Abstract
Background:
Delirium is a common postoperative complication. Many studies have found that dexmedetomidine is associated with a reduced incidence of postoperative delirium (POD). This meta-analysis aimed to analyze the effects of dexmedetomidine on POD incidence among elderly patients undergoing general anesthesia.
Methods:
We searched 4 electronic databases (i.e., Pubmed, Embase, Cochrane, and Web of Science) from inception to November 30, 2020, for randomized controlled trials that evaluated the effects of dexmedetomidine in preventing the occurrence of POD in elderly patients (aged ≥60 years). The study protocol was registered in PROSPERO (CRD42020192114).
Results:
14 studies with 4173 patients showed that dexmedetomidine was significantly associated with a decreased POD incidence among elderly patients (relative risk [RR] = 0.58; 95% confidence interval [CI] = 0.44–0.76). The incidence of POD was significantly reduced in the noncardiac surgery group (RR 0.51; 95% CI 0.37–0.72), when dexmedetomidine was applied during the postoperative period (RR = 0.53; 95% CI = 0.40–0.70), and in patients received low-doses (RR = 0.54; 95% CI = 0.34–0.87) and normal-doses (RR = 0.59; 95% CI = 0.42–0.83). There were no significant differences in POD incidence in the cardiac surgery group (RR = 0.71; 95% CI = 0.45–1.11), and when dexmedetomidine was applied during the intra- (RR = 0.55; 95% CI = 0.29–1.01) or perioperative period (RR = 0.95; 95% CI = 0.64–1.40).
Conclusions:
Our meta-analysis suggests that dexmedetomidine may significantly reduce POD incidence in elderly noncardiac surgery patients and when applied during the postoperative period, in addition, both low- and normal-doses of dexmedetomidine may reduce POD incidence. However, its use in cardiac surgery patients and during the intra- or perioperative period may have no significant effects on POD incidence.
Keywords: dexmedetomidine, elderly patient, general anesthesia, postoperative delirium
1. Introduction
Delirium is an acute and fluctuating alteration in mental status that results in reduced awareness and attention disturbances.[1] It is a common postoperative complication, with a reported range in incidence of 10% to 53%.[2–8] Elderly patients are vulnerable to delirium, especially when undergoing major surgery. Postoperative delirium (POD) not only prolongs the length of hospital stay[9] and increases postoperative mortality,[10,11] but also increases the risk of subsequent dementia,[12,13] which greatly reduces the quality of life of the elderly.
Dexmedetomidine is a highly selective α2-adrenergic receptor agonist with sedative, anti-anxiety, and anti-sympathetic effects, widely used in various stages of the perianesthetic period.[14] Some meta-analyses have investigated the benefits of dexmedetomidine in preventing POD in elderly patients undergoing noncardiac surgery and found that dexmedetomidine may reduce POD incidence in this population.[15–17] A 2018 meta-analysis published by Duan et al[18] concluded that dexmedetomidine had a protective effect on the occurrence of POD among adult patients who underwent cardiac or noncardiac surgery. However, this meta-analysis was limited by the inclusion of 7 studies whose primary endpoints were not the incidence of delirium and some studies with a high risk of bias. Our meta-analysis re-pooled the randomized controlled trials (RCTs) that investigated dexmedetomidine's effects among elderly patients and whose primary outcome was POD. In addition, the results of 2 newly published RCTs suggested that dexmedetomidine does not reduce the incidence of POD among elderly surgical patients.[19,20] One of these studies utilized low-dose dexmedetomidine (0.1 μg/kg/h) during the postoperative period.[19] A few meta-analyses of elderly patients that underwent cardiac surgery and previous studies found that age and surgery type were both risk factors for POD.[1] Our meta-analysis focused on assessing the effects of dexmedetomidine on POD among elderly patients undergoing cardiac or noncardiac surgery and estimating the effects of different doses and timing of dexmedetomidine administration on POD incidence.
2. Methods
2.1. Protocol and registration
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42020192114). This study did not contain any participates and ethics approval and informed consent are not applicable.
2.2. Eligibility criteria
Inclusion criteria: RCTs, patients aged ≥60 years, general anesthesia patients, the intervention was intravenous dexmedetomidine, and the primary outcome was POD. Exclusion criteria: patients with serious preoperative mental illness, delirium, or severe dementia; failure to assess POD with the confusion assessment method (CAM) or confusion assessment method-intensive care unit (CAM-ICU). Since many RCTs used the cut-off ≥60 years to define elderly, our meta-analysis included all studies in which patients were aged ≥60 years.
2.3. Search strategy
We searched 4 electronic databases (i.e., Pubmed, Embase, Cochrane, and Web of Science) from database inception to November 30, 2020, for all RCTs that evaluated the effect of dexmedetomidine in preventing the occurrence of POD in elderly patients. The same keywords were searched as MeSH and free-text terms in each database: “Dexmedetomidine,” “aged, “delirium,” and “RCT.” There was no time restriction, but only English or Chinese language articles and human studies were included. A sample search strategy for PubMed is found in Figure S1, Supplemental Digital Content.
2.4. Data extraction
Two investigators (Youran W and Xinyi B) independently collected and charted the following data from each included study according to PICO model described previously: first author, year of publication, sex, age, surgery type, sample size, duration of surgery or anesthesia, dosage and timing of dexmedetomidine perfusion, methods used in the control group, methods used to assess delirium, the primary and secondary outcomes. These are summarized in Table 1. Discrepancies were discussed and adjudicated by a third investigator (Yali G).
Table 1.
