Abstract
During the past decade, emergence of direct oral anticoagulants (DOACs) has drastically improved the prevention of thrombosis. However, several unmet needs prevail in the field of thrombosis prevention, even in the DOACs’ era. The use of DOACs is still constrained and the drugs cannot be administered in every clinical scenario, such as an increased anticoagulant-associated bleeding risk, particularly in some specific populations (cancer – notably those with gastrointestinal or genitourinary cancer – and frail patients), the impossibility to be used in certain patients (eg, end-stage kidney failure during hemodialysis, pregnancy and breastfeeding), and their lack of efficacy in certain clinical scenarios (eg, mechanical heart valves, triple-positive antiphospholipid syndrome). Efforts to find a factor that upon antagonization prevents thrombosis but spares haemostasis have resulted in the identification of coagulation factor XI (FXI) as a therapeutic target. After briefly recapitulating the role of factor XI in the balance of haemostasis, we propose a narrative review of the key data published to date with compounds targeting factor XI to prevent thrombosis as well as the main ongoing clinical studies, opening up prospects for improving the care of patients requiring thrombosis prevention.
Keywords: FXI inhibitor, venous thromboembolic events, thromboprophylaxis, anticoagulant, clinical trials
Introduction
During the past decade, emergence of direct oral anticoagulants like rivaroxaban, apixaban and edoxaban (DOACs) has drastically improved the prevention of thrombosis.1 DOACs were found to be as efficient as conventional therapy in the prevention of recurrent venous thrombotic events, while they were associated with a decreased risk of major bleeding. Alongside with low-molecular weight heparin (LMWH) or fondaparinux, DOACs has become a new option in VTE prevention for total knee or hip replacements. To prevent thromboembolic events in patients with non-valvular atrial fibrillation (AF), DOACs have demonstrated their efficacy and safety as compared to vitamin-K antagonists (VKAs). Hence, DOACs are currently recommended as the main option of therapy in most situations with an indication of anticoagulant therapy,2–4 and they have replaced VKAs in the majority of patients with VTE5 or AF.6
However, many unmet needs prevail in the field of thrombosis prevention, even in the DOACs’ era.7 The use of DOACs is still constrained. In increased anticoagulant-associated bleeding risk population (such as cancer – in particularly those with gastrointestinal or genitourinary cancer – and frail patients), DOACs cannot be administered. Moreover, in certain patients DOACs use is either not recommended nor even impossible (eg end-stage kidney failure, pregnancy and breastfeeding) or either inefficient (eg mechanical heart valves, triple-positive antiphospholipid syndrome).8–13 Therefore, there is still a need for new and improved anticoagulant drugs. All the current anticoagulants antagonize activated factor X (FXa) and/or thrombin function, either directly or indirectly. These two proteases play an essential role for haemostasis and therefore administration of current anticoagulants coincide with an increased risk of bleeding. Efforts to find a factor that upon antagonization prevents thrombosis but spares haemostasis have resulted in the identification of coagulation factor XI (FXI) as a therapeutic target.14
After briefly recapitulating the role of factor XI in the balance of haemostasis, we propose a narrative review of the key data published to date with compounds targeting factor XI to prevent thrombosis as well as the main ongoing clinical studies, opening up prospects for improving the care of patients requiring thrombosis prevention.
Factor XI in the Coagulation Cascade: The Perfect Target?
In the traditional representation of the coagulation cascade, comprised of the intrinsic, extrinsic, and the common pathway, FXI is placed in the intrinsic “arm” or contact activation pathway of coagulation.15,16 FXI was not considered as a serious therapeutic target to prevent thrombosis, since its deficiency only causes a mild bleeding phenotype (hemophilia C). This means that antagonizing the coagulation factor would be safe without severe bleeding as a side effect, but thrombosis prevention would not be as effective as traditional anticoagulants such as LMWHs or heparin. In humans, deficiencies for contact activation proteins (coagulation factor XII (FXII), prekallikrein (PK), and high-molecular-weight kininogen) do not coincide with a bleeding phenotype.17,18 For this reason, contact activation was long regarded as irrelevant for human thrombosis, although direct activation of FXII using an artificial negatively charged surface leads to clotting of plasma. Based on that principle, the APTT (activated partial thromboplastin time) test measures plasma clotting time upon activation of FXII using an artificial contact activator such as silica or ellagic acid, and the relatively simple procedure of the test allows a physician to detect abnormalities regarding the plasma levels of certain coagulation factors.19 FXI was not considered as a serious therapeutic target to prevent thrombosis, since its deficiency only causes a mild bleeding phenotype (hemophilia C).20 This means that antagonizing the coagulation factor would be safe without severe bleeding as a side effect, but thrombosis prevention would not be as effective as traditional anticoagulants such as LMWHs or heparin.
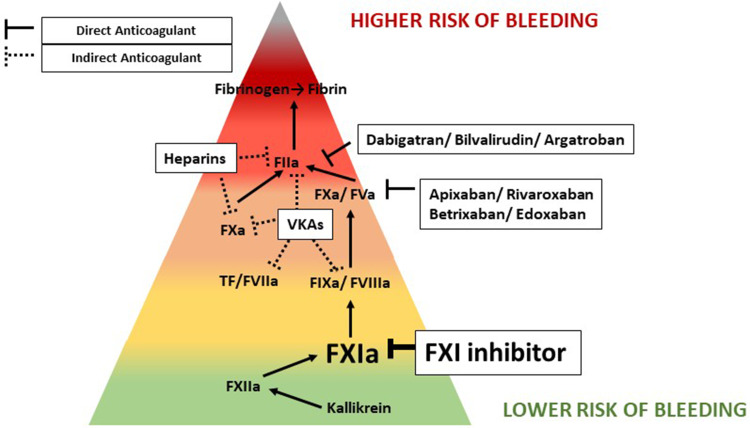
Recently, the interest for FXI has increased dramatically, since it has been suggested that FXI is more important for thrombosis than for hemostasis. This would make FXI an ideal target to prevent venous thrombosis while minimizing the risk for (severe) bleeding (Figure 1). Using a thrombosis mouse model it was shown that FXII and FXI deficient mice are resistant to thrombosis.21 Interestingly, deficiency in either of these factors did not coincide with bleeding. Accordingly, using compounds that antagonize FXI function, such as antisense oligonucleotides22 or blocking antibodies,23,24 thrombosis was reduced in preclinical animal models. In recent years, several contact activators involved in venous thrombosis onset have been proposed to serve as physiological FXII activators, such as DNA,25 RNA,26 platelet polyphosphates,27 and misfolded proteins.28 Based on the data from these preclinical studies, FXI could be regarded to serve as a substrate exclusively of FXIIa and thus only involved in coagulation as a consequence of contact activation. However, this does not translate to the human situation in which FXI deficiency, unlike FXII and PK, coincides with a bleeding phenotype and, unlike for FXI plasma levels, FXII and PK levels do not appear to be correlated with risk of VTE.29 This discrepancy is most likely explained by the discovery that FXI is also activated by thrombin, the key mediator of coagulation.30 This means that upon thrombin formation coagulation can be maintained via a positive feedback loop by thrombin-mediated activation of FXI. The extent to which the proteases FXIIa and thrombin contribute to FXI activation in humans is not entirely clear and most likely depends on the specific biological circumstances.
