Abstract
PURPOSE:
Patients who carry reduced-activity DPYD polymorphisms have increased fluoropyrimidine (FP) toxicity risk. Although pretreatment DPYD testing is recommended throughout most of Europe, it is not recommended in the United States, and adoption has been limited. The objective of this survey was to describe the current practice in the United States regarding pretreatment DPYD testing and understand the factors deterring oncologists from ordering testing.
METHODS:
Survey invitations were e-mailed to 325 medical oncologists practicing in the United States who are members of the SWOG Cancer Research Network Gastrointestinal Cancer, Breast Cancer, or Early Therapeutics Committees. Descriptive statistics were used to evaluate survey responses.
RESULTS:
Responses were collected from 59 (18.2%) US medical oncologists, of whom 98% strongly or somewhat agree that patients with dihydropyrimidine dehydrogenase (DPD) deficiency have increased toxicity risk and 96% would modify FP dosing for a patient with known DPD deficiency. However, only 32% strongly or somewhat agree that pretreatment DPYD testing is useful to inform FP treatment, 20% have ever ordered pretreatment testing, and 3% order testing for at least 10% of their FP-treated patients. The most important factors that deter oncologists from ordering testing were low prevalence of DPD deficiency (54%) and lack of clinical practice guideline recommendations (48%).
CONCLUSION:
Clinical adoption of pretreatment DPYD testing is extremely limited in the United States. Utilization may be substantially increased by inclusion in the oncology clinical practice guideline recommendations, coverage through health insurance, and potentially education of medical oncologists regarding available treatment modification guidelines.
INTRODUCTION
The fluoropyrimidines (FP), fluorouracil and its oral analog capecitabine, are antimetabolite chemotherapy agents widely used for the treatment of various solid tumors and have served as a backbone of chemotherapy regimens for more than 60 years.1 These agents are recommended within National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for the management of colon, rectal, pancreatic, biliary tract, gastric, head and neck, ovarian, and breast cancers.2-8 Although generally well tolerated, up to 30% of patients treated with FP experience severe toxicity such as diarrhea, nausea, mucositis, myelosuppression, neurotoxicity, and hand-foot syndrome, which can lead to treatment-related death in up to 1% of patients.9
Dihydropyrimidine dehydrogenase (DPD) is the primary enzyme responsible for metabolic elimination of the FP.1 Polymorphisms in DPYD, the gene encoding DPD, reduce DPD activity. Patients who carry pathogenic DPYD variants have partial or complete loss of DPD activity and are at increased risk of severe FP toxicity. In Caucasians, approximately 6% of patients inherit at least one nonfunctional or reduced-function allele, leading to partial DPD deficiency and approximately 0.2% inherit two nonfunctional alleles leading to complete DPD deficiency, whereas about approximately 8% of African Americans have partial or complete DPD deficiency.10,11
More than 300 DPYD variants have been identified12 and at least four variants (DPYD*2A [c.1905+1G>A], DPYD*13 [c1679T>G], p.D949V [c.2846A>T], and HapB3 [c.1129-5923C>G, c.1236G>A]) have been confirmed to increase risk of severe FP toxicity.13,14 The increased risk of FP toxicity is acknowledged in the US Food and Drug Administration (FDA) labeling of these drugs15,16 and in the NCCN guidelines for colon cancer treatment.2 FP dose reduction in carriers of DPYD variants has been prospectively demonstrated to reduce severe toxicity and health care costs.17-19 The Clinical Pharmacogenetics Implementation Consortium (CPIC), an interdisciplinary group that develops evidence-based clinical practice guidelines for pharmacogenetic-guided medication therapy, recommends 50% dose reduction in DPYD intermediate metabolizers and recommends > 90% reduction or avoiding FP therapy in poor metabolizers.20
On the basis of the strong evidence of clinical benefit, pretreatment DPYD testing is recommended by European Society of Medical Oncology (ESMO) guidelines21 and is recommended or required throughout most of Europe.22-24 However, pretreatment DPYD testing is not recommended by the FDA15,16 or any national oncology practice guidelines in the United States such as NCCN and ASCO,2,22 and thus, test adoption is believed to be limited.25 The objective of this survey was to identify US medical oncologists' current practices and beliefs regarding pretreatment DPYD testing and understand the factors deterring medical oncologists from ordering the test.