Characteristics of all included studies.
| Studies | Age (yr) (Dex/Oth) | Sex (male/ female) | No. (Dex/Oth) | Surgery | Research center | Delirium assessment | Medicine administration |
| Jun Hu 2020[25] | 69.6/69.1 | None | 77/75 | Noncardiac surgery (Open transthoracic oesophagectomy) | Single-center | CAM | Dex: A loading dose of dexmedetomidine, 0.4 μg/kg, bolus was administered over 15 min immediately prior to induction of anaesthesia, followed by a maintenance dexmedetomidine infusion of 0.1 μg/kg/h until 1 h before the anticipated end of surgery. |
| Shokri 2020[26] | 63.8/64.4 | Dex: 77/67Clo: 62/80 | 144/142 | CABG | Single-center | CAM-ICU | Dex: 0.7–1.2 μg/kg/h after arriving on ICU, if the RASS score ranged from +1 to +4, the infusion rate of dexmedetomidine was increased up to the maximum dose of 1–1.4 μg/kg/h. Clo: 0.5 μg/kg intravenous infusion over a period of 10–15 min, followed by a continuous intravenous infusion of 1–2 μg/kg/h until extubation. |
| Li 2020[27] | 69.0/69.0 | Dex: 126/183Con: 120/190 | 309/310 | Noncardiac surgery | Single-center | CAM/CAM-ICU | Dex: 0.6 μg/kg loading dose before induction, followed by 0.5 μg/kg/h until 1h before the end of surgery. |
| Shi 2019[20] | 74.7/74.2 | Dex: 63/21Con: 56/24 | 84/80 | Cardiac surgery | Multi-center | CAM | Dex: 0.4–0.6 μg/kg/h until the end of surgery. |
| Sun 2019[19] | 68.0/69.0 | Dex: 161/120Con: 154/122 | 281/276 | Noncardiac Surgery (spine, orthopedic, urologic, thoracic, general surgery) | Single-center | CAM/CAM-ICU | Dex: 0.1 μg/kg/h immediately after surgery, the total duration <48 h. |
| Azeem 2018[28] | 65.3/66.7 | Dex: 17/13Con: 15/15 | 30/30 | Cardiac surgery | Single-center | CAM-ICU | Dex: loading dose of 1 μg/kg dexmedetomidine infused over 10 min immediately postoperative, followed by continuous infusion of 0.2–0.7 μg/kg/h, maintained <24 h after extubation. Com: Morphine in a dose of 10–50 μg/kg/h as an analgesic with midazolam in a dose of 0.05 mg/kg up to 0.2 mg/kg repeated as needed, and stopped before extubation. |
| Lee 2018[29] | 72.2/73.8 | Dex1: 44/51NS: 47/62 | 95/109 | Noncardiac surgery (Laparoscopic or robotic-assisted radical cystectomy/ partial or total nephrectomy/colorectal) | Single-center | CAM | Dex1: 1 μg/kg bolus followed by 0.2–0.7 μg/kg/h infusion from induction of anesthesia to the end of surgery; NS: received an equivalent volume of saline 15 min before the end of surgery. |
| Li 2017[30] | 66.4/67.5 | Dex: 95/47Con: 102/41 | 142/143 | Cardiac surgery | Two-center | CAM/CAM-ICU | Dex: 0.6 μg/kg loading dose once the intravenous access was established, followed by 0.4 μg/kg/h until the end of surgery, then received 0.1 μg/kg/h until the end of MV. |
| Deiner 2017[31] | 74.0/74.0 | Dex: 92/97Con: 98/103 | 189/201 | Noncardiac Surgery (spine, orthopedic, urologic, thoracic, general surgery) | Multi-center | CAM/CAM-ICU | Dex: 0.5 μg/kg/h on entering the operating room and was continued until 2 h into recovery. |
| Su 2016[32] | >65 | Non | 350/350 | Noncardiac Surgery (abdominal, thoracic, Spinal, Superficial and transurethral) | Two-center | CAM-ICU | Dex: 0.1 μg/kg/h within 1 h after ICU admission until 08:00 in the morning on the first day after surgery. |
| Liu 2016[33] | 71.2/72.8 | Dex: 26/34Con: 29/29 | 60/58 | Noncardiac surgery (hip, knee, or shoulder joint replacement) | Single-center | CAM | Dex: 0.2 - 0.4 μg/kg/h throughout the surgery and be stopped 20 min before the end of surgery. |
| Djaiani 2016[34] | 72.7/72.4 | Dex: 68/23Pro: 70/22 | 91/92 | Cardiac surgery | Single-center | CAM/CAM-ICU | Dex: 0.4 μg/kg bolus followed by 0.2–0.7 μg/kg/h infusion until arrive in ICU for 24 h, or not discontinued before extubation; Pro: received propofol infusion 25 to 50 μg/kg/min until extubation. |
| Guo 2015[35] | 71.9/70.7 | Dex: 41/37Con: 39/39 | 78/78 | Oral cancer | Single-center | CAM-ICU | Dex: 0.2 μg/kg/h after arrive on ICU for 12 h. |
| Shehabi 2009[36] | 71.5/71.0 | Dex: 114/38Mor: 111/36 | 152/147 | Cardiac surgery | Two-center | CAM-ICU | Dex: 0.1–0.7 μg/kg/h after arriving on ICU until removal of chest drain, when ready to discharge from ICU, or for up to 48 h of MV; Mor: 10–70 μg/kg/h same as Dexmedetomidine group. |
2.5. Outcome measures
The primary outcome was POD, which included participants who completed all trials. For POD classification, we required that patients be diagnosed by CAM or CAM-ICU within 7 days post-surgery. We also performed subgroup analyses by surgery type, Dexmedetomidine administration time, and ICU admission. Secondary outcomes included the duration of POD, extubation time, length of ICU and hospital stay, postoperative nausea or vomiting (PONV) rate, and mortality rate. Adverse outcomes of interest were bradycardia and hypotension. Both secondary outcomes and adverse effects were defined by each trial.
2.6. Risk of bias in individual
The Cochrane Risk of Bias Tool was used to assess the risk of bias for each RCT included in this meta-analysis. This assessment was performed independently by the same 2 authors who completed the data extraction. Seven domains were classified as having high, unclear, or low risk of bias. A graph and summary of the risk of bias analysis are shown in Figure S2, Supplemental Digital Content.
