Abstract
BACKGROUND:
Men are 4 to 8 times more likely to develop hepatocellular carcinoma (HCC) than women. Preclinical models have suggested a role for sex hormones in the development of HCC. In the current study, the authors investigated the impact of age, sex, race, and ethnicity on the survival of patients with HCC using the Surveillance, Epidemiology, and End Results (SEER) database.
METHODS:
Patients diagnosed with HCC from 1988 through 2010 were identified from the SEER registry. Hazard ratios (HR) for overall survival (OS) were derived using the Cox regression model adjusted for race, year of diagnosis, marital status, treatment, birthplace, tumor differentiation, and tumor size.
RESULTS:
A total of 39,345 patients were identified; 76% were men and 34% were women (50% white, 12% African American, 21% Asian, 16% Hispanic, and 1% Native American). The median age at the time of diagnosis was 61 years for men and 67 years for women. Approximately 84% of patients had liver-limited disease and 16% had metastatic disease. Treatment information was available for patients diagnosed after 1998 (34,674 patients): 11% received liver-directed therapy, 11% underwent surgical resection, and 7% underwent liver transplantation. The HR for the OS of women versus men was 0.83 (95% confidence interval [95% CI], 0.77–0.88) for patients aged <55 years. The protective effect of sex on OS was found to be greatest in patients aged 18 to 44 years (HR, 0.75; 95% CI, 0.65–0.86 [P<.001]), especially those with surgically resected tumors (HR, 0.68; 95% CI, 0.54–0.86 [P=.001]) and those who were African American (HR, 0.85; 95% CI, 0.78–0.92 [P<.001]). There was no survival difference between sexes noted among Hispanics or patients aged >65 years.
CONCLUSIONS:
Sex appears to be associated with survival in patients with HCC. The role of androgens and estrogens in the development and progression of HCC warrants further investigation.
Keywords: hepatocellular carcinoma, sex, survival, androgens, estrogens
INTRODUCTION
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related mortality, with approximately 600,000 deaths occurring each year worldwide.1,2 In the United States, HCC is the fifth and ninth leading cause of cancer-related death among men and women, respectively.3 HCC, the predominant histological subtype of liver cancer, is associated with hepatitis B virus (HBV) or hepatitis C virus, alcohol abuse, nonalcoholic fatty liver disease (NAFLD), diabetes mellitus, obesity, iron storage diseases, and aflatoxin B1 exposure.1 Regardless of predisposing factors or geographic location, a striking sex disparity in the incidence of HCC has been consistently demonstrated, with men found to be 4 to 8 times more likely to develop the disease than women.4 It is from such epidemiologic observations that sex hormones were first surmised to modulate hepatocellular carcinogenesis nearly 5 decades ago.5,6 Since then, androgens and estrogens have been shown to mediate infectious hepatitis,7,8 hepatic fibrosis,9 NAFLD,10 and cirrhosis,11 in addition to HCC.12 For example, androgens have been linked to hepatic tumorigenesis in mice models13 and a predilection for HBV-related liver cancer in men.14 Conversely, estrogen has been shown to repress HCC growth in vitro and in vivo by upregulation of the JAK-STAT (Janus kinase/signal transducers and activators of transcription) pathway,15 suppression of interleukin-6 secretion from Kupffer cells,16,17 decreased hepatocyte growth factor production,17 and NF-κB (nuclear factor kappalight chain-enhancer of activated B cells) inhibition.18
Sex-related discrepancies in the incidence and survival of other gastrointestinal tumors, including esophageal,19 gastric,20 and colorectal cancers,21 have previously been demonstrated. Further supporting the protective role of estrogen is the consistent age-dependent relationship between sex and outcomes in these malignancies, with premenopausal women having improved survival compared with younger men. Moreover, racial/ethnic disparities in HCC survival have been reported,22 most likely reflecting differences in endemic risk factors, socioeconomic status, and/or health care practices across populations. In the current study, we explored the hypothesis that sex is a prognostic indicator in patients with HCC, and that this relationship is further influenced by age, race/ethnicity, and disease stage.
MATERIALS AND METHODS
Study Design
The Surveillance, Epidemiology, and End Results (SEER) research database for 1973 through 2010 (April 2013 version) was retrieved for the current study. The SEER program, which is sponsored by the National Cancer Institute, collects information regarding cancer incidence and survival from 18 population-based cancer registries, covering 28% of the US population (seer.cancer.gov/about/overview.html). The demographics, primary tumor site, tumor morphology, stage of disease at diagnosis, first course of radiation, surgical intervention, and survival of all patients newly diagnosed with cancer were systematically compiled by each registry. The SEER program oversampled Hispanics, Native Americans, and Asians/Pacific Islanders.
Study Population
We first identified patients diagnosed with HCC (International Classification of Diseases for Oncology, 3rd Edition [ICD-O-3] site code C22.0 and histologic type ICD-O-3 codes 8170–8175) between 1988 and 2010 from the SEER database (58,883 patients). Those patients diagnosed between 1973 and 1987 (4879 patients) were excluded due to insufficient information regarding staging. Disease stage was assessed by established prognostic criteria,23 with categorization based on liver-limited disease (further classified by the number of lesions, the presence/absence of vascular invasion, and primary lesion size [<3 cm, 3–5 cm, or >5 cm]) versus metastatic disease. A total of 3611 patients with only a clinical diagnosis without radiographic or pathologic confirmation were excluded. Patients with SEER historic stage A (in situ or unstaged disease; 6464 patients), fibrolamellar histology (268 patients), diagnosis made at the time of autopsy or death (694 patients), unknown ethnicity (144 patients), unknown survival time (8040 patients), and those diagnosed at age <18 years or >90 years (616 patients) were excluded. Only patients with complete information available regarding sex, age, ethnic background, and staging were incorporated into the final analysis. Detailed exclusion criteria are shown in Figure 1. A total of 39,345 patients with HCC matching the specified eligibility criteria were included in the final analysis (Fig. 1).
Figure 1.

The subject selection algorithm is shown. Note that some patients met >1 exclusion criteria. SEER indicates Surveillance, Epidemiology, and End Results; HCC, hepatocellular carcinoma.
