Abstract
Background
a suspected urinary tract infection (UTI) is the most common reason to prescribe antibiotics in a frail older patient. Frequently, antibiotics are prescribed unnecessarily. To increase appropriate antibiotic use for UTIs through antibiotic stewardship interventions, we need to thoroughly understand the factors that contribute to these prescribing decisions.
Objectives
(1) to obtain insight into factors contributing to antibiotic prescribing for suspected UTIs in frail older adults. (2) To develop an overarching model integrating these factors to guide the development of antibiotic stewardship interventions for UTIs in frail older adults.
Methods
we conducted an exploratory qualitative study with 61 semi-structured interviews in older adult care settings in Poland, the Netherlands, Norway and Sweden. We interviewed physicians, nursing staff, patients and informal caregivers.
Results
participants described a chain of decisions by patients, caregivers and/or nursing staff preceding the ultimate decision to prescribe antibiotics by the physician. We identified five themes of influence: (1) the clinical situation and its complexity within the frail older patient, (2) diagnostic factors, such as asymptomatic bacteriuria, (3) knowledge (gaps) and attitude, (4) communication: interprofessional, and with patients and relatives and (5) context and organisation of care, including factors such as availability of antibiotics (over the counter), antibiotic stewardship efforts and factors concerning out-of-hours care.
Conclusions
decision-making on suspected UTIs in frail older adults is a complex, multifactorial process. Due to the diverse international setting and stakeholder variety, we were able to provide a comprehensive overview of factors to guide the development of antibiotic stewardship interventions.
Keywords: urinary tract infections, older adults, antibiotic stewardship, decision-making, qualitative research, older people
Key Points
Antibiotics for suspected urinary tract infections (UTIs) are the result of multiple decisions by the physician, nursing staff, patient and/or caregiver.
Knowledge of differentiating between UTIs and asymptomatic bacteriuria needs to be strengthened.
Good communication is essential for appropriate prescribing but often impeded by high workload and lack of continuity of care.
Patients report to trust health care professionals in decision-making on UTIs; however, patients are not always involved.
We recommend antibiotic stewardship interventions to be multidisciplinary, multifaceted and tailored to the specific setting.
Introduction
Frail older adults are frequently prescribed antibiotics, in majority for suspected urinary tract infections (UTIs) [1]. Many of these prescriptions are given inappropriately [1]. Improving appropriate antibiotic use is key to slowing the development of antibiotic resistance, which is a major threat to global health [2, 3]. To design effective antibiotic stewardship interventions (ASIs), a thorough understanding of the antibiotic prescribing decision for suspected UTIs is needed.
Traditionally, a wide range of non-specific symptoms, such as cloudy urine or a change in mental status, have been linked to UTIs in frail older adults [4, 5]. Recently, criteria for starting empiric antibiotic treatment have changed [6]. New guidelines recommend restricting antibiotic use for UTIs to patients with specific symptoms localised to the urinary tract or systemic symptoms [7]; guideline adherence thus requires a major behavioural change from health care professionals (HCPs). ASIs are needed to actively implement these guidelines, and they need to be tailored to the factors specifically relevant in suspected UTIs in older adults.
Extensive research in primary care and hospital-settings has established that antibiotic prescribing is determined by many factors: at the level of HCPs, patients and organisation of care [8–12]. In recent years, studies on antibiotic prescribing decisions for older adults confirmed this complexity [13–16]. A higher risk of complications, advance care plans and the importance of nursing staff are examples of factors relevant in the older patient population [13–18]. In case of suspected UTIs, the presence of non-specific symptoms and use of urine tests are known to be important drivers of inappropriate antibiotic prescriptions [1, 17–22]. However, extreme variability in antibiotic consumption in long-term care facilities is reported between and within countries [23]. Factors underlying these differences may be due to local practices and organisation of care and undoubtedly affect antibiotic prescribing decisions [23]. This heterogeneity in the older adult care setting thus calls for a deeper understanding of the prescribing decision.
We therefore formed a European research consortium in Poland, the Netherlands, Norway and Sweden to improve antibiotic prescribing for UTIs in frail older adults (ImpresU) [24]. In these countries, organisation of care, guidelines and progress of antibiotic stewardship differ [25–29]. We set out to perform an in-depth qualitative exploration with HCPs, patients and informal caregivers (ICGs) in a variety of older adult care settings. Our objectives were (1) to identify relevant factors that contribute to antibiotic prescribing for UTIs in frail older adults, and (2) to integrate these into an overarching model to guide the development of effective ASIs in clinical practice.
