Abstract
PURPOSE:
Time from diagnosis to treatment has been associated with worse survival outcomes in non–small-cell lung cancer (NSCLC). However, little is known about the impact of delay in time to diagnosis. We aimed to evaluate the impact of time from radiographic suspicion to histologic diagnosis on survival outcomes using the US SEER-Medicare population database.
METHODS:
We identified patients from the SEER-Medicare data set diagnosed with any stage NSCLC between January 1, 2011, and December 31, 2015, who received stage-appropriate treatment and had a computed tomography scan within 1 year of diagnosis. Time to confirmation was determined as the interval between most recent computed tomography imaging and date of histologic diagnosis. Our primary outcome was overall survival (OS).
RESULTS:
In total, 10,824 eligible patients were identified. The median time to confirmation was 20 (range 0-363) days. Using multivariate Cox regression models, longer time to confirmation was associated with improved OS in all comers driven by stage IV patients after adjustment for age, sex, diagnosis year, histology, and comorbidity index. In a separate landmark analysis excluding patients deceased within 6 months of diagnosis, the association between time to diagnosis and survival was no longer evident.
CONCLUSION:
Time to confirmation of NSCLC was inversely associated with OS in this US SEER population study. This association was lost when patients deceased within 6 months of diagnosis were excluded, suggesting that retrospective registry-claims databases may not be the optimal data source to study time to diagnosis as a quality metric because of the unaccounted confounding effects of tumor behavior. Prospective evaluations of clinically enriched data sources may better serve this purpose.
INTRODUCTION
As the United States places increasing emphasis on quality improvement in health care, there has been a growing interest in defining high-quality and timely cancer care.1,2 Despite lung cancer remaining the leading cause of cancer-related mortality in the United States, quality metrics in non–small-cell lung cancer (NSCLC) have lagged behind other common cancer types, including breast and colon cancers.1,3 One challenge in improving quality care in NSCLC is defining timeliness of care. Work has been done demonstrating significant variability in time from first suspicion to treatment initiation.2,4 Previous studies demonstrated associations between prolonged time from diagnosis to treatment initiation and inferior patient outcomes in early- and advanced-stage NSCLC.5-9 Delays from histologic diagnoses to treatment have been increasing for all major cancer types,7 and although some degree of variation exists across institution types in time to treatment, previous research has clearly identified sociodemographic predictors of such delays.4,7,10,11
Although previous studies have evaluated the impact of delay from diagnosis to treatment initiation in NSCLC, little is known about the time interval from a suspected diagnosis of lung cancer to histologic confirmation and how variations in that interval affect survival outcomes among patients who receive appropriate oncologic therapy. To improve care delivery, providers need further insights into the magnitude of variation in this time interval and the extent to which delays in diagnostic workup independently affect survival. We hypothesize that prolonged intervals from a suspected diagnosis of NSCLC to histologic confirmation (time to diagnosis) is associated with inferior overall survival (OS) across all disease stages regardless of timely treatment initiation.
Using the population-based SEER registry linked to Medicare claims, we conducted a retrospective cohort study to pursue the following goals: (1) to characterize the time intervals from radiographic suspicion to histologic confirmation of NSCLC and (2) to test the associations of time intervals from radiographic suspicion to histologic confirmation with OS, after accounting for patient confounding characteristics and receipt of oncologic treatments.
METHODS
Data Source and Study Cohort
The study leverages the National Cancer Institute SEER-Medicare database, which links SEER records of incidental cancer cases to Medicare health care claims.12-14 The SEER records provide patient-level information on sociodemographic characteristics, tumor (eg, histologic type and stage), and OS.15 Medicare claims provide longitudinal information on resource utilization, including diagnostic tests and procedures, surgery, radiation, chemotherapy, outpatient and emergency department visits, hospitalizations, skilled nurse facility, home health, hospice, and durable medical equipment.14,16,17
Eligible patients were age 67 years or older and had histologically confirmed NSCLC of any subtype (adenocarcinoma, squamous cell carcinoma, large cell carcinoma, or non—small-cell carcinoma not otherwise specified) diagnosed at any stage between January 1, 2011, and December 31, 2015. All patients had at least one computed tomography of the chest within 1 year before histologic diagnosis and were required to start stage-appropriate treatment within 6 months of diagnosis.
