vasques-Yanes C (1984) Light environments of tropical forests. In: Medina E, Mooney HA, Vazquez-Yanes C (eds) Physiological ecology of plants of the wet tropics. Springer, Dordrecht, Hague, The Netherlands, pp 27–36. [Google Scholar]
- Clark DA, Clark DB (1992) Life-history diversity of canopy and emergent trees in a neotropical rain-forest. Ecol Monogr 62:315–344. [Google Scholar]
- Clark DA, Clark DB (2001) Getting to the canopy: tree height growth in a neotropical rain forest. Ecology 82:1460–1472. [Google Scholar]
- Cook ER, Peters K (1980) The smoothing spline: a new approach to standardizing forest interior tree-ring width series for dendroclimatic studies. Tree Ring Bull 41:45–53. [Google Scholar]
- Coste S, Roggy J-C, Sonnier G, Dreyer E (2010) Similar irradiance-elicited plasticity of leaf traits in saplings of 12 tropical rainforest tree species with highly different leaf mass to area ratio. Funct Plant Biol 37:342–355. [Google Scholar]
- Dawson TE (1996) Determining water use by trees and forests from isotopic, energy balance and transpiration analyses: the roles of tree size and hydraulic lift. Tree Physiol 16:263–272. [DOI] [PubMed] [Google Scholar]
- DeSoto L, Cailleret M, Sterck F et al. (2020) Low growth resilience to drought is related to future mortality risk in trees. Nat Commun 11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duursma R, Marshall J (2006) Vertical canopy gradients in δ13C correspond with leaf nitrogen content in a mixed-species conifer forest. Trees 20:496–506. [Google Scholar]
- Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol 11:539–552. [Google Scholar]
- Farquhar GD, Oleary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the inter-cellular carbon-dioxide concentration in leaves. Aust J Plant Physiol 9:121–137. [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537. [Google Scholar]
- Fauset S, Freitas HC, Galbraith DR, Sullivan MJ, Aidar MP, Joly CA, Phillips OL, Vieira SA, Gloor MU (2018) Differences in leaf thermoregulation and water use strategies between three co-occurring Atlantic forest tree species. Plant Cell Environ 41:1618–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francey R, Allison C, Etheridge D, Trudinger C, Enting I, Leuenberger M, Langenfelds R, Michel E, Steele L (1999) A 1000-year high precision record of δ13C in atmospheric CO2. Tellus B 51:170–193. [Google Scholar]
- Franks PJ, Adams MA, Amthor JS et al. (2013) Sensitivity of plants to changing atmospheric CO2 concentration: from the geological past to the next century. New Phytol 197:1077–1094. [DOI] [PubMed] [Google Scholar]
- Gessler A, Cailleret M, Joseph J et al. (2018) Drought induced tree mortality–a tree-ring isotope based conceptual model to assess mechanisms and predispositions. New Phytol 219:485–490. [DOI] [PubMed] [Google Scholar]
- Gagen M, McCarroll D, Robertson I, Loader NJ, Jalkanen R (2008) Do tree ring delta C-13 series from Pinus sylvestris in northern Fennoscandia contain long-term non-climatic trends? Chem Geol 252:42–51. [Google Scholar]
- Gessler A, Ferrio J, Hommel R, Treydte K, Werner R, Monson R (2014) Stable isotopes in tree rings: towards a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood. Tree Physiol 34:796–818. [DOI] [PubMed] [Google Scholar]
- Giuggiola A, Ogée J, Rigling A, Gessler A, Bugmann H, Treydte K (2016) Improvement of water and light availability after thinning at a xeric site: which matters more? A dual isotope approach. New Phytol 210:108–121. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Wong SC, Stuart-Williams H, Holloway-Phillips M, Farquhar GD (2014) Determinants of maximum tree height in eucalyptus species along a rainfall gradient in Victoria, Australia. Ecology 95:2991–3007. [Google Scholar]
- Granato-Souza D, Stahle DW, Barbosa AC, Feng S, Torbenson MC, de Assis Pereira G, Schöngart J, Barbosa JP, Griffin D (2019) Tree rings and rainfall in the equatorial Amazon. Clim Dyn 52:1857–1869. [Google Scholar]
