Abstract
Purpose
To determine the rate of carriage of multidrug-resistant organisms (MDROs) between 2015 and 2019 among patients admitted to the National Institute of Infectious Diseases “Prof. Dr. Matei Balș,” from Bucharest, Romania.
Methods
Nasal, throat, and rectal/perirectal screening swabs were collected either immediately or during the first 24 hours of admission and sent to the microbiology laboratory where the following MDROs were identified: methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), extended-spectrum β-lactamase (ESBL)-producing Enterobacterales, carbapenem-resistant/carbapenemase-producing Enterobacterales (CRE/CPE), multidrug-resistant/extended drug-resistant Acinetobacter baumannii (MDR/XDR-AB), and multidrug-resistant/extended drug-resistant Pseudomonas aeruginosa (MDR/XDR-PA).
Results
A total of 5083 unique patients were screened for MRSA and 5008 for VRE, ESBL/CRE/CPE, MDR/XDR-AB, and MDR/XDR-PA. MRSA was detected in 8.24% of patients, VRE in 17.67%, ESBL Enterobacterales in 25.85%, and CPE in 6.13%. MDR/XDR-AB was found in 1.59% and MDR/XDR-PA in 1.91% of patients. The rates of carriage increased between 2015 and 2019 for MRSA (7.23–7.6%), VRE (9–16.68%), CPE (1.15–6.77%), MDR/XDR-PA (1.15–1.91%), and MDR/XDR-AB (1.15–2.04%). OXA-48-type carbapenemase was predominant in Klebsiella pneumoniae (68.62%) and Escherichia coli (89.47%). CPE bacteria other than Klebsiella pneumoniae and Escherichia coli identified in our study carried mostly metallo-beta-lactamase (n = 28, 84.85%).
Conclusion
In this study, 37% of the unique patients screened over five years were found to be MDRO carriers. The proportion of VRE and CPE rectal carriers increased significantly between 2015 and 2019. The most frequently isolated carbapenemase was the OXA-48 type.
Keywords: surveillance, carriage, screening, multidrug-resistant organisms, MDRO, Romania
Introduction
Antimicrobial resistance is one of the greatest threats nowadays, and drug-resistant diseases could cause 10 million deaths each year by 2050 if no action is taken.1
Multidrug-resistant organisms (MDROs) are microorganisms that are resistant to several classes of antibiotics, and some of them are responsible for the majority of healthcare-associated infections (HAI) and are capable of “escaping” the actions of antimicrobial agents. These are the ESKAPE pathogens: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.2
In 2017, the World Health Organization (WHO) listed the ESKAPE pathogens among the 12 bacterial species against which new antimicrobials are urgently needed. The following Gram-negative rods appear in the critical priority list of pathogens: carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa, and carbapenem-resistant and/or extended-spectrum β-lactamase (ESBL)-producing Enterobacterales (eg, Klebsiella pneumoniae and Enterobacter spp.). Vancomycin-resistant Enterococcus faecium (VRE) and methicillin-resistant Staphylococcus aureus (MRSA) are on the list of the high-priority group.3
In Europe, over 33,000 deaths and 671,000 infections are attributed to antibiotic-resistant infections (including ESKAPE infections) each year (with 63.5% HAI).4 According to a study carried out in an infectious disease hospital in Romania, out of 4293 bacterial strains isolated between 2016 and 2020, 97% were ESKAPE pathogens.5 In another study conducted in Hungary between 2010 and 2020, 70,099 bacterial strains were tested and the prevalence of ESKAPE pathogens, especially Klebsiella pneumoniae and Pseudomonas aeruginosa, increased during that period.6
The prevalence of severe infections caused by multidrug-resistant organisms (MDROs) has increased constantly over years. International travel, migration, and the transfer of patients from one country to another have increased the risk of spreading these infections; so, HAIs are a cause of the increasing morbidity and mortality worldwide.7 It is difficult to prevent the spread of some resistance determinants that are found in mobile elements, such as ESBL-encoding genes and carbapenemase-encoding genes. These plasmid-encoded resistance genes are transmitted horizontally between various strains of Gram-negative rods, especially among members of the Enterobacterales order, which makes carbapenemase-producing Enterobacterales (CPE) germs of utmost interest, especially from the public health perspective. Also, if infections caused by ESBL-producing bacteria can usually be treated with carbapenems, infections with CPE or carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa are difficult to treat since these microorganisms are resistant to antibiotics of the last line of defense, contributing to high morbidity and mortality rates, especially in healthcare settings.