METHODS
Survey Development
The initial draft survey to collect information about DPYD testing practices and beliefs was created in Qualtrics (Provo, UT) by the study team. Questions and response choices were reviewed by medical oncologists within the University of Michigan Rogel Cancer Center and revised on the basis of feedback. The revised survey was submitted to the SWOG Survey Committee for further review and revision before final approval.
Sample Selection and Survey Distribution
The SWOG Cancer Research Network is composed of more than 12,000 members of diverse oncology professions practicing in a variety of oncology practice settings. On April 5, 2021, the SWOG Operations Office sent e-mail survey invitations to 325 US-based medical oncologists who are members of the Gastrointestinal Cancer, Breast Cancer, or Early Therapeutics Committees. Two e-mail reminders were sent approximately 3 weeks apart, before closing the survey on May 28, 2021. This study was exempt from human subject research by the University of Michigan Institutional Review Board (IRBMED). All participants agreed to participate by completing the survey. No compensation was offered for survey completion.
Survey Analysis
Descriptive statistics were calculated to evaluate survey responses. The results are reported as n (%) or median (interquartile range [IQR], range). Exploratory subgroup analysis was conducted to compare the difference in responses to selected questions between academic and nonacademic practices using the chi-square test using GraphPad Prism (San Diego, CA).
RESULTS
Survey Respondents
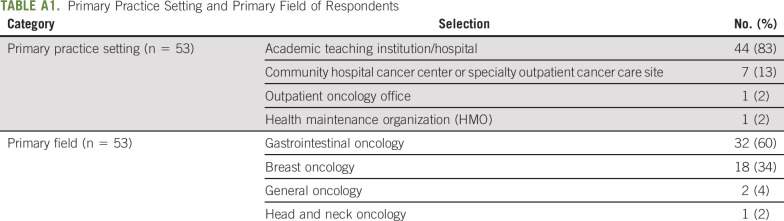
A total of 59 survey responses were initiated and partially completed and 54 completed surveys were collected, with an overall response rate of 18.2%. The primary practice settings of the responding oncologists were academic teaching institution/hospital (83%) or community hospital cancer center or specialty outpatient cancer care site (13%; Appendix Table A1, online only). The most common primary field of practice was gastrointestinal oncology (60%) followed by breast oncology (34%). The median number of years in practice was 13.5 years (IQR: 14 years, range: 2-39 years). The median number of patients the medical oncologist started on a new chemotherapy regimen containing either 5-FU or capecitabine in the past 6 months was 20 (IQR: 37, range: 0-100).
DPYD Testing Before or After Starting Fluoropyrimidine-Based Chemotherapy
Twenty percent of oncologists (n = 12) indicated they had ever ordered DPYD testing before starting FP chemotherapy, including 17% (n = 10) who rarely order pretreatment testing (< 10% of patients) and 3% (n = 2) who order testing for 10%-49% of patients. Alternatively, 78% of oncologists have ordered testing after starting FP treatment.
Perspective on DPD Deficiency
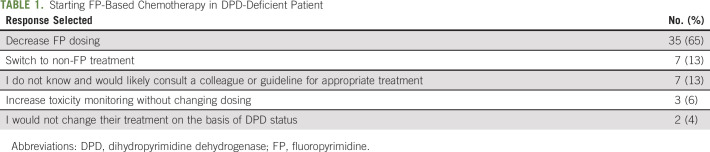
There was general agreement among the oncologists that patients with DPD deficiency are at increased risk of toxicity from FP chemotherapy. The majority of oncologists strongly agree (72%) with the statement, and no oncologists selected somewhat disagree or strongly disagree (Fig 1A). There was also general agreement that DPD deficiency is actionable information. For a patient starting FP chemotherapy who was known to be DPD-deficient, 65% of medical oncologists would decrease dosing, whereas only 4% would continue with treatment without any modification (Table 1).
