Abstract
PURPOSE:
Many older patients with advanced lung cancer have functional limitations and require skilled nursing home care. Function, assessed using activities of daily living (ADL) scores, may help prognostication. We investigated the relationship between ADL impairment and overall survival among older patients with advanced non–small-cell lung cancer (NSCLC) receiving care in nursing homes.
METHODS:
Using the SEER-Medicare database linked with Minimum Data Set assessments, we identified patients age 65 years and older with NSCLC who received care in nursing homes from 2011 to 2015. We used Cox regression and Kaplan-Meier survival curves to examine the relationship between ADL scores and overall survival among all patients; among patients who received systemic cancer chemotherapy or immunotherapy within 3 months of NSCLC diagnosis; and among patients who did not receive any treatment.
RESULTS:
We included 3,174 patients (mean [standard deviation] age, 77 [7.4] years [range, 65-102 years]; 1,664 [52.4%] of female sex; 394 [12.4%] of non-Hispanic Black race/ethnicity), 415 (13.1%) of whom received systemic therapy, most commonly with carboplatin-based regimens (n = 357 [86%] patients). The median overall survival was 3.1 months for patients with ADL score < 14, 2.8 months for patients with ADL score between 14 and 17, 2.3 months for patients with ADL score between 18-19, and 1.8 months for patients with ADL score 20+ (log-rank P < .001). The ADL score was associated with increased risk of death (hazard ratio [HR], 1.20; 95% CI, 1.16 to 1.25 per standard deviation). One standard deviation increase in the ADL score was associated with lower overall survival rate among treated (HR, 1.14; 95% CI, 1.02 to 1.27) and untreated (HR, 1.20; 95% CI, 1.15 to 1.26) patients.
CONCLUSION:
ADL assessment stratified mortality outcomes among older nursing home adults with NSCLC, and may be a useful clinical consideration in this population.
INTRODUCTION
Lung cancer incidence and mortality rates are highest among older adults, with a median age of diagnosis at 71 years and median age at death of 72 years in the United States.1 Although many factors contribute to high mortality rates observed among older adults with cancer, there is evidence linking impaired functional status with higher mortality.2,3 Currently, leading organizations such as the National Comprehensive Cancer Network (NCCN) and ASCO recommend routine geriatric assessment in the care of older adults with cancer.4,5 The rationale for these assessments is their ability to identify deficits in a patient's functional ability, physical health, cognition and mental health, and socioenvironmental circumstances.
Activities of daily living (ADL) are an integral component of geriatric assessments and closely related to a patient's performance status scale, which is extensively used in oncologic care.6 However, the latter lacks the granularity that can be achieved when considering many ADLs jointly. Similar to decreased performance status, impairments in ADLs are associated with worse survival among hospitalized patients with advanced cancers7; however, some studies have differed, showing nonsignificant results.8,9 Importantly, most of what we currently know about the association between functional impairments and patient outcomes is based on data from community-dwelling adults among whom these7-9 and other10 studies were conducted. Although many patients with advanced lung cancer receive nursing home care during the course of their illness, there is a paucity of research specific to nursing home patients.11,12 These patients differ from those living in the community by virtue of the fact that they require skilled nursing care for their substantial functional limitations and comorbidities.
Because nursing home patients are clinically heterogeneous compared with those living in the community,13,14 the magnitude of the association between ADL impairments and overall survival remains unclear. To address this gap, we analyzed a large, national population–based registry aiming to determine whether ADL impairments stratify survival outcomes as expected during the first year after diagnosis among older adults with advanced non–small-cell lung cancer (NSCLC) who receive care in a nursing home.
METHODS
Data Source
We used secondary health data that are routinely collected for administrative and disease surveillance purposes. No informed consent was required. The study was reviewed by the Brown University Institutional Review Board, which determined it to be exempt from the regulations of 45 CFR 46 regarding the inclusion of human participants in research.
We used data from the National Cancer Institute's (NCI’s) SEER-Medicare database linked with Minimum Data Set (MDS) 3.0 assessment data.12 SEER data are derived from population-based cancer registries representing more than 30% of the US population; they include mandatory reporting on all incident diagnoses of malignant tumors along with demographic, clinical, and survival information. Medicare data include demographic and vital status information on all Medicare beneficiaries along with administrative claims on health care services provided in the inpatient and outpatient settings including skilled nursing care and cancer therapies. Recently, the SEER-Medicare data set was enriched with MDS assessment data, which capture health services utilization, physical and mental health, and physical and cognitive function for all individuals who received care in Medicare and/or Medicaid-certified nursing homes.
Eligibility Criteria
We included all fee-for-service Medicare beneficiaries age 65 years and older with pathologically confirmed advanced NSCLC (stage IIIB-IV) diagnosed in SEER from 2011 to 2015 (ie, the most recently available data at the time of the study) who received care in a nursing home within 30 days after cancer diagnosis, or up to 15 days before, and had available MDS assessment data (Data Supplement, online only). We selected stages IIIB and IV owing to their similar prognosis15 and the fact that treatment goals for both stages are primarily palliative with principally systemic therapies; by contrast, earlier-stage disease (stages I, II, and IIIA) is potentially resectable and treated with curative goals using different paradigms (particularly with regard to the use of systemic therapy). Of note, we did not include stage IIIC as the dates for the data occurred before the American Joint Committee on Cancer version 8 and therefore only included stage IIIA and IIIB.16 We excluded patients with no continuous enrollment in Medicare parts A/B for 12 months before cancer diagnosis, enrollment in managed care plans in the year following diagnosis, diagnosis at autopsy, or enrollment in hospice care at the time of nursing home admission. We also excluded patients who received systemic therapy before MDS assessment because it may affect functional status.17,18 This study was deemed exempt by the Brown University Institutional Review Board.
