Abstract
Application of animal manures to soil as crop fertilizers is an important means for recycling the nitrogen and phosphorus which the manures contain. Animal manures also contain bacteria, including many types of pathogens. Manure pathogen levels depend on the source animal, the animal's state of health, and how the manure was stored or treated before use. Rainfall may result in pathogen spread into soil by runoff from stored or unincorporated manure or by leaching through the soil profile. Steady rainfall consisting of 16.5 mm h−1 was applied to 100-mm disturbed soil cores that were treated with manure and inoculated with Escherichia coli O157:H7 strain B6914. The level of B6914 in leachate was near the inoculum level each hour for 8 h, as was the level of B6914 at several soil depths after 24 h, indicating that there was a high rate of growth. Bacterial movement through three different types of soil was then compared by using disturbed (tilled) and intact (no-till) soil cores and less intense rainfall consisting of 25.4 mm on 4 consecutive days and then four more times over a 17-day period. Total B6914 levels exceeded the inoculum levels for all treatments except intact clay loam cores. B6914 levels in daily leachate samples decreased sharply with time, although the levels were more constant when intact sandy loam cores were used. The presence of manure often increased total B6914 leachate and soil levels in intact cores but had the opposite effect on disturbed soil cores. Ammonia and nitrate levels correlated with B6914 and total coliform levels in leachate. We concluded that tillage practice, soil type, and method of pathogen delivery affect but do not prevent vertical E. coli O157:H7 and coliform transport in soil and that soluble nitrogen may enhance transport.
Contamination of food and water by microorganisms from animal manures has become a topic of concern recently. Non-point-source contamination from manure may result from pastured animals, from roaming wild animals, or from manure intentionally spread onto fields as fertilizer or waste. Point sources of manure contamination include animal feedlots, animal housing facilities, and manure storage areas, such as lagoons. A point source may also lead to non-point-source spread of manure or pathogens by runoff or leaching that spreads to fields and water supplies.
Movement of bacteria through soil from point sources has been studied extensively by using septic tank effluent (1, 13) and municipal sewage treatment effluent (15, 20). Coliform bacteria have been reported to move through soil from 0.9 to 456 m depending on the soil type (10). Coliforms from septic tank effluent were transported at rates between 102 and 106 cells per day through 60-cm packed loamy sand soil columns subjected to unsaturated flow conditions over a period of 200 days, which represented a 92% removal rate (21). Infiltration basins over loamy sand were shown to transport as much as 100 times more fecal coliforms to groundwater following rainfall than during dry spells (9).
Non-point-source pathogen contamination resulting from animal manure has garnered increasing attention recently (17). Poultry manure applied to intact silt loam soil blocks that were 32.5 cm square by 42.5 cm long led to between 103 and 105 fecal coliform cells ml−1 in leachate with unsaturated flow and generally greater numbers and faster breakthrough of coliforms with sod-covered soil than with bare soil (14). The influence of cattle grazing on silt loam soils with shallow aquifers was shown by the 51% increase in fecal coliform-positive well samples obtained after cattle grazed on several different fields for the first time (11). A direct relationship with cattle grazing was shown in a study in which coliform transport through the top 25 cm of a sandy loam soil was measured; there was increased coliform contamination in surface layers during grazing months and at greater soil depths after cattle were no longer using the field (6).
In the United States, current federal environmental regulations do not consider pathogens from animal manure directly, although the Clean Water Act (40 CFR 122) covers pollutants from point sources, especially concentrated feedlots, under the National Pollutant Discharge Elimination System (NPDES) permit program. This regulation is in effect while manure is stored, but regulation stops when manure is spread onto a field. Generally, if uncovered manure storage is used, it must contain the runoff from a once-in-25-year storm and be outside any flood plain and the manure must not come into direct contact with the high groundwater table for the area. Current NPDES permitting is carried out by individual states and generally incorporates best management practices designed to limit nitrogen to amounts that crops can readily utilize during a growing season.
Although the regulations applicable to manure are regulations for nutrients, pathogens have recently become an issue since several disease outbreaks have been traced to livestock; these outbreaks involved Escherichia coli O157:H7, Salmonella species, Listeria monocytogenes, Mycobacterium paratuberculosis, and several enteric viruses and protozoans (16). Although the U.S. Environmental Protection Agency sets limits on the allowable concentrations of coliform bacteria in drinking water (0 CFU in 100 ml) and primary contact water (200 CFU in 100 ml), it is unclear how often well water or surface waters on farms are checked for contamination. There are no regulations concerning the pathogen content of soil, although human sewage sludge must be treated to reduce the pathogen content prior to use on fields (Clean Water Act 40 CFR 503). In addition, in Canada, packaged animal manures must not be detrimental to plants, animals, or public health (4). Pathogens that reach groundwater or surface water on a farm may be recycled by crop irrigation and may infect animals and humans through drinking water or ingestion of a crop. It is possible that pathogens that are present at low levels in this water multiply when they are exposed to favorable environmental conditions or available nutrients. In fact, it has been shown that the levels of members of several genera of pathogenic bacteria decrease only slightly during 100 days in groundwater alone (7), and several studies have shown that sediments serve as reservoirs for fecal pathogens (2, 5).