FIG 1.
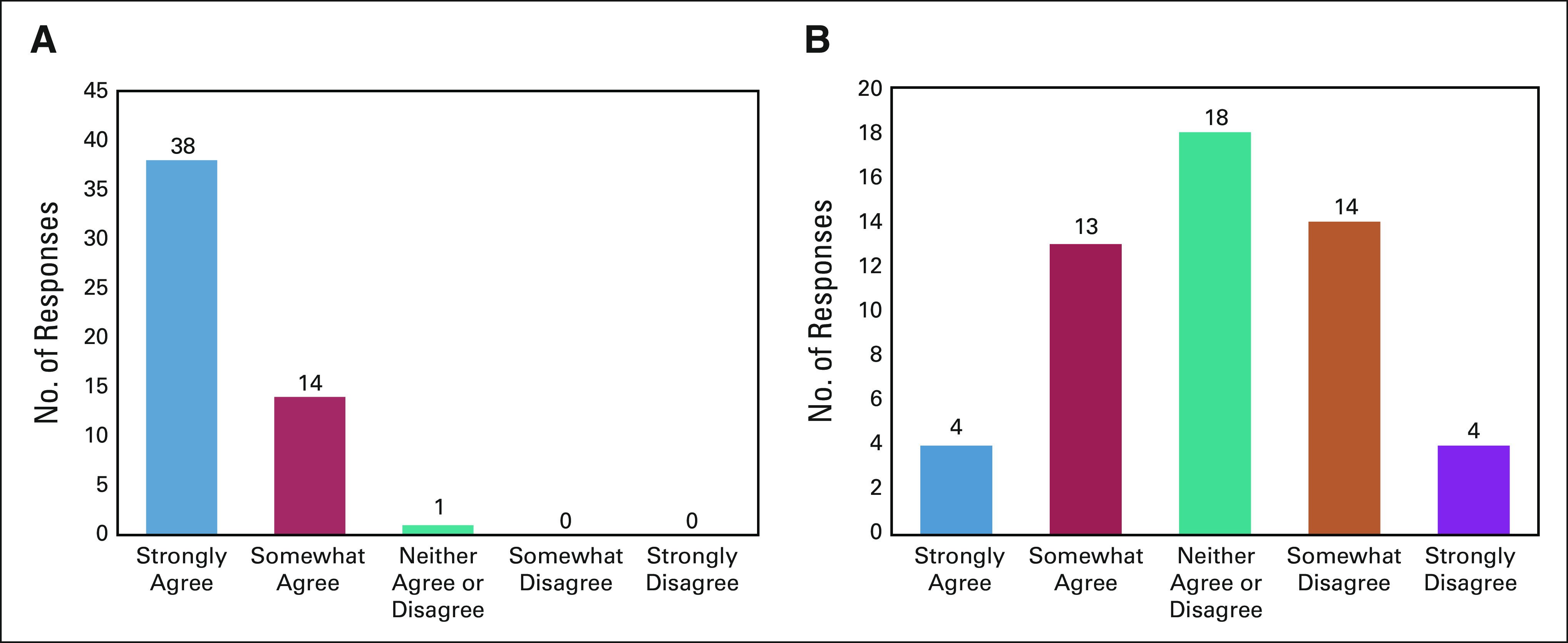
Perspective of oncologists on DPD deficiency and pretreatment DPYD testing. Histogram of agreement to the prompts: (A) patients with DPD deficiency are at increased risk of toxicity from fluoropyrimidine chemotherapy and (B) pretreatment DPD testing is useful. DPD, dihydropyrimidine dehydrogenase.
