Abstract
At 22°C a flagellin mutant of Listeria monocytogenes was found to attach to stainless steel at levels 10-fold lower than wild-type cells, even under conditions preventing active motility. At 37°C, when flagella are not produced, attachment of both strains was identical. Therefore, flagella per se facilitate the early stage of attachment.
Communities of microorganisms which adhere to various surfaces form biofilms (3, 5); these sessile microorganisms have advantages in both growth and survival compared with their planktonic forms (6, 8, 25). Several factors are thought to affect surface attachment, including extracellular material, nutrients, and temperature (7, 11, 20). It has been suggested that flagellation and motility play a role at the cell level in several stages of biofilm formation (13, 16), and motility has been shown to facilitate attachment to both biotic (2, 14, 18) and abiotic (12, 13, 17, 23) surfaces. Flagella, in addition to being the locomotive organelles of bacteria, have also been reported to serve as an adhesive structure (16); however, no evidence has demonstrated that flagella alone are involved in attachment.
To investigate the importance of flagella, in the absence of motility, for initial attachment to stainless steel surfaces, we have used the food-borne pathogen Listeria monocytogenes, wild-type strain NCTC 7973 (serotype 1/2a), and a nonflagellated (fla2) mutant. The wild-type bacterium is peritrichous in a temperature-dependent manner, being flagellated and motile at 20 to 25°C but nonmotile and with very few flagella above 35°C (21, 22). The nonflagellated (fla2) mutant was constructed by insertion of the luxAB genes (10) into the flaA gene by double homologous recombination using the temperature-sensitive shuttle vector pAUL-A (4). The mutant produces a 7-kDa truncate representing the N-terminal end of the flagellin protein. The use of a mutant which lacks the ability to produce flagella allowed us both to investigate the role of the flagellum in attachment and to separate this from the effect of temperature on the ability of Listeria to attach to a surface.
To confirm that the conditions to be used in attachment studies did not allow the cells to be motile, cell motility was first examined. Cultures were grown in brain heart infusion broth (Oxoid, Ltd., Hampshire, United Kingdom), incubated statically overnight at either 22 or 37°C, and then centrifuged at 2,000 × g (Centaur 2; Fisons, Leicester, United Kingdom) for 20 min at room temperature. Cells were washed twice with phosphate-buffered saline (PBS) (pH 7.2; Oxoid), centrifuged at 2,000 × g for 15 min, and resuspended in PBS to ∼8 × 108 CFU ml−1 (A600 = 0.5). Motility was observed by dark-field microscopy at a ×400 magnification. When suspended in PBS to provide nutrient-limiting conditions, both the wild-type and mutant strains of L. monocytogenes grown at 22°C were not motile, although the wild type was flagellated. As expected, neither strain was motile at 37°C because of the temperature-dependent flagellum production in Listeria spp. At 22°C motility in the wild-type strain could be restored for at least 24 h by adding an equivalent volume of brain heart infusion broth to the cell suspension and incubating the suspension at room temperature for 10 to 15 min. Conditions in the PBS thus prevented motility by nutrient limitation. While the motility of wild-type cells was restored, the mutant cells remained nonmotile, as they are unable to synthesize flagellin protein. Thus, under the experimental conditions used, attachment behavior would result not from an effect of motility but from the presence or absence of flagella per se.
Attachment studies were carried out using stainless steel coupons (type 304 polished finished, 1.5 by 1.5 cm; Ohw Heap Shang Hang Ltd., Bangkok, Thailand), which had been cleaned and dry sterilized as previously described by Dhir and Dodd (6). Coupons were immersed vertically in 10 ml of PBS-cell suspension using cultures grown overnight at either 22 or 37°C in 30-ml universal tubes (Bibby Sterilin, Ltd., Staffordshire, United Kingdom) and incubated at 22 or 37°C, as appropriate. Coupons were removed at various intervals, and unattached cells were removed by rinsing the coupons four times with 10 ml of PBS.
To determine the level of attachment by viable count, the rinsed coupons were placed in 5 ml of maximum recovery diluent (Oxoid) in a universal tube and vortexed (Whirlimixer; Fisons) at maximum speed for 2 min to dislodge the cells (modification of the method of Dhir and Dodd) (6). The detached cell suspensions were serially diluted in maximum recovery diluent, and viable cell counts were determined using the method of Miles et al. (15) by spotting appropriate dilutions (10 μl) onto plate count agar (Oxoid) and incubating them at 37°C for 24 h before enumeration. Each observation used at least duplicate stainless steel samples and was repeated twice. An analysis of variance was performed using Data Analysis Tool Pack from Excel 5.0. Assessment was based on a 95% confidence limit. Data are mean values with standard deviations.