Since SEER defines the date of diagnosis on the basis of either clinical or histologic criteria, we applied the following algorithm to define date of histologic diagnosis: (1) we used International Classification of Diseases code (ICD)-9, ICD-10, and Healthcare Common Procedure Coding System codes to identify the procedures that commonly characterize diagnostic biopsies in NSCLC and extracted their respective dates; (2) we identified the closest biopsy procedure in time relative to the SEER diagnostic date; (3) if the date of the biopsy matched the date of SEER diagnosis, this date constituted the histologic confirmation date; (4) if the date of the biopsy differed from the SEER date of diagnosis (either before or after), the date in the biopsy claim served as proxy of the histologic confirmation date. For patients who underwent surgical resection upfront without previous biopsy, the date of surgery constituted the date of histologic confirmation. We excluded patients without continuous enrollment in Medicare parts A and B for 2 years before diagnosis and 6 months after diagnosis, those with prior or concurrent cancers (except nonmelanoma cutaneous cancers), those enrolled in Medicare as a result of end-stage renal disease or disability, and those who died within 2 months of diagnosis. This study was approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center.
Stage-Appropriate Treatment
We defined stage-appropriate treatment within 180 days from the date of histologic confirmation of diagnosis as follows: Stage I patients underwent surgical resection (lobectomy, pneumonectomy, or segmentectomy) or stereotactic body radiotherapy. Stage II patients underwent surgical resection as defined above with or without adjuvant chemotherapy involving a platinum agent (carboplatin or cisplatin). Stage III treatment included surgical resection followed by platinum-based adjuvant chemotherapy, concurrent chemoradiation therapy also involving a platinum agent, or neoadjuvant platinum–based chemotherapy followed by surgical resection. Stage IV patients underwent systemic therapy characterized by the receipt of any form of oral (for those with Medicare Part D) or IV-based chemotherapy that received approval from the US Food and Drug Administration before or during the study observation period for use in first-line treatment of NSCLC and/or programmed cell death 1 or programmed cell death ligand-1 immune checkpoint inhibitors (immunotherapy). Neoadjuvant therapy was defined as chemotherapy regimens followed by surgery where the surgery date must fall within 3 months of the last chemotherapy billing claim. Adjuvant chemotherapy was defined as chemotherapy starting within 12 weeks of the time of surgery. Concurrent chemoradiotherapy required that at least one chemotherapy claim fell within the time of radiation therapy and/or started 2 weeks before radiation therapy start dates. We included all codes for external beam radiotherapy to ascertain the use of radiation therapy. For stage II and III patients who received sequential treatment modalities (eg, surgery followed by adjuvant chemotherapy), we considered the date of the first treatment modality in determining the date of treatment initiation.
Exposure and Outcome Measures
The exposure consisted of the time to diagnosis, defined as the interval in weeks between the last computed tomography of the chest and the date of histologic confirmation of diagnosis. We categorized patients into quartiles of time to confirmation (Q1 < Q2 < Q3 < Q4). To account for variations in the time from histologic confirmation of NSCLC to treatment initiation, we categorized patients as starting treatment ≤ 6 weeks or > 6 weeks from histologic confirmation. Our primary outcome was OS, defined as the time interval between the date of histologic confirmation of NSCLC to death of any cause. We censored patients who were alive at the last date of the observation period (December 31, 2017).
Statistical Analysis
We conducted descriptive analysis reporting medians with an interquartile range for continuous variables, and proportions and percentages for categorical variables.
We applied the Kaplan-Meier product limit method to estimate overall and used the log-rank test for unadjusted comparisons. We used a multivariate Cox proportional hazards regression model to estimate the effect of increasing quartiles of time intervals from suspicion to histologic confirmation of NSCLC on the risk of death from any cause for each stage of NSCLC, after adjustment for baseline confounding patient characteristics.
Sensitivity Analysis
To account for the use of oral therapies in advanced stage, we estimated the effects of increasing quartiles of diagnostic intervals on survival in a subset of patients who had Medicare part D coverage and stage IV NSCLC. To untangle associations of prolonged intervals from suspicion to diagnostic confirmation from prolonged time intervals from diagnosis to treatment initiation, we repeated all stage-specific analyses in a subset of patients who initiated oncologic therapy ≤ 6 weeks from the date of histologic confirmation of NSCLC.