TABLE 1.
Starting FP-Based Chemotherapy in DPD-Deficient Patient
Beliefs About Pretreatment DPYD Testing
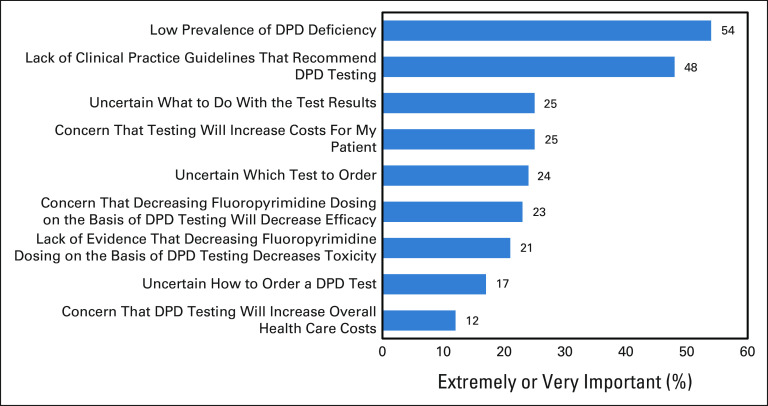
There was less agreement among oncologists regarding the usefulness of pretreatment DPYD testing, with only 32% strongly or somewhat agreeing (Fig 1B). The two most important factors that deter oncologists from ordering pretreatment DPYD testing were low prevalence of DPD deficiency (54% indicated this factor was extremely or very important) and lack of clinical practice guidelines that recommend testing (48%; Fig 2). Exploratory subgroup analysis did not find any differences in these reasons between medical oncologists who worked in academic versus nonacademic settings (all P > .05, data not shown).
FIG 2.
Factors deterring oncologists from ordering DPYD testing. Factors that deter oncologists from ordering DPYD testing before starting fluoropyrimidine chemotherapy in descending order of importance (combined percentage of extremely or very important). Multiple options could be selected. DPD, dihydropyrimidine dehydrogenase.
DISCUSSION
Patients who carry reduced-activity DPYD polymorphisms have a higher risk of severe toxicity from FP chemotherapy.13,14,17 Despite the known clinical benefits of pretreatment DPYD testing in reducing severe FP toxicity and decrease in overall health care costs,18,19 testing has not been recommended in the United States. The objective of this survey was to describe the current practice of US-based medical oncologists regarding pretreatment DPYD testing and understand the factors preventing oncologists from ordering testing. As expected, adoption of pretreatment DPYD testing is limited; only 20% of oncologists have ever ordered testing before FP treatment and only 3% routinely do so in their practices. Although there was near-uniform agreement that DPYD genotype information is actionable, pretreatment testing is not believed to be useful because of the low prevalence of DPD deficiency and lack of clinical practice guidelines that recommend testing. Although complete DPD deficiency is rare (approximately 0.2%), partial DPD deficiency is common (approximately 6%) and is also associated with unacceptable rates of severe (> 50%) and fatal (2%-4%) toxicity.10,26
In this survey, lack of clinical practice guidelines that recommend testing was the second most important factor preventing pretreatment DPYD testing. The significance of this factor has been found in surveys conducted in other countries. In a survey conducted in France before a national requirement for pretreatment testing, lack of recommendations from medical societies/health authorities was one of the main arguments for limited DPYD screening, along with delays in obtaining results and lack of adequate reimbursement by the health insurance system.27 In the Netherlands, national guidelines for colon cancer were updated in 2017 to recommend pretreatment DPYD genotyping. This update to treatment guidelines increased the percentage of patients who received pretreatment DPYD testing from 1% to 87%.28 Clinical adoption of pretreatment DPYD testing in the United States would be expected to substantially increase if national oncology practice guidelines such as NCCN and ASCO recommended testing.