Statistical Analysis
The primary endpoint was overall survival (OS), defined as the interval from the date of diagnosis to the date of death. OS was censored at date of last contact, December 31, 2010, or 5 years after diagnosis, whichever came first. For censored subjects (10,608 patients), the median follow-up was 27 months, and 43% of the patients within this group had a follow-up of at least 3 years. The primary hypothesis was that women survive longer than men after a diagnosis of HCC, especially women aged <55 years. Based on previous studies,19,21 we used an age of 55 years as a surrogate for menopause. A Cox proportional hazards regression model was performed to evaluate the impact of sex on OS when adjusting for other potential prognostic factors in the SEER database. We considered age at diagnosis, race/ethnicity, marital status, birthplace, tumor stage, tumor size, tumor grade, surgical procedure, use of radiotherapy, SEER registry site, and year of diagnosis in the multivariable Cox regression model. Detailed definitions of each variable are shown in Tables 1 and 2. A likelihood ratio test was used to test the interactions between sex, age, race, stage of disease, and treatment on OS. Departures from the proportional hazards assumption for the model were examined graphically using smoothed plots of weighted Schoenfeld residuals. No violation was detected. All analyses were performed using SAS statistical software (version 9.3; SAS Institute Inc, Cary, NC). Results were considered significant at P=.05 using 2-sided tests.
TABLE 1.
Variable Definitions and Categories
| Variable | Definitions and Categories |
|---|---|
|
| |
| Sex | Male and female |
| Age, y | 18–44, 45–54, 55–64, 65–74, and ≥75 |
| Race/ethnicity | White, African American, Asian/Pacific Islander, Hispanic (identified as having Spanish/Hispanic surname or of Spanish origin regardless the category in race/ethnicity), and Native American |
| Marital status at diagnosis | Not married (including never married, separated, divorced, widowed, and unknown) and married (including common-law marriage) |
| Birthplace | US-born, abroad low-incidence region, abroad high-incidence region, and unknown |
| Tumor stage | Single lesion in 1 lobe, multiple tumors, vascular invasion, and metastatic disease |
| Tumor size, cm | <3, 3 to <5 cm, ≥5 cm, and unknown |
| AJCC tumor grade/differentiation | Well differentiated (grade 1), moderately differentiated (grade 2), poorly differentiated or undifferentiated (grade 3 or 4), and not determined |
| Initial surgical procedure | None or unknown, local therapy (photodynamic therapy, electrocautery, fulguration, cryosurgery, laser, percutaneous ethanol injection, heat-radiofrequency ablation, or other), resection (wedge or segmental resection, lobectomy, or hepatectomy), and liver transplantation |
| Initial radiation | No, yes, and unknown |
| Year of diagnosis | 4 periods: 1988 to 1997, 1998 to 2002, 2003 to 2005, and 2006 to 2010 |
| SEER registry | 18 SEER registry sites (seer.cancer.gov/registries/list.html) |
Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
TABLE 2.
HCC Staging Definitions
| Stage | Description | Code |
|---|---|---|
|
| ||
| Single lesion in 1 lobe | Single lesion (1 lobe) | EOD extension: 10 CS extension: 100 |
| Multiple tumors or >1 lobe involved | Multiple (satellite) tumors/nodules (1 lobe) without intrahepatic vascular invasion, including NOS; confined to liver, NOS; localized, NOS | EOD extension: 30, or 50 CS extension: 150, 170, 250, 270, and 390 |
| Vascular invasion | Lesion(s) with intrahepatic vascular invasion or extension to extrahepatic blood vessel(s) | EOD extension: 20, 40, 60, 61, 62, 65, 70, 75, and 80 CS extension: 350, 370, 380, 400, 420, 440, 460, 600, 630, 635, 638, 639, 640, 645, 660, 700, 750, 755, 770, 800, and 810 |
| Metastatic disease | Metastasis or distant lymph node involvement | EOD extension: 85 or EOD lymph node involvement: 6 or 7 CS metastases at diagnosis: 10–60 or CS lymph nodes: 200–800 |
Abbreviations: CS, collaborative stage; EOD, extent of disease, HCC, hepatocellular carcinoma; NOS, not otherwise specified.
RESULTS
Patient, Tumor, and Treatment Characteristics
Among the 39,345 subjects in the current study, 76% were men and 24% were women (male:female ratio of 3.1:1) (Table 3). The distribution by race/ethnicity included 50% white, 12% African American, 21% Asian, 16% Hispanic, and 1% Native American. With regard to disease burden, 84% of patients had liver-limited disease, 27% had unifocal disease, 20% had multiple lesions or multilobar involvement by a single lesion, and 36% had tumors with macrovascular invasion. Approximately 16% of patients had metastatic disease (Table 3). The distribution of tumor burden was similar between men and women, except for a slightly higher incidence of single lesions noted among women and of vascular invasion noted among men (both P<.001).
TABLE 3.
Demographic, Clinicopathologic, and Treatment Characteristics by Sex in Patients With HCC: SEER Data 1988 Through 2010
| Characteristics | Total (N=39,345) | Men (N=29,788; 76%) | Women (N=9557; 24%) | P a |
|---|---|---|---|---|
|
| ||||
| Median age (range), y | 62 (18–89) | 61 (18–89) | 67 (18–89) | <.001 |
| Age group, y | ||||
| 18–44 | 1888 (5%) | 1455 (5%) | 433 (5%) | |
| 45–54 | 8168 (21%) | 6848 (23%) | 1320 (14%) | |
| 55–64 | 11,941 (30%) | 9656 (32%) | 2285 (24%) | <.001 |
| 65–74 | 9902 (25%) | 7064 (24%) | 2838 (30%) | |
| ≥75 | 7446 (19%) | 4765 (16%) | 2681 (28%) | |
| Race | ||||
| White | 19,586 (50%) | 15,067 (51%) | 4519 (47%) | |
| African American | 4826 (12%) | 3696 (12%) | 1130 (12%) | |
| Asian | 8293 (21%) | 6015 (20%) | 2278 (24%) | <.001 |
| Hispanic | 6236 (16%) | 4719 (16%) | 1517 (16%) | |
| Native American | 404 (1%) | 291 (1%) | 113 (1%) | |
| Stage | ||||
| Liver-limited disease | ||||
| Single lesion in one lobe | 10,780 (27%) | 7762 (26%) | 3018 (32%) | |
| Tumor size, cm | ||||
| <3 | 3151 (29%) | 2249 (29%) | 902 (30%) | .78b |
| 3–5 | 3021 (28%) | 2178 (28%) | 843 (28%) | |
| ≥5 | 3682 (34%) | 2660 (34%) | 1022 (34%) | |
| Unknown | 926 (9%) | 675 (9%) | 251 (8%) | |
| Multiple tumors/multilobar disease | 8037 (20%) | 6104 (20%) | 1933 (20%) | <.001c |
| Vascular invasion | 14,297 (36%) | 11,007 (37%) | 3290 (34%) | |
| Metastatic disease | 6231 (16%) | 4915 (16%) | 1316 (14%) | |
| Treatmentd | ||||
| None or unknown | 24,225 (70%) | 18,633 (71%) | 5592 (68%) | |
| Liver-directed therapy | 3921 (11%) | 2969 (11%) | 952 (12%) | |
| Surgical resection | 3960 (11%) | 2782 (11%) | 1178 (14%) | <.001 |
| Liver transplantation | 2568 (7%) | 2029 (8%) | 539 (7%) | |
Abbreviations: HCC, hepatocellular carcinoma; SEER, Surveillance, Epidemiology, and End Results.