In the present study, we evaluated two types of soil cores to study the extent to which efflux resulting from fresh manure (including pathogens and nutrients) traveled through intact and disturbed (sieved, packed) soil cores, which simulated no-till soil and conventional-tillage soil treatments, respectively. The risks which we assessed included the ability of potential pathogens (E. coli O157:H7 and total coliforms) to travel through soils with different textures. We also assessed whether manure trapped pathogens at the soil surface or was a source of nutrients that enhanced pathogen survival. It is possible that pathogens that reach the groundwater are involved in an on-farm cycle of reinfection that spreads pathogens among animal populations. As the groundwater is recycled for crop irrigation, for vegetable cleansing, or as drinking water, pathogens may then directly affect the human population.
MATERIALS AND METHODS
Inoculum preparation.
All cores were inoculated with a rifamycin-resistant derivative of E. coli O157:H7 strain B6914 containing plasmid pGFP with genes for green fluorescent protein and ampicillin resistance (8). B6914 was grown to the mid-log phase in Luria-Bertani broth (Gibco BRL, Gaithersburg, Md.) containing 100 μg of both ampicillin and rifamycin SV per ml, chilled rapidly to 4°C, pelleted by centrifugation at 5,000 × g, and resuspended to a Klett reading of 100 in sterile, chilled, reverse osmosis grade (RO) water. The inoculum used for the preliminary experiment contained 4.018 × 107 CFU ml−1, the inoculum used for disturbed soil cores contained 4.697 × 107 CFU ml−1, and the inoculum used for intact cores contained 3.015 × 107 CFU ml−1. Fresh manure was obtained from the University of Maryland Western Maryland Research and Education Center dairy farm in Clarksville, Md. (Table 1). For each core that received manure, 1 ml of inoculum was mixed with 50 g of manure, which was then spread evenly over the surface of the core. For each core that did not receive manure, 1 ml of inoculum was spread evenly over the soil surface. Rainfall treatments began immediately after manure application or inoculation.
TABLE 1.
Soil and manure characteristics prior to inoculation, rainfall, and leaching
| Soil or manure | Bulk density (g/cm3) | Pore density (g/cm3) | % Solids | Pore space (%) | % Organic mattera | % Sandb | % Siltb | % Clayb | NO3 content (μg/g) | NH4 content (μg/g) | PO4 content (μg/g) | Total organic carbon content (μg/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dairy manure | NDc | ND | ND | ND | ND | ND | ND | ND | 0.98 | 11.90 | 34.05 | 2.21 |
| Sandy loam | 1.08 | 2.36 | 45.90 | 54.10 | 2.33 | 69.30 | 9.32 | 21.38 | 8.43 | 1.34 | 0.69 | 1.42 |
| Silt loam | 0.86 | 1.98 | 43.48 | 56.52 | 4.45 | 19.53 | 53.14 | 27.33 | 22.19 | 1.53 | 1.22 | 1.26 |
| Clay loam | 1.08 | 2.34 | 46.10 | 53.90 | 2.86 | 27.85 | 41.31 | 21.49 | 21.49 | 4.23 | 2.85 | 1.10 |
Determined by the oven method.
Sand, silt, and clay contents were determined by the hydrometer method after oxidation with H2O2; this and other soil analysis methods used are described in Methods of Soil Analysis (19).
ND, not done or not applicable.
Intact and disturbed soil cores.
The following three types of soil were obtained fresh and used to prepare cores: a clay loam soil from Clarksville, Md., under conventionally tilled corn; a sandy loam soil from Upper Marlboro, Md., under conventionally tilled tobacco; and a silt loam soil from Beltsville, Md., under no-till hay (Table 1). Intact cores were prepared by using sections of polyvinyl chloride plumbing pipe (inside diameter, 102 mm; length, 177.5 mm) with a 45° bevel at one end. Each pipe section was driven into the soil by placing a steel plate over the end without a bevel and striking the plate with a hammer until 152.5 mm of the pipe was in the soil. An intact core was removed by excavating around the pipe and then cutting the bottom of the soil profile cleanly. The intact core was kept in the pipe and was placed on filter paper inside a 133-mm-diameter plastic Buchner funnel (Fisher Scientific), and the bottom of the pipe was caulked with silicone sealant. Anchoring cement (Quikrete Corp., Atlanta, Ga.) was then poured between the pipe and the funnel walls and allowed to dry overnight. To obtain disturbed soil cores, we used the same soils, but the soils were first air dried and sieved through a 5-mm mesh. Disturbed soil cores were prepared by using aluminum flashing that was cut and taped together to form cylinders which were the same size as the polyvinyl chloride cores. A piece of filter paper was placed at the bottom of each cylinder inside a 133-mm-diameter plastic Buchner funnel; soil was then added 15 mm at a time and tamped repeatedly to promote settling and remove air pockets, until a final depth of 152.5 mm was reached. All of the cores were then saturated by immersing them in RO water to just below the soil line and allowing capillary action to draw up water until the soil surface was wet. Then the aluminum cylinder was carefully removed from each disturbed soil core, a second cylinder was placed around the outside of the Buchner funnel so that it extended 50 mm above the soil, and a third cylinder which was 50 mm tall whose circumference was 5 mm less than the soil core circumference was placed on top and pressed slightly into the soil surface. Anchoring cement was poured between the soil and the outer aluminum cylinder to within a few millimeters of the top edge of the upper cylinder. After the cement had dried, the outer cylinder was removed, which left a soil column whose edges were encased in concrete and the top aluminum cylinder in place so that it could catch rainfall on the soil surface. Since the sides of the disturbed soil core were encased in cement, potential preferential flow or so-called edge effects were eliminated. The disturbed soil cores used for the preliminary experiment were identical except they were 102 mm long and 102 mm wide and were composed of a silty clay loam soil and a sandy loam soil obtained from different sources in Beltsville, Md.