Functional Status
Functional status was measured using the validated Morris ADL scale derived from the MDS.19 The MDS ADL score ranges from 0-28 and represents a composite score relaying the level of assistance needed for dressing, eating, toileting, hygiene, transfers, bed mobility, and locomotion on unit; the Data Supplement shows the Likert-scale scoring methodology for each ADL. Higher ADL score values indicate worse functional status. Each component receives a score from 0 to 4 as follows: 0—total independence, no help or staff oversight; 1—supervision provided three or more times in last 7 days; 2—limited assistance by staff, resident highly involved in the activity; 3—extensive assistance by staff with resident performing part of the activity; and 4—total dependence and full staff participation in the activity during entire 7 days.
Follow-Up Time
Because our interest was in outcomes within the first year of diagnosis, each patient was followed up for 1 year from the time of diagnosis, until the date of death, or the administrative end of follow-up on December 31, 2016, whichever occurred first.
Outcome
Overall survival was the outcome of interest because it is typically the primary end point is oncologic trials, is most often used for therapeutic decision making, and is not subject to measurement error because of misclassification. It was defined as the time from cancer diagnosis until the end of the follow-up. We ascertained the date of death from Medicare's Master Beneficiary Summary File, which includes vital status validated by the National Death Index.
Additional Variables
We ascertained the following variables at the time of diagnosis, including age (continuous), sex (male or female), race/ethnicity (non-Hispanic White, non-Hispanic Black, or other), histology (squamous cell carcinoma [SCC], adenocarcinoma, adenosquamous carcinoma, lepidic adenocarcinoma, carcinoid tumor, malignant non–small-cell carcinoma not otherwise specified, and carcinoma not otherwise specified), whether the patient had received treatment with systemic cancer chemotherapy or immunotherapy within 3 months of diagnosis (see the Data Supplement for a detailed list of regimens), whether the patient was a long-stay (defined as a stay > 90 consecutive days) or a short-stay (defined as a stay of 90 or fewer consecutive days) nursing home (NH) resident, receipt of palliative radiation, cancer surgery, and the NCI comorbidity index, ie, a cancer-specific version of the Charlson comorbidity score calculated using Medicare claims from 1 year before NSCLC diagnosis.20
Statistical Analysis
We estimated the overall survival probability for patients in each quartile of the MDS score (< 14, 14-17, 18-19, ≥ 20) using the Kaplan-Meier estimator and compared overall survival rates across quartiles with the log-rank test; we also calculated the median and 1-year survival for each MDS quartile. To define ADL score cutpoints, we used the quartiles of the ADL distribution following previous work.21,22 Our rationale for using cutpoints over the continuous score was that they are useful for the visualization of the survival curves and their application to a clinical context, eg, for risk stratification purposes; this operationalization is similar to how a biomarker is used as a dichotomous variable (expressed v not expressed) over its absolute values.23
We fitted Cox proportional hazard ratio models on time since diagnosis to calculate hazards ratios (HR) and corresponding 95% CI for the association between ADL and survival. Models were adjusted for factors presumed to correlate with the independent variable (ADL) and outcome (survival), ie, age, sex, race/ethnicity, histology, whether the patient had received treatment with systemic cancer chemotherapy or immunotherapy within 3 months of diagnosis, whether the patient was a long-stay or a short-stay NH resident, receipt of palliative radiation, cancer surgery, and the NCI comorbidity index; all factors were determined a priori on the basis of clinical knowledge as recommended in the statistical modeling literature.24 The proportionality of hazards assumption was verified by Schoenfeld residuals (Data Supplement). Parameterization of the ADL was selected on the basis of the Akaike information criterion, ie, we fitted models where ADL was parameterized as a continuous variable and models where it was parameterized as a categorical variable and selected the continuous parameterization because it resulted in the lowest Akaike information criterion. This linearity in the ADL score documented by this parameterization further supported the notion that quartiles are a reasonable approach to identify groups on the basis of their gradient of risk.
To assess the consistency of the association between ADL and overall survival across levels of major and clinically relevant patient characteristics, we performed subgroup analyses by receipt of systemic therapy (yes v no), histology (SCC, adenocarcinoma, or other), NCI comorbidity index (0-1 v ≥ 2), length of nursing home admission (< 90 v ≥ 90 consecutive days), sex, and age (65-75 years old v > 75 years old). We did not test for effect moderation by means of statistical interactions because our interest was in assessing whether ADL is consistently associated with overall survival in each subgroup rather than identifying patients for whom ADL may have the highest prognostic value.
All analyses were performed using SAS 9.4. P values are two-tailed at a type I error rate α = .05.