Based on Wilcoxon or chi-square test whenever appropriate.
For comparison of tumor size between men and women among subjects with a single lesion in 1 lobe.
For comparison of liver-limited disease (including a single lesion in 1 lobe, multiple tumors/multilobar disease, and vascular invasion) and metastatic disease between men and women.
Among patients diagnosed in 1998 or later (n=34,674).
The median age at the time of diagnosis was 67 years in women and 61 years in men (P<.001) (Table 3). Among individuals between the ages of 18 to 54 years, the incidence of HCC was higher in men compared with women (28% vs 19%; P<.001). The age at diagnosis was significantly different by race (Figs. 2A and 2B) among both women and men (both P<.001). African American patients (median age of 58 years for men and 62 years for women) and Hispanic patients (median age of 59 years for men and 66 years for women) were younger at the time of diagnosis compared with white patients (median age of 62 years for men and 69 years for women) and Asian patients (median age of 62 years for men and 68 years for women) (Figs. 2A and 2B).
Figure 2.
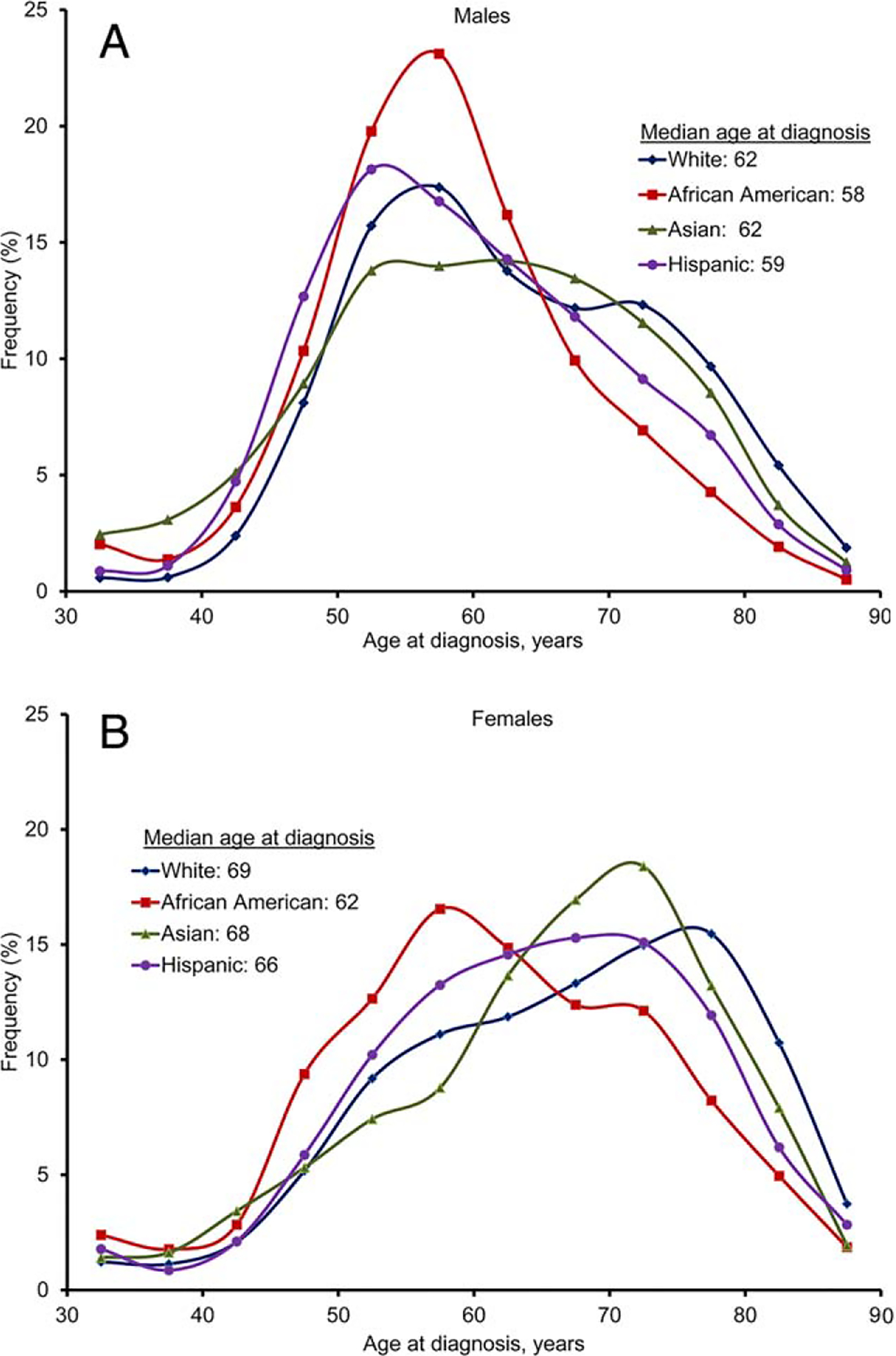
Distribution of age at diagnosis is shown by race in (A) men and (B) women.
Detailed information regarding initial treatment was available for patients diagnsed after 1998 (34,674 patients). Of these patients, 11% received liver-directed therapy including photodynamic therapy, cryosurgery, percutaneous ethanol injection, or heat-radiofrequency ablation. Approximately 11% of patients underwent surgical resection (including wedge or segmental resection, lobectomy, or hepatectomy) and 7% underwent liver transplantation (Tables 1 and 3). The distribution of treatment options was found to be similar between men and women except for surgical resection, which was more frequent among women (44% of women vs 36% of men; P<.001) (Table 3).
