Abstract
In fasted and fed states, blood insulin levels are oscillatory. While this phenomenon is well studied at high glucose levels, comparatively little is known about its origin under basal conditions. We propose a possible mechanism for basal insulin oscillations based on oscillations in glycolysis, demonstrated using an established mathematical model. At high glucose, this is superseded by a calcium-dependent mechanism.
Keywords: bursting electrical activity, calcium, metabolism, pulsatile insulin secretion
Introduction
In response to a meal, blood glucose levels rise, and this is sensed by the β-cells of the islets of Langerhans distributed sparsely throughout the pancreas, which then secrete the hormone insulin (1). Once entering the circulation, insulin acts to increase glucose uptake into muscle and adipose tissues while it inhibits hepatic glucose production (2). Glucose-induced insulin secretion is thus a key player in whole body glucose homeostasis, and its dysregulation is a major contributor to type 2 diabetes (3).
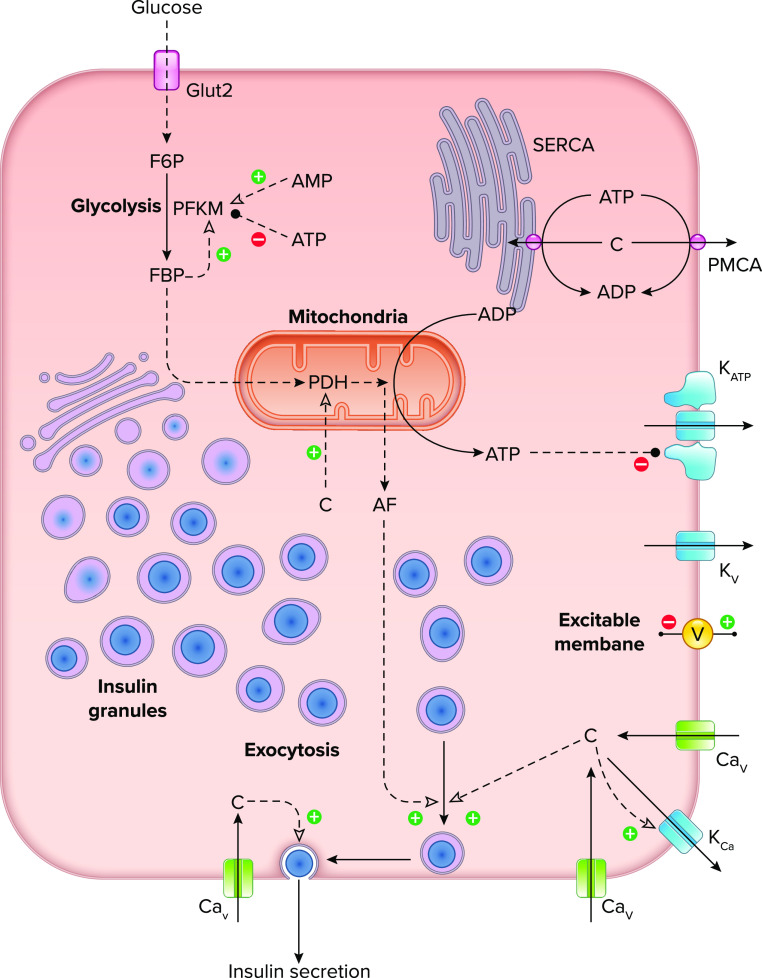
The insulin secretory component of this homeostatic control system is regulated by an exquisite set of mechanisms in β-cells (1) (FIGURE 1). In contrast to many other hormones, insulin secretion is regulated directly by the rate of glucose metabolism, which serves as a surrogate for the concentration of glucose in the blood (4). Glucose enters the β-cells through glucose transporters and is metabolized in glycolysis and then by mitochondrial respiration, increasing the ATP/ADP ratio. Insulin secretion is then stimulated via two major pathways: the triggering pathway, which mediates the rise in intracellular Ca2+ needed to trigger exocytosis, and the amplifying pathway, which increases docking and priming of insulin-containing granules and brings them into close proximity to CaV channels, enhancing the efficacy of Ca2+ in driving secretion.
FIGURE 1.
Diagram illustrating the key cellular components and molecular mechanisms involved in oscillations of Ca2+ and insulin secretion in the pancreatic β-cell Glucose enters the cell through glucose transporters and is metabolized in glycolysis and the mitochondria. ATP, mostly produced by the mitochondria, closes KATP channels in the plasma membrane, leading to depolarization. Additional ion channels, including voltage-gated Ca2+ (CaV) and K+ channels (KV) and Ca2+-activated K+ channels (KCa), support excitability of the plasma membrane potential (V) and underlie bursting activity at postprandial glucose levels. Depolarization of the membrane leads to Ca2+ (c) influx through CaV channels, while cytosolic Ca2+ levels are reduced by activity of Ca2+ ATPase pumps [sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) and plasma membrane Ca2+-ATPase (PMCA)]. Exocytosis of insulin-containing granules is triggered by Ca2+ and amplified by one or more metabolic signaling factors (AF) that promote the movement of insulin granules towards a releasable state. F6P, fructose-6-phosphate; FBP, fructose-1,6-bisphosphate; PDH, pyruvate dehydrogenase; PFKM, phosphofructokinase, muscle.
Central to the triggering pathway are ATP-sensitive potassium (KATP) channels, which close in response to increases in the cytosolic ATP/ADP ratio. KATP channels are open in low glucose, maintaining the cells at a negative membrane potential, but when they close, the cells depolarize, opening voltage-dependent calcium (CaV) channels and initiating Ca2+ entry (c; FIGURE 1). The reciprocal activation of CaV and voltage-dependent potassium (KV) channels generates action potentials (spiking), much like the excitable membranes of neurons and muscle cells. Ca2+ then triggers the exocytosis of insulin granules, primarily those located near the CaV channels.
In addition to generating ATP, glucose metabolism gives rise to one or more metabolic signals, called amplification factors (AF; FIGURE 1), that mediate the amplifying pathway introduced above. The identity of the AF remains unclear, but some of the suggested candidates include ATP, glutamine, and NADPH (4, 5). A recent review highlights possible roles of reactive oxygen species, lipids and phosphoenolpyruvate (6). The AF is physiologically important, as it is responsible for about half the insulin secretion (7).