RESULTS
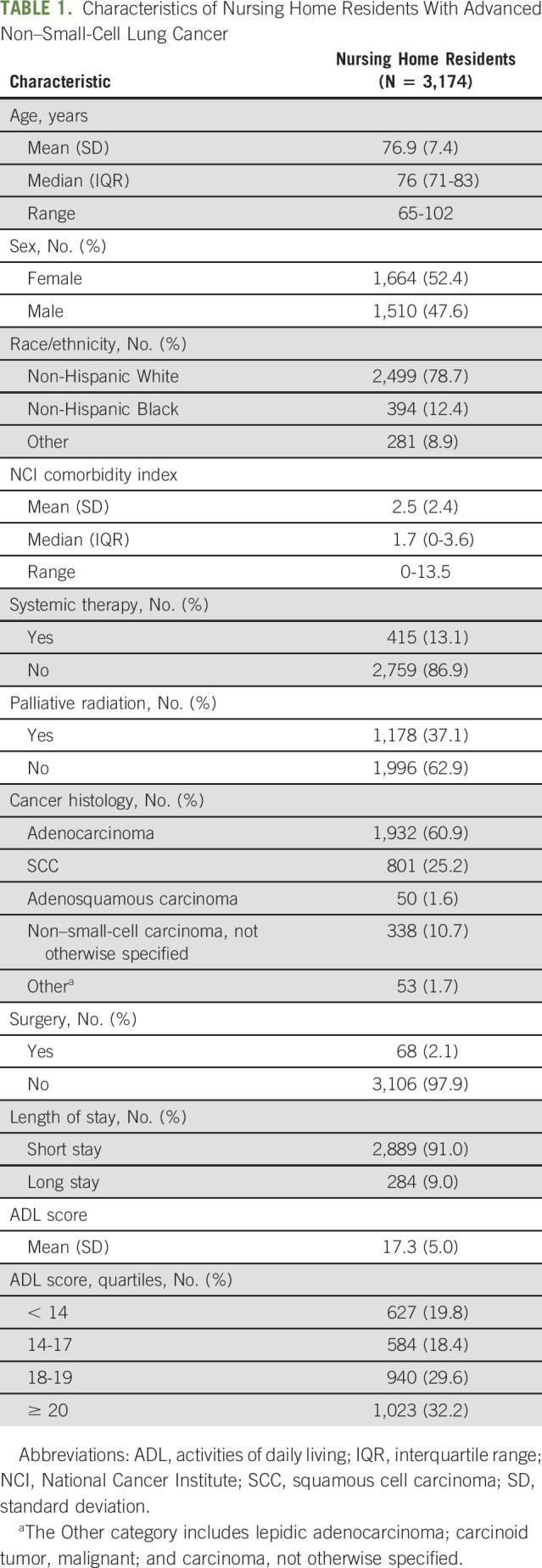
We identified 3,174 patients with advanced NSCLC who met our eligibility criteria (Data Supplement). The mean age was 76.9 (standard deviation [SD], 7.4) years; 1,664 (52.4%) patients were of female sex and 394 (12.4%) were of non-Hispanic Black race/ethnicity (Table 1). The majority (91%) of patients were short-stay nursing home residents, and the median (interquartile range) length of stay during the follow-up period was 22 (11-51) days. The mean ADL score was 17.3 (SD, 5.0), and the mean NCI Comorbidity Index was 2.5 (SD, 2.4). A total of 415 (13.1%) patients received systemic chemotherapy or immunotherapy within 3 months of diagnosis (Data Supplement). The median follow-up time was 69 days, and 2,863 (90.2%) patients died during the follow-up.
TABLE 1.
Characteristics of Nursing Home Residents With Advanced Non–Small-Cell Lung Cancer
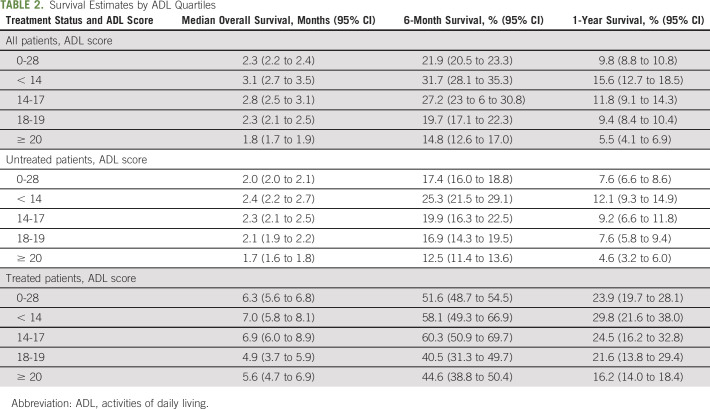
As shown in Table 2, the median overall survival among all patients was 2.3 months (95% CI, 2.2 to 2.4) and it was higher among patients receiving any cancer systemic therapy (6.3 months; 95% CI, 5.6 to 6.8), than those who did not (2 months; 95% CI, 2 to 2.1). The 6-month and 1-year overall survival rates were, respectively, 21.9% (95% CI, 20.5 to 23.3) and 9.8% (95% CI, 8.8 to 10.8) among all patients; 17.4% (95% CI, 16.0 to 18.8) and 7.6% (95% CI, 6.6 to 8.6) among patients not receiving treatment; and 51.6% (95% CI, 48.7 to 54.5) and 23.9% (95% CI, 19.7 to 28.1) among patients who received treatment.