Methods
Design and setting
We conducted a qualitative study using semi-structured interviews in Poland (PL), the Netherlands (NL), Norway (NO) and Sweden (SE) in the care setting for frail older adults with varying care dependency levels in GP practices, home care, residential care homes and nursing homes. This study informed the development and tailoring of an ASI that was implemented in a subsequent cluster randomised controlled trial. The protocol describing the design of this qualitative study and subsequent trial has been published previously [24]. The consolidated criteria for reporting qualitative research (COREQ) checklist is provided in Appendix 1 [30].
Study participants and recruitment
We performed interviews with three stakeholder groups: (1) physicians, (2) nursing staff and (3) patients and ICGs. Participants had to be able and willing to provide written informed consent and communicate personal thoughts in the local language. Patients had to be 70 years or older and were not recruited during acute illness. Recruitment took place through the networks of the research teams. HCPs were approached via e-mail or telephone, and patients and ICGs through nursing staff. We used purposive sampling to ensure variation within each stakeholder group (e.g. in gender, years of experience for HCPs). The sampling process was discussed during regular international conference calls.
Data collection and management
Topic lists (Appendix 2) and interview guides were designed based on literature [15] and the researchers’ (clinical) experience. In interviews with physicians and nursing staff, topics included challenges and considerations in clinical practice regarding decision-making to prescribe antibiotics or not, their own role, and roles of others. In interviews with patients and ICGs, the focus was on their views and expectations concerning UTIs and possible treatments. The topic lists were translated and back-translated in each country for verification.
Interviews were conducted by EH, SHO, PS, IS, ESA, AK and AM between January and November 2019 in the native language, audio-recorded and transcribed verbatim. Basic demographic participant data and transcripts were pseudonymised. Because Dutch researchers performed the coding of transcripts, the Polish, Norwegian and Swedish interviews were translated into English by the local researchers and verified by a professional language editor. Three Dutch interviews (one per stakeholder) were translated into English to facilitate information exchange within the research team.
Data analysis
Data were analysed using the framework method [31]. EH and AM independently coded twelve interviews and formed a codebook with preliminary themes through consensus. Next, EH, AM and WG applied this codebook on the remaining interviews using ATLAS.ti software (V9.0.22.0) and discussed the generation of new codes or modifications. The codebook was organised using the conceptual model previously described by Van Buul et al. [15]. Regular conference calls were held within the international research team to discuss the findings and resolve issues on translations or interpretation in context. Ultimately, we reorganised the data in framework matrices and subsequently built a model of factors [31].
Ethics
Ethical approval was given by the Committee of Bioethics of the Medical University of Lodz, Poland (RNN/381/18/KE), the Regional Committee for Medical and Health Research Ethics in Norway (2018/2191/REK sør-øst A), the Swedish Ethical Review Authority (2019-00504). Approval was not required in the Netherlands as established by the Medical Ethics Review Committee of VU University Medical Centre (2018.500).
Results
In total, 61 interviews (15 PL, 15 NO, 15 SE, 16 NL) were conducted with 20 physicians, 21 nurses and nurse assistants, 16 patients and 4 ICGs (Table 1). Amongst all participants, 46 were female and 15 were male; the majority of nursing staff and patients were female. The median age was 47 for HCPs (range 27–69), and 86 (range 70–97) for patients. Work experience of HCPs widely varied (<5 to ≥21 years). Settings included GP practices, nursing homes, residential care homes and home care. We experienced some challenges in recruiting patients; we understood from nursing staff that some were hesitant to talk with an academic researcher or believed they did not have enough knowledge on the topic.
Table 1.
