Abstract
Erythrocytosis, or increased production of red blood cells, is one of the most well-documented physiological traits that varies within and among in high-altitude populations. Although a modest increase in blood O2-carrying capacity may be beneficial for life in highland environments, erythrocytosis can also become excessive and lead to maladaptive syndromes such as chronic mountain sickness (CMS).
Keywords: chronic hypoxia, chronic mountain sickness, excessive erythrocytosis, high-altitude erythrocytosis
Introduction
Increased production of red blood cells (RBCs), or erythrocytosis, has been considered a hallmark response of acclimatization in lowlanders at high altitude since its first description by Viault in 1890 (1). Hematocrit, the ratio of RBC volume to the total volume of blood generally reaches a moderately high steady-state value after a few weeks and remains stable during the length of altitude exposure. In high-altitude populations, hematocrit and hemoglobin concentrations ([Hb]) have become oversimplified markers of successful adaptation to highland environments around the world; however, the ability to offset challenges of decreased oxygen (O2) availability depends on an integrated system of O2 transport and cellular responses to hypoxia (2, 3). One hypothesis regarding increased RBC production at altitude is that the erythropoietic response to hypoxia arose in evolution to correct anemia (i.e., hemorrhage), compensating for decreased blood O2-carrying capacity and correcting tissue hypoxia (4–7). However, at high altitude, tissue hypoxia cannot be fully corrected by simply increasing O2-carrying capacity due to low inspired Po2. Questions regarding the beneficial effects of increased RBCs for life at high altitude are still debatable due to the occurrence of some maladaptive outcomes (8–12).
Populations with generations of high-altitude ancestry differ greatly in their hematological characteristics, features of O2 transport, and genetic factors that likely contribute to distinct physiological responses to hypobaric hypoxia. For example, erythrocytosis can become excessive in some highlanders and give rise to maladaptive syndromes such as chronic mountain sickness (CMS) or Monge’s disease, which is more common among Andeans than Tibetans (12). [Hb] has a genetic basis in Tibetans (13–15), and CMS is associated with key genetic factors in Andeans (16–19). In addition, epigenetic changes could be associated with key traits that remain to be comprehensively examined and are likely further compounded by age, sex, and multiple systemic factors (Yu et al., unpublished observations). While these findings suggest populations with relatively lower [Hb] at high altitude may be better adapted, the differences in genetic backgrounds, environmental and epigenetic factors, and feedback between erythrocytosis and O2 transport and delivery remain unclear.
Efforts to understand the cause-and-effect relationship between the hypoxic stimulus and the erythropoietic response at a given altitude are active areas of study. For instance, the point at which hematocrit and [Hb] are truly “excessive,” and how best to define this with regard to other factors, are yet to be determined. Furthermore, whether this designation should be determined based on altitude or set at a physiological, altitude-independent threshold remains to be established. How these values impact the O2 transport cascade and adaptation and maladaptation at individual and population levels requires additional systematic investigation. This review will address mechanisms of high-altitude erythrocytosis, genetic evidence for associations with [Hb] that provide clues into adaptive and maladaptive pathways, and detailed consideration of thresholds commonly used for excessive erythrocytosis and CMS scores.
Mechanisms of High-Altitude Erythrocytosis
Hypoxemia, or low blood Po2, stimulates erythropoiesis. Although increased hematocrit and [Hb] values are observed in high-altitude populations, “normal” values vary greatly and depend on unique adaptations as well as the altitude of residence. Many Tibetans exhibit relatively lower [Hb] and CMS prevalence relative to Andeans at comparable altitudes and are often categorized as a population well adapted to the highland environment. [Hb] in healthy Tibetan women and men typically falls within ∼14–16 g/dl at ∼4,000 m compared with ∼17–19 g/dl in Andeans (20, 21). At the systemic level, compared with Andean highlanders, Tibetans tend to exhibit elevated pulmonary ventilation and lower end-tidal Pco2 (a proxy for higher alveolar ventilation) and a small alveolar-arterial Po2 difference (22–24), suggesting a high lung diffusing capacity due to greater total lung volumes that may be attributed to developmental factors (25, 26). The combination of these physiological characteristics suggests that Tibetans might have an advantage in defending arterial Po2 and O2 saturation from dropping dramatically. However, despite these characteristics, Po2 in arterial blood in Tibetans at ∼3,700 m tends to be slightly lower compared with Andean high-altitude natives at the same altitude (24, 27), and [Hb] in arterial blood is less saturated with O2 among Tibetans relative to their Andean counterparts (28, 29). Rightward as well as leftward shifts of the Hb-O2 dissociation curve have been reported in native highlanders, but at present, there is limited consensus across studies. Even with very similar arterial Po2, arterial blood O2 saturation (SaO2) has generally been reported to be higher in Andeans than Tibetans (20, 30–34).
Given these findings, it is likely other mechanisms could underlie suitable oxygenation in Tibetans to sustain normal aerobic metabolism or improved O2 utilization. This may include increased blood flow, which may be attributed to changes in nitric oxide (NO) metabolism (20, 35, 36), and O2 diffusion from the bloodstream to cells. Having a denser capillary network could potentially improve perfusion and O2 delivery, with each capillary supplying a smaller area of tissue and a shorter O2 diffusion distance. Tibetans have higher capillary density in muscles as compared with Andean high-altitude natives (37); thus this adaptive feature may help overcome low arterial O2 content with lower [Hb] as it allows a high rate of diffusion to tissues. When [Hb] is reduced, the time to diffusive equilibration between microcirculatory vessels and cells is shortened (38). Piiper and Scheid (39) showed that the compound constant D/(β · Q) determined the degree of diffusion equilibration to be expected in muscle (D is muscle diffusing capacity, β is the average slope of the O2-Hb dissociation curve, and Q is blood flow). Since β must decrease when total [Hb] decreases, D/(β · Q) must rise, assuming no change, or even a modest increase in Q, diffusion equilibration would take less time. As a result, tissue O2 extraction increases when [Hb] is lower, and O2 delivery is improved.
Ethiopians generally exhibit [Hb] comparable to Tibetans at high altitude (37, 40, 41) and are also believed to be well adapted to the high-altitude environment. This group does not show elevated pulmonary ventilation but maintains a relatively high SaO2 at similar altitudes compared with Tibetans and Andeans (42, 43). Although various physiological features in Ethiopian highlanders have yet to be studied, it seems low [Hb] does not enhance convective O2 transport but prevents an increase in blood viscosity and the impairment of vascular and hemodynamic function. What underlies low [Hb] values and whether it is the result of blunted erythropoiesis or other physiological adjustments remain to be determined.
In Tibetans, lower [Hb] is associated with polymorphisms in genes involved in O2 sensing and response. Genome-wide analyses revealed positive selection in key hypoxia-inducible factor (HIF) pathway genes such as EPAS1 (13–15) and EGLN1 (14, 15), as well as PPARA (14), which encode HIF-2α, HIF-prolyl-hydroxylase 2 (PHD2), and peroxisome proliferator-activated receptor-α, respectively. Variants within these putatively adaptive gene regions are also associated with decreased [Hb] in Tibetans (reviewed in Ref. 44) and the latter with more efficient O2 utilization (45, 46). High-frequency missense mutations identified in the EGLN1 gene encoding the oxygen sensor PHD2 results in a lower Km value for O2, suggesting increased HIF degradation under hypoxic conditions (47) but a loss-of-function in other contexts (48). Interestingly, variants in the EPAS1 locus in Tibetans are most similar to archaic DNA sequence relative to other populations, suggesting adaptive introgression at this genomic region (49, 50). These findings highlight the importance of considering distinct evolutionary histories among highland populations and their effects on genetic background and, therefore, physiology. While these findings provide a step forward in understanding associations, further studies are needed to determine their functional relevance and if [Hb] is the direct target of selection or secondary to other adaptive factors (44, 51).
Recent studies suggest decreased erythropoiesis might not be the only mechanism involved in maintaining relatively low [Hb] values at a given altitude. Hematocrit and [Hb] are also determined by plasma volume as well as RBC destruction. A comparison between Sherpas and Andeans indicates that lower [Hb] in the former is the result of considerably larger plasma volume and a modestly increased packed red blood cell mass (52); therefore, Sherpas have an equivalent blood volume to Andeans but a lower [Hb]. This difference is critically important, as it means Sherpa are able to benefit from the increased O2-carrying capacity that comes from an expansion of their Hb mass but are not restricted by an increase in blood viscosity that affects vascular function and hemodynamics (53–55). Our recent studies indicate Tibetans with relatively lower [Hb] also exhibit slightly elevated endogenous carbon monoxide (CO) (Moya et al., unpublished observations; Gu et al., unpublished observations), which is a natural by-product of hemolysis and could underlie a reduction in [Hb]. Increased CO levels are also noted among Andeans with excessive erythrocytosis (EE) (56); however, this is likely due to the excessive RBCs produced and then destroyed as part of the RBC lifecycle (56). Thus these findings suggest that hematocrit in high-altitude populations with relatively low [Hb] is not regulated only through the control of erythropoiesis, but perhaps through water volume regulation via a feedback loop based on renal Po2 (52) and potentially differences in RBC destruction and/or life span versus production.
Pathology and Risk Factors of High-Altitude Erythrocytosis
The most prominent manifestation of the overproduction of RBCs is excessive erythrocytosis (EE), the hallmark feature of CMS, a highly prevalent and incapacitating syndrome in Andeans and other high-altitude populations across the world (9, 57). EE coincides with severe hypoxemia, neurological deficits, and sleep disorders (12) and is often associated with pulmonary hypertension, myocardial infarction, and stroke owing to blood hyperviscosity predisposing to thrombophilia (8–10). It is estimated that 5–10% of the world’s population living at high-altitude may develop EE (9), and its prevalence increases with altitude and age (58–65). Above 4,300 m in the central Andes of Peru, more than 30% of highlanders by their mid-50s develop EE (59–61, 66). The prevalence of EE varies considerably within particular populations and also between men and women. Our group has shown that while hematocrit increases uniformly with age in men, this is further compounded in women owing in part to a more pronounced increase after menopause due to the combined reduction in progesterone and estradiol concentrations (59, 66). Female hormones protect women against the development of EE through their stimulatory effect on pulmonary ventilation and through the inhibition of erythropoiesis (18, 66). In addition, menstruation acts as a regular natural phlebotomy that prevents hematocrit from rising excessively.
