Abstract
Introduction:
Long term management of patients with stable coronary artery disease of >1 year after myocardial infarction (MI) or percutaneous coronary intervention and atrial fibrillation is unclear. Current guidelines recommend using oral anti-coagulation (OAC) alone although the recommendation is weak and there is low quality evidence. Two new randomized control trials (RCTs) were published recently. We conducted an updated meta-analysis to evaluate the effect of these studies on patient outcomes
Objective:
To conduct a systematic review and meta-analysis of published RCTs and observational studies to compare OAC alone versus OAC plus single anti-platelet therapy.
Methods:
Electronic searches were conducted using appropriate terms from 3 databases. Relevant studies included. Data extracted and analysis were performed using STATA.
Measurements:
Summary statistics were pooled and measured for primary and secondary outcomes of both treatment arms.
Main results:
Eight studies involving 10,120 patients were included for the analysis. Five thousand two hundred thirty-seven patients were on combination therapy while 4883 were on OAC alone. There was no statistically significant difference in the primary outcome of major adverse cardiac events (hazard ratio [HR] 1.067; 95% confidence interval [CI] 0.912–1.249; P value .417). There was no statistically significant difference even in the measured secondary outcomes namely all cause mortality (HR 1.048; 95% CI 0.830–1.323; P value .695), cardiovascular mortality (HR 0.863; 95% CI 0.593–1.254; P value .439). However, we found statistically significant difference between the 2 groups in the incidence of MI with higher incidence in mono therapy group (HR 1.229; 95% CI 1.011–1.495; P value .039) and higher incidence of major bleeding in the combination therapy group in the subgroup analysis (HR 0.649; 95% CI 0.464–0.907; P value .011).
Conclusion:
We found no reduction of major adverse cardiac event between combination therapy and mono therapy. Although mono therapy showed increased risk of major bleeding overall, subgroup analysis of the RCTs showed increased risk of major bleeding in the combination therapy group. MI was higher in the mono therapy group compared to the combination therapy group, however this outcome was not reproducible in the subgroup analysis of the RCTs.
Keywords: anti-platelet meta-analysis, atrial fibrillation, oral anti-coagulation, stable CAD/coronary artery disease
1. Introduction
Coronary artery disease (CAD) has a significant and independent association with atrial fibrillation (AF).[1] Nearly 30% of the patients with CAD have associated AF.[2] Although data from Danish registries suggest that prior myocardial infarction (MI) is an independent risk factor for stroke,[3] AF augments the risk further in such patients.[4] The cornerstone of AF management is ischemic stroke prevention with anti-coagulation. Long-term use of aspirin is essential in reducing the risk of major cardiovascular events by nearly 25% in patients with CAD.[5] This poses a challenge in balancing bleeding risk versus insufficient anti-thrombosis. As non-modifiable risk factors such as age pose a major challenge to avert risk of bleeding as well as stroke prevention, it is essential to address the factors that can be modified.[6] Concomitant use of anti-platelet therapy is one such modifiable factor. Current guidelines from CHEST society suggest using oral anti-coagulation (OAC) therapy alone rather than a combination of OAC therapy and single anti-platelet combination therapy (SAPT), however this recommendation remains weak with low quality evidence since the majority of the data on this topic comes from observational and prospective cohort studies.[7] Recently 2 randomized control trials (RCTs) have been conducted assessing OAC alone versus OAC + SAPT for management of stable CAD with AF. The OAC-ALONE trial,[8] which was conducted in Japan, was an underpowered RCT leading to inconclusive outcomes. The AFIRE trial,[9] which was also conducted in Japan, is a recent addition to the available data. Hence, we conducted a systematic review and performed an updated meta-analysis of the available data from observational studies and RCTs to analyze the safety profile and efficacy between OAC mono therapy and combined OAC and anti-platelet therapy.
2. Methods
Standard method was followed to conduct systematic review and meta-analysis.
Data sources & search strategy (Table 1, Supplemental Fig. 1 ). A database search of all original research articles was conducted using PubMed, Ovid/Embase, and the Cochrane Library until June 11, 2020 using the following search terms: “stable coronary artery disease”, “stable coronary”, “coronary artery disease” OR “CAD”, “atrial fibrillation” OR “a-fib”, “a fib”, “afib”, “anti thrombotic” “oral anticoagulation” OR “OAC”, “anti platelet” OR “aspirin”. No limitation to language, study type was implemented. Species was limited to humans. All search results were compiled in a citation program, Mendeley© (Mendeley Ltd), and filtered for duplicates.
