Abstract
Escherichia coli, total coliforms, fecal coliforms, and sulfite-reducing anaerobic spore formers from different polluted sites in a tropical environment were determined in order to test for their indication ability for fecal contamination. Quantification of E. coli contamination with Chromocult coliform agar proved to be efficient and feasible for determining fecal pollutions in the investigated area within 24 h. The other microbial parameters showed a lower ability to differentiate sites and cannot be recommended for monitoring fecal pollution in the studied tropical surface waters.
As a means for assessing fecal pollution in environmental freshwaters in temperate regions like Europe and North America, the determination of fecal indicators, such as fecal coliforms (FC) or Escherichia coli, is widely accepted (25). In contrast, application of these monitoring techniques in tropical countries has yielded questionable results, and the indication value of such parameters is doubted (5, 6, 12, 13, 15, 16, 20, 22–24). There is some evidence that standard fecal indicators (e.g., FC) may originate from sources other than enteric ones, survive significantly longer in tropical waters than in temperate ones, or even become part of the aquatic microbial community (13, 25). However, comprehensive investigations which take into account the indication value of microbial indicators for fecal pollution in tropical regions are scarce (13).
Like many developing nations, Uganda faces a high population density accompanied by a relatively poor infrastructure. Especially in the urban centers, the available sanitary facilities cannot sustain the population, leading to contamination of surface water sources with fecal material. As waterborne diseases such as cholera and typhoid fever have been rampant (18), there is need for appropriate, cheap, and feasible methods of detecting fecal contamination. The aim of this work was to analyze and compare the discrimination ability of different internationally recommended microbial indicators for fecal pollution on an existing contamination gradient (from highly polluted waters to waters of minimal human impact) in the area of Kampala, Uganda.
Study area and sampling.
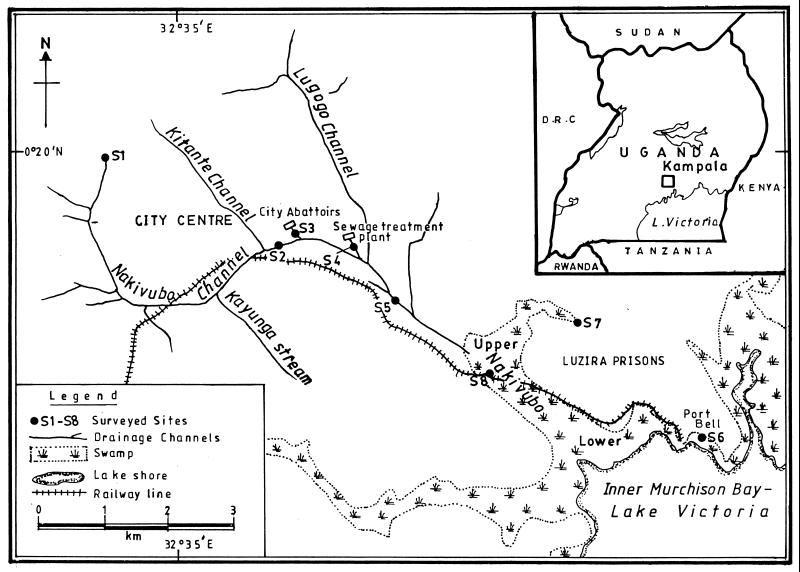
The main study area, the Nakivubo channel, is a manmade stream that drains Kampala and its suburbs. It discharges into Lake Victoria at the Inner Murchison Bay (Fig. 1). The channel receives raw sewage from slums, industrial effluents, and discharge from a sewage treatment plant and from a complex of slaughterhouses. The Nakivubo channel passes through a swamp before discharging into the lake. Eight sampling sites were selected (Fig. 1). Four sites were chosen along the channel. Station S1, the source of the channel, receives domestic waste, residential sewage, and discharge from Makerere Kivulu, a slum of the capital. Station S2 is located downstream of the city center and is influenced mainly by commercial, industrial, and residential establishments. Station S5 is downstream of two main effluents, one from the abattoirs which discharges a mixture of untreated animal waste and water (S3) and the other from a sewage treatment plant with a trickling filter mechanism (S4). Station S8 is located at a railway crossing, after the Nakivubo channel has crossed the upper Nakivubo swamp. The two other sites are a protected water spring (S7) and a site on the shores of Lake Victoria at Port Bell (S6). Six samples were taken at each location from July to September 1998 (twice a month), during a time of year when a mixture of rainy and dry patterns is evident.