Rainfall and leachate collection.
The rainfall in the preliminary experiment consisted of continuously formed droplets of tap water that were applied at a rate of approximately 16 mm per h for 8 h; each core received a total of 128 mm (1,038 ml) of rainfall. A rainfall rate of 25.4 mm over a 4-h period was used in continuing experiments; three 70-ml portions of RO water (total, 210 ml) were applied at hourly intervals to each core surface (area, 81.1 cm2). Leachate was collected in sterile beakers, and samples were collected each hour in the preliminary experiment. For long-term experiments, rainfall was applied daily for 4 days, and then at 3- to 4-day intervals four additional times (eight rainfall events). In the long-term experiments leachate samples were obtained once after each rainfall, when gravity draining from all of the cores had ceased (within 8 h).
Selective media.
Samples were serially diluted in isotonic saline-phosphate buffer (pH 8.0) and then plated onto Luria-Bertani agar supplemented with 100 μg of ampicillin per ml, 100 μg of rifamycin SV per ml, and 100 μg of cycloheximide per ml (for strain B6914), MacConkey agar (for coliforms) or RIM agar (3) (for total heterotrophs). Higher dilutions were plated onto selective media by using a spiral plating system (Autoplate 3000; Spiral Systems, Inc.), while 100- or 1,000-μl portions of lower dilutions were plated directly onto replicate plates. The plates were inverted after 30 min and incubated at 37°C for 48 h. The bacteria on spiral plates were counted by using a vendor-supplied spiral grid and volume table, while all of the bacteria on replicate plates were counted and the values obtained for replicates were averaged. B6914 colony counts were confirmed by placing the plates on a long-wavelength (310-nm) UV light box and disregarding any colonies that did not glow green. The same media and dilutions were used for soil samples, except that soil samples were diluted 1:10 in sterile water and were extracted by using a Waring blender with a 1-liter glass container that was centrifuged at the top speed (22,000 rpm) for 1 min; after this the contents were allowed to settle for 1 min before dilution and plating from the middle fraction in the blender container.
Sampling schedule and experimental design.
In the preliminary experiment leachate samples were collected each hour for 8 h during continuous rainfall, and a final sample was collected after the cores had drained overnight; thus, a total of nine leachate samples were collected from each core. The soil samples consisted of 10 g of well-sieved soil from five different depths and the manure layer (total, six samples) from a single core for each soil. In the long-term experiments, daily leachate samples were collected on four consecutive days, and then four additional samples spaced 3 or 4 days apart were collected; thus, a total of eight leachate samples were collected over a 17-day period. Soil samples were collected after 18 days at three evenly spaced depths and from the manure layer if the core had one; thus, three samples were collected from cores that did not receive manure, and four samples were collected from cores that received manure. Intact core and disturbed soil core inoculation and sampling were performed independently by using the same parameters and sampling schedules. Replicate cores were prepared (disturbed soil cores) or obtained (intact soil cores) fresh, and treatments were randomly assigned in duplicate to similar cores. The two treatments were (i) inoculated soil and (ii) inoculated soil with added manure. For both treatments intact and disturbed soil cores were replicated and three different soils were used; a total of 24 cores were used for the long-term experiment.
RESULTS
A preliminary experiment showed that the levels of B6914 in leachates from disturbed soil cores exceeded the inoculum level (4.018 × 107 CFU ml−1) after 2 h of 16.6-mm h−1 rainfall with a silty clay loam soil and approached the inoculum level after 3 h with a sandy loam soil (Table 2). After an initial lag, the level of leached B6914 in hourly samples remained near the inoculum level until rainfall was stopped after 8 h. Overnight gravity draining of cores resulted in leachate B6914 levels that were several times greater than the inoculum level and total B6914 levels at six soil depths that were near or greater than the inoculum level (Table 2). B6914 was fairly evenly distributed vertically in both soil types, although the concentrations of B6914 were slightly higher near the tops of the cores (Table 2). The total amount of B6914 recovered from soil and leachate was 15.37 times greater than the inoculum level with the sandy loam soil and 15.91 times greater than the inoculum level with the silty clay loam soil.
TABLE 2.