OS by Sex, Race, Disease Burden, and Treatment
Survival differences by sex
On multivariate analysis, women were found to have a significantly greater median OS compared with men, independent of age, race, disease stage, or treatment (11 months vs 10 months; hazard ratio [HR], 0.93 [95% CI, 0.91–0.96]; P<.001) (Table 4). The impact of sex on OS was further investigated by age group and captured in adjusted survival curves (Table 4) (Fig. 3). Women aged 18 to 64 years had significantly longer OS than men of the same age group (Pinteraction<.001) (Table 4). The HR for the OS of women versus men was 0.83 (95% CI, 0.77–0.88) for patients aged <55 years. The largest difference in OS was noted among the cohort of patients aged 18 to 44 years, in which women had a 4-month survival benefit (median OS, 14 months) relative to age-matched men (median OS, 10 months; HR, 0.75 [95% CI, 0.65–0.86]; P<.001) (Table 4). In contrast, there was no survival difference noted by sex among patients aged ≥65 years.
TABLE 4.
Association Between Sex and OS on Multivariate Analysis
| Adjusted Median OS (95% CI), Months | HR (95% CI)a | P a | ||
|---|---|---|---|---|
|
| ||||
| Subjects | Male | Female | ||
| All | 10 (10–10) | 11 (11–12) | 0.93 (0.91–0.96) | <.001 |
| Age group, y | ||||
| 18–44 | 10 (9–11) | 14 (12–16) | 0.75 (0.65–0.86) | <.001 |
| 45–54 | 11 (11–12) | 13 (12–15) | 0.86 (0.79–0.92) | <.001 |
| 55–64 | 12 (11–12) | 14 (13–15) | 0.86 (0.81–0.91) | <.001 |
| 65–74 | 10 (10–11) | 11 (10–12) | 0.97 (0.92–1.02) | .19 |
| ≥75 | 8 (7–8) | 7 (7–8) | 1.04 (0.99–1.10) | .15 |
| P interaction | <.001 | |||
| Race | ||||
| White | 10 (10–10) | 11 (10–11) | 0.93 (0.89–0.96) | <.001 |
| African American | 8 (7–8) | 9 (9–10) | 0.85 (0.78–0.92) | <.001 |
| Asian | 12 (12–13) | 13 (12–14) | 1.00 (0.94–1.06) | .87 |
| Hispanic | 10 (10–11) | 11 (10–12) | 0.99 (0.92–1.07) | .79 |
| P interaction | .017 | |||
| Stage | ||||
| Single lesion | 26 (25–27) | 29 (27–31) | 0.95 (0.90–1.01) | .086 |
| Multiple tumors | 13 (13–14) | 14 (13–15) | 0.96 (0.90–1.03) | .24 |
| Vascular invasion | 8 (8–9) | 9 (9–10) | 0.91 (0.87–0.95) | <.001 |
| Metastatic disease | 4 (3–4) | 4 (3–4) | 0.94 (0.88–1.00) | .055 |
| P interaction | .036 | |||
| Treatment | ||||
| None or unknown | 7 (6–7) | 7 (7–7) | 0.96 (0.93–0.99) | .017 |
| Liver-directed therapy | 27 (25–28) | 27 (25–30) | 0.99 (0.89–1.09) | .82 |
| Surgical resection | 44 (40–46) | 48 (44–54) | 0.87 (0.78–0.96) | .008 |
| Liver transplantation | 60b | 60b | 1.06 (0.86– 1.29) | .60 |
| P interaction | .045 | |||
Abbreviations: 95% CI, 95% confidence interval; HR, hazard ratio; OS, overall survival.
Men as the reference group (HR, 1).
Estimates were not reached.
Figure 3.
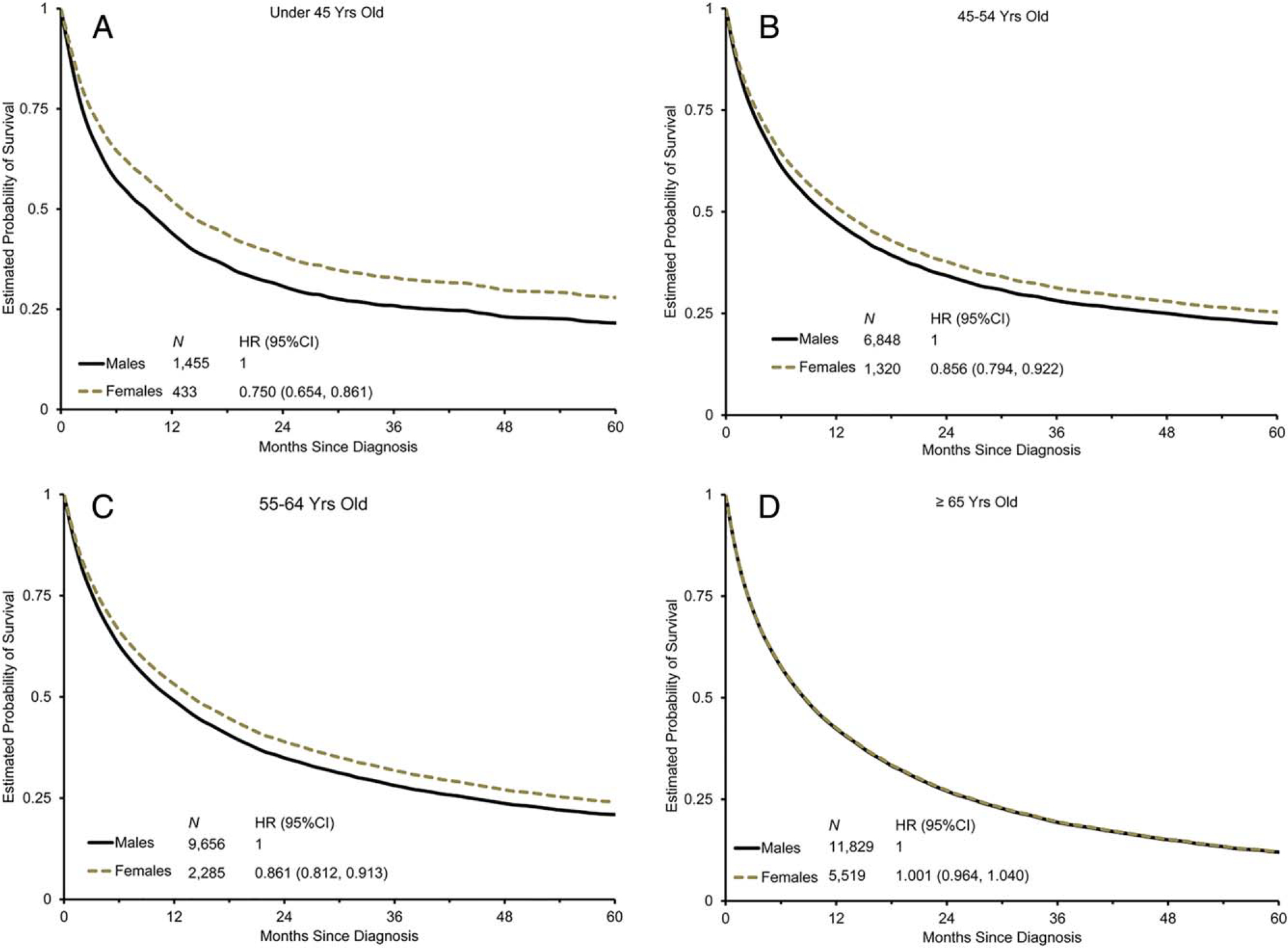
Overall survival is shown by sex and age. Survival probability curves are shown in patients aged (A) 18 to 44 years, (B) 45 to 54 years, (C) 55 to 64 years, and (D) ≥65 years. Men were used as the reference group for hazard ratios (HRs), which were adjusted for race, stage of disease, marital status, birthplace, tumor size, tumor grade, initial surgical procedure, initial radiation, and year of diagnosis and stratified by the Surveillance, Epidemiology, and End Results (SEER) registry. 95% CI indicates 95% confidence interval.