FIG. 1.
Map showing study area and sampling sites. D.R.C., Democratic Republic of Congo.
Chemophysical parameters.
Electrical conductivity (EC) and temperature were measured in situ with an LF-96 calibrated at 25°C (WTW, Vienna, Austria). Five days' biochemical oxygen demand (BOD), pH, and total suspended solids (TSS) were determined in the laboratory according to American Public Health Association standards (2).
Bacteriological parameters.
All samples were collected in sterile glass bottles, immediately placed into dark cooling boxes, and processed within 6 h of collection. The most-probable-number (MPN) technique (2), with five tubes per water sample dilution (10−1 to 10−7), was used for total coliforms (TCM), FC, and sulfite-reducing anaerobic spore formers (SASF) by using lauryl sulfate broth, EC medium, and differential reinforced clostridial medium broth (all media from Merck, Darmstadt, Germany), respectively (2, 11). Bottles (10 ml) containing the media and inverted Durham tubes (for TCM and FC) were inoculated with 1-ml volumes of the respective dilutions. TCM bottles were incubated at 37°C in a dry incubator; FC bottles were incubated at 44°C in a water bath. Gas and turbidity production within 48 h was considered to be a positive response. For detection of SASF the inoculated differential reinforced clostridial media were covered with a paraffin oil layer (4 mm) and pasteurized for 25 min at 75°C, followed by incubation at 37°C for 2 days. Bottles that turned black due to sulfite reduction were considered to contain samples that were positive for SASF (11). The surface plate technique was used for simultaneous detection of total coliforms (TCC) and E. coli with Chromocult coliform agar (CCA) (1, 10, 19) which was enriched with 5 mg of cefsulodin (Sigma, Vienna, Austria) per ml. Portions (100 μl) of the respective sample dilutions (10−1 to 10−4) were applied to plates (triplicate plates for each dilution step) and incubated at 37°C for 24 h. Pink colonies resulting from salmon-galactoside cleavage by β-d-galactosidase were classified as TCC, whereas dark blue colonies resulting from salmon-galactoside and X-glucuronide cleavage by β-d-galactosidase and β-d-glucuronidase were classified as presumptive E. coli colonies. For samples of S6 and S7, 100-ml sample enrichments were performed by means of membrane filtration with cellulose nitrate filters (pore size, 0.45 μm; Sartorius, Vienna, Austria). The membranes were placed on CCA plates and incubated as described above.
Chemophysical sampling site characterization.
The sampling sites showed distinct patterns of EC, TSS, and BOD values (Table 1), differing significantly from each other (P < 0.001; by the Kruskal-Wallis test, n = 3 × 8 × 6). In addition, a significant correlation between EC, TSS, and BOD values was observed (Table 2). Temperature and pH values were uniform for all sampling stations, except that the pH values of the protected spring water site were lower, most likely due to CO2 saturation (Table 1). The pronounced differences of EC, TSS, and BOD values at the respective sampling sites correspond highly to infrastructural conditions and to the various kinds of usage of the water. As a consequence, sampling sites could be ranked in the following sequence, with habitats showing a gradual decrease in the level of anthropogenic influence: S3 and S4 (slaughterhouse and sewage treatment plant effluent, respectively) > S1 (channel source) > S2 and S5 (main channel stations) > S8 (channel station after swamp) > S7 and S8 (protected spring and Lake Victoria shore site, respectively). This ranking was the basis for testing the discrimination ability of selected microbial indicators for fecal pollution.
TABLE 1.