In the absence of chronic pulmonary diseases (pulmonary emphysema, chronic bronchitis, bronchiectasis, cystic fibrosis, lung cancer, etc.) or other underlying chronic medical conditions that worsen hypoxemia, potential risk factors for the development of EE include perinatal adverse events (67, 68), the natural age-dependent reduction in pulmonary ventilation, the consequent accentuation of arterial hypoxemia (63), as well as an increased occurrence of sleep-disordered breathing with bouts of intermittent hypoxia and nocturnal hypoxemia (69–72) (FIGURE 1). Higher central and peripheral chemoreflex set points might lead to hypoventilation at a given Pco2, resulting in lower arterial O2 saturation and increased erythropoietic response. Frequently, individuals with CMS have lower ventilatory sensitivities to CO2 compared with non-CMS Andean controls (73, 74). Also, ventilatory sensitivities to O2 and CO2 play a key role in sleep-disordered breathing in highlanders (70, 75), and low ventilatory sensitivity to hypoxia or CO2 can lead to more severe desaturation during sleep and/or prolonged desaturation periods (76). Recent studies have shown that sleep-disordered breathing is more prevalent in Peruvian highlanders than in lowlanders at sea level (71), and nocturnal hypoxemia and sleep apnea events are independently associated with EE. We have recently shown that lower pulse O2 saturation (SpO2) during sleep and during the day are associated with higher hematocrit in Andean men and women. When adjusting for age and SpO2, obstructive apnea index and apnea-hypopnea index also predict higher hematocrit and CMS scores in highlander men. While the hypoxic ventilatory response is blunted in Andeans with and without EE, lower hypoxic chemosensitivity is associated with lower daytime SpO2 and may therefore play a role in the development of EE (69).
FIGURE 1.
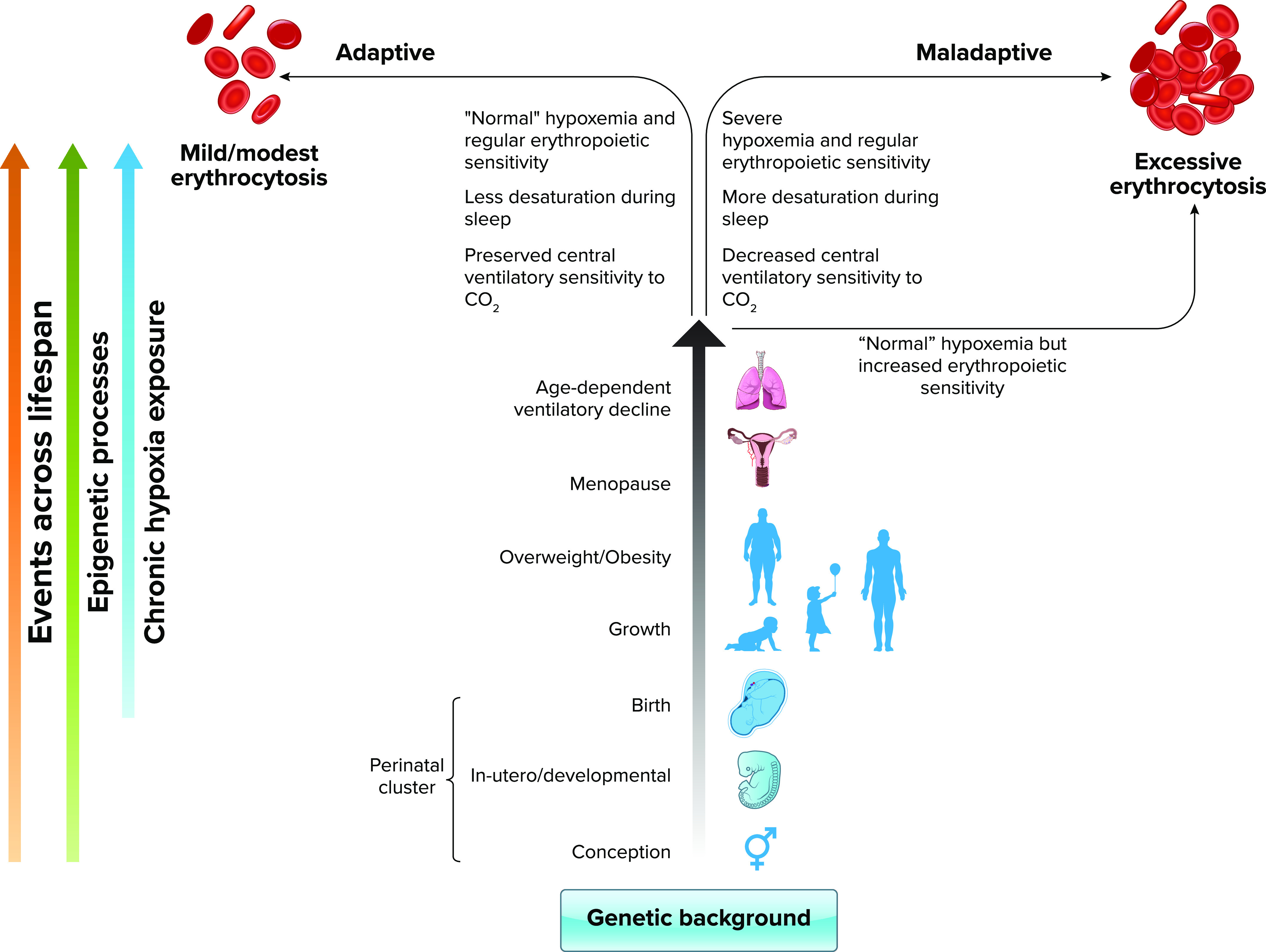
Schematic diagram showing the expression of the adaptive and maladaptive erythropoietic phenotypes as the result of genetic background, epigenomic processes, and physiological responses to chronic hypoxia across the life span In recent years, an increasing amount of evidence has accumulated to support the genetic basis of the adaptive and maladaptive erythropoietic responses to chronic hypoxia. The genetic background of an individual will ultimately determine the development of a moderate or an excessive erythrocytosis (EE) in response to the different stimuli, epigenomic processes, and physiological events throughout the life span. In utero and perinatal events can condition the erythropoietic response in later life. As individuals age, they go through several physiological processes during adulthood such as menopause and the age-dependent decline in ventilation, and can also develop conditions such as obesity; all recognized risk factors for the development of EE. Thus, what determines that some individuals end up taking the adaptive or maladaptive path? Our genetic background, together with epigenetic modifications accumulated through life will determine the impact of these physiological or pathophysiological changes on predetermined characteristics such as ventilatory control or erythropoietic sensitivity. Individuals with an adaptive genetic background and favorable epigenetic changes for life at high altitude will have a preserved central ventilatory sensitivity to CO2, hence maintaining adequate ventilation during day and night; will have fewer and/or less prolonged respiratory events during sleep, therefore maintaining proper oxygenation; and will have a regular erythropoietic sensitivity of erythroid progenitors. All these characteristics assure maintaining a moderate hemoglobin concentration ([Hb]) at a sufficient level for their altitude of residence. On the other hand, individuals with a maladaptive genetic background may express maladaptive traits early in life and develop EE. These individuals may show adequate responses during young age but reach a tipping point at some stage in their lives in which maladaptive responses become manifest (i.e., decreased ventilatory central sensitivity to CO2, increased sleep disorders, and severe hypoxemia) resulting in a stronger stimulus for erythropoiesis. Alternatively, they may have increased erythropoietic sensitivity, even with “normal” hypoxemia (usual SpO2 range for a given altitude in healthy individuals), and without any respiratory or sleep alterations, they will develop EE over time. RBC, red blood cell. Image created with BioRender.com and used with permission.
While various physiological mechanisms are potentially involved in the etiopathogenesis of EE, once EE has developed, excess RBCs might impair oxygenation. We can say that at some point during life at high altitude, chronic hypoxemia initiates a vicious cycle in which hematocrit starts rising, causing abnormal blood rheology and imposing a burden on vasculature. This in turn could contribute to impairments in the distribution of pulmonary blood flow, ventilation/perfusion ratio, and O2 diffusion. Impaired pulmonary gas exchange further augments the degree of arterial hypoxemia (6, 77, 78), which would in the end stimulate further erythropoiesis (FIGURE 1).
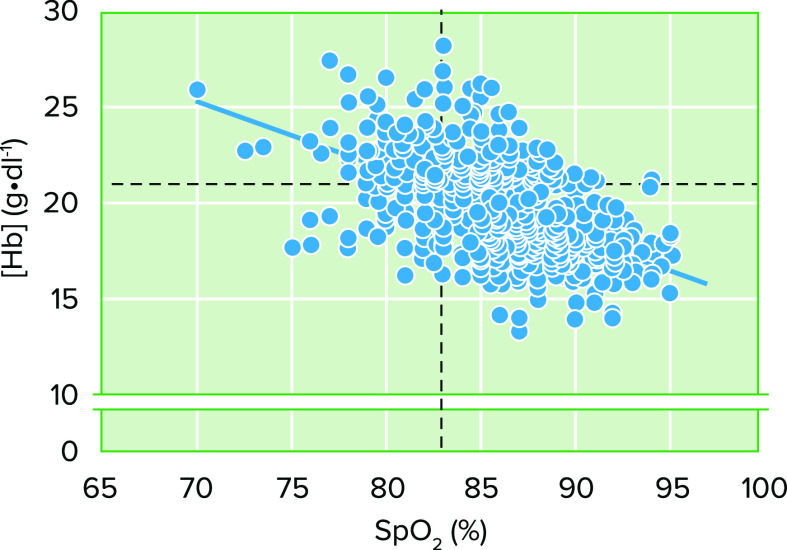
Although at present the pathophysiology of EE is sufficiently described to provide plausible mechanistic explanations, it is necessary to recognize the paradoxes between what is actually known about this high-altitude disease and how that knowledge is interpreted. It is commonly believed that severe hypoxemia and/or increased erythropoietin (EPO) concentration underlies an exacerbated erythropoietic response in highlanders. However, this is not always the case. Although the relative risk (RR) of having EE with severe hypoxemia [SpO2 <83% (60)] at 4,340 m in Cerro de Pasco, Peru is 2.66 [95% confidence interval (CI), 2.29–3.08, P < 0.0001, n = 965; Villafuerte FC, unpublished observations)], ∼27% of highlanders with SpO2 values above 83% have EE. On the other hand, 28% of highlanders with SpO2 below 83% have [Hb] within the standard range relative to the altitude of residence (FIGURE 2). Highlanders with serum EPO > 1 standard deviation (SD) of the average value of healthy individuals at the same altitude (79) show a RR of 1.63 of having EE (95% CI: 1.23–2.17, P = 0.0007, n =146; Villafuerte FC, unpublished observations). Important to note, however, is that 47% of individuals without EE have high serum EPO (79). Therefore, this variety of phenotypes shows that severe hypoxemia or high EPO levels are not always necessary to develop EE. These observations highlight the individual variability and the potential role for the modulation of EPO signaling and the hypoxia- or EPO-sensitivity of erythroid progenitors. We have shown that EE is associated with decreased soluble EPO receptor (sEPOR) levels and with a higher EPO-to-sEPOR ratio during day and sleep, which implies a stronger erythropoietic stimulus at similar EPO concentrations (80). Our results suggest that sEPOR could act as an extracellular regulator of erythropoiesis and mechanistic link for the development of EE. We have also recently shown that erythroid progenitors derived from highlanders with EE show an increased proliferative response under hypoxic conditions and upregulated expression of proerythropoietic genes (17). Thus, besides any extracellular modulation of the erythropoietic signal, it can be hypothesized that erythroid progenitors derived from highlanders with CMS are genetically determined for exacerbated proliferation, which could be further enhanced by poor systemic oxygenation during daytime or sleep as a consequence of altered respiratory control or sleep-disordered breathing, or by normal physiological processes such as menopause (18, 70, 75, 81).