Table 1.
Demographics.
| PUBMED | EMBASE | COCHRANE LIBRARY | |
| (1) Atrial Fibrillation | 56,873 | 174,466 | 12,221 |
| (2) A fib | 1696 | 7954 | 356 |
| (3) A-Fib | 66 | 474 | 27 |
| (4) afib | 56,903 | 1380 | 83 |
| (5) Stable Coronary Artery Disease | 8471 | 5753 | 3350 |
| (6) Stable Coronary | 26,771 | 7411 | 3877 |
| (7) Stable CAD | 2882 | 3162 | 949 |
| (8) CAD | 33,094 | 100,344 | 4609 |
| (9) Coronary Artery Disease | 116,742 | 252,101 | 23,577 |
| (10) Single antiplatelet therapy | 1653 | 2255 | 2126 |
| (11) Antiplatelet therapy | 18,364 | 19,812 | 5101 |
| (12) Aspirin | 37,925 | 118,937 | 13,643 |
| (13) Clopidogrel | 10,886 | 61,490 | 5506 |
| 14 Plavix | 10,918 | 3368 | 242 |
| 15 Py2Y12 inhibitors | 2435 | 1747 | 781 |
| 16 Py2Y12 inhibitor | 2435 | 2360 | 781 |
| 17 Antithrombotic therapy | 10,128 | 21,004 | 1907 |
| 18 antithromb∗ | 31,364 | 97,975 | 5134 |
| 19 DAPT | 1419 | 4726 | 5134 |
| 20 [#5 or #6 or #7] and [#1 or #2 or #3 or #4] and [#11] | 1557 | 1360 | 21 |
Study selection: Inclusion criteria for the studies were as follows. The study should include patients with stable CAD and AF. The study design should have included a follow up of at least 1 year. A comparison of safety and efficacy between OAC and OAC + SAPT be reported. Primary and secondary clinical outcomes explicitly mentioned or presented in a derivable way. Studies that measured acute management of CAD with AF immediately after percutaneous intervention were excluded, we also excluded studies that did not clearly define study arms and did not include both arms of treatment. Literature reviews, case reports, case studies were also excluded. Heterogeneity was anticipated considering different study designs, study population of the included studies.
Screening: After duplicates were removed, 2 authors (Srikanth Malladi & Kewan Hamid) independently screened titles and abstracts by following the PRISMA IPD flow diagram.[10] If the full abstract was not available or was not clear, the full article was obtained and reviewed for possible inclusion. References of select studies were also searched to find studies relevant to our meta-analysis. Corresponding authors were contacted for important data that were not available in the published article or supplement. The principal investigator (AS) resolved any disagreements between the authors.
Data extraction: Two independent reviewers (Srikanth Malladi & Nitin Pendyala) extracted the estimates of hazard ratio (HR) with 95% confidence interval (CI). In addition, study design, size, setting, patient population; all primary and secondary outcomes that were clearly reported were also extracted.
Statistical analyses: Statistical analyses were performed using STATA© IC/64 software (version 15, College Station, TX). Statistics were pooled using a random effect model with inverse variance. We calculated a pooled HR with 95% CI with Der Simonian- Laird method. We measured 2-sided P values for each outcome; statistical significance was determined by a 2-tailed P value <.05. Heterogeneity among studies is reported using the Cochrane Q, I2, modified H2, and tau2.