TABLE 2.
Survival Estimates by ADL Quartiles
Among all patients, overall survival was lower in patients with higher compared to those with lower ADL scores (log-rank P < .001; Fig 1). The median overall survival was 3.1 months (95% CI, 2.7 to 3.5) for patients with ADL score < 14, 2.8 months (95% CI, 2.5 to 3.1) for patients with ADL score between 14 and 17, 2.3 months (95% CI, 2.1 to 2.5) for patients with ADL score between 18 and19, and 1.8 months (95% CI, 1.7 to 2.0) for patients with ADL score 20+. As shown in Table 2, the 6-month and 1-year survival rates, respectively, were 31.7% and 15.6% for ADL score < 14, 27.2% and 11.8% for ADL score between 14 and 17%, 19.7% and 9.4% for ADL score between 18 and19%, and 14.8% and 5.5% for ADL score 20+. In adjusted Cox models, a standard deviation increase in the ADL score was associated with 1.2-fold lower overall survival rate (HR, 1.20; 95% CI, 1.15 to 1.25).
FIG 1.
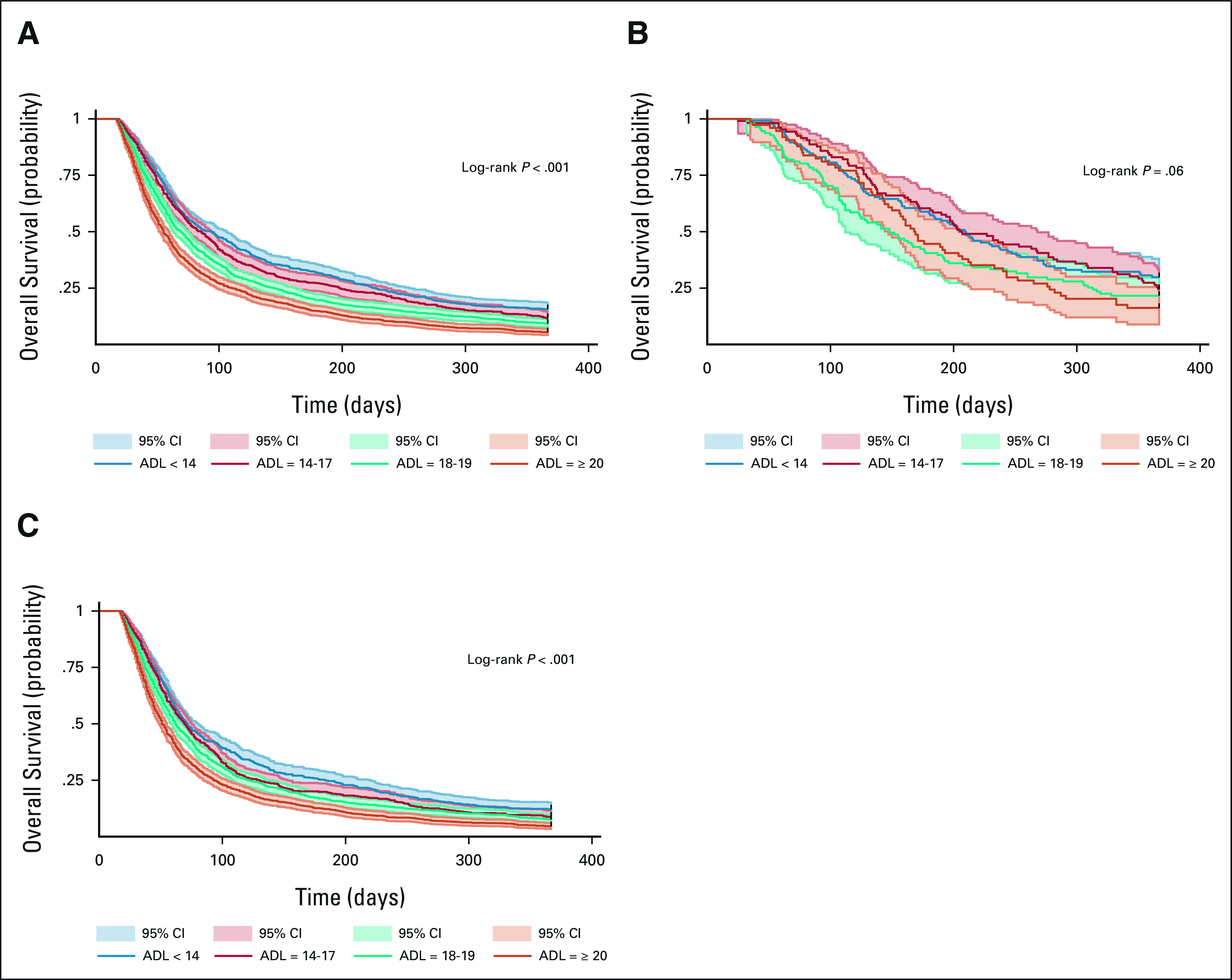
Kaplan-Meier overall survival curves by ADL quartile for (A) all patients, (B) patients receiving treatment, and (C) patients not receiving treatment. ADL, activities of daily living.