Recovery of viable E. coli O157:H7 strain B6914 from leachate and soil during a preliminary experiment in which there was 8 h of rainfall at a rate of 1.65 cm h−1a
| Expt | Soil | Treatment
|
B6914 concn (log CFU g−1) | Vol (ml) | Wt (g) | Total amt (log CFU) | |
|---|---|---|---|---|---|---|---|
| Length of rainfall (h) | Soil depth (cm) | ||||||
| Recovery from hourly leachate samples | Sandy loamb | 1 | 3.682 (0.0000)c | 45 | 5.335 | ||
| 2 | 4.454 (0.0635) | 111 | 6.450 | ||||
| 3 | 5.456 (0.0513) | 100 | 7.456 | ||||
| 4 | 5.385 (0.0251) | 86 | 7.319 | ||||
| 5 | 5.454 (0.0251) | 72 | 7.312 | ||||
| 6 | 5.382 (0.0475) | 74 | 7.251 | ||||
| 7 | 5.334 (0.0000) | 86 | 7.269 | ||||
| 8 | 5.443 (0.0090) | 66 | 7.263 | ||||
| Drain overnight | 5.526 (0.0373) | 800 | 8.429 | ||||
| Silty clay loamd | 1 | 2.716 (0.3010) | 41 | 4.329 | |||
| 2 | 5.529 (0.0042) | 160 | 7.733 | ||||
| 3 | 5.793 (0.0023) | 120 | 7.872 | ||||
| 4 | 5.625 (0.0390) | 106 | 7.650 | ||||
| 5 | 5.672 (0.0236) | 112 | 7.721 | ||||
| 6 | 5.533 (0.0021) | 126 | 7.633 | ||||
| 7 | 5.641 (0.0057) | 146 | 7.805 | ||||
| 8 | 5.401 (0.0085) | 152 | 7.583 | ||||
| Drain overnight | 5.444 (0.0283) | 400 | 8.046 | ||||
| Recovery from different soil depths after overnight drainage | Sandy loame | 0 (manure) | 5.686 (0.0170) | 40 | 7.319 | ||
| 2 | 5.403 (0.0282) | 261 | 7.830 | ||||
| 4 | 5.148 (0.0229) | 293 | 7.690 | ||||
| 6 | 4.940 (0.1374) | 371 | 7.522 | ||||
| 8 | 4.773 (0.0121) | 332 | 7.330 | ||||
| 10 | 4.751 (0.0095) | 353 | 7.310 | ||||
| Silty clay loamf | 0 (manure) | 5.396 (0.1039) | 43 | 6.998 | |||
| 2 | 5.430 (0.0226) | 267 | 7.846 | ||||
| 4 | 5.024 (0.0152) | 348 | 7.491 | ||||
| 6 | 4.841 (0.0387) | 382 | 7.410 | ||||
| 8 | 4.320 (0.0000) | 360 | 6.841 | ||||
| 10 | 4.469 (0.0668) | 362 | 7.017 | ||||
The concentration of strain B6914 in the inoculum was 7.600 log CFU g−1. For the sandy loam soil the amount of B6914 recovered from the leachate and soil over a 24-h period was 15.37 times the amount inoculated, and for the silty clay loam soil the amount of B6914 recovered from the leachate and soil over a 24-h period was 16.01 times the amount inoculated.
The total amount of B6914 recovered from sandy loam soil in this experiment (concentration multiplied by volume of leachate) was 8.60 log CFU, and the ratio of the amount recovered to the amount inoculated was 10.00.
The values in parentheses are standard errors, which were calculated from the values for replicate plates prepared by using single soil cores.
The total amount of B6914 recovered from silty clay loam soil in this experiment (concentration multiplied by volume of leachate) was 8.68 log CFU, and the ratio of the amount recovered to the amount inoculated was 12.02.
The total amount of B6914 recovered from sandy loam soil in this experiment (concentration multiplied by soil weight) was 8.33 log CFU, and the ratio of the amount recovered to the amount inoculated was 5.37.
The total amount of B6914 recovered from silty clay loam soil in this experiment (concentration multiplied by soil weight) was 8.19 log CFU, and the ratio of the amount recovered to the amount inoculated was 3.89.
The conditions used in the preliminary experiment were extreme compared to average seasonal field conditions in the area studied, so reduced rainfall consisting of 25.4 mm over a 4-h period was utilized in a long-term experiment along with larger cores, three different types of soil, and different soil matrices. B6914 traveled through all of the soils in leachate and was detected at all sampling times over 18 days. For most of the cores the total amount of B6914 in leachate ranged from 105 to 108 CFU on the day of inoculation and from 104 to 106 CFU after 18 days. The only exceptions were the intact clay loam soil cores; in 75% of these cores leaching ceased after 3 days due to clogging. In the long-term experiments, the B6914 data for leachate and soil showed that for all but one treatment, the number of viable B6914 CFU recovered was more than the number inoculated; the number of CFU recovered ranged from 0.64 to 30.97 times more than the number of CFU in the inoculum (Table 3). Overall, there was not a significant difference (α = 0.05) between B6914 levels in leachate based on core type (intact and disturbed soil cores). In all cases, the leachate levels of B6914 for disturbed soil cores without manure were greater than the leachate levels for the same cores with manure added (Table 3). For intact soil cores, replication and vertical movement were often much greater when 50 g of fresh manure solids was added to the core (Table 3).