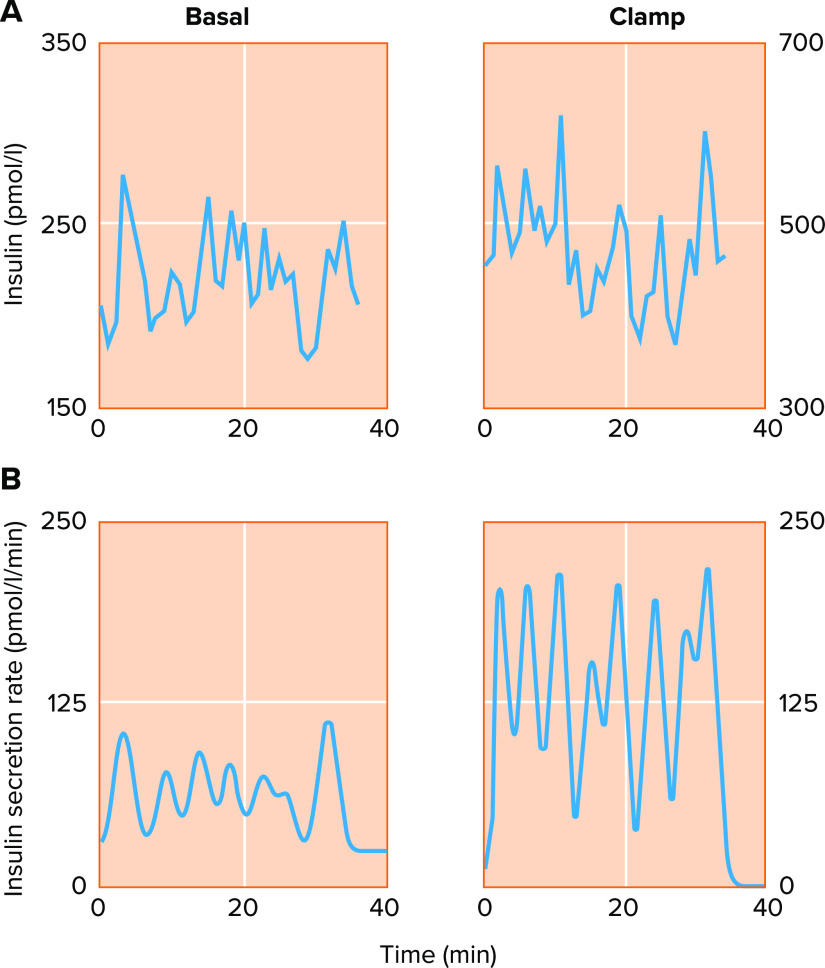
The picture described above contains the basic information needed to understand β-cell function but is incomplete, as blood insulin levels in vivo are not steady but oscillatory with a period of about 5 min, as has been observed in humans, rodents, nonhuman primates, and canines (8–11). FIGURE 2 shows an example of insulin levels recorded from the portal vein in a human (FIGURE 2A), with insulin secretion rate reconstructed by deconvolution, as shown below the raw secretory data (FIGURE 2B) (12). Similar data have been recorded from the portal vein in rats (13) and in dogs (14). The latter study looked at oscillations before and after glucose ingestion, indicating an average increase of 400% in pulse amplitude and 40% in frequency. The pulsatile nature of insulin release, which resembles in a general manner that of other hormones (15), is believed to be necessary for the efficacious action of the hormone (16); for a review, see Ref. 17.
FIGURE 2.
Basal and glucose-stimulated insulin secretion are oscillatory in humans
A: insulin oscillations measured from the hepatic portal vein at basal glucose (4.4 mM; left) and high glucose imposed via hyperglycemic clamp (nominally 8–9 mM; right). The ∼5-min period of the oscillations does not change dramatically in response to glucose, but insulin rises about 2-fold in the higher glucose condition (note the difference in scale). B: deconvolution analysis resolves the underlying pulsatile nature of the insulin secretion rate, demonstrating that glucose stimulates a large increase in insulin pulse mass. Reproduced from Ref. 12, with permission from Journal of Clinical Endocrinology & Metabolism.
The insulin oscillations observed in the circulation are driven by insulin secreted in pulses from the islets. Pulsatile insulin secretion from islets in vitro has been shown for both humans and mice (18–24). Notably, the oscillations of isolated islets have the correct period and respond to increasing glucose concentration with increases in insulin pulse amplitude. Despite the pulse-generating capability of individual islets, important questions remain about how the hundreds of thousands of islets within the intact pancreas synchronize their secretory output to generate the insulin pulses of portal blood. Suggested synchronizing signals include acetylcholine, ATP, and nitric oxide, which have been studied by pharmacologically inhibiting the neurons that innervate the pancreas or by vagotomy, but these studies failed to reach a consensus (25–28). While it is also possible that interislet synchronization is different under basal conditions, we assume in the absence of evidence to the contrary that the core oscillation mechanism still resides within individual islets. We proceed to focus here as a first step on the oscillation mechanisms of individual islets.
At higher glucose levels (7–15 mM) corresponding to postprandial or diabetic conditions, oscillations in islet insulin secretion are driven in large part by oscillations in cytosolic free Ca2+ (29). These Ca2+ oscillations arise in turn from a second role of Ca2+ in addition to stimulating the exocytosis of insulin granules shown in FIGURE 1, namely the negative feedback exerted by Ca2+ on its own entry. This occurs by two complementary mechanisms. The first mechanism proposed was activation of KCa channels, similar to the situation in many other endocrine cells and neurons. This causes the spikes to cluster into bursts rather than occurring continuously [see FIGURE 4 showing simulation of membrane potential (V) and concentration (c)] and was the basis of the earliest mathematical models of Ca2+ oscillations in β-cells (30). A number of other models were quickly proposed based on this idea or variations on it, as reviewed in Refs. 31, 32.
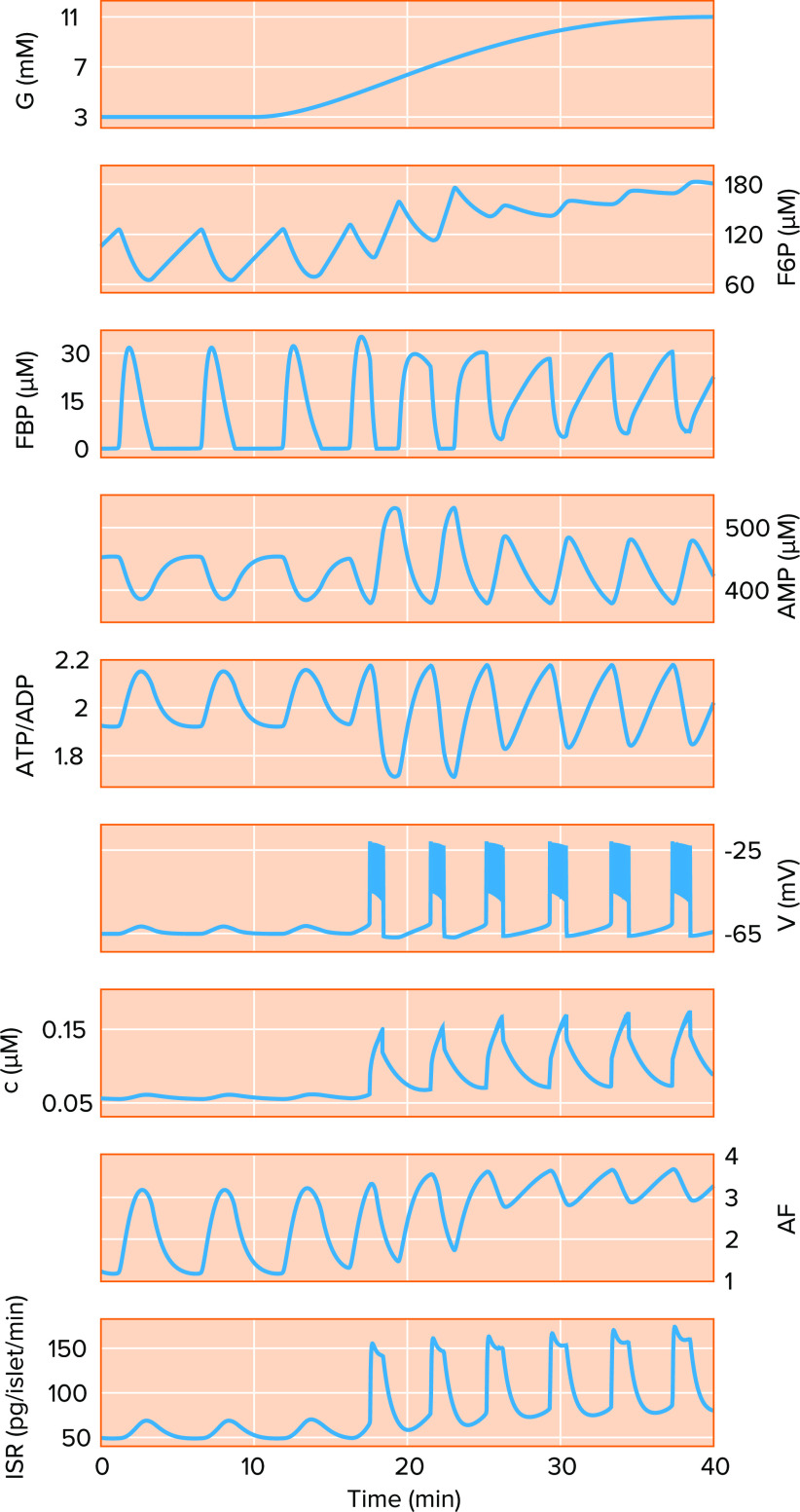
FIGURE 4.