As shown in Figure 1, overall survival was lower with increasing ADL scores in both patients who received systemic cancer treatment (log-rank P = .06) and those who did not receive it (log-rank P < .001). Among patients receiving treatment with ADL < 14, 14-17, 18-19, and ≥ 20, the median overall survival was 7.0 months (95% CI, 5.8 to 8.1), 6.9 months (95% CI, 6.0 to 8.9), 4.9 months (95% CI, 3.7 to 5.9), and 5.6 months (95% CI, 4.9 to 6.9), respectively. The corresponding numbers for patients who did not receive any treatment were 2.4 months (95% CI, 2.2 to 2.7), 2.3 months (95% CI, 2.1 to 2.5), 2.1 months (95% CI, 1.9 to 2.2), and 1.7 months (95% CI, 1.6 to 1.8). Survival rates at 6 months and 1 year are shown in Table 2. In adjusted Cox models, one standard deviation increase in the ADL score was associated with 1.14-fold lower overall survival rate among patients receiving treatment (HR, 1.14; 95% CI, 1.02 to 1.28) and 1.2-fold lower rate among those not receiving any therapy (HR, 1.20; 95% CI, 1.15 to 1.26).
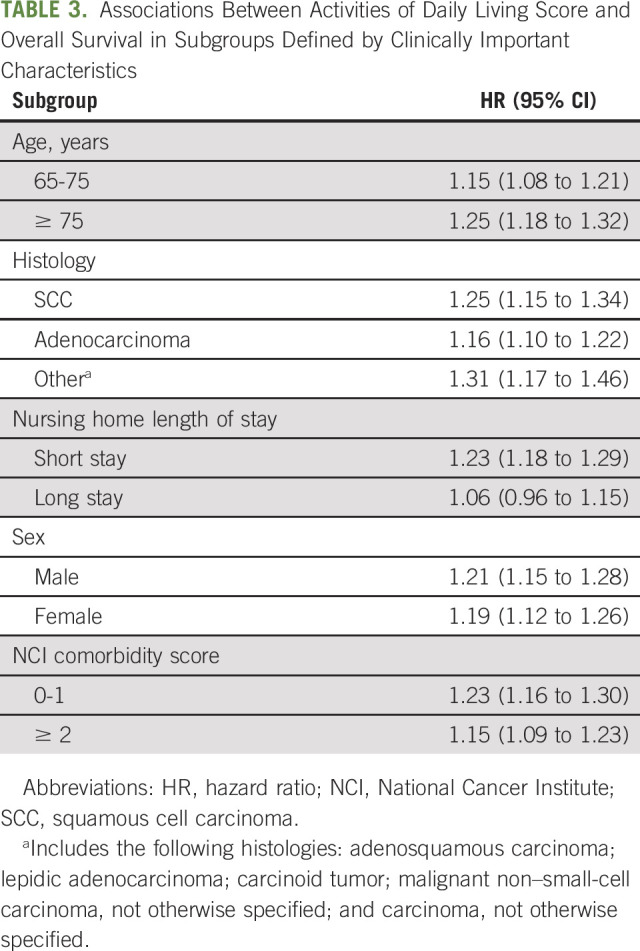
The results were similar when we stratified our analyses by histology, NCI comorbidity index, length of nursing home admission, sex, and age groups (Table 3).
TABLE 3.
Associations Between Activities of Daily Living Score and Overall Survival in Subgroups Defined by Clinically Important Characteristics
DISCUSSION
We examined a large national cohort of older adults with advanced NSCLC who received care in nursing homes, the majority of whom did not receive cancer-directed treatment. One-year overall survival was < 10%, which is substantially lower than that in the general population; for context, in the latter group, 1-year survival for late-stage SCC and adenocarcinoma is 27.8% and 34.7%, respectively.25 Although overall survival was low, it was markedly decreased among patients with the highest degree of functional limitations, 50% of whom survived for < 2 months compared with over 3 months for those with lower impairment. Functional limitations, as measured by the ADL score, independently stratified mortality outcomes in these patients with up to 1.2-fold higher mortality rates among patients per standard deviation of ADL deficit. The probability of survival was strongly related to ADL limitations in both treated and untreated individuals, and the association was consistent across different histology types.
Our findings are consistent with a recent study showing an association between the presence of ADL impairment and survival in patients with advanced cancers.6 However, other studies have not demonstrated a relationship between ADLs and clinical outcomes.7,8 These differences in findings may be attributed to the differences in populations studied, as community-dwelling cohorts from prior investigations often are higher-functioning with little or no ADL limitations; by contrast, but in accordance with our hypothesis, most nursing home patients in our study had ADL limitations. Additionally, we find an association between progressive ADL limitations and mortality, as survival linearly decreased with increasing levels of impairment. Even among nursing home patients with advanced lung cancer, there was a nearly three-fold 1-year survival difference between the first and last quartiles of ADL limitations. Although the baseline ADL categories do separate the survival curves, they do not do so drastically since the differences in OS across ADL quartiles are small in absolute terms (ie, 3.1 months v 2.8 months v 2.3 months v 1.8 months for each quartile increase in the ADL score). This finding could potentially be attributed to the fact that 87% of the patients did not receive any anticancer therapy and therefore the short overall survival is determined by their cancer progressing uniformly.