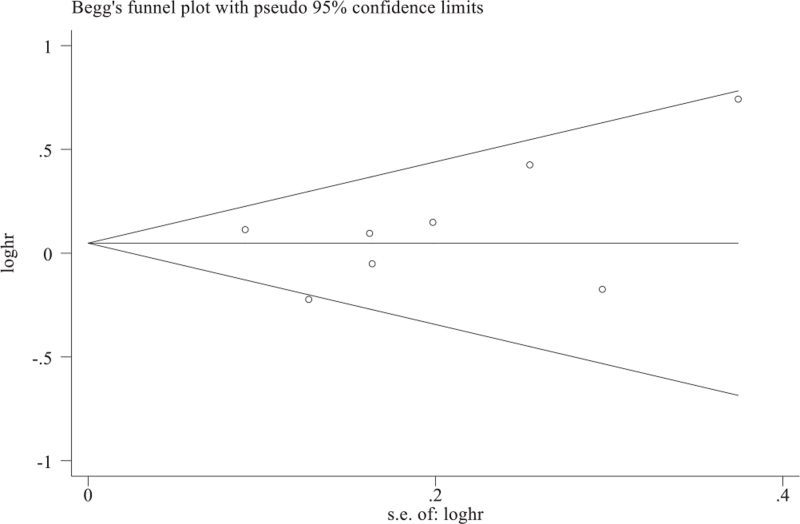
Publication bias of the studies included was assessed using a funnel plot with Begs and Egger test for quantitative assessment. A value of 0.05 or less combined with asymmetry in the funnel plot would indicate publication bias (Fig. 1). Primary outcome was major adverse cardiac event (MACE), which can be defined as a composite of all cause death, MI, stroke or systemic embolism. Secondary outcomes measured were all cause mortality, MI, major bleeding, cardiovascular death, and systemic thromboembolism. Since our meta-analysis included both observational and RCTs, subgroup analysis of 2 RCTs were obtained. Incidence of hemorrhagic and ischemic stroke, which was not clearly identified in the observational studies, was analyzed in the RCTs.
Figure 1.
Publication bias assessment for major adverse cardiac events. Begg funnel with pseudo 95% confidence limits. Funnels plot is symmetrical and infers no publication bias and low heterogeneity with P value using Egger test.
3. Results
A total of (n = 404) studies were identified through electronic database searches. Four hundred three studies were left after duplicates were removed, 382 were excluded based on title and abstract, 21 were completely revised for inclusion, and 8 studies, 6 observational,[8,11–15] 2 RCTs[8,16] with total of 10,120 patients were included. Study characteristics of studies included are shown in (Table 2). Five thousand two hundred thirty-seven patients were on combination therapy while 4883 were on OAC alone. With demographics showed 73.8% males and 26.2% females with mean age of 73.25 years (Table 2). All 5 observational studies used vitamin K antagonist as OAC with or without anti-platelet therapy based on study arm, anti-platelet used were either aspirin or clopidogrel, mean duration of follow up was approximately 1.7 years. While RCTs used either vitamin K antagonists or newer OAC with or without above anti-platelet therapy, with a mean follow up duration of 6.1 years, approximately 50% patients with paroxysmal AF.
Table 2.
General characteristics of the studies included.
| First author of study | Study period | Study design | OAC alone (n = no. of patients) | OAC + APT (n = no. of patients) | Type of OAC | Type of SAPT | Definition of MACE | Definition of Bleeding | Definition of stroke | Mean age (yrs) | Male | Type of stents | Follow up duration |
| Lamberts (2014) (aspirin) | 2002–2011 | Observational registry | 950 | 1471 | VKA | Aspirin | MI/coronary death | ISTH major | Ischemic + systemic thromboembolism | 73.4 | 66.10% | NR | 1 yr |