RESULTS
Patient Characteristics
A total of 10,824 eligible patients were identified in the US SEER database and included in this study. Patient baseline characteristics are shown in Table 1. Patients were evenly distributed across age range and year of diagnosis, with females comprising 52.2% of the cohort. The majority of patients were White (86.5%) and from urban areas (60.2%). Stage I and IV patients made up the bulk of the cohort (with 42.9% and 35.6%, respectively). With a median follow-up of 808 days, there were 6,356 total deaths (58.7%). Median survival by stage was as follows: 706 days for stage I, 616 days for stage II, 460 days for stage III, and 262 days for stage IV.
TABLE 1.
Patient Characteristics (N = 10,824)
Time to Diagnosis and Survival Outcomes
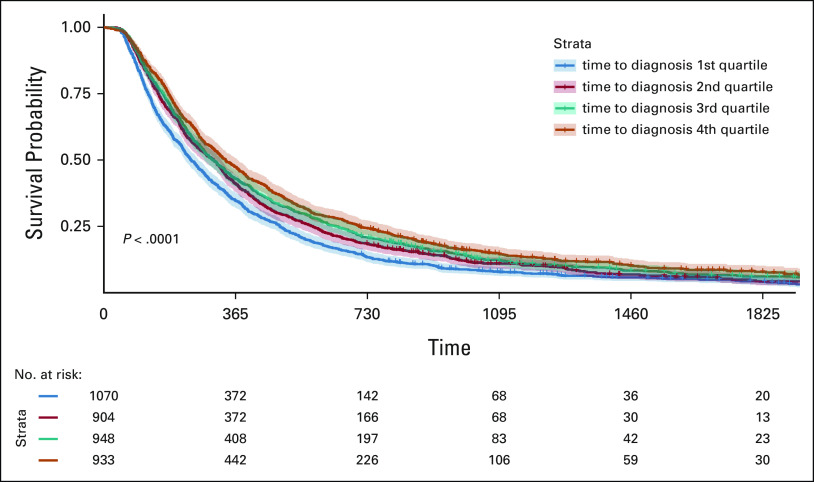
The median time to diagnosis for this cohort was 20 (range 0-363, standard deviation 52, interquartile range 5-48) days. Time to diagnosis varied across stage groups, with a longer median time seen in stage I patients (30 days) compared with stage II-IV patients (28, 19, and 7 days, respectively). In an unadjusted analysis, shorter time to diagnosis was significantly associated with