2.7. Statistical analysis
Meta-analyses were conducted using Review Manager version 5.3 (Cochrane Informatics and Knowledge Management Department, Denmark) and Stata version 15.1 (Stata Press, College Station, TX). Heterogeneity was quantified by the I2 statistic and classified as low (0%–40%), moderate (30%–60%), substantial (50%–90%), and considerable (75%–100%). According to the Cochrane Handbook,[21] the I2 statistic describes the percentage of variability in an effect estimate that is due to heterogeneity rather than sample size or chance. We applied fixed-effect models to estimate effect sizes when I2 ≤ 50% and random-effect models in analyses with high heterogeneity (I2 > 50%). Publication bias was assessed by examining asymmetry in the funnel plot, Begg test, and Egger test (STATA 15.1). Sensitivity analyses were performed by successively excluding each study to identify whether the included RCTs contributed to the heterogeneity. We formed subgroups to determine the source of heterogeneity and explore the impact of other risk factors on the primary outcome. For dichotomous data, we extracted the number of events and reported effect sizes as relative risks (RRs) with 95% confidence intervals (95% CIs). For continuous outcome data, we collected means and standard deviations (SDs) from each group. When studies reported continuous data as medians and interquartile ranges, we converted these data to means and SDs using the methods proposed by Wan et al[22] and Lou et al[23] If continuous data were reported as medians and 95% CIs, we calculated SDs using the formula ×(upper limit-low limit)/3.92. The effect sizes of continuous outcomes were reported as standard mean differences (SMDs, for data with different units) or mean differences (MDs, for data with the same unit) with 95% CIs.
2.8. Trial sequence analysis
Trial sequence analysis (TSA) aims to reduce the risk of false-positive results and false-negative results by adjusting the statistical significance boundary and determining the required information size.[24] In this meta-analysis, TSA was applied to each primary and secondary outcome. We set up the conventional boundary for 2-sided tests and used α = 0.05 to determine significance. We constructed the adjusted trial sequential monitoring boundary (TSMB) based on the O’Brien-Tleming alpha-spending method and calculated the required information size simultaneously, with a type-I error of 0.05 and power of 0.8. For dichotomous outcomes, we set the intervention arm's incidence as the incidence of POD calculated in each subgroup. The incidence of events in the control arm was computed from the control group of each outcome. For continuous outcomes, MD and variance were set as low bias. These analyses were performed by TSA 0.9.5.10 Beta software (http://www.ctu.dk/tsa).
2.9. Grading of recommendations, assessment, development and evaluations
We used the grading of recommendations, assessment, development, and evaluations (GRADE) framework (http://gradepro.org/) to assess the quality of evidence for all primary and secondary outcomes. The quality of evidence was classified as high, moderate, low, or very low.
3. Results
3.1. Search results
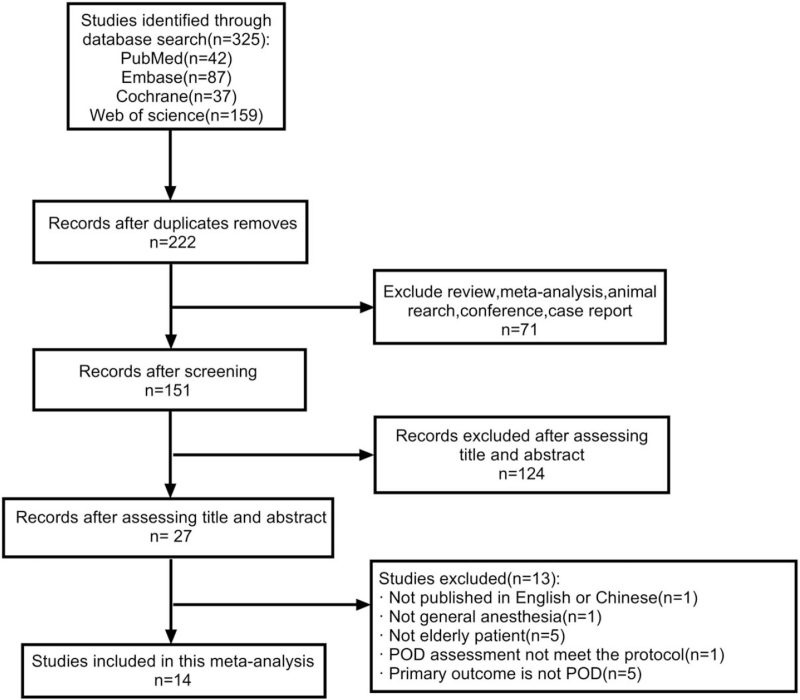
We carried out the study selection process according to pre-determined PICOS strategies. Twelve studies were included, as detailed in the flow chart of study selection (Fig. 1).
Figure 1.
Flow chart of study selection.
3.2. Study characteristics
As shown in Table 1, the timing and dosage of dexmedetomidine administration varied across studies. Six of 14 studies[25,27–30,34] used a maintenance rate of 0.1–0.7 μg/kg/h with a loading dose 0.4–1.0 μg/kg. The remaining 8 studies[19,20,26,31–33,35,36] infused dexmedetomidine at a speed of 0.1–1.2 μg/kg/h without loading doses. In 5 studies[20,25,27,29,33] dexmedetomidine was only administered during the intraoperative period; 7 studies[19,26,28,32,34–36] patients received dexmedetomidine after arrival at the ICU and were continuously infused until the end of mechanical ventilation (MV), the next morning, or 48 hours after surgery; and for the remaining 2 studies,[30,31] dexmedetomidine was administered throughout the perioperative period and up to 2 hours after surgery or before extubation. The control group was administered normal saline in 8 studies[25,27,29–33,35] and clonidine,[26] propofol,[34] morphine,[36] and morphine combined with midazolam,[28] respectively, in 3 studies. In the remaining 2 studies,[19,20] the control group did not receive any drugs or normal saline.
Six studies involved cardiac surgery[20,26,28,30,34,36] and 8 studies[19,25,27,29,31–33,35] noncardiac surgery patients. All of the cardiac surgeries were cardiopulmonary bypasses. The noncardiac surgeries included different types of surgeries, such as major abdominal, orthopedic, oral cavity, and general surgery. Finally, 9 trials[19,25–29,33–35] were single-centre studies, 3 trials[30,32,36] were 2-centre studies, and the remaining 2 trials[20,31] were multi-centre studies.
3.3. Risk of bias and publication bias
All of the included studies were at low risk of bias. Figure S2, Supplemental Digital Content provides the details of the risk of bias for each trial. A funnel plot was constructed to determine the publication bias (see Figure S3, Supplemental Digital Content). Begg test and Egger test (see Figure S4, Supplemental Digital Content) provided P-values of .381 > .1 and .201 > .1, respectively, suggesting that the funnel plot was symmetrical and there was no publication bias overall.
