Abstract
Bacterial wilt and canker caused by Clavibacter michiganensis (Cm) inflict considerable damage in tomato‐growing regions around the world. Cm has a narrow host range and can cause disease in tomato but not in many eggplant varieties. The pathogenicity of Cm is dependent on secreted serine proteases, encoded by the chp/tomA pathogenicity island (PI), and the pCM2 plasmid. Screening combinations of PI deletion mutants and plasmid‐cured strains found that Cm‐mediated hypersensitive response (HR) in the Cm‐resistant eggplant variety Black Queen is dependent on the chp/tomA PI. Singular reintroduction of PI‐encoded serine proteases into Cm∆PI identified that the HR is elicited by the protease ChpG. Eggplant leaves infiltrated with a chpG marker exchange mutant (CmΩchpG) did not display an HR, and infiltration of purified ChpG protein elicited immune responses in eggplant but not in Cm‐susceptible tomato. Virulence assays found that while wild‐type Cm and the CmΩchpG complemented strain were nonpathogenic on eggplant, CmΩchpG caused wilt and canker symptoms. Additionally, bacterial populations in CmΩchpG‐inoculated eggplant stem tissues were c.1000‐fold higher than wild‐type and CmΩchpG‐complemented Cm strains. Pathogenicity tests conducted in multiple Cm‐resistance eggplant varieties demonstrated that immunity to Cm is dependent on ChpG in all tested varieties, indicating that ChpG‐recognition is conserved in eggplant. ChpG‐mediated avirulence interactions were disabled by alanine substitution of serine231 of the serine protease catalytic triad, suggesting that protease activity is required for immune recognition of ChpG. Our study identified ChpG as a novel avirulence protein that is recognized in resistant eggplant varieties and restricts the host range of Cm.
Keywords: avirulence protein, bacterial wilt and canker of tomato, Clavibacter, eggplant, host specificity, plant immunity, serine protease
The secreted serine protease ChpG of Clavibacter michiganensis functions as an avirulence protein that elicits a hypersensitive response and restricts the pathogen from causing disease in resistant eggplant varieties.

1. INTRODUCTION
Clavibacter is a genus of plant‐associated actinobacteria (Li et al., 2018). The genus is composed of multiple pathogenic species, which usually possess high host specificity and are restricted to a small number of host plant species (Nandi et al., 2018). Diseases caused by Clavibacter spp. include bacterial wilt and canker of tomato (C. michiganensis), potato ring rot (C. sepedonicus), Gross's wilt of maize (C. nebraskensis), bacterial canker of pepper (C. capsici), bacterial wilt of alfalfa (C. insidiosus), bacterial mosaic of wheat (C. tessellarius), bean leaf yellowing (C. phaseoli), and leaf brown spot of barley (C. zhangzhiyongii) (Nandi et al., 2018; Tian et al., 2021). In particular, Clavibacter‐mediated diseases significantly affect the tomato, maize, and potato industries, resulting in substantial yield losses worldwide (Hukkanen et al., 2005; Jackson et al., 2007; Peritore‐Galve et al., 2021). Despite their economic importance, pathogenicity and host specificity determinants of these bacteria are not well characterized and resistance loci that confer immunity to Clavibacter spp. have yet to be identified.
Bacterial wilt and canker caused by C. michiganensis (Cm) is one of the most important diseases of tomato (Solanum lycopersicum). The disease is characterized by the appearance of spreading canker lesions in the main stem and secondary branches, leaf wilting, leaf tissue necrosis, and the appearance of “bird's‐eye” spotting on fruit (Peritore‐Galve et al., 2021). Vascular collapse, which results in death, is frequent in heavily infected plants (Peritore‐Galve et al., 2021). Latent infection, which enables further spread into neighbouring plants and contaminated seeds, is common as well (Gitaitis et al., 1991). Cm spreads throughout the plant by systemically colonizing host xylem vessels and bacteria can reach 1010 colony‐forming units (cfu) per gram plant tissue (Chalupowicz et al., 2012), which results in vascular collapse and leaf wilting due to blockage of water‐conducting elements. Cm bacteria are usually introduced into fields or greenhouses through contaminated seed lots and spread to neighbouring plants through water splash or human‐based transfer of Cm‐contaminated guttation fluid released by the hydathodes (Sharabani et al., 2013; Tsiantos, 1987). Because no commercial Cm‐resistant tomato varieties are available, the disease is mainly controlled by horticultural practices and extensive screening for seed contamination (Blank et al., 2016; de León et al., 2011).
Multiple wild tomato varieties and other nonhost solanaceous plants exhibit resistance or high tolerance to Cm. Resistance quantitative trait loci (QTLs) were identified in Solanum peruvianum and Solanum habrochaites (Coaker & Francis, 2004; van Heusden et al., 1999). However, backcross attempts into domesticated tomatoes only conferred partial resistance to the pathogen (Coaker & Francis, 2004; van Heusden et al., 1999). A recent study aimed at characterizing eggplant (Solanum melongena) susceptibility to Cm identified that numerous eggplant varieties demonstrate moderate to high resistance to Cm (Boyaci et al., 2021). In addition, reciprocal crosses between susceptible and resistant varieties followed by segregation analyses identified that resistance to Cm is a dominant trait that follows Mendelian‐like segregation, indicating that eggplant resistance to Cm is likely to be linked to a single locus (Boyaci et al., 2021).
The main virulence determinants encoded by Cm are localized in the 129 kb chp/tomA pathogenicity island (PI) and two plasmids, pCM1 (c.27 kb) and pCM2 (c.70 kb) (Gartemann et al., 2008; Meletzus & Eichenlaub, 1991). The chp/tomA PI is composed of two genomic regions: the 79 kb chp subregion has a low gene density (putative coding capacity of 45.3%) and mainly encodes secreted hydrolases such as proteases, pectinases, and glycosyl hydrolases (Gartemann et al., 2008). The 50 kb tomA subregion has a high gene density (putative coding capacity of 96.3%) and mostly encodes genes associated with perception and uptake of metabolites and carbohydrates (Gartemann et al., 2008). Two independent clones harbouring a deletion of the entire chp/tomA PI were identified in Cm NCPPB 382 (Chalupowicz et al., 2010; Gartemann et al., 2008). Both clones are unable to properly colonize or cause symptoms in tomato (Chalupowicz et al., 2010; Gartemann et al., 2008). Curing of pCM1 or pCM2 does not significantly affect localized colonization at the inoculation site but has a major effect on distal colonization and symptom development, which indicates that pCM1‐ and pCM2‐encoded virulence factors are required for xylem spread of the bacterium (Chalupowicz et al., 2012; Meletzus et al., 1993). Multiple genomic studies identified that the chp/tomA PI and pCM1‐like plasmid occur in all currently sequenced pathogenic Cm clones, further validating their importance of virulence (Méndez et al., 2020; Thapa et al., 2017). Cm lacks any known translocation apparatus and mainly depends on Sec‐dependent secreted carbohydrate‐active enzymes and serine proteases to facilitate pathogenic interaction with the host (Chalupowicz et al., 2017; Jahr et al., 2000; Thapa et al., 2017). A conserved family of putative secreted serine proteases that share homology to the pCM2‐encoded Pat‐1 protein play an important role in the virulence of Cm. Pat‐1 homologs are of c.280 amino acids and harbour an N‐terminal signal peptide and a C‐terminal serine proteases trypsin‐type domain (Stork et al., 2008). Cm NCPPB 382 encodes seven functional Pat‐1 homologs: three are encoded in the pCM2 plasmid (Pat‐1, PhpA, PhpB) and four (ChpC, ChpE, ChpF, ChpG) are encoded by the chp subregion of the chp/tomA PI (Gartemann et al., 2008). Pat‐1 homologs have been found to be enriched in the secretome of Cm grown in rich and xylem‐mimicking media, which indicates they are indeed secreted proteins (Savidor et al., 2012). In addition, Pat‐1 homologs are transcriptionally and translationally induced in planta and in xylem‐mimicking media and additively contribute to the virulence of Cm (Chalupowicz et al., 2010, 2017; Savidor et al., 2012, 2014). Pat‐1 and the Pat‐1 homolog ChpC are particularly important for the virulence of Cm. A chpC inactivation mutant is impaired in host colonization and is unable to cause disease symptoms (Stork et al., 2008) while Pat‐1 is the main pCM2‐encoded virulence factor and is required for symptom development and systemic colonization of tomato (Chalupowicz et al., 2012; Dreier et al., 1997). Pat‐1 homologs function as virulence factors in other Clavibacter spp. and ChpG of C. capsici and Chp‐7 C. sepedonicus play a significant role in pathogenesis on pepper and eggplant (Hwang et al., 2020; Nissinen et al., 2009). Besides their role in virulence, Pat‐1 homologs have been reported to elicit localized cell death in nonhost plants. Specifically, Chp‐7 of C. sepedonicus and ChpG of Cm elicit hypersensitive response (HR)‐like cell death on Nicotiana tabacum and Mirabilis jalapa (Lu et al., 2015; Nissinen et al., 2009; Stork et al., 2008). This indicates that these proteases potentially play a role in host specificity through their recognition in nonhost plants.
In this study, we identified that the Pat‐1 homolog ChpG functions as a host specificity determinant that restricts Cm from colonizing certain eggplant varieties through elicitation of host‐specific immune responses.
2. RESULTS
2.1. The chp/tomA PI is required for immune recognition of Cm in eggplant
The host range of Cm is mostly restricted to tomato and pepper; the bacteria cannot cause disease in potato and in numerous varieties of eggplant (Boyaci et al., 2021; Yim et al., 2012). We speculated that immune recognition plays a role in determining the host range of Cm. To test this, we examined whether Cm induces selective HR localized cell death, a hallmark of immune activation, in eggplant. Tomato (cv. Moneymaker), pepper (cv. Early California Wonder), and eggplant (cv. Black Queen) leaves were infiltrated with suspensions of Cm and monitored for the appearance of HR and disease symptoms. HR was not observed as of 72 h postinoculation (hpi) in tomato and pepper (Table 1 and Figure S1), and infiltrated tissues developed chlorotic symptoms that later became necrotic between 5 and 10 days postinoculation (dpi), similar to the leaf symptoms reported by Chalupowicz et al. (2017). On the other hand, eggplant leaves inoculated with Cm developed HR‐like localized cell death between 36 and 72 hpi (Table 1 and Figure S1).
TABLE 1.
Induction of hypersensitive response (HR) on solanaceous crops by Clavibacter michiganensis mutant strains
| Genotype | Strain name | pCM1 | pCM2 | PI | Tomato | Pepper | Eggplant |
|---|---|---|---|---|---|---|---|
| Cm | NCPPB 382 | + a | + | + | N b | N | HR b |
| Cm−pCM2 | CMM101 | + | − | + | N | N | HR |
| Cm−pCM1 | CMM102 | − a | + | + | N | N | HR |
| Cm−pCM1,−pCM2 | CMM100 | − | − | + | N | N | HR |
| Cm∆PI , −pCM2 | CMM30‐18 | + | − | − | N | N | N |
| Cm∆PI | CMM27 | + | + | − | N | N | N |
+ or − indicates the presence/absence of the pCM1, pCM2, and chp/tomA pathogenicity island (PI) in the strain.
HR, localized cell death was observed within 72 h after infiltrating OD600 = 0.1 of the indicated strain; N, no response was observed.