Survival differences by sex and race
Among all patients, the survival difference between sexes persisted in white patients (median OS of 10 months for men vs 11 months for women; P<.001) and African American patients (median OS of 8 months for men vs 9 months for women; P<.001) but not Asian (median OS of 12 months for men vs 13 months for women; P=.87) or Hispanic (median OS of 10 months for men vs 11 months for women; P=.79) patients (Table 4).
Women aged 18 to 54 years had significantly longer OS in every racial/ethnic group except for Hispanics (Table 5). Among patients aged ≥55 years, only African American women were found to have significantly longer OS compared with their male counterparts (HR, 0.86 [95% CI, 0.78–0.94]; P=.001). There were no significant differences noted with respect to sex and OS among Hispanic patients across all age groups.
TABLE 5.
Association Between Sex and OS Across Age Groups by Race, Stage of Disease, and Treatment on Multivariate Analysis
| Subjects | No. Men/Women | Women Aged 18 to 54 Years |
No. Men/Women | Women Aged ≥55 Years |
||
|---|---|---|---|---|---|---|
| HR (95% CI)a | P a | HR (95% CI)a | P a | |||
|
| ||||||
| All | 8303/1753 | 0.83 (0.77–0.88) | <.001 | 21,485/7804 | 0.96 (0.93–1.00) | .024 |
| Race | ||||||
| White | 3574/742 | 0.75 (0.68–0.83) | <.001 | 11,493/3777 | 0.97 (0.92–1.01) | .13 |
| African American | 1213/294 | 0.82 (0.71–0.96) | .013 | 2483/836 | 0.86 (0.78–0.94) | .001 |
| Asian | 1816/408 | 0.85 (0.74–0.97) | .015 | 4199/1870 | 1.04 (0.97–1.12) | .22 |
| Hispanic | 1608/288 | 1.12 (0.96–1.32) | .16 | 3111/1229 | 0.96 (0.89–1.05) | .38 |
| Stage of disease | ||||||
| Single lesion | 2065/519 | 0.89 (0.77–1.03) | .11 | 5697/2499 | 0.97 (0.91–1.03) | .29 |
| Multiple tumors | 1625/314 | 0.90 (0.76–1.06) | .19 | 4479/1619 | 0.98 (0.91–1.05) | .52 |
| Vascular invasion | 3163/663 | 0.80 (0.72–0.88) | <.001 | 7844/2627 | 0.95 (0.90–1.00) | .044 |
| Metastatic disease | 1450/257 | 0.78 (0.68–0.90) | <.001 | 3465/1059 | 0.99 (0.92–1.07) | .78 |
| Treatment | ||||||
| None or unknown | 5095/904 | 0.87 (0.80–0.94) | <.001 | 13,538/4688 | 0.98 (0.94–1.02) | .35 |
| Liver-directed therapy | 777/148 | 0.87 (0.68–1.11) | .26 | 2192/804 | 1.00 (0.89–1.12) | .96 |
| Surgical resection | 710/274 | 0.68 (0.54–0.86) | .001 | 2072/904 | 0.93 (0.83–1.05) | .26 |
| Liver transplantation | 900/188 | 1.06 (0.75–1.51) | .74 | 1129/351 | 1.00 (0.78–1.29) | .99 |
Abbreviations: 95% CI, 95% confidence interval; HR, hazard ratio; OS, overall survival.
Men as the reference group (HR, 1), adjusted for age, race, stage of disease, marital status, birthplace, tumor size, tumor grade, initial surgical procedure, initial radiation, and year of diagnosis and stratified by Surveillance, Epidemiology, and End Results (SEER) registry data among all patients.
Survival differences by sex and disease burden and treatment
The only disease burden category in which women were found to have a significantly better survival than men when including all age groups was vascular invasion (median OS of 9 months vs 8 months; P<.001) (Table 4). However, when stratified by age, women aged 18 to 54 years were found to have superior survival to men in the cohorts with vascular invasion (HR, 0.80; 95% CI, 0.72–0.88 [P<.001]) and metastatic disease (HR, 0.78; 95% CI, 0.68–0.90 [P<.001]) (Table 5).
With regard to treatment, women who underwent surgical resection were found to have a significantly improved OS compared with men (median OS of 48 months vs 44 months; HR, 0.87 [95% CI 0.78–0.96]; P=.01) (Table 4). This difference retained statistical significance at the time of stratification by age among patients with resected tumors, with women aged 18 to 54 years living longer than age-matched men (HR, 0.68; 95% CI, 0.54–0.86 [P=.001]) (Table 5). There was no difference in survival noted between sexes among those who received liver-directed therapy or transplantation (Table 4).