| Lamberts (2014) (clopidogrel) | 2002–2011 | Observational registry | 950 | 322 | VKA | Clopidogrel | MI/coronary death | ISTH major | Ischemic + systemic thromboembolism | 73 | 64.20% | NR | 1 yr |
| Hamon (2014) | 2010–2011 | Prospective cohort | 119 | 342 | VKA | Aspirin or clopidogrel | Cardiovascular death/MI/non-hemorrhagic stroke | BARC≥3 | Not reported | 66.9 | 77.8 | BMS or DES | 2 yrs |
| Lemesle (2017) | 2003–2004 | Prospective cohort | 1481 | 866 | VKA | Aspirin or clopidogrel | Cardiovascular death/MI/stroke | Requiring hospitalization/transfusion | Not reported | 73.2 | 71.20% | NR | 4 yrs |
| Fischer (2018) | 2010–2015 | Observational registry | 172 | 434 | VKA or DOAC | Aspirin or clopidogrel | Cardiovascular death/MI/ischemic stroke | TIMI bleeding requiring medical attention | Not reported | 76 | 68.90% | BMS or DES | 2.8 yrs |
| Patti (2018) | 2012–2016 | Observational registry | 710 | 348 | VKA or DOAC | Aspirin or clopidogrel | Acute coronary syndrome | ISTH major | Not reported | 74.1 | 78.60% | BMS or DES | 1 yr |
| Matsumura-Nakano (2018) | 2013–2016 | Randomized control trial | 344 | 346 | VKA or DOAC | Aspirin or clopidogrel | Cardiovascular death/MI/ischemic stroke/systemic embolism | ISTH major | Stroke or systemic embolism | 75.1 | 85.20% | BMS or DES | 2.5 yrs |
| Yasuda (2019) | 2015–2017 | Randomized control trial | 1107 | 1108 | DOAC | Aspirin or P2Y12 | Cardiovascular and non- cardiovascular death/MI/ischemic stroke/systemic embolism | ISTH major | Ischemic + systemic thromboembolism | 74.3 | 79.00% | BMS or DES | 23 mos |
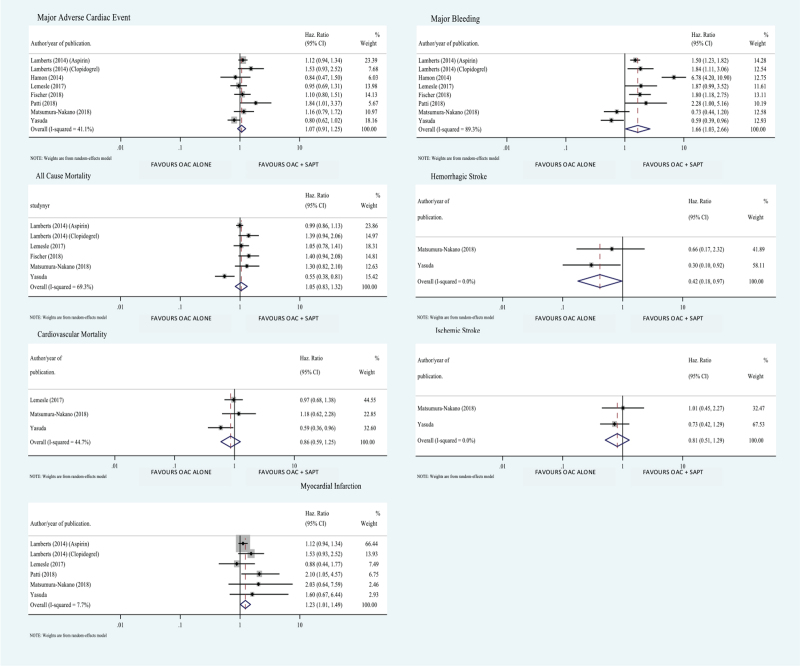
Our analysis showed higher incidence of hemorrhagic stroke when OAC was combined with an anti-platelet drug (HR 0.417; 95% CI 0.179–0.973; P value .043) (Fig. 2). We also found higher incidence of bleeding when OAC was combined with an anti-platelet drug (HR 1.656; 95% CI 1.03–2.663; P value .038) (Fig. 2) which was also found in the subgroup analysis of the 2 RCTs (HR 0.649; 95% CI 0.464–0.907; P value .011) (Fig. 3). We found higher incidence MI in OAC alone group (HR 1.229; 95% CI 1.011–1.495; P value .039) (Fig. 2) but this difference was not seen in the subgroup analysis of the 2 RCTs included in the study (HR 1.783; 95% CI 0.774–4.109; P value .174) (Fig. 1).
Figure 2.
Forest plot comparing measured outcome between OAC alone versus OAC and SAPT combination. Values less than 1 favor oral anti-coagulation alone therapy (OAC). Values greater 1 favor oral anti-coagulation + single anti-platelet combination therapy (OAC + SAPT).
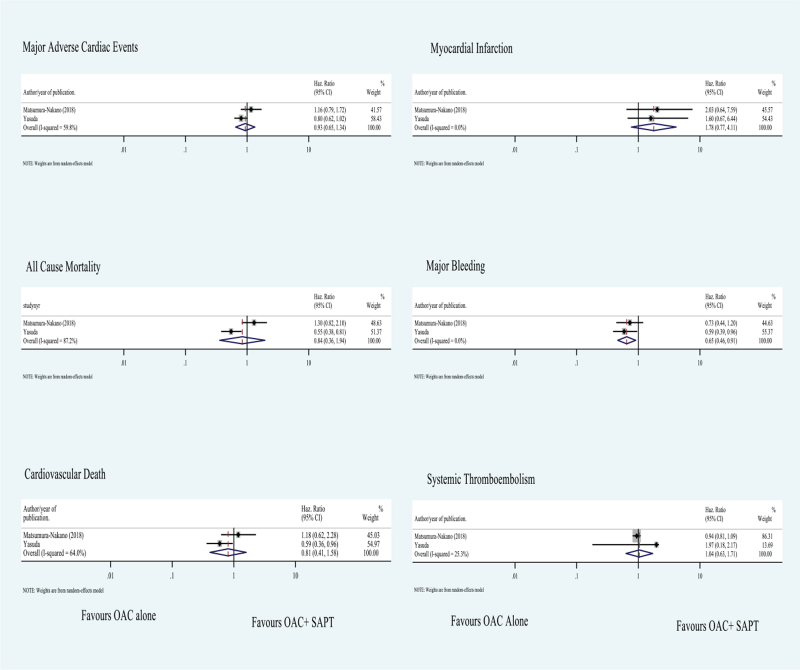
Figure 3.