Host‐specific immune responses are usually facilitated through recognition of secreted virulence effectors. Most secreted virulence effector proteins of Cm are encoded within the chp/tomA PI and the plasmids pCM1 and pCM2 (Gartemann et al., 2008). Therefore, we hypothesized that these genomic regions are likely to encode an avirulence effector. To test this, eggplant leaves were infiltrated with a series of Cm mutant strains, carrying combinations of deletions of the chp/tomA PI and curing of pCM1 or pCM2 plasmids (Table 1 and Figure S1), and monitored for HR. The occurrence of pCM1 or pCM2 did not affect HR induction in eggplant. On the other hand, two independent deletion mutant strains of the chp/tomA PI failed to elicit HR in eggplant (Table 1 and Figure S1). Similar to the wild‐type Cm strain, none of the mutants affected HR elicitation in tomato or pepper (Table 1 and Figure S1). The analyses suggests that immunity of eggplant to Cm is mediated by recognition of a chp/tomA PI‐encoded protein.
2.2. The protease effector ChpG induces host‐specific HR in eggplant
The chp region of the chp/tomA PI encodes a large number of secreted hydrolases that potentially could be recognized in eggplant as immune elicitors. In particular, it encodes four secreted proteases of the Pat‐1 family (chpC, chpE, chpF, and chpG; Figure 1a), of which specific members were previously reported to elicit HR‐like cell death in certain nonhost genotypes (Lu et al., 2015; Nissinen et al., 2009; Stork et al., 2008). Elicitation of an HR in eggplant by the four Pat‐1 homologs was examined by singular introduction of each homolog into the chp/tomA PI deletion strain CMM30‐18 (Cm∆PI) and using marker exchange mutant strains of the Pat‐1 homologs in the background of CMM101 (referred to throughout the manuscript as Cm). The four Pat‐1 homologs along with their putative promoter regions (250–1000 bp upstream of the start codon) were amplified from Cm, cloned into the pHN216 Escherichia coli–Clavibacter shuttle vector (Laine et al., 1996), and introduced into Cm∆PI. Transcript accumulation of the introduced Pat‐1 homologs in Cm∆PI was confirmed by reverse transcription (RT)‐PCR (Figure S2). Infiltration of eggplant leaves with Cm∆PI carrying a plasmid encoding ChpG restored the ability to elicit an HR (Figure 1b). Cm∆PI carrying ChpC, ChpE or ChpF failed to elicit an HR in eggplant in a similar manner to Cm∆PI (Figure 1b). Eggplant leaves infiltrated with chpC, chpE and chpF marker‐exchanged mutants developed an HR in the same intensity and kinetics as the wild‐type Cm (Figure 1c) while eggplant leaves infiltrated with the chpG marker‐exchanged mutant (CmΩchpG) failed to develop an HR by 72 hpi (Figures 1c and 2a). In addition, CmΩchpG infiltrated leaves exhibited chlorotic symptoms that later became water‐soaked and necrotic around 6–10 dpi (Figure S3). HR cell death was further validated by quantification of ion leakage (Figure 2b).
FIGURE 1.
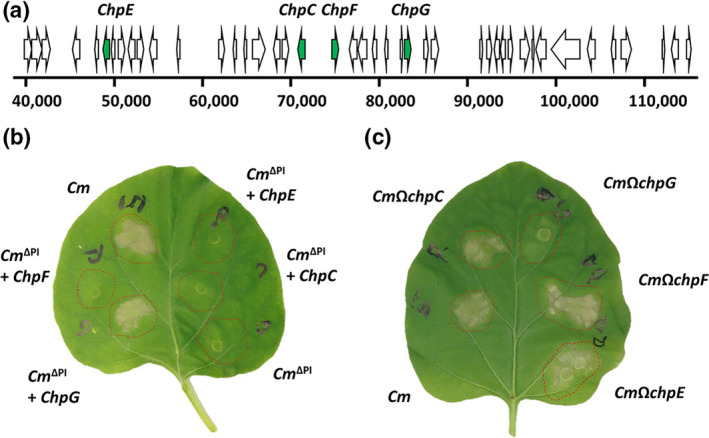
Hypersensitive response (HR) in eggplant is mediated by a pathogenicity island (PI)‐encoded Pat‐1 homolog. (a) Physical map of the chp genomic island of Cm NCPPB 382 (38,000–117,000 region). Open reading frames encoding Pat‐1 homologs are marked in green. (b) Clavibacter michiganensis (Cm) CMM30‐18 (Cm∆PI), a chp/tomA island deletion strain, carrying pHN216 plasmids encoding for the indicted Pat‐1 homologs were syringe infiltrated (108 cfu/ml) into Black Queen eggplant. Pictures were taken 72 h later. (c) CMM101 (Cm) strains harbouring insertional inactivation of the indicated Pat‐1 homologs were syringe infiltrated (108 cfu/ml) into Black Queen eggplant. Pictures were taken 72 h later. Data was repeated in three independent experiments. Five plants were used for each experimental repeat
FIGURE 2.
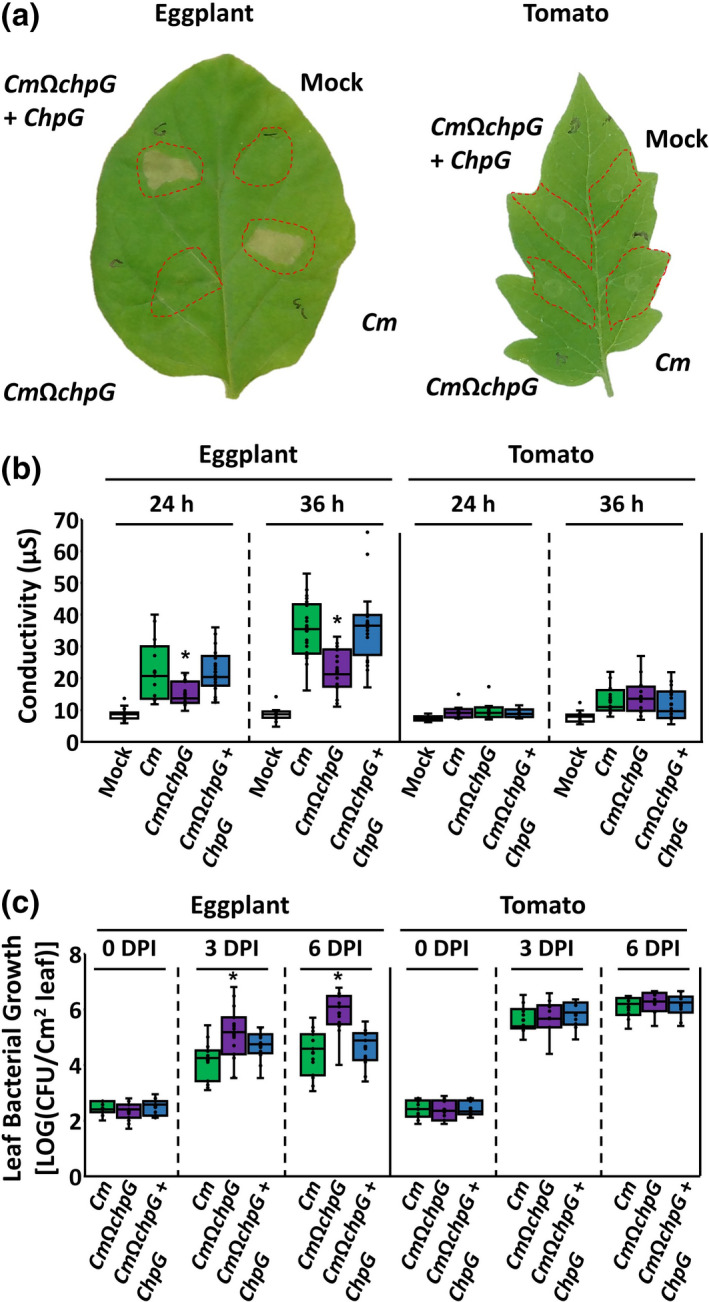
ChpG is required for elicitation of hypersensitive response (HR) in eggplant. Black Queen eggplant and Moneymaker tomato leaves were infiltrated (108 cfu/ml for a and b, 104 cfu/ml for c) with Clavibacter michiganensis wild‐type CMM101 (Cm), CMM101 chpG inactivation mutant (CmΩchpG), and CmΩchpG carrying pHN216:ChpG (CmΩchpG + ChpG). (a) Leaves were photographed 72 h after infiltration. (b) Cell death was quantified by ion leakage at 24 and 36 h postinfiltration. Lower and upper quartiles are marked at the margins of the boxes. Central lines and “o” represent medians and data points of at least 15 (for eggplant) or 10 (for tomato) biological repeats collected from two (tomato at 24 h) or three independent experiments. * indicates significant difference (Mann–Whitney U test, p ≤ 0.05) from Cm. (c) Leaf apoplast bacterial populations of the indicated Cm strains were quantified at 0, 3, and 6 days postinoculation (dpi). Lower and upper quartiles are marked at the margins of the boxes. Central lines and “o” represent medians and data points of 15 (for eggplant) or 10 (for tomato) biological repeats collected from two (tomato) or three (eggplant) independent experiments. * indicates significant difference (Mann–Whitney U test, p ≤ 0.05) from Cm
Elicitation of immune responses that result in HR usually correlates to the restriction of bacterial spread (Balint‐Kurti, 2019), therefore we monitored leaf bacterial colonization in eggplant and identified that CmΩchpG populations were c.50‐fold higher at 6 dpi compared to the wild‐type Cm strain (Figure 2c). Introduction of pHN216:ChpG complementation vector into CmΩchpG reduced bacterial leaf colonization to the levels of the wild‐type Cm strain and restored HR elicitation in eggplant (Figure 2a–c). To confirm that the data represent a host‐specific avirulent interaction, leaf infiltration tests were repeated in susceptible tomato. The experiments showed that inactivation of chpG affected neither HR elicitation nor leaf colonization in tomato (Figure 2a–c). The data suggest that ChpG is an elicitor of host‐specific immune responses in eggplant.
2.3. Cm chpG mutant is pathogenic on eggplant
Immune recognition of secreted virulence effectors is common in resistant plants. However, effector recognition is not the only factor that determines host range, which can be dictated by a combination of numerous factors such as host physiology, metabolic state, production of secondary chemical compounds, structural variations in target tissues, and altered host physical barriers (van den Bosch et al., 2020; Favaro et al., 2014; Pizarro et al., 2020; Stice et al., 2020; Sun et al., 2011).
We aimed to decipher whether ChpG recognition is the main determinant that restricts Cm from causing disease in eggplant. To do so, eggplant and susceptible tomato plants were inoculated with Cm, CmΩchpG, and the complemented strain and monitored for disease development and systemic colonization. Four‐leaf stage eggplant and tomato plants were inoculated with the aforementioned Cm strains by puncturing the main stem area between the cotyledons with a wooden toothpick soaked in Cm suspensions (5 × 107 cfu/ml). Inoculated plants were monitored for a duration of 10 days and quantified for the severity of wilting (according to the percentage of wilted/leaf blotched leaves), canker development (according to the speared of the canker lesion), and stem bacterial colonization.
Tomato plants inoculated with the three strains developed wilt and canker symptoms, and stem bacterial populations 1 cm above the puncher site reached 109–1010 cfu/g stem at 10 dpi (Figure S4). No significant difference was observed in the severity of disease symptoms and bacterial colonization between the chpG mutant and the wild‐type strain in tomato (Figure S4).