The integrated oscillator model with modification can account for basal insulin oscillations A model simulation of a ramped increase in glucose (G) from basal (3 mM) to postprandial to the high levels typically studied in vitro (11 mM). Key dynamic variables shown include the glycolytic metabolites fructose 6 phosphate (F6P) and fructose 1,6 bisphosphate (FBP), AMP, ATP/ADP ratio, membrane potential (V), cytosolic Ca2+ concentration (c), an amplifying factor (AF) that enhances exocytosis, and the insulin secretion rate (ISR).
A second, more subtle form of negative feedback was subsequently appreciated: Ca2+ entry and accumulation activate Ca2+ pumps in the endoplasmic reticulum (ER) and plasma membranes [sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) and plasma membrane Ca2+-ATPase (PMCA), as shown in FIGURE 1]. These pumps restore cytosolic Ca2+ levels in between bursts of spikes but also consume ATP; see Ref. 33 for experimental evidence that raising Ca2+ lowers ATP/ADP. This would reopen some of the KATP channels that were closed when glucose was elevated and could contribute to the termination of each burst of spikes, as first proposed in Ref. 33. This is a much slower process than activation of KCa channels and is a more appropriate explanation of the 5-min oscillations of insulin observed in the circulation. Current models in which KATP channels either drive Ca2+ oscillations (34) or are limited to setting the glucose threshold for oscillations driven by other ion channels (35, 36) are reviewed and contrasted in Refs. 37, 38. A further point, which will take on heightened significance below, is that if metabolism oscillates in phase with Ca2+, the AF will also oscillate and reinforce the pulses of secretion (39).
We now come to the heart of the matter: oscillations in secretion are also seen in basal glucose in vivo (4–5 mM) (12, 14, 40, 41) and from isolated human (24) and mouse islets in vitro (42, 43). In fact, it has been reported that as much as 70% of the total insulin secreted from the pancreas under basal conditions, where both constant and pulsatile release are observed, occurs in pulses (44). Yet, in vitro data from islets exposed to these low glucose concentrations indicate that the β-cells are not electrically active and do not exhibit large amplitude oscillations in cytosolic free Ca2+ concentration in this case. This raises the question: what is the mechanism for oscillatory insulin secretion at basal glucose levels? This is not a purely academic question, because blood glucose is within its basal range most of the time. The high concentrations commonly used in in vitro experiments are typically only experienced by people or animals with diabetes.
Regulation of basal insulin secretion has clinical significance, as elevated basal insulin is a good predictor of future diabetes (45). It occurs years before basal glucose increases and is a marker of insulin resistance, as embodied in the widely used indexes homeostatic model assessment for insulin resistance (HOMA-IR) and quantitative insulin-sensitivity check index (QUICKI) (46, 47). It has also been suggested that in addition to being a marker of insulin resistance, elevated basal insulin may be a cause of insulin resistance (48, 49) as well as obesity (6, 50). A recent review, however, concludes that the preponderance of evidence is against hyperinsulinemia as a primary cause of diabetes in most cases; a good introduction to this debate is the review in Ref. 51 and the commentary responding to it in Refs. 52, 53.
While the mechanism of elevated basal insulin secretion and its contribution to diabetes is not established, various hypotheses have been proposed. Examples include dysregulated basal insulin release due to the interaction between reactive oxygen species (ROS) and long-chain fatty acids (as reviewed in Ref. 6), abnormal levels of cardiolipin in mitochondria due to its altered biosynthesis with concomitant changes in mitochondrial respiration (54), changes in the regulation of sweet taste receptors on the β-cell that normally act as a brake on basal secretion (55), elevation in basal secretion due to fatty acids that does not involve ATP synthesis, mitochondrial lipid oxidation or ROS but does involve Ca2+ (56), increased proton leak across the mitochondrial inner membrane mediated by fatty acids independently of ATP synthesis (57), and lastly, a novel Ca2+ influx pathway activated by ER stress under low glucose conditions, leading to more insulin secretion (58).
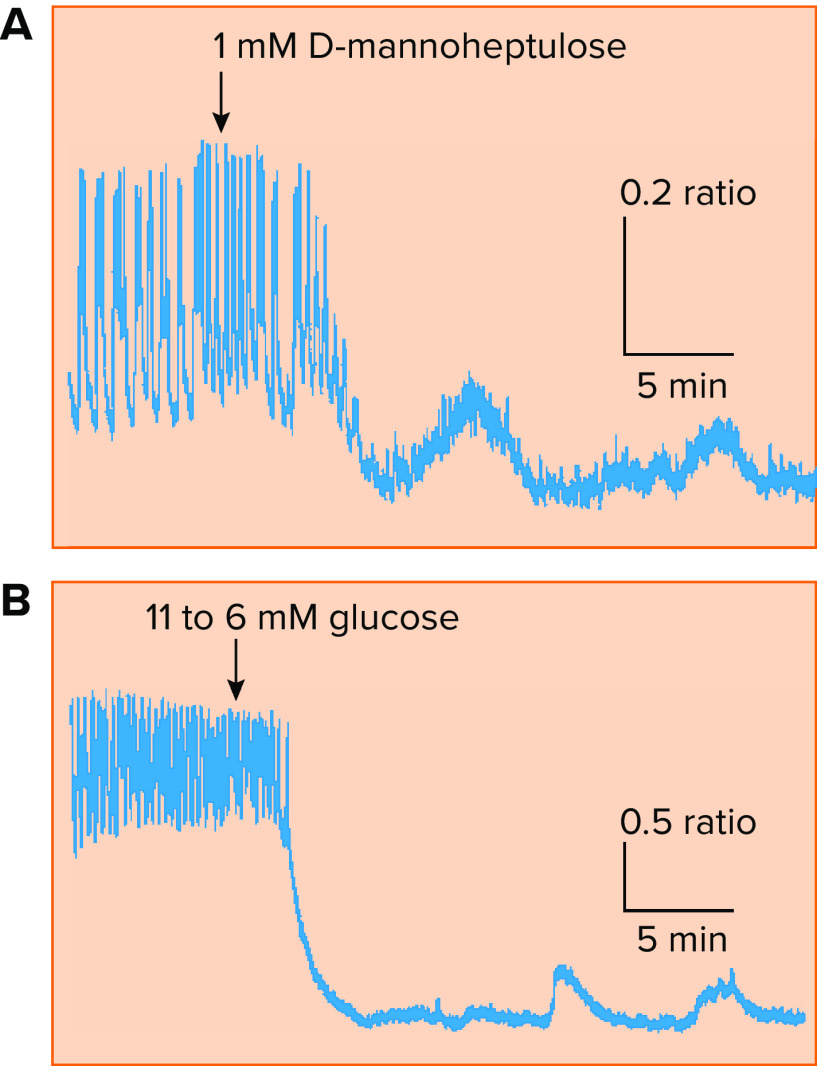
Calcium oscillations have in fact been seen in low glucose (e.g., at 6 mM, just below threshold levels of glucose needed to trigger full blown oscillatory activity) and in islets treated with high glucose and mannoheptulose to inhibit glycolysis (FIGURE 3) (59). However, these small-amplitude Ca2+ oscillations are too small to plausibly engage the Ca2+ feedback mechanisms acting on KCa or KATP channels oscillations described above, and it is unlikely that they alone could be the driver of insulin oscillations in basal glucose.