Figure 1.
Anticoagulant associated bleeding risk.
The reason why FXI seems to be pivotal in separating hemostasis from thrombosis could be found in the availability of the major driver to start coagulation, tissue factor (TF). When hemostasis needs to be restored, usually in the case of a vascular injury, large quantities of extravascular TF are exposed to the circulation. Substantial TF exposure and according FVIIa formation cause a rapid propagation of coagulation leading to fibrin formation mediated by the dominant extrinsic pathway. In contrast, thrombosis is usually not caused by vascular injury but by a more modest trigger, for instance upon exposure of the circulation to compounds associated with endothelial cells, such as TF+ monocytes,31 microvesicles,32 neutrophil extracellular traps,33 or platelet polyphosphates.27 As a consequence, the initial burst of thrombin formation is significantly lower and for that reason feedback mechanisms, including thrombin-mediated FXI activation, to maintain a steady flow of freshly generated thrombin may play a more significant role. When coagulation is initiated by the introduction of an artificial contact activation surface, such as placement of a central venous catheter34 or extracorporeal membrane oxygenation (ECMO)35 treatment, it is not difficult to envision the benefit of FXI inhibition to reduce thrombosis risk while sparing the patient from (severe) bleeding.
Targeting Factor XI in Patients: What Do We Currently Know?
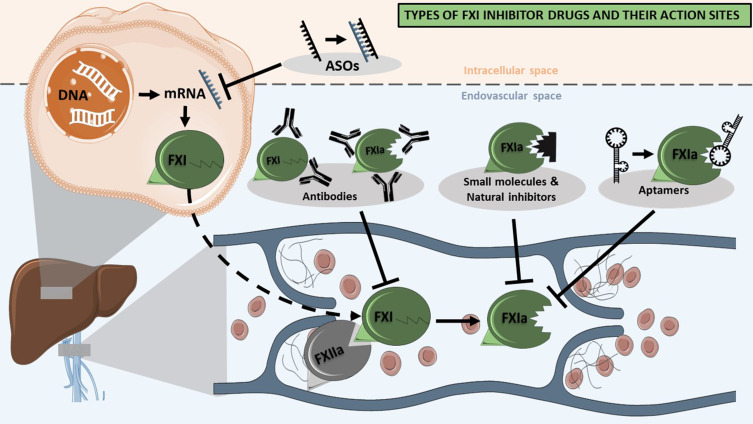
Several therapeutic approaches to target the FXI protein in humans have been proposed, including the use of antisense oligonucleotides (ASOs), aptamers, monoclonal antibodies, natural peptide inhibitors, and synthetic small peptidomimetic molecules (Figure 2). The main pharmacological characteristics of each type of drugs are summarized in Table 1. Natural inhibitors, such as Ir-CPI, seem to be the least feasible option to use as a therapeutic due to their slow on- and offset, and the relatively high risk of negative side effects.36 Small peptidomimetic molecules, such as Milvexian/BMS-986177 or BAY 2433334, are currently the only class of drugs that can be delivered orally. They have a short half-life and a renal clearance similar to DOACs.37,38 In contrast, ASOs (such as Factor XI LICA, IONIS FXI-LRx/ISIS 416858) and monoclonal antibodies (such as Xisomab 3G3/AB023, Osocimab/BAY1213790 or Abelacimab/MAA868) have longer half-lives for up to 30 days, which enables a decreased frequency of administration and thus may increase the patient adherence and compliance.39–41 Antibodies and ASOs approaches to target FXI differ in their onset of action; upon administration, monoclonal antibodies lower FXI function after hours, while the effect of ASOs only becomes apparent after several weeks. Most likely for this reason, inhibition of FXI using monoclonal antibodies are currently more explored.
Figure 2.
Types of FXI inhibitor drugs and their action sites.
Table 1.
Pharmacokinetics and Pharmacodynamics Parameters of FXI Inhibitors
| Drug Name | Antisense Oligonucleotides or ASO | Aptamers | Monoclonal Antibodies | Natural Inhibitors | Small Peptidomimetic Molecules |
|---|---|---|---|---|---|
| Target | FXI mRNA | FXIa | FXI or FXI synthesis | FXIa or FXIa + FXIIa | FXIa or FXI+plasma kallicrein |
| Mechanism | Specific protein synthesis blocking | Specific protein binding | Specific protein binding and decrease its concentration | Specific protein binding | Specific protein binding |
| Oral galenic formulation | No | No | No | No | Yes |
| Administration route | Administration route SC | Administration route IV or SC | Administration route IV or SC | Administration route IV | Administration route IV or oral |
| Half life | Long: Weekly administration | Short: Daily administration | Long: Monthly administration | Short: Daily administration | Short: Daily administration |
| Action | Slow and long acting | Fast and short acting | Fast and long-acting | Fast and short acting | Fast and short acting |
| Renal excretion | No | No | No | n/d | Biliary and 15% renal excretion |
| CYP metabolism | No | No | No | No | CYP3A4 |
| Potential for drug-drug interactions | No | No | No | n/d | Midazolam Rifampicin Verapamil, Ketoconazole … |
| Need and availability of antidote or of reversion strategy | Yes: FXI replacement | No | Yes: not existing failure of FXI replacement | No | No |
Several FXI inhibitors are being developed and tested on humans. Published Phase I trials do not report any safety concerns for FXI drugs (Table 2).