It has been shown that comprehensive geriatric assessments (CGAs) better predict poor outcomes in older adults with cancer, compared with oncologists' clinical judgment or performance status.26 However, CGA requires time and training and, to date, has been rarely implemented by oncologists.27,28 Identifying components of the CGA that are most associated with outcomes may facilitate routine adoption of CGA into oncology practice. One such component is a patient's ADL status, and this element is captured in nursing homes through mandated, routinely performed MDS assessments and can provide prognostic information for nursing home patients with advanced NSCLC, as our findings indicate. ADL assessment is simple, fast, and familiar to most physicians such that it can easily be incorporated directly into the oncologists' history-taking. Importantly, ADL measures are validated and comparable to physical therapist evaluations in patients with cancer.29,30 Although the relationship between ADLs and mortality is logical, such a validated scale may also be helpful in goals-of-care discussions for the nursing home patients who are physiologically frail and vulnerable after an acute hospitalization.
Our results can be used to provide realistic prognostication for NH residents with cancer and clinicians caring for them. In particular, the ADL score for patients with advanced NSCLC who do leave the skilled nursing facility and receive treatment can be useful to determine guidelines for clinicians regarding the tradeoffs between treatment and hospice. This information can be helpful because of the fallacy that care in a skilled nursing facility can make patients stronger to receive additional chemotherapy, while in fact those with a high ADL score may have little to no survival benefit from nursing home care.31 Notably, as immunotherapies are increasingly becoming common in the treatment of NSCLC and other malignancies, our results could be compared in future studies of novel immunotherapies to evaluate whether more nursing home patients are able to receive therapy and whether the outcomes of patients receiving treatment are improved with these lower-toxicity agents.
Except for patients receiving treatment who had ADL score < 14, all other patients in our study had median survival < 6 months, and they thus meet the qualifying estimated survival for hospice. This finding has implications for delivery of palliative care within the nursing home setting, ie, all patients diagnosed with advanced NSCLC may benefit from a palliative care consultation to address their symptoms, help with goals of care, and support them through treatment, should they opt to receive it.32,33 Therefore, ADL measurements from MDS assessments are readily available when nursing home–residing patients present to the oncology clinic and can be added to other evaluations to guide clinicians and patients in their decision making for treatment, supportive care, and end-of-life care.34
Nevertheless, because for every ADL category, patients receiving treatment had better outcomes than untreated patients, our results also indicate that ADLs alone should not be used to disqualify a patient from systemic therapy, but need to be interpreted in a larger geriatric context considering the findings of a full CGA, patient's comorbidities, polypharmacy, cognitive impairments, and psychosocial support. As our study shows, older patients with advanced NSCLC who receive nursing home care have short overall survival. In this context, ADL deficits identified through CGA could not only be used to assess prognosis but may inform other decisions related to the care of these patients. For example, recent data35 demonstrate that increasing CGA domain deficits are associated with increasing burden for family caregivers and in this context, a CGA could be meaningful when considering a transition of a patient with advanced NSCLC from the nursing home setting to home. In addition, a CGA could also help identify needs for formal palliative care for nursing home residents with advanced NSCLC, especially those whose prognosis is poor regardless of whether they receive anticancer therapy.
Our study has some limitations. First, our findings may have limited generalizability to community-dwelling older adults outside of the nursing home population because, despite having some functional limitations, the latter have little to no skilled care needs and are less vulnerable. Second, SEER-Medicare does not include information on certain tumor characteristics (eg, EGFR mutations) that have prognostic value and could also inform treatment. Third, although our findings suggest that ADL impairment is a prognostic factor in nursing home residents, its predictive value to identify patients who will benefit from treatment should be examined in future prospective studies specific to this population. Importantly, decision making around treatment options for patients with advanced NSCLC should consider patient preferences, as some patients (especially those with short life expectancy) may opt to forego treatments that may have toxic effects. However, patient preferences and decision making are not directly measured in most real-world, routinely collected health data including SEER-Medicare. Fourth, our cohort included patients diagnosed with NSCLC up to 2015, ie, the most recent year of SEER data available at the time of the study; given that treatment of NSCLC has changed over the past few years with increased use of immunotherapy and personalized therapy guided by next-generation sequencing, our findings need to be confirmed in future studies using contemporary cohorts. Fifth, targeted oral therapies (such as EGFR inhibitors and ALK inhibitors) are not identifiable in administrative data for nursing home residents. However, these agents are applicable to only approximately 20% of patients with adenocarcinoma,36 only a small fraction of whom receives them in the real world37; thus, given our sensitivity analyses with stratification by histology showing no differences, it is unlikely that the overall survival results would have been different, had we had access to data on these targeted therapies. Last, we did not examine the relationships of ADL impairments with other outcomes, including hospitalization, intensive care unit admission, or hospice use, because these outcomes were not available to us at the time of this analysis.