FIGURE 3.
Oscillations in intracellular Ca2+ concentration, the main trigger for insulin exocytosis, are observed in islets in vitro A; upon partial block of glycolysis using d-mannoheptulose, large amplitude glucose-stimulated Ca2+ oscillations in a mouse islet give way to small amplitude oscillations unlikely to be sustained by ionic mechanisms alone. B: a similar result observed in response to a reduction in glucose from a suprathreshold (11 mM) to a subthreshold (6 mM) concentration. Reproduced with permission from Ref. 59, with permission from Biophysical Journal.
We hypothesize instead that metabolism can oscillate at the level of glycolysis despite low levels of Ca2+, as described in detail below. The possibility that glycolytic oscillations can occur in low glucose is supported by recordings of oscillations in KATP channel conductance at 3 mM glucose (60) as well as by simulations carried out using mathematical models (61). We further hypothesize that when subthreshold, small-amplitude Ca2+ oscillations (henceforth referred to here as subthreshold oscillations) are coupled to coincident oscillations in metabolism, their effect is amplified sufficiently by the AF to produce small amplitude secretory oscillations. Oscillations in secretion driven by oscillations in metabolism with Ca2+ fixed, albeit at high-glucose levels, have been seen experimentally (62), making this plausible. Finally, we demonstrate here using a current mathematical model of oscillatory activity in mouse islets that as glucose is increased, the oscillations in the free cytosolic Ca2+ concentration, membrane potential, and insulin secretion transform naturally into the patterns that are observed at high glucose. Although our goal is to explain basal insulin oscillations in humans, the model for mouse is the best developed for addressing the interplay between oscillations driven by metabolism versus Ca2+, and we expect the general principles to apply.
The Integrated Oscillator Model
Over the past two decades we have developed a mathematical model that can account for most of the oscillatory activity patterns observed in β-cells. This model, the integrated oscillator model (IOM) (37, 63, 64), has been very helpful for generating hypotheses that were subsequently tested in experiments and in the design of those experiments. Here, we use the IOM to illustrate our hypothesis for the origin of oscillatory insulin secretion at basal glucose levels and demonstrate its feasibility. An earlier version of the model demonstrated that subthreshold Ca2+ oscillations were indeed possible and would convert to full amplitude oscillations at higher glucose (59, 61), but we did not examine their relationship to secretion oscillations using that model. The model used in this article is closely related to the version previously described in Ref. 64, with the addition only of a previously published set of equations to translate oscillations in Ca2+ and metabolism into oscillations in insulin secretion (39). Computer codes for the model are available at https://www.math.fsu.edu/~bertram/software/islet/ (also see Supplementary Material available at https://doi.org/10.6084/m9.figshare.17063984).
We Hypothesize That Glycolytic Oscillations Drive Pulsatile Insulin Secretion at Basal Glucose Levels
Using the IOM, we demonstrate the transitions in electrical activity and secretion that occur as glucose is ramped from basal to suprathreshold levels (FIGURE 4). At subthreshold glucose it is possible to produce oscillations, albeit small, in insulin secretion (see the first 15 min of the ISR in FIGURE 4). In this case, the model β-cell is nearly electrically silent, so the dramatic opening and closing of ion channels that would occur when the cell is electrically active do not take place. In fact, the Ca2+ channels that allow Ca2+ entry into the cell are mostly closed since the membrane potential (V) is relatively hyperpolarized, so the small changes in the Ca2+ concentration (c) observed in this scenario reflect small fluctuations in V and associated changes in the driving force for Ca2+ current. These Ca2+ oscillations are not by themselves sufficient for basal insulin secretion oscillations.
In the model, the oscillations in the insulin secretion rate (ISR) are driven instead by oscillations in glycolysis, represented here by the intermediate metabolites fructose 6 phosphate (F6P) and fructose 1,6 bisphosphate (FBP). These lead to oscillations in the ATP/ADP ratio and in turn to small oscillations in V and c via changes in KATP channel conductance. Additionally, the oscillations in glucose metabolism lead to robust oscillations in the metabolic amplification factor described above (AF; FIGURES 1 AND 4), which enhances the efficacy of Ca2+ to trigger the exocytosis of insulin granules. The model calculation in FIGURE 4 suggests that even small excursions of the Ca2+ concentration, when combined with large pulses in AF, can result in meaningful oscillations in the secretion rate.
Insulin Pulse Amplitude Increases With Glucose and Activity Patterns Change
As glucose increases above the threshold for electrical activity (∼5 mM), repetitive bursts of action potentials appear (starting at around 17 min; FIGURE 4), mediating large oscillations in the intracellular Ca2+ concentration (c; FIGURE 4). The oscillations in FBP and AF persist but do not increase dramatically in amplitude; the large increase in ISR seen here is due mainly to the increased amplitude of the Ca2+ oscillations.
As glucose rises further toward the range usually studied in vitro (8–11 mM), oscillations in V, c, and ISR continue but intensify. The active, spiking phases of the bursts become longer, which increases the average level of Ca2+, which in turn combines with glucose-dependent increases in AF to increase the amplitude of the ISR pulses. Note that oscillation frequency does not change much, consistent with the experimental data in FIGURE 2.
A subtle but important change in the character of the FBP oscillations in FIGURE 4 also appears at these higher glucose levels: instead of a discrete pulse that precedes each burst and falls nearly to 0, the FBP oscillations become sawtooth in shape, rising slowly throughout the silent phase and falling during the active phase. This is a good sign of the fidelity of the model, as measurements of FBP oscillations at higher glucose levels generally have a sawtooth shape (65, 66). Closer examination elsewhere (37, 63) has revealed that the pulse-like metabolic oscillations do not require oscillations in Ca2+, whereas the sawtooth oscillations cease if Ca2+ is held constant. We denote these as active metabolic oscillations (AMOs) and passive metabolic oscillations (PMOs), respectively, to distinguish the two cases as previously described in Refs. 37, 63. The existence of AMOs at basal glucose is critical to our hypothesis, as PMOs require large amplitude Ca2+ oscillations to occur that would not be possible at basal glucose.
How Do β-Cells Orchestrate Such a Wide Range of Activity Patterns?