Wild‐type Cm and the ChpG‐complemented strain (CmΩchpG + ChpG) were nonpathogenic on eggplant and bacterial colonization only reached c.106 cfu/g stem (Figure 3a,b). The strains did not cause any significant wilting or leaf blotch symptoms (Figure 3c,d), and canker lesions were localized to the inoculated areas and did not significantly spread throughout the main stem (Figure 3e,f). On the other hand, eggplants inoculated with CmΩchpG developed wilt and leaf blotch symptoms in 50%–100% of the leaves (Figure 3a,c,d), large expanded canker lesions (Figure 3e,f), and eruptions of independent canker lesions on the upper stem regions and leaf petioles. In addition, CmΩchpG populations in the stem area 1 cm above the puncher site were around c.1000‐higher than the wild‐type and complemented strains and reached 5 × 108–1010 cfu/g stem (Figure 3b).
FIGURE 3.
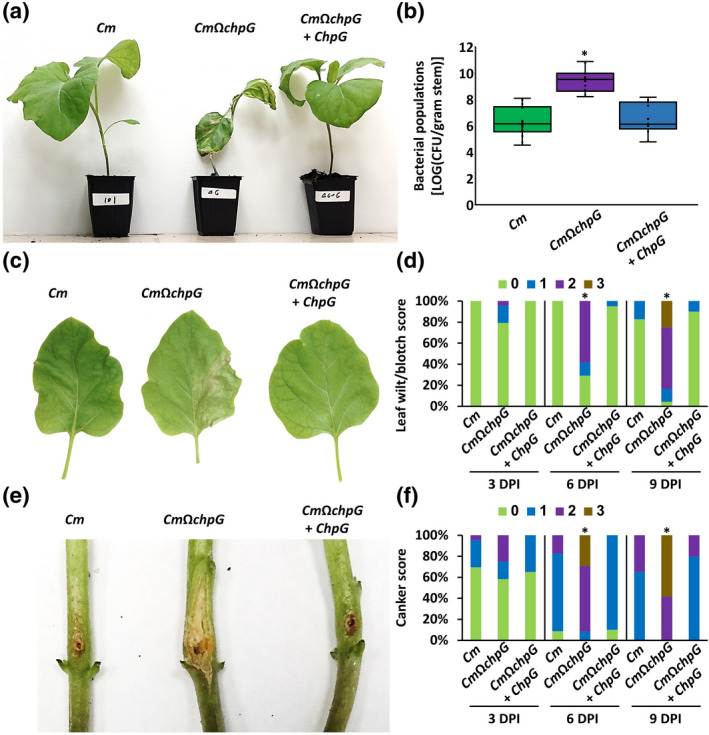
chpG inactivation mutant is pathogenic on eggplant. Four‐leaf stage Black Queen eggplants were inoculated with Clavibacter michiganensis wild‐type CMM101 (Cm), CMM101 chpG inactivation mutant (CmΩchpG), and CmΩchpG carrying pHN216:ChpG (CmΩchpG + ChpG) by puncturing the stem area between the cotyledons with a wooden toothpick soaked in Cm solution (5 × 107 cfu/ml). (a) Picture was taken 10 days postinoculation (dpi). (b) Stem bacterial populations 1 cm above the inoculation site at 10 dpi. Lower and upper quartiles are marked at the margins of the boxes. Central lines and “o” represent medians and data points of 14 biological repeats collected from four independent experiments. * indicates significant difference (Mann–Whitney U test, p ≤ 0.05) from Cm. (c) Leaves from the second rosette were photographed at 10 dpi. (d) Leaf wilt and blotch symptoms in each plant were scored according to the percentage of symptomatic leaves (0 = no leaf wilt/blotch, 1 = 1%–25%, 2 = 26%–50%, 3 = 51%–100%) at 3, 6, and 9 dpi. Bar charts represent the distribution of scores of 23 (Cm), 24 (CmΩchpG) or 20 (CmΩchpG + ChpG) plants pooled from four experimental repeats. * indicates the score distribution is different from Cm (Pearson's chi‐squared test, p ≤ 0.05). (e) Canker lesions were photographed at 10 dpi. (f) Canker symptoms of each plant were scored according to length of the canker lesion (0 = no canker, 1 = <0.1 cm, 2 = 0.1–1 cm, 3 = >1 cm) at 3, 6, and 9 dpi. Bar charts represent the distribution of scores of 23 (Cm), 24 (CmΩchpG) or 20 (CmΩchpG + ChpG) plants pooled from four experimental repeats. * indicates the score distribution is different from Cm (Pearson's chi‐squared test, p ≤ 0.05)
Taken together, the results demonstrate that ChpG is a bona fide avirulence gene that restricts the host range of Cm on eggplant.
2.4. Immune recognition of ChpG is dependent on its catalytic activity
Recognition of avirulent proteins is usually associated with plant immune receptors that can directly recognize the effector through protein–protein interaction or indirectly recognize it through its activity on a guardee or a decoy target (Kourelis & van der Hoorn, 2018). The following experiments aimed to identify the mode of ChpG‐mediated immune recognition in eggplant. First, recognition of ChpG as a standalone elicitor was examined. ChpG was fused to a maltose‐binding protein (MBP) tag and purified from Escherichia coli (Figure 4a, western blot validation is presented in Figure S5). Eggplant and tomato leaves were infiltrated with purified MBP or MBP‐ChpG and monitored for elicitation of immune responses through visual inspection of localized cell death, quantification of cell death by ion leakage, and quantification of secreted peroxidase (POX) activity, which was previously reported to be induced in response to innate immune activation in plants (Daudi et al., 2012; Felix et al., 1999; Seto et al., 2020). MBP‐ChpG‐infiltrated but not MBP‐infiltrated eggplant leaf areas developed localized cell death 24–48 h postinfiltration (Figure 4b), which was accompanied by increased ion leakage and POX activity (Figure 4c,d). No difference was observed between MBP and MBP‐ChpG infiltrated regions in tomato (Figure 4b–d). Next, we tested whether ChpG recognition is mediated by its catalytic activity. Sequence‐based function and structure modulation analyses conducted using NCBI conserved domain (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) and SWISS MODEL (https://swissmodel.expasy.org/) predicted ChpG to be a trypsin‐like serine protease that harbours an Asp/His/Ser catalytic triad, which was conserved among the other six Cm‐encoded Pat‐1 homologs (Figure S6). To inactivate the putative catalytic activity of ChpG, the conserved catalytic Ser231 residue was substituted with alanine, and protein was purified (Figure 4a) and infiltrated into eggplant and tomato leaves. The S231A substitution abolished immune elicitation by ChpG and MBP‐ChpGS231A neither caused cell death nor promoted POX activity (Figure 4b–d), which indicates that catalytic activity is required for immune recognition of ChpG.
FIGURE 4.
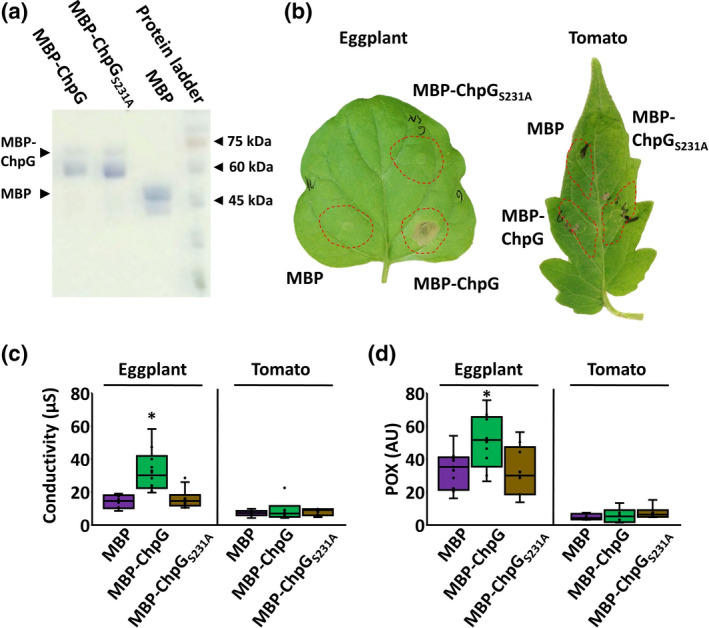
The catalytic activity of ChpG is required for its immune recognition. (a) Wild‐type and catalytic site mutant (S231A) ChpG variants were fused to a maltose‐binding protein (MBP) tag, purified from Escherichia coli, and visualized by SDS‐PAGE. (b–d) Purified proteins (0.02 µg/ml) were infiltrated into Black Queen eggplant and Moneymaker tomato leaves. (b) Leaves were photographed 72 h postinfiltration. (c) Cell death was quantified by ion leakage at 36 h. (d) Peroxidase (POX) activity was quantified at 36 h. (c, d) Lower and upper quartiles are marked at the margins of the boxes. Central lines and “o” represent medians and data points of 10 (for eggplant) or 6 (for tomato) biological repeats collected from two (tomato) or three (eggplant) independent experiments. * indicates significant difference (Mann–Whitney U test, p ≤ 0.05) from MBP control
We further validated our results by the introduction of catalytically inactive ChpG S231A into CmΩchpG and monitoring complementation through HR elicitation, symptom development, and stem bacterial colonization. ChpG and ChpG S231A fused to HA‐tag under the control of the pCMP1 promoter, which was previously reported to confer strong constitutive expression in Cm (Chalupowicz et al., 2012; Stevens et al., 2021), were introduced into CmΩchpG. Western blot analysis validated that ChpGHA and ChpGHA S231A were expressed and accumulated in similar amounts in CmΩchpG (Figure 5a). As expected, the introduction of ChpG HA into CmΩchpG restored the ability to elicit HR upon infiltration into eggplant leaves similarly to wild‐type Cm whereas the introduction of ChpG HA S231A failed to do so (Figure 5b,c). Additionally, expression of ChpG HA but not ChpG HA S231A in CmΩchpG hindered its ability to cause disease symptoms and colonize eggplant on stem inoculations (Figure 5d–f). Similar to the overexpression experiments, introduction of ChpG S231A expressed under its native promoter into CmΩchpG failed to elicit an immune response, and bacteria did not cause an HR on leaf infiltration (Figure S6a,b) and were as pathogenic as CmΩchpG on stem puncher inoculations (Figure S6c–e).
FIGURE 5.