Table 2.
Published Phase I Trial for FXI Inhibitors
| Registration Number | Patient Characteristics | Study | Drug Name | N | Endpoints | Conclusion | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibodies | |||||||||||
| NCT03097341 | Healthy adult volunteers aged 18–48 yo |
Lorentz et al 201939 | Xisomab 3G3 (AB023) | 21 N=16 active N= 5 placebo |
Safety (Abnormal Vital Signs, Abnormal Electrocardiogram, Abnormal Injection Site Reaction, Abnormal Laboratory Values, Immunogenicity, death) Tolerability Pharmacokinetic parameters Pharmacodynamic parameters |
There were no serious adverse events (SAEs) experienced in this study. No subjects were removed from the study due to adverse events (AEs). Overall, 10 of 21 (48%) subjects experienced a total of 20 TEAEs in this study, with 7 of 16 (44%) subjects following active treatment and 3 of 5 (60%) subjects following placebo | |||||
| / | ANT-003 Healthy adult volunteers + obese patients (BMI ≥ 35 kg/m2) aged 18-60 yo ANT-004 patients with atrial fibrillation or flutter CHA2DS2-VASc of 0–1 for men and 1–2 for women aged 18–85 yo |
Yi et al 202240 ANT-003 ANT-004 |
Abelacimab (MAA868) | 32 ANT-003=24 ANT-004=8 |
As vital signs, adverse event assessments, laboratory tests, Pharmacokinetic parameters Pharmacodynamic parameters Anti-drug antibodies (ADA) assessment |
The limitations of these studies include, by design, the single dose exposure in ANT-003 which limits the ability to detect clinically relevant safety signals. In addition, due to the COVID-19 pan -demic, enrollment in the ANT-004 study was stopped early and thus the fully planned data set of PK, PD, and immunogenicity data for monthly subcutaneous administration of abelacimab could not be collectedParenteral administration of abelacimab demonstrated a fa-vorable safety profile with no clinically relevant bleeding events | |||||
| Antisens oligonucleotides or ASO | |||||||||||
| / | Healthy subjects aged 18–65 yo | Liu et al. 201157 | ISIS-FXIRx | Single dose SC 50 mg/kg n=8 100 mg/kg n=8 200 mg/kg n=16 300 mg/kg n=8 8x SC injections n=12 |
Safety (adverse event (AE) clinical and laboratory tests safety, tolerability, pharmacokinetics and pharmacodynamics | No study drug related bleeding events were reported. No clinical or biological significant modification occured One serious AE (allergic reaction) occured | |||||
| Small molecule | |||||||||||
| NCT03919890 | Part A enrolled men only, Part B enrolled men or women of non-childbearing potential aged 18–55 yo | Beale et al 202158 | ONO-7684 | 72 Part A n= 36 active n= 12 Part B n=24 |
Safety (adverse event (AE) reporting using the Medical Dictionary for Regulatory Activities (version 22.0), clinical laboratory tests (biochemistry, haematology and urinalysis), vital signs, electrocardiograms (ECGs), physical examination and cardiac telemetry) Tolerability Pharmacokinetic parameters Pharmacodynamic parameters |
No signal on safety total of eight (16.7%) out of 48 fasted subjects and one (12.5%) out of eight fed subjects reported treatment-emergent adverse events (TEAEs) in Part A, and three (12.5%) of 24 subjects reported TEAEs in Part B (Table 2). There were no severe or serious TEAEs and no TEAEs that led to treatment discontinuation or interruption. | |||||
| / | Healthy adult caucasian men Aged 18–45 yo |
Thomas et al 202159 | Asundexian (BAY2433334) | 70 Part 1 n=56 active n=14 placebo; Part 2 n=16 | Safety (symptomatic bleeding, signs of hepatobiliary dysfunction or pancreatic disorders, Tolerability Pharmacokinetic parameters Pharmacodynamic parameters |
BAY 2433334 exhibited favorable safety and tolerability. BAY 2433334 dose-dependently inhibited FXIa activity and increased activated partial thromboplastin time. |
|||||
| / | Healthy adult caucasian men Aged 18–45 yo | Kubitza et al 202238 | Asundexian (BAY2433334) | 96 Parts A and B: n= 36 active; n=12 to placebo. Part C: n= 48 active plus midazolam | Safety (physical examination, vital signs, 12-lead electrocardiogram (ECG) and clinical laboratory evaluation) Tolerability Pharmacokinetic parameters Pharmacodynamic parameters Interaction with Midazolam |
Multiple dosing of BAY 2433334 in healthy volunteers was well tolerated, with a predictable pharmacokinetic/pharmacodynamic profile and no clinically relevant CYP3A4 induction or inhibition. | |||||
| / | Healthy adult volunteers: Aged 18–45 yo |
Perera et al 202137 | Milvexian (BMS-986177) (JNJ70033093) | 48 part A of the study (n = 24 active, n = 8 placebo). Part B (n = 32 active, n = 8 placebo) | Safety a medical review of adverse event (AE) reports and the results of clinical laboratory tests, vital sign measurements, ECGs, and physical examinations up to 48 h postdose. Events of special interest included clinically and nonclinically significant bleeding. AEs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA), version 20.0. Pharmacokinetic parameters Pharmacodynamic parameters |
Administration were generally safe and well-tolerated. No deaths or other Severe AEs occurred during the study. All Treatment-emergent AEs were mild in severity. | |||||
Completed Phase II trials exist with ASOs, monoclonal antibodies, and small molecules (Table 3). There are no published data regarding the use of natural inhibitors in clinical trials. The vast majority of patients included in phase II trials to assess anti-FXI therapy in prevention of VTE were patients undergoing total knee replacements.
Table 3.