FIGURE 2.
Relationship between Hb concentration ([Hb]) and blood oxygenation Shown is the inverse relationship between [Hb] and SpO2 in male highlanders from Cerro de Pasco, Peru at 4,340 m (r = −0.55, P < 0.0001, n = 965; Villafuerte FC, unpublished observations). The dotted lines represent the thresholds of [Hb] to determine excessive erythrocytosis (EE; ≥21 g/dl) and of SpO2 (<83%) to determine severe hypoxemia. The different phenotypes on these variables can be observed from the 4 quadrants. Although it is expected that individuals with severe hypoxemia develop EE (relative risk of 2.66, 95% confidence interval of 2.29–3.08, P < 0.0001), about one-third of highlanders with SpO2 values >83% have EE. On the other hand, also a third of highlanders with SpO2 <83% have [Hb] within the usual range relative to the altitude of residence.
In Andeans, genetic variants, at least partly, underlie the regulation of erythropoiesis. Whole-genome and genotyping studies have shown an association between the sentrin-specific protease 1 (SENP1) allelic variant rs7963934 and the EE phenotype among Andeans (16, 82, 83), yet the precise functional variant(s) remain unknown. Recent studies suggest SENP1, which regulates the activity of transcription factors such as HIF and GATA-binding factor 1 (GATA1), plays a central role in the excessive production of RBCs, possibly by modulating different stages of erythropoiesis, including steps of the EPO signaling pathway and apoptosis in erythroid progenitors (84–86) (FIGURE 3). Under in vitro hypoxic conditions, CMS erythroid progenitors derived from human-induced pluripotent stem cells, obtained from skin fibroblasts of Andean highlanders, show increased proliferation and upregulation of SENP1 expression, which is associated with stabilization and upregulation of GATA1 and GATA1-responsive genes such as the mitochondrial anti-apoptotic factor Bcl-xL (84). These findings suggest a crucial role for SENP1 in erythroid proliferation seen in CMS. We have confirmed the upregulation of SENP1, GATA1, and EPOR expression in erythroid progenitors derived from peripheral blood mononuclear cells (PBMCs) of highlanders with EE (17). We have also recently shown, through a genome-wide association study (89), associations between CMS and a variant of the AEBP2 gene, an epigenetic regulator for neural crest cells (90), the calpastatin gene CAST, which has an inhibiting effect on calpain and has been associated with the progression of pulmonary hypertension (91), and MCTP2, which has been associated with body fat levels and obesity in other populations (92).
FIGURE 3.
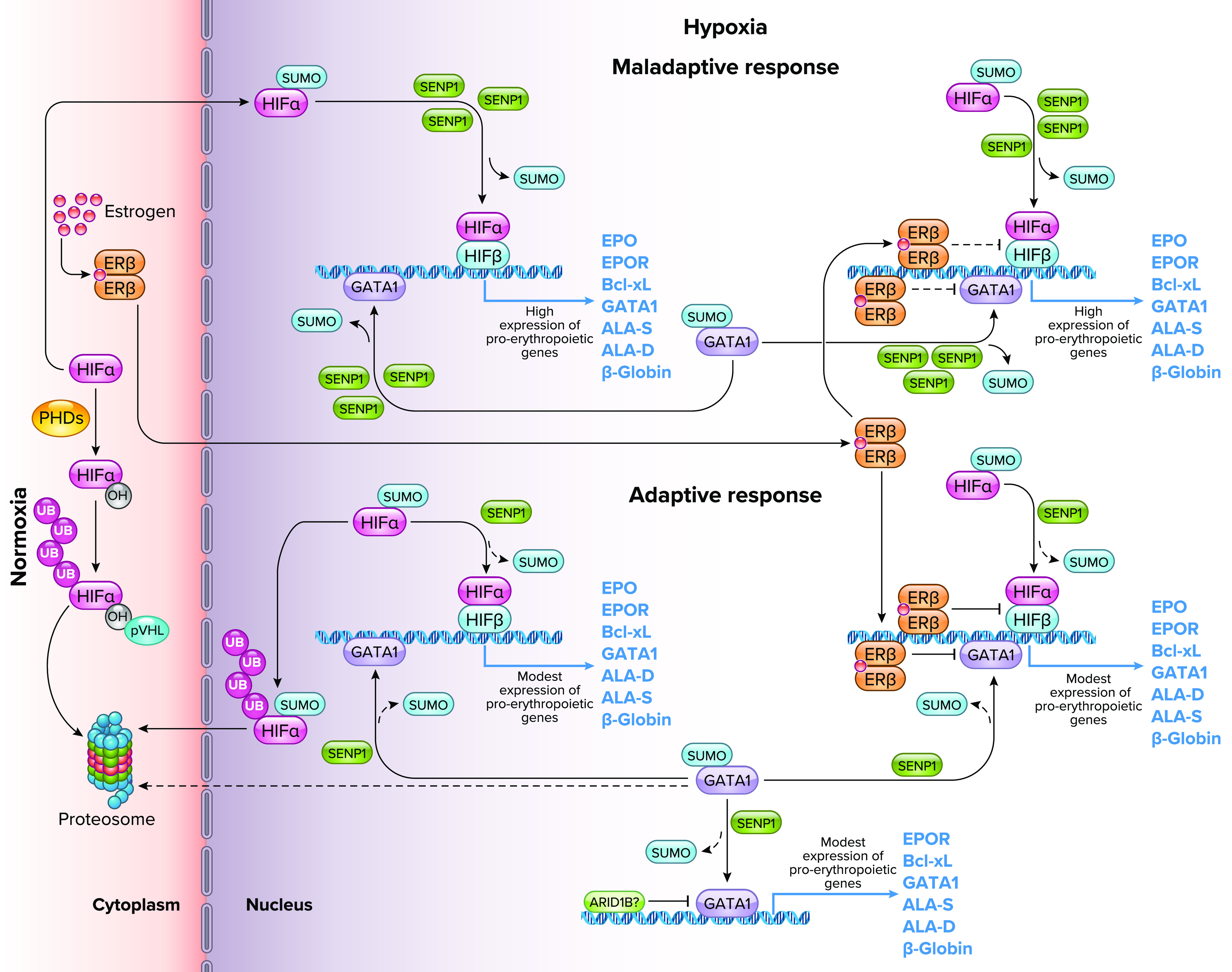
Role of sentrin-specific protease 1 (SENP1) and GATA-binding factor 1 (GATA1) in the maladaptive and adaptive erythropoietic response to chronic hypoxia Whole-genome sequencing analysis revealed single nucleotide polymorphisms in the SENP1 gene in Andean highlanders (16) that explain part of the maladaptive and adaptive erythopoietic responses to life at high altitude. Under normoxia, the hypoxia-inducible factor-α (HIFα) subunit is hydroxylated by prolyl hydroxylase enzymes (PHDs), targeted for ubiquitylation (UB) by the von Hippel-Lindau protein (pVHL), and subsequently degraded in the proteasome (87). Under hypoxia, the activity of PHDs decreases due to low Po2, and HIFα escapes hydroxylation and undergoes SUMOylation. SENP1-dependent deSUMOylation rescues HIFα from ubiquitylation/degradation and allows its association with nuclear HIFβ. The heterodimer binds to a core consensus sequence at the promoters of HIF-responsive genes and, upon binding to coactivators, initiates transcription. SENP1 plays a critical role in the deSUMOylation and stabilization of HIFα and GATA1 by preventing their ubiquitylation and subsequent degradation, and enhancing their transcriptional activity (85, 86). Individuals with a reduced frequency of the SENP1 gene allelic variant rs7963934, showed increased expression of the SENP1 protein under hypoxic conditions and a maladaptive erythropoietic response (16, 82). The overexpression of SENP1 increases the deSUMOylation activity and favors the stabilization of HIF and GATA1, resulting in an increased expression of hypoxia responsive genes and genes that regulate the erythropoietic process. These, in turn, promote an exaggerated proliferation of erythroid cells (84). On the other hand, individuals with an increased frequency of the rs7963934 variant show an adaptive erythropoietic response to hypoxia, which favors the ubiquitination and degradation of SUMOylated HIFα and GATA1, keeping the response to hypoxia and the proliferation of erythroid cells under a normal range relative to the altitude of residence. Estrogen also plays an adaptive role as a protective factor against excessive erythrocytosis by binding to its β-receptor, causing a decrease in the expression of the HIF complex and GATA1, and increasing apoptosis in erythroid cells (18). After menopause, however, estrogen levels decrease (59, 66), which allows an increase in the transcriptional activity of HIF and GATA1, which leads to an exaggerated proliferation of erythroid cells. This eventually might lead to a maladaptive outcome such as excessive erythrocytosis in women. Also, recently, ARID1B, a chromatin remodeling factor, has been suggested to act as an adaptive regulator of erythropoiesis in Andean highlanders by modulating the expression of GATA1 and maintaining erythroid cell proliferation within the normal high-altitude range (88). ER, estrogen receptor; EPO, erythropoietin; EPOR, EPO receptor; Bcl-xL, B-cell lymphoma-extra large anti-apoptotic factor; ALA-D, delta-aminolevulinic acid dehydratase; ALA-S, delta-aminolevulinic acid synthase. Image created with BioRender.com and used with permission.