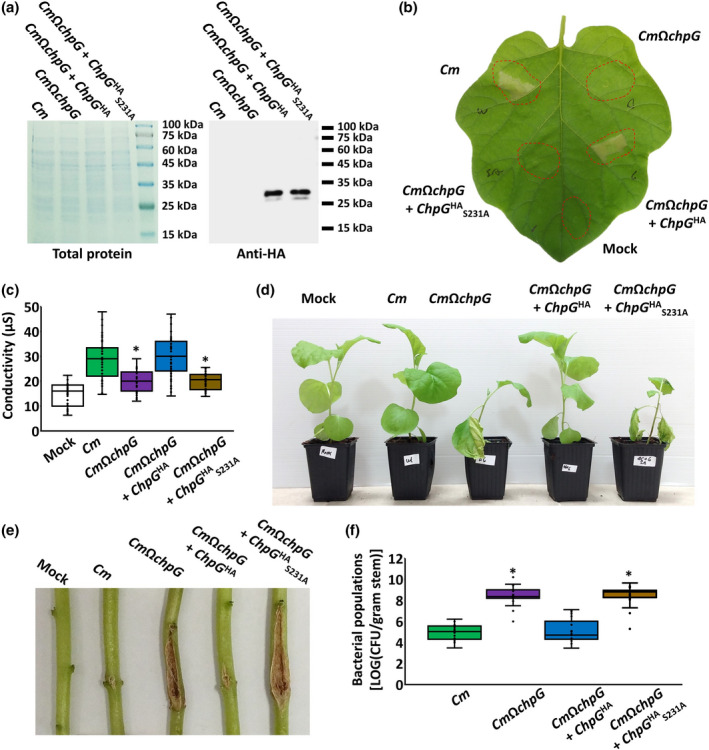
Immune recognition of ChpG expressed by Clavibacter michiganensis (Cm) is dependent on its catalytic activity. (a) Total protein was extracted from CMM101 (Cm), CmΩchpG, and CmΩchpG carrying pHN216 plasmids expressing ChpG or ChpG S231A fused to HA‐tag under the control of the pCMP1 promoter. Samples were separated on SDS‐PAGE and gels were either stained with Coomassie brilliant blue (left panel) or immunoblotted with anti‐HA antibody (right panel). (b, c) The indicated bacterial cultures (108 cfu/ml) were infiltrated into Black Queen eggplant leaves. (b) Picture was taken 48 h after infiltration. (c) Cell death was quantified by ion leakage 36 h postinfiltration. Lower and upper quartiles are marked at the margins of the boxes. Central lines and “o” represent medians and data points of at least 34 biological repeats collected from four independent experiments. * indicates significant difference (Mann–Whitney U test, p ≤ 0.05) from Cm. (d–f) Four‐leaf stage Black Queen eggplant plants were inoculated with the indicated Cm strains or water control (mock) by puncturing the stem area between the cotyledons with a wooden toothpick soaked in Cm solution (5 × 107 cfu/ml). Plants (d) and canker lesions (e) were photographed at 12 days postinfiltration (dpi). (f) Stem bacterial populations 1 cm above the inoculation site at 12 dpi. Lower and upper quartiles are marked at the margins of the boxes. Central lines and “o” represent medians and data points of at least 19 biological repeats collected from four independent experiments. * indicates significant difference (Mann–Whitney U test, p ≤ 0.05) from Cm
These experiments show that the protease activity of ChpG is required for its recognition in eggplant.
2.5. Immune recognition of ChpG is conserved in multiple eggplant varieties
A recent screen conducted by Boyaci and colleagues identified that numerous eggplant varieties demonstrate moderate to high Cm resistance and classified only three out of 46 screened varieties as susceptible to the bacteria (Boyaci et al., 2021). We hypothesized that resistance to Cm, which is abundant in eggplants, is mediated through immune recognition of ChpG. To test this, we monitored HR elicitation and disease development by either wild‐type Cm or CmΩchpG in 12 eggplant varieties (Figure 6). These included 10 heirloom varieties and two commercial varieties (Tudla and Aragon). On leaf infiltration, wild‐type Cm elicited an HR in all tested varieties within 36–72 hpi (Figure 6a). An HR was not observed in CmΩchpG‐infiltrated leaves, which demonstrated weak to moderate chlorosis within 72–96 hpi (Figure 6a). Next, we inoculated eight varieties with wild‐type Cm or CmΩchpG using stem puncture and monitored plants for disease symptoms and bacterial colonization (Figure 6b,c). Seven out of the eight eggplant varieties inoculated with wild‐type Cm did not develop significant wilting or leaf blotch symptoms while CmΩchpG‐inoculated plants showed wilting/leaf blotch symptoms in 50%–100% of the leaves (Figure 6b). Some cv. Japanese Finger eggplants inoculated with wild‐type Cm developed wilting symptoms and significantly larger canker lesions compared to other varieties (not shown). This phenomenon was not consistent between biological repeats. Symptom severity caused by CmΩchpG ranged dramatically between different varieties. However, it is difficult to assess if these differences occurred due to immune elicitation or to plant vigour because varieties also demonstrated differential growth rates that might affect symptom development. When monitored for stem bacterial colonization all varieties inoculated with CmΩchpG had significantly higher stem bacterial titre compared to wild‐type Cm within the same variety (Figure 6c). Stem bacterial populations of wild‐type Cm differed between eggplant varieties. In particular, stem colonization of the five oval fruit eggplant varieties used in this study (Aragon, Baladi, Bianca White Snowy, Black Queen, Tudla) ranged from 5 × 104 to 106 cfu/g and demonstrated lower variance between biological repeats (Figure 6c). In contrast, bacterial populations of wild‐type Cm were higher and more variable in the three tested slender fruit varieties (Japanese Finger, Little Finger, Thai Long Green) and ranged from 105 to 5 × 107 cfu/g (Figure 6c). These data suggest that immune recognition of ChpG is conserved in different eggplant varieties. However, the quantitative contribution of ChpG recognition to Cm resistance varies between varieties.
FIGURE 6.
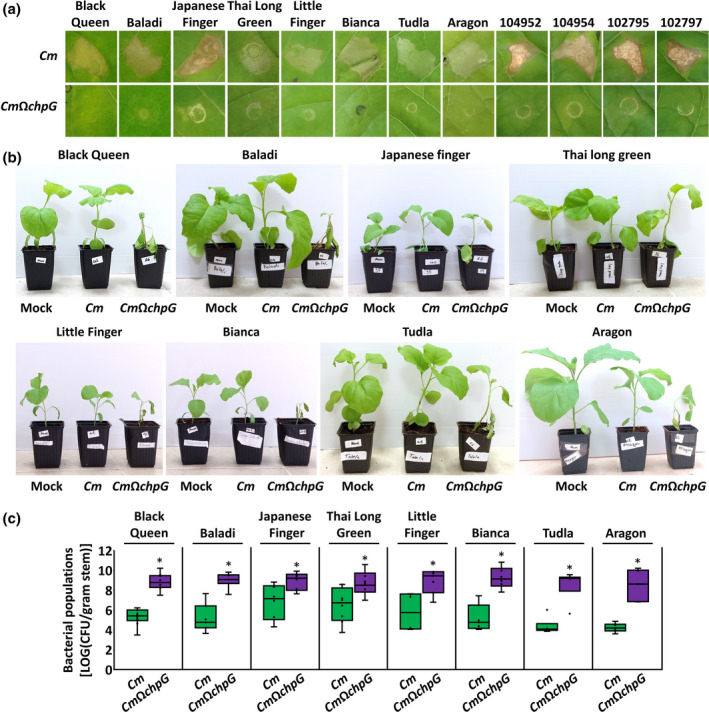
ChpG functions as an avirulence factor in multiple eggplant varieties. (a) Leaves of the indicated eggplant varieties were infiltrated (108 cfu/ml) with Clavibacter michiganensis wild‐type CMM101 (Cm) and CMM101 chpG inactivation mutant (CmΩchpG). Pictures were taken 96 h later. Representative pictures were taken out of at least 10 biological repeats conducted in two independent experiments. (b, c) The indicated eggplant varieties were inoculated with Cm, CmΩchpG or water control (mock) by puncturing the stem area between the cotyledons with a wooden toothpick soaked in Cm solution (5 × 107 cfu/ml). (b) Pictures were taken 14 days postinoculation (dpi). (c) Stem bacterial populations 1 cm above the inoculation site at 12 (Black Queen) or 14 dpi. Lower and upper quartiles are marked at the margins of the boxes. Central lines and “o” represent medians and data points of at least six biological repeats collected from two independent experiments. * indicates significant differences (Mann–Whitney U test, p ≤ 0.05) from Cm in the same eggplant variety
3. DISCUSSION
Most plant bacterial diseases are associated with specific genera or species, such as Xanthomonas, Pseudomonas syringae, Ralstonia, Erwinia, and Clavibacter. While bacteria that belong to these genera cause disease in diverse plant hosts, the majority of subspecies harbour a very narrow host range and are confined to a small number of crops. In the majority of cases, the bacterial and plant factors that dictate host range are unknown. Such interactions are likely to be multifaceted and depend on host tissue structure, secondary metabolites, and elicitation or avoidance of host immune responses. With that in mind, immune recognition of specific secreted bacterial effectors was found to be the sole determinant that controls host range in several pathosystems (Leach & White, 1996; Saur & Hückelhoven, 2021). This study identified the protease ChpG of Cm as the sole determinant through its immune recognition in resistant eggplant varieties.
Clavibacter spp. rely on Sec‐dependent hydrolases to promote disease. Accordingly, Pat‐1 homologs play a central role in the virulence of these bacteria and specific proteases were found to be crucial for the pathogenicity of different Clavibacter spp. on tomato, pepper, and eggplant (Dreier et al., 1997; Hwang et al., 2020; Nissinen et al., 2009; Stork et al., 2008). While relatively conserved as a protein family, specific Pat‐1 homologs demonstrate differential distribution between Clavibacter spp. (Figure 7), which suggests that these proteases function in a host‐specific manner. In particular, ChpG is conserved in most Cm and C. capsici genomes, but is absent from C. sepedonicus (Figure 7). Moreover, previous studies have reported that eggplants are susceptible to C. sepedonicus whereas a large number of eggplant varieties are resistant to Cm (Bishop & Slack, 1987; Boyaci et al., 2021; Nissinen et al., 2001) and that ChpG contributes to virulence on pepper, which serves as a main and alternative host of C. capsici and Cm, respectively (Hwang et al., 2020; Oh et al., 2016; Yim et al., 2012). Altogether, these findings suggest that loss and/or acquisition of ChpG in the genome through horizontal gene transfer plays a key role in the differentiation of these bacteria into host‐specialized pathogens. Interestingly, even though this recognition was not directly linked to virulence, ChpG has been reported to specifically trigger an HR in the nonhost plants N. tabacum and M. jalapa (Lu et al., 2015; Stork et al., 2008). This indicates that ChpG is immune recognized in a number of plant species, which suggests that its role in determining the host range of Clavibacter pathogens might extend beyond restricting Cm from eggplant varieties.
FIGURE 7.