- Groenendijk P (2010) Growth Sensitivity of Cedrela salvadorensis to climate and its potential responses to future climate changes in southern Mexico. Utrecht University, Utrecht, The Netherlands. [Google Scholar]
- Guzmán JA, Cordero RA, Corea E (2016) Biomass allocation and gas exchange are affected by light conditions in endangered Cedrela salvadorensis (Meliaceae) seedlings. Rev Biol Trop 64:1143–1154. [DOI] [PubMed] [Google Scholar]
- Harris PP, Huntingford C, Cox PM, Gash JHC, Malhi Y (2004) Effect of soil moisture on canopy conductance of Amazonian rainforest. Agric For Meteorol 122:215–227. [Google Scholar]
- Houter NC, Pons TL (2012) Ontogenetic changes in leaf traits of tropical rainforest trees differing in juvenile light requirement. Oecologia 169:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DJ, Needham J, Xu C, Massoud EC, Davies SJ, Anderson-Teixeira KJ, Bunyavejchewin S, Chambers JQ, Chang-Yang C-H, Chiang J-M (2018) Climate sensitive size-dependent survival in tropical trees. Nat Ecol Evol 2:1436. [DOI] [PubMed] [Google Scholar]
- Keeling RF, Graven HD, Welp LR, Resplandy L, Bi J, Piper SC, Sun Y, Bollenbacher A, Meijer HA (2017) Atmospheric evidence for a global secular increase in carbon isotopic discrimination of land photosynthesis. Proc Natl Acad Sci USA 114:10361–10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kira T, Yoda K (1989) Vertical stratification in microclimate. In: Leith H, Werger MJA (eds) Tropical rain forest ecosystems. pp. 59–71. [Google Scholar]
- Kira T, Shinizaki K, Hosumi K (1969) Structure of forest canopies as related to their primary productivity. Plant Cell Physiol 10:129–142. [Google Scholar]
- Klein T, Randin C, Körner C (2015) Water availability predicts forest canopy height at the global scale. Ecol Lett 18:1311–1320. [DOI] [PubMed] [Google Scholar]
- Klesse S, Weigt R, Treydte K, Saurer M, Schmid L, Siegwolf RT, Frank DC (2018) Oxygen isotopes in tree rings are less sensitive to changes in tree size and relative canopy position than carbon isotopes. Plant Cell Environ 41:2899–2914. [DOI] [PubMed] [Google Scholar]
- Koch GW, Sillett SC, Jennings GM, Davis SD (2004) The limits to tree height. Nature 428:851–854. [DOI] [PubMed] [Google Scholar]
- Kunert N, Schwendenmann L, Holscher D (2010) Seasonal dynamics of tree sap flux and water use in nine species in Panamanian forest plantations. Agric For Meteorol 150:411–419. [Google Scholar]
- Leavitt SW, Woodhouse CA, Castro CL, Wright WE, Meko DM, Touchan R, Griffin D, Ciancarelli B (2011) The North American monsoon in the US southwest: potential for investigation with tree-ring carbon isotopes. Quat Int 235:101–107. [Google Scholar]
- Lloyd J, Farquhar GD (2008) Effects of rising temperatures and [CO2] on the physiology of tropical forest trees. Philos Trans R Soc Lond B Biol Sci 363:1811–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López L, Villalba R (2011) Climate influences on the radial growth of Centrolobium microchaete, a valuable timber species from the tropical dry forests in Bolivia. Biotropica 43:41–49. [Google Scholar]
- McCarroll D, Duffy JE, Loader NJ, Young GH, Davies D, Miles D, Bronk Ramsey C (2020) Are there enormous age-trends in stable carbon isotope ratios of oak tree rings? Holocene 30:1637–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell B, Bond H, Hubbard I, Köstner M, Marshall M, Phillips R, Whitehead D (2002) The relationship between tree height and leaf area: sapwood area ratio. Oecologia 132:12–20. [DOI] [PubMed] [Google Scholar]
- McDowell NG, Allen CD (2015) Darcy's law predicts widespread forest mortality under climate warming. Nat Clim Chang 5:669. [Google Scholar]
- McDowell NG, Bond BJ, Dickman LT, Ryan MG, Whitehead D (2011) Relationships between tree height and carbon isotope discrimination. Size-and age-related changes in tree structure and function. Eds (FC Meinzer, B Lachenbruch, TE Dawson). Springer, Dordrecht, pp 255–286. [Google Scholar]