Subgroup analysis of measured outcomes. Values less than 1 favor oral anti-coagulation alone therapy (OAC). Values greater 1 favor oral anti-coagulation + single-anti-platelet combination therapy (OAC + SAPT).
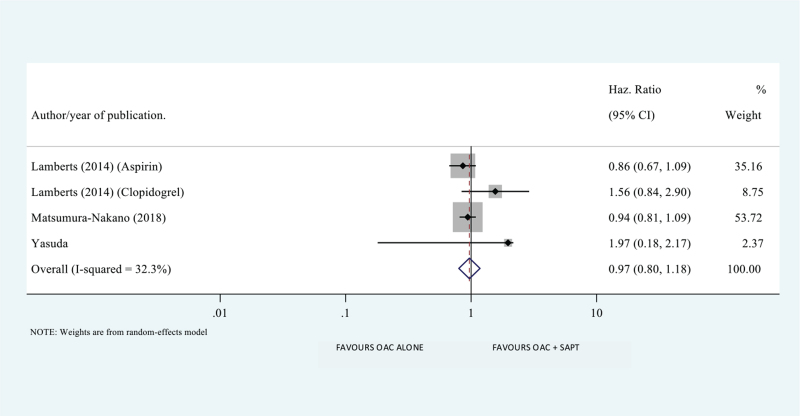
We found no difference between the 2 treatment groups in the incidence of MACEs (HR 1.067; 95% CI 0.912–1.249; P value .417), all-cause mortality (HR 1.048; 95% CI 0.830–1.323; P value .695), cardiovascular mortality (HR 0.863; 95% CI 0.593–1.254; P value .439), systemic embolism (HR 0.969; 95% CI 0.798–1.177; P value .753), and ischemic stroke (HR 0.811; 95% CI 0.512–1.286; P value .374) (Figs. 2 and 4).
Figure 4.
Systemic embolism in oral anti-coagulation alone therapy (OAC) alone versus oral anti-coagulation + single anti-platelet combination therapy (OAC + SAPT). Values less than 1 favor oral anti-coagulation alone therapy (OAC). Values greater 1 favor oral anti-coagulation + single anti-platelet combination therapy (OAC + SAPT).
In the subgroup analysis of 2 included RCTs also we found no significant difference in MACE (HR 0.934; 95% CI 0.652–1.337; P value .708), all-cause mortality (HR 0.836; 95% CI 0.360–1.941; P value .676), cardiovascular mortality (HR 0.806; 95% CI 0.410–1.585; P value .532), and systemic embolism (HR 1.04; 95% CI 0.632–1.712; P value 0.877) (Fig. 3).
Stent thrombosis was only reported in 2 studies with negligible numbers indicating comparable efficiency between both arms of treatment.
4. Discussion
The purpose of this updated systematic review and meta-analysis was to compare combination OAC + SAPT versus OAC mono therapy in patients with chronic stable CAD and AF. Our analysis showed no difference between the 2 groups in regards to primary measured outcome (MACE), these results were unchanged in subgroup analysis of 2 included RCTs (Figs. 1 and 2). This finding is consistent with all the studies included in our meta-analysis except Patti et al[15] (HR 1.84; 95% CI 1.01–3.37; P = .048). Since basic patient characteristics that were receiving combination OAC + SAPT were not clear in this study, it is uncertain whether patients who were on combination OAC + SAPT were sicker with multiple co-morbidities.[15] This could further be explained by the fact that bleeding precluded continued usage of medication, which puts the patients at thrombotic risk.[15] Secondary measured outcomes included; all-cause mortality, cardiovascular mortality, stroke and systemic embolism, MI, major bleeding.