In addition to these genes, other key pathway and HIF-regulated genes have been identified as potential targets of selection in Andeans, including those related to cardiovascular function (93). While limited data are available regarding genotype-phenotype relationships, our recent work suggests HIF-related genes, similar to those reported in Tibetans (94, 95), are under selection in Andeans with likely distinct functional variants (96). Recent studies also indicate differential methylation at the EGLN1 locus in Andeans with and without EE (97) and differential methylation patterns at various sites across Andean genomes, including reduced methylation at EPAS1 (98, 99). These findings suggest epigenetic mechanisms could underlie developmental plasticity and long-term effects at high-altitude that warrant additional investigation.
Thus these recent findings provide insight into the multiple risk factors and potential mechanisms of EE in terms of adaptive and maladaptive outcomes. However, longitudinal studies with integrative translational approaches should be conducted to obtain a deep appreciation of the natural history of the disorder on an individual basis.
Definition of Excessive Erythrocytosis and the Qinghai CMS Score
The presence and severity of CMS are assessed through the Qinghai Score, a system based on the presence of EE ([Hb] ≥21 g/dl in men and ≥19 g/dl in women) and a group of signs and symptoms agreed by consensus at the International Working Group for the study of CMS that formed in 1998 and established the diagnostic guide principles for this syndrome (9) (Table 1). According to the sum of individual scores, the presence and severity of primary CMS are determined. It is important to point out that EE is the defining feature of CMS. Thus it is possible to have EE and not have CMS, but it is not possible to have CMS without EE. Therefore, [Hb] must be measured as the first step in diagnosing CMS, as a high score without EE rules out the presence of the syndrome.
TABLE 1.
Assessments through the Qinghai Score
| Points | Sign or Symptom |
|---|---|
| Hemoglobin concentration | |
| Men | |
| 0 | [Hb] <21 g/dl |
| 3 | Excessive erythrocytosis, [Hb] ≥21 g/dl |
| Women | |
| 0 | [Hb] <19 g/dl |
| 3 | Excessive erythrocytosis, [Hb] ≥19 g/dl |
| Breathlessness and/or palpitations | |
| 0 | No breathlessness/palpitations |
| 1 | Mild breathlessness/palpitations |
| 2 | Moderate breathlessness/palpitations |
| 3 | Severe breathlessness/palpitations |
| Sleep disturbance | |
| 0 | Slept as well as usual |
| 1 | Did not sleep as well as usual |
| 2 | Woke many times, poor night’s sleep |
| 3 | Could not sleep at all |
| Cyanosis | |
| 0 | No cyanosis |
| 1 | Mild cyanosis |
| 2 | Moderate cyanosis |
| 3 | Severe cyanosis |
| Dilatation of veins | |
| 0 | No dilatation of veins |
| 1 | Mild dilatation of veins |
| 2 | Moderate dilatation of veins |
| 3 | Severe dilatation of veins |
| Paresthesia | |
| 0 | No paresthesia |
| 1 | Mild paresthesia |
| 2 | Moderate paresthesia |
| 3 | Severe paresthesia |
| Headache | |
| 0 | No headache |
| 1 | Mild headache symptoms |
| 2 | Moderate headache |
| 3 | Severe headache, incapacitating |
| Tinnitus | |
| 0 | No tinnitus |
| 1 | Mild tinnitus |
| 2 | Moderate tinnitus |
| 3 | Severe tinnitus |
The Qinghai score (9) has been designed to assess the presence and severity of chronic mountain sickness (CMS) based on hemoglobin concentration ([Hb]) and a group of signs and symptoms at the altitude of residence. The addition of points for each sign or symptom category results in the total CMS score. CMS absent: score = 0–5; mild: score = 6–10; moderate: score =11–14; and severe: score >15.
There is an ongoing controversy and discussion regarding the use of a single threshold value for determining EE and the use of the scoring system. One issue is that the [Hb] threshold value for diagnosing EE is altitude independent, and thus the same value would be used to define EE either at 3,400, 4,400, or 5,100 m. This single value was obtained from the largest epidemiological study in the early 1990s conducted in Cerro de Pasco, Peru, at 4,340 m. [Hb] was determined to be excessive when it was higher than 2 SD above the mean [Hb] value of young healthy highlanders at that altitude (58, 61). At the time, Cerro de Pasco was the largest and highest demographically stable population in the world and, therefore, if an individual was determined to have EE at that altitude, it was assumed definitively excessive at lower altitudes. If we accept that the “normal” [Hb] value increases with altitude, having a threshold based on “normal” [Hb] at 4,340 m would result in an underestimation of the prevalence of EE if we apply this value to a population living at a lower altitude. Conversely, now that large semistable populations are inhabiting higher altitudes (i.e., La Rinconada, Peru, 5,100 m) (100, 101), applying this value would result in an overestimation of EE prevalence. Accordingly, recent studies have shown that at La Rinconada, the presence of EE does not necessarily correlate with a high CMS score and that sometimes a high CMS score is observed in highlanders without EE (100, 102). It would be straightforward to think that if [Hb] increases with altitude, the current [Hb] threshold at 5,100 m would no longer define EE. Thus 21 g/dl or 19 g/dl in men and women, respectively, would not necessarily be excessive. This has been wrongly interpreted as EE not being the main sign of CMS at extreme altitudes and led to questioning of the validity of the Qinghai CMS score.
In cases of threshold-based diagnosis of EE and low CMS scores (i.e., <6 points; absence of CMS), it is necessary to consider the age of individuals, the time of residence at a given altitude, the age of EE onset, and the frequency of migration to and from lower altitudes. For example, the longer amount of time an individual has had an excessive hematocrit, the more severe its effects on vasculature. Hence, it is possible that some young or middle-aged individuals with EE and relatively few years living at an extreme altitude, and whose migratory patterns to low altitude are frequent, do not present marked symptoms. A recent study at 5,100 m (102) showed a significant number of highlanders with high hematocrit values and relatively young age (64–76%, 36–47 yr, respectively) but apparently without symptoms of CMS. In fact, although these high-altitude natives showed a low CMS score, the prognosis for this high [Hb] level over time may be a higher score and the occurrence of thromboses and hemorrhages, as it has been observed in the Cerro de Pasco population, where the prevalence of stroke is associated with a high prevalence of EE (103).
In cases of the absence of EE but a high CMS score (i.e., ≥6 points) it is necessary to consider the typical clinical picture of the syndrome, first described by Carlos Monge-M in 1925 (104–107), where the excessive count of RBCs is central in the definition of CMS. In other words, there is no CMS without EE. Therefore, a high CMS score (≥6 points) without EE is just an addition of nonspecific symptoms. Bloodletting and hemodilution studies at high altitude, as well regular phlebotomies (common practice of highlanders with EE to manage the condition), have shown that once hematocrit is reduced, CMS signs and symptoms disappear rapidly, which confirms that these are secondary to EE (Refs. 6, 108–110; Anza-Ramírez C, et al., unpublished observations).
Thus, from an epidemiological point of view, a critical reappraisal of the consensus criteria might consider population studies at different altitudes to estimate normal and excessive [Hb] values and either increase or decrease the [Hb] threshold value to define EE. However, from the physiological point of view, it is possible that above certain value, [Hb] becomes detrimental irrespective of the altitude of residence. Recent evidence from our group has provided a molecular basis to explain the hemodynamic impairments observed in EE owing to the associated exponential increases in blood viscosity and shear stress which can collectively induce vascular endothelial dysfunction (11, 54, 55, 111). Impaired vascular endothelial function has been associated with a free radical-mediated induction of inflammation and scavenging of vascular NO bioavailability, a metabolic cascade collectively termed oxidative-inflammatory-nitrosative stress (OXINOS) (53, 111, 112). Bailey et al have consistently demonstrated systemic OXINOS to be selectively elevated in CMS patients compared with age-matched non-CMS controls (53, 112). Elevated OXINOS was associated with more pronounced impairments in systemic and cerebrovascular endothelial function in the form of elevated arterial stiffness, cerebral hypoperfusion, blunted cerebral vasoreactivity, cognitive impairment, and depression (53, 112, 113). Thus physiologically, it is also possible to establish a threshold [Hb] value based on longitudinal studies of cerebrovascular function as a function of increasing [Hb], and its relationship with the neurological symptoms associated with EE, which defines the full clinical picture of CMS. This information coupled with an individual’s life history and tracking of biomarkers across the life span (or at least part of it) would provide much needed insight into the development of EE and CMS and combinations thereof that develop in some but not all individuals.
Clinical Significance of Research in the Field
EE and CMS are major public health problems in high-altitude populations around the world not only because their incapacitating nature, but also because their association with cardiovascular and cerebrovascular disease. We and others have shown that EE associates with increased cardiovascular disease risk and cardiometabolic disorders (114–117) in various high-altitude regions across the world (118–121). Importantly, we have identified independent associations between EE and 24-h ambulatory hypertension including systolic-diastolic and isolated diastolic hypertension (114, 122) and a significant proportion of masked hypertension, the latter linked to increased cardiovascular morbidity and mortality in lowlanders (123). Our data also show that the incidence of ambulatory hypertension in highlanders with EE is greater compared with non-EE individuals across age groups. We have identified independent associations between EE, insulin resistance, hyperglycemia, and dyslipidemia (114) and shown relationships between EE, control of breathing, and sleep phenotypes (69), which have major impacts on cardiometabolic health. These findings agree with other studies at high altitude in the Peruvian Andes that have identified independent relationships between EE and hypertension, hypertriglyceridemia (103, 116, 118), and metabolic syndrome (115). Importantly, EE may have a more adverse impact on cardiovascular and cerebrovascular disease risk in women, especially following menopause.