The NCCN colon cancer guidelines panel does not support universal pretreatment DPYD genotyping because of a concern that FP dose reduction in DPYD carriers may reduce treatment efficacy.29,30 Our survey found that concern of decreasing efficacy is not a major concern of medical oncologists regarding pretreatment DPYD testing (23% extremely/very important). This may be due to evidence that FP dose reduction in patients with DPD deficiency results in comparable exposure to wild-type patients receiving standard FP doses,18,19 and no evidence of efficacy reduction has been detected.31,32 Nevertheless, further validation of the clinical utility of pretreatment DPYD testing, specifically the noninferiority of efficacy, within prospective randomized-controlled trials may be necessary for national oncology practice guidelines to support pretreatment testing.
The third most important factor preventing pretreatment testing was the lack of understanding of what to do with the test results (25% extremely/very important). CPIC publishes expert consensus treatment guidelines for dosing FP in patients with known DPYD genotype. Most medical oncologists indicated they would decrease dosing (65%) or switch to a non-FP (13%) in a DPD-deficient patient, consistent with the guideline recommendations. However, it is unclear whether medical oncologists are aware of CPIC guidelines, as prior studies have found low levels of awareness of CPIC guidelines across medical specialities.33-35 The other two education-related factors, including which test to order (24%) and how to order the test (17%), were somewhat lower in the rankings of importance, potentially because of medical oncologists' familiarity and prior experience with ordering DPYD testing after treatment started (78%). The National Center for Biotechnology Information Genetic Testing Registry is a searchable resource to find companies offering Clinical Laboratory Improvement Amendments–approved DPYD testing options.
Increasing costs for patients was the fourth most important factor (25%), whereas increasing overall health care costs was the least important factor. Pretreatment DPYD testing is slightly cost-saving (∼$50-60 US dollars/patient),18,19 primarily because of the prevention of severe toxicity that can require costly hospital admissions.36 Pretreatment testing is covered by Medicare across most of the United States and by some, but not all, private insurance providers.37 Increasing insurance coverage of DPYD testing could further increase clinician acceptance of pretreatment testing.
This was the first survey, to our knowledge, to describe the current practice and perspectives of US medical oncologists regarding DPYD testing. Distribution via SWOG enabled broad inclusion of oncologists from across the United States in various practice settings and specialties in which FP treatments are indicated. However, the results of this survey are limited by the number of respondents (n = 54) and the low overall response rate (18.2%), which may over-represent medical oncologists who have strong beliefs about or understanding of ordering DPYD testing. The survey also represents views of oncologists who are SWOG members, who may be more familiar with clinical practice guidelines or in some other way different from the general population of medical oncologists in the United States. Additionally, these results may not be representative of all oncology specialties or practice settings, as 83% of respondents practiced in academic teaching hospitals. Finally, to minimize survey burden, the survey questions did not differentiate between DPYD genotype testing and DPD phenotype testing or between complete and partial DPD deficiency, which may have affected some responses and complicated interpretation of some questions.
In conclusion, DPYD testing before FP treatment in the United States is limited, despite evidence of clinical benefit, reduction in overall health care costs, and acceptance by international clinical guidelines. Adoption in the United States would likely be substantially increased by inclusion of pretreatment DPYD testing within the oncology clinical practice guidelines. However, this may require further validation of clinical utility and possibly confirmation of noninferiority in DPYD variant carriers who receive reduced FP dose within prospective randomized clinical trials. Uniformity in insurance coverage and clinical education regarding DPYD testing options and interpretation would also enhance adoption of pretreatment testing in the United States to ensure safe and effective FP treatment in patients with cancer.
ACKNOWLEDGMENT
The authors would like to thank the members of the SWOG Gastrointestinal Cancer, Breast Cancer, and Early Therapeutics Committees who completed our survey.