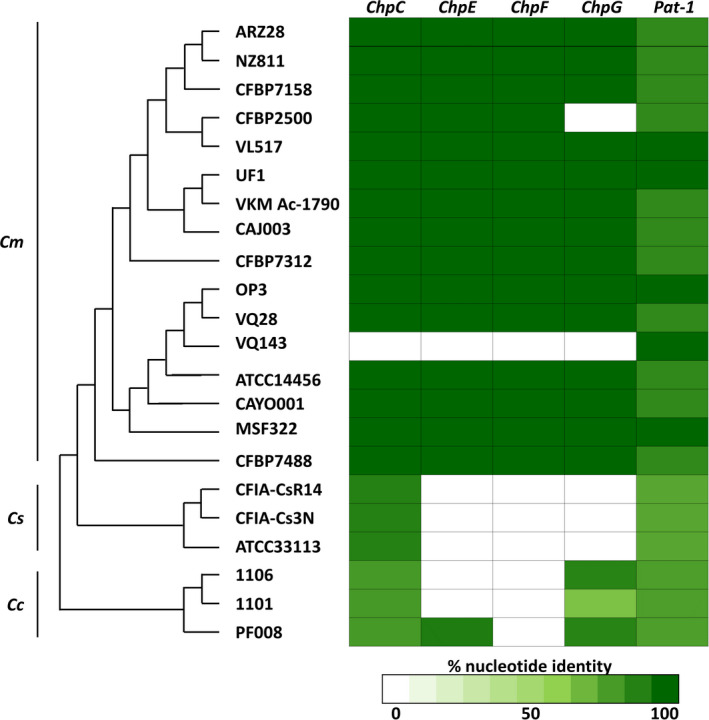
Distribution of Pat‐1 homologs among Clavibacter spp. Figure states the distribution of Pat‐1 homologs of Clavibacter michiganensis (Cm) NCPPB 382 [ChpC (CMM_0052), ChpE (CMM_0039), ChpF (CMM_0053), ChpG (CMM_0059), and Pat‐1 (pCM2_0054)] within NCBI genome deposits of Cm, C. sepedonicus (Cs), and C. capsici (Cc). Left, multilocus sequence typing phylogenetic analysis conducted by AutoMLST (https://automlst.ziemertlab.com/analyze); right, percentage nucleotide identity according to NCBI blastn suite (https://blast.ncbi.nlm.nih.gov/Blast.cgi, cut off set at E‐value of e−6) of the five Pat‐1 homologs in the Cm, Cs, and Cc genomes marked by green colour intensity according to the scale located at the bottom of the figure
The findings presented in this manuscript show that immune recognition of ChpG in certain eggplant varieties restricts them from being a host of Cm, which classifies it as an avirulence gene under Flor's gene‐for‐gene model (Flor, 1971). Numerous secreted apoplastic effectors or hydrolases have been classified as avirulence proteins in fungi and oomycetes (Tyler & Rouxel, 2012). However, with the exception of RaxX in rice (Pruitt et al., 2015), all reported avirulence factors that define the host range of plant‐pathogenic bacteria are translocated type III secretion system effectors (Khan et al., 2016; Leach & White, 1996). Nevertheless, secreted apoplastic hydrolases have been characterized as immune elicitors. Such immune elicitation is hypothesized to be mainly mediated through recognition of damage‐associated molecular patterns (Hou et al., 2019; Sinha et al., 2013). Specific secreted hydrolases such as Xanthomonas XagP, Pseudomonas cichorii HrpW, and Clavibacter Pat‐1 protease homologs promote nonhost‐specific HR but inactivation of these elicitors does not expand the host range of these pathogens (Kaewnum et al., 2006; Kajihara et al., 2012; Lu et al., 2015; Nissinen et al., 2009; Stork et al., 2008). Unlike the aforementioned examples, recognition of ChpG dictates the host range of Cm and restricts it from causing disease in numerous eggplant varieties. This suggests that immune recognition of apoplastic hydrolases such as ChpG, in addition to recognition of translocated type III secretion system effectors, plays a role in determining the host range of plant‐pathogenic bacteria.
Eggplant genes associated with immunity to Cm are yet to be identified. A recent screen identified that a large number of eggplant varieties are resistant to Cm and that this resistance is likely to be associated with a single dominant locus (Boyaci et al., 2021). Considering that ChpG is conserved in most Cm strains (Figure 7), and that all eggplant varieties used in this study displayed ChpG‐dependent immune elicitation that resulted in reduced disease symptoms and bacterial colonization (Figure 6), it is reasonable to hypothesize that the putative aforementioned resistance locus facilitates the recognition of ChpG. If that is indeed the case, the locus is likely to encode a transmembrane receptor that mediates immunity in the presence of ChpG. Therefore, ChpG can be used as a screening marker for future backcross lines, which will greatly assist in the identification of the immune locus that recognizes it.
Immune recognition of ChpG was abolished when its catalytic active site was mutated. Therefore, its recognition is likely to be indirect and dependent on catalytic modification of a plant protein. Such recognition could be mediated through modification of a specific guarded or decoy target by ChpG, which is later recognized by a transmembrane receptor‐like kinase or a receptor‐like protein in a similar manner to the Avr2‐RCR3‐Cf2 resistance model (Rooney et al., 2005; Shabab et al., 2008). Alternatively, ChpG might target structural cell wall components, which could result in accumulation of a damage‐associated molecular pattern elicitor recognized by an receptor‐like kinase in a similar manner to the oligogalacturonides‐WAK1 model (Brutus et al., 2010). Because immune recognition is based on the unique perception of a single Pat‐1 homolog but not by other family members, the decoy‐type model is more plausible. The biological functions and plant cleavage targets of ChpG, or any other Pat‐1 homolog, are unknown. Elucidating the molecular mechanisms that facilitate the virulence contribution of ChpG and other Pat‐1 homologs will provide further insights into the mechanisms of perception. Additionally, further functional analyses of the involvement of plant immune components in the perception of ChpG, such as the BAK1 and SOBIR1 co‐receptors (Gust & Felix, 2014), will pinpoint if this recognition is indeed mediated by a transmembrane receptor or through an unknown mechanism.
In summary, this study identified the protease ChpG as a novel avirulence protein that functions as a host specificity determinant by prohibiting Cm from colonizing and causing disease in eggplant varieties. In addition, the study indicates that immune recognition of ChpG in eggplant is dependent on its catalytic activity, which suggests that perception of ChpG by a potential immune receptor is indirect. This is the first evidence of secreted extracellular bacterial hydrolase that functions as a sole host‐specificity factor in plant‐pathogenic bacteria.
4. EXPERIMENTAL PROCEDURES
4.1. Bacterial strains and plant material
Bacterial strains and plasmids used in the study are listed in Table S1. Cm and E. coli were grown in Luria Bertani (LB) broth at 28 or 37°C, respectively. When required, media were supplemented with 10 µg/ml nalidixic acid, 10 µg/ml chloramphenicol, 50 µg/ml neomycin, 50 µg/ml kanamycin or 100 µg/ml ampicillin.
Plant cultivars used in this study were tomato Moneymaker, pepper Early California Wonder, eggplant heirloom varieties Baladi, Bianca White Snowy, Black Queen, Japanese Finger, Little Finger, Thai Long Green, 104952, 104954, 102796, and 102797, and commercial eggplant varieties Aragon and Tudla. Varieties 104952, 104954, 102796, and 102797 were supplied by the Israel Plant Gene Bank (IGB, https://igb.agri.gov.il/web/?lang=en&page=0). Plants were grown in a 25°C temperature‐controlled growth room under a 16 h light/8 h dark day cycle.
4.2. Plasmid construction and bacterial transformation
The oligonucleotides used in this study are listed in Table S2. For Clavibacter‐mediated expression of Pat‐1 homologs, DNA fragments carrying ChpE (CMM_0039), ChpF (CMM_0053), and ChpG (CMM_0059) and their upstream regulatory regions (as specified in Table S1) were amplified from genomic DNA of Cm strain CMM101 using Q5 high‐fidelity DNA polymerase (NEB) and cloned into the E. coli–Clavibacter shuttle vector pHN216 (Laine et al., 1996). Serine231 of the predicted catalytic triad of ChpG was substituted to alanine using the QuikChange II kit (Agilent Technologies, Inc.). For constitutive overexpression of ChpG fused to HA‐tag, the 801 bp fragment located upstream to GFP, which contains the 254 bp pCMP1 promoter (Chalupowicz et al., 2012), and the open reading frames of ChpG or ChpG S231A were amplified from pK2‐22, pHN216:ChpG, and pHN216:ChpG S231A, respectively, and sequentially subcloned into a pBBR1MCS‐2 derivative containing an HA tag (kindly provided by Guido Sessa). Next, pCMP1:ChpG‐HA fragments were amplified as one unit and cloned into pHN216 (Figure S8).
pHN216 derivatives were introduced into Cm∆PI (CMM30‐18) or CmΩchpG by electroporation as previously described (Kirchner et al., 2001) with slight modifications. Briefly, stationary phase Cm cultures were diluted to OD600 = 0.1 and grown in 100 ml of LB medium until the mid‐log phase was reached (OD600 = 0.5–0.7) at 25°C on an orbital shaker (120 rpm). Medium was supplemented with 20% glycine solution to a final concentration of 2.5% glycine and bacteria were kept at 25°C on an orbital shaker for 2 h. Bacteria were pelleted at 4°C and washed three times with 25 ml of ice‐cold 10% glycerol solution and finally resuspended in 1 ml of ice‐cold 15% glycerol. Electroporation was performed in the following conditions: 25 µF, 12.5 kV/cm, and 600 Ω using the Gene Pulser Electroporation System (Bio‐Rad). After electroporation, bacteria were supplemented with 1 ml of LB medium, incubated for 5 h at 25°C, and plated on LB agar supplemented with neomycin and incubated for 4 days at 28°C. Neomycin‐resistant colonies were validated by PCR using gene‐specific primers.
Protein accumulation of ChpG‐HA derivatives expressed in Cm under the control of the pCMP1 promoter was validated by western blot analysis as described: overnight Cm cultures were diluted to OD600 = 0.3 and lysed by SDS gel‐loading buffer followed by 10 min of incubation in 95°C. Equal volumes were separated in parallel on two SDS‐PAGE gels: one gel was stained with Coomassie brilliant blue to visualize protein loading. The second gel was used for western blot analysis using anti‐HA‐tag (F‐7) mouse monoclonal antibody (Santa Cruz Biotechnology) as described by Sambrook and Russell (2006) and according to the manufacturer's instructions.
4.3. RNA purifications and RT‐PCR
Bacterial cultures were grown for 24 h in M9 medium supplemented with tomato xylem sap (Savidor et al., 2012). Total RNA was purified from culture pellets using GeneMATRIX Universal RNA Purification Kit (EURx), treated with DNase I (Hylabs), and reverse transcribed to cDNA using a qScript cDNA Synthesis Kit (Quantabio) according to the manufacturer's instructions. Pat‐1 homologs and gyrA (positive control) were PCR amplified from 1‐µl cDNA or control RNA samples using gene‐specific primers (Table S2).
4.4. Expression and purification of MBP fusion proteins in E. coli
The 112–831 bp fragments containing the ChpG open reading frame minus its signal peptide‐coding region (predicted by SignalP‐5.0 Server; http://www.cbs.dtu.dk/services/SignalP/) were amplified from pHN216:ChpG and pHN216:ChpG S231A, cloned into pMALc2× (NEB) and plasmids were introduced into E. coli Rosetta (Merck). Bacterial cultures were grown in an orbital shaker at 37°C to OD600 = 0.4–0.6, supplemented with 0.1 mM isopropyl β‐d‐1‐thiogalactopyranoside, and incubated for 3 h at 37°C. Bacteria were pelleted and resuspended in ice‐cold buffer solution (20 mM Tris‐HCl, 200 mM NaCl, 1 mM EDTA, pH 7.4) and lysed using a SONIC‐150W ultrasonic processor (MRC Labs). MBP‐fused proteins were purified from supernatants using amylose resin (NEB) according to the manufacturer's instructions. Purified proteins were quantified by Bradford protein assay kit (Bio‐Rad) and validated by SDS‐PAGE followed by staining with Coomassie brilliant blue (Sambrook & Russell, 2006). Protein accumulation in total bacterial extracts and in the purified fractions was confirmed with western blot using anti‐MBP tag (8G1) mouse monoclonal antibody (Cell Signaling) according to the manufacturer's instructions (Figure S5).
4.5. Leaf infiltrations, ion leakage, and peroxidase activity measurements, and quantification of leaf bacterial populations
Purified proteins or bacterial suspensions were infiltrated to fully expanded upper rosette leaves of the four‐ to six‐leaf stage Black Queen eggplant and six‐leaf stage Moneymaker tomato using a needleless syringe. For protein infiltration, purified MBP, MBP‐ChpG, and MBP‐ChpGS231A proteins were diluted to a concentration of c.0.02 µg/ml in sterile distilled water prior to infiltration. For bacterial suspensions, Cm bacteria were scraped from fresh 2‐day‐old cultures grown on LB agar and diluted to a concentration of 108 (OD600 = 0.2, used for HR visualization, ion leakage, and POX activity measurements) or 104 cfu/ml (used for quantification of leaf bacterial populations) in sterile distilled water prior to infiltration.