Conclusions
Values of [Hb] and hematocrit are commonly used to characterize an individual’s physiological response to chronic high-altitude hypoxia. However, mechanisms underlying differences in this response are multifaceted and should be evaluated in the context of other O2 transport components that assess physiological impact, genetic and life history information, and altitude of residence. Thresholds used to define EE and CMS are currently under discussion and would benefit from thorough considerations of these factors, as well as much-needed, carefully planned, longitudinal assessments that aim to determine the effects of elevated [Hb] at the individual and population levels.
Acknowledgments
F.C.V. was supported by a Wellcome Trust Grant 107544/Z/15/Z. T.S.S. is supported by National Heart, Lung, and Blood Institute of Health Grant R01HL145470, the National Geographic Explorer Award, and the John B. West Endowed Chair in Respiratory Physiology
No conflicts of interest, financial or otherwise, are declared by the authors
F.C.V., T.S.S., and F.L.-V. conceived and designed research; F.C.V. analyzed data; F.C.V., T.S.S., and F.L.-V. interpreted results of experiments; F.C.V. and D.B. prepared figures; F.C.V., T.S.S., D.B. and F.L.-V. drafted manuscript; F.C.V., T.S.S., D.B. and F.L.-V. edited and revised manuscript; F.C.V., T.S.S., D.B., and F.L.-V. approved final version of manuscript.
References
- 1.Viault F. Sur l‘augmentation considerable du nombre des globules rouges dans le sang chez les habitants des hautes plateaux de l‘Amerique du sud. Compt Rend Soc Biol (Paris) 30: 917–918, 1890. [Google Scholar]
- 2.Azad P, Stobdan T, Zhou D, Hartley I, Akbari A, Bafna V, Haddad GG. High-altitude adaptation in humans: from genomics to integrative physiology. J Mol Med (Berl) 95: 1269–1282, 2017. doi: 10.1007/s00109-017-1584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monge C, Leon-Velarde F. Physiological adaptation to high altitude: oxygen transport in mammals and birds. Physiol Rev 71: 1135–1172, 1991. doi: 10.1152/physrev.1991.71.4.1135. [DOI] [PubMed] [Google Scholar]
- 4.Monge-C C, Leon-Velarde F, Villafuerte FC. The function of blood in tissue oxygenation (In Spanish). In: El Reto Fisiologico de Vivir en los Andes, edited by Monge-C C, Leon-Velarde F.. Lima, Peru: Institute Francaise d´ Etudes Andines. Universidad Peruana Cayetano Heredia, 2003. [Google Scholar]
- 5.Wagner PD. Altitude physiology then (1921) and now (2021): meat on the bones. Physiol Rev 102: 323–332, 2021. doi: 10.1152/physrev.00033.2021. [DOI] [PubMed] [Google Scholar]
- 6.Winslow R, Monge-C C. Hypoxia, Polycythemia, and Chronic Mountain Sickness. Baltimore, MD: Johns Hopkins University Press, 1987. [Google Scholar]
- 7.Winslow RM. The role of polycythemia in adaptation to high altitude. In: Adjustment to High Altitude, edited by Chamberlayne EC, Condliffe PG.. Bethesda, MD: U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, 1981. [Google Scholar]
- 8.Arias-Stella J. Chronic mountain sickness: pathology and definition. In: High Alltitude Physiology: Cardiac and Respiratory Aspects, edited by Porter R, Knight J.. London: Churchill Livingstone, 1971, p. 31–40. [Google Scholar]
- 9.Leon-Velarde F, Maggiorini M, Reeves JT, Aldashev A, Asmus I, Bernardi L, Ge RL, Hackett P, Kobayashi T, Moore LG, Penaloza D, Richalet JP, Roach R, Wu T, Vargas E, Zubieta-Castillo G, Zubieta-Calleja G. Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol 6: 147–157, 2005. doi: 10.1089/ham.2005.6.147. [DOI] [PubMed] [Google Scholar]
- 10.Penaloza D, Arias-Stella J. The heart and pulmonary circulation at high altitudes: healthy highlanders and chronic mountain sickness. Circulation 115: 1132–1146, 2007. doi: 10.1161/CIRCULATIONAHA.106.624544. [DOI] [PubMed] [Google Scholar]
- 11.Rimoldi SF, Rexhaj E, Pratali L, Bailey DM, Hutter D, Faita F, Salmon CS, Villena M, Nicod P, Allemann Y, Scherrer U, Sartori C. Systemic vascular dysfunction in patients with chronic mountain sickness. Chest 141: 139–146, 2012. doi: 10.1378/chest.11-0342. [DOI] [PubMed] [Google Scholar]
- 12.Villafuerte FC, Corante N. Chronic mountain sickness: clinical aspects, etiology, management, and treatment. High Alt Med Biol 17: 61–69, 2016. doi: 10.1089/ham.2016.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Li JC, Liang Y, McCormack M, Montgomery HE, Pan H, Robbins PA, Shianna KV, Tam SC, Tsering N, Veeramah KR, Wang W, Wangdui P, Weale ME, Xu Y, Xu Z, Yang L, Zaman MJ, Zeng C, Zhang L, Zhang X, Zhaxi P, Zheng YT. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci U S A 107: 11459–11464, 2010. doi: 10.1073/pnas.1002443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simonson TS, Yang Y, Huff CD, Yun H, Qin G, Witherspoon DJ, Bai Z, Lorenzo FR, Xing J, Jorde LB, Prchal JT, Ge R. Genetic evidence for high-altitude adaptation in Tibet. Science 329: 72–75, 2010. doi: 10.1126/science.1189406. [DOI] [PubMed] [Google Scholar]
- 15.Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZX, Pool JE, , et al. Sequencing of 50 human exomes reveals adaptation to high altitude. Science (New York, NY) 329: 75–78, 2010. doi: 10.1126/science.1190371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou D, Udpa N, Ronen R, Stobdan T, Liang J, Appenzeller O, Zhao HW, Yin Y, Du Y, Guo L, Cao R, Wang Y, Jin X, Huang C, Jia W, Cao D, Guo G, Gamboa JL, Villafuerte F, Callacondo D, Xue J, Liu S, Frazer KA, Li Y, Bafna V, Haddad GG. Whole-genome sequencing uncovers the genetic basis of chronic mountain sickness in Andean highlanders. Am J Hum Genet 93: 452–462, 2013. doi: 10.1016/j.ajhg.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bermudez D, Azad P, Figueroa-Mujica R, Vizcardo-Galindo G, Corante N, Guerra-Giraldez C, Haddad GG, Villafuerte FC. Increased hypoxic proliferative response and gene expression in erythroid progenitor cells of Andean highlanders with chronic mountain sickness. Am J Physiol Regul Integr Comp Physiol 318: R49–R56, 2020. doi: 10.1152/ajpregu.00250.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azad P, Villafuerte FC, Bermudez D, Patel G, Haddad GG. Protective role of estrogen against excessive erythrocytosis in Monge’s disease. Exp Mol Med 53: 125–135, 2021. doi: 10.1038/s12276-020-00550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stobdan T, Akbari A, Azad P, Zhou D, Poulsen O, Appenzeller O, Gonzales GF, Telenti A, Wong EH, Saini S, Kirkness EF, Venter JC, Bafna V, Haddad GG. New insights into the genetic basis of Monge’s disease and adaptation to high-altitude. Mol Biol Evol 34: 3154–3168, 2017. doi: 10.1093/molbev/msx239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beall CM. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci U S A 104, Suppl 1: 8655–8660, 2007. doi: 10.1073/pnas.0701985104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beall CM, Brittenham GM, Strohl KP, Blangero J, Williams-Blangero S, Goldstein MC, Decker MJ, Vargas E, Villena M, Soria R, Alarcon AM, Gonzales C. Hemoglobin concentration of high-altitude Tibetans and Bolivian Aymara. Am J Phys Anthropol 106: 385–400, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 22.Schoene RB, Roach RC, Lahiri S, Peters RM Jr, Hackett PH, Santolaya R. Increased diffusion capacity maintains arterial saturation during exercise in the Quechua Indians of Chilean Altiplano. Am J Hum Biol 2: 663–668, 1990. doi: 10.1002/ajhb.1310020609. [DOI] [PubMed] [Google Scholar]
- 23.Wagner PD, Araoz M, Boushel R, Calbet JA, Jessen B, Radegran G, Spielvogel H, Sondegaard H, Wagner H, Saltin B. Pulmonary gas exchange and acid-base state at 5,260 m in high-altitude Bolivians and acclimatized lowlanders. J Appl Physiol (1985) 92: 1393–1400, 2002. doi: 10.1152/japplphysiol.00093.2001. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang J, Droma T, Sutton JR, Groves BM, McCullough RE, McCullough RG, Sun S, Moore LG. Smaller alveolar-arterial O2 gradients in Tibetan than Han residents of Lhasa (3658 m). Respir Physiol 103: 75–82, 1996. doi: 10.1016/0034-5687(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 25.Brutsaert TD, Araoz M, Soria R, Spielvogel H, Haas JD. Higher arterial oxygen saturation during submaximal exercise in Bolivian Aymara compared with European sojourners and Europeans born and raised at high altitude. Am J Phys Anthropol 113: 169–181, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 26.Droma T, McCullough RG, McCullough RE, Zhuang JG, Cymerman A, Sun SF, Sutton JR, Moore LG. Increased vital and total lung capacities in Tibetan compared with Han residents of Lhasa (3,658 m). Am J Phys Anthropol 86: 341–351, 1991. doi: 10.1002/ajpa.1330860303. [DOI] [PubMed] [Google Scholar]
- 27.Winslow RM, Chapman KW, Gibson CC, Samaja M, Monge CC, Goldwasser E, Sherpa M, Blume FD, Santolaya R. Different hematologic responses to hypoxia in Sherpas and Quechua Indians. J Appl Physiol (1985) 66: 1561–1569, 1989. doi: 10.1152/jappl.1989.66.4.1561. [DOI] [PubMed] [Google Scholar]