APPENDIX
TABLE A1.
Primary Practice Setting and Primary Field of Respondents
N. Lynn Henry
Research Funding: Blue Note Therapeutics (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/27894/summary
Vaibhav Sahai
Consulting or Advisory Role: Celgene, Halozyme, Newlink Genetics, Ipsen, Incyte, QED Therapeutics, Klus Pharma, AstraZeneca, GlaxoSmithKline, Rafael Pharmaceuticals, Histosonics
Research Funding: Celgene (Inst), Celgene (Inst), Bristol Myers Squibb (Inst), Agios (Inst), Incyte (Inst), Clovis Oncology (Inst), Debiopharm Group (Inst), FibroGen (Inst), Halozyme (Inst), MedImmune (Inst), Rafael Pharmaceuticals (Inst), Ipsen (Inst), Exelixis (Inst), Syros Pharmaceuticals (Inst), Relay Therapeutics (Inst), Pancreatic Cancer Action Network (Inst), Actuate Therapeutics (Inst)
Travel, Accommodations, Expenses: ASCO, FibroGen, FibroGen
Daniel L. Hertz
Research Funding: Disarm Therapeutics
Uncompensated Relationships: PEPID, Saladax Biomedical
No other potential conflicts of interest were reported.
DISCLAIMER
The funding bodies played no role in the design of the study, analysis, and interpretation of data and in writing the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or SWOG.
PRIOR PRESENTATION
Presented in preliminary form at Pharmacogenomics Global Research Network-American Society of Human Genetics (PGRN-ASHG) Symposium (virtual), October 8, 2021; American Society of Health-System Pharmacists (ASHP) Midyear Conference (virtual), December 8, 2021.
SUPPORT
This survey was developed in collaboration with the SWOG Symptom Control and Quality of Life and Digital Engagement Committees and SWOG Survey Subcommittee, which are supported in part by NIH/NCI grants CA180888 and CA180819.
AUTHOR CONTRIBUTIONS
Conception and design: Daniel L. Hertz
Collection and assembly of data: Kyoin Koo, Amy L. Pasternak, Daniel L. Hertz
Data analysis and interpretation: Kyoin Koo, N. Lynn Henry, Vaibhav Sahai
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Survey of US Medical Oncologists' Practices and Beliefs Regarding DPYD Testing Before Fluoropyrimidine Chemotherapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
N. Lynn Henry
Research Funding: Blue Note Therapeutics (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/27894/summary
Vaibhav Sahai
Consulting or Advisory Role: Celgene, Halozyme, Newlink Genetics, Ipsen, Incyte, QED Therapeutics, Klus Pharma, AstraZeneca, GlaxoSmithKline, Rafael Pharmaceuticals, Histosonics
Research Funding: Celgene (Inst), Celgene (Inst), Bristol Myers Squibb (Inst), Agios (Inst), Incyte (Inst), Clovis Oncology (Inst), Debiopharm Group (Inst), FibroGen (Inst), Halozyme (Inst), MedImmune (Inst), Rafael Pharmaceuticals (Inst), Ipsen (Inst), Exelixis (Inst), Syros Pharmaceuticals (Inst), Relay Therapeutics (Inst), Pancreatic Cancer Action Network (Inst), Actuate Therapeutics (Inst)
Travel, Accommodations, Expenses: ASCO, FibroGen, FibroGen
Daniel L. Hertz
Research Funding: Disarm Therapeutics
Uncompensated Relationships: PEPID, Saladax Biomedical
No other potential conflicts of interest were reported.