Chemophysical sampling site characterizationa
| Sampling site | WTEMP (°C)
|
pH
|
EC (μS/cm)
|
TSS concn (mg/dm3)
|
BOD (mg of O2/dm3)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | R | M | R | M | R | M | R | M | R | |
| S1 | 23.9 | 23.4–25.3 | 6.4 | 6.1–6.5 | 465 | 421–620 | 950 | 716–1,385 | 917 | 353–1,428 |
| S2 | 24.6 | 23.6–24.9 | 6.6 | 6.5–6.9 | 371 | 323–387 | 84 | 26–320 | 77 | 48–181 |
| S3 | 24.9 | 24.0–25.0 | 6.2 | 6.3–6.9 | 741 | 632–850 | 1,090 | 770–6,820 | 2,040 | 1,000–3,823 |
| S4 | 24.6 | 24.2–24.8 | 6.9 | 6.9–7.1 | 786 | 654–896 | 69 | 41–222 | 132 | 78–338 |
| S5 | 24.1 | 23.5–24.9 | 6.7 | 6.4–6.9 | 382 | 343–462 | 100 | 77–180 | 74 | 8.8–125 |
| S6 | 25.6 | 24.3–25.6 | 6.4 | 6.1–6.5 | 109 | 90–290 | 13 | 6.2–47 | 27 | 8.8–91 |
| S7 | 24.0 | 23.9–24.1 | 5.4 | 5.2–5.7 | 102 | 85–111 | 2.5 | 1.6–8.5 | 16 | 2.9–35 |
| S8 | 23.1 | 22.7–23.3 | 6.4 | 6.1–6.5 | 465 | 454–550 | 17 | 8.8–29 | 29 | 5.9–36 |
Values are the results of testing of six samples. Abbreviations: M, median; R, range; WTEMP, water temperature; EC, electric conductivity; TSS, total suspended solids; BOD, 5 days' biochemical oxygen demand. Sampling sites: S1, channel source; S2, channel before abattoirs' effluent; S3, abattoirs' effluent; S4, sewage treatment plant effluent; S5, channel after effluent loads; S6, Lake Victoria shore site; S7, protected spring; S8, channel after crossing part of the swamp.
TABLE 2.
Correlation half-matrix of EC, TSS, BOD, and microbial indicatorsa
| Parameter | Spearman's rank correlation coefficient for:
|
||||||
|---|---|---|---|---|---|---|---|
| EC | BOD | TSS | TCC | TCM | E. coli | FC | |
| EC | 1.00 | ||||||
| BOD | 0.65 | 1.00 | |||||
| TSS | 0.62 | 0.82 | 1.00 | ||||
| TCC | 0.70 | 0.83 | 0.82 | 1.00 | |||
| TCM | 0.58 | 0.65 | 0.65 | 0.79 | 1.00 | ||
| E. coli | 0.78 | 0.79 | 0.68 | 0.87 | 0.78 | 1.00 | |
| FC | 0.56 | 0.60 | 0.63 | 0.74 | 0.74 | 0.85 | 1.00 |
| SASF | 0.62 | 0.56 | 0.67 | 0.64 | 0.64 | 0.61 | 0.68 |
Spearman's rank correlation coefficient (r) of 48 samples per parameter. All values are significant (P < 0.05). See Table 1 and text for explanations of abbreviations.
Discrimination ability of fecal pollution indicators.
Microbial indicator concentrations observed at the different polluted sites are given in Table 3. Although all indicator concentrations correlated significantly with EC, TSS, and BOD values (Table 2), remarkable differences in the fecal pollution indicator discrimination potential between selected sampling sites could be detected. Pairwise comparisons of detectable and nondetectable differences between representative sampling sites or habitat types (i.e., pulled data sets from comparable sampling locations, e.g., channel stations) for the selected microbial parameters revealed in a high discrimination ability for E. coli contamination as determined with CCA (Table 4). E. coli concentrations showed significant differences between 8 out of the 10 pairs of stations or habitats, whereas TCC, TCM, FC, and SASF showed significant differences for only six, four, three, and two pairs, respectively. The high discrimination ability of E. coli concentrations as determined by CCA is further supported by the fact that E. coli contamination could not be detected at the least-influenced sampling station, S6, whereas the other indicators were detected at all stations during the whole study (Table 3). These results provide good evidence that the extent of E. coli contamination as determined by CCA is highly efficient in discriminating between waters influenced by different levels of anthropogenic activity. This fact was further underlined by the maximum-to-minimum ratios of the observed microbial indicator concentrations (i.e., the highest observed value divided by the lowest observed value of pulled data sets), showing ratios of 107 for E. coli, 106 for TCC, 106 for FC, 105 for TCM, and 105 for SASF.