3.4. Primary outcome
Fourteen studies[19,20,25–36] of 4173 patients were included in our meta-analysis (see Table S1, Supplemental Digital Content, which is a summary of primary outcomes). The pooled primary outcome revealed that dexmedetomidine was associated with a decreased incidence of POD among elderly patients (RR = 0.58; 95% CI = 0.44–0.76; P < .0001; I2 = 65%; see Figure S5, Supplemental Digital Content, which illustrates that dexmedetomidine can significant reduce the POD incidence). We performed sensitivity analyses due to substantial heterogeneity and found that 2 multi-center studies were the source of heterogeneity.[20,31] Therefore, a subgroup analysis of different research center types was performed with 2335 patients in 9 single-center studies,[19,25–29,33–35] 1284 patients in 3, 2-center studies,[30,32,36] and 554 patients in 2, multi-center studies.[20,31] The forest plot (see Figure S6, Supplemental Digital Content, which shows the effect of dexmedetomidine on the incidence of POD as related to the number of centers) revealed low or moderate heterogeneity in each subgroup, and considerable differences among the 3 groups demonstrated that research center type was the source of heterogeneity. Moreover, the single-center and 2-center studies found a significant decrease in POD, while nonsignificant findings occurred in the multi-center studies. This was confirmed by the TSA results in the 3 groups. Specifically, the TSA indicated that the number of participants reached the required information size, and the Z-curve crossed the TSMB in the single-center group. The required information size was reached according to sample size, but the Z-curve did not cross the TSMB in the 2-center group, which indicated that this group was at risk of a false-positive result. Finally, in the multi-center group, the number of participants did not reach the required information size, and the cumulative Z-curve neither crossed the conventional boundary and TSMB nor crossed the futility boundary, which indicated the possibility of false-negative results. Therefore, the TSA revealed that more RCTs should be included in further analyses. Grade scores for the primary outcomes ranged from very low to high, the details of the overall and subgroup analyses are provided in Table S2, Supplemental Digital Content, which shows the GRADE levels for POD incidence of every subgroup.
3.5. Cardiac or noncardiac surgery
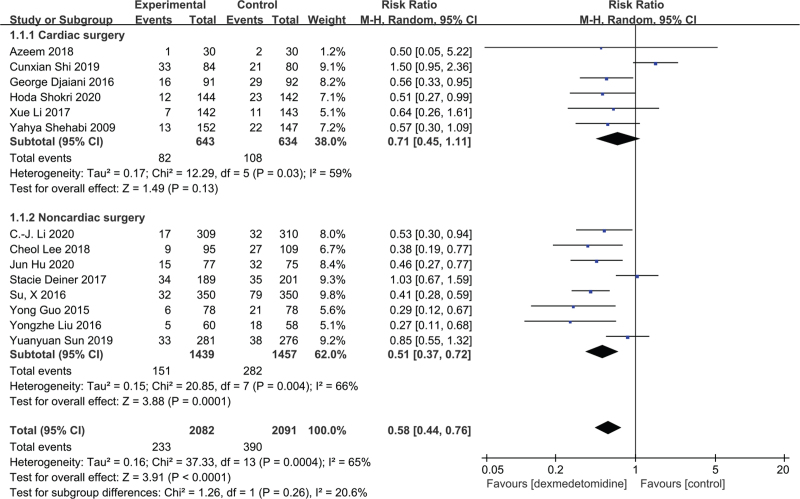
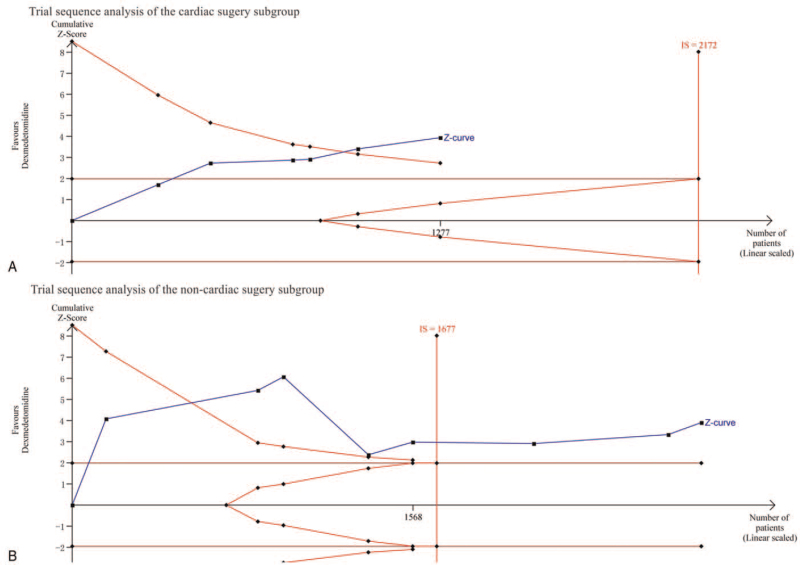
Subgroup analyses by surgery type included 1277 cardiac surgery patients in 6 studies[20,26,28,30,34,36] and 2896 noncardiac surgery patients in 8 studies.[19,25,27,29,31–33,35] The forest plot (Fig. 2) found no significant effects in the cardiac surgery group (RR = 0.71; 95% CI = 0.45–1.11; P = .13; I2 = 59%), whereas POD incidence was significantly reduced in the noncardiac surgery group (RR = 0.51; 95% CI = 0.37–0.72; P = .0001; I2 = 66%). The TSA confirmed the findings in each group (Fig. 3). Specifically, the cumulative Z-curve crossed the TSMB in both cardiac and noncardiac groups, while the required information size was not reached in the cardiac surgery group and reached in the noncardiac surgery group. The evidence was graded as low due to inconsistencies and imprecision in the cardiac surgery group, and graded as moderate due to inconsistencies in the noncardiac surgery group (see Table S2, Supplemental Digital Content).
Figure 2.
The effect of dexmedetomidine on incidence of postoperative delirium following cardiac and noncardiac surgery.
Figure 3.
(A) Trial sequence analysis of the cardiac surgery subgroup: error α = 0.05, β = 0.2, incidence in the intervention arm: 12.75%, incidence in the control arm: 17.03%. (B) Trial sequence analysis of the noncardiac surgery subgroup: error α = 0.05, β = 0.2, incidence in the intervention arm: 10.49%, incidence in the control arm: 19.35%.