Participant characteristics
| Stakeholders (total n = 61) | Countrya | Sex | Age (years) | Experience | Details |
|---|---|---|---|---|---|
| Physicians (n = 20) | 5 PL, 5 NL, 5 NO, 5 SE | 10 female, 10 male | 27–69 (median 45) | ≤5 years (n = 5) 6–20 years (n = 9) ≥21 years (n = 6) |
15 general practitioners, 5 nursing home doctorsb |
| Nursing staff (n = 21) | 5 PL, 6 NL, 5 NO, 5 SE | 19 female, 2 male | 27–61 (median 48) | ≤5 years (n = 2) 6–20 years (n = 9) ≥21 years (n = 10) |
15 nurses, 6 nurse assistants |
| Patients (n = 16) and informal caregivers (ICGs) (n = 4) | Patients: 4 PL, 3 NL, 4 NO, 5 SE ICGs: 1 PL, 2 NL, 1 NO |
Patients: 14 female, 2 male ICGs: 3 female, 1 male |
Patients: 70–97 (median 86) ICGs: 51–74 (median 62) |
N/A | Patients: 9 in a nursing home, 1 in a residential care home, 5 in home care, 1 in rotational nursing / home care. ICGs: 2 daughters, 1 brother, 1 cousin |
aPL = Poland, NL = the Netherlands, NO = Norway, SE = Sweden.
bIn Norway, medical care in nursing homes is provided by nursing home doctors with various medical backgrounds, often in general practice.
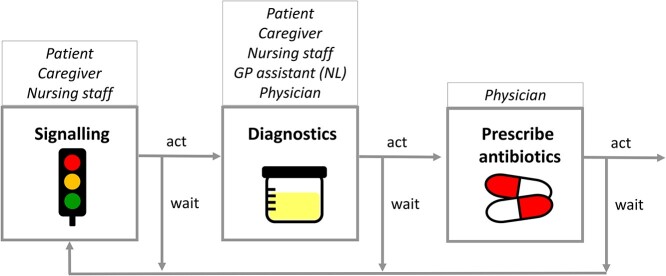
The interviewees elaborately described the roles of the different stakeholders in the process, that appeared to consist of a chain of multiple decisions and actions preceding the final prescribing decision. Next to the physician, the patient, ICG, nursing staff and the assistant of the general practitioner (GP) may be involved in these decisions to act, i.e. to signal or perform diagnostics, or to wait and monitor. An antibiotic prescription thus requires multiple people to take action (‘Well, I notify the staff. It would be the assistant nurse who in turn informs the nurse. And I usually get fast help with this. Sending in a culture, they then contact the doctor, and then I get Kåvepenin [phenoxymethylpenicillin], which I believe is usual. And then both the doctor and I are satisfied.’ SE_P_01). This process is illustrated in Figure 1 and Appendix 3.
Figure 1.
Chain of decision-making by multiple stakeholders.
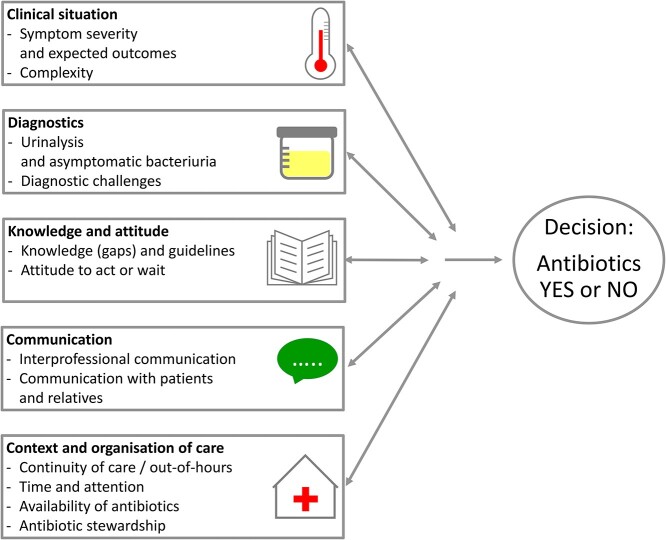
We identified many factors reported to influence the antibiotic prescribing decision and organised these in five themes, visualised in Figure 2: the clinical situation of the patient, diagnostics, knowledge and attitudes, communication, and context and organisation of care. However, we wish to note that reality is complex. While we address these themes separately, the multidirectional arrows in our model indicate that factors vary in their impact and may interact differently across situations and settings. Moreover, the order in which we present the themes does not reflect their relative importance nor their chronologic order in the decision-making. Representative quotes are numbered and presented in Appendix 4 (q1–157).
Figure 2.