To determine the percentage of coverage visually, duplicate coupons were stained with acridine orange (0.01%, wt/vol) for 3 min, excess dye was rinsed off with water, and the coupons were air dried. The stained coupons were observed under an epifluorescent microscope (Axiovert 135 TV; Carl Zeiss Ltd., Welyn Garden City, Herfordshire, United Kingdom) connected to a charge-coupled-device camera (EG&G Berthold, Bad Wildbad, Germany). Images of 15 microscopic fields per coupon were saved using WinLight software (EG&G Berthold). Pixels of the biofilm area were selected using Adobe Photoshop 3.0 software, and the percentage of coverage (total pixels of biofilms in each field compared with total pixels of the whole area of a microscopic field) was obtained by an image analysis software program (provided by Christine Onyango, Silsoe Research Institute, Bedford, United Kingdom). As previously, each observation used at least duplicate stainless steel samples and was repeated twice; analysis of variance was performed as described above.
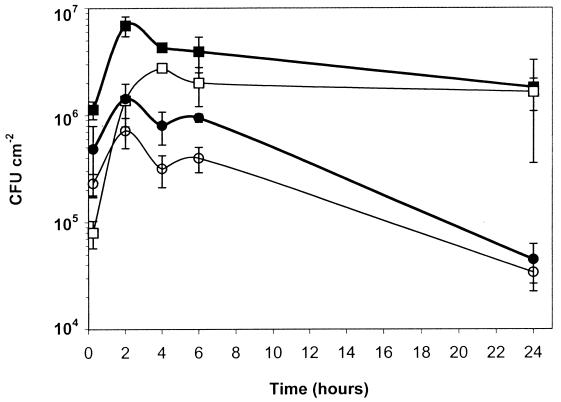
As shown in Fig. 1, at 22°C the number of wild-type cells (flagellated and nonmotile) attaching to the coupons gradually increased from 1 to 4 h of incubation and remained at the same level afterwards for up to 24 h. This was up to 10-fold higher than attachment levels of the nonflagellated mutant over the first 4 h of incubation (significance, P ≤ 0.05). After longer incubation (6 to 24 h) at 22°C, the cell numbers of the wild type showed no significant difference from those of the mutant. In contrast, at 37°C there was no significant difference (P ≥ 0.05) between the number of attached cells of the wild type (limited flagellation) and those of the mutant (nonflagellated), but the numbers were lower than those achieved at 22°C. The attachment of wild-type cells and the attachment of mutant cells were similarly reduced at 24 h (4.62 ± 0.18 and 4.51 ± 0.15, respectively). At 24 h the percentages of coverage of wild-type and mutant cells incubated at 22°C were 14.4 ± 2.5 and 8.8 ± 2.2, respectively, whereas the percentages of coverage of the wild type and mutant incubated at 37°C declined to 0.5 ± 0.2 and 0.6 ± 0.3, respectively. Hence, the presence or absence of the flagella did not influence final levels of attachment achieved over longer periods of time. Also, at 37°C the mutant gave a final level of attachment similar to that of the parental wild type, but these levels were lower than those achieved at 22°C. The results indicate that the higher incubation temperature does diminish the overall levels of the attachment of the wild-type cells over 24 h, since both the viable count and the percentage of coverage decreased. These results suggest that changes in surface structures other than flagella contribute to attachment. The decline in cell numbers remaining attached at 37°C is not likely to be due to cell death; it is more likely that the same changes which result in lower levels of cell attachment when cells are grown at 37°C also allow cells to detach from the surface more readily.
FIG. 1.
Viable cell numbers in L. monocytogenes biofilm for up to 24 h. ■ and ●, wild type incubated at 22 and 37°C, respectively; □ and ○, nonflagellated mutant incubated at 22 and 37°C, respectively. The initial concentration was 8.5 × 108 CFU ml−1. Error bars show the standard deviations of six values from triplicate samples.