3.6. Administration time of dexmedetomidine
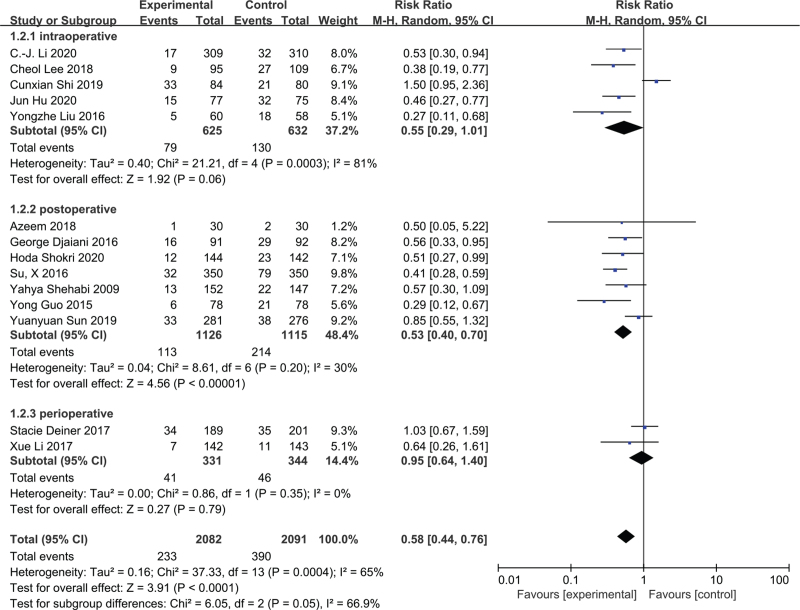
Further subgroup analyses were performed for administration time of dexmedetomidine (Fig. 4). The subgroups consisted of 5 studies[20,25,27,29,33] comprising 1257 patients who received dexmedetomidine during the intraoperative period, 7 studies[19,26,28,32,34–36] comprising 2241 patients infused after surgery, and 2 studies[30,31] including 675 patients administered dexmedetomidine throughout the perioperative period (during intra- to postoperative periods). The forest plot indicated that there were no significant differences in POD incidence in the intraoperative (RR = 0.55; 95% CI = 0.29–1.01; P = .06; I2 = 81%) and perioperative period (RR = 0.95; 95% CI = 0.64–1.40; P = .79; I2 = 0%) groups. However, POD was significantly reduced in the postoperative period group (RR = 0.53; 95% CI = 0.40–0.70; P < .00001; I2 = 30%). Based on the TSA, firm evidence was only found in the postoperative period group (see Figure S7B, Supplemental Digital Content, which shows TSA for the postoperative period subgroup.) and the quality of evidence of this group was graded as high (see Table S2, Supplemental Digital Content ). The TSA revealed an absence of evidence in the other 2 groups (see Figures S7A and S7C, Supplemental Digital Content, which shows TSA for the intra- and perioperative period subgroup) and their GRADE assessments are provided in Table S2, Supplemental Digital Content.
Figure 4.
The effect of the timing of dexmedetomidine administration on incidence of postoperative delirium.
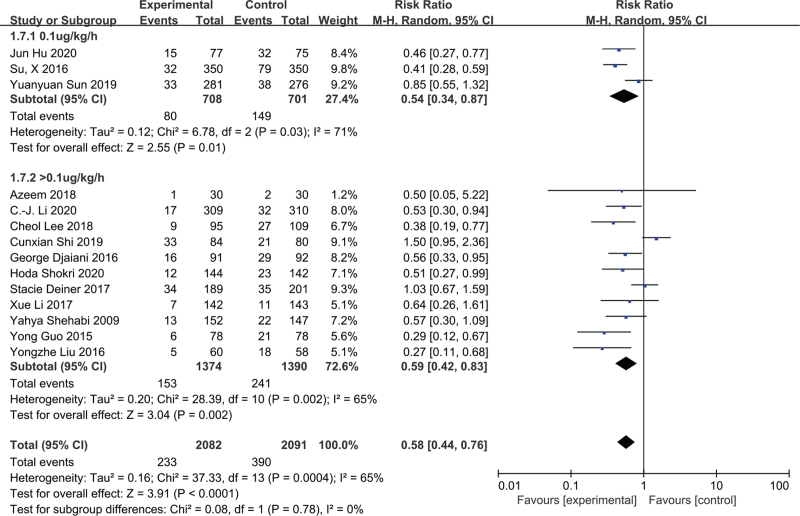
3.7. Dexmedetomidine dosage
We created 2 subgroups according to dexmedetomideine infusion rate. These included a low dose (i.e., 0.1 μg/kg/h) and normal dose (>0.1 μg/kg/h) group. The low-dose group was comprised of 3 studies[19,25,32] and 1409 patients, and the normal-dose group consisted of 11 studies and 2764 patients.[20,26–31,33–36] Dexmedetomidine was associated with a significantly reduced POD incidence in both 2 groups (RR = 0.54; 95% CI = 0.34–0.87; P = .01; I2 = 71%; and RR = 0.59; 95% CI = 0.42–0.83; P = .002; I2 = 65%; Fig. 5). The TSA indicated firm evidence for both the low- and normal-dose groups (see Figure S8, Supplemental Digital Content, which shows TSA for the low-does and the normal-does subgroup), a low quality of evidence that was downgraded by inconsistency and imprecision in the low-dose group, and a moderate quality of evidence that was downgraded by inconsistency in the normal-dose group (see Table S2, Supplemental Digital Content).
Figure 5.
The effect of the dose of dexmedetomidine on incidence of postoperative delirium.