For ion leakage measurements and POX activity, 1.5‐cm (for eggplant) or 1‐cm (for tomato) diameter leaf disks were sampled from the inoculation sites, transferred to flasks containing 10 ml of distilled water, and incubated on an orbital shaker (50 rpm) for 4 h at room temperature. Electrolyte leakage was quantified in water solutions using a conductivity meter (MRC Labs). Secreted POX activity quantification was modified from Mott et al. (2018). One hundred microlitres of diluted supernatants from each sample was incubated with 100 μl of freshly prepared POX assay solution (1 mg/ml 5‐aminosalicylic acid, 0.01% H2O2, pH 6.0) and timed. Reactions were stopped with 40 μl of 2 M NaOH once brown pigmentation started to appear and quantified in optical density of 595 nm using a Multiskan FC Microplate Photometer (Thermo Fisher). POX activity was determined in arbitrary units (AU) and calculated as 1000 × (OD595/[time (min)]).
Leaf bacterial population was quantified in each plant in two pooled 0.2‐cm diameter leaf disks from different inoculated leaves. Samples were homogenized in 1 ml of sterile water and bacterial numbers were determined per cm2 leaf areas by plating 10 μl of 10‐fold serial dilutions and counting the resulting colonies.
4.6. Plant inoculations, disease severity assessments, and quantification of stem bacterial populations
Stem inoculations were conducted by a single punctures of the stem areas between the cotyledons of four‐leaf stage eggplants or Moneymaker tomatoes with Cm‐contaminated toothpicks. Contaminated toothpicks were prepared as follows: Cm bacteria were scraped from fresh 2‐day‐old cultures grown on LB agar and diluted to 5 × 107 cfu/ml in distilled water in 1.5‐ml tubes. Wooden toothpicks were soaked in solutions for at least 10 min and each toothpick was then used for a single inoculation. After inoculations, plants were kept at 25°C in a greenhouse and wilt/leaf blotch and canker symptoms were determined and scored at 3, 6, and 9 dpi.
Wilting/leaf blotch symptoms were scored in each plant as the percentage of leaves demonstrating wilting and/or necrotic blotch symptoms according to the following scale: 0 = no wilt or leaf blotch, 1 = 1%–25%, 2 = 26%–50%, 3 = 51%–100%. The length of the canker lesion expanding from the inoculation areas was measured with a ruler and given a score according to the following scale: 0 = no canker, 1 = <0.1 cm, 2 = 0.1–1 cm, 3 = >1 cm. During virulent interactions, additional canker lesions appeared in different areas of the stem and leaf petioles. Due to high plant‐to‐plant variations in the quantity and size of cankers, these lesions were not included in canker scoring.
Bacterial populations were quantified in 1‐mm stem areas taken 1 cm above the inoculation sites. Samples were weighed and supplemented with 1 ml of sterile distilled water. Samples were homogenized and bacterial numbers per gram of tissue were determined by plating 10 μl of 10‐fold serial dilutions and counting the resulting colonies.
4.7. Phylogenetic analysis and determining the distribution of Pat‐1 homologs in Clavibacter spp.
Phylogenetic analysis was conducted on available NCBI genome deposits of C. michiganensis, C. sepedonicus, and C. capsici by multilocus sequence typing analysis with AutoMLST online server (Alanjary et al., 2019) (https://automlst.ziemertlab.com/) using the fast alignment mode feature; the genes used are found in File S1. To determine the distribution of Pat‐1 homologs, the nucleotide identity of the closest homologs of Cm NCPPB 382 ChpC (CMM_0052), ChpE (CMM_0039), ChpF (CMM_0053), ChpG (CMM_0059), and Pat‐1 (pCM2_0054) found in Clavibacter genomes depicted in Figure 7 was determined NCBI blastn suite (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Cutoff was set at E‐value of e−6 and minimum coverage of 80%.
CONFLICT OF INTEREST
The author declares that there is no conflict of interests.
Supporting information
FIGURE S1 Elicitation of hypersensitive response (HR) on solanaceous crops by Clavibacter michiganensis (Cm). Bacterial cultures (108 cfu/ml) of the indicated Cm NCPPB 382‐based strains were infiltrated into leaves of Moneymaker tomato, Early California Wonder pepper, and Black Queen eggplant. Pictures were taken 48 h after infiltration. The HR pattern was repeated in at least four independent experiments containing five internal biological repeats
FIGURE S2 Transcriptional expression of Pat‐1 homologs introduced into Cm∆PI. Total RNA was isolated from Clavibacter michiganensis (Cm), Cm∆PI, and Cm∆PI harbouring pHN216 encoding the indicated Pat‐1 homologs grown in M9 medium supplemented with tomato xylem sap and transcribed into cDNA. cDNA and RNA samples were used as a template for PCR amplification of chpC (CMM_0052), chpE (CMM_0039), chpF (CMM_0053), chpG (CMM_0059), and gyrA (CMM_0007, positive control). Samples were separated on 1% agarose gel containing BGSafe Stain and visualized by blue light transilluminator
FIGURE S3 Leaf symptoms caused by Clavibacter michiganensis (Cm) inoculation. Black Queen eggplant and Moneymaker tomato leaves were infiltrated (108 cfu/ml) with wild‐type CMM101 (Cm), CMM101 chpG inactivation mutant (CmΩchpG), and CmΩchpG carrying pHN216:ChpG (CmΩchpG + ChpG). Leaves were photographed 6 days after infiltration
FIGURE S4 ChpG does not significantly contribute to virulence on tomato. Four‐leaf stage Moneymaker tomato plants were inoculated with wild‐type Clavibacter michiganensis CMM101 (Cm), CMM101 chpG inactivation mutant (CmΩchpG), CmΩchpG carrying pHN216:ChpG (CmΩchpG + ChpG) or water solution (mock) by puncturing the stem area between the cotyledons with a wooden toothpick soaked in Cm solution (5 × 107 cfu/ml). Symptomatic plants (a) and canker lesions (b) were photographed at 14 days postinoculation (dpi). (c) Stem bacterial populations 1 cm above the inoculation site at 10 dpi. Lower and upper quartiles are marked at the margins of the boxes. Central lines and “o” represent medians, means, and data points of 13 biological repeats collected from three independent experiments
FIGURE S5 Immunoblot of maltose‐binding protein (MBP) fusion constructs. Total protein extract from Escherichia coli expressing the indicated MBP fusion proteins (a) and purified MBP fusion proteins (b; c.1 µg total protein) were separated on SDS‐PAGE and gels were either stained with Coomassie brilliant blue (left panel) or transferred to nitrocellulose membrane and immunoblotted with anti‐MBP antibody (right panel)
FIGURE S6 Sequence alignment of Pat‐1 homologs. The seven Pat‐1 homologs of Clavibacter michiganensis (Cm) NCPPB 382 ChpC (CMM_0052), ChpE (CMM_0039), ChpF (CMM_0053), ChpG (CMM_0059), Pat‐1 (pCM2_0054), PhpA (pCM2_0053), PhpB (pCM2_0052), and Escherichia coli serine protease DegQ (D9D84_09960) were aligned by the Clustal Omega multiple sequence alignment tool (https://www.ebi.ac.uk/Tools/msa/clustalo/) using default features. Histidine, aspartate, and serine residues of the predicted serine protease catalytic triad are marked in red
FIGURE S7 Catalytically inactive ChpG fails to complement chpG inactivation mutant. (a, b) Black Queen eggplant leaves were infiltrated (108 cfu/ml) with Clavibacter michiganensis wild‐type CMM101 (Cm), CMM101 chpG inactivation mutant (CmΩchpG), CmΩchpG carrying pHN216:chpG (CmΩchpG + ChpG), and CmΩchpG carrying pHN216:ChpG S231A (CmΩchpG + ChpG S231A). (a) Leaves were photographed 72 h after infiltration. (b) Cell death was quantified by ion leakage 36 h postinfiltration. Lower and upper quartiles are marked at the margins of the boxes. Central lines and “o” represent medians and data points of 12 biological repeats collected from three independent experiments. * indicates significant difference (Mann–Whitney U test, p ≤ 0.05) from Cm. (c–f) Four‐leaf stage Black Queen eggplant plants were inoculated with the indicated Cm strains by puncturing the stem area between the cotyledons with a wooden toothpick soaked in Cm solution (5 × 107 cfu/ml). Canker lesions (c), blotch symptoms in leaves from the second rosette (d), and symptomatic plants (e) were photographed at 10 (c) or 14 (d, e) days postinoculation (dpi). (f) Stem bacterial populations 1 cm above the inoculation site at 10 dpi. Lower and upper quartiles are marked at the margins of the boxes. Central lines and “o” represent medians and data points of 12 biological repeats collected from three independent experiments. * indicates significant difference (Mann–Whitney U test, p ≤ 0.05) from Cm
FIGURE S8 Physical map of ChpG HA overexpression plasmid. Sequence map of the Escherichia coli–Clavibacter shuttle vector pHN216 carrying ChpG HA or ChpG HA S231A expressed under the control of the pCMP1 promoter. Map indicating the replication region of the plasmid pCM2 (pCM2 rep), origin of replication of the vector pBR322 (ori pBR), neomycin phosphotransferase gene (Neo), the pCMP1 promoter cloned from the bacteriophage CMP1 (pCMP1), and ChpG fused to an HA‐tag. The sequence of the DNA region between the EcoRI and HindIII sites is presented above the plasmid map. The pCMP1 promoter region is marked by a magenta background, the open reading frame of ChpG (CMM_0059) is marked by a red background, the HA tag sequence is marked by an aqua background, and the yellow font marks position 691 in the ChpG open reading frame represented by thymine in wild‐type ChpG and guanine in ChpG S231A. Restriction sites used for cloning are marked by a yellow background
TABLE S1 Bacterial strains and plasmids used in this study
TABLE S2 Primers used during this study
FILE S1 Genes used for multilocus sequence typing analysis
ACKNOWLEDGEMENTS
The work was supported through the core start‐up funding provided by the Agricultural Research Organization (ARO), Volcani Institute. Special thanks go to Professor Guido Sessa (Tel Aviv University), Dr Shulamit Manulis‐Sasson (ARO, retiree), and Professor Rudolf Eichenlaub (Bielefeld University) for providing bacterial strains and plasmids.
Verma, R.K. & Teper, D. (2022) Immune recognition of the secreted serine protease ChpG restricts the host range of Clavibacter michiganensis from eggplant varieties. Molecular Plant Pathology, 23, 933–946. 10.1111/mpp.13215
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable requests.