Work dating back to the 1970s has demonstrated that metabolic oscillations could be produced in glycolysis, the first stage of glucose metabolism that precedes aerobic respiration. First in yeast (67), and later in muscle extracts (68, 69), it was shown that glycolysis can sustain long-term oscillations. These oscillations are believed to be due to the actions of a key allosteric enzyme, phosphofructokinase (PFK), that converts the substrate fructose-6-phosphate (F6P) into fructose-1,6-bisphosphate (FBP). Importantly, the product FBP is a positive regulator of PFK, and that positive feedback causes a buildup of FBP at the expense of the substrate F6P. At the same time, AMP, which increases the affinity of PFK for F6P, also falls due to increased ATP production downstream in glycolysis and the mitochondria. When F6P and AMP fall too low, PFK activity largely shuts down, ending the positive feedback cycle and resetting the system. This is the basis of the FBP oscillations in FIGURE 4 that underlie oscillatory insulin secretion at low glucose levels. This oscillation mechanism was first proposed in the context of stimulatory glucose levels (69), but we believe that it is actually most important at subthreshold levels.
The PFK isoform that has highest affinity for FBP, PFKM, was long assumed to be the critical player in this scenario, but we and others have found that slow Ca2+ oscillations persist in PFKM knockout mice (70, 71). Furthermore, model simulations suggested that other PFK isoforms can take over in the absence of PFKM (70). Regardless of the details regarding this enzyme, as long as glycolytic oscillations can occur independent of Ca2+, our basic hypothesis that they are responsible for subthreshold oscillations in insulin secretion would remain viable.
Although we have described how glycolytic oscillations can produce Ca2+ oscillations, the reverse can also happen: Ca2+ can influence glycolytic oscillations. This is the key to the transformation from AMOs to PMOs as glucose increases, as illustrated in FIGURE 4 and discussed above. At high glucose, glycolysis loses the ability to oscillate independently of Ca2+. This is partly due to the intrinsic glucose sensitivity of glycolysis: at high glucose the substrate F6P remains high, so PFK activity remains high, and oscillations are lost. In addition, Ca2+ activation of pyruvate dehydrogenase (PDH; FIGURE 1) contributes by increasing the consumption of pyruvate by PDH, which in turn increases the consumption rates of glycolytic metabolites, including FBP. This shuts down the positive feedback of FBP onto PFK and inhibits glycolytic oscillations. The signature of this in FIGURE 4 is the conversion of the FBP waveform from pulses into a sawtooth, as FBP slowly responds to the rise and fall of Ca2+.
Nonetheless, oscillations in metabolites, such as FBP and ATP/ADP, can still occur, by a mechanism discussed in the Introduction. When Ca2+ is high, ATP is consumed by Ca2+ pumps, including the sarco/endoplasmic reticulum Ca2+ ATPase and plasma membrane Ca2+ ATPase (labeled as SERCA and PMCA in FIGURE 1). This lowers ATP/ADP, allowing some KATP channels to reopen, which turns off Ca2+ entry. The drop in Ca2+ allows ATP/ADP to recover, which again closes KATP channels, and electrical activity resumes. Glycolytic metabolites, such as FBP, also oscillate because of the Ca2+ dependence of PDH mentioned above—when Ca2+ is high, flux through glycolysis and cellular respiration is increased—and also because of feedback of ATP and AMP onto PFK. These repeated cycles of ATP consumption and production underlie the oscillations in Ca2+, metabolites, and insulin secretion at high glucose, shown in the simulation in FIGURE 4 starting at around 25 min. In vitro data support this mechanism for PMOs (65).
Summary and Future Directions
We have presented here the hypothesis that oscillations of basal insulin secretion are driven by metabolic oscillations, specifically, oscillations in glycolysis that do not require, but can be modified by, Ca2+. We have used model simulations of oscillations in membrane potential, Ca2+, and insulin secretion to illustrate and support the feasibility of the hypothesis. Models in which oscillations of metabolism only occur secondary to oscillations in Ca2+, which likely occur for the secretory oscillations produced at elevated glucose, cannot account for oscillations observed under basal glucose.
The hypothesis could be tested in islets in vitro by looking for small amplitude oscillations in Ca2+ at low glucose, say in the range of 3–7 mM, while simultaneously recording oscillations in metabolites, such as ATP/ADP, FBP, or NAD(P)H. It may take some trial and error to find the right conditions, as the simulations show that the prevailing Ca2+ levels may determine whether metabolic oscillations occur at a particular level of glucose.
There are ample data in the literature demonstrating oscillations at stimulatory glucose levels in multiple metabolites, including oxygen consumption (72), mitochondrial membrane potential (73), ATP/ADP (74), and NAD(P)H (75), which are synchronized with membrane potential and Ca2+ (65). In addition, a fluorescence resonance energy transfer (FRET) sensor has been developed called the pyruvate kinase activity reporter (PKAR), based on the glycolytic enzyme pyruvate kinase (PK). PK is allosterically activated by FBP, and PKAR FRET is thus an assay to dynamically measure FBP concentration. Fluorescence measurements from islets showed that at stimulatory glucose levels PKAR FRET responses were oscillatory (76) and coincident with Ca2+ oscillations (65). Furthermore, metabolic oscillations persisted under conditions where oscillations in membrane potential and Ca2+ were abolished with diazoxide, demonstrating the existence of AMOs at high glucose (65). No study has yet been performed using PKAR to look for AMOs at basal glucose levels, so it is not known if oscillations exist in this case. We predict that they would exist for cases in which basal insulin secretion oscillations are present. We also predict that the concentration of ATP would oscillate. As an alternative to PKAR, the fluorescent reporter Perceval-HR, which provides a readout of the cytosolic ATP/ADP ratio, could be used to demonstrate metabolic oscillations in basal glucose.
In addition to offering a plausible mechanism for oscillations in basal insulin secretion, the simulations described here also help resolve a conundrum about how the IOM works. Slow oscillations in Ca2+ in the model can result from either AMOs or PMOs. The simulations here suggest that these two modes of operation are most appropriate at different glucose levels. AMOs are the only candidate mechanism of which we are aware that can generate secretory oscillations in basal glucose, and they transition in an orderly way to PMOs as glucose is increased. This was not apparent from previous modeling studies in which glucose was held fixed while other parameters were varied. It is still not apparent why AMOs give way to PMOs at higher glucose, as either mechanism could operate effectively in this range in theory, raising the question of what benefit islets might derive from having two seemingly redundant mechanisms. Nonetheless, AMOs appear to persist in high glucose in at least some islets, as demonstrated by the PKAR measurements mentioned above (65), as well as by the existence of compound oscillations (e.g., slow oscillations that have superimposed fast bursts), which so far have only been plausibly explained by AMOs. However, the observed and detailed characteristics of the slow oscillations indicate that PMOs predominate at higher glucose levels (63, 77).
An analogy for the division of labor between AMOs and PMOs that we find useful is the gas-electric hybrid car, which has two motors. At low speeds, the car is powered by the battery, which energizes the car’s electric motor, while at higher speeds, typically 25–40 mph, depending on the rate of acceleration and battery capacity, the internal combustion engine takes over. This arrangement seems complicated at first glance but is an effective way to exploit the characteristics of each type of engine to produce high fuel efficiency. If pulsatility is important for the efficiency of insulin action (17), it would seem appropriate to maintain such pulsatility over a range of glucose.