3.8. Secondary results
Six studies[20,26,29,30,34,36] of 223 patients revealed that dexmedetomidine shortened the duration of POD (days, MD = −1.24; 95% CI = -−1.91 to −0.57; P = 0.0003; I2 = 80%). Eight studies[20,25,26,28,30,32,34,36] with 2129 patients described the extubation time and revealed that dexmedetomidine may shorten the length of MV (SMD −0.59; 95% CI −1.17 to −0.01; P = .05; I2 = 97%). Length of ICU stay was described in 8 studies[20,26–28,30,32,34,36] of 2596 patients, and was significantly shortened by administration of dexmedetomidine (SMD = −0.28; 95% CI = −0.48 to −0.08; P = 0.006; I2 = 84%). Analysis of 3483 patients in 9 studies[19,20,26,27,30–32,34,36] found no significant difference in the length of hospital stay (days, MD = −1.53; 95% CI = 3.06 to 0.00; P = 0.05; I2 = 97%). Six studies[19–20,25,27,35,36] with 1947 patients indicated that dexmedetomidine may curtail the PONV rate (RR = 0.75; 95% CI = 0.59–0.97; P = 0.03; I2 = 45%). An analysis of 3319 patients from 8 studies[19,26,27,30–32,34,36] revealed that dexmedetomidine was associated with a decreased mortality rate (RR = 0.45; 95% CI = 0.23–0.90; P = 0.02; I2 = 0%). Further details are provided in Table S3, Supplemental Digital Content which is a summary of secondary outcomes.
The results for extubation time and length of ICU stay were confirmed by the TSA, and the GRADE level was very low. Other secondary outcomes such as duration of POD, or hospital stay, PONV, and mortality rate were not supported by the TSA. Additionally, with the exception of mortality rate (which was graded as moderate), the quality of evidence underlying these secondary outcomes was rated as low or very low by GRADE (see Table S4, Supplemental Digital Content, which shows the GRADE levels of secondary outcomes).
3.9. Adverse effects
An analysis of 9 studies[19,25–27,30–32,35,36] with 3444 patients found that the incidence of bradycardia was increased in the dexmedetomidine group (RR = 1.47; 95% CI = 1.23–1.75; P < .0001; I2 = 22%). The TSA indicated the presence of firm evidence, and GRADE suggested high-quality evidence. Conversely, the same 8 studies did not find a significant difference in the incidence of hypotension (RR = 1.07; 95% CI = 0.96–1.19; P = .24; I2 = 49%), while the TSA revealed the absence of evidence to support this result and the GRADE quality was low (see Table S4, Supplemental Digital Content).
3.10. Other secondary outcomes
Other outcomes such as postoperative pain score and consumption of opioids decreased in the dexmedetomidine group, and the sleep quality score after surgery was improved. However, more RCTs are needed for meta-analyses to confirm these results.
4. Discussion
The overall pooled results of our meta-analysis indicated that dexmedetomidine may significantly reduce POD occurrence. In contrast to the study by Duan et al,[18] our meta-analysis found that dexmedetomidine only reduced POD incidence in patients undergoing noncardiac surgery and had no significant association with POD occurrence in cardiac surgery. A TSA confirmed these results. Probable explanations for the lack of association in the cardiac surgery group include the low GRADE level of the cardiac surgery subgroup, which was downgraded by substantial heterogeneity, and the cardiac surgery subgroup's sample size not meeting the required information size in our meta-analysis. Both indicate the need to carry out more high-quality RCTs to confirm our meta-analysis results in cardiac surgery.
Unlike propofol,[37] dexmedetomidine's sedation process is more in line with the physiological state.[38] It is widely used for procedural sedation in ICU patients[39] and as an adjuvant anesthetics during surgery.[40] We conducted a subgroup analysis of the treatment time of dexmedetomidine and found that postoperative administration of dexmedetomidine may significantly reduce POD occurrence in elderly patients, but the associations with intraoperative or perioperative administration were not apparent. A TSA was conducted on the 3 groups. The results confirmed the findings of the postoperative administration group (i.e., the Z-curve crossed the TSMB and the sample size reached the required information size) and indicated an absence of evidence in the intraoperative and perioperative administration groups (i.e., the sample sizes did not reach the required information sizes and Z-curves did not cross the conventional threshold and TSMB, suggesting that the traditional meta-analysis may have given a false negative conclusion). Additionally, the GRADE levels of the 2 groups were very low and low, respectively. Therefore, the results obtained in these 2 groups cannot confirm the effects of dexmedetomidine during the intraoperative or perioperative period. More RCTs are needed to establish the effects of intraoperative or perioperative administration of dexmedetomidine on POD in elderly patients.
The optimal dose of dexmedetomidine for decreasing the incidence of POD is unknown. A dexmedetomidine infusion rate of 0.2 to 0.7 μg/kg/h is generally recommended for maintenance.[41] Previous studies came to conflicting conclusions about the effects of different doses of dexmedetomidine, especially at a low-dose rate of 0.1 μg/kg/h.[42] In our meta-analysis, we performed a subgroup analysis to investigate whether dexmedetomidine dose was associated with POD incidence. Our results indicated that both low and normal-doses of dexmedetomidine may reduce the incidence of POD. Both groups’ pooled results displayed substantial heterogeneity (I2 = 71% and 65%, respectively). However, the results of the TSA and GRADE classification indicated that the normal-dose group's results were reliable. The TSA results also confirmed the findings of the low-dose group (i.e., the Z-curve crossed the TSMB, although the sample size did not reach the required information size), but the GRADE level was low, which indicated that no more RCTs are needed to further investigate the reliability of these results.
Similar to previous studies,[43–47] our meta-analysis found that dexmedetomidine significantly reduced the duration of POD, length of postoperative MV, length of ICU stay, incidence of PONV, and postoperative mortality. However, dexmedetomidine was not significantly associated with length of hospital stay, which the TSA suggested may be a false-negative result. Moreover, the low or very low GRADE level indicated that more RCTs are needed to confirm these findings.
Bradycardia and hypotension are common adverse effects of dexmedetomidine caused by the pharmacological properties of the α2-adrenergic receptor agonists.[15] A retrospective study found that perioperative hypotension was also a risk factor for delirium.[48] In our meta-analysis, analyses of adverse effects indicated that dexmedetomidine may increase the incidence of bradycardia and have no significant effects on the incidence of hypotension. However, a TSA suggested the absence of evidence to support these findings, and the GRADE evidence level was very low.