TABLE 3.
Microbial indicator levelsa
| Sampling site |
E. coli concn (CFU/100 ml)
|
FC concn (MPN/100 ml)
|
TCC concn (CFU/100 ml)
|
TCM concn (MPN/100 ml)
|
SASF concn (MPN/100 ml)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | R | M | R | M | R | M | R | M | R | |
| S1 | 2.2 × 106 | 0.5 × 106–95 × 106 | 3.5 × 105 | 1.7 × 105–50 × 105 | 4.7 × 107 | 2.4 × 107–26 × 107 | 1.1 × 107 | 0.2 × 107–8.0 × 107 | 1.8 × 105 | 0.02 × 104–80 × 104 |
| S2 | 2.0 × 106 | 0.3 × 106–6.7 × 106 | 5.0 × 105 | 4.0 × 105–110 × 105 | 1.8 × 107 | 0.2 × 107–3.8 × 107 | 3.0 × 106 | 0.1 × 106–50 × 106 | 9.0 × 104 | 3.0 × 104–300 × 104 |
| S3 | 7.0 × 106 | 3.8 × 106–24 × 106 | 2.3 × 105 | 2.2 × 105–23 × 105 | 4.4 × 107 | 1.8 × 107–4.7 × 107 | 2.3 × 106 | 0.3 × 106–7.0 × 106 | 1.7 × 105 | 1.1 × 105–11 × 105 |
| S4 | 9.2 × 106 | 6.5 × 106–11 × 106 | 7.0 × 105 | 3.0 × 105–20 × 105 | 2.0 × 107 | 1.9 × 107–4.0 × 107 | 8.0 × 106 | 5.0 × 106–8.2 × 106 | 2.8 × 105 | 0.5 × 105–11 × 105 |
| S5 | 3.7 × 106 | 0.4 × 106–7.0 × 106 | 3.0 × 105 | 0.7 × 105–23 × 105 | 1.9 × 107 | 0.6 × 107–4.1 × 107 | 3.0 × 106 | 0.6 × 106–8.0 × 106 | 8.0 × 104 | 2.3 × 104–30 × 104 |
| S6 | NCD | NCD | 8.0 × 101 | 1.1 × 101–50 × 101 | 6.0 × 104 | 2.0 × 104–23 × 104 | 1.1 × 103 | 0.3 × 103–8.0 × 103 | 3.3 × 102 | 0.4 × 102–13 × 102 |
| S7 | 1.2 × 101 | 0.4 × 101–5.0 × 101 | 5.5 × 101 | 2.0 × 101–13 × 101 | 3.7 × 102 | 1.1 × 102–8.9 × 102 | 1.5 × 102 | 1.3 × 102–25 × 102 | 4.0 × 101 | 3.8 × 101–4.2 × 101 |
| S8 | 4.0 × 105 | 1.0 × 105–9.0 × 105 | 8.0 × 104 | 3.0 × 104–22 × 104 | 2.4 × 106 | 0.9 × 106–7.6 × 106 | 2.8 × 105 | 0.2 × 105–5.0 × 105 | 2.3 × 104 | 0.8 × 104–8.0 × 104 |
Values are the result of six samples. Abbreviations: M, median; R, range, NCD, no colonies detected; FC, fecal coliform (concentrations determined with EC media; TCC, total coliform (concentrations determined with Chromocult coliform agar); TCM, total coliform (concentrations determined with lauryl sulfate broth); SASF, sulfite-reducing anaerobic spore formers (concentrations determined with reinforced clostridial medium). For explanation of sampling sites see Table 1.
TABLE 4.