DISCUSSION
The higher risk of HCC in men compared with women is well established. However, to the best of our knowledge, there is limited information regarding the impact of sex on survival in patients with HCC. Using the SEER database, we evaluated the effect of sex on OS among patients with HCC, while accounting for other demographic variables and prognostic factors. Our analysis revealed an association between female sex and improved survival in patients with HCC. Although the improved survival observed in women compared with men was modest when including all age groups, the sex effect was more pronounced in younger cohorts. In the cohort of patients aged 18 to 44 years, the OS difference between men and women was >25%; this difference decreased gradually in the cohorts aged 45 to 54 years and 55 to 64 years and disappeared in patients aged ≥65 years. Although women were more likely to undergo surgical resection compared with men, women still retained a significant survival advantage compared with age-matched men in the subgroup of surgically resected patients, especially among those aged 18 to 54 years (HR, 0.68). The association between sex and survival in the absence of major differences in disease burden or broad treatment categories suggests an underlying physiologic explanation.
To our knowledge to date, the evidence regarding the influence of hormones and reproductive factors on liver cancer has been mixed. For example, although smaller clinical studies have postulated a causative role for oral contraceptives (OCPs) in the development of HCC,24–27 other larger-scale and international investigations have failed to detect any significant relationship.28,29 In one retrospective analysis evaluating >3000 patients, OCP use was actually found to be associated with improved survival in women with HCC,30 and a recent meta-analysis has deemed the association between OCPs and HCC inconclusive.31 Likewise, the roles of parity,32–35 age at menarche and menopause,35–37 and age at first birth32,35 remain unclear, with studies both supporting34,35,37 and refuting32,33,36 a correlation between estrogen exposure and the development of liver cancer.
The findings of the current study suggest that estrogen may protect against hepatocarcinogenesis and promote a more favorable biology once HCC develops. Specifically, the observed superior survival in women versus men among patients aged 18 to 44 years and 45 to 54 years suggests that menopausal status may correlate with outcomes in patients with HCC, and is concordant with other investigations revealing an inverse relationship between age at menopause and HCC risk.36 Menopause, defined as 12 months of amenorrhea in women aged ≥45 years, occurs at an average age of 51.3 years, with only 10% of women becoming menopausal between ages 40 and 45 years or after age 55 years.38 The survival advantage in women aged 55 to 64 years may reflect the lasting effect of estrogen on the biology of HCC, a generally indolent tumor with a prolonged period of carcinogenesis. Furthermore, hormone replacement therapy has been correlated with a lower risk of developing HCC,36,39,40 and may contribute to the sex effect on survival in this age group. The lack of information regarding menopausal status or use of hormone therapy in the SEER database limits our ability to reach definitive conclusions in this regard.
Estrogen exerts its effects through 3 estrogen receptors (ERs): ER-alpha (ERα), ER-beta (ERβ), and G protein-coupled ER.41 Genome-wide expression and microRNA analyses in patients with HCC have shown ERα to be a tumor suppressor protein whose expression is inversely correlated with the presence of HBV infection, tumor size, and disease stage.42 Conversely, derangements in ER structure have been linked to the development of cirrhosis11 and HCC12 in Chinese carriers of HBV, although it should be noted that studies examining such genetic single nucleotide polymorphisms have yielded inconsistent results. Nonetheless, others have demonstrated that variant ER expression predominates in male patients with HCC and predicts worse survival, especially in those infected with HBV.43,44 At the mechanistic level, preclinical models reveal that loss of ERα accelerates the development of diethylnitrosamine-induced HCC by promoting hepatocyte necrosis over apoptosis; the compensatory hepatocyte proliferation is concomitant with increased NF-κB-dependent transcriptional control of cytokine expression in Kupffer cells.45 Another potential explanation is that estrogens repress HCC growth by inhibiting tumor-associated macrophages and preventing ERβ from interacting with ATP5J (also known as ATPase-coupling factor 6), a part of ATPase, thus inhibiting the JAK1-STAT6 pathway.45 Although a detailed review of the biologic impact of estrogen on HCC is beyond the scope of this article, investigations to validate these mechanisms may offer critical information regarding novel therapeutic opportunities in this disease.
Another hypothesis for the association between female sex and improved prognosis in HCC may reside in the tumor-promoting impact of androgens. Androgens have demonstrated a synergistic oncogenic effect with HBV in men,46 but not women,47 with HCC. The androgen receptor (AR), located on the X chromosome, is found in higher concentrations in malignant compared with surrounding tissue, and peritumoral48 and intratumoral49 AR expression has been inversely correlated with recurrence rates after surgical resection of localized disease in patients with HCC.48 Increased AR levels also have been linked to higher metastatic potential in HCC cell lines.50 Moreover, patients with AR-negative HCC are more likely to be women and have significantly better 5-year OS.51 A possible mechanism through which androgen effects may be exerted is the Wnt signaling cascade,52 as evidenced by gene expression analyses53 and mouse models.54 Further investigations are warranted to elucidate the exact pathways, especially as Wnt inhibitors enter into clinical practice.
In addition to the impact of sex on survival, we explored potential interactions between sex and ethnicity. In the current analysis, women aged <55 years had superior survival to men across all ethnicities except Hispanics. This could reflect disparities in health care access and treatment patterns. One SEER investigation, for example, demonstrated that Hispanic individuals were 24% to 27% less likely than white individuals to undergo surgery for localized HCC.55 When taking into account both local therapies and transplantation, another study found that Hispanic patients had the lowest administration of any invasive intervention.56 Another possible explanation may be related to the difference in HCC etiology among Hispanic individuals. Hispanic patients tend to have less hepatitis-associated HCC and more hepatomas related to alcoholic cirrhosis and NAFLD,57 which may be influenced to a different extent and manner by androgens and estrogens.
The current study has its limitations, the first of which is its retrospective nature. Moreover, the lack of information in the SEER database regarding HCC predisposing factors (eg, HBV, hepatitis C virus, NAFLD, etc), other comorbidities, socioeconomic status, and treatment rendered (other than crude initial surgical procedure and radiation) limits the strength of our conclusions because any of these factors could differ by sex. Importantly, we cannot determine the effect of menopausal status or exogenous estrogen supplementation on outcomes because this information was not available.
The results of the current study demonstrate that sex influences survival among patients with HCC. This is in agreement with preclinical data confirming androgens and estrogens as modulators of hepatic fibrosis, cirrhosis, and progression to HCC. It is also consistent with epidemiologic studies demonstrating superior outcomes in younger women with other gastrointestinal cancers. The findings of the current study should be validated in prospective studies that allow for better control of confounding factors and may present unique opportunities for biomarker development and novel therapeutics.