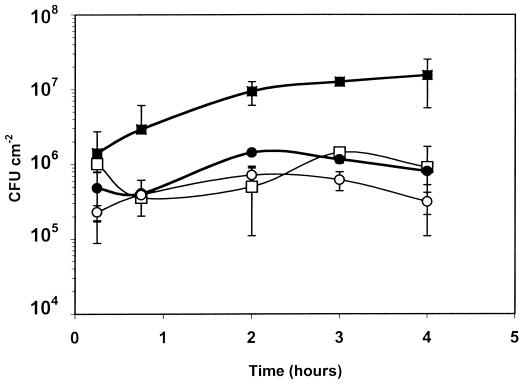
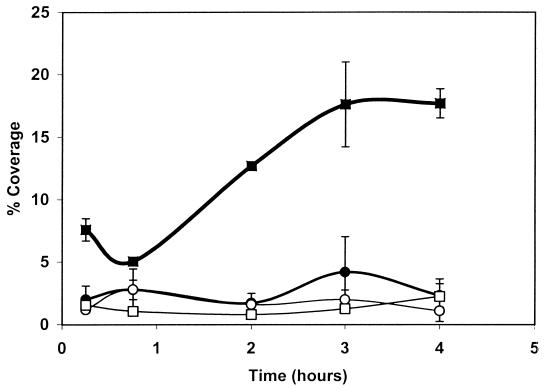
Since the initial stages of attachment appeared to be especially affected by the presence or absence of flagella, the first 4 h of attachment were examined in more detail. The results (Fig. 2) showed that the number of viable detached cells was significantly higher (P ≤ 0.05) for the wild type incubated at 22°C than for the mutant incubated at 22 or 37°C or for the wild type incubated at 37°C. However, there was no significant difference (P ≥ 0.05) between the attachment of the wild-type strain at 37°C and that of the mutant incubated at either temperature. The use of acridine orange staining showed that the area of stainless steel covered by the wild type when incubated at 22°C increased between 15 min and 3 h (Fig. 3) and was almost 10-fold higher after 3 h than the area colonized by the nonflagellated mutant grown at either temperature or the wild type incubated at 37°C; the latter also showed no significant difference from the mutant incubated at either temperature. This suggests that the difference in attachment of the wild type at 22°C is due primarily to the presence of flagella; it is only this initial attachment that is affected, however, since after longer periods surface coverage by nonflagellated cells at 22°C achieved nearly the same level as that of flagellated cells.
FIG. 2.
Viable cell numbers of L. monocytogenes biofilm over a 4-h period. The initial concentration was 8.6 × 108 CFU ml−1. ■ and ●, wild type incubated at 22 and 37°C, respectively; □ and ○, nonflagellated mutant incubated at 22 and 37°C, respectively. Error bars show the standard deviations of six values from triplicate samples.
FIG. 3.
Percentage of coverage by L. monocytogenes biofilm on stainless steel over a 4-h period, determined by staining with acridine orange. Fifteen microscopic fields of each coupon were analyzed. The area covered by the bacteria on each field was compared to the total area of a microscopic field as an absolute value (100%). ■ and ●, wild type incubated at 22 and 37°C, respectively; □ and ○, nonflagellated mutant incubated at 22 and 37°C, respectively. Error bars show standard deviations of values from triplicate coupons.
Our data suggest that the flagella, independent of cell motility, do indeed act as an adhesive structure during early stages of attachment under static conditions. In the environment, and in particular in areas of food production, temperatures of 22°C and below are frequently encountered; under these conditions L. monocytogenes will be flagellated, and this will aid attachment to stainless steel processing surfaces even if the surrounding environment does not provide enough nutrients to allow the organism to be fully motile. In addition, changes in surface structure other than the presence or absence of the flagella also favor attachment of cells to stainless steel at low temperatures.
Acknowledgments
We thank Christine Onyango, Silsoe Research Institute, for her help with image analysis software.