Discrimination potential of microbial indicators by pairwise comparison of representative sampling sites and habitat typesa
| Sampling sites used for pairwise comparisons | Result for:
|
||||
|---|---|---|---|---|---|
| E. coli | FC | TCC | TCM | SASF | |
| S2, S3 | + | − | + | − | − |
| S2, S4 | + | − | − | − | − |
| S2, S5 | − | − | − | − | − |
| S3, S4 | − | − | − | − | − |
| S5, S8 | + | − | + | + | − |
| S6, S7 | + | − | + | − | + |
| S8, S(6,7) | + | + | + | + | − |
| S(1,2,5), S(3,4) | + | − | − | − | − |
| S(1,2,5), S(6,7) | + | + | + | + | + |
| S(1,2,5), S8 | + | + | + | + | − |
Tests for differences by pairwise comparison were performed by Wilcoxon test. + or −, significant or nonsignificant difference, respectively (P < 0.05). Number of samples was 48 per station. S(1,2,5), S(3,4), and S(6,7) indicate pooled values from channel stations S1, S2, and S5, effluent stations S3 and S4, and stations with low pollution levels S6 and S7, respectively.
E. coli detection with CCA.
Recently, application of defined substrate medium technology with particular selective growth conditions and the simultaneous detection of β-d-galactosidase and β-d-glucuronidase activity have become widespread tools for the detection of E. coli in water and wastewater (3, 4, 7, 21, 26). In fact, CCA has proven to be efficient for E. coli detection in temperate regions (1, 10, 14, 19). The results of this study proved that CCA could be applied successfully to tropical waters as well (i.e., in Kampala, Uganda). Using appropriate dilution steps of various samples of polluted waters, presumptive E. coli colonies could be readily counted on the CCA after 24 h of incubation. Overgrowth of competitive microorganisms was not observed. The surface plating technique could be successfully applied for spreading respective dilutions of water samples on CCA, hence saving expensive membrane filters and reducing costs dramatically. In practice, E. coli determination with CCA took significantly less processing time and was less prone to cross-contamination than MPN methodology. In order to further characterize the presumptive E. coli colonies from CCA, indole testing was performed on 290 randomly selected presumptive E. coli colonies from CCA, resulting in 281 indole-positive colonies. According to these results, an error of 3% could be estimated, suggesting that reliable simultaneous E. coli isolation and identification by CCA could be carried out in tropical waters of this kind.
Sampling stations from moderately to highly polluted sites yielded concentrations for E. coli, as determined with CCA, that were consistently higher than FC concentrations in EC medium. In contrast, low-impact sites such as S7, the protected spring site, and S6, the sampling site on Lake Victoria, yielded higher values for FC. These observations may be explained by the following: CCA seems to favor the growth of E. coli at 37°C more effectively than EC medium does for FC. This hypothesis is strengthened by the findings of Mercado and Hazen (17), who reported that values obtained by MPN were lower than those obtained by four different membrane filtration methods for FC isolation. In contrast, at less influenced and low-pollution sites, FC values were significantly higher than counts of E. coli obtained with CCA; this was true for the spring site (S7), and in particular for the Lake Victoria shore (S6), where no E. coli could be detected with CCA. This could be due either to the fact that no E. coli cells were present at the Lake Victoria sampling site and the recorded FC were composed of bacteria other than E. coli or to the fact that the high values were caused by false-positive FC, i.e., low specificity of testing with EC medium. Mercado and Hazen (17) previously suggested that in tropical waters there would be more bacteria of types other than E. coli that would yield a positive FC reaction for MPN methods than in temperate waters. Since the ambient water temperature in tropical waters is much higher than that in temperate climates, more thermotolerant bacteria can be expected as background flora. This is also supported by Evison and James (9), who reported a higher proportion of 44°C E. coli II, Citrobacter freundii II, and Klebsiella aerogenes I isolated from samples in Kenya than from samples in the United Kingdom. It is rather unlikely that the use of CCA resulted in a failure to detect E. coli cells that were members of the FC fraction at the Lake Victoria station, as CCA clearly proved more efficient in isolating E. coli in highly polluted sites than EC medium (Table 3).