Acknowledgments
R.B. was partially supported by National Science Foundation Grant DMS 1853342. L.S.S. was partially supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants RO1 DK46409 and U01 DK127747. P.A.F. and A.S.S. were supported by the Intramural Research Program of the NIDDK. I.M. acknowledges financial support from the University of Birmingham Dynamic Investment Fund.
No conflicts of interest, financial or otherwise, are declared by the authors.
P.A.F. performed mathematical simulations and analysis; I.M. provided conceptual advice on the model; P.A.F. prepared figures; A.S.S., L.S.S., and R.B. provided resources and supervision; P.A.F., I.M., R.B., L.S.S., and A.S.S. edited and revised manuscript; P.A.F., I.M., R.B., L.S.S., and A.S.S. approved final version of manuscript.
References
- 1.Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol 54: 87–143, 1989. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37: 667–687, 1988. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 3.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 46: 3–19, 2003. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 4.Rustenbeck I, Schulze T, Morsi M, Alshafei M, Panten U. What is the metabolic amplification of insulin secretion and is it (still) relevant? Metabolites 11: 355, 2021. doi: 10.3390/metabo11060355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferdaoussi M, Dai X, Jensen MV, Wang R, Peterson BS, Huang C, Ilkayeva O, Smith N, Miller N, Hajmrle C, Spigelman AF, Wright RC, Plummer G, Suzuki K, Mackay JP, van de Bunt M, Gloyn AL, Ryan TE, Norquay LD, Brosnan MJ, Trimmer JK, Rolph TP, Kibbey RG, Manning Fox JE, Colmers WF, Shirihai OS, Neufer PD, Yeh ET, Newgard CB, MacDonald PE. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional beta cells. J Clin Invest 125: 3847–3860, 2015. doi: 10.1172/JCI82498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corkey BE, Deeney JT, Merrins MJ. What regulates basal insulin secretion and causes hyperinsulinemia? Diabetes 70: 2174–2182, 2021. doi: 10.2337/dbi21-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49: 1751–1760, 2000. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 8.Laurenti MC, Dalla Man C, Varghese RT, Andrews JC, Rizza RA, Matveyenko A, De Nicolao G, Cobelli C, Vella A. Diabetes-associated genetic variation in TCF7L2 alters pulsatile insulin secretion in humans. JCI Insight 5: e136136, 2020. doi: 10.1172/jci.insight.136136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shukla N, Kadam S, Padinhateeri R, Kolthur-Seetharam U. Continuous variable responses and signal gating form kinetic bases for pulsatile insulin signaling and emergence of resistance. Proc Natl Acad Sci U S A 118: e2102560118, 2021. doi: 10.1073/pnas.2102560118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurenti MC, Matveyenko A, Vella A. Measurement of pulsatile insulin secretion: rationale and methodology. Metabolites 11: 409, 2021. doi: 10.3390/metabo11070409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porksen N, Nyholm B, Veldhuis JD, Butler PC, Schmitz O. In humans at least 75% of insulin secretion arises from punctuated insulin secretory bursts. Am J Physiol Ednocrinol Metab 273: E908–E914, 1997. doi: 10.1152/ajpendo.1997.273.5.E908. [DOI] [PubMed] [Google Scholar]
- 12.Song SH, McIntyre SS, Shah H, Veldhuis JD, Hayes PC, Butler PC. Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J Clin Endocrinol Metab 85: 4491–4499, 2000. doi: 10.1210/jcem.85.12.7043. [DOI] [PubMed] [Google Scholar]
- 13.Matveyenko AV, Veldhuis JD, Butler PC. Measurement of pulsatile insulin secretion in the rat: direct sampling from the hepatic portal vein. Am J Physiol Endocrinol Metab 295: E569–E574, 2008. doi: 10.1152/ajpendo.90335.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porksen N, Munn S, Steers J, Veldhuis JD, Butler PC. Effects of glucose ingestion versus infusion on pulsatile insulin secretion. The incretin effect is achieved by amplification of insulin secretory burst mass. Diabetes 45: 1317–1323, 1996. doi: 10.2337/diabetes.45.10.1317,10.2337/diab.45.10.1317. doi:. [DOI] [PubMed] [Google Scholar]
- 15.Nunemaker CS, Satin LS. Episodic hormone secretion: a comparison of the basis of pulsatile secretion of insulin and GnRH. Endocrine 47: 49–63, 2014. doi: 10.1007/s12020-014-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matveyenko AV, Liuwantara D, Gurlo T, Kirakossian D, Dalla Man C, Cobelli C, White MF, Copps KD, Volpi E, Fujita S, Butler PC. Pulsatile portal vein insulin delivery enhances hepatic insulin action and signaling. Diabetes 61: 2269–2279, 2012. doi: 10.2337/db11-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satin LS, Butler PC, Ha J, Sherman AS. Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes. Mol Aspects Med 42: 61–77, 2015. doi: 10.1016/j.mam.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunemaker CS, Zhang M, Wasserman DH, McGuinness OP, Powers AC, Bertram R, Sherman A, Satin LS. Individual mice can be distinguished by the period of their islet calcium oscillations: is there an intrinsic islet period that is imprinted in vivo? Diabetes 54: 3517–3522, 2005. doi: 10.2337/diabetes.54.12.3517. [DOI] [PubMed] [Google Scholar]
- 19.Martin F, Soria B. Glucose-induced [Ca2+]i oscillations in single human pancreatic islets. Cell Calcium 20: 409–414, 1996. doi: 10.1016/S0143-4160(96)90003-2. [DOI] [PubMed] [Google Scholar]
- 20.Riz M, Braun M, Pedersen MG. Mathematical modeling of heterogeneous electrophysiological responses in human beta-cells. PLoS Comput Biol 10: e1003389, 2014. doi: 10.1371/journal.pcbi.1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misun PM, Yesildag B, Forschler F, Neelakandhan A, Rousset N, Biernath A, Hierlemann A, Frey O. In vitro platform for studying human insulin release dynamics of single pancreatic islet microtissues at high resolution. Adv Biosyst 4: e1900291, 2020. doi: 10.1002/adbi.201900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchetti P, Scharp DW, McLear M, Gingerich R, Finke E, Olack B, Swanson C, Giannarelli R, Navalesi R, Lacy PE. Pulsatile insulin secretion from isolated human pancreatic islets. Diabetes 43: 827–830, 1994. doi: 10.2337/diab.43.6.827. [DOI] [PubMed] [Google Scholar]
- 23.Lin JM, Fabregat ME, Gomis R, Bergsten P. Pulsatile insulin release from islets isolated from three subjects with type 2 diabetes. Diabetes 51: 988–993, 2002. doi: 10.2337/diabetes.51.4.988. [DOI] [PubMed] [Google Scholar]