We found no statistically significant differences between the 2 groups in all-cause mortality, cardiovascular mortality, ischemic stroke and systemic embolism, these findings are generally consistent with the studies included, with the exceptions of AFIRE. AFIRE study showed significant increase in mortality with OAC + SAPT group.[9] This could be because the number of non-cardiac deaths in the combination therapy group was twice the number of non-cardiac deaths in the mono therapy group. We do not know the cause of death in these patients, and could be due to other causes like occult cancer.[16] Importantly, the AFIRE trial found an increase in cardiovascular mortality in patients with OAC + SAPT combination, which is in contrast with the existing data and is currently under investigation in the AFIRE trial.
There were statistically significant differences in other secondary measured outcomes including MI and major bleeding. These results were reproducible in subgroup analysis of the 2 included RCTs for major bleeding but not for MI (Fig. 1). In case of major bleeding these findings are observed in all the studies included. The OAC-ALONE trial had more than 5 times higher incidence of major bleeding in combination therapy group compared with OAC mono therapy. Similarly, AFIRE trial also revealed higher risk of major bleeding in combination therapy versus OAC mono therapy (2.67% vs 1.62% events per patient-year) (P = .01). Patti et al[15] showed bleeding profile was 2.3 times higher in patients who received combination OAC + SAPT versus OAC mono therapy no matter the baseline bleeding profile.
Hemorrhagic stroke was reported in the included RCTs only, updated meta-analysis showed increased risk of hemorrhagic stroke in combination therapy versus OAC mono therapy, which is in line with the AFIRE trail. However, OAC-ALONE trial did not increase hemorrhagic stroke possibly because stringent anti-coagulation was not reported in this group when compared to the OAC mono therapy group as it was an open-label trial.
Anti-thrombotic strategy in AF and stable CAD requires a balance between effective stroke prevention and avoiding stent thrombosis while carefully balancing the risk of bleeding. Available data are limited to 4 observational registries, 2 prospective cohorts, and 2 RCTs. Additionally, prior meta-analysis conducted by Lee et al[17], published previously in 2019, contained inaccuracies in data extraction of Patti et al and Matsumura et al in the categories of MACEs, major bleeding, all cause death variable, resulting in potentially erroneous conclusion[15] which is why we believed it is imperative to conduct an updated systematic review and meta-analysis.
Our study has several limitations. Firstly, our findings are significantly limited by the limitations of the studies included, such as observational nature of some of the studies and their lack of randomization. Two arms of the treatment in most of the studies included were not equal, which may have affected the observed outcomes.
Another limitation is observed heterogeneity; some degree of heterogeneity is certain in meta-analysis and can pose a challenge in interpretation of results since patient demographics included in the studies were different. In conclusion, our findings suggest a similar outcome between OAC mono therapy compared with OAC + SAPT with lower risk of major bleeding and hemorrhagic stroke in patients with stable CAD and AF. This is in line with the current recommended guidelines.
Supplemental Digital Content.
Author contributions
Literature search and review (Kewan Hamid, Srikanth Malladi, Smit Deliwala).
Data extraction (Srikanth Malladi, Vijaysai Veerapaneni, Nitin Chandra Pendyala, Smit Deliwala).
Data analysis (Kewan Hamid, Donald Dubre).
Initial Draft (Kewan Hamid, Srikanth Malladi, Vijaysai Veerapaneni).
Final draft (Srikanth Malladi, Kewan Hamid, Samir Elian, Adiraj Singh).
Conceptualization: Srikanth Malladi.
Data curation: Srikanth Malladi, Vijaysai Veerapaneni, Nitin Chandra Pendyala, Smit Deliwala.
Formal analysis: Kewan Hamid.
Methodology: Srikanth Malladi.
Writing – original draft: Srikanth Malladi.
Writing – review & editing: Donald Dubre, Samir A Elian, Adiraj Singh.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AF = atrial fibrillation, CAD = coronary artery disease, CI = confidence interval, HR = hazard ratio, MACE = major adverse cardiac event, MI = myocardial infarction, OAC = oral anti-coagulation, RCTs = randomized control trials, SAPT = single anti-platelet therapy.
How to cite this article: Malladi S, Hamid K, Pendyala NC, Veerapaneni V, Deliwala S, Dubre D, Elian SA, Singh A. Management of stable coronary artery disease and atrial fibrillation with anti-thrombotic therapy: a systematic review and meta-analysis. Medicine. 2021;100:48(e27498).