Overall, our study provides evidence that routinely measured ADLs among nursing home residents with NSCLC may be useful to guide prognostication and postacute care transitions. Considering that other geriatric elements (eg, depression and cognitive function) are included in MDS assessments, future research should assess their prognostic information when considered jointly with ADLs.
ACKNOWLEDGMENT
The authors dedicate this paper to the memory of our friend, colleague, and coauthor Jessica Ogarek who was instrumental in all aspects of the current work.
Jessica Ogarek
Other Relationship: Personal Feels from American Hospital Association
Elizabeth Wulff-Burchfield
Stock and Other Ownership Interests: Nektar, Immunomedics
Consulting or Advisory Role: Astellas Scientific and Medical Affairs Inc, Exelixis, Bristol Myers Squibb, Janssen Oncology
Research Funding: Pfizer
Adam Olszewski
Research Funding: Genentech/Roche (Inst), TG Therapeutics (Inst), Spectrum Pharmaceuticals (Inst), Celldex (Inst), Adaptive Biotechnologies, Precision Biosciences (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/111328
Orestis A. Panagiotou
Consulting or Advisory Role: International Consulting Associates
No other potential conflicts of interest were reported.
DISCLAIMER
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
The ideas and opinions expressed herein are those of the author(s), and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
SUPPORT
Supported in part by an Agency for Healthcare Research and Quality National Research Service Award grant 5 T32 HS000011–33 to T.K., and a Center on Health Services Training and Research fellowship funded by the Foundation for Physical Therapy Research to T.K. O.A.P. was supported in part by grants 5P01 AG027296–10 and R01 AG054656–01 from the National Institute on Aging. E.B. was supported in part by grant 5P01 AG027296–10 from the National Institute on Aging. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement U58DP003862–01 awarded to the California Department of Public Health.
DATA SHARING STATEMENT
Patient-level data can be obtained from the National Cancer Institute, which maintains the SEER-Medicare database. Analytical code is available and can be requested from the corresponding author.
AUTHOR CONTRIBUTIONS
Conception and design: Michael A. Liu, Humera Khurshid, Adam Olszewski, Orestis A. Panagiotou
Administrative support: Humera Khurshid
Provision of study materials or patients: Emmanuelle Bélanger
Collection and assembly of data: Michael A. Liu, Jessica Ogarek, Adam Olszewski, Emmanuelle Bélanger, Orestis A. Panagiotou
Data analysis and interpretation: Michael A. Liu, Tamra Keeney, Alexa Papaila, Jessica Ogarek, Elizabeth Wulff-Burchfield, Adam Olszewski, Emmanuelle Bélanger, Orestis A. Panagiotou
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Functional Status and Survival in Older Nursing Home Residents With Advanced Non–Small-Cell Lung Cancer: A SEER-Medicare Analysis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jessica Ogarek
Other Relationship: Personal Feels from American Hospital Association
Elizabeth Wulff-Burchfield
Stock and Other Ownership Interests: Nektar, Immunomedics
Consulting or Advisory Role: Astellas Scientific and Medical Affairs Inc, Exelixis, Bristol Myers Squibb, Janssen Oncology
Research Funding: Pfizer
Adam Olszewski
Research Funding: Genentech/Roche (Inst), TG Therapeutics (Inst), Spectrum Pharmaceuticals (Inst), Celldex (Inst), Adaptive Biotechnologies, Precision Biosciences (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/111328
Orestis A. Panagiotou
Consulting or Advisory Role: International Consulting Associates
No other potential conflicts of interest were reported.
REFERENCES
- 1.National Cancer Institute . Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Lung and Bronchus Cancer; Rockville, MD: 2020. [Google Scholar]
- 2.Klepin H, Mohile S, Hurria A.Geriatric assessment in older patients with breast cancer J Natl Compr Canc Netw 7226–2362009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cesari M, Cerullo F, Zamboni V, et al. Functional status and mortality in older women with gynecological cancer J Gerontol A Biol Sci Med Sci 681129–11332013 [DOI] [PubMed] [Google Scholar]
- 4.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer J Clin Oncol 322595–26032014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dotan E, Walter LC, Browner IS, et al. NCCN Guidelines® Insights: Older adult oncology, version 1.2021 J Natl Compr Canc Netw 191006–10192021 [DOI] [PubMed] [Google Scholar]
- 6.Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology J Clin Oncol 362326–23472018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lage DE, El-Jawahri A, Fuh CX, et al. Functional impairment, symptom burden, and clinical outcomes among hospitalized patients with advanced cancer J Natl Compr Canc Netw 18747–7542020 [DOI] [PubMed] [Google Scholar]
- 8.DuMontier C, Liu MA, Murillo A, et al. Function, survival, and care utilization among older adults with hematologic malignancies J Am Geriatr Soc 67889–8972019 [DOI] [PubMed] [Google Scholar]
- 9.Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: A prognostic analysis of the multicenter Italian lung cancer in the elderly study J Clin Oncol 236865–68722005 [DOI] [PubMed] [Google Scholar]
- 10.Neo J, Fettes L, Gao W, et al. Disability in activities of daily living among adults with cancer: A systematic review and meta-analysis Cancer Treat Rev 6194–1062017 [DOI] [PubMed] [Google Scholar]