Infection prevention and control (IPC) is strongly recommended nowadays, and there are national and international guidelines to follow. Some scientists propose the use of active/routine surveillance systems while many others suggest the testing of “at-risk” patients (ie, those with a history of antimicrobial treatment and long-term stay in a healthcare facility) to have cost-effective results of the surveillance and control of HAIs.8–12 Since the main reservoir of Enterobacterales is the gut microbiota, stool samples and rectal swabs are the most suitable specimens for performing screening not only for VRE, ESBL-producing Enterobacterales, and/or carbapenem-resistant/carbapenemase-producing Enterobacterales (CRE/CPE) but also for carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Nasal swabs are used to collect samples to screen for MRSA.13
The microorganisms we are looking for when identifying asymptomatic carriers are the ESKAPE pathogens with specific antimicrobial resistance determinants. Thus, it is important to prevent the spread of such bacteria in healthcare settings because the infections they cause are hard to treat and are often fatal.
Many studies conducted in Romania report highly resistant microorganisms isolated in healthcare facilities, such as Acinetobacter baumannii, Klebsiella pneumoniae, and other carbapenemase-producing Enterobacterales.14–19 However, there is still a paucity of data on asymptomatic carriers of multidrug-resistant organisms (MDROs), and most of the existing ones are either six-month studies or surveys.20–22 Thus, we conducted this study to determine the rate of carriage of MDROs over five years in an infectious disease hospital and to determine the extent to which carbapenem resistance in enterobacteria was determined by carbapenemase production.
Materials and Methods
Study Design
We conducted a cross-sectional study aimed at identifying asymptomatic carriers of MDROs in an infectious disease hospital with 700 beds in Bucharest, Romania. We analyzed the data collected in the microbiology laboratory of this hospital between January 1, 2015, and December 31, 2019. The study was conducted in accordance with the principles of the declaration of Helsinki. The surveillance of throat/nasal and rectal swab cultures were introduced in the routine admission procedures as part of the IPC measures of the National Institute of Infectious Diseases “Prof. Dr. Matei Balș” in Bucharest, Romania, and all patients who participated in the study gave their written informed consent.
All patients who were considered “at-risk” according to our national and facility regulations were screened for MDROs on admission. These include (a) any patient transferred from another healthcare facility (acute or long-term), (b) any patient transferred to the intensive care unit, (c) any patient known to have stayed in a healthcare facility in the last six months, and (d) any patient who underwent antibiotic treatment in the last six months. MDROs included methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), extended-spectrum β-lactamase (ESBL)-producing Enterobacterales, carbapenem-resistant/carbapenemase-producing Enterobacterales (CRE/CPE), multidrug-resistant/extended drug-resistant Acinetobacter baumannii (MDR/XDR-AB), and multidrug-resistant/extended drug-resistant Pseudomonas aeruginosa (MDR/XDR-PA). Multidrug resistance (MDR) was defined as resistance to at least three classes of tested antibiotics and extended drug resistance (XDR) as resistance to all but two classes of antibiotics.23
Laboratory Procedures
Bacterial Isolation and Identification
Simple nasal, throat, and rectal/perirectal screening swabs were collected either immediately or during the first 24 hours of admission and sent to the microbiology laboratory. These specimens were processed according to microbiology laboratory standard operating procedures by plating on screening mediums. Nasal and throat swabs were plated on ChromID MRSA while rectal/perirectal swabs were plated on ChromID VRE and ChromID ESBL (bioMérieux, La Balme-les-Grottes, France) for 18–24 hours at 37°C. Bacterial colonies grown on screening mediums were further identified by matrix-assisted laser desorption ionization-time of flight mass spectrometry (Biotyper, Bruker Daltonics GmbH & Co, Bremen, Germany).
Antimicrobial Susceptibility Testing
Resistance to cefoxitin (for Staphylococcus aureus) and vancomycin (for Enterococcus faecium and Enterococcus faecalis) was detected using cefoxitin (30 μg) and vancomycin (5 μg) (Thermo Scientific™, Oxoid™, UK) via the disk diffusion method according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) standards which did not change over time for these antimicrobials.24
Antimicrobial susceptibility testing (AST) for identifying MDR/XDR-AB and MDR/XDR-PA was performed using an automated system known as the Vitek 2 Compact (bioMérieux, Inc, Hazelwood, Mo, USA).
Confirmation of ESBL Production
The confirmation of the ESBL drug resistance phenotype was done via the double-disk synergy test (DDST) using disks of ceftazidime (10 μg), cefepime (30 μg), and amoxicillin/clavulanic acid (30 μg) (Thermo Scientific™, Oxoid™, UK) according to the EUCAST guidelines for the detection of resistance mechanisms.25 When a zone of synergy between any cephalosporin and amoxicillin-clavulanic acid was observed, ESBL was confirmed. If no inhibition zone was observed around cephalosporin and no synergy zone was seen with the DSST, the strain was tested with a combination of the disk test using tablets containing the cephalosporin alone (cefotaxime, ceftazidime, cefepime – 30 μg each) and clavulanic acid (Total ESBL Confirm Kit, Rosco Diagnostica A/S, Taastrup, Denmark). The test was considered positive if the inhibition zone was ≥ 5 mm larger around the disk with clavulanic acid than around the disk with the cephalosporin alone.