REFERENCES
- 1. Wigle TJ, Tsvetkova EV, Welch SA, et al. DPYD and fluorouracil-based chemotherapy: Mini review and case report. Pharmaceutics. 2019;11:199. doi: 10.3390/pharmaceutics11050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NCCN . Clinical Practice Guidelines in Oncology. Colon Cancer, Version 3.2021. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf [Google Scholar]
- 3.NCCN . Clinical Practice Guidelines in Oncology. Rectal Cancer, Version 2.2021. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf [Google Scholar]
- 4.NCCN . Clinical Practice Guidelines in Oncology. Pancreatic Cancer, Version 2.2021. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf [Google Scholar]
- 5.NCCN . Clinical Practice Guidelines in Oncology. Gastric Cancer, Version 5.2021. https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf [Google Scholar]
- 6.NCCN . Clinical Practice Guidelines in Oncology. Head and Neck Cancer, Version 3.2021. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf [Google Scholar]
- 7.NCCN . Clinical Practice Guidelines in Oncology. Ovarian Cancer, Version 3.2021. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf [Google Scholar]
- 8.NCCN . Clinical Practice Guidelines in Oncology. Colon Cancer, Version 8.2021. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf [Google Scholar]
- 9.Lunenburg CATC, Henricks LM, Guchelaar H-J, et al. Prospective DPYD genotyping to reduce the risk of fluoropyrimidine-induced severe toxicity: Ready for prime time Eur J Cancer 5440–482016 [DOI] [PubMed] [Google Scholar]
- 10.Dean L, Kane M. Fluorouracil therapy and DPYD genotype. In: Pratt VM, Scott SA, Pirmohamed M, et al., editors. Medical Genetics Summaries. Bethesda, MD: National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- 11.Mattison LK, Fourie J, Desmond RA, et al. Increased prevalence of dihydropyrimidine dehydrogenase deficiency in African-Americans compared with caucasians Clin Cancer Res 125491–54952006 [DOI] [PubMed] [Google Scholar]
- 12.DPYD[gene]-ClinVar-NCBI. www.ncbi.nlm.nih.gov/clinvar/?term=DPYD%5Bgene%5D [Google Scholar]
- 13.Meulendijks D, Henricks LM, Sonke GS, et al. Clinical relevance of DPYD variants c.1679T>G, c.1236G>A/HapB3, and c.1601G>A as predictors of severe fluoropyrimidine-associated toxicity: A systematic review and meta-analysis of individual patient data Lancet Oncol 161639–16502015 [DOI] [PubMed] [Google Scholar]
- 14.Shakeel F, Fang F, Kwon JW, et al. Patients carrying DPYD variant alleles have increased risk of severe toxicity and related treatment modifications during fluoropyrimidine chemotherapy Pharmacogenomics 22145–1552021 [DOI] [PubMed] [Google Scholar]
- 15.Xeloda [package insert] South San Francisco, CA: Hoffmann-La Roche; 2021. [Google Scholar]
- 16.Fluorouracil [package insert] Irvine, CA: Spectrum Pharmaceuticals; 2016. [Google Scholar]
- 17. Lee AM, Shi Q, Pavey E, et al. DPYD variants as predictors of 5-fluorouracil toxicity in adjuvant colon cancer treatment (NCCTG N0147) J Natl Cancer Inst. 2014;106:dju298. doi: 10.1093/jnci/dju298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deenen MJ, Meulendijks D, Cats A, et al. Upfront genotyping of DPYD*2A to individualize fluoropyrimidine therapy: A safety and cost analysis J Clin Oncol 34227–2342015 [DOI] [PubMed] [Google Scholar]
- 19.Henricks LM, Lunenburg CATC, de Man FM, et al. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: A prospective safety analysis Lancet Oncol 191459–14672018 [DOI] [PubMed] [Google Scholar]
- 20.Amstutz U, Henricks LM, Offer SM, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing: 2017 update Clin Pharmacol Ther 103210–2162018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Argilés G, Tabernero J, Labianca R, et al. Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up Ann Oncol 311291–13052020 [DOI] [PubMed] [Google Scholar]