TABLE 3.
Recovery of viable E. coli O157:H7 strain B6914 from leachate and soil during two 18-day experiments in which intact (no-till) and disturbed (tilled) soil cores were useda
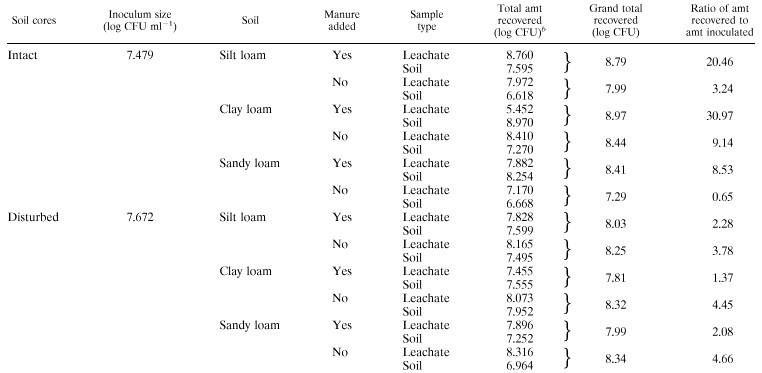 |
All values are means based on two replicates.
In each experiment the total amount for leachate samples was calculated by adding the amounts obtained after eight 25.4-mm rainfall events over a 17-day period (Fig. 1 and 2). The total amount for soil samples was calculated by adding the amounts obtained for three 50-mm layers plus the amount obtained for a manure layer if applicable; samples were obtained 18 days after inoculation.
Least-significant-difference tests (Table 4) revealed that significantly more nitrate leached through cores to which manure was added, and the highest nitrate levels leached from the sandy loam and silt loam soils. Disturbed soil core leachates contained more ammonia and total organic carbon and were more turbid than intact soil core leachates (Table 4); turbidity was greatest in sandy loam leachate, followed by the silt loam leachate. Slightly more phosphate leached through the silt loam and sandy loam soil cores than through the clay loam soil cores (Table 4). Comparisons of levels of B6914, total coliforms, and total heterotrophs with levels of nutrients and turbidity in leachates revealed that nitrate and ammonia levels were positively correlated with B6914 and total coliform levels (Table 5). Phosphate levels and turbidity did not correlate, and total organic carbon content was negatively correlated with B6914 and total coliform levels in leachates.
TABLE 4.
Least-significant differences for nutrients and turbidity for soil column leachates during 18-day experiments
| Variable compared | Type | Significancea
|
||||
|---|---|---|---|---|---|---|
| Water-soluble nitrate | Water-soluble ammonia | Water-soluble phosphate | Total organic carbon | Turbidity (optical density at 405 nm) | ||
| Days | —b | —c | —c | —c | —c | |
| Core type | Intact | NS | B | NS | B | B |
| Disturbed | NS | A | NS | A | A | |
| Soil type | Sandy loam | A | NS | AB | NS | A |
| Silt loam | A | NS | A | NS | B | |
| Clay loam | B | NS | B | NS | C | |
| Manure | Present | A | NS | NS | NS | NS |
| Absent | B | NS | NS | NS | NS | |
Different letters indicate that differences were significant. All replicates and time points were compared by using a linear model to determine overall treatment effects. NS, not significantly different.
Leachate values were generally greatest after inoculation and decreased over time.
Leachate values were generally lowest after inoculation and increased over time.
TABLE 5.
Correlation of nutrient levels and turbidity with total coliform, E. coli O157:H7 strain B6914, and total heterotroph levels in leachates collected from intact and disturbed soil columns over a 17-day perioda
| Organism(s) | NO3
|
NH4
|
PO4
|
Total organic carbon
|
Turbidity
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Correlation | Probability | Correlation | Probability | Correlation | Probability | Correlation | Probability | Correlation | Probability | |
| Total coliforms | 0.15047b | 0.1569 | 0.11821 | 0.2671 | 0.19634 | 0.0636 | −0.01026 | 0.9235 | 0.17995 | 0.0897 |
| E. coli O157:H7 strain B6914 | 0.16867 | 0.1120 | 0.06548 | 0.5398 | 0.20552 | 0.0520 | −0.08795 | 0.4098 | −0.22412 | 0.0337 |
| Total heterotrophs | 0.0543 | 0.6112 | −0.00692 | 0.9484 | 0.04626 | 0.6650 | −0.10878 | 0.3075 | −0.20565 | 0.0518 |
A positive correlation with a high probability (α > 0.10) indicates that the parameters compared occurred together; a negative correlation with a high probability indicates that the parameters did not occur together; and a low probability was inconclusive (there was no pattern). Coliform and B6914 levels were positively correlated with nitrate and ammonia levels in leachate and negatively correlated with total organic carbon levels.