Confirmation of Carbapenemase Production in Enterobacterales
Since there is no guideline on the optimal microbiological method of detecting CRE carriage, any sensitive method that yields good results in a timely manner can be used to facilitate the prompt implementation of IPC measures.10 On the same day as ESBL production, strains were also tested for carbapenem-resistance in Enterobacterales using disks of ertapenem, meropenem, imipenem (10 μg each), and piperacillin-tazobactam (36 μg) performing an AST according to the EUCAST guidelines.26 A screening cut-off value of < 25 mm for meropenem (and meropenem < 28 mm if the strain was resistant to piperacillin-tazobactam) was chosen as an indicator of possible carbapenemase production according to the EUCAST guidelines for the detection of resistance mechanisms.25 To test for carbapenemase production, we used the following neosensitab combinations: meropenem (10 μg), meropenem with boronic acid for detecting Klebsiella pneumoniae carbapenemase (KPC), meropenem with dipicolinic acid for detecting metallo-β-lactamase (MBL), meropenem with cloxacillin (as AmpC inhibitor), and temocillin (30 μg) for detecting oxacillinase type 48 (OXA-48) (KPC, MBL, and OXA-48 Confirm Kit, Rosco Diagnostica A/S, Taastrup, Denmark), per the manufacturer’s instructions.
Statistical Analysis
During data analysis, the ESBL and CRE/CPE categories were mutually exclusive. The rate of MDRO carriage was compared between the first and the last year of the study using the Chi-square test, and a p-value of < 0.05 was considered statistically significant. All p-values were two-tailed.
Results
From January 2015 to December 2019, 5083 unique patients were screened for MRSA and 5008 for VRE, ESBL/CRE/CPE, MDR/XDR-AB, and MDR/XDR-PA. MRSA was detected in 419 (8.2%) individuals, VRE in 885 (17.7%), ESBL Enterobacterales in 1295 (25.9%), and CPE in 307 (6.1%). MDR/XDR-AB was found in 80 (1.6%) and MDR/XDR-PA in 96 (1.9%) of the screened patients.
Temporal Evolution
The temporal evolution of screened patients with positive rectal swabs and MDRO prevalence are presented in Table 1. Table 2 presents the five-year evolution of MRSA carriers.
Table 1.
Rectal Swab-Screened Patients. Trends in VRE, ESBL, CPE, and MDR/XDR Pseudomonas aeruginosa and Acinetobacter baumannii Carriage Between 2015 and 2019
| Period Patients |
Total n (%) | 2015 n (%) | 2016 n (%) | 2017 n (%) | 2018 n (%) | 2019 n (%) | p -value (2015–2019) |
|---|---|---|---|---|---|---|---|
| Screened patients | 5008 | 433 | 704 | 1095 | 1212 | 1564 | ↑ |
| Positive patients | 1853 (37%) | 121 (27.94%) | 236 (33.52%) | 399 (36.43%) | 551 (45.46%) | 546 (34.91%) | ↑ <0.05 |
| VRE+ | 885 (17.67%) | 39 (9%) | 100 (14.2%) | 207 (18.9%) | 278 (22.93%) | 261 (16.68%) | ↑ <0.001 |
| E. coli ESBL+ | 730 (14.57%) | 61 (14.08%) | 109 (15.48%) | 160 (14.61%) | 200 (16.5%) | 200 (12.78%) | ↓ 0.47 |
| E. coli carbapenemase+ | 19 (0.37%) | 0 | 1 (0.14%) | 1 (0.09%) | 9 (0.74%) | 8 (0.51%) | ↑ 0.19 |
| K. pneumoniae ESBL+ | 408 (8.14%) | 33 (7.62%) | 63 (8.94%) | 97 (8.85%) | 98 (8.08%) | 117 (7.48%) | ↓ 0.92 |
| K. pneumoniae carbapenemase+ | 255 (5.09%) | 5 (1.15%) | 17 (2.41%) | 64 (5.84%) | 91 (7.5%) | 78 (4.98%) | ↑ <0.001 |
| Other ESBL+ Enterobacterales | 157 (3.13%) | 19 (4.38%) | 16 (2.27%) | 57 (5.2%) | 18 (1.48%) | 47 (3%) | ↓ 0.15 |
| Other carbapenemase+ Enterobacterales | 33 (0.65%) | 0 | 1 (0.14%) | 5 (0.45%) | 7 (0.57%) | 20 (1.27%) | ↑ <0.05 |
| P. aeruginosa MDR/XDR | 96 (1.91%) | 5 (1.15%) | 15 (2.13%) | 14 (1.27%) | 32 (2.64%) | 30 (1.91%) | ↑ 0.28 |
| A. baumannii MDR/XDR | 80 (1.59%) | 5 (1.15%) | 12 (1.7%) | 10 (0.91%) | 21 (1.73%) | 32 (2.04%) | ↑ 0.22 |
Notes: + = positive, ↑= increase, ↓= decrease, numbers in bold means statistically significant.