Published Phase II Trial for FXI Inhibitors
| DOAC short coming in VTE |
Registration number | Study | Drug name | Compator | N | End Point | Conclusion |
|---|---|---|---|---|---|---|---|
| Total Knee Replacement | Anti-sens oligonucleotides | ||||||
| NCT01713361 / EudraCT: 2012-001836-72 | Büller et al42 2015 FXI-ASO TKA trial |
IONIS FXI-LRx (ISIS 416858) 200 mg or 300 mg SC |
Enoxaparin 40 mg |
412 | All DVT symptomatic PE, fatal PE, and unexplained death |
Incidence of VTE - 27% in the 200-mg dose of FXI-ASO - 4% in the 300-mg dose of FXI-ASO, -30% of who received enoxaparin Bleeding - 3% in the 200-mg dose of FXI-ASO - 3% in the 300-mg dose of FXI-ASO, -8% of who received enoxaparin |
|
| Small molecule | |||||||
| NCT03891524 / CR108600 / EudraCT: 2018-004237-32 | Weitz et al44 2021 AXIOMATIC-TKR trial | Milvexian (BMS-986177) (JNJ70033093) oral 5 mg, 50 mg, 100 mg, or 200 mg twice daily or 25 mg, 50 mg, or 200 mg once daily | Enoxaparin 40 mg SC |
1242 | VTE, Bleeding Event, Major, CRNM and minimal bleeding Deaths |
Incidence of VTE among those receiving Milvexian twice daily : -21% taking 25 mg -11% taking 50 mg -9% taking 100 mg -8% taking 200 mg. Incidence of VTE among those receiving Milvexian once daily: - 25% taking 25 mg - 24% taking 50 mg - 7% taking 200 mg - 21% taking enoxaparin. Any bleeding 4% taking milvexian 4% taking enoxaparin Any major bleeding or CRNMB -1% taking milvexian -2% taking enoxaparin Serious adverse events -2% taking milvexian -4% taking enoxaparin |
|
| Antibody | |||||||
| NCT03276143/ Sponsor: 17664 / EudraCT: 2016-002681-31 | Weitz et al41 2020 FOXTROT trial | Osocimab (BAY1213790) Single IV postoperative doses of 0.3 mg/kg, 0.6 mg/kg, 1.2 mg/kg, or 1.8 mg/kg Single IV preoperative doses of 0.3 mg/kg or 1.8 mg/kg |
40 mg of SC enoxaparin once daily or 2.5 mg of oral apixaban twice daily for at least 10 days or until venography | 813 | Composite endpoint of VTE events Composite endpoint of major and clinically relevant non-major bleeding |
Incidence of VTE among patients taking Osocimab postoperative -23.7% receiving 0.3 mg/kg -15.7% receiving 0.6 mg/kg -16.5% receiving 1.2 mg/kg -17.9% receiving 1.8 mg/kg taking Osocimab preoperative -29.9% receiving 0.3 mg/kg -11.3% receiving 1.8 mg/kg Taking enoxaparin -26.3% receiving enoxaparin Taking apixaban -14.5% receiving Major or CRNMB -4.7% receiving Osocimab -5.9% receiving enoxaparin -2% receiving apixaban |
|
| Sponsor : 129008 / EudraCT: 2019-003756-37 | Verhamme et al45 2021 ANT-005 TKA trial |
Abelacimab (MAA868) 30 mg, 75 mg, or 150 mg | Enoxaparin 40 mg SC |
412 | Composite endpoint of VTE events (asymptomatic DVT, symptomatic VTE, inexplicable death) | Incidence of VTE -13% in the 30-mg abelacimab group -5% in the 75-mg abelacimab group -4% in the 150-mg abelacimab group -22% in the enoxaparin group Bleeding 2% in the 30-mg 2% in the 75-mg of the patients in the abelacimab group No bleeding in the other group |
|
| End Stage Renal Disease | Antibody | ||||||
| NCT03612856 | Lorentz et al 2021 | Xisomab 3G3 (AB023) VTE prophylaxis during hemodialysis 0.25 mg/kg or 0.50 mg/kg |
placebo | 27 | Bleeding of vascular access site | Bleeding 0% Receiving 0.25 mg.kg 62% Receiving 0.5 mg/kg 38% Receiving placebo |
|
| Small Molecule | |||||||
| NCT03000673 | / | Milvexian (BMS-986177) (JNJ70033093) VTE prophylaxis during hemodialysis 100 mg or 300 mg |
UFH IV infusion enoxaparin 40 mg by SC |
32 | Adverse Events (AEs), Serious AEs (SAEs), AEs Leading to Discontinuation and Death | AE UFH IV infusion 6.25% enoxaparin 40 mg by SC 9.7% Milvexian 100mg 12.5% Milvexian 300 mg 13% |
|
| Anti-sens oligonucleotides | |||||||
| NCT03358030 / EudraCT Number 2017-002165-21 | / ESMERALD trial | IONIS FXI-LRx (ISIS 416858) VTE prophylaxis during hemodialysis 200 mg, 250 mg or 300 mg |
placebo | 213 | Safety, Tolerability Incidence of clinically relevant and non-major bleeding |
No results posted | |
In the FXI-ASO TKA study,42 Büller et al randomised 300 patients undergoing total knee arthroplasty to receive one of two doses of an ASO against FXI (IONIS FXI-LRx 200 mg or 300 mg) or 40 mg of enoxaparin once daily. The primary efficacy outcome was a composite of objectively documented symptomatic VTE or asymptomatic DVT. The principal safety outcome was clinically relevant bleeding, a composite of major and clinically relevant non-major bleeding, up to day 30 after surgery. All the events of interest were adjudicated by an independent adjudication committee, blinded to the treatment arm. At 200-mg, FXI-ASO was non-inferior (27%), while the 300-mg was superior (4%) to enoxaparin (30%) (P<0.001). Rates of bleeding were inferior in the FXI-ASO groups (both 3%) than in the enoxaparin group (8%).
The ANT-005 TKA43 study was a non-inferiority phase II trial, comparing the post-operative admission of 3 unique doses of the FXIa antibody abelacimab (30, 75, or 150 mg), to one daily subcutaneous enoxaparin (40 mg) in patients undergoing total knee arthroplasty. The primary outcome was a composite of objectively documented symptomatic VTE or asymptomatic DVT (on systematic venography realized between day 8 to 12). The principal safety outcome was clinically relevant bleeding, a composite of major and clinically relevant non-major bleeding, up to day 30 after surgery. All the events of interest were adjudicated by an independent adjudication committee, blinded to the treatment arm. Abelacimab (at the two highest doses) was significantly non-inferior to enoxaparin, in terms of rates of VTE (4% and 5%, compared with 22% with enoxaparin).