Overview of themes and factors that interact in the decision to prescribe antibiotics for UTIs in the frail older adult.
Clinical situation
Symptom severity and expected outcomes for the patient
The clinical situation of the patient was identified as a major theme. Patients, ICGs and HCPs highlighted the burden or severity of UTIs (‘it’s so important because I see how distressed Dad is when he has one’, NO_ICG_01). The wish to relieve symptoms or pain was stated as an important reason to prescribe, whereas mild symptoms may allow a wait-and-see approach (q1, q2). Furthermore, anticipated outcomes for the patient were frequently reported to affect decision-making. HCPs, patients and ICGs described the risk of complications, such as urosepsis, delirium or falling (q3–5). HCPs reported that several patient characteristics (medical history, frailty, comorbidity, male sex) impact their decision through influencing the perceived risk of complications (q6–9). At the same time, several GPs pointed out that a lower UTI is usually self-limiting and that they saw overtreatment as a risk (q10, q137). Furthermore, expected positive or negative effects of antibiotics on quality of life can play a role in the HCP’s decisions (q11). End-of-life care and advance care plans were frequently stated as reasons to refrain from antibiotics; although some mentioned that antibiotics were still prescribed for suspected UTIs in the last phase of life (q12, q13).
Complexity in the clinical situation
HCPs shared many difficulties in the recognition of UTIs in frail older adults. They describe that many older patients, especially when suffering from dementia, cannot clearly express the symptoms; a UTI—or urosepsis—may present with non-typical symptoms, or symptoms may be difficult to recognise due to incontinence or other comorbidity (q14–19). When discussing symptoms, most patients and caregivers mentioned dysuria and frequent urination, but non-specific symptoms were also described (‘Yes. The stench, the smell, to say it in a more proper way’, NL_P_01) (q20–23). Physicians varied in their decision whether non-specific symptoms justify an antibiotic prescription or not (q24–26). The situation may be further complicated in case of allergies, drug interactions, side effects, or patient difficulties with swallowing tablets. These may influence the prescribing decision or the choice of antibiotics (q27–29). Furthermore, HCPs stated that the risk of antibiotic resistance influenced the prescribing decision. This risk was described at the population-level, leading to a general awareness to be cautious with antibiotics, and at the patient-level, leading to more strictness in prescribing in patients carrying multi-resistant bacteria (q30–33). Patients and ICGs also emphasised the risk of resistance as a reason to be careful with antibiotic use (q34, q59).
Diagnostics
Urine tests and asymptomatic bacteriuria
Next to the clinical situation, HCPs described to rely on the results of urine tests for UTI diagnosis to determine the need for antibiotics: positive results confirm a UTI and negative results rule it out. Much variation in practices was described. Some reported to decide based on urine dipstick results, or to wait for urine culture results, depending on the clinical situation. Dutch GPs reported using a dipslide test next to dipsticks (q35–39). In Poland, urine dipsticks were often reported to be unavailable; urine is tested by the laboratory. Nursing staff often initiate urine testing, but patients or ICGs may also do this themselves (Appendix 3). Furthermore, some HCPs described using urine tests to evaluate whether the UTI was “flushed away” through increased fluid intake, or whether antibiotic treatment was effective (q40, q41). While urine tests were described to influence the decision, several HCPs brought up their limitations. In all countries, asymptomatic bacteriuria was mentioned by HCPs (not by patients or ICGs) as a reason to refrain from prescribing antibiotics (‘I don’t want to remove bacteriuria because I know it will come back in a week’, SE_GP_01). Accordingly, many HCPs reported that urine test results are less important for the decision than the patient’s symptoms (q42–46).
Diagnostic challenges
HCPs described several issues leading to diagnostic uncertainty influencing the prescribing decision (q47, q48). Both physicians and nurses described situations where urine testing was initiated by others for unknown reasons, leaving them with the dilemma on how to proceed (q49). Furthermore, diagnostics or test results are not always (immediately) available, for example, due to costs or out-of-hours logistics (q50). Additionally, challenges and burdens were described in obtaining a urine sample from a frail older—often incontinent—patient, adding uncertainty to the quality of the sample (q51). Some HCPs mentioned that testing for C-reactive protein is helpful in the prescribing decision as an indicator of systemic infection (q43).