- 22.Innocenti F, Mills SCB, Sanoff HM, et al. All you need to know about DPYD genetic testing for patients treated with fluorouracil and capecitabine: A practitioner-friendly guide JCO Oncol Pract 16793–7982020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lunenburg CATC, van der Wouden CH, Nijenhuis M, et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene–drug interaction of DPYD and fluoropyrimidines Eur J Hum Genet 28508–5172020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-Alfonso P, Saiz-Rodríguez M, Mondéjar R, et al. Consensus of experts from the Spanish Pharmacogenetics and Pharmacogenomics Society and the Spanish Society of Medical Oncology for the genotyping of DPYD in cancer patients who are candidates for treatment with fluoropyrimidines Clin Transl Oncol 24483–4942022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hertz DL, Sahai V.Including DPYD on cancer genetic panels to prevent fatal fluoropyrimidine toxicity J Natl Compr Cancer Netw 18372–3742020 [DOI] [PubMed] [Google Scholar]
- 26.Sharma BB, Rai K, Blunt H, et al. Pathogenic DPYD variants and treatment-related mortality in patients receiving fluoropyrimidine chemotherapy: A systematic review and meta-analysis Oncologist 261008–10162021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loriot M-A, Masskouri F, Carni P, et al. Dihydropyrimidine dehydrogenase deficiency screening for management of patients receiving a fluoropyrimidine: Results of two national practice surveys addressed to clinicians and biologists [in French] Bull Cancer 106759–7752019 [DOI] [PubMed] [Google Scholar]
- 28. Martens FK, Huntjens DW, Rigter T, et al. DPD testing before treatment with fluoropyrimidines in the Amsterdam UMCs: An evaluation of current pharmacogenetic practice. Front Pharmacol. 2020;10:1609. doi: 10.3389/fphar.2019.01609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Message in Our Mailbox: Letter from NCCN (National Comprehensive Cancer Network) 2021. https://www.ismp.org/acute-care/medication-safety-alert-july-29-2021 [Google Scholar]
- 30.Screening for Dihydropyrimidine Dehydrogenase (DPD) Deficiency in Fluorouracil Patients: Why Not? Institute for Safe Medication Practices. 2021. https://www.ismp.org/resources/screening-dihydropyrimidine-dehydrogenase-dpd-deficiency-fluorouracil-patients-why-not [Google Scholar]
- 31.Henricks LM, van Merendonk LN, Meulendijks D, et al. Effectiveness and safety of reduced-dose fluoropyrimidine therapy in patients carrying the DPYD 2A variant: A matched pair analysis Int J Cancer 1442347–23542019 [DOI] [PubMed] [Google Scholar]
- 32.Launay M, Dahan L, Duval M, et al. Beating the odds: Efficacy and toxicity of dihydropyrimidine dehydrogenase-driven adaptive dosing of 5-FU in patients with digestive cancer Br J Clin Pharmacol 81124–1302016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dressler LG, Deal AM, Patel J, et al. Cancer pharmacogenomics, adoption by oncologists and patient benefit Per Med 11143–1532014 [DOI] [PubMed] [Google Scholar]
- 34.Stanek EJ, Sanders CL, Taber KAJ, et al. Adoption of pharmacogenomic testing by US physicians: Results of a nationwide survey Clin Pharmacol Ther 91450–4582012 [DOI] [PubMed] [Google Scholar]
- 35.Rahawi S, Naik H, Blake KV, et al. Knowledge and attitudes on pharmacogenetics among pediatricians J Hum Genet 65437–4442020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toffoli G, Innocenti F, Polesel J, et al. The genotype for DPYD risk variants in patients with colorectal cancer and the related toxicity management costs in clinical practice Clin Pharmacol Ther 105994–10022019 [DOI] [PubMed] [Google Scholar]
- 37.Empey PE, Pratt VM, Hoffman JM, et al. Expanding evidence leads to new pharmacogenomics payer coverage Genet Med 23830–8322021 [DOI] [PMC free article] [PubMed] [Google Scholar]