- 28.Beall CM, Almasy LA, Blangero J, Williams-Blangero S, Brittenham GM, Strohl KP, Decker MJ, Vargas E, Villena M, Soria R, Alarcon AM, Gonzales C. Percent of oxygen saturation of arterial hemoglobin among Bolivian Aymara at 3,900-4,000 m. Am J Phys Anthropol 108: 41–51, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 29.Beall CM, Strohl KP, Blangero J, Williams-Blangero S, Decker MJ, Brittenham GM, Goldstein MC. Quantitative genetic analysis of arterial oxygen saturation in Tibetan highlanders. Hum Biol 69: 597–604, 1997. [PubMed] [Google Scholar]
- 30.Gilbert-Kawai ET, Milledge JS, Grocott MP, Martin DS. King of the mountains: Tibetan and Sherpa physiological adaptations for life at high altitude. Physiology (Bethesda) 29: 388–402, 2014. doi: 10.1152/physiol.00018.2014. [DOI] [PubMed] [Google Scholar]
- 31.Moore LG. Comparative human ventilatory adaptation to high altitude. Respir Physiol 121: 257–276, 2000. doi: 10.1016/S0034-5687(00)00133-X. [DOI] [PubMed] [Google Scholar]
- 32.Petousi N, Robbins PA. Human adaptation to the hypoxia of high altitude: the Tibetan paradigm from the pregenomic to the postgenomic era. J Appl Physiol (1985) 116: 875–884, 2014. doi: 10.1152/japplphysiol.00605.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu T, Kayser B. High altitude adaptation in Tibetans. High Alt Med Biol 7: 193–208, 2006. doi: 10.1089/ham.2006.7.193. [DOI] [PubMed] [Google Scholar]
- 34.Simonson TS, Wei G, Wagner HE, Wuren T, Bui A, Fine JM, Qin G, Beltrami FG, Yan M, Wagner PD, Ge RL. Increased blood-oxygen binding affinity in Tibetan and Han Chinese residents at 4200 m. Exp Physiol 99: 1624–1635, 2014. doi: 10.1113/expphysiol.2014.080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beall CM, Laskowski D, Erzurum SC. Nitric oxide in adaptation to altitude. Free Radic Biol Med 52: 1123–1134, 2012. doi: 10.1016/j.freeradbiomed.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoit BD, Dalton ND, Erzurum SC, Laskowski D, Strohl KP, Beall CM. Nitric oxide and cardiopulmonary hemodynamics in Tibetan highlanders. J Appl Physiol (1985) 99: 1796–1801, 2005. doi: 10.1152/japplphysiol.00205.2005. [DOI] [PubMed] [Google Scholar]
- 37.Gassmann M, Mairbaurl H, Livshits L, Seide S, Hackbusch M, Malczyk M, Kraut S, Gassmann NN, Weissmann N, Muckenthaler MU. The increase in hemoglobin concentration with altitude varies among human populations. Ann N Y Acad Sci 1450: 204–220, 2019. doi: 10.1111/nyas.14136. [DOI] [PubMed] [Google Scholar]
- 38.Wagner PD. A theoretical analysis of factors determining VO2 MAX at sea level and altitude. Respir Physiol 106: 329–343, 1996. doi: 10.1016/S0034-5687(96)00086-2. [DOI] [PubMed] [Google Scholar]
- 39.Piiper J, Scheid P. Model for capillary-alveolar equilibration with special reference to O2 uptake in hypoxia. Respir Physiol 46: 193–208, 1981. doi: 10.1016/0034-5687(81)90121-3. [DOI] [PubMed] [Google Scholar]
- 40.Beall CM. Andean, Tibetan, and Ethiopian patterns of adaptation to high-altitude hypoxia. Integr Comp Biol 46: 18–24, 2006. doi: 10.1093/icb/icj004. [DOI] [PubMed] [Google Scholar]
- 41.Moore LG. Measuring high-altitude adaptation. J Appl Physiol (1985) 123: 1371–1385, 2017. doi: 10.1152/japplphysiol.00321.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beall CM, Decker MJ, Brittenham GM, Kushner I, Gebremedhin A, Strohl KP. An Ethiopian pattern of human adaptation to high-altitude hypoxia. Proc Natl Acad Sci U S A 99: 17215–17218, 2002. doi: 10.1073/pnas.252649199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoit BD, Dalton ND, Gebremedhin A, Janocha A, Zimmerman PA, Zimmerman AM, Strohl KP, Erzurum SC, Beall CM. Elevated pulmonary artery pressure among Amhara highlanders in Ethiopia. Am J Hum Biol 23: 168–176, 2011. doi: 10.1002/ajhb.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simonson TS. Altitude adaptation: a glimpse through various lenses. High Alt Med Biol 16: 125–137, 2015. doi: 10.1089/ham.2015.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horscroft JA, Kotwica AO, Laner V, West JA, Hennis PJ, Levett DZ, Howard DJ, Fernandez BO, Burgess SL, Ament Z, Gilbert-Kawai ET, Vercueil A, Landis BD, Mitchell K, Mythen MG, Branco C, Johnson RS, Feelisch M, Montgomery HE, Griffin JL, Grocott MP, Gnaiger E, Martin DS, Murray AJ. Metabolic basis to Sherpa altitude adaptation. Proc Natl Acad Sci U S A 114: 6382–6387, 2017. doi: 10.1073/pnas.1700527114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Brien KA, Simonson TS, Murray AJ. Metabolic adaptation to high altitude. Cur Opin Endo Metab Res 11: 33–41, 2020. doi: 10.1016/j.coemr.2019.12.002. [DOI] [Google Scholar]
- 47.Lorenzo FR, Huff C, Myllymaki M, Olenchock B, Swierczek S, Tashi T, Gordeuk V, Wuren T, Ri-Li G, McClain DA, Khan TM, Koul PA, Guchhait P, Salama ME, Xing J, Semenza GL, Liberzon E, Wilson A, Simonson TS, Jorde LB, Kaelin WG Jr, Koivunen P, Prchal JT. A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet 46: 951–956, 2014. doi: 10.1038/ng.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song D, Navalsky BE, Guan W, Ingersoll C, Wang T, Loro E, Eeles L, Matchett KB, Percy MJ, Walsby-Tickle J, McCullagh JS, Medina RJ, Khurana TS, Bigham AW, Lappin TR, Lee FS. Tibetan PHD2, an allele with loss-of-function properties. Proc Natl Acad Sci U S A 117: 12230–12238, 2020. doi: 10.1073/pnas.1920546117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu H, Petousi N, Glusman G, Yu Y, Bohlender R, Tashi T, Downie JM, Roach JC, Cole AM, Lorenzo FR, Rogers AR, Brunkow ME, Cavalleri G, Hood L, Alpatty SM, Prchal JT, Jorde LB, Robbins PA, Simonson TS, Huff CD. Evolutionary history of Tibetans inferred from whole-genome sequencing. PLoS Genet 13: e1006675, 2017. doi: 10.1371/journal.pgen.1006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huerta-Sanchez E, Jin X, Asan Bianba Z, Peter BM, Vinckenbosch N, Liang Y, Yi X, He M, Somel M, Ni P, Wang B, Ou X, Luosang H, Cuo J, Li ZX, Gao K, Yin G, Wang Y, Zhang W, Xu X, Yang X, Li H, Wang Y, Wang J, Nielsen JR. Altitude adaptation in Tibetans caused by introgression of Denisovan-like DNA. Nature 512: 194–197, 2014. doi: 10.1038/nature13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Storz JF. Evolution. Genes for high altitudes. Science 329: 40–41, 2010. doi: 10.1126/science.1192481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stembridge M, Williams AM, Gasho C, Dawkins TG, Drane A, Villafuerte FC, Levine BD, Shave R, Ainslie PN. The overlooked significance of plasma volume for successful adaptation to high altitude in Sherpa and Andean natives. Proc Natl Acad Sci U S A 116: 16177–16179, 2019. doi: 10.1073/pnas.1909002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bailey DM, Brugniaux JV, Filipponi T, Marley CJ, Stacey B, Soria R, Rimoldi SF, Cerny D, Rexhaj E, Pratali L, Salmon CS, Murillo Jauregui C, Villena M, Smirl JD, Ogoh S, Pietri S, Scherrer U, Sartori C. Exaggerated systemic oxidative-inflammatory-nitrosative stress in chronic mountain sickness is associated with cognitive decline and depression. J Physiol 597: 611–629, 2019. doi: 10.1113/JP276898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tremblay JC, Coombs GB, Howe CA, Vizcardo-Galindo GA, Figueroa-Mujica RJ, Bermudez D, Tymko MM, Villafuerte FC, Ainslie PN, Pyke KE. Global Reach 2018: reduced flow-mediated dilation stimulated by sustained increases in shear stress in high-altitude excessive erythrocytosis. Am J Physiol Heart Circ Physiol 317: H991–H1001, 2019. doi: 10.1152/ajpheart.00316.2019. [DOI] [PubMed] [Google Scholar]
- 55.Tremblay JC, Hoiland RL, Howe CA, Coombs GB, Vizcardo-Galindo GA, Figueroa-Mujica RJ, Bermudez D, Gibbons TD, Stacey BS, Bailey DM, Tymko MM, MacLeod DB, Gasho C, Villafuerte FC, Pyke KE, Ainslie PN. Global REACH 2018: high blood viscosity and hemoglobin concentration contribute to reduced flow-mediated dilation in high-altitude excessive erythrocytosis. Hypertension 73: 1327–1335, 2019, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12780. [DOI] [PubMed] [Google Scholar]
- 56.Tift MS, Alves de Souza RW, Weber J, Heinrich EC, Villafuerte FC, Malhotra A, Otterbein LE, Simonson TS. adaptive potential of the heme oxygenase/carbon monoxide pathway during hypoxia. Front Physiol 11: 886, 2020. doi: 10.3389/fphys.2020.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leon-Velarde F, Rivera-Ch M, Huicho L, Villafuerte FC. Chronic mountain sickness. In: High Altitude Human Adaptation to Hypoxia, edited by Swenson ER, Bartsch P.. New York: Springer, 2014. [Google Scholar]
- 58.Leon-Velarde F, Arregui A, Monge C, Ruiz y Ruiz H. Aging at high altitudes and the risk of chronic mountain sickness. J Wild Med 4: 183–188, 1993. doi: 10.1580/0953-9859-4.2.183. [DOI] [Google Scholar]