This meta-analysis has several limitations. First, in 2018, postoperative cognitive dysfunction was renamed perioperative neurocognitive disorders. The diagnostic criteria for POD were re-defined within the Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition as occurring within 1 week after surgery or before discharge, meaning that the follow-up time was longer than before. The studies included in our meta-analysis only followed patients up to 5 days after surgery. Consequently, delirium that occurred during postoperative days 5-to-7 may have been missed. Second, in the meta-analysis, there was a lot of heterogeneity in the cardiac and noncardiac surgery groups, intraoperative administration group, and ICU and non-ICU admission groups (I2 > 50%). The GRADE level of evidence was only high or moderate in the noncardiac surgery, postoperative administration, normal-dose dexmedetomidine, and bradycardia groups. The quality of the evidence of the other groups was graded as low or very low, indicating that more RCTs are needed to confirm the reliability of these results. Third, postoperative pain may also increase the occurrence of POD. However, the type and timing of pain assessments used in the studies included in our meta-analysis were diverse. Therefore, we were not able to perform a meta-analysis of this outcome.
5. Conclusion
Our meta-analysis suggested that dexmedetomidine may reduce the incidence of POD after noncardiac, but not cardiac, surgery. Furthermore, postoperative administration of dexmedetomidine, and low- or normal-doses of dexmedetomidine, may also reduce POD incidence. However, use of dexmedetomidine during the intra- or perioperative period may have no significant effects on POD incidence.
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.com/) for the expert linguistic services provided.
Author contributions
Conceptualization: Youran Wang, Yali Ge.
Data curation: Youran Wang, Xinyi Bu.
Formal analysis: Youran Wang.
Software: Youran Wang, Na Zhao, Shuxia Wang.
Supervision: Yali Ge, Honggang Yi.
Verifying Statistical Method: Honggang Yi.
Writing – original draft: Youran Wang.
Writing – review & editing: Xinyi Bu, Xiaoliang Wang, Yali Ge, Honggang Yi.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: 95% CIs = 95% confidence intervals, CAM = confusion assessment method, CAM-ICU = confusion assessment method-intensive care unit, GRADE = grading of recommendations, assessment, development and evaluations, MDs = mean differences, POD = postoperative delirium, PONV = postoperative nausea or vomiting, RCTs = randomized controlled trials, RRs = relative risks, SDs = standard deviations, SMDs = standard mean differences, TSA = trial sequence analysis, TSMB = trial sequential monitoring boundary.
How to cite this article: Wang Y, Bu X, Zhao N, Wang S, Wang X, Ge Y, Yi H. Dexmedetomidine effect on delirium in elderly patients undergoing general anesthesia: a protocol for systematic review and meta-analysis. Medicine. 2021;100:48(e27782).
The datasets generated during and/or analyzed during the current study are publicly available.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article.
Age was expressed as mean.
CAM = confusion assessment method, CAM-ICU = confusion assessment method in the ICU, Clo = clonidine, Dex = dexmedetomidine, Mor = morphine, MV = mechanical ventilation, Pro = propofol.
References
- [1].Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol 2017;34:192–214. [DOI] [PubMed] [Google Scholar]
- [2].Bruce AJ, Ritchie CW, Blizard R, Lai R, Raven P. The incidence of delirium associated with orthopedic surgery: a meta-analytic review. Int Psychogeriatr 2007;19:197–214. [DOI] [PubMed] [Google Scholar]
- [3].Janssen TL, Steyerberg EW, Faes MC, et al. Risk factors for postoperative delirium after elective major abdominal surgery in elderly patients: a cohort study. Int J Surg 2019;71:29–35. [DOI] [PubMed] [Google Scholar]
- [4].Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg 2009;249:173–8. [DOI] [PubMed] [Google Scholar]
- [5].Chaiwat O, Chanidnuan M, Pancharoen W, et al. Postoperative delirium in critically ill surgical patients: incidence, risk factors, and predictive scores. BMC Anesthesiol 2019;19:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ansaloni L, Catena F, Chattat R, et al. Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. Br J Surg 2010;97:273–80. [DOI] [PubMed] [Google Scholar]
- [7].Mu DL, Wang DX, Li LH, et al. High serum cortisol level is associated with increased risk of delirium after coronary artery bypass graft surgery: a prospective cohort study. Crit Care 2010;14:R238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sanson G, Khlopenyuk Y, Milocco S, Sartori M, Dreas L, Fabiani A. Delirium after cardiac surgery. Incidence, phenotypes, predisposing and precipitating risk factors, and effects. Heart Lung 2018;47:408–17. [DOI] [PubMed] [Google Scholar]
- [9].Edelstein DM, Aharonoff GB, Karp A, Capla EL, Zuckerman JD, Koval KJ. Effect of postoperative delirium on outcome after hip fracture. Clin Orthop Relat Res 2004;422:195–200. [DOI] [PubMed] [Google Scholar]
- [10].Sanders RD, Pandharipande PP, Davidson AJ, Ma D, Maze M. Anticipating and managing postoperative delirium and cognitive decline in adults. BMJ 2011;343:d4331. [DOI] [PubMed] [Google Scholar]
- [11].Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010;304:443–51. [DOI] [PubMed] [Google Scholar]
- [12].Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults. Lancet Neurol 2015;14:823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Inouye SK, Marcantonio ER, Kosar CM, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement 2016;12:766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bao N, Tang B. Organ-protective effects and the underlying mechanism of dexmedetomidine. Mediators Inflamm 2020;2020:6136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shen QH, Li HF, Zhou XY, Yuan XZ. Dexmedetomidine in the prevention of postoperative delirium in elderly patients following non-cardiac surgery: a systematic review and meta-analysis. Clin Exp Pharmacol Physiol 2020;47:1333–41. [DOI] [PubMed] [Google Scholar]
- [16].Pan H, Liu C, Ma X, Xu Y, Zhang M, Wang Y. Perioperative dexmedetomidine reduces delirium in elderly patients after non-cardiac surgery: a systematic review and meta-analysis of randomized-controlled trials. Can J Anaesth 2019;66:1489–500. [DOI] [PubMed] [Google Scholar]