- McGregor IR, Helcoski R, Kunert N et al. (2021) Tree height and leaf drought tolerance traits shape growth responses across droughts in a temperate broadleaf forest. New Phytol 231:601–616. [DOI] [PubMed] [Google Scholar]
- Mencuccini M, Grace J (1995) Climate influences the leaf area/sapwood area ratio in scots pine. Tree Physiol 15:1–10. [DOI] [PubMed] [Google Scholar]
- Mendivelso HA, Camarero JJ, Gutiérrez E, Zuidema PA (2014) Time-dependent effects of climate and drought on tree growth in a Neotropical dry forest: short-term tolerance vs. long-term sensitivity. Agric For Meteorol 188:13–23. [Google Scholar]
- Mérian P, Lebourgeois F (2011) Size-mediated climate–growth relationships in temperate forests: a multi-species analysis. For Ecol Manage 261:1382–1391. [Google Scholar]
- Montgomery RA, Chazdon RL (2001) Forest structure, canopy architecture, and light transmittance in tropical wet forests. Ecology 82:2707–2718. [Google Scholar]
- Olson ME, Soriano D, Rosell JA et al. (2018) Plant height and hydraulic vulnerability to drought and cold. Proc Natl Acad Sci USA 115:7551–7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter L, Hayashida-Oliver Y (2000) Effects of seasonal drought on gap and understorey seedlings in a Bolivian moist forest. J Trop Ecol 16:481–498. [Google Scholar]
- Poorter L, Bongers F, Sterck F, Woll H (2005) Beyond the regeneration phase: differentiation of height-light growth trajectories among tropical tree species. J Ecol 93:256–267. [Google Scholar]
- Prentice IC, Meng T, Wang H, Harrison SP, Ni J, Wang G (2011) Evidence of a universal scaling relationship for leaf CO2 drawdown along an aridity gradient. New Phytol 190:169–180. [DOI] [PubMed] [Google Scholar]
- Rijkers T, Pons TL, Bongers F (2000) The effect of tree height and light availability on photosynthetic leaf traits of four neotropical species differing in shade tolerance. Funct Ecol 14:77–86. [Google Scholar]
- Ryan MG, Yoder BJ (1997) Hydraulic limits to tree height and tree growth. Bioscience 47:235–242. [Google Scholar]
- Ryan MG, Phillips N, Bond BJ (2006) The hydraulic limitation hypothesis revisited. Plant Cell Environ 29:367–381. [DOI] [PubMed] [Google Scholar]
- Saurer M, Borella S, Schweingruber F, Siegwolf R (1997) Stable carbon isotopes in tree rings of beech: climatic versus site-related influences. Trees Struct Funct 11:291–297. [Google Scholar]
- Saurer M, Siegwolf RTW, Schweingruber FH (2004) Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years. Glob Chang Biol 10:2109–2120. [Google Scholar]
- Schubert BA, Jahren AH (2015) Global increase in plant carbon isotope fractionation following the last glacial maximum caused by increase in atmospheric pCO2. Geology 43:435–438. [Google Scholar]
- Schulze E-D, Turner NC, Nicolle D, Schumacher J (2006) Leaf and wood carbon isotope ratios, specific leaf areas and wood growth of Eucalyptus species across a rainfall gradient in Australia. Tree Physiol 26:479–492. [DOI] [PubMed] [Google Scholar]
- Seibt U, Rajabi A, Griffiths H, Berry JA (2008) Carbon isotopes and water use efficiency: sense and sensitivity. Oecologia 155:441–454. [DOI] [PubMed] [Google Scholar]
- Slot M, Janse-ten Klooster SH, Sterck FJ, Sass-Klaassen U, Zweifel R (2012) A lifetime perspective of biomass allocation in Quercus pubescens trees in a dry, alpine valley. Trees 26:1661–1668. [Google Scholar]
- Steppe K, Niinemets Ü, Teskey RO (2011) Tree size-and age-related changes in leaf physiology and their influence on carbon gain. In: Size- and age related changes in tree structure and function. Eds (FC Meinzer, B Lachenbruch, TE Dawson). Springer, Dordrecht, pp 235–253. [Google Scholar]
- Tao S, Guo Q, Li C, Wang Z, Fang J (2016) Global patterns and determinants of forest canopy height. Ecology 97:3265–3270. [DOI] [PubMed] [Google Scholar]