In conclusion, the results of this study recommend the determination of E. coli contamination with CCA for the detection of fecal pollution in the area of Kampala, Uganda. All other microbial indicators were less efficient in detecting and discriminating selected tropical waters bearing diverse contamination, and therefore cannot be recommended for monitoring fecal pollution. The high fecal indication value revealed for E. coli in this study is in contradiction to the results of former investigations carried out in other tropical environments. There, E. coli concentrations did not seem to coincide with known sources of fecal pollution (13), and furthermore, E. coli could even be isolated from pristine sites of a tropical rain forest (22). However, it is important to note that there are major differences between Uganda and other tropical countries, especially the lower temperature range due to the high altitude of the country. For example, Oluwande et al. (20) and Collazo et al. (8) reported water temperatures of up to 32.0 and 33.4°C in Nigerian streams and Puerto Rican waters, respectively. In this study, a water temperature range of about 23 to 26°C was observed. Furthermore, there exist also methodological differences, as previous investigations used detection media which identified TCC or FC colonies as a first step and then deduced E. coli concentrations from isolation and identification of representative colonies. In our study we used CCA for direct quantification of E. coli CFU, leading to statistically sound numbers. The results of this study strongly call for further evaluation of our approach in different tropical regions, especially in developing countries.
Acknowledgments
This research was funded by a grant (612-01-96) from the Austrian government awarded to Dennis Byamukama.
We thank the staff of the National Water and Sewerage Corporation Central Laboratories, Kampala, Uganda, for their assistance. Many thanks also to G. Winkler, C. Beiwl, L. Sebela, and G. Kavka for helpful discussions, and to S. Grillenberger for proofreading the manuscript.
REFERENCES
- 1.Alonso J L, Soriano K, Amoros I, Ferrus M A. Quantitative determination of E. coli and fecal coliforms in water using a chromogenic medium. J Environ Sci Health. 1998;33:1229–1248. [Google Scholar]
- 2.American Public Health Association. Standard methods for the examination of water and wastewater. 19th ed. Washington, D.C.: American Public Health Association; 1995. [Google Scholar]
- 3.Brenner K P, Rankin C C, Roybal Y R, Stelma G N, Jr, Scarpino P V, Dufour A P. New medium for the simultaneous detection of total coliforms and Escherichia coli. Appl Environ Microbiol. 1993;59:3534–3544. doi: 10.1128/aem.59.11.3534-3544.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner K P, Rankin C C, Sivaganesan M, Scarpino P V. Comparison of the recoveries of Escherichia coli and total coliforms from drinking water by the MI agar method and the U.S. Environmental Protection Agency-approved membrane filter method. Appl Environ Microbiol. 1996;62:203–208. doi: 10.1128/aem.62.1.203-208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burlingame G A, McElhaney J, Pipes W O. Bacterial interference with coliform colony sheen production on membrane filters. Appl Environ Microbiol. 1984;47:56–60. doi: 10.1128/aem.47.1.56-60.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carillo M, Estrada E, Hazen T C. Survival and enumeration of the fecal indicators Bifidobacterium adolescentis and Escherichia coli in a tropical rain forest watershed. Appl Environ Microbiol. 1985;50:468–476. doi: 10.1128/aem.50.2.468-476.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark D L, Milner B B, Stewart M H, Wolfe R L, Olson B H. Comparative study of commercial 4-methylumbelliferyl-β-d-glucuronide preparations with the Standard Methods membrane filtration fecal coliform test for the detection of Escherichia coli in water samples. Appl Environ Microbiol. 1991;57:1528–1534. doi: 10.1128/aem.57.5.1528-1534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collazo L V, Shultz J A, Hazen T C. Survival of Candida albicans in tropical marine fresh waters. Appl Environ Microbiol. 1987;53:1762–1767. doi: 10.1128/aem.53.8.1762-1767.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evison L M, James A. A comparison of the distribution of intestinal bacteria in British and East African waters. J Appl Bacteriol. 1973;36:109–118. doi: 10.1111/j.1365-2672.1973.tb04078.x. [DOI] [PubMed] [Google Scholar]