worse survival outcomes for all stages combined (P < .0001). When stratified by stage, this trend held within stage IV patients but not earlier stage patients (Kaplan-Meier survival curve for stage IV patients shown in Figure 1).
FIG 1.
5-Year OS by time to diagnosis quartile in stage IV patients.
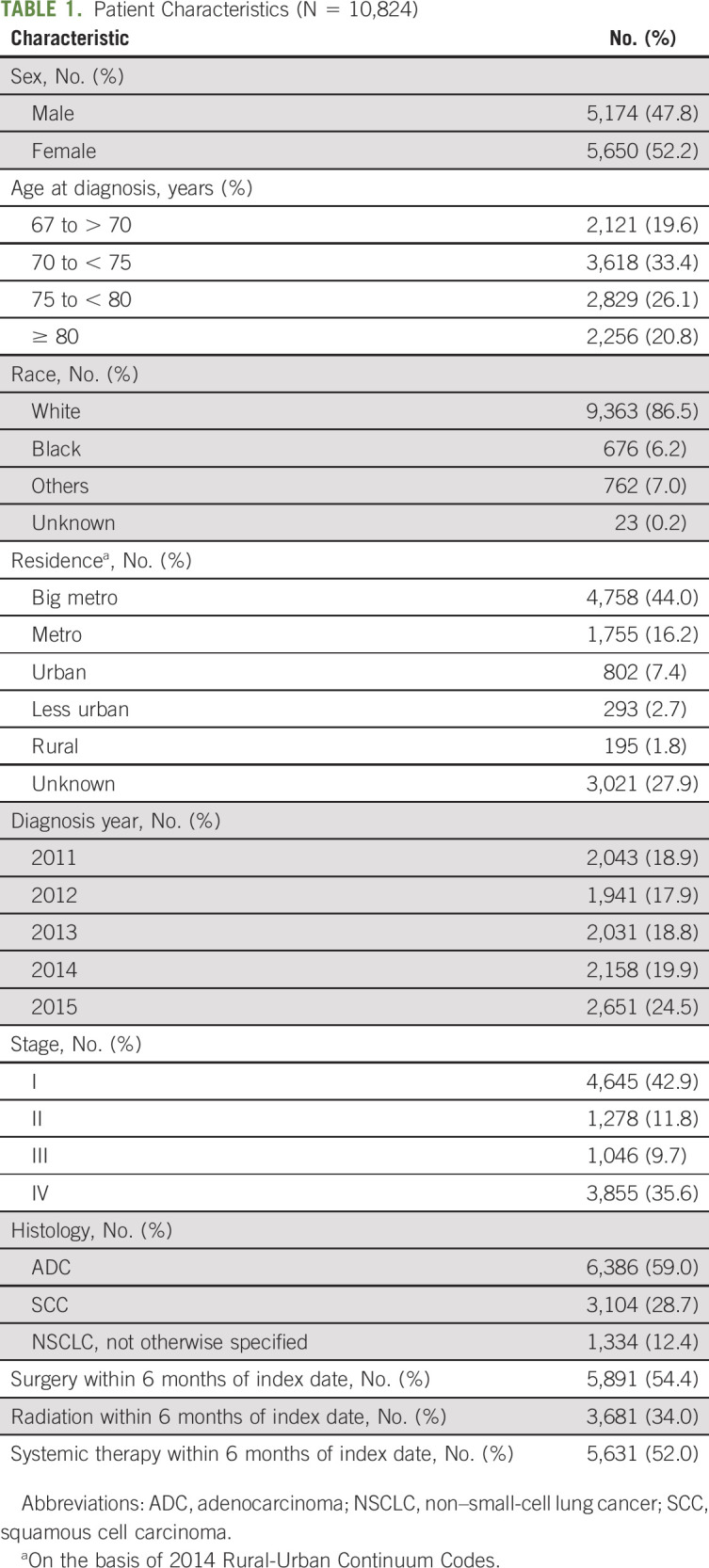
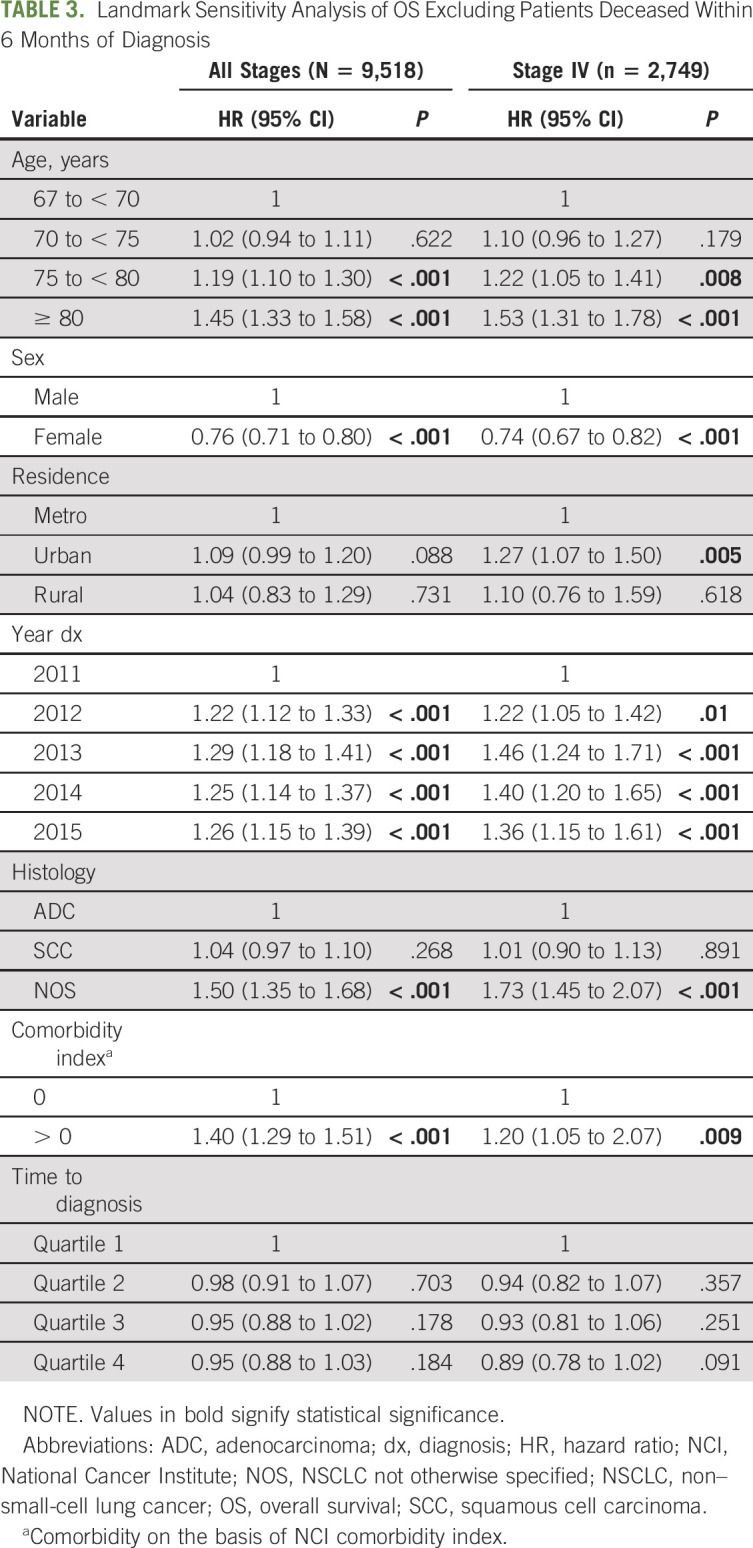
Multivariate survival analysis for all stages combined and by stage is shown in Table 2. In the entire cohort and within each stage, age, sex, diagnosis year, histology, and comorbidity index were statistically associated with OS. Longer time to confirmation was independently associated with improved survival outcomes for all patients combined, driven by the subset of patients with stage IV disease. In a secondary landmark analysis excluding patients deceased within 6 months after diagnosis, a shorter time to confirmation was no longer associated with worse survival outcomes in all comers or stage IV patients (P = .65 and .065, respectively, Table 3).
TABLE 2.
Cox Multivariate Analysis for OS
TABLE 3.
Landmark Sensitivity Analysis of OS Excluding Patients Deceased Within 6 Months of Diagnosis
Sensitivity Analysis
In two separate sensitivity analyses, we analyzed the impact of delay in time to diagnosis on OS in stage IV patients with Medicare part D alone and in patients treated within 6 weeks of diagnosis. In both cases, the trend toward better survival outcomes in those with a longer time to diagnosis held although the association was not as large as in the original analysis (data not shown).
DISCUSSION
Leveraging the US SEER-Medicare data set, this study demonstrates that the median time from first radiographic suspicion of lung cancer to diagnosis in our study population was 20 days. This compares favorably with previous studies evaluating time to diagnosis and time to treatment in lung cancer.4,11,18,19 Similarly, this study demonstrated shorter time to diagnosis in patients with more advanced-stage disease, a trend previously shown in time to treatment studies in NSCLC.20 Interestingly, the median time to diagnosis for patients with stage IV disease was 7 days, which was shorter than we anticipated. This finding suggests that recent efforts to increase efficiency in the diagnostic workup may be shortening the time to diagnosis of lung cancer, particularly for stage IV disease.