Supplementary Material
FUNDING SUPPORT
The project described was supported by award number P30CA014089 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Dr. El-Khoueiry has received personal fees from Bayer, Roche Genentech, Sanofi, GlaxoSmithKline, Exelixis, and Medimmune and received a grant from Astex Pharmaceuticals for work performed outside of the current study.
Additional Supporting Information may be found in the online version of this article.
Presented at the 49th Annual Meeting of the American Society of Clinical Oncology; May 31-June 4, 2013; Chicago, IL.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- 4.Ananthakrishnan A, Gogineni V, Saeian K. Epidemiology of primary and secondary liver cancers. Semin Intervent Radiol. 2006;23:47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagasue N, Kohno H, Chang YC, et al. Androgen and estrogen receptors in hepatocellular carcinoma and the surrounding liver in women. Cancer. 1989;63:112–116. [DOI] [PubMed] [Google Scholar]
- 6.Weisburger JH, Yamamoto RS, Korzis J, Weisburger EK. Liver cancer: neonatal estrogen enhances induction by a carcinogen. Science. 1966;154:673–674. [DOI] [PubMed] [Google Scholar]
- 7.Deng G, Zhou G, Zhai Y, et al. Association of estrogen receptor alpha polymorphisms with susceptibility to chronic hepatitis B virus infection. Hepatology. 2004;40:318–326. [DOI] [PubMed] [Google Scholar]
- 8.Murakami Y, Fukasawa M, Kaneko Y, Suzuki T, Wakita T, Fukazawa H. Selective estrogen receptor modulators inhibit hepatitis C virus infection at multiple steps of the virus life cycle. Microbes Infect. 2013;15:45–55. [DOI] [PubMed] [Google Scholar]
- 9.Di Martino V, Lebray P, Myers RP, et al. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology. 2004;40:1426–1433. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Liu Y, Wang L, et al. Differential effects of estrogen/androgen on the prevention of nonalcoholic fatty liver disease in the male rat. J Lipid Res. 2013;54:345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan Z, Tan W, Xu B, et al. A cis-acting regulatory variation of the estrogen receptor α(ESR1) gene is associated with hepatitis B virus-related liver cirrhosis. Hum Mutat. 2011;32:1128–1136. [DOI] [PubMed] [Google Scholar]
- 12.Zhai Y, Zhou G, Deng G, et al. Estrogen receptor alpha polymorphisms associated with susceptibility to hepatocellular carcinoma in hepatitis B virus carriers. Gastroenterology. 2006;130:2001–2009. [DOI] [PubMed] [Google Scholar]
- 13.Ma WL, Hsu CL, Yeh CC, et al. Hepatic androgen receptor suppresses hepatocellular carcinoma metastasis through modulation of cell migration and anoikis. Hepatology. 2012;56:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu CM, Yeh SH, Chen PJ, et al. Hepatitis B virus X protein enhances androgen receptor-responsive gene expression depending on androgen level. Proc Natl Acad Sci U S A. 2007;104:2571–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou J, Xu J, Jiang R, et al. Estrogen-sensitive PTPRO expression represses hepatocellular carcinoma progression by control of STAT3. Hepatology. 2013;57:678–688. [DOI] [PubMed] [Google Scholar]
- 16.Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. [DOI] [PubMed] [Google Scholar]
- 17.Wang YC, Xu GL, Jia WD, et al. Estrogen suppresses metastasis in rat hepatocellular carcinoma through decreasing interleukin-6 and hepatocyte growth factor expression. Inflammation. 2012;35:143–149. [DOI] [PubMed] [Google Scholar]
- 18.Xu H, Wei Y, Zhang Y, et al. Oestrogen attenuates tumour progression in hepatocellular carcinoma. J Pathol. 2012;228:216–229. [DOI] [PubMed] [Google Scholar]
- 19.Bohanes P, Yang D, Chhibar RS, et al. Influence of sex on the survival of patients with esophageal cancer. J Clin Oncol. 2012;30:2265–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang D, Hendifar A, Lenz C, et al. Survival of metastatic gastric cancer: significance of age, sex and race/ethnicity. J Gastrointest Oncol. 2011;2:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendifar A, Yang D, Lenz F, et al. Gender disparities in metastatic colorectal cancer survival. Clin Cancer Res. 2009;15:6391–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Artinyan A, Mailey B, Sanchez-Luege N, et al. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010;116:1367–1377. [DOI] [PubMed] [Google Scholar]
- 23.Poon RT, Ng IO, Fan ST, et al. Clinicopathologic features of long-term survivors and disease-free survivors after resection of hepatocellular carcinoma: a study of a prospective cohort. J Clin Oncol. 2001; 19:3037–3044. [DOI] [PubMed] [Google Scholar]
- 24.Fiel MI, Min A, Gerber MA, Faire B, Schwartz M, Thung SN. Hepatocellular carcinoma in long-term oral contraceptive use. Liver. 1996;16:372–376. [DOI] [PubMed] [Google Scholar]
- 25.De Benedetti VM, Welsh JA, Yu MC, Bennett WP. p53 mutations in hepatocellular carcinoma related to oral contraceptive use. Carcinogenesis. 1996;17:145–149. [DOI] [PubMed] [Google Scholar]
- 26.Hsing AW, Hoover RN, McLaughlin JK, et al. Oral contraceptives and primary liver cancer among young women. Cancer Causes Control. 1992;3:43–48. [DOI] [PubMed] [Google Scholar]
- 27.Tao LC. Oral contraceptive-associated liver cell adenoma and hepatocellular carcinoma. Cytomorphology and mechanism of malignant transformation. Cancer. 1991;68:341–347. [DOI] [PubMed] [Google Scholar]
- 28.Waetjen LE, Grimes DA. Oral contraceptives and primary liver cancer: temporal trends in three countries. Obstet Gynecol. 1996;88:945–949. [DOI] [PubMed] [Google Scholar]
- 29.Mant JW, Vessey MP. Trends in mortality from primary liver cancer in England and Wales 1975–92: influence of oral contraceptives. Br J Cancer. 1995;72:800–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam CM, Yong JL, Chan AO, et al. Better survival in female patients with hepatocellular carcinoma: oral contraceptive pills related? J Clin Gastroenterol. 2005;39:533–539. [DOI] [PubMed] [Google Scholar]