- 11.Panagiotou OA, Keeney T, Ogarek JA, et al. Prevalence of functional limitations and their associations with systemic cancer therapy among older adults in nursing homes with advanced non-small cell lung cancer J Geriatr Oncol 12765–7702021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas KS, Boyd E, Mariotto AB, et al. New opportunities for cancer health services research: Linking the SEER-Medicare data to the nursing home Minimum data Set Med Care 56e90–e962018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunnicutt JN, Tjia J, Lapane KL.Hospice use and pain management in elderly nursing home residents with cancer J Pain Symptom Manage 53561–5702017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodin MB.Cancer patients admitted to nursing homes: What do we know? J Am Med Dir Assoc 9149–1562008 [DOI] [PubMed] [Google Scholar]
- 15.Koul R, Rathod S, Dubey A, et al. Comparison of 7th and 8th editions of the UICC/AJCC TNM staging for non-small cell lung cancer in a non-metastatic North American cohort undergoing primary radiation treatment Lung Cancer 123116–1202018 [DOI] [PubMed] [Google Scholar]
- 16.Giroux DJ, Rami-Porta R, Chansky K, et al. The IASLC Lung Cancer Staging Project: Data elements for the prospective project J Thorac Oncol 4679–6832009 [DOI] [PubMed] [Google Scholar]
- 17.Hung R, Krebs P, Coups EJ, et al. Fatigue and functional impairment in early-stage non-small cell lung cancer survivors J Pain Symptom Manage 41426–4352011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loh KP, Lam V, Webber K, et al. Characteristics associated with functional changes during systemic cancer treatments: A systematic review focused on older adults J Natl Compr Canc Netw 191055–10622021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris JN, Fries BE, Morris SA.Scaling ADLs within the MDS J Gerontol A Biol Sci Med Sci 54M546–M5531999 [DOI] [PubMed] [Google Scholar]
- 20.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data J Clin Epidemiol 531258–12672000 [DOI] [PubMed] [Google Scholar]
- 21.Kosar CM, Thomas KS, Gozalo PL, et al. Higher level of obesity is associated with intensive personal care assistance in the nursing home J Am Med Dir Assoc 191015–10192018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang V, Zhao S, Boscardin J, et al. Functional status and survival after breast cancer surgery in nursing home residents JAMA Surg 1531090–10962018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pezo R, Bedard P. Definition: Translational and Personalised Medicine, Biomarkers, Pharmacodynamics. ESMO Handbook of Translational Research; Lugano, Switzerland: 2015. [Google Scholar]
- 24.Harrell JFE.Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis, Springer Series in Statistics ed 2ChamImprint, Springer International PublishingSpringer; 2015. pp 1 [Google Scholar]
- 25.Adenocarcinoma of the Lung and Bronchus . SEER Survival Rates by Time Since Diagnosis, 2004-2017. Rockville, MD: National Cancer Institute; [Google Scholar]
- 26.Kirkhus L, Saltyte Benth J, Rostoft S, et al. Geriatric assessment is superior to oncologists' clinical judgement in identifying frailty Br J Cancer 117470–4772017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dale W, Williams GR, MacKenzie AR, et al. How is geriatric assessment used in clinical practice for older adults with cancer? A survey of cancer providers by the American Society of Clinical Oncology JCO Oncol Pract 17336–3442021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamaker ME, Wildes TM, Rostoft S.Time to stop saying geriatric assessment is too time consuming J Clin Oncol 352871–28742017 [DOI] [PubMed] [Google Scholar]
- 29.Pavon JM, Sloane R, Morey MC, et al. Inpatient mobility measures as useful predictors of discharge destination in hospitalized older adults J Am Geriatr Soc 65224–2262017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lage DE, Nipp RD, D'Arpino SM, et al. Predictors of posthospital transitions of care in patients with advanced cancer J Clin Oncol 3676–822018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh S, Eguchi M, Min SJ, et al. Outcomes of patients with cancer discharged to a skilled nursing facility after acute care hospitalization J Natl Compr Canc Netw 18856–8652020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update J Clin Oncol 3596–1122017 [DOI] [PubMed] [Google Scholar]
- 33.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer N Engl J Med 363733–7422010 [DOI] [PubMed] [Google Scholar]
- 34. Baronner A, MacKenzie A. Using geriatric assessment strategies to lead end-of-life care discussions. Curr Oncol Rep. 2017;19:75. doi: 10.1007/s11912-017-0631-4. [DOI] [PubMed] [Google Scholar]
- 35.Xu H, Kadambi S, Mohile SG, et al. Caregiving burden of informal caregivers of older adults with advanced cancer: The effects of rurality and education J Geriatr Oncol 121015–10212021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer N Engl J Med 361958–9672009 [DOI] [PubMed] [Google Scholar]
- 37. Vashistha V, Armstrong J, Winski D, et al. Barriers to prescribing targeted therapies for NSCLC patients with highly actionable gene variants in the VA National Precision Oncology Program. J Clin Oncol. 2020;38 doi: 10.1200/OP.20.00703. suppl; abstr 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Patient-level data can be obtained from the National Cancer Institute, which maintains the SEER-Medicare database. Analytical code is available and can be requested from the corresponding author.