Contrary to our initial hypothesis, longer time to diagnosis was in fact associated with improved survival outcomes, driven by patients with stage IV NSCLC. This association held when evaluating patients with only part D Medicare, ensuring that access to oral directed therapy was not affecting our results. Given previous work demonstrating an association between time to treatment and survival outcomes in NSCLC, a second sensitivity analysis was performed including only those patients with treatment initiation within 6 weeks of diagnosis with similar results.5-9,21 Although our results do not support the use of time to diagnosis as a quality metric, we are not postulating that prolonged diagnostic workup of NSCLC improves OS. More likely, biases inherent to registry-claims database studies confounded the analysis of the association of time to diagnosis with OS. Specifically, SEER-Medicare does not include variables that accurately reflect tumor biologic behavior. We postulate that health care professionals appropriately react to patients presenting with clinical, radiographic, and laboratory characteristics suggestive of aggressive disease by expediting the diagnostic workup. Alternatively, longer time to diagnosis may be a proxy for a more thorough workup, including broad genomic profiling in stage IV NSCLC. We are unable to verify the latter explanation as our analysis did not capture details related to the procedures performed as part of the diagnostic workup. Further research may require the use of clinically enriched electronic health record–derived databases to tease out the elements of diagnostic workup that contribute to time to treatment initiation and survival.
To address the possible inherent bias of individual tumor biology, we used a conditional landmark analysis approach. The landmark method, first described in 1983, attempts to address immortal time bias, or the bias favoring the group treated or exposed in survival analyses, when the exposure or treatment may occur at a time point after study entry.22 By excluding patients deceased within 6 months of diagnosis, we aimed to re-evaluate our model in patients with inherently less aggressive tumor biology. In our conditional landmark analysis, the association of shorter time to diagnosis with inferior survival was no longer significant, suggesting that immortal time bias partially accounted for our results.
Finally, it is worth noting that this study included more stage I than stage IV patients, contrary to known epidemiologic NSCLC stage trends.23 This likely reflects our exclusion of stage IV patients who did not receive palliative systemic therapy. It is possible that this exclusion affected the results of our study by including patients with a more favorable underlying disease. Similarly, this study is inherently limited by the years of patient inclusion. Given the rapid pace of treatment advances since 2015, particularly within the field of immunotherapy, it is likely that we excluded advanced-stage patients for not receiving palliative therapy who may be considered candidates for newer treatments, affecting the generalizability of our results to a more contemporary patient population.
In conclusion, this study demonstrates that time to diagnosis of NSCLC in appropriately treated patients is inversely associated with survival outcomes in this US SEER-Medicare database population. This association was lost when patients deceased within 6 months of diagnosis were excluded, suggesting that time to diagnosis reflects underlying tumor behavior, although further prospective work is needed to evaluate this. Although limited by its retrospective nature, this study does not support the use of time to diagnosis as a quality metric, but may indicate that the oncology workforce is appropriately expediting the workup for patients presenting with more aggressive disease behavior.
Keith D. Eaton
Consulting or Advisory Role: Lilly
Research Funding: Mirati Therapeutics (Inst)
Renato G. Martins
Honoraria: Roche/Genentech
Research Funding: Lilly (Inst), Eisai (Inst), Pfizer (Inst), Merck Sharp & Dohme (Inst), Genentech (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by the NCI under award No. T32CA009515.
AUTHOR CONTRIBUTIONS
Conception and design: Perrin E. Romine, Mariel Tang, Keith D. Eaton, Bernardo H.L. Goulart, Renato G. Martins
Collection and assembly of data: Perrin E. Romine, Catherine Fedorenko, Li Li
Data analysis and interpretation: Perrin E. Romine, Qin Sun, Li Li, Keith D. Eaton, Bernardo H.L. Goulart
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of Diagnostic Delays on Lung Cancer Survival Outcomes: A Population Study of the US SEER-Medicare Database
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Keith D. Eaton
Consulting or Advisory Role: Lilly