Abbreviations: n, number; VRE, vancomycin-resistant enterococci; ESBL, extended-spectrum β-lactamase; CPE, carbapenemase-producing Enterobacterales; MDR/XDR, multidrug resistant/extended drug resistant.
Table 2.
Trends in the Proportion of MRSA Carriers Between 2015 and 2019
| Period Patients |
Total n (%) | 2015 n (%) | 2016 n (%) | 2017 n (%) | 2018 n (%) | 2019 n (%) | p -value (2015–2019) |
|---|---|---|---|---|---|---|---|
| Screened patients | 5083 | 470 | 738 | 1093 | 1218 | 1564 | ↑ |
| MRSA positive | 419 (8.24%) | 34 (7.23%) | 44 (5.96%) | 119 (10.89%) | 103 (8.45%) | 119 (7.6%) | ↑ 0.78 |
Note: ↑= increase.
Abbreviations: n, number; MRSA, methicillin resistant Staphylococcus aureus.
The rates increased between 2015 and 2019 for MRSA, VRE, CPE, MDR/XDR-PA, and MDR/XDR-AB but not for ESBL Enterobacterales. The trend was undulating over the years, except for CPE other than Escherichia coli and Klebsiella pneumoniae, for which the trend was continuously increasing. It is worth noting that in 2015, only isolates of Klebsiella pneumoniae were detected as being carbapenemase-producing among Enterobacterales bacteria (n = 5/433, 1.2%). The increasing trend in the proportion of VRE and CPE rectal carriers between 2015 and 2019 was statistically significant (Table 1).
Carbapenemase Types
Carbapenemase types are presented in Table 3. OXA-48-type carbapenemase was predominant in Klebsiella pneumoniae (n = 175/255, 68.6%) and Escherichia coli (n = 17/19, 89.4%). The CPE bacteria other than Klebsiella pneumoniae and Escherichia coli identified in our study were Enterobacter cloacae, Enterobacter kobei, Klebsiella aerogenes, Providencia stuartii, Morganella morganii, Serratia marcescens, and Proteus mirabilis. For all these microorganisms, MBL was the most isolated carbapenemase (n = 28/33, 84.9%).
Table 3.
Carbapenemase-Producing Microorganisms and Carbapenemase Types
| Carba Bacteria |
OXA-48 n (%) | MBL n (%) | KPC n (%) | MBL+KPC n (%) | OXA-48 +MBL n (%) | OXA-48 +KPC+MBL n (%) |
|---|---|---|---|---|---|---|
| Kpn | 175 | 28 | 35 | 1 | 13 | 3 |
| Eco | 17 | 1 | 1 | |||
| Ecl | 2 | 6 | ||||
| Ekob | 1 | |||||
| Kaer | 1 | |||||
| Pst | 19 | 1 | ||||
| Mmo | 1 | |||||
| Pmir | 1 | |||||
| Sma | 1 | |||||
| Total (307) | 195 (63.52%) | 57 (18.57%) | 36 (11.73%) | 2 (0.65%) | 14 (4.56%) | 3 (0.97%) |
Abbreviations: n, number; OXA, oxacillinase; MBL, metallo-β-lactamase; KPC, Klebsiella pneumoniae carbapenemase; Kpn, Klebsiella pneumoniae; Eco, Escherichia coli; Ecl, Enterobacter cloacae; Ekob, Enterobacter kobei; Kaer, Klebsiella aeruginosa; Pst, Providencia stuartii; Mmo, Morganella morganii; Pmir, Proteus mirabilis; Sma, Serratia marcescens.