- 59.Leon-Velarde F, Ramos MA, Hernandez JA, De Idiaquez D, Munoz LS, Gaffo A, Cordova S, Durand D, Monge C. The role of menopause in the development of chronic mountain sickness. Am J Physiol Regul Comp Integr Physiol 272: R90–R94, 1997. doi: 10.1152/ajpregu.1997.272.1.R90. [DOI] [PubMed] [Google Scholar]
- 60.Monge-C C, Arregui A, Leon-Velarde F. Pathophysiology and epidemiology of chronic mountain sickness. Int J Sports Med 13: S79–81, 1992. doi: 10.1055/s-2007-1024603. [DOI] [PubMed] [Google Scholar]
- 61.Monge-C C, Leon-Velarde F, Arregu A. Increasing prevalence of excessive erythrocytosis with age among healthy high-altitude miners. N Engl J Med 321: 1271, 1989. [DOI] [PubMed] [Google Scholar]
- 62.Sahota IS, Panwar NS. Prevalence of Chronic Mountain Sickness in high altitude districts of Himachal Pradesh. Indian J Occup Environ Med 17: 94–100, 2013. doi: 10.4103/0019-5278.130839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sime F, Monge C, Whittembury J. Age as a cause of chronic mountain sickness (Monge’s disease). Int J Biometeorol 19: 93–98, 1975. doi: 10.1007/BF01463864. [DOI] [PubMed] [Google Scholar]
- 64.Whittembury J, Mong CC. High altitude, haematocrit and age. Nature 238: 278–279, 1972. doi: 10.1038/238278b0. [DOI] [PubMed] [Google Scholar]
- 65.Wu T, Li W, Li Y, Ri-Li G, Cheng Q, Wang S, Zhao G, Wei L, Jin Y, Don G. Epidemiology of chronic mountain sickness: ten years’ study in Qinghai-Tibet. In: Progress in Mountain Medicine and High Altitude Physiology, edited by Ohno H, Kobayashi T, Masuyama S, Nakashima M. Matsumoto,. Japan: Press Committee of the 3rd World Congress on Mountan Medicine and High Altitude Physiology, 1998, p. 120–125. [Google Scholar]
- 66.Leon-Velarde F, Rivera-Chira M, Tapia R, Huicho L, Monge-C C. Relationship of ovarian hormones to hypoxemia in women residents of 4,300 m. Am J Physiol Regul Integr Comp Physiol 280: R488–R493, 2001. doi: 10.1152/ajpregu.2001.280.2.R488. [DOI] [PubMed] [Google Scholar]
- 67.Julian CG, Gonzales M, Rodriguez A, Bellido D, Salmon CS, Ladenburger A, Reardon L, Vargas E, Moore LG. Perinatal hypoxia increases susceptibility to high-altitude polycythemia and attendant pulmonary vascular dysfunction. Am J Physiol Heart Circ Physiol 309: H565–H573, 2015. doi: 10.1152/ajpheart.00296.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moore LG, Niermeyer S, Vargas E. Does chronic mountain sickness (CMS) have perinatal origins?. Respir Physiol Neurobiol 158: 180–189, 2007. doi: 10.1016/j.resp.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 69.Heinrich EC, Orr JE, Gilbertson D, Anza-Ramirez C, DeYoung PN, Djokic MA, Corante N, Vizcardo-Galindo G, Macarlupu JL, Gaio E, Powell FL, Malhotra A, Villafuerte FC, Simonson TS. Relationships between chemoreflex responses, sleep quality, and hematocrit in Andean men and women. Front Physiol 11: 437, 2020. doi: 10.3389/fphys.2020.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Julian CG, Vargas E, Gonzales M, Davila RD, Ladenburger A, Reardon L, Schoo C, Powers RW, Lee-Chiong T, Moore LG. Sleep-disordered breathing and oxidative stress in preclinical chronic mountain sickness (excessive erythrocytosis). Respir Physiol Neurobiol 186: 188–196, 2013. doi: 10.1016/j.resp.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pham LV, Meinzen C, Arias RS, Schwartz NG, Rattner A, Miele CH, Smith PL, Schneider H, Miranda JJ, Gilman RH, Polotsky VY, Checkley W, Schwartz AR. Cross-sectional comparison of sleep-disordered breathing in native peruvian highlanders and lowlanders. High Alt Med Biol 18: 11–19, 2017. doi: 10.1089/ham.2016.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pham LV, Miele CH, Schwartz NG, Arias RS, Rattner A, Gilman RH, Miranda JJ, Polotsky VY, Checkley W, Schwartz AR. Cardiometabolic correlates of sleep disordered breathing in Andean highlanders. Eur Respir J 49: 1601705, 2017. doi: 10.1183/13993003.01705-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fatemian M, Gamboa A, Leon-Velarde F, Rivera-Ch M, Palacios JA, Robbins PA. Selected contribution: ventilatory response to CO2 in high-altitude natives and patients with chronic mountain sickness. J Appl Physiol (1985) 94: 1279–1287, 2003. doi: 10.1152/japplphysiol.00859.2002. [DOI] [PubMed] [Google Scholar]
- 74.Leon-Velarde F, Gamboa A, Rivera-Ch M, Palacios JA, Robbins PA. Selected contribution: Peripheral chemoreflex function in high-altitude natives and patients with chronic mountain sickness. J Appl Physiol 94: 1269–1278, 2003. doi: 10.1152/japplphysiol.00858.2002. [DOI] [PubMed] [Google Scholar]
- 75.Spicuzza L, Casiraghi N, Gamboa A, Keyl C, Schneider A, Mori A, Leon-Velarde F, Di Maria GU, Bernardi L. Sleep-related hypoxaemia and excessive erythrocytosis in Andean high-altitude natives. Eur Respir J 23: 41–46, 2004. doi: 10.1183/09031936.03.00000703. [DOI] [PubMed] [Google Scholar]
- 76.Azarbarzin A, Sands SA, Taranto-Montemurro L, Redline S, Wellman A. Hypoxic burden captures sleep apnoea-specific nocturnal hypoxaemia. Eur Heart J 40: 2989–2990, 2019. doi: 10.1093/eurheartj/ehz274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cruz JC, Diaz C, Marticorena E, Hilario V. Phlebotomy improves pulmonary gas exchange in chronic mountain polycythemia. Respiration 38: 305–313, 1979. doi: 10.1159/000194097. [DOI] [PubMed] [Google Scholar]
- 78.Manier G, Guenard H, Castaing Y, Varene N, Vargas E. Pulmonary gas exchange in Andean natives with excessive polycythemia–effect of hemodilution. J Appl Physiol (1985) 65: 2107–2117, 1988. doi: 10.1152/jappl.1988.65.5.2107. [DOI] [PubMed] [Google Scholar]
- 79.Villafuerte FC, Macarlupu JL, Anza-Ramirez C, Corrales-Melgar D, Vizcardo-Galindo G, Corante N, Leon-Velarde F. Decreased plasma soluble erythropoietin receptor in high-altitude excessive erythrocytosis and Chronic Mountain Sickness. J Appl Physiol (1985) 117: 1356–1362, 2014. doi: 10.1152/japplphysiol.00619.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Villafuerte FC, Corante N, Anza-Ramirez C, Figueroa-Mujica R, Vizcardo-Galindo G, Mercado A, Macarlupu JL, Leon-Velarde F. Plasma soluble erythropoietin receptor is decreased during sleep in Andean highlanders with Chronic Mountain Sickness. J Appl Physiol 121: 53–58, 2016. doi: 10.1152/japplphysiol.00107.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leon-Velarde F, Richalet JP. Respiratory control in residents at high altitude: physiology and pathophysiology. High Alt Med Biol 7: 125–137, 2006. doi: 10.1089/ham.2006.7.125. [DOI] [PubMed] [Google Scholar]
- 82.Cole AM, Petousi N, Cavalleri GL, Robbins PA. Genetic variation in SENP1 and ANP32D as predictors of chronic mountain sickness. High Alt Med Biol 15: 497–499, 2014. doi: 10.1089/ham.2014.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hsieh MM, Callacondo D, Rojas-Camayo J, Quesada-Olarte J, Wang X, Uchida N, Maric I, Remaley AT, Leon-Velarde F, Villafuerte FC, Tisdale JF. SENP1, but not fetal hemoglobin, differentiates Andean highlanders with chronic mountain sickness from healthy individuals among Andean highlanders. Exp Hematol 44: 483–490.e2, 2016. doi: 10.1016/j.exphem.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Azad P, Zhao HW, Cabrales PJ, Ronen R, Zhou D, Poulsen O, Appenzeller O, Hsiao YH, Bafna V, Haddad GG. Senp1 drives hypoxia-induced polycythemia via GATA1 and Bcl-xL in subjects with Monge’s disease. J Exp Med 213: 2729–2744, 2016. doi: 10.1084/jem.20151920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheng J, Kang X, Zhang S, Yeh ET. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell 131: 584–595, 2007. doi: 10.1016/j.cell.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu L, Ji W, Zhang H, Renda MJ, He Y, Lin S, Cheng EC, Chen H, Krause DS, Min W. SENP1-mediated GATA1 deSUMOylation is critical for definitive erythropoiesis. J Exp Med 207: 1183–1195, 2010. doi: 10.1084/jem.20092215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Samanta D, Prabhakar NR, Semenza GL. Systems biology of oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med 9: 10.1002/wsbm.1382, 2017. doi: 10.1002/wsbm.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Azad P, Haddad G. Molecular basis of hypoxia‐induced excessive erythrocytosis of high altitude. FASEB J 32: lb405–lb405, 2018. doi: 10.1096/fasebj.2018.32.1_supplement.lb405. [DOI] [Google Scholar]
- 89.Gazal S, Espinoza JR, Austerlitz F, Marchant D, Macarlupu JL, Rodriguez J, Ju-Preciado H, Rivera-Chira M, Hermine O, Leon-Velarde F, Villafuerte FC, Richalet JP, Gouya L. The genetic architecture of chronic mountain sickness in Peru. Front Genet 10: 690, 2019. doi: 10.3389/fgene.2019.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim H, Kang K, Ekram MB, Roh TY, Kim J. Aebp2 as an epigenetic regulator for neural crest cells. PLoS One 6: e25174, 2011. doi: 10.1371/journal.pone.0025174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wan F, Letavernier E, Abid S, Houssaini A, Czibik G, Marcos E, Rideau D, Parpaleix A, Lipskaia L, Amsellem V, Gellen B, Sawaki D, Derumeaux G, Dubois-Rande JL, Delcroix M, Quarck R, Baud L, Adnot S. Extracellular calpain/calpastatin balance is involved in the progression of pulmonary hypertension. Am J Respir Cell Mol Biol 55: 337–351, 2016. doi: 10.1165/rcmb.2015-0257OC. [DOI] [PubMed] [Google Scholar]