- 24.Ritzel RA, Veldhuis JD, Butler PC. Glucose stimulates pulsatile insulin secretion from human pancreatic islets by increasing secretory burst mass: dose-response relationships. J Clin Endocrinol Metab 88: 742–747, 2003. doi: 10.1210/jc.2002-021250. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen MG, Bertram R, Sherman A. Intra- and inter-islet synchronization of metabolically driven insulin secretion. Biophys J 89: 107–119, 2005. doi: 10.1529/biophysj.104.055681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gylfe E, Tengholm A. Neurotransmitter control of islet hormone pulsatility. Diabetes Obes Metab 16, Suppl 1: 102–110, 2014. doi: 10.1111/dom.12345. [DOI] [PubMed] [Google Scholar]
- 27.Stagner JI, Samols E. Role of intrapancreatic ganglia in regulation of periodic insular secretions. Am J Physiol Endocrinol Metab 248: E522–E530, 1985. doi: 10.1152/ajpendo.1985.248.5.E522. [DOI] [PubMed] [Google Scholar]
- 28.Matthews DR, Lang DA, Burnett MA, Turner RC. Control of pulsatile insulin secretion in man. Diabetologia 24: 231–237, 1983. [DOI] [PubMed] [Google Scholar]
- 29.Beauvois MC, Merezak C, Jonas JC, Ravier MA, Henquin JC, Gilon P. Glucose-induced mixed [Ca2+]c oscillations in mouse beta-cells are controlled by the membrane potential and the SERCA3 Ca2+-ATPase of the endoplasmic reticulum. Am J Physiol Cell Physiol 290: C1503–C1511, 2006. doi: 10.1152/ajpcell.00400.2005. [DOI] [PubMed] [Google Scholar]
- 30.Chay TR, Keizer J. Minimal model for membrane oscillations in the pancreatic beta-cell. Biophys J 42: 181–190, 1983. doi: 10.1016/S0006-3495(83)84384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherman A. Contributions of modeling to understanding stimulus-secretion coupling in pancreatic beta-cells. Am J Physiol Endocrinol Metab 271: E362–E372, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Felix-Martinez GJ, Godinez-Fernandez JR. Mathematical models of electrical activity of the pancreatic beta-cell: a physiological review. Islets 6: e949195, 2014. doi: 10.4161/19382014.2014.949195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Detimary P, Gilon P, Henquin JC. Interplay between cytoplasmic Ca2+ and the ATP/ADP ratio: a feedback control mechanism in mouse pancreatic islets. Biochem J 333: 269–274, 1998. doi: 10.1042/bj3330269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertram R, Sherman A. A calcium-based phantom bursting model for pancreatic islets. Bull Math Biol 66: 1313–1344, 2004. doi: 10.1016/j.bulm.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Cha CY, Nakamura Y, Himeno Y, Wang J, Fujimoto S, Inagaki N, Earm YE, Noma A. Ionic mechanisms and Ca2+ dynamics underlying the glucose response of pancreatic beta cells: a simulation study. J Gen Physiol 138: 21–37, 2011. doi: 10.1085/jgp.201110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fridlyand LE, Ma L, Philipson LH. Adenine nucleotide regulation in pancreatic beta-cells: modeling of ATP/ADP-Ca2+ interactions. Am J Physiol Endocrinol Metab 289: E839–E848, 2005. doi: 10.1152/ajpendo.00595.2004. [DOI] [PubMed] [Google Scholar]
- 37.Marinelli I, Fletcher PA, Sherman AS, Satin LS, Bertram R. Symbiosis of electrical and metabolic oscillations in pancreatic beta-cells. Front Physiol 12: 781581, 2021. doi: 10.3389/fphys.2021.781581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marinelli I, Thompson BM, Parekh VS, Fletcher PA, Gerardo-Giorda L, Sherman AS, Satin LS, Bertram R. Oscillations in K(ATP) conductance drive slow calcium oscillations in pancreatic beta-cells. Biophys J 121: 1449–1464, 2022. doi: 10.1016/j.bpj.2022.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen YD, Wang S, Sherman A. Identifying the targets of the amplifying pathway for insulin secretion in pancreatic beta-cells by kinetic modeling of granule exocytosis. Biophys J 95: 2226–2241, 2008. doi: 10.1529/biophysj.107.124990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodner CJ, Walike BC, Koerker DJ, Ensinck JW, Brown AC, Chideckel EW, Palmer J, Kalnasy L. Insulin, glucagon, and glucose exhibit synchronous, sustained oscillations in fasting monkeys. Science 195: 177–179, 1977. doi: 10.1126/science.401543. [DOI] [PubMed] [Google Scholar]
- 41.Lang DA, Matthews DR, Peto J, Turner RC. Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N Engl J Med 301: 1023–1027, 1979. doi: 10.1056/NEJM197911083011903. [DOI] [PubMed] [Google Scholar]
- 42.Westerlund J, Bergsten P. Glucose metabolism and pulsatile insulin release from isolated islets. Diabetes 50: 1785–1790, 2001. doi: 10.2337/diabetes.50.8.1785. [DOI] [PubMed] [Google Scholar]
- 43.Hellman B, Salehi A, Grapengiesser E, Gylfe E. Isolated mouse islets respond to glucose with an initial peak of glucagon release followed by pulses of insulin and somatostatin in antisynchrony with glucagon. Biochem Biophys Res Commun 417: 1219–1223, 2012. doi: 10.1016/j.bbrc.2011.12.113. [DOI] [PubMed] [Google Scholar]
- 44.Porksen N, Munn S, Steers J, Vore S, Veldhuis J, Butler P. Pulsatile insulin secretion accounts for 70% of total insulin secretion during fasting. Am J Physiol Endocrinol Metab 269: E478–E488, 1995. doi: 10.1152/ajpendo.1995.269.3.E478. [DOI] [PubMed] [Google Scholar]
- 45.Dankner R, Chetrit A, Shanik MH, Raz I, Roth J. Basal-state hyperinsulinemia in healthy normoglycemic adults is predictive of type 2 diabetes over a 24-year follow-up: a preliminary report. Diabetes Care 32: 1464–1466, 2009. doi: 10.2337/dc09-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 47.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85: 2402–2410, 2000. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 48.Erion K, Corkey BE. Beta-cell failure or beta-cell abuse? Front Endocrinol (Lausanne) 9: 532, 2018. doi: 10.3389/fendo.2018.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care 31, Suppl 2: S262–268, 2008. [DOI] [PubMed] [Google Scholar]
- 50.Page MM, Johnson JD. Mild suppression of hyperinsulinemia to treat obesity and insulin resistance. Trends Endocrinol Metab 29: 389–399, 2018. doi: 10.1016/j.tem.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 51.Esser N, Utzschneider KM, Kahn SE. Early beta cell dysfunction vs insulin hypersecretion as the primary event in the pathogenesis of dysglycaemia. Diabetologia 63: 2007–2021, 2020. doi: 10.1007/s00125-020-05245-x. [DOI] [PubMed] [Google Scholar]
- 52.Esser N, Utzschneider KM, Kahn SE. On the causal relationships between hyperinsulinaemia, insulin resistance, obesity and dysglycaemia in type 2 diabetes: reply to Johnson JD. Diabetologia 64: 2345–2347, 2021. doi: 10.1007/s00125-021-05511-6. [DOI] [PubMed] [Google Scholar]
- 53.Johnson JD. On the causal relationships between hyperinsulinaemia, insulin resistance, obesity and dysglycaemia in type 2 diabetes. Diabetologia 64: 2138–2146, 2021. doi: 10.1007/s00125-021-05505-4. [DOI] [PubMed] [Google Scholar]