In the FOXTROT Randomized Clinical Trial,41 investigators randomized 813 patients to receive either single intravenous Osocimab postoperative doses of 0.3 mg/kg (n = 107), 0.6 mg/kg (n = 65), 1.2 mg/kg (n = 108), or 1.8 mg/kg (n = 106); preoperative doses of 0.3 mg/kg (n = 109) or 1.8 mg/kg (n = 108); or 40 mg of subcutaneous enoxaparin once daily (n = 105) or 2.5 mg of oral apixaban twice daily (n = 105) for at least 10 days (or until venography) after knee arthroplasty. The primary efficacy outcome was venous thromboembolism (assessed by mandatory bilateral venography performed 10 to 13 days after surgery or confirmed symptomatic deep vein thrombosis or pulmonary embolism). The primary safety outcome was a composite of major or clinically relevant nonmajor bleeding, assessed until 10 to 13 days postoperatively. Of note, only 600 of the 813 randomized patients were included in the per-protocol analysis. Only preoperative dose of 1.8 mg/kg was found to be superior to enoxaparin (risk difference of 15.1%; 2-sided 90% CI, 4.9% to 25.2%). No regimen of Osocimab was found to be superior to apixaban.
The AXIOMATIC-TKR44 study assessed the efficacy and safety of the oral FXIa inhibitor Milvexian, in patients undergoing total knee arthroplasty. Among the 1242 patients randomized to receive one of seven postoperative regimens of milvexian (25 mg, 50 mg, 100 mg, or 200 mg twice daily or 25 mg, 50 mg, or 200 mg once daily) or enoxaparin (40 mg once daily), the primary efficacy outcome was a composite endpoint (including asymptomatic deep-vein thrombosis, confirmed symptomatic venous thromboembolism, or death from any cause), while the principal safety outcome was clinically relevant bleeding, a composite of major and clinically relevant non-major bleeding. In the milvexian twice daily arms, venous thromboembolism developed in 27 of 129 (21%) taking 25 mg, in 14 of 124 (11%) taking 50 mg, in 12 of 134 (9%) taking 100 mg, and in 10 of 131 (8%) taking 200 mg. In patients who received milvexian once daily, venous thromboembolism developed in 7 of 28 (25%) taking 25 mg, in 30 of 127 (24%) taking 50 mg, and in 8 of 123 (7%) taking 200 mg, as compared with 54 of 252 patients (21%) taking enoxaparin. No differences were found in terms of bleeding (4% in patients receiving milvexian and 4% in those taking enoxaparin). The rates of adverse events were similar in the milvexian (39%) and enoxaparin (38%) groups, while the rates of serious adverse events were reported in 2% of the milvexian group and 4% of the enoxaparin group. The authors concluded that oral milvexian was effective for the prevention of venous thromboembolism, particularly with twice-daily regimen, and associated with a low risk of bleeding.
Ongoing Studies
Based on the encouraging results of phase II trials performed in patients undergoing total-knee replacement surgery, FXI drugs are currently being evaluated in situations where the use of DOACs is discouraged,36,45–49 particularly in patients with End-Stage Renal Disease and patients with Cancer-associated thrombosis.
The small molecule Milvexian is registered in 22 trials. Within these trials, especially patients with End-Stage Renal Disease (ESRD) under hemodialysis have been included (NCT03196206, NCT02902679).50 These patients have an increased risk for VTE and are usually contraindicated for DOACs, because of drug bioaccumulation in patients with high bleeding risk.51 Indeed, in five other registered studies ESDR patients are included for this reason: Asundexian (BAY2433334), Factor XI LICA (BAY 2976217), IONIS FXI-LRx (ISIS 416858), MK-2060 and Osocimab (BAY1213790).
Abelacimab is the first FXIa Inhibitor to be tested in Phase III studies (NCT05171049, NCT05171075), and both studies are conducted in patients with Cancer-associated thrombosis (CAT). CAT patients are both at an increased risk of recurrent VTE and anticoagulation-associated bleeding. Current guidelines advise cautious treatment of CAT patients with DOACs (apixaban, rivaroxaban or edoxaban), due to the increased bleeding risk, or to settle altogether for LMWH therapy.52 The increased risk for anticoagulant-associated bleeding in CAT patients makes it apparent that there is a dire need for safer anticoagulants, and FXIa inhibitors could substantially improve patient care in this clinical scenario. In addition, some oncologic patients suffer from variable oral route availability due to difficulties in swallowing or vomiting, which impairs correct administration of DOACs. Monoclonal antibodies such as Abelacimab could provide a solution in these cases because of its monthly parenteral administration. CAT patients with cancer involving mucosa, such as gastrointestinal or genitourinary cancers, are at an even higher risk of bleeding than patients with other cancer types, which has been taken into account by Abelacimab researchers by dedicating one phase III trials to this cohort (NCT05171075).53 Another scenario in which FXI inhibition could be beneficial is that of cancer patients with Catheter-Related Thrombosis (CRT). The monoclonal antibody against FXIa, Xisomab 3G3 (AB023), has a Phase II trial ongoing to address the prevention of CRT in cancer patients (NCT04465760).
Table 4 summarises the most expected ongoing and non-published completed trials, particularly in patients with ESRD or CAT, situations in which FXI drugs seem to have a great potential. FXI inhibitors Milvexian (BMS-986177, JNJ70033093) and Abelacimab (MAA868) can be regarded as the most advanced drugs, regarding their clinical development.
Table 4.