Research Funding: Mirati Therapeutics (Inst)
Renato G. Martins
Honoraria: Roche/Genentech
Research Funding: Lilly (Inst), Eisai (Inst), Pfizer (Inst), Merck Sharp & Dohme (Inst), Genentech (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Lennes IT, Lynch TJ.Quality indicators in cancer care: Development and implementation for improved health outcomes in non-small-cell lung cancer Clin Lung Cancer 10341–3462009 [DOI] [PubMed] [Google Scholar]
- 2.Ost DE, Jim Yeung S-C, Tanoue LT, et al. Clinical and organizational factors in the initial evaluation of patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines Chest 143e121S–e141S2013suppl 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A.Cancer statistics, 2020 CA Cancer J Clin 707–302020 [DOI] [PubMed] [Google Scholar]
- 4.Vidaver RM, Shershneva MB, Hetzel SJ, et al. Typical time to treatment of patients with lung cancer in a multisite, US-based study J Oncol Pract 12e643–6532016 [DOI] [PubMed] [Google Scholar]
- 5.Coughlin S, Plourde M, Guidolin K, et al. Is it safe to wait? The effect of surgical wait time on survival in patients with non-small cell lung cancer Can J Surg 58414–4182015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez DR, Liao K-P, Swisher SG, et al. Time to treatment as a quality metric in lung cancer: Staging studies, time to treatment, and patient survival Radiother Oncol 115257–2632015 [DOI] [PubMed] [Google Scholar]
- 7. Khorana AA, Tullio K, Elson P, et al. Time to initial cancer treatment in the United States and association with survival over time: An observational study. PLoS One. 2019;14:e0213209. doi: 10.1371/journal.pone.0213209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samson P, Patel A, Garrett T, et al. Effects of delayed surgical resection on short-term and long-term outcomes in clinical stage I non-small cell lung cancer Ann Thorac Surg 991906–19122015discussion 1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tørring ML, Frydenberg M, Hansen RP, et al. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: A cohort study in primary care Eur J Cancer 492187–21982013 [DOI] [PubMed] [Google Scholar]
- 10.Shugarman LR, Mack K, Sorbero MES, et al. Race and sex differences in the receipt of timely and appropriate lung cancer treatment Med Care 47774–7812009 [DOI] [PubMed] [Google Scholar]
- 11.Zullig LL, Williams CD, Fortune-Britt AG.Lung and colorectal cancer treatment and outcomes in the Veterans Affairs health care system Cancer Manag Res 719–352015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hankey BF, Ries LA, Edwards BK.The surveillance, epidemiology, and end results program: A national resource Cancer Epidemiol Biomarkers Prev 81117–11211999 [PubMed] [Google Scholar]
- 13.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database Med Care 31732–7481993 [PubMed] [Google Scholar]
- 14.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-medicare data: Content, research applications, and generalizability to the United States elderly population Med Care 40IV-3-182002 [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute . SEER Program Overview. National Cancer Institute; 2011. http://seer.cancer.gov/ [Google Scholar]
- 16. Lavery JA, Lipitz-Snyderman A, Li DG, et al. Identifying cancer-directed surgeries in Medicare claims: A validation study using SEER-Medicare data. JCO Clin Cancer Inform. 2019;3:124. doi: 10.1200/CCI.18.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamont EB, Herndon JE, Weeks JC, et al. Criterion validity of Medicare chemotherapy claims in Cancer and Leukemia Group B breast and lung cancer trial participants J Natl Cancer Inst 971080–10832005 [DOI] [PubMed] [Google Scholar]
- 18.Lo DS, Zeldin RA, Skrastins R, et al. Time to treat: A system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis J Thorac Oncol 21001–10062007 [DOI] [PubMed] [Google Scholar]
- 19.Riedel RF, Wang X, McCormack M, et al. Impact of a multidisciplinary thoracic oncology clinic on the timeliness of care J Thorac Oncol 1692–6962006 [PubMed] [Google Scholar]
- 20.Powell AA, Schultz EM, Ordin DL, et al. Timeliness across the continuum of care in veterans with lung cancer J Thorac Oncol 3951–9572008 [DOI] [PubMed] [Google Scholar]
- 21. Tsai C-H, Kung P-T, Kuo W-Y, et al. Effect of time interval from diagnosis to treatment for non-small cell lung cancer on survival: A national cohort study in taiwan. BMJ Open. 2020;10:e034351. doi: 10.1136/bmjopen-2019-034351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson JR, Cain KC, Gelber RD.Analysis of survival by tumor response J Clin Oncol 1710–7191983 [DOI] [PubMed] [Google Scholar]
- 23.Morgensztern D, Ng SH, Gao F, et al. Trends in stage distribution for patients with non-small cell lung cancer: A National Cancer Database survey J Thorac Oncol 529–332010 [DOI] [PubMed] [Google Scholar]