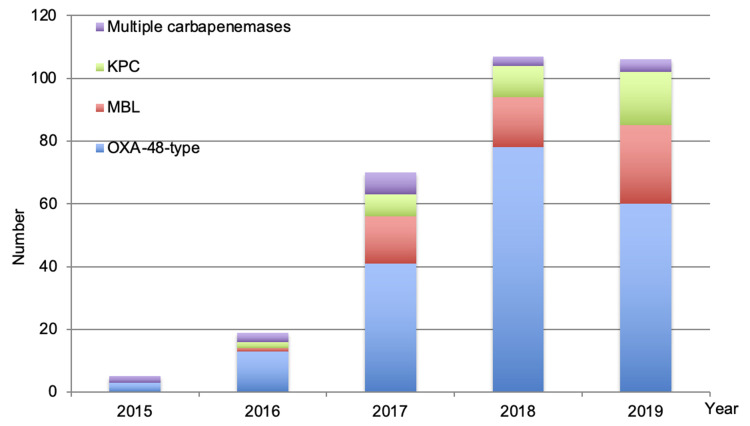
Figure 1 presents the evolution of carbapenemases over time. The OXA-48 type of carbapenemase increased significantly over the years from 3 in 2015 to 60 in 2019 (p = 0.006) while MBLs and KPCs also increased from 2016 to 2019; however, the increase was not statistically significant.
Figure 1.
Carbapenemases evolution over time (2015–2019).
Abbreviations: OXA, oxacillinase; MBL, metallo-β-lactamase; KPC, Klebsiella pneumoniae carbapenemase.
Discussion
We conducted this study to determine the rate of carriage of MDROs over five years and to determine the extent to which carbapenem resistance in enterobacteria was determined by carbapenemase production. Our results showed an overall rate of MRSA carriage of 8.2% and MDROs rectal carriage of 37%. The proportion of CPE carriers was 6.1% in five years, with Klebsiella pneumoniae as the predominant carbapenemase-producing strain (83.06%). OXA-48-type was the most isolated carbapenemase (69.05%), followed by MBL (24.75%).
According to our results, 37% of the unique patients screened over five years tested positive for rectal swabs, which is higher than the 23% reported by van Hout et al.27 We observed an increasing trend in the proportion of positive rectal-screened patients between 2015 and 2019; however, this may be due to the increased compliance with national and facility regulations for active screening of at-risk patients admitted to our hospital.
The overall rate of MRSA carriage in this study was 8.2%, ranging from 6% (44/738) in 2016 to 10.9% (119/1093) in 2017, which is much less than the rates in other studies conducted in Northeastern Romania where the proportion of carriers was 34% (17/50) in one year (2012); however, our findings are similar to those of a study conducted over six months in 2014 in a hospital in Central Romania, which reported a rate of 11.8%.20,28 MRSA prevalence at admission in this study was higher than the prevalence reported by studies conducted in other Northwestern European countries, the United States (range: 0.13% to 7.3%), smaller than that reported by a one-day point-prevalence survey conducted in Albania (14.2%), and similar to that of a study carried out in Tanzania in 2017 (8.5%).29–34
The proportion of VRE carriers was almost 18% in our study, which is lower than the 29.4% reported in a hospital in Central Romania but similar to the VRE colonization rate in Western European countries: 24.1% in the United Kingdom and 23.8% in Germany.20,35,36
Overall, the proportion of ESBL Enterobacterales carriers was approximately 26%, which is higher than the proportions reported by Vidal-Navarro et al in a French hospital (15.8%) and Platteel et al in a Dutch hospital (8.2%); however, it is lower than those reported by studies conducted in Guinea-Bissau (32.6%), Albania (41.3%), and Morocco (42.9%).33,37–40 The higher proportion of ESBL carriers in Romania than in other Western European countries may be because Romania has a high rate of ESBL carriage in its healthy population, which was 28% in a study by Rodríguez-Molina et al.41
The overall proportion of CPE carriers in our study was 6.1% (307 out of 5008) in five years, which is similar to the findings of a study conducted in France (5.3%) by Vidal-Navarro et al.37 This is the first study conducted in Romania on asymptomatic carriers of MDROs; so, we do not have other local data to compare our findings to; however, in a pilot study conducted in 2015 in three other hospitals, the CPE samples (including rectal swabs) were 22 out of 522 (4.2%) in a hospital in the North-Central part of the country and 28 out of 187 (15%) in a hospital in Northeast Romania.21
The predominant CPE in our study was Klebsiella pneumoniae, and OXA-48-type was the most isolated carbapenemase (2/3), a finding that is similar to those of another study by López-González et al in Spain (56.7%).42
The prevalence of fecal contamination with MDR/XDR-AB (1.5%) and MDR/XDR-PA (2%) seems not to be a major problem for now; however, in another hospital in Bucharest, carbapenem-resistant Pseudomonas spp, and Acinetobacter baumannii were both isolated in 3.6% of patients in the first semester of 2019.22 In a study conducted in a community in the United Kingdom and The Netherlands in 2005, 0.8% of Acinetobacter spp. carriage in Leiden and 1% in Nottingham were reported. The authors concluded that the human intestine is not a major community reservoir for this microorganism. However, in the ICU, a study conducted in Spain by Corbella et al reported 41% fecal colonization with multidrug-resistant Acinetobacter baumannii.43,44
Conclusion
The continuously increasing proportion of CPE other than Klebsiella pneumoniae or Escherichia coli is worrisome because it may be due to genetic recombination and the acquisition of new resistance gene determinants by other bacteria found in the intestinal tract, the reservoir of Enterobacterales. Implementing a program of surveillance of “at-risk” patients followed by IPC is necessary to reduce the spread of MDROs and, subsequently, HAI, especially with ESKAPE pathogens.