- 54.Cole LK, Agarwal P, Doucette CA, Fonseca M, Xiang B, Sparagna GC, Seshadri N, Vandel M, Dolinsky VW, Hatch GM. Tafazzin deficiency reduces basal insulin secretion and mitochondrial function in pancreatic islets from male mice. Endocrinology 162: bqab102, 2021. doi: 10.1210/endocr/bqab102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kyriazis GA, Smith KR, Tyrberg B, Hussain T, Pratley RE. Sweet taste receptors regulate basal insulin secretion and contribute to compensatory insulin hypersecretion during the development of diabetes in male mice. Endocrinology 155: 2112–2121, 2014. doi: 10.1210/en.2013-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barlow J, Jensen VH, Jastroch M, Affourtit C. Palmitate-induced impairment of glucose-stimulated insulin secretion precedes mitochondrial dysfunction in mouse pancreatic islets. Biochem J 473: 487–496, 2016. doi: 10.1042/BJ20151080. [DOI] [PubMed] [Google Scholar]
- 57.Taddeo EP, Alsabeeh N, Baghdasarian S, Wikstrom JD, Ritou E, Sereda S, Erion K, Li J, Stiles L, Abdulla M, Swanson Z, Wilhelm JJ, Bellin MD, Kibbey RG, Liesa M, Shirihai OS. Mitochondrial proton leak regulated by cyclophilin D elevates insulin secretion in islets at nonstimulatory glucose levels. Diabetes 69: 131–145, 2020. doi: 10.2337/db19-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang IX, Ren J, Vadrevu S, Raghavan M, Satin LS. ER stress increases store-operated Ca(2+) entry (SOCE) and augments basal insulin secretion in pancreatic beta cells. J Biol Chem 295: 5685–5700, 2020. doi: 10.1074/jbc.RA120.012721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nunemaker CS, Bertram R, Sherman A, Tsaneva-Atanasova K, Daniel CR, Satin LS. Glucose modulates [Ca2+]i oscillations in pancreatic islets via ionic and glycolytic mechanisms. Biophys J 91: 2082–2096, 2006. doi: 10.1529/biophysj.106.087296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dryselius S, Lund PE, Gylfe E, Hellman B. Variations in ATP-sensitive K+ channel activity provide evidence for inherent metabolic oscillations in pancreatic beta-cells. Biochem Biophys Res Commun 205: 880–885, 1994. doi: 10.1006/bbrc.1994.2746. [DOI] [PubMed] [Google Scholar]
- 61.Bertram R, Satin L, Zhang M, Smolen P, Sherman A. Calcium and glycolysis mediate multiple bursting modes in pancreatic islets. Biophys J 87: 3074–3087, 2004. doi: 10.1529/biophysj.104.049262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ravier MA, Gilon P, Henquin JC. Oscillations of insulin secretion can be triggered by imposed oscillations of cytoplasmic Ca2+ or metabolism in normal mouse islets. Diabetes 48: 2374–2382, 1999. doi: 10.2337/diabetes.48.12.2374. [DOI] [PubMed] [Google Scholar]
- 63.Bertram R, Satin LS, Sherman AS. Closing in on the mechanisms of pulsatile insulin secretion. Diabetes 67: 351–359, 2018. doi: 10.2337/dbi17-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marinelli I, Vo T, Gerardo-Giorda L, Bertram R. Transitions between bursting modes in the integrated oscillator model for pancreatic beta-cells. J Theor Biol 454: 310–319, 2018. doi: 10.1016/j.jtbi.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 65.Merrins MJ, Poudel C, McKenna JP, Ha J, Sherman A, Bertram R, Satin LS. Phase analysis of metabolic oscillations and membrane potential in pancreatic islet beta-cells. Biophys J 110: 691–699, 2016. doi: 10.1016/j.bpj.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lewandowski SL, Cardone RL, Foster HR, Ho T, Potapenko E, Poudel C, VanDeusen HR, Sdao SM, Alves TC, Zhao X, Capozzi ME, de Souza AH, Jahan I, Thomas CJ, Nunemaker CS, Davis DB, Campbell JE, Kibbey RG, Merrins MJ. Pyruvate kinase controls signal strength in the insulin secretory pathway. Cell Metab 32: 736–750, 2020. doi: 10.1016/j.cmet.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Higgins J. A chemical mechanism for oscillation of glycolytic intermediates in yeast cells. Proc Natl Acad Sci U S A 51: 989–994, 1964. doi: 10.1073/pnas.51.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tornheim K, Lowenstein JM. The purine nucleotide cycle. 3. Oscillations in metabolite concentrations during the operation of the cycle in muscle extracts. J Biol Chem 248: 2670–2677, 1973. doi: 10.1016/S0021-9258(19)44058-1. [DOI] [PubMed] [Google Scholar]
- 69.Tornheim K. Are metabolic oscillations responsible for normal oscillatory insulin secretion? Diabetes 46: 1375–1380, 1997. doi: 10.2337/diab.46.9.1375,10.2337/diabetes.46.9.1375. [DOI] [PubMed] [Google Scholar]
- 70.Marinelli I, Parekh V, Fletcher P, Thompson B, Ren J, Tang X, Saunders TL, Ha J, Sherman A, Bertram R, Satin LS. Slow oscillations persist in pancreatic beta cells lacking phosphofructokinase M. Biophys J 121: 692–704, 2022. doi: 10.1016/j.bpj.2022.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Richard AM, Webb DL, Goodman JM, Schultz V, Flanagan JN, Getty-Kaushik L, Deeney JT, Yaney GC, Dunaway GA, Berggren PO, Tornheim K. Tissue-dependent loss of phosphofructokinase-M in mice with interrupted activity of the distal promoter: impairment in insulin secretion. Am J Physiol Endocrinol Metab 293: E794–E801, 2007. doi: 10.1152/ajpendo.00168.2007. [DOI] [PubMed] [Google Scholar]
- 72.Jung SK, Aspinwall CA, Kennedy RT. Detection of multiple patterns of oscillatory oxygen consumption in single mouse islets of Langerhans. Biochem Biophys Res Commun 259: 331–335, 1999. doi: 10.1006/bbrc.1999.0784. [DOI] [PubMed] [Google Scholar]
- 73.Krippeit-Drews P, Dufer M, Drews G. Parallel oscillations of intracellular calcium activity and mitochondrial membrane potential in mouse pancreatic B-cells. Biochem Biophys Res Commun 267: 179–183, 2000. doi: 10.1006/bbrc.1999.1921. [DOI] [PubMed] [Google Scholar]
- 74.Li J, Shuai HY, Gylfe E, Tengholm A. Oscillations of sub-membrane ATP in glucose-stimulated beta cells depend on negative feedback from Ca(2+). Diabetologia 56: 1577–1586, 2013. doi: 10.1007/s00125-013-2894-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luciani DS, Misler S, Polonsky KS. Ca2+ controls slow NAD(P)H oscillations in glucose-stimulated mouse pancreatic islets. J Physiol 572: 379–392, 2006. doi: 10.1113/jphysiol.2005.101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Merrins MJ, Van Dyke AR, Mapp AK, Rizzo MA, Satin LS. Direct measurements of oscillatory glycolysis in pancreatic islet beta-cells using novel fluorescence resonance energy transfer (FRET) biosensors for pyruvate kinase M2 activity. J Biol Chem 288: 33312–33322, 2013. doi: 10.1074/jbc.M113.508127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McKenna JP, Ha J, Merrins MJ, Satin LS, Sherman A, Bertram R. Ca2+ effects on ATP production and consumption have regulatory roles on oscillatory islet activity. Biophys J 110: 733–742, 2016. doi: 10.1016/j.bpj.2015.11.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]