Boldface type indicates correlations that did not have a significantly different (high) probability.
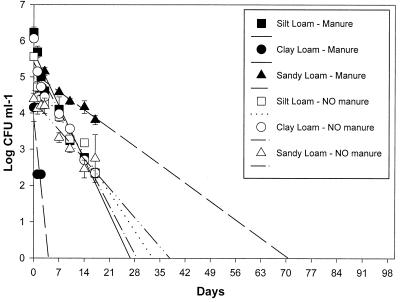
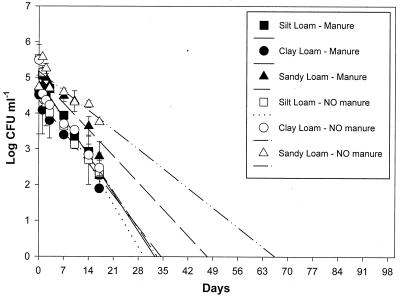
A regression analysis (Table 6 and Fig. 1 and 2) was performed with daily leachate concentrations of B6914 measured over 17 days. A linear regression analysis revealed that B6914 was expected to continue leaching through most soils and soils subjected to most tillage treatments for about 30 days (except for the sandy loam soil) based on a best-fit regression line extended to the x axis. The daily levels of B6914 in leachates from sandy loam intact soil cores treated with manure and disturbed soil cores not treated manure were less than 1 log lower than the initial leachate levels (Fig. 1 and 2), with x-axis intercepts of 64 to 74 days (Table 6). For other soils (both core types), the B6914 levels in leachate were higher initially but declined to lower values over approximately 30 days (Fig. 1 and 2 and Table 6). The only exception was intact clay loam cores, which became clogged after 3 days, although disturbed clay loam soil cores had normal infiltration rates throughout the experiment. If tillage and manure treatments are disregarded, B6914 should leach from the clay loam soil for 4 to 34 days (confidence interval, up to 55 days), from the silt loam soil for 27 to 33 days (confidence interval, up to 40 days), and from the sandy loam soil for 38 to 76 days (confidence interval, up to 127 days). Based on r2 (regression fit) values (Table 6) and graphical representations of measured values (Fig. 1 and 2), linear regression gave an accurate indication of future leaching trends for B6914.
TABLE 6.
Linear regression parameters: E. coli O157:H7 strain B6914 concentrations in leachate over a 17-day perioda
| Manure | Soil | Intact soil cores
|
Disturbed soil cores
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| y intercept | Slopeb | r2c | x intercept | x intercept UCLd | x intercept LCLe | y intercept | Slopeb | r2c | x intercept | x intercept LCLd | x intercept LCLe | ||
| None | Clay loam | 5.407 | −0.193 | 0.890 | 28f | 35 | 24 | 4.885 | −0.147 | 0.882 | 33 | 40 | 28 |
| Silt loam | 4.966 | −0.151 | 0.894 | 33 | 40 | 28 | 4.982 | −0.168 | 0.787 | 30 | 40 | 24 | |
| Sandy loam | 4.332 | −0.115 | 0.796 | 38 | 51 | 31 | 5.168 | −0.078 | 0.726 | 66 | 98 | 50 | |
| Fresh dairy manure solids | Clay loam | 3.986 | −1.010 | 0.751 | 4f | 2 | −24 | 4.364 | −0.127 | 0.644 | 34 | 55 | 25 |
| Silt loam | 5.683 | −0.212 | 0.922 | 27 | 31 | 24 | 4.915 | −0.150 | 0.927 | 33 | 38 | 29 | |
| Sandy loam | 5.018 | −0.066 | 0.677 | 76 | 127 | 55 | 5.013 | −0.106 | 0.763 | 47 | 66 | 37 | |
See Fig. 1 and 2. Leachate samples were collected from duplicate intact and disturbed soil cores after each rainfall event, a total of eight times. One-half of the cores were inoculated, and one-half of the cores were inoculated and amended with 50 g of manure tilled into the soil surface.
Slope of a linear regression line through all data points extending to both axes.
Fit of the regression line to the data points, where 1.000 is a perfect fit.
UCL, upper confidence interval.
LCL, lower confidence interval.
One-half of the clay loam soil cores that did not receive manure and all of the clay loam soil cores that received manure became clogged after 3 days.
FIG. 1.
Concentrations of E. coli O157:H7 recovered in intact soil core leachate samples after eight 25-mm day−1 rainfall events for three soil types. Duplicate cores received either manure with inoculum or inoculum alone. The standard error bars represent means of two replicates for a total of 12 intact soil cores. Both clay loam intact cores with manure and one core without manure became clogged after 3 days, which resulted in leachate levels of B6914 that were lower than the levels for other soils.
FIG. 2.
Concentrations of E. coli O157:H7 recovered in disturbed soil core leachates after eight 25-mm day−1 rainfall events for three soil types. Duplicate cores received either manure with inoculum or inoculum alone. The standard error bars represent means of two replicates for a total of 12 disturbed soil cores. Disturbed soil cores did not become clogged, and leachate flowed evenly throughout the 17-day experiment.