Acknowledgments
We thank our collaborators, Ioana Bădicuț, Emilia Bălulescu, and Andreea Mihaela Sandu, for collecting the data. We are grateful to the Romanian Society of Microbiology for helping to publish this paper.
Funding Statement
The authors received funding for publication from the Romanian Society of Microbiology.
Abbreviations
MDRO, multidrug-resistant organism; ESKAPE, Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci; ESBL, extended-spectrum β-lactamase; CRE/CPE, carbapenem-resistant/carbapenemase-producing Enterobacterales; MDR/XDR-AB, multidrug-resistant/extended drug-resistant Acinetobacter baumannii; MDR/XDR-PA, multidrug-resistant/extended drug-resistant Pseudomonas aeruginosa; HAI, healthcare-associated infections; IPC, infection prevention and control; ICU, intensive care unit; EUCAST, European Committee on Antimicrobial Susceptibility Testing; DDST, double-disk synergy test; AST, antimicrobial susceptibility testing; OXA, oxacillinase; KPC, Klebsiella pneumoniae carbapenemase; MBL, metallo-β-lactamase.
Data Sharing Statement
All relevant data and methods are reported in the main text. Additional datasets used during this study are available from the corresponding author upon reasonable request.
Ethical Approval
This study, which was conducted in accordance with the principles of the Declaration of Helsinki, was approved by the Ethics Committee of the National Institute of Infectious Diseases “Prof. Dr. Matei Balș,” Bucharest, Romania, by decision number C09689/22.07.2021. The Ethics Committee did not require patients’ informed consent because it was given on admission to the hospital.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
- 1.UN Ad hoc Interagency Coordinating Group on Antimicrobial Resistance. New report calls for urgent action to avert antimicrobial resistance crisis. Available from: https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis. Accessed March 22, 2022.
- 2.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197:1079–1081. doi: 10.1086/533452 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO priority pathogens list for R&D of new antibiotics. Available from: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. Accessed March 22, 2022.
- 4.Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbune M, Gurau G, Niculet E, et al. Prevalence of antibiotic resistance of ESKAPE pathogens over five years in an infectious diseases hospital from south-east of Romania. Infect Drug Resist. 2021;14:2369–2378. doi: 10.2147/IDR.S312231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orosz L, Lengyel G, Ánosi N, et al. Changes in resistance pattern of ESKAPE pathogens between 2010 and 2020 in the clinical center of University of Szeged, Hungary. Acta Microbiol Immunol Hung. 2022;69(1):27–34. doi: 10.1556/030.2022.01640 [DOI] [PubMed] [Google Scholar]
- 7.Mutters NT, Tacconelli E. Infection prevention and control in Europe – the picture in the mosaic. Clin Microbiol Infect. 2015;21:1045–1046. doi: 10.1016/j.cmi.2015.06.012 [DOI] [PubMed] [Google Scholar]
- 8.Tacconelli E, Cataldo MA, Dancer SJ, et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014;20(Suppl. 1):1–55. doi: 10.1111/1469-0691.12427 [DOI] [PubMed] [Google Scholar]
- 9.Centers of Disease Control and Prevention. Management of multidrug-resistant organisms in healthcare settings; 2017:1–74. Available from: https://www.cdc.gov/infectioncontrol/guidelines/mdro/. Accessed August 31, 2021.