Ongoing Studies Assessing Anti-FXI Drugs in the Prevention of Thrombosis
| Setting | Trial ID | Phase | Molecule Name | Study Name | Molecule Type | Regimen | Comparator | N | Primary Endpoints | Status |
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer-associated thrombosis | NCT05171075 | III | Abelacimab (MAA868) | MAGNOLIA trial | Ab | I.V/S.C | Dalteparin | 1020 | Time to VTE | Upcoming |
| NCT05171049 | III | Abelacimab (MAA868) | ASTER trial | Ab | I.V/S.C | Apixaban | 1655 | Time to VTE | Upcoming | |
| NCT04465760 | II | Xisomab 3G3 (AB023) | / | Ab | I.V | None | 50 | Incidence of CRT | Ongoing | |
| End-stage renal disease | NCT04534114 | II | Factor XI LICA (BAY 2976217) | / | ASO | S.C | Placebo | 305 | Major and CRNM bleeding events | Upcoming |
| NCT02553889 | II | IONIS FXI-LRx (ISIS 416858) | / | ASO | S.C | Placebo | 49 | Frequency and severity of Adverse events (including bleeding events) | Completed | |
| NCT03196206 | I | Milvexian (BMS-986177) | / | Small molecule | ORALLY | Enoxaparin | 33 | PK parameters | Completed | |
| NCT02902679 | I | Milvexian (BMS-986177) | Small molecule | ORALLY | None | 6 | Number of subjects with Adverse events (AEs) | Completed | ||
| NCT03873038 | I | MK-2060 | MK-2060-004 | Ab | I.V | Placebo | 38 | Percentage of Participants with Any Adverse Event | Completed | |
| NCT05027074 | II | MK-2060 | MK-2060-007 | Ab | I.V | Placebo | 489 | Time to thrombosis of the arteriothrombosis graft | Ongoing | |
| NCT03787368 | I | Osocimab (BAY1213790) | / | Ab | I.V | Placebo | 55 | Major and CRNM bleeding events | Completed | |
| NCT04523220 | II | Osocimab (BAY1213790) | / | Ab | S.C | Placebo | 686 | Major and CRNM bleeding events | Upcoming | |
| NCT04510987 | I | BAY2433334 (Asundexian) | / | Small molecule | ORALLY | None | 48 | PK parameters | Completed |
Abbreviations: Ab, antibodies; I.V, intravenous; S.C, subcutaneous; CRNM, clinically relevant non major; PK, pharmacokinetic.
Perspectives
Phase III trials on so-called frail patients, such as elderly, critical care, or septic patients have not yet been put in motion. These patients suffer from an increased anticoagulant-related bleeding, so they could benefit from safer profile anticoagulants as FXIa inhibitors.54 Despite their common referral as frail patients, these groups also differ in drug pharmacokinetic profile needs. Elderly patients would benefit from a drug with a non-aggressive metabolic impact on the blockage of the coagulation balance in order to avoid adverse effects (AEs) derived from pharmacodynamic (PD) and pharmacokinetic (PK) changes that characterize aged population. These PD and PK changes include an increased sensitivity to anticoagulants on the one hand, and a diminished renal and hepatic clearance on the other, thus why perhaps a drug with a “Slow onset – Fast offset” pharmacokinetic (PK) profile could improve the safety profile of DOACs. Perhaps in the development of new FXIa inhibitors, a good candidate will arise that allows for a progressive introduction and easy reversal in case of bleeding to avoid adverse effects. In contrast, critical care and septic patients require a “Fast onset - Fast offset” PK profile. Aptamers and small molecules that inhibit FXIa display this specific profile while administration of the drug is parenteral, overcoming oral route unavailability that is frequently present in these patients. However, it is unclear whether FXIa inhibitors would be beneficial over the current standard of care, Heparins, that already have a fitting PK and safety profile, and parenteral administration. Dedicated monitoring would be needed.
For pregnant and breastfeeding VTE patients, another group in which DOACs are contraindicated, the use of FXI inhibitors should be explored. Known problematic scenarios regarding the inefficacy of DOACS, such as mechanical heart valve, cardiac assist devices, ECMO therapy and noncancer CRT patients, are englobed under the term “Artificial Contact Surfaces Associated Thrombosis” (ACSAT). These patients may benefit profoundly from an anticoagulant strategy based on inhibition of FXI, since these drugs target the factor that is activated as a direct result of contact activation. For ACSAT, researchers have even advocated the use of FXIIa inhibitors over FXIa because of their negligible associated risk for bleeding. However, additional research is needed to test the efficacy of FXII inhibitors to prevent VTE in these specific clinical scenarios.48,49
FXI antagonizing drugs may also become an interesting option as “One Shot” therapies in situations where a short period of treatment is desired, as an alternative to a prophylactic or intermediate dose of daily Fondaparinux or LWMH injections (e.g superficial venous thrombosis, post-operative thromboprophylaxis).47,55 Some FXIa inhibitors like monoclonal antibodies could be active for 30–45 days after being administered once subcutaneously.