- 31.Maheshwari S, Sarraj A, Kramer J, El-Serag HB. Oral contraception and the risk of hepatocellular carcinoma. J Hepatol. 2007;47:506–513. [DOI] [PubMed] [Google Scholar]
- 32.Wu CH, Chan TF, Changchien CC, Yang CY. Parity, age at first birth, and risk of death from liver cancer: evidence from a cohort in Taiwan. J Gastroenterol Hepatol. 2011;26:334–339. [DOI] [PubMed] [Google Scholar]
- 33.Lambe M, Trichopoulos D, Hsieh CC, Ekbom A, Pavia M. Parity and hepatocellular carcinoma. A population-based study in Sweden. Int J Cancer. 1993;55:745–747. [DOI] [PubMed] [Google Scholar]
- 34.Hsing AW, McLaughlin JK, Hoover RN, Co Chien HT, Blot WJ, Fraumeni JF Jr. Parity and primary liver cancer among young women. J Natl Cancer Inst. 1992;84:1118–1119. [DOI] [PubMed] [Google Scholar]
- 35.La Vecchia C, Negri E, Franceschi S, D’Avanzo B. Reproductive factors and the risk of hepatocellular carcinoma in women. Int J Cancer. 1992;52:351–354. [DOI] [PubMed] [Google Scholar]
- 36.Yu MW, Chang HC, Chang SC, et al. Role of reproductive factors in hepatocellular carcinoma: impact on hepatitis B- and C-related risk. Hepatology. 2003;38:1393–1400. [DOI] [PubMed] [Google Scholar]
- 37.Mucci LA, Kuper HE, Tamimi R, Lagiou P, Spanos E, Trichopoulos D. Age at menarche and age at menopause in relation to hepatocellular carcinoma in women. BJOG. 2001;108:291–294. [DOI] [PubMed] [Google Scholar]
- 38.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 2008;61:4–16. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez E, Gallus S, Bosetti C, Franceschi S, Negri E, La Vecchia C. Hormone replacement therapy and cancer risk: a systematic analysis from a network of case-control studies. Int J Cancer. 2003;105:408–412. [DOI] [PubMed] [Google Scholar]
- 40.Tavani A, Negri E, Parazzini F, Franceschi S, La Vecchia C. Female hormone utilisation and risk of hepatocellular carcinoma. Br J Cancer. 1993;67:635–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang B, Wu ZY. Estrogen derivatives: novel therapeutic agents for liver cirrhosis and portal hypertension. Eur J Gastroenterol Hepatol. 2013;25:263–270. [DOI] [PubMed] [Google Scholar]
- 42.Hishida M, Nomoto S, Inokawa Y, et al. Estrogen receptor 1 gene as a tumor suppressor gene in hepatocellular carcinoma detected by triple-combination array analysis. Int J Oncol. 2013;43:88–94. [DOI] [PubMed] [Google Scholar]
- 43.Villa E, Grottola A, Colantoni A, et al. Hepatocellular carcinoma: role of estrogen receptors in the liver. Ann NY Acad Sci. 2002;963:37–45. [PubMed] [Google Scholar]
- 44.Villa E, Moles A, Ferretti I, et al. Natural history of inoperable hepatocellular carcinoma: estrogen receptors’ status in the tumor is the strongest prognostic factor for survival. Hepatology. 2000;32:233–238. [DOI] [PubMed] [Google Scholar]
- 45.Hong EJ, Levasseur MP, Dufour CR, Perry MC, Giguere V. Loss of estrogen-related receptor αpromotes hepatocarcinogenesis development via metabolic and inflammatory disturbances. Proc Natl Acad Sci U S A. 2013;110:17975–17980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu MW, Cheng SW, Lin MW, et al. Androgen-receptor gene CAG repeats, plasma testosterone levels, and risk of hepatitis B-related hepatocellular carcinoma. J Natl Cancer Inst. 2000;92:2023–2028. [DOI] [PubMed] [Google Scholar]
- 47.Yu MW, Yang YC, Yang SY, et al. Androgen receptor exon 1 CAG repeat length and risk of hepatocellular carcinoma in women. Hepatology. 2002;36:156–163. [DOI] [PubMed] [Google Scholar]
- 48.Boix L, Castells A, Bruix J, et al. Androgen receptors in hepatocellular carcinoma and surrounding liver: relationship with tumor size and recurrence rate after surgical resection. J Hepatol. 1995;22:616–622. [DOI] [PubMed] [Google Scholar]
- 49.Nagasue N, Yu L, Yukaya H, Kohno H, Nakamura T. Androgen and oestrogen receptors in hepatocellular carcinoma and surrounding liver parenchyma: impact on intrahepatic recurrence after hepatic resection. Br J Surg. 1995;82:542–547. [DOI] [PubMed] [Google Scholar]
- 50.Ao J, Meng J, Zhu L, et al. Activation of androgen receptor induces ID1 and promotes hepatocellular carcinoma cell migration and invasion. Mol Oncol. 2012;6:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagasue N, Chang YC, Hayashi T, et al. Androgen receptor in hepatocellular carcinoma as a prognostic factor after hepatic resection. Ann Surg. 1989;209:424–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lustig B, Jerchow B, Sachs M, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng H, Cheng AS, Tsang DP, et al. Cell cycle-related kinase is a direct androgen receptor-regulated gene that drives β-catenin/T cell factor-dependent hepatocarcinogenesis. J Clin Invest. 2011;121:3159–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liao YJ, Liu SP, Lee CM, et al. Characterization of a glycine N-methyltransferase gene knockout mouse model for hepatocellular carcinoma: implications of the gender disparity in liver cancer susceptibility. Int J Cancer. 2009;124:816–826. [DOI] [PubMed] [Google Scholar]
- 55.Sonnenday CJ, Dimick JB, Schulick RD, Choti MA. Racial and geographic disparities in the utilization of surgical therapy for hepatocellular carcinoma. J Gastrointest Surg. 2007;11:1636–1646; discussion 1646. [DOI] [PubMed] [Google Scholar]
- 56.Wong RJ, Corley DA. Survival differences by race/ethnicity and treatment for localized hepatocellular carcinoma within the United States. Dig Dis Sci. 2009;54:2031–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fong Y, Kemeny N, Lawrence TS. In: Devita VT, Hellman S, Rosenberg SA, editor. Cancer: Principles and Practice of Oncology. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2001. Cancer of the liver and biliary tree. pp. 1162–1189. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