- 10.Magiorakos AP, Burns K, Rodríguez Baño J, et al. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: guidance from the European Centre for Disease Prevention and Control. Antimicrob Resist Infect Control. 2017;6:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in health care facilities. World Health Organization; 2017. Available from: https://apps.who.int/iris/handle/10665/259462. Accessed August 31, 2021. [PubMed] [Google Scholar]
- 12.European Centre for Disease Prevention and Control. Carbapenem-resistant Enterobacteriaceae. Stockholm: ECDC; 2019:1–17. [Google Scholar]
- 13.Biehl LM, Bertz H, Bogner J, et al. Screening and contact precautions – a survey on infection control measures for multidrug-resistant bacteria in German university hospitals. Antimicrob Resist Infect Control. 2017;6:37. doi: 10.1186/s13756-017-0191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surleac M, Czobor Barbu IC, Paraschiv S, et al. Whole genome sequencing snapshot of multidrug resistant Klebsiella pneumoniae strains from hospitals and receiving wastewater treatment plants in Southern Romania. PLoS One. 2020;15(1):e0228079. doi: 10.1371/journal.pone.0228079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gheorghe I, Czobor Barbu I, Surleac M, et al. Subtypes, resistance and virulence platforms in extended-drug resistant Acinetobacter baumannii Romanian isolates. Springer Nature. 2021;11:13288. doi: 10.1038/s41598-021-92590-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Székely E, Damjanova I, Jánvári L, et al. First description of blaNDM-1, blaOXA-48, blaOXA-181 producing Enterobacteriaceae strains in Romania. Int J Med Microbiol. 2013;303(8):697–700. doi: 10.1016/j.ijmm.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 17.Lixandru EL, Cotar AI, Straut M, et al. Carbapenemase-producing Klebsiella pneumoniae in Romania: a six-month survey. PLoS One. 2015;10(11):e0143214. doi: 10.1371/journal.pone.0143214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rafila A, Talapan D, Dorobăț OM, et al. Emergence of carbapenemase-producing Enterobacteriaceae, a public health threat: a Romanian infectious disease hospital based study. Rev Romana Med Lab. 2015;23(3):295–301. [Google Scholar]
- 19.Popa LI, Gheorghe I, Barbu IC, et al. Multidrug resistant Klebsiella pneumoniae ST101 clone survival chain from inpatients to hospital effluent after chlorine treatment. Front Microbiol. 2021;11:610296. doi: 10.3389/fmicb.2020.610296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lupșe M, Flonta M, Herbel L, et al. Is carriage of multidrug-resistant organisms a risk factor for nosocomial infections in an infectious diseases ICU? [abstract]. Critical Care. 2015;19(Suppl 1):S32 (P93). doi: 10.1186/cc14173 [DOI] [Google Scholar]
- 21.Timofte D, Panzaru CV, Maciuca IE, et al. Active surveillance scheme in three Romanian hospitals reveals a high prevalence and variety of carbapenemase-producing Gram-negative bacteria: a pilot study, December 2014 to May 2015. Euro Surveil. 2016;21(25):30262. doi: 10.2807/1560-7917.ES.2016.21.25.30262 [DOI] [PubMed] [Google Scholar]
- 22.Nedelcu NI, Iacob CP. Predictors for MDRO carriage in adult patients of a infectious diseases clinic from Bucharest, Romania. Int J Infect Dis. 2020;101(1):25–26. doi: 10.1016/j.ijid.2020.09.103 [DOI] [Google Scholar]
- 23.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 24.The European Committee on Antimicrobial Susceptibility Testings. Breakpoint tables for interpretation of MICs and zone diameters. EUCAST, version 5.0-9.0, 2015-2019. Available from: https://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/. Accessed September 9, 2021.
- 25.The European Committee on Antimicrobial Susceptibility Testing. EUCAST guidelines for detection of resistance mechanisms and specific resistance of clinical and/or epidemiological importance. EUCAST, version 2.0; 2017. Available from: https://www.eucast.org/resistance_mechanisms/. Accessed September 9, 2021.
- 26.Matuschek E, Brown DFJ, Kahlmeter G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin Microbiol Infect. 2014;20:255–266. doi: 10.1111/1469-0691.12373 [DOI] [PubMed] [Google Scholar]
- 27.van Hout D, Bruijning-Verhagen P, Blok H, Troelstra A, Bonten M. Universal risk assessment upon hospital admission for screening of carriage with multidrug-resistant microorganisms (MDRO) in a Dutch tertiary care centre (2016–2019). J Hosp Infect. 2021;109:32–39. doi: 10.1016/j.jhin.2020.12.007 [DOI] [PubMed] [Google Scholar]
- 28.Monecke S, Müller E, Dorneanu OS, Vremera T, Ehricht R. Molecular typing of MRSA and of clinical Staphylococcus aureus isolates from Iași, Romania. PLoS One. 2014;9(5):e97833. doi: 10.1371/journal.pone.0097833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hidron AI, Kourbatova EV, Halvosa JS, et al. Risk factors for colonization with methicillin-resistant Staphylococcus aureus (MRSA) in patients admitted to an urban hospital: emergence of community-associated MRSA nasal carriage. Clin Infect Dis. 2005;41:159–166. doi: 10.1086/430910 [DOI] [PubMed] [Google Scholar]