Conclusion
Based on available data, FXI drugs show great promise. However, confirmation of their efficacy in dedicated randomized controlled trials is needed. Importantly, if the expected advantage of FXIa inhibitors is to be safer than DOACs, they are not exempt from bleeding risk, and dedicated reversal strategies are needed.56 Especially for “slow offset” molecules like antibodies or ASOs, development of a reliable reversal strategy based on antidote drugs is required. To our knowledge, only a single suspended trial on Milvexian antidote research has been registered so far (NCT04543383). Most of the cited studies are either completed with no published results or scheduled to be completed as late as 2024. It is therefore not unreasonable that we will incorporate FXIa inhibitors into daily clinical practice in the following 3–4 years, and that we can expect changes in VTE clinical guidelines accordingly.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Weitz JI, Jaffer IH, Fredenburgh JC. Recent advances in the treatment of venous thromboembolism in the era of the direct oral anticoagulants. F1000Research. 2017;6. doi: 10.12688/F1000RESEARCH.11174.1/DOI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hindricks G, Potpara T, Dagres N, et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2020;2020:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 3.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543–603. doi: 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 4.Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report – executive summary. Chest. 2021;160:e545–e608. doi: 10.1016/j.chest.2021.07.056 [DOI] [PubMed] [Google Scholar]
- 5.Bertoletti L, Gusto G, Khachatryan A, et al. Effectiveness and safety of oral anticoagulants in the treatment of acute venous thromboembolism: a nationwide comparative cohort study in France. Thromb Haemost. 2022. doi: 10.1055/a-1731-3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozieł M, Teutsch C, Bayer V, et al. Changes in anticoagulant prescription patterns over time for patients with atrial fibrillation around the world. J Arrhythmia. 2021;37(4):990–1006. doi: 10.1002/joa3.12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertoletti L, Ollier E, Duvillard C, et al. Direct oral anticoagulants: current indications and unmet needs in the treatment of venous thromboembolism. Pharmacol Res. 2017;118:33–42. doi: 10.1016/j.phrs.2016.06.023 [DOI] [PubMed] [Google Scholar]
- 8.Al-Samkari H, Connors J. The role of direct oral anticoagulants in treatment of cancer-associated thrombosis. Cancers. 2018;10(8):271. doi: 10.3390/cancers10080271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levi M. Management of bleeding in patients treated with direct oral anticoagulants. Crit Care. 2016;20(1):249. doi: 10.1186/s13054-016-1413-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mavrakanas TA, Samer CF, Nessim SJ, Frisch G, Lipman ML. Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol. 2017;28(7):2241–2248. doi: 10.1681/ASN.2016090980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speed V, Roberts LN, Patel JP, Arya R. Venous thromboembolism and women’s health. Br J Haematol. 2018;183(3):346–363. doi: 10.1111/bjh.15608 [DOI] [PubMed] [Google Scholar]
- 12.Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214. doi: 10.1056/NEJMoa1300615 [DOI] [PubMed] [Google Scholar]
- 13.Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132(13):1365–1371. doi: 10.1182/blood-2018-04-848333 [DOI] [PubMed] [Google Scholar]
- 14.Gailani D, Renné T. The intrinsic pathway of coagulation: a target for treating thromboembolic disease? J Thromb Haemost. 2007;5(6):1106–1112. doi: 10.1111/j.1538-7836.2007.02446.x [DOI] [PubMed] [Google Scholar]
- 15.Davie EW, Ratnoff OD. Waterfall sequence for intrinsic blood clotting. Science. 1964;145(3638):1310–1312. doi: 10.1126/science.145.3638.1310 [DOI] [PubMed] [Google Scholar]
- 16.Wolberg AS, Rosendaal FR, Weitz JI, et al. Venous thrombosis. Nat Rev Dis Prim. 2015;1:15006. doi: 10.1038/nrdp.2015.6 [DOI] [PubMed] [Google Scholar]
- 17.Tillman BF, Gruber A, McCarty OJT, Gailani D. Plasma contact factors as therapeutic targets. Blood Rev. 2018;32(6):433–448. doi: 10.1016/j.blre.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maas C, Oschatz C, Renné T. The plasma contact system 2.0. Semin Thromb Hemost. 2011;37(04):375–381. doi: 10.1055/s-0031-1276586 [DOI] [PubMed] [Google Scholar]
- 19.Fritsma GA, Dembitzer FR, Randhawa A, et al. Recommendations for appropriate activated partial thromboplastin time reagent selection and utilization. Am J Clin Pathol. 2012;137(6):904–908. doi: 10.1309/AJCP3J1ZKYBFQXJM [DOI] [PubMed] [Google Scholar]
- 20.Lewandowska MD, Connors JM. Factor XI Deficiency. Hematol Oncol Clin North Am. 2021;35(6):1157–1169. doi: 10.1016/j.hoc.2021.07.012 [DOI] [PubMed] [Google Scholar]
- 21.Renné T, Pozgajová M, Grüner S, et al. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202(2):271–281. doi: 10.1084/jem.20050664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Löwenberg EC, Crosby JR, et al. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: a novel antithrombotic strategy with lowered bleeding risk. Blood. 2010;116(22):4684–4692. doi: 10.1182/blood-2010-04-277798 [DOI] [PubMed] [Google Scholar]
- 23.Cheng Q, Tucker EI, Pine MS, et al. A role for factor XIIa–mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116(19):3981–3989. doi: 10.1182/blood-2010-02-270918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.David T, Kim YC, Ely LK, et al. Factor XIa–specific IgG and a reversal agent to probe factor XI function in thrombosis and hemostasis. Sci Transl Med. 2016;8:353. doi: 10.1126/scitranslmed.aaf4331 [DOI] [PubMed] [Google Scholar]
- 25.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci. 2010;107(36):15880–15885. doi: 10.1073/pnas.1005743107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kannemeier C, Shibamiya A, Nakazawa F, et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci. 2007;104(15):6388–6393. doi: 10.1073/pnas.0608647104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller F, Mutch NJ, Schenk WA, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139(6):1143–1156. doi: 10.1016/j.cell.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maas C, Govers-Riemslag JWP, Bouma B, et al. Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest. 2008. doi: 10.1172/JCI35424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller F, Gailani D, Renné T. Factor XI and XII as antithrombotic targets. Curr Opin Hematol. 2011;18(5):349–355. doi: 10.1097/MOH.0b013e3283497e61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gailani D, Broze GJ. Factor XI activation in a revised model of blood coagulation. Science. 1991;253(5022):909–912. doi: 10.1126/science.1652157 [DOI] [PubMed] [Google Scholar]