REFERENCES
- Alanjary, M. , Steinke, K. & Ziemert, N. (2019) AutoMLST: an automated web server for generating multi‐locus species trees highlighting natural product potential. Nucleic Acids Research, 47, W276–W282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint‐Kurti, P. (2019) The plant hypersensitive response: concepts, control and consequences. Molecular Plant Pathology, 20, 1163–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, A.L. & Slack, S.A. (1987) Effect of inoculum dose and preparation, strain variation, and plant growth conditions on the eggplant assay for bacterial ring rot. American Potato Journal, 64, 227–234. [Google Scholar]
- Blank, L. , Cohen, Y. , Borenstein, M. , Shulhani, R. , Lofthouse, M. , Sofer, M. et al. (2016) Variables associated with severity of bacterial canker and wilt caused by Clavibacter michiganensis subsp. michiganensis in tomato greenhouses. Phytopathology, 106, 254–261. [DOI] [PubMed] [Google Scholar]
- van den Bosch, T.J.M. , Niemi, O. & Welte, C.U. (2020) Single gene enables plant pathogenic Pectobacterium to overcome host‐specific chemical defence. Molecular Plant Pathology, 21, 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyaci, H.F. , Kabas, A. , Aysan, Y. & Prohens, J. (2021) Screening of eggplant genotypes for resistance to bacterial wilt disease caused by Clavibacter michiganensis subsp. michiganensis . Plant Protection Science, 57, 112–121. [Google Scholar]
- Brutus, A. , Sicilia, F. , Macone, A. , Cervone, F. & De Lorenzo, G. (2010) A domain swap approach reveals a role of the plant wall‐associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proceedings of the National Academy of Sciences of the United States of America, 107, 9452–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupowicz, L. , Barash, I. , Reuven, M. , Dror, O. , Sharabani, G. , Gartemann, K.‐H. et al. (2017) Differential contribution of Clavibacter michiganensis ssp. michiganensis virulence factors to systemic and local infection in tomato. Molecular Plant Pathology, 18, 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupowicz, L. , Cohen‐Kandli, M. , Dror, O. , Eichenlaub, R. , Gartemann, K.‐H. , Sessa, G. et al. (2010) Sequential expression of bacterial virulence and plant defense genes during infection of tomato with Clavibacter michiganensis subsp. michiganensis . Phytopathology, 100, 252–261. [DOI] [PubMed] [Google Scholar]
- Chalupowicz, L. , Zellermann, E.‐M. , Fluegel, M. et al. (2012) Colonization and movement of GFP‐labeled Clavibacter michiganensis subsp. michiganensis during tomato infection. Phytopathology, 102, 23–31. [DOI] [PubMed] [Google Scholar]
- Coaker, G.L. & Francis, D.M. (2004) Mapping, genetic effects, and epistatic interaction of two bacterial canker resistance QTLs from Lycopersicon hirsutum . Theoretical and Applied Genetics, 108, 1047–1055. [DOI] [PubMed] [Google Scholar]
- Daudi, A. , Cheng, Z. , O’Brien, J.A. , Mammarella, N. , Khan, S. , Ausubel, F.M. et al. (2012) The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern‐triggered immunity. The Plant Cell, 24, 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier, J. , Meletzus, D. & Eichenlaub, R. (1997) Characterization of the plasmid encoded virulence region pat‐1 of phytopathogenic Clavibacter michiganensis subsp. michiganensis . Molecular Plant‐Microbe Interactions, 10, 195–206. [DOI] [PubMed] [Google Scholar]
- Favaro, M.A. , Micheloud, N.G. , Roeschlin, R.A. , Chiesa, M.A. , Castagnaro, A.P. , Vojnov, A.A. et al. (2014) Surface barriers of Mandarin ‘Okitsu’ leaves make a major contribution to canker disease resistance. Phytopathology, 104, 970–976. [DOI] [PubMed] [Google Scholar]
- Felix, G. , Duran, J.D. , Volko, S. & Boller, T. (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. The Plant Journal, 18, 265–276. [DOI] [PubMed] [Google Scholar]
- Flor, H.H. (1971) Current status of the gene‐for‐gene concept. Annual Review of Phytopathology, 9, 275–296. [Google Scholar]
- Gartemann, K.‐H. , Abt, B. , Bekel, T. et al. (2008) The genome sequence of the tomato‐pathogenic actinomycete Clavibacter michiganensis subsp. michiganensis NCPPB382 reveals a large island involved in pathogenicity. Journal of Bacteriology, 190, 2138–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitaitis, R.D. , Beaver, R.W. & Voloudakis, A.E. (1991) Detection of Clavibacter michiganensis subsp. michiganensis in symptomless tomato transplants. Plant Disease, 75, 834. [Google Scholar]
- Gust, A.A. & Felix, G. (2014) Receptor like proteins associate with SOBIR1‐type of adaptors to form bimolecular receptor kinases. Current Opinion in Plant Biology, 21, 104–111. [DOI] [PubMed] [Google Scholar]
- van Heusden, A.W. , Koornneef, M. , Voorrips, R.E. , Brüggemann, W. , Pet, G. , Vrielink‐van Ginkel, R. et al. (1999) Three QTLs from Lycopersicon peruvianum confer a high level of resistance to Clavibacter michiganensis ssp. michiganensis . Theoretical and Applied Genetics, 99, 1068–1074. [Google Scholar]
- Hou, S. , Liu, Z. , Shen, H. & Wu, D. (2019) Damage‐associated molecular pattern‐triggered immunity in plants. Frontiers in Plant Science, 10, 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkanen, A. , Karjalainen, R. , Nielsen, S. & van der Wolf, J.M. (2005) Epidemiology of Clavibacter michiganensis subsp. sepedonicus in potato under European conditions: population development and yield reduction. Journal of Plant Diseases and Protection, 112, 88–97. [Google Scholar]
- Hwang, I.S. , Lee, H.M. , Oh, E. , Lee, S. , Heu, S. & Oh, C. (2020) Plasmid composition and the chpG gene determine the virulence level of Clavibacter capsici natural isolates in pepper. Molecular Plant Pathology, 21, 808–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, T.A. , Harveson, R.M. & Vidaver, A.K. (2007) Reemergence of Goss’s wilt and blight of corn to the central High Plains. Plant Health Progress, 8, 44. [Google Scholar]
- Jahr, H. , Dreier, J. , Meletzus, D. , Bahro, R. & Eichenlaub, R. (2000) The endo‐β‐1,4‐glucanase CelA of Clavibacter michiganensis subsp. michiganensis is a pathogenicity determinant required for induction of bacterial wilt of tomato. Molecular Plant‐Microbe Interactions, 13, 703–714. [DOI] [PubMed] [Google Scholar]
- Kaewnum, S. , Prathuangwong, S. & Burr, T.J. (2006) A pectate lyase homolog, xagP, in Xanthomonas axonopodis pv. glycines is associated with hypersensitive response induction on tobacco. Phytopathology, 96, 1230–1236. [DOI] [PubMed] [Google Scholar]
- Kajihara, S. , Hojo, H. , Koyanagi, M. , Tanaka, M. , Mizumoto, H. , Ohnishi, K. et al. (2012) Implication of hrpW in virulence of Pseudomonas cichorii . Plant Pathology, 61, 355–363. [Google Scholar]
- Khan, M. , Subramaniam, R. & Desveaux, D. (2016) Of guards, decoys, baits and traps: pathogen perception in plants by type III effector sensors. Current Opinion in Microbiology, 29, 49–55. [DOI] [PubMed] [Google Scholar]
- Kirchner, O. , Gartemann, K.‐H. , Zellermann, E.‐M. , Eichenlaub, R. & Burger, A. (2001) A highly efficient transposon mutagenesis system for the tomato pathogen Clavibacter michiganensis subsp. michiganensis . Molecular Plant‐Microbe Interactions, 14, 1312–1318. [DOI] [PubMed] [Google Scholar]
- Kourelis, J. & van der Hoorn, R.A.L. (2018) Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. The Plant Cell, 30, 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine, M.J. , Nakhei, H. , Dreier, J. , Lehtilä, K. , Meletzus, D. , Eichenlaub, R. et al. (1996) Stable transformation of the gram‐positive phytopathogenic bacterium Clavibacter michiganensis subsp. sepedonicus with several cloning vectors. Applied and Environmental Microbiology, 62, 1500–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach, J.E. & White, F.F. (1996) Bacterial avirulence genes. Annual Review of Phytopathology, 34, 153–179. [DOI] [PubMed] [Google Scholar]
- de León, L. , Siverio, F. , López, M.M. & Rodríguez, A. (2011) Clavibacter michiganesis subsp. michiganensis, a seedborne tomato pathogen: healthy seeds are still the goal. Plant Disease, 95, 1328–1338. [DOI] [PubMed] [Google Scholar]
- Li, X. , Tambong, J. , Yuan, K. , Chen, W. , Xu, H. , Lévesque, C.A. et al. (2018) Re‐classification of Clavibacter michiganensis subspecies on the basis of whole‐genome and multi‐locus sequence analyses. International Journal of Systematic and Evolutionary Microbiology, 68, 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. , Hatsugai, N. , Katagiri, F. , Ishimaru, C.A. & Glazebrook, J. (2015) Putative serine protease effectors of Clavibacter michiganensis induce a hypersensitive response in the apoplast of Nicotiana species. Molecular Plant‐Microbe Interactions, 28, 1216–1226. [DOI] [PubMed] [Google Scholar]
- Meletzus, D. , Bermphol, A. , Dreier, J. & Eichenlaub, R. (1993) Evidence for plasmid‐encoded virulence factors in the phytopathogenic bacterium Clavibacter michiganensis subsp. michiganensis NCPPB382. Journal of Bacteriology, 175, 2131–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletzus, D. & Eichenlaub, R. (1991) Transformation of the phytopathogenic bacterium Clavibacter michiganense subsp. michiganense by electroporation and development of a cloning vector. Journal of Bacteriology, 173, 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez, V. , Valenzuela, M. , Salvà‐Serra, F. , Jaén‐Luchoro, D. , Besoain, X. , Moore, E.R.B. et al. (2020) Comparative genomics of pathogenic Clavibacter michiganensis subsp. michiganensis strains from Chile reveals potential virulence features for tomato plants. Microorganisms, 8, 1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott, G.A. , Desveaux, D. & Guttman, D.S. (2018) A high‐sensitivity, microtiter‐based plate assay for plant pattern‐triggered immunity. Molecular Plant‐Microbe Interactions, 31, 499–504. [DOI] [PubMed] [Google Scholar]
- Nandi, M. , Macdonald, J. , Liu, P. , Weselowski, B. & Yuan, Z.‐C. (2018) Clavibacter michiganensis ssp. michiganensis: bacterial canker of tomato, molecular interactions and disease management. Molecular Plant Pathology, 19, 2036–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissinen, R. , Kassuwi, S. , Peltola, R. & Metzler, M. (2001) In planta‐complementation of Clavibacter michiganensis subsp. sepedonicus strains deficient in cellulase production or HR induction restores virulence. European Journal of Plant Pathology, 107, 175–182. [Google Scholar]
- Nissinen, R. , Xia, Y. , Mattinen, L. , Ishimaru, C.A. , Knudson, D.L. , Knudson, S.E. et al. (2009) The putative secreted serine protease Chp‐7 is required for full virulence and induction of a nonhost hypersensitive response by Clavibacter michiganensis subsp. sepedonicus . Molecular Plant‐Microbe Interactions, 22, 809–819. [DOI] [PubMed] [Google Scholar]
- Oh, E.‐J. , Bae, C. , Lee, H.‐B. et al. (2016) Clavibacter michiganensis subsp. capsici subsp. nov., causing bacterial canker disease in pepper. International Journal of Systematic and Evolutionary Microbiology, 66, 4065–4070. [DOI] [PubMed] [Google Scholar]
- Peritore‐Galve, F.C. , Tancos, M.A. & Smart, C.D. (2021) Bacterial canker of tomato: revisiting a global and economically damaging seedborne pathogen. Plant Disease, 105, 1581–1595. [DOI] [PubMed] [Google Scholar]