- Tardieu F, Davies W (1993) Integration of hydraulic and chemical signalling in the control of stomatal conductance and water status of droughted plants. Plant Cell Environ 16:341–349. [Google Scholar]
- Thomas SC (2011) Age-related changes in tree growth and functional biology: the role of reproduction. In: Meinzer F, Lachenbruch B, Dawson T (eds) Size and age-related changes in tree structure and function. Springer, The Netherlands, pp 33–64. [Google Scholar]
- Trouillier M, van der Maaten-Theunissen M, Scharnweber T, Würth D, Burger A, Schnittler M, Wilmking M (2019) Size matters—a comparison of three methods to assess age-and size-dependent climate sensitivity of trees. Trees 33:183–192. [Google Scholar]
- Turner IM (2002) The ecology of trees in the tropical rainforest. Cambridge University Press, Cambridge. [Google Scholar]
- Vadeboncoeur MA, Jennings KA, Ouimette AP, Asbjornsen H (2020) Correcting tree-ring δ13C time series for tree-size effects in eight temperate tree species. Tree Physiol 40:333–349. [DOI] [PubMed] [Google Scholar]
- van der Sleen P, Groenendijk P, Vlam M, Anten NP, Boom A, Bongers F, Pons TL, Terburg G, Zuidema PA (2015) No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nat Geosci 8:4. [Google Scholar]
- van der Sleen P, Soliz-Gamboa C, Helle G, Pons T, Anten N, Zuidema P (2014) Understanding causes of tree growth response to gap formation: ∆13C-values in tree rings reveal a predominant effect of light. Trees 28:439–448. [Google Scholar]
- Vlam M, Baker PJ, Bunyavejchewin S, Zuidema PA (2014) Temperature and rainfall strongly drive temporal growth variation in Asian tropical forest trees. Oecologia 174:1449–1461. [DOI] [PubMed] [Google Scholar]
- Vlam M, van der Sleen P, Groenendijk P, Zuidema PA (2017) Tree age distributions reveal large-scale disturbance-recovery cycles in three tropical forests. Front Plant Sci 7:1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker SL, Meinzer FC, Lachenbruch B, Brooks JR, Guyette RP (2014) Drivers of radial growth and carbon isotope discrimination of bur oak (Quercus macrocarpa Michx.) across continental gradients in precipitation, vapour pressure deficit and irradiance. Plant Cell Environ 37:766–779. [DOI] [PubMed] [Google Scholar]
- Voelker SL, Brooks JR, Meinzer FC et al. (2016) A dynamic leaf gas-exchange strategy is conserved in woody plants under changing ambient CO2: evidence from carbon isotope discrimination in paleo and CO2 enrichment studies. Glob Chang Biol. 22:889–902. [DOI] [PubMed] [Google Scholar]
- Wagner FH, Hérault B, Bonal D et al. (2016) Climate seasonality limits leaf carbon assimilation and wood productivity in tropical forests. Biogeosciences 13:2537–2562. [Google Scholar]
- Wieloch T, Helle G, Heinrich I, Voigt M, Schyma P (2011) A novel device for batch-wise isolation of α-cellulose from small-amount wholewood samples. Dendrochronologia 29:115–117. [Google Scholar]
- Wilmking M, van der Maaten-Theunissen M, van der Maaten E et al. (2020) Global assessment of relationships between climate and tree growth. Glob Chang Biol 26:3212–3220. [DOI] [PubMed] [Google Scholar]
- Worbes M (1999) Annual growth rings, rainfall-dependent growth and long-term growth patterns of tropical trees from the Caparo Forest Reserve in Venezuela. J Ecol 87:391–403. [Google Scholar]
- Young GH, McCarroll D, Loader NJ, Kirchhefer AJ (2010) A 500-year record of summer near-ground solar radiation from tree-ring stable carbon isotopes. Holocene 20:315–324. [Google Scholar]
- Zuidema PA, Heinrich I, Rahman M, Vlam M, Zwartsenberg SA, van der Sleen P (2020) Recent CO2 rise has modified the sensitivity of tropical tree growth to rainfall and temperature. Glob Chang Biol. 26:4028–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data and codes used in this publication are available from the authors upon request.