- 30.Cunha BA, Schoch P, Abruzzo ED. Clinical- and cost-ineffectiveness of targeted methicillin-resistant Staphylococcus aureus screening of high-risk patients admitted to a low-prevalence teaching hospital. Am J Infect Control. 2013;41:1136–1146. doi: 10.1016/j.ajic.2013.03.297 [DOI] [PubMed] [Google Scholar]
- 31.Otter JA, Herdman MT, Williams B, Tosas O, Edgeworth JD, French GL. Low prevalence of methicillin-resistant Staphylococcus aureus carriage at hospital admission: implications for risk-factor-based vs universal screening. J Hosp Infect. 2013;83:114–121. doi: 10.1016/j.jhin.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 32.Weterings V, Veenemans J, van Rijen M, Kluytmans J. Prevalence of nasal carriage of methicillin-resistant Staphylococcus aureus in patients at hospital admission in The Netherlands, 2010–2017: an observational study. Clin Microbiol Infect. 2019;25:1428e1–1428e5. doi: 10.1016/j.cmi.2019.03.012 [DOI] [PubMed] [Google Scholar]
- 33.Parascandalo FA, Zarb P, Tartari E, et al. Carriage of multidrug-resistant organisms in a tertiary university hospital in Albania – a point prevalence survey. Antimicrob Resist Infect Control. 2016;5:29. doi: 10.1186/s13756-016-0128-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joachim A, Moyo SJ, Nkinda L, et al. Prevalence of methicillin-resistant Staphylococcus aureus carriage on admission among patients attending regional hospitals in Dar es Salaam, Tanzania. BMC Res Notes. 2017;10:417. doi: 10.1186/s13104-017-2668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson HJ, Khokhar F, Enoch DA, et al. Point-prevalence survey of carbapenemase-producing Enterobacteriaceae and vancomycin-resistant enterococci in adult inpatients in a university teaching hospital in the UK. J Hosp Infect. 2018;100:35–39. doi: 10.1016/j.jhin.2018.06.024 [DOI] [PubMed] [Google Scholar]
- 36.Chhatwal P, Ebadi E, Thol F, et al. Prospective infection surveillance and systematic screening for vancomycin-resistant enterococci in hematologic and oncologic patients – findings of a German tertiary care center. J Glob Antimicrob Resist. 2020;22:102–105. doi: 10.1016/j.jgar.2020.02.012 [DOI] [PubMed] [Google Scholar]
- 37.Vidal-Navarro L, Pfeiffer C, Bouziges N, Sotto A, Lavigne JP. Faecal carriage of multidrug-resistant Gram-negative bacilli during a non-outbreak situation in a French university hospital. J Antimicrob Chemother. 2010;65:2455–2458. doi: 10.1093/jac/dkq333 [DOI] [PubMed] [Google Scholar]
- 38.Platteel TN, Leverstein-van Hall MA, Cohen Stuart JW, et al. Predicting carriage with extended-spectrum beta-lactamase-producing bacteria at hospital admission: a cross-sectional study. Clin Microbiol Infect. 2015;21:141–146. doi: 10.1016/j.cmi.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 39.Isendahl J, Turlej-Rogacka A, Manjuba C, Rodrigues A, Giske CG, Nauclér P. Fecal carriage of ESBL-producing E. coli and K. pneumoniae in children in Guinea-Bissau: a hospital-based cross-sectional study. PLoS One. 2012;7(12):e51981. doi: 10.1371/journal.pone.0051981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Girlich D, Bouihat N, Poirel L, Benouda A, Nordmann P. High rate of faecal carriage of extended-spectrum β-lactamase and OXA-48 carbapenemase-producing Enterobacteriaceae at a university hospital in Morocco. Clin Microbiol Infect. 2014;20:350–354. doi: 10.1111/1469-0691.12325 [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez-Molina D, Berglund F, Blaak H, et al. Carriage of ESBL-producing Enterobacterales in wastewater treatment plant workers and surrounding residents – the AWARE Study. Eur J Clin Microbiol Infect Dis. 2021:1–16. doi: 10.1007/s10096-021-04387-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.López-González L, Viñuela-Prieto JM, Rodriguez-Avial I, Manzano R, Candel FJ. Description of carbapenemase-producing Enterobacteriaceae isolates in a Spanish tertiary hospital. Epidemiological analysis and clinical impact. Rev Esp Quimioter. 2019;32(3):254–262. [PMC free article] [PubMed] [Google Scholar]
- 43.Dijkshoorn L, van Aken E, Shunburne L, et al. Prevalence of Acinetobacter baumannii and other Acinetobacter spp. in faecal samples from non-hospitalised individuals. Clin Microbiol Infect. 2005;11:329–332. doi: 10.1111/j.1469-0691.2005.01093.x [DOI] [PubMed] [Google Scholar]
- 44.Corbella X, Pujol M, Ayats J, et al. Relevance of digestive tract colonization in the epidemiology of nosocomial infections due to multiresistant Acinetobacter baumannii. Clin Infect Dis. 1996;23:329–334. doi: 10.1093/clinids/23.2.329 [DOI] [PubMed] [Google Scholar]