- Pizarro, L. , Leibman‐Markus, M. , Gupta, R. , Kovetz, N. , Shtein, I. & Bar, E. et al. (2020) A gain of function mutation in SlNRC4a enhances basal immunity resulting in broad‐spectrum disease resistance. Communications Biology, 3, 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt, R.N. , Schwessinger, B. , Joe, A. , Thomas, N. , Liu, F. , Albert, M. et al. (2015) The rice immune receptor XA21 recognizes a tyrosine‐sulfated protein from a Gram‐negative bacterium. Science Advances, 1, e1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney, H.C.E. , Van’t Klooster, J.W. , van der Hoorn, R.A.L. , Joosten, M.H.A.J. , Jones, J.D.G. & de Wit, P.J.G.M. (2005) Cladosporium Avr2 inhibits tomato Rcr3 protease required for Cf‐2‐dependent disease resistance. Science, 308, 1783–1786. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. & Russell, D.W. (2006) SDS‐polyacrylamide gel electrophoresis of proteins. Cold Spring Harbor Protocols, 2006, 281–283. [Google Scholar]
- Saur, I.M.L. & Hückelhoven, R. (2021) Recognition and defence of plant‐infecting fungal pathogens. Journal of Plant Physiology, 256, 153324. [DOI] [PubMed] [Google Scholar]
- Savidor, A. , Chalupowicz, L. , Teper, D. , Gartemann, K.‐H. , Eichenlaub, R. , Manulis‐Sasson, S. et al. (2014) Clavibacter michiganensis subsp. michiganensis Vatr1 and Vatr2 transcriptional regulators are required for virulence in tomato. Molecular Plant‐Microbe Interactions, 27, 1035–1047. [DOI] [PubMed] [Google Scholar]
- Savidor, A. , Teper, D. , Gartemann, K.‐H. , Eichenlaub, R. , Chalupowicz, L. , Manulis‐Sasson, S. et al. (2012) The Clavibacter michiganensis subsp. michiganensis–tomato interactome reveals the perception of pathogen by the host and suggests mechanisms of infection. Journal of Proteome Research, 11, 736–750. [DOI] [PubMed] [Google Scholar]
- Seto, D. , Laflamme, B. , Guttman, D.S. & Desveaux, D. (2020) The Arabidopsis ZED1‐related kinase genomic cluster is specifically required for effector‐triggered immunity. Plant Physiology, 184, 1635–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabab, M. , Shindo, T. , Gu, C. , Kaschani, F. , Pansuriya, T. , Chintha, R. et al. (2008) Fungal effector protein AVR2 targets diversifying defense‐related Cys proteases of tomato. The Plant Cell, 20, 1169–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharabani, G. , Manulis‐Sasson, S. , Borenstein, M. , Shulhani, R. , Lofthouse, M. , Chalupowicz, L. et al. (2013) The significance of guttation in the secondary spread of Clavibacter michiganensis subsp. michiganensis in tomato greenhouses. Plant Pathology, 62, 578–586. [Google Scholar]
- Sinha, D. , Gupta, M.K. , Patel, H.K. , Ranjan, A. & Sonti, R.V. (2013) Cell wall degrading enzyme induced rice innate immune responses are suppressed by the type 3 secretion system effectors XopN, XopQ, XopX and XopZ of Xanthomonas oryzae pv. oryzae . PLoS One, 8, e75867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, D.M. , Tang, A. & Coaker, G. (2021) A genetic toolkit for investigating Clavibacter species: markerless deletion, permissive site identification, and an integrative plasmid. Molecular Plant‐Microbe Interactions, 34, 1336–1345. [DOI] [PubMed] [Google Scholar]
- Stice, S.P. , Thao, K.K. , Khang, C.H. , Baltrus, D.A. , Dutta, B. & Kvitko, B.H. (2020) Thiosulfinate tolerance is a virulence strategy of an atypical bacterial pathogen of onion. Current Biology, 30, 3130–3140.e6. [DOI] [PubMed] [Google Scholar]
- Stork, I. , Gartemann, K.‐H. , Burder, A. & Eichenlaub, R. (2008) A family of serine proteases of Clavibacter michiganensis subsp. michiganensis: chpC plays a role in colonization of the host plant tomato. Molecular Plant Pathology, 9, 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Q. , Greve, L.C. & Labavitch, J.M. (2011) Polysaccharide compositions of intervessel pit membranes contribute to Pierce’s disease resistance of grapevines. Plant Physiology, 155, 1976–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa, S.P. , Pattathil, S. , Hahn, M.G. , Jacques, M.‐A. , Gilbertson, R.L. & Coaker, G. (2017) Genomic analysis of Clavibacter michiganensis reveals insight into virulence strategies and genetic diversity of a gram‐positive bacterial pathogen. Molecular Plant‐Microbe Interactions, 30, 786–802. [DOI] [PubMed] [Google Scholar]
- Tian, Q. , Chuan, J. , Sun, X. , Zhou, A. , Wang, L. , Zou, J. et al. (2021) Description of Clavibacter zhangzhiyongii sp. nov., a phytopathogenic actinobacterium isolated from barley seeds, causing leaf brown spot and decline. International Journal of Systematic and Evolutionary Microbiology, 71, 004786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiantos, J. (1987) Transmission of bacterium Corynebacterium michiganense pv. michiganense by seeds. Journal of Phytopathology, 119, 142–146. [Google Scholar]
- Tyler, B.M. & Rouxel, T. (2012) Effectors of fungi and oomycetes: their virulence and a virulence functions and translocation from pathogen to host cells. In: Sessa, G. (Ed.) Molecular plant immunity. Oxford, UK: Wiley‐Blackwell, pp. 123–167. [Google Scholar]
- Yim, K.‐O. , Lee, H.‐I. , Kim, J.‐H. , Lee, S.‐D. , Cho, J.‐H. & Cha, J.‐S. (2012) Characterization of phenotypic variants of Clavibacter michiganensis subsp. michiganensis isolated from Capsicum annuum . European Journal of Plant Pathology, 133, 559–575. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Elicitation of hypersensitive response (HR) on solanaceous crops by Clavibacter michiganensis (Cm). Bacterial cultures (108 cfu/ml) of the indicated Cm NCPPB 382‐based strains were infiltrated into leaves of Moneymaker tomato, Early California Wonder pepper, and Black Queen eggplant. Pictures were taken 48 h after infiltration. The HR pattern was repeated in at least four independent experiments containing five internal biological repeats
FIGURE S2 Transcriptional expression of Pat‐1 homologs introduced into Cm∆PI. Total RNA was isolated from Clavibacter michiganensis (Cm), Cm∆PI, and Cm∆PI harbouring pHN216 encoding the indicated Pat‐1 homologs grown in M9 medium supplemented with tomato xylem sap and transcribed into cDNA. cDNA and RNA samples were used as a template for PCR amplification of chpC (CMM_0052), chpE (CMM_0039), chpF (CMM_0053), chpG (CMM_0059), and gyrA (CMM_0007, positive control). Samples were separated on 1% agarose gel containing BGSafe Stain and visualized by blue light transilluminator
FIGURE S3 Leaf symptoms caused by Clavibacter michiganensis (Cm) inoculation. Black Queen eggplant and Moneymaker tomato leaves were infiltrated (108 cfu/ml) with wild‐type CMM101 (Cm), CMM101 chpG inactivation mutant (CmΩchpG), and CmΩchpG carrying pHN216:ChpG (CmΩchpG + ChpG). Leaves were photographed 6 days after infiltration
FIGURE S4 ChpG does not significantly contribute to virulence on tomato. Four‐leaf stage Moneymaker tomato plants were inoculated with wild‐type Clavibacter michiganensis CMM101 (Cm), CMM101 chpG inactivation mutant (CmΩchpG), CmΩchpG carrying pHN216:ChpG (CmΩchpG + ChpG) or water solution (mock) by puncturing the stem area between the cotyledons with a wooden toothpick soaked in Cm solution (5 × 107 cfu/ml). Symptomatic plants (a) and canker lesions (b) were photographed at 14 days postinoculation (dpi). (c) Stem bacterial populations 1 cm above the inoculation site at 10 dpi. Lower and upper quartiles are marked at the margins of the boxes. Central lines and “o” represent medians, means, and data points of 13 biological repeats collected from three independent experiments
FIGURE S5 Immunoblot of maltose‐binding protein (MBP) fusion constructs. Total protein extract from Escherichia coli expressing the indicated MBP fusion proteins (a) and purified MBP fusion proteins (b; c.1 µg total protein) were separated on SDS‐PAGE and gels were either stained with Coomassie brilliant blue (left panel) or transferred to nitrocellulose membrane and immunoblotted with anti‐MBP antibody (right panel)
FIGURE S6 Sequence alignment of Pat‐1 homologs. The seven Pat‐1 homologs of Clavibacter michiganensis (Cm) NCPPB 382 ChpC (CMM_0052), ChpE (CMM_0039), ChpF (CMM_0053), ChpG (CMM_0059), Pat‐1 (pCM2_0054), PhpA (pCM2_0053), PhpB (pCM2_0052), and Escherichia coli serine protease DegQ (D9D84_09960) were aligned by the Clustal Omega multiple sequence alignment tool (https://www.ebi.ac.uk/Tools/msa/clustalo/) using default features. Histidine, aspartate, and serine residues of the predicted serine protease catalytic triad are marked in red
FIGURE S7 Catalytically inactive ChpG fails to complement chpG inactivation mutant. (a, b) Black Queen eggplant leaves were infiltrated (108 cfu/ml) with Clavibacter michiganensis wild‐type CMM101 (Cm), CMM101 chpG inactivation mutant (CmΩchpG), CmΩchpG carrying pHN216:chpG (CmΩchpG + ChpG), and CmΩchpG carrying pHN216:ChpG S231A (CmΩchpG + ChpG S231A). (a) Leaves were photographed 72 h after infiltration. (b) Cell death was quantified by ion leakage 36 h postinfiltration. Lower and upper quartiles are marked at the margins of the boxes. Central lines and “o” represent medians and data points of 12 biological repeats collected from three independent experiments. * indicates significant difference (Mann–Whitney U test, p ≤ 0.05) from Cm. (c–f) Four‐leaf stage Black Queen eggplant plants were inoculated with the indicated Cm strains by puncturing the stem area between the cotyledons with a wooden toothpick soaked in Cm solution (5 × 107 cfu/ml). Canker lesions (c), blotch symptoms in leaves from the second rosette (d), and symptomatic plants (e) were photographed at 10 (c) or 14 (d, e) days postinoculation (dpi). (f) Stem bacterial populations 1 cm above the inoculation site at 10 dpi. Lower and upper quartiles are marked at the margins of the boxes. Central lines and “o” represent medians and data points of 12 biological repeats collected from three independent experiments. * indicates significant difference (Mann–Whitney U test, p ≤ 0.05) from Cm
FIGURE S8 Physical map of ChpG HA overexpression plasmid. Sequence map of the Escherichia coli–Clavibacter shuttle vector pHN216 carrying ChpG HA or ChpG HA S231A expressed under the control of the pCMP1 promoter. Map indicating the replication region of the plasmid pCM2 (pCM2 rep), origin of replication of the vector pBR322 (ori pBR), neomycin phosphotransferase gene (Neo), the pCMP1 promoter cloned from the bacteriophage CMP1 (pCMP1), and ChpG fused to an HA‐tag. The sequence of the DNA region between the EcoRI and HindIII sites is presented above the plasmid map. The pCMP1 promoter region is marked by a magenta background, the open reading frame of ChpG (CMM_0059) is marked by a red background, the HA tag sequence is marked by an aqua background, and the yellow font marks position 691 in the ChpG open reading frame represented by thymine in wild‐type ChpG and guanine in ChpG S231A. Restriction sites used for cloning are marked by a yellow background
TABLE S1 Bacterial strains and plasmids used in this study
TABLE S2 Primers used during this study
FILE S1 Genes used for multilocus sequence typing analysis
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable requests.