S. Vatanyoopaisarn was supported by a Thai government studentship.
REFERENCES
- 1.Blackman I C, Frank J F. Growth of Listeria monocytogenes as a biofilm on various food-processing surfaces. J Food Prot. 1996;59:827–831. doi: 10.4315/0362-028X-59.8.827. [DOI] [PubMed] [Google Scholar]
- 2.Butler J L, Stewart J C, Vanderzant C, Carpenter Z L, Smith G C. Attachment of microorganisms to pork skin and surfaces of beef and lamb carcasses. J Food Prot. 1979;42:401–406. doi: 10.4315/0362-028X-42.5.401. [DOI] [PubMed] [Google Scholar]
- 3.Carpentier B, Cerf O. Biofilms and their consequences, with hygiene in the food industry. J Appl Bacteriol. 1993;75:499–511. doi: 10.1111/j.1365-2672.1993.tb01587.x. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty T, Leimeister-Wächter M, Domann E, Hartl M, Goebel W, Nichterlein T, Notermans S. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J Bacteriol. 1992;174:568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton J W, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 6.Dhir V K, Dodd C E R. Susceptibility of suspended and surface-attached Salmonella enteritidis to biocides and elevated temperatures. Appl Environ Microbiol. 1995;61:1731–1738. doi: 10.1128/aem.61.5.1731-1738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher M. The effect of culture concentration, age, time and temperature on bacterial attachment to polystyrene. Can J Microbiol. 1977;23:1–6. [Google Scholar]
- 8.Frank J F, Koffi R A. Surface-adherent growth of Listeria monocytogenes is associated with increased resistance to surfactant sanitizers and heat. J Food Prot. 1990;53:550–554. doi: 10.4315/0362-028X-53.7.550. [DOI] [PubMed] [Google Scholar]
- 9.Griffin A M, Robbins M L. The flagellation of Listeria monocytogenes. J Bacteriol. 1944;48:114–115. doi: 10.1128/jb.48.1.114-115.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill P J, Swift S, Stewart G S A B. PCR based gene engineering of the Vibrio-harveyi-lux operon and the Escherichia-coli-trp operon provides for biochemically functional native and fused gene products. Mol Gen Genet. 1991;226:41–48. doi: 10.1007/BF00273585. [DOI] [PubMed] [Google Scholar]
- 11.Kim K Y, Frank J F. Effect of nutrients on biofilm formation by Listeria monocytogenes on stainless steel. J Food Prot. 1995;58:24–28. doi: 10.4315/0362-028X-58.1.24. [DOI] [PubMed] [Google Scholar]
- 12.Korber D R, Lawrence J R, Caldwell D E. Effect of motility on surface colonization and reproductive success of Pseudomonas fluorescens in dual-dilution continuous culture and batch culture systems. Appl Environ Microbiol. 1994;60:1421–1429. doi: 10.1128/aem.60.5.1421-1429.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korber D R, Lawrence J R, Sutton B, Caldwell D E. Effect of laminar flow velocity on the kinetics of surface recolonization by mot+ and mot−Pseudomonas fluorescens. Microb Ecol. 1989;18:1–19. doi: 10.1007/BF02011692. [DOI] [PubMed] [Google Scholar]
- 14.Lillard H S. Role of fimbriae and flagella in the attachment of Salmonella typhimurium to poultry skin. J Food Sci. 1986;51:54–56. [Google Scholar]
- 15.Miles A A, Misra S S, Irwin J O. The estimation of the bactericidal power of blood. J Hyg. 1938;38:733–749. doi: 10.1017/s002217240001158x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moens S, Vanderleyden J. Functions of bacterial flagella. Crit Rev Microbiol. 1996;22:67–100. doi: 10.3109/10408419609106456. [DOI] [PubMed] [Google Scholar]
- 17.O'Toole G A, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 18.Piette J-P G, Idziak E S. Role of flagella in adhesion of Pseudomonas fluorescens to tendon slices. Appl Environ Microbiol. 1991;57:1635–1639. doi: 10.1128/aem.57.6.1635-1639.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prentice P A, Neaves P. The identification of Listeria species. In: Board R G, Jones D, Skinner F A, editors. Applied bacterial symposium. Oxford, England: Blackwell Science Ltd.; 1992. pp. 283–296. [Google Scholar]
- 20.Sasahara K C, Zottola E A. Biofilm formation by Listeria monocytogenes utilizes a primary colonizing microorganism in flowing systems. J Food Prot. 1993;56:1022–1028. doi: 10.4315/0362-028X-56.12.1022. [DOI] [PubMed] [Google Scholar]
- 21.Seeliger H P R. Listeriosis. New York, N.Y: Hafner Publishing Co.; 1961. [Google Scholar]
- 22.Seeliger H P R, Jones D. Genus Listeria Pirie 1940, 383AL. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. pp. 1235–1245. [Google Scholar]
- 23.Stanley P M. Factors affecting the irreversible attachment of Pseudomonas aeruginosa to stainless steel. Can J Microbiol. 1983;29:1493–1499. doi: 10.1139/m83-230. [DOI] [PubMed] [Google Scholar]
- 24.Wirtanen G, Mattila-Sandholm T. Epifluorescence image analysis and cultivation of foodborne biofilm bacteria grown on stainless steel surfaces. J Food Prot. 1993;56:678–683. doi: 10.4315/0362-028X-56.8.678. [DOI] [PubMed] [Google Scholar]
- 25.Zottola E A. Microbial attachment and biofilm formation: a new problem for the food industry? Food Technol. 1994;48:107–114. [Google Scholar]