- 92.Bouchard L, Bouchard C, Chagnon YC, Perusse L. Evidence of linkage and association with body fatness and abdominal fat on chromosome 15q26. Obesity (Silver Spring) 15: 2061–2070, 2007. doi: 10.1038/oby.2007.245. [DOI] [PubMed] [Google Scholar]
- 93.Crawford JE, Amaru R, Song J, Julian CG, Racimo F, Cheng JY, Guo X, Yao J, Ambale-Venkatesh B, Lima JA, Rotter JI, Stehlik J, Moore LG, Prchal JT, Nielsen R. Natural selection on genes related to cardiovascular health in high-altitude adapted Andeans. Am J Hum Genet 101: 752–767, 2017. doi: 10.1016/j.ajhg.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bigham A, Bauchet M, Pinto D, Mao X, Akey JM, Mei R, Scherer SW, Julian CG, Wilson MJ, Lopez Herraez D, Brutsaert T, Parra EJ, Moore LG, Shriver MD. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet 6: e1001116, 2010. doi: 10.1371/journal.pgen.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Foll M, Gaggiotti OE, Daub JT, Vatsiou A, Excoffier L. Widespread signals of convergent adaptation to high altitude in Asia and america. Am J Hum Genet 95: 394–407, 2014. doi: 10.1016/j.ajhg.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heinrich EC, Wu L, Lawrence ES, Cole AM, Anza-Ramirez C, Villafuerte FC, Simonson TS. Genetic variants at the EGLN1 locus associated with high-altitude adaptation in Tibetans are absent or found at low frequency in highland Andeans. Ann Hum Genet 83: 171–176, 2019. doi: 10.1111/ahg.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Julian CG. Epigenomics and human adaptation to high altitude. J Appl Physiol (1985) 123: 1362–1370, 2017. doi: 10.1152/japplphysiol.00351.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Childebayeva A, Goodrich JM, Leon-Velarde F, Rivera-Chira M, Kiyamu M, Brutsaert TD, Dolinoy DC, Bigham AW. Genome-wide epigenetic signatures of adaptive developmental plasticity in the Andes. Genome Biol Evol 13: evaa239, 2021. doi: 10.1093/gbe/evaa239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Childebayeva A, Jones TR, Goodrich JM, Leon-Velarde F, Rivera-Chira M, Kiyamu M, Brutsaert TD, Dolinoy DC, Bigham AW. LINE-1 and EPAS1 DNA methylation associations with high-altitude exposure. Epigenetics 14: 1–15, 2019. doi: 10.1080/15592294.2018.1561117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hancco I, Bailly S, Baillieul S, Doutreleau S, Germain M, Pepin JL, Verges S. Excessive erythrocytosis and chronic mountain sickness in dwellers of the highest city in the world. Front Physiol 11: 773, 2020. doi: 10.3389/fphys.2020.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.West JB. Highest permanent human habitation. High Alt Med Biol 3: 401–407, 2002. doi: 10.1089/15270290260512882. [DOI] [PubMed] [Google Scholar]
- 102.Oberholzer L, Lundby C, Stauffer E, Ulliel-Roche M, Hancco I, Pichon A, Lundby AM, Villafuerte FC, Verges S, Robach P. Reevaluation of excessive erythrocytosis in diagnosing chronic mountain sickness in men from the world’s highest city. Blood 136: 1884–1888, 2020. doi: 10.1182/blood.2019004508. [DOI] [PubMed] [Google Scholar]
- 103.Leon-Velarde F, Arregui A. Desadaptacion a la Vida en las Grandes Alturas. Lima, Peru: Institut Français d’Etudes Andines (IFEA), 1994, p. 145. [Google Scholar]
- 104.Monge-M C. Sobre un caso de enfermedad de Vaquez. In: Comunicacion Presentada a la Academia Nacional de Medicina. Lima, Peru: Sanmarti y Cía, 1925. [Google Scholar]
- 105.Monge-M C. La enfermedad de los Andes. In: Anales de la Facultad de Medicina. Lima, Peru: Universidad de Lima, 1928, p. 1-–309. [Google Scholar]
- 106.Monge-M C. Life in the Andes and Chronic Mountain Sickness. Science 95: 79–84, 1942. doi: 10.1126/science.95.2456.79. [DOI] [PubMed] [Google Scholar]
- 107.Monge-M C. Chronic Mountain Sickness. Physiol Rev 23: 166–184, 1943. doi: 10.1152/physrev.1943.23.2.166. [DOI] [Google Scholar]
- 108.Rivera-Ch M, Leon-Velarde F, Huicho L. Treatment of chronic mountain sickness: critical reappraisal of an old problem. Respir Physiol Neurobiol 158: 251–265, 2007. doi: 10.1016/j.resp.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 109.Winslow RM, Monge CC, Brown EG, Klein HG, Sarnquist F, Winslow NJ, McKneally SS. Effects of hemodilution on O2 transport in high-altitude polycythemia. J Appl Physiol (1985) 59: 1495–1502, 1985. doi: 10.1152/jappl.1985.59.5.1495. [DOI] [PubMed] [Google Scholar]
- 110.Klein H. Isovolemic hemodilution in high-altitude polycythemia. In: Proceedings of the International Symposium on Acclimatization, Adaptation, and Tolerance to High Altitude. Washington, DC: US Department of Health and Human Services, 1983, p. 47–51. [Google Scholar]
- 111.Tymko MM, Tremblay JC, Bailey DM, Green DJ, Ainslie PN. The impact of hypoxaemia on vascular function in lowlanders and high altitude indigenous populations. J Physiol 597: 5759–5776, 2019. doi: 10.1113/JP277191. [DOI] [PubMed] [Google Scholar]
- 112.Bailey DM, Rimoldi SF, Rexhaj E, Pratali L, Salinas Salmon C, Villena M, McEneny J, Young IS, Nicod P, Allemann Y, Scherrer U, Sartori C. Oxidative-nitrosative stress and systemic vascular function in highlanders with and without exaggerated hypoxemia. Chest 143: 444–451, 2013. doi: 10.1378/chest.12-0728. [DOI] [PubMed] [Google Scholar]
- 113.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Ej B. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121: 505–511, 2010. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Corante N, Anza-Ramirez C, Figueroa-Mujica R, Macarlupu JL, Vizcardo-Galindo G, Bilo G, Parati G, Gamboa JL, Leon-Velarde F, Villafuerte FC. Excessive erythrocytosis and cardiovascular risk in Andean highlanders. High Alt Med Biol 19: 221–231, 2018. doi: 10.1089/ham.2017.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.De Ferrari A, Miranda JJ, Gilman RH, Dávila-Román VG, León-Velarde F, Rivera-Ch M, Huicho L, Bernabé-Ortiz A, Wise RA, Checkley W. Prevalence, clinical profile, iron status, and subject-specific traits for excessive erythrocytosis in andean adults living permanently at 3825 meters above sea level. Chest 146: 1327–1336, 2014. doi: 10.1378/chest.14-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jefferson JA, Escudero E, Hurtado ME, Kelly JP, Swenson ER, Wener MH, Burnier M, Maillard M, Schreiner GF, Schoene RB, Hurtado A, Johnson RJ. Hyperuricemia, hypertension, and proteinuria associated with high-altitude polycythemia. Am J Kidney Dis 39: 1135–1142, 2002. doi: 10.1053/ajkd.2002.33380. [DOI] [PubMed] [Google Scholar]
- 117.Miele CH, Schwartz AR, Gilman RH, Pham L, Wise RA, Davila-Roman VG, Jun JC, Polotsky VY, Miranda JJ, Leon-Velarde F, Checkley W. Increased cardiometabolic risk and worsening hypoxemia at high altitude. High Alt Med Biol 17: 93–100, 2016. doi: 10.1089/ham.2015.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gonzales GF, Tapia V. Association of high altitude-induced hypoxemia to lipid profile and glycemia in men and women living at 4,100m in the Peruvian Central Andes. Endocrinol Nutr 60: 79–86, 2013.doi: 10.1016/j.endoen.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 119.Okumiya K, Sakamoto R, Fukutomi E, Kimura Y, Ishimoto Y, Chen WL, Ishikawa M, Hozo R, Otsuka K, Matsubayashi K, Wada T, Inamura T, Lazo M, Lu JP, Garcia PJ. Strong association between polycythemia and glucose intolerance in older adults living at high altitudes in the Andes. J Am Geriatr Soc 59: 1971–1973, 2011. doi: 10.1111/j.1532-5415.2011.03610_8.x. [DOI] [PubMed] [Google Scholar]
- 120.Okumiya K, Sakamoto R, Kimura Y, Ishimoto Y, Wada T, Ishine M, Ishikawa M, Nakajima S, Hozo R, Ge RL, Norboo T, Otsuka K, Matsubayashi K. Strong association between polycythemia and glucose intolerance in elderly high-altitude dwellers in Asia. J Am Geriatr Soc 58: 609–611, 2010. doi: 10.1111/j.1532-5415.2010.02753.x. [DOI] [PubMed] [Google Scholar]
- 121.Sherpa LY, Stigum H, Chongsuvivatwong V, Luobu O, Thelle DS, Nafstad P, Bjertness E. Lipid profile and its association with risk factors for coronary heart disease in the highlanders of Lhasa, Tibet. High Alt Med Biol 12: 57–63, 2011.doi: 10.1089/ham.2010.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bilo G, Acone L, Anza-Ramirez C, Macarlupu JL, Soranna D, Zambon A, Vizcardo-Galindo G, Pengo MF, Villafuerte FC, Parati G, on behalf of HIGHCARE-ANDES Highlanders Study Investigators. Office and ambulatory arterial hypertension in highlanders. Hypertension 76: 1962–1970, 2020. doi: 10.1161/HYPERTENSIONAHA.120.16010. [DOI] [PubMed] [Google Scholar]
- 123.Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta analysis. Am J Hypertens 24: 52–58, 2011. doi: 10.1038/ajh.2010.203. [DOI] [PubMed] [Google Scholar]