- [17].Zeng H, Li Z, He J, Fu W. Dexmedetomidine for the prevention of postoperative delirium in elderly patients undergoing noncardiac surgery: a meta-analysis of randomized controlled trials. PLoS One 2019;14:e0218088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Duan X, Coburn M, Rossaint R, Sanders RD, Waesberghe JV, Kowark A. Efficacy of perioperative dexmedetomidine on postoperative delirium: systematic review and meta-analysis with trial sequential analysis of randomised controlled trials. Br J Anaesth 2018;121:384–97. [DOI] [PubMed] [Google Scholar]
- [19].Sun Y, Jiang M, Ji Y, Sun Y, Liu Y, Shen W. Impact of postoperative dexmedetomidine infusion on incidence of delirium in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. Drug Des Devel Ther 2019;13:2911–22. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [20].Shi C, Jin J, Qiao L, Li T, Ma J, Ma Z. Effect of perioperative administration of dexmedetomidine on delirium after cardiac surgery in elderly patients: a double-blinded, multi-center, randomized study. Clin Interv Aging 2019;14:571–5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [21].Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons; 2011. [Google Scholar]
- [22].Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785–805. [DOI] [PubMed] [Google Scholar]
- [24].Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis (TSA), Vol. 1. 2011;Copenhagen, Denmark: Copenhagen Trial Unit, Centre for Clinical Intervention Research, 1–115. [Google Scholar]
- [25].Hu J, Zhu M, Gao Z, et al. Dexmedetomidine for prevention of postoperative delirium in older adults undergoing oesophagectomy with total intravenous anaesthesia: A double-blind, randomised clinical trial. Eur J Anaesthesiol 2020;38:S9–17. [DOI] [PubMed] [Google Scholar]
- [26].Shokri H, Ali I. A randomized control trial comparing prophylactic dexmedetomidine versus clonidine on rates and duration of delirium in older adult patients undergoing coronary artery bypass grafting. J Clin Anesth 2020;61:109622. [DOI] [PubMed] [Google Scholar]
- [27].Li CJ, Wang BJ, Mu DL, et al. Randomized clinical trial of intraoperative dexmedetomidine to prevent delirium in the elderly undergoing major non-cardiac surgery. Br J Surg 2020;107:e123–32. [DOI] [PubMed] [Google Scholar]
- [28].Azeem TMA, Yosif NE, Alansary AM, Esmat IM, Mohamed AK. Dexmedetomidine vs morphine and midazolam in the prevention and treatment of delirium after adult cardiac surgery; a randomized, double-blinded clinical trial. Saudi J Anaesth 2018;12:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lee C, Lee CH, Lee G, Lee M, Hwang J. The effect of the timing and dose of dexmedetomidine on postoperative delirium in elderly patients after laparoscopic major non-cardiac surgery: a double blind randomized controlled study. J Clin Anesth 2018;47:27–32. [DOI] [PubMed] [Google Scholar]
- [30].Li X, Yang J, Nie XL, et al. Impact of dexmedetomidine on the incidence of delirium in elderly patients after cardiac surgery: a randomized controlled trial. PLoS One 2017;12:e0170757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Deiner S, Luo X, Lin HM, et al. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. JAMA Surg 2017;152:e171505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016;388:1893–902. [DOI] [PubMed] [Google Scholar]
- [33].Liu Y, Ma L, Gao M, Guo W, Ma Y. Dexmedetomidine reduces postoperative delirium after joint replacement in elderly patients with mild cognitive impairment. Aging Clin Exp Res 2016;28:729–36. [DOI] [PubMed] [Google Scholar]
- [34].Djaiani G, Silverton N, Fedorko L, et al. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology 2016;124:362–8. [DOI] [PubMed] [Google Scholar]
- [35].Guo Y, Sun LL, Chen ZF, Li QF, Jiang H. Preventive effect of dexmedetomidine on postoperative delirium in elderly patients with oral cancer. Shanghai Kou Qiang Yi Xue 2015;24:236–9. [PubMed] [Google Scholar]
- [36].Shehabi Y, Grant P, Wolfenden H, et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study). Anesthesiology 2009;111:1075–84. [DOI] [PubMed] [Google Scholar]
- [37].Guldenmund P, Vanhaudenhuyse A, Sanders RD, et al. Brain functional connectivity differentiates dexmedetomidine from propofol and natural sleep. Br J Anaesth 2017;119:674–84. [DOI] [PubMed] [Google Scholar]
- [38].Sanders RD, Maze M. Contribution of sedative-hypnotic agents to delirium via modulation of the sleep pathway. Can J Anaesth 2011;58:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shehabi Y, Howe BD, Bellomo R, et al. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med 2019;380:2506–17. [DOI] [PubMed] [Google Scholar]
- [40].Kamibayashi T, Maze M. Clinical uses of alpha2-adrenergic agonists. Anesthesiology 2000;93:1345–9. [DOI] [PubMed] [Google Scholar]
- [41].Li X, Wang X, Jin S, Zhang D, Li Y. The safety and efficacy of dexmedetomidine-remifentanil in children undergoing flexible bronchoscopy: a retrospective dose-finding trial. Medicine (Baltimore) 2017;96:e6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lee H, Yang SM, Chung J, et al. Effect of perioperative low-dose dexmedetomidine on postoperative delirium after living-donor liver transplantation: a randomized controlled trial. Transplant Proc 2020;52:239–45. [DOI] [PubMed] [Google Scholar]
- [43].Peng K, Ji FH, Liu HY, Zhang J, Chen QC, Jiang YH. Effects of perioperative dexmedetomidine on postoperative mortality and morbidity: a systematic review and meta-analysis. Clin Ther 2019;41:138–54.e4. [DOI] [PubMed] [Google Scholar]
- [44].Sampson EL, West E, Fischer T. Pain and delirium: mechanisms, assessment, and management. Eur Geriatr Med 2020;11:45–52. [DOI] [PubMed] [Google Scholar]
- [45].Blaudszun G, Lysakowski C, Elia N, Tramèr MR. Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology 2012;116:1312–22. [DOI] [PubMed] [Google Scholar]
- [46].Liang X, Zhou M, Feng JJ, et al. Efficacy of dexmedetomidine on postoperative nausea and vomiting: a meta-analysis of randomized controlled trials. Int J Clin Exp Med 2015;8:8450–71. [PMC free article] [PubMed] [Google Scholar]
- [47].Ji F, Li Z, Nguyen H, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation 2013;127:1576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Maheshwari K, Ahuja S, Khanna AK, et al. Association between perioperative hypotension and delirium in postoperative critically ill patients: a retrospective cohort analysis. Anesth Analg 2020;130:636–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.