Ethics approval and consent to participate is not applicable. This is a systematic review and meta-analysis, no direct patient contacts were made.
Consent for publication has been obtained.
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
APT = anti-platelet therapy, BARC = Bleeding Academic Research Consortium, BMS = bare-metal stent, DES = drug-eluting stent, DOAC = direct oral anti-coagulant, ISTH = International Society on Thrombosis and Hemostasis, MACE = major adverse cardiac event, MI = myocardial infarction, NR = not reported, OAC = oral anti-coagulation, SAPT = single anti-platelet therapy, VKA = vitamin K antagonist.
References
- [1].Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994;271:840–4. [PubMed] [Google Scholar]
- [2].Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet (London, England) 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- [3].Nielsen PB, Skjøth F, Rasmussen LH, Larsen TB, Lip GYH. Using the CHA2DS2-VASc score for stroke prevention in atrial fibrillation: a focus on vascular disease, women, and simple practical application. Can J Cardiol 2015;31:820.e9–10. [DOI] [PubMed] [Google Scholar]
- [4].Lip G, Freedman B, De Caterina R, Potpara TS. Stroke prevention in atrial fibrillation: past, present and future. Comparing the guidelines and practical decision-making. Thromb Haemost 2017;117:1230–9. [DOI] [PubMed] [Google Scholar]
- [5].Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chao T-F, Lip GYH, Lin Y-J, et al. Incident risk factors and major bleeding in patients with atrial fibrillation treated with oral anticoagulants: a comparison of baseline, follow-up and Delta HAS-BLED scores with an approach focused on modifiable bleeding risk factors. Thromb Haemost 2018;118:768–77. [DOI] [PubMed] [Google Scholar]
- [7].Inaba K, Doi A, Nisida I. Purification and some characteristics of liver cytosol cornin, an antimitotic substance from rat liver cytosol. Acta Med Okayama 1977;31:203–9. [PubMed] [Google Scholar]
- [8].Matsumura-Nakano Y, Shizuta S, Komasa A, et al. Open-label randomized trial comparing oral anticoagulation with and without single antiplatelet therapy in patients with atrial fibrillation and stable coronary artery disease beyond 1 year after coronary stent implantation. Circulation 2019;139:604–16. [DOI] [PubMed] [Google Scholar]
- [9].Yasuda S, Kaikita K, Akao M, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med 2019;381:1103–13. [DOI] [PubMed] [Google Scholar]
- [10].Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535.doi:10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lamberts M, Gislason GH, Olesen JB, et al. Oral anticoagulation and antiplatelets in atrial fibrillation patients after myocardial infarction and coronary intervention. J Am Coll Cardiol 2013;62:981–9. [DOI] [PubMed] [Google Scholar]
- [12].Hamon M, Lemesle G, Tricot O, et al. Incidence, source, determinants, and prognostic impact of major bleeding in outpatients with stable coronary artery disease. J Am Coll Cardiol 2014;64:1430–6. [DOI] [PubMed] [Google Scholar]
- [13].Lemesle G, Ducrocq G, Elbez Y, et al. Vitamin K antagonists with or without long-term antiplatelet therapy in outpatients with stable coronary artery disease and atrial fibrillation: association with ischemic and bleeding events. Clin Cardiol 2017;40:932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fischer Q, Georges JL, Le Feuvre C, et al. Optimal long-term antithrombotic treatment of patients with stable coronary artery disease and atrial fibrillation: “OLTAT registry”. Int J Cardiol 2018;264:64–9. [DOI] [PubMed] [Google Scholar]
- [15].Patti G, Pecen L, Lucerna M, et al. Outcomes of anticoagulated patients with atrial fibrillation treated with or without antiplatelet therapy - a pooled analysis from the PREFER in AF and PREFER in AF PROLONGATON registries. Int J Cardiol 2018;270:160–6. [DOI] [PubMed] [Google Scholar]
- [16].Yasuda S, Ogawa H. AFIRE Investigators. Antithrombotic therapy for atrial fibrillation with stable coronary disease. Reply. N Engl J Med 2019;381:2481.doi:10.1056/NEJMc1914049. [DOI] [PubMed] [Google Scholar]
- [17].Lee S-R, Rhee T-M, Kang D-Y, Choi E-K, Oh S, Lip GYH. Meta-analysis of oral anticoagulant monotherapy as an antithrombotic strategy in patients with stable coronary artery disease and nonvalvular atrial fibrillation. Am J Cardiol 2019;124:879–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.