DISCUSSION
Due to the somewhat unrealistic conditions used in the preliminary experiment, we designed longer-term experiments in which we used less rainfall (lower volume and intensity), larger cores, and extended sampling periods (more than 24 h). In addition to reduced rainfall, we used intermittent periods of wetting and drying throughout the experiment and tried to determine whether the presence of manure influenced survival and leaching of B6914 and nutrients. Rainfall at a rate of 6.35 mm per h for 4 h (total, 25.4 mm) was a more realistic situation, since it simulated the average once-a-month storm for the area studied. Rainfall was initially applied daily and then spaced several days apart so the soil cores were not saturated all of the time. The intermittent wetting and drying did not seem to affect the daily leachate level trends for B6914 (Fig. 1 and 2), although we expected leachate levels to decrease sharply when the soil dried between rainfall events. It is probable that the available moisture remaining in the soil between rainfall events was adequate for bacterial survival and that rainfall events led to high rates of B6914 growth. High levels of B6914 remaining in the soil indicated that B6914 was able to attach to microsites and may have replicated by budding off cells when water and nutrients (especially nitrogen) flowed past microcolonies (12).
Not as much total B6914 was recovered from disturbed soil cores in the long-term experiments as in the preliminary experiment, probably because the amount of B6914 remaining in the soil (measured after 18 days instead of after 24 h) was much lower (Tables 3 and 4); using larger cores also may have decreased transport. Soil type and manure effects were not so easily explained. In some cases the soil B6914 levels for the same core-manure treatment were similar for different core types (silt loam soil with manure, sandy loam soil without manure) but the leachate levels were different, while in other cases the leachate levels were similar (silt loam soil without manure, sandy loam soil with manure) but the soil levels were different. B6914 generally replicated better in disturbed soil cores without manure, while in intact soil cores replication and recovery of B6914 were much greater when manure was added. Nutrient limitation for both indigenous and inoculated microbes seemed to be why manure had a greater effect on intact soil core B6914 replication. Since disturbed soil cores were well mixed and often more nutrients leached out of these soil cores (Table 4), B6914 and coliforms probably had to compete more with the soil microflora for available nutrients that were in short supply. In intact soil cores, microsites were left intact, and B6914 and coliforms were probably better able to avoid competition and predation by seeking refuge and therefore could take better advantage of available nutrients and replicate faster. Compared to the conditions in disturbed soil, the physical and biological conditions in intact soil were better for survival and replication of B6914 and coliforms.
Turbidity in leachate samples was not correlated with bacterial movement overall, and there was a negative correlation between B6914 levels and turbidity (Table 5), indicating that the organisms probably were not attached to leaching particles and probably did not leach as if they were particles. The lack of correlation between B6914 and coliform levels with phosphate content in leachate confirmed that these organisms did not behave as particulates since phosphate often moves with particulates in soil. B6914 and coliform levels were positively correlated with nitrogen (ammonia and nitrate) levels in leachate; these organisms may follow nitrogen sources through soil by using chemotaxis or may simply survive longer or replicate faster in the presence of available nitrogen.
Clearly, if E. coli O157:H7 strain B6914 reached soil, whether it was applied in manure or came from runoff from a point source, it could survive, replicate, and move vertically for some time, posing a continuing threat to nontarget environments. In the case of intact clay loam cores, which became clogged shortly after inoculation, high levels of recovery of B6914 in soil and low leachate levels showed that population growth could occur if there were minimal leaching losses. Saturated soil conditions may also enhance growth of coliforms compared with the indigenous flora, since coliforms are facultative anaerobes and oxygen is limited in a saturated soil. Low infiltration rates and saturated soil conditions may also lead to increased spread in runoff during rainfall events. Low levels of survival and recovery of B6914 in the sandy loam soil cores reflected the opposite effect. Most often, less B6914 was recovered from sandy loam leachate than from leachate from other soils, but the leachate rate was more consistent and leaching continued longer (38 to 76 days) than for the other soils (4 to 34 days) (Table 6).
We concluded that the presence of manure enhances the survival of E. coli in no-till soils, probably due to enhanced microsite habitat and the addition of nitrogen. However, similarly high levels of replication and leaching from no-till soil without manure and from tilled soil represented about the same level of risk. Risk is therefore present when coliforms, specifically E. coli O157:H7, reach the soil as runoff from stored manure or when manure is applied directly to fields, if rainfall or irrigation provides the mechanism for dispersion. Data indicate that if soil pores do not become clogged, E. coli O157:H7 can travel below the top layers of soil for more than 2 months after the initial application.
Since B6914 levels were correlated with nitrogen levels in leachate, nutrient management through NPDES permits may be effective for controlling pathogens by limiting the amount of nitrogen applied to fields to the amount which a crop can utilize in a season. However, to manage potential problems with crop, surface, and groundwater contamination, some form of on-farm pathogen management in addition to NPDES permit requirements may be necessary before manure is spread onto agricultural fields.
ACKNOWLEDGMENTS
We thank Assad Rouhi and Dan Shelton for their assistance designing and setting up soil cores for the preliminary experiment.