- 10.Frampton E W, Restaino L, Blaszko N. Evaluation of the β-glucuronidase substrate 5-bromo-4-chloro-3-indolyl-β-d-glucuronide (X-GLUC) in a 24 h direct plating method for Escherichia coli. J Food Prot. 1988;51:402–404. doi: 10.4315/0362-028X-51.5.402. [DOI] [PubMed] [Google Scholar]
- 11.Freame B, Fitzpatrick B W F. The use of differential reinforced clostridial medium for the isolation and enumeration of clostridia from food. In: Shapton D A, Board R G, editors. Isolation of anaerobes. New York, N.Y: Academic Press; 1972. pp. 48–55. [Google Scholar]
- 12.Fujioka R S, Narikawa O T. Effect of sunlight on the enumeration of fecal indicator bacteria under field conditions. Appl Environ Microbiol. 1982;44:395–401. doi: 10.1128/aem.44.2.395-401.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazen T C, Toranzos G A. Tropical source water. In: McFeters G A, editor. Drinking water microbiology: progress and recent developments. Berlin, Germany: Springer-Verlag KG; 1990. pp. 32–53. [Google Scholar]
- 14.Manafi M, Kneifel W. A combined chromogenic fluorogenic medium for the simultaneous detection of total coliforms and E. coli in water. Zentbl Hyg Umweltmed. 1989;189:225–234. [PubMed] [Google Scholar]
- 15.McCambridge J, McMeekin T A. Effect of solar radiation and predacious microorganisms on survival of fecal and other bacteria. Appl Environ Microbiol. 1981;41:1083–1087. doi: 10.1128/aem.41.5.1083-1087.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Means E G, Olson B H. Coliform inhibition by bacteriocin-like substances in drinking water distribution systems. Appl Environ Microbiol. 1981;42:506–512. doi: 10.1128/aem.42.3.506-512.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercado J S, Hazen T C. Comparison of four membrane filter methods for fecal coliform enumeration in tropical waters. Appl Environ Microbiol. 1987;53:2922–2928. doi: 10.1128/aem.53.12.2922-2928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of Natural Resources Uganda. State of the environment report for Uganda. Kampala, Uganda: Ministry of Natural Resources Uganda; 1996. [Google Scholar]
- 19.Ogden I D, Brown G C, Gallacher S, Garthwalle P H, Gennari M, Gonzalez M P, Jorgensen L B, Lunestad B T, Macrae M, Nunes M C, Petersen A C, Rosnes J T, Vlingenthart J. An interlaboratory study to find an alternative to the MPN technique for enumerating Escherichia coli in shellfish. J Food Microbiol. 1998;40:57–64. doi: 10.1016/s0168-1605(98)00016-6. [DOI] [PubMed] [Google Scholar]
- 20.Oluwande P A, Sridhar M K C, Bammeke A O, Okubadejo A O. Pollution levels in some Nigerian streams. Water Res. 1983;17:957–963. [Google Scholar]
- 21.Rice E W, Allen M J, Edberg S C. Efficacy of β-glucuronidase assay for identification of Escherichia coli by the defined-substrate technology. Appl Environ Microbiol. 1990;56:1203–1205. doi: 10.1128/aem.56.5.1203-1205.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivera S C, Hazen T C, Toranzos G A. Isolation of fecal coliforms from pristine sites in a tropical rain forest. Appl Environ Microbiol. 1988;54:513–517. doi: 10.1128/aem.54.2.513-517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson J S, Croll J M, James A, Gay J. Pollution of underground water from pea silage. Mon Bull Minist Health Public Health Lab Serv Directed Med Res Counc. 1966;25:172. [PubMed] [Google Scholar]
- 24.Roll B M, Fujioka R S. Sources of fecal indicator bacteria in a brackish, tropical stream and their impact on recreational water quality. Water Sci Technol. 1997;35:179–186. [Google Scholar]
- 25.Toranzos G A, McFeters G A. Detection of indicator microorganisms in environmental fresh waters. In: Hurst C J, Knudsen G R, McInerney M J, Stetzenbach L D, Walter M V, editors. Manual of environmental microbiology. Washington, D.C.: American Society for Microbiology; 1997. pp. 184–194. [Google Scholar]
- 26.Venkateswaran K, Murakoshi A, Satake M. Comparison of commercially available kits with standard methods for the detection of coliforms and Escherichia coli in foods. Appl Environ Microbiol. 1996;62:2236–2243. doi: 10.1128/aem.62.7.2236-2243.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]