- 31.von Brühl M-L, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209(4):819–835. doi: 10.1084/jem.20112322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geddings JE, Hisada Y, Boulaftali Y, et al. Tissue factor-positive tumor microvesicles activate platelets and enhance thrombosis in mice. J Thromb Haemost. 2016;14(1):153–166. doi: 10.1111/jth.13181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood. 2014;123(18):2768–2776. doi: 10.1182/blood-2013-10-463646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sridhar DC, Abou-Ismail MY, Ahuja SP. Central venous catheter-related thrombosis in children and adults. Thromb Res. 2020;187:103–12. doi: 10.1016/j.thromres.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 35.Olson SR, Murphree CR, Zonies D, et al. Thrombosis and bleeding in extracorporeal membrane oxygenation (ECMO) without anticoagulation: a systematic review. ASAIO J. 2021;67(3):290–296. doi: 10.1097/MAT.0000000000001230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fredenburgh JC, Weitz JI. New anticoagulants: moving beyond the direct oral anticoagulants. J Thromb Haemost. 2021;19(1):20–29. doi: 10.1111/jth.15126 [DOI] [PubMed] [Google Scholar]
- 37.Perera V, Wang Z, Luettgen J, et al. First‐in‐human study of milvexian, an oral, direct, small molecule factor XIa inhibitor. Clin Transl Sci. 2021;15:330–342. doi: 10.1111/cts.13148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubitza D, Heckmann M, Distler J, Koechel A, Schwers S, Kanefendt F. Pharmacokinetics, pharmacodynamics and safety of BAY 2433334, a novel activated factor XI inhibitor, in healthy volunteers: a randomized Phase 1 multiple‐dose study. Br J Clin Pharmacol. 2022. doi: 10.1111/bcp.15230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorentz CU, Verbout NG, Wallisch M, et al. Contact activation inhibitor and factor xi antibody, AB023, produces safe, dose-dependent anticoagulation in a phase 1 first-in-human trial. Arterioscler Thromb Vasc Biol. 2019;39(4):799–809. doi: 10.1161/ATVBAHA.118.312328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi BA, Freedholm D, Widener N, et al. Pharmacokinetics and pharmacodynamics of Abelacimab (MAA868), a novel dual inhibitor of factor XI and factor XIa. J Thromb Haemost. 2022;20(2):307–315. doi: 10.1111/jth.15577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weitz JI, Bauersachs R, Becker B, et al. Effect of osocimab in preventing venous thromboembolism among patients undergoing knee arthroplasty. JAMA. 2020;323(2):130. doi: 10.1001/jama.2019.20687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Büller HR, Bethune C, Bhanot S, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372(3):232–240. doi: 10.1056/NEJMoa1405760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verhamme P, Yi BA, Segers A, et al. Abelacimab for prevention of venous thromboembolism. N Engl J Med. 2021;385(7):609–617. doi: 10.1056/NEJMoa2105872 [DOI] [PubMed] [Google Scholar]
- 44.Weitz JI, Strony J, Ageno W, et al. Milvexian for the prevention of venous thromboembolism. N Engl J Med. 2021;385(23):2161–2172. doi: 10.1056/NEJMoa2113194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Horani RA. Factor XI(a) inhibitors for thrombosis: an updated patent review (2016-present). Expert Opin Ther Pat. 2020;30(1):39–55. doi: 10.1080/13543776.2020.1705783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Horani RA. Targeting factor XI(a) for anticoagulation therapy: a patent landscape. Pharm Pat Anal. 2020;9(1):3–5. doi: 10.4155/ppa-2020-0002 [DOI] [PubMed] [Google Scholar]
- 47.Mackman N, Bergmeier W, Stouffer GA, Weitz JI. Therapeutic strategies for thrombosis: new targets and approaches. Nat Rev Drug Discov. 2020;19(5):333–352. doi: 10.1038/s41573-020-0061-0 [DOI] [PubMed] [Google Scholar]
- 48.Srivastava P, Gailani D. The rebirth of the contact pathway: a new therapeutic target. Curr Opin Hematol. 2020;27(5):311–319. doi: 10.1097/MOH.0000000000000603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weitz JI, Chan NC. Novel antithrombotic strategies for treatment of venous thromboembolism. Blood. 2020;135(5):351–359. doi: 10.1182/blood.2019000919 [DOI] [PubMed] [Google Scholar]
- 50.Ribic C, Crowther M. Thrombosis and anticoagulation in the setting of renal or liver disease. Hematology. 2016;2016(1):188–195. doi: 10.1182/asheducation-2016.1.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Catella J, Bertoletti L, Mismetti P, et al. Severe renal impairment and risk of bleeding during anticoagulation for venous thromboembolism. J Thromb Haemost. 2020;18:1728–1737. doi: 10.1111/jth.14837 [DOI] [PubMed] [Google Scholar]
- 52.Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38(5):496–520. doi: 10.1200/JCO.19.01461 [DOI] [PubMed] [Google Scholar]
- 53.Kraaijpoel N, van Es N, Bleker S, et al. Clinical impact and course of anticoagulant-related major bleeding in cancer patients. Thromb Haemost. 2018;118(01):174–181. doi: 10.1160/TH17-04-0274 [DOI] [PubMed] [Google Scholar]
- 54.Decousus H, Tapson VF, Bergmann JF, et al. Factors at admission associated with bleeding risk in medical patients: findings from the improve investigators. Chest. 2011;139(1):69–79. doi: 10.1378/chest.09-3081 [DOI] [PubMed] [Google Scholar]
- 55.Decousus H, Prandoni P, Mismetti P, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med. 2010;363(13):1222–1232. doi: 10.1056/NEJMoa0912072 [DOI] [PubMed] [Google Scholar]
- 56.Salomon O, Gailani D. A proposal for managing bleeding in patients on therapeutic factor XI(a) inhibitors. J Thromb Haemost. 2022;20(1):32–38. doi: 10.1111/jth.15579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Q, Bethune C, Dessouki E, Grundy J, Monia BP, Bhanot S. ISIS-FXIRx, A novel and specific antisense inhibitor of factor XI, caused significant reduction in FXI antigen and activity and increased aPTT without causing bleeding in healthy volunteers. Blood. 2011;118(21):209. doi: 10.1182/BLOOD.V118.21.209.209 [DOI] [Google Scholar]
- 58.Beale D, Dennison J, Boyce M, et al. ONO‐7684 a novel oral FXIa inhibitor: safety, tolerability, pharmacokinetics and pharmacodynamics in a first‐in‐human study. Br J Clin Pharmacol. 2021;87(8):3177–3189. doi: 10.1111/bcp.14732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas D, Kanefendt F, Schwers S, Unger S, Yassen A, Boxnick S. First evaluation of the safety, pharmacokinetics, and pharmacodynamics of BAY 2433334, a small molecule targeting coagulation factor XIa. J Thromb Haemost. 2021;19(10):2407–2416. doi: 10.1111/jth.15439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lorentz CU, Tucker EI, Verbout NG, et al. The contact activation inhibitor AB023 in heparin-free hemodialysis: results of a randomized Phase 2 clinical trial. Blood. 2021;138(22):2173–2184. doi: 10.1182/BLOOD.2021011725 [DOI] [PMC free article] [PubMed] [Google Scholar]