REFERENCES
- 1.Berry D F, Hagedorn C. Soil and groundwater transport of microorganisms. Bio/Technology. 1991;15:57–73. doi: 10.1016/b978-0-409-90199-3.50010-3. [DOI] [PubMed] [Google Scholar]
- 2.Burton G A, Jr, Gunnison D, Lanza G R. Survival of pathogenic bacteria in various freshwater sediments. Appl Environ Microbiol. 1987;53:633–638. doi: 10.1128/aem.53.4.633-638.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buyer J S. A soil and rhizosphere microorganism isolation and enumeration medium that inhibits Bacillus mycoides. Appl Environ Microbiol. 1995;61:1839–1842. doi: 10.1128/aem.61.5.1839-1842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canadian Food Inspection Agency. Canadian Food Inspection Agency Acts SOR/93–232, s.4; SOR/95–548, s.3; and SOR/95–548, s.3. Ottawa, Ontario, Canada: Canadian Food Inspection Agency; 1999. [Google Scholar]
- 5.Crabill C, Donald R, Snelling J, Foust R, Southam G. The impact of sediment fecal coliform reservoirs on seasonal water quality in Oak Creek, Arizona. Water Res. 1998;33:2163–2171. [Google Scholar]
- 6.Faust M A. Relationship between land-use practices and fecal bacteria in soils. J Environ Qual. 1982;11:141–146. [Google Scholar]
- 7.Filip Z, Kaddu-Mulindwa D, Milde G. Survival of some pathogenic and facultative pathogenic bacteria in groundwater. Water Sci Technol. 1988;20:227–231. [Google Scholar]
- 8.Fratamico P M, Deng M Y, Strobaugh T P, Palumbo S A. Construction and characterization of Escherichia coli O157:H7 strains expressing firefly luciferase and green fluorescent protein and their use in survival studies. J Food Prot. 1997;60:1167–1173. doi: 10.4315/0362-028X-60.10.1167. [DOI] [PubMed] [Google Scholar]
- 9.Gerba C P, Bitton G. Microbial pollutants; their survival and transport pattern to groundwater. In: Bitton G, Gerba C P, editors. Groundwater pollution microbiology. New York, N.Y: John Wiley and Sons; 1984. pp. 65–88. [Google Scholar]
- 10.Gerba C P, Wallis C, Melnick J L. Fate of wastewater bacteria and viruses in soil. J Irrigat Drainage Eng. 1975;101:157–174. [Google Scholar]
- 11.Howell J M, Coyne M S, Cornelius P. Fecal bacteria in agricultural waters of the bluegrass region of Kentucky. J Environ Qual. 1995;24:411–419. [Google Scholar]
- 12.Lawrence J R, Delaquis P J, Korber D R, Caldwell D E. Behavior of Pseudomonas fluorescens within the hydrodynamic boundary layers of surface microenvironments. Microb Ecol. 1987;14:1–14. doi: 10.1007/BF02011566. [DOI] [PubMed] [Google Scholar]
- 13.McCoy E L, Hagedorn C. Quantitatively tracing bacterial transport in saturated soil systems. Water Air Soil Pollut. 1979;11:467–479. [Google Scholar]
- 14.McMurry S W, Coyne M S, Perfect E. Fecal coliform transport through intact soil blocks amended with poultry manure. J Environ Qual. 1998;27:86–92. [Google Scholar]
- 15.Miettinen I T, Vartiainen T, Martikainen P J. Changes in water microbial quality during bank filtration of lake water. Can J Microbiol. 1997;43:1126–1132. doi: 10.1139/m97-161. [DOI] [PubMed] [Google Scholar]
- 16.Pell A N. Manure and microbes: public and animal health problem? J Dairy Sci. 1997;80:2673–2681. doi: 10.3168/jds.S0022-0302(97)76227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sims J T. Characteristics of animal wastes and waste-amended soils: an overview of the agricultural and environmental issues. In: Steele K, editor. Animal waste and the land-water interface. Proceedings of a conference held in Fayetteville, AR, July 16–19, 1995. Boca Raton, Fla: Lewis Publishers; 1995. pp. 1–14. [Google Scholar]
- 18.U.S. Environmental Protection Agency Office of Research and Development, Municipal Environmental Research Laboratory. Management of small waste flows. Publication USEPA-600/2-78-173. Springfield, Va: National Technical Information Service; 1978. [Google Scholar]
- 19.Weaver R W, Angle J S, Bottomley P S. Microbiological and biochemical properties. Madison, Wis: Soil Science Society of America, Inc.; 1994. Methods of soil analysis, part 2. [Google Scholar]
- 20.Wilson L G, Amy G L, Gerba C P, Gordon H, Johnson B, Miller J. Water quality changes during soil aquifer treatment of tertiary effluent. Water Environ Res. 1995;67:372–376. [Google Scholar]
- 21.Ziebell W A. Removal of fecal bacteria from wastewater of individual homes during treatment by conventional and experimental methods. M.S. thesis. University of Wisconsin, Madison; 1975. [Google Scholar]