Knowledge and attitude
Knowledge (gaps) and guidelines
Physicians and nurses frequently pointed out that colleagues lacked knowledge, with inappropriate antibiotic treatment as a possible consequence. We indeed observed knowledge gaps across interviews (q53–57). For example, HCPs, patients and ICGs linked non-specific symptoms to UTIs and misinterpreted a positive urine stick/culture as confirmation of UTI, while this may indicate asymptomatic bacteriuria. Notably, not all Polish patients and nursing staff appeared to be aware that furazidine, available over the counter, is an antibiotic (‘first such mild remedies, as I said, Furagina type drugs, […]and then, indeed, if these symptoms do not subside, then you have to step in with an antibiotic. But this is the last resort’, PL_N_04). Generally, patients and ICGs described the value of antibiotics but were also aware of their negative consequences. Furthermore, they expressed the burden of side effects (q58–61). However, several patients reported not to know how a UTI can be treated, what antibiotics are, or what antibiotic resistance is (q62–64).
Physicians reported using guidelines in their prescribing decision, with variation in how much they relied on them. Some reported finding them clear, supporting (uniform) decision-making, or useful for GP assistants. On the other hand, some GPs in the Netherlands and Poland described deviating from guidelines as they felt they were not tailored to the frail older adult (q65–69).
Attitude to act or to wait
HCPs described different attitudes (in oneself, in colleagues and in patients) to influence decision-making on antibiotic prescribing. On one hand, an (over)alertness on UTIs was described; a UTI is often the first thing on the mind to confirm or rule out with a urine dipstick (‘like a reflex by our assistant’, NL_GP_04). The urge for quick action in case of a UTI suspicion was indeed expressed across interviews with HCPs, patients and ICGs (q70–73). HCPs also expressed the wish to act just for the sake of doing something (q74, q75). On the other hand, HCPs and patients described taking a monitoring approach with observation and increased fluid intake, often combined with alternative remedies such as cranberry products. Furthermore, HCPs stressed the importance of prevention, looking at the overall picture, other potential causes of the symptoms, and underlying causes of recurrent UTIs (q76–83).
HCPs connected this attitude to act or wait to the responsibility and accountability for their decision (in the chain in Figure 1). To act may entail that the responsibility moves further in the chain (‘the nursing assistants take the test […] and then they come with the note “Yes, and now it’s your responsibility” ’, NO_N_01). This process may often lead to an antibiotic prescription (q84, q85). Fear of mistakes was mentioned as a reason to act and prescribe antibiotics. Furthermore, clinical experience was described to influence the HCP’s attitude and thus the prescribing decision (q86–88).
Communication
Interprofessional communication
The physician’s prescribing decision was reported to be strongly dependent on information from others, especially nursing staff (q89, q90). Physicians described many challenges in acquiring all necessary information for a good decision. Reported factors include previously described knowledge gaps, many intermediaries (nurse, nursing assistant or GP assistant), different communication channels (e.g. phone, fax, message books, electronic referrals), language barriers, and problems with medical records (q91–95). Physicians also reported pressure from the nursing staff to prescribe antibiotics (q96). A reason for nursing staff to exert pressure may be in attempt to represent the patient (q97). Good teamwork, trust in colleagues and good communication between HCPs were described to affect the prescribing decision, and to be essential for a watchful waiting approach (q98–100).
Communication with patients and ICGs
Diverse scenarios regarding communication between HCPs, patients and ICGs were shared in the interviews. Across all countries, HCPs reported to have felt pressured to perform urine tests or to prescribe antibiotics. Often, HCPs noted to experience pressure mostly from relatives, and to a lesser extent from patients themselves. In many older adults, they observed a compliant attitude (q101–104). Indeed, while some patients voiced the expectation to receive antibiotics in case of a UTI suspicion (‘you should stick to what works’, SE_P_01), many patients and ICGs reported to have trust in the HCPs, also in case of refraining from treatment (q105–107). Beyond this, several patients reported not to participate in the decision-making (‘I don’t discuss drugs because I don’t know. I take what the nurses give me’, PL_P_02). Possibly, the patient may not always be involved due to all intermediaries in the communication, or because a cystitis may be considered a triviality (q108–111). Moreover, it was pointed out that HCPs sometimes forget to inform relatives when their family member has a UTI, which may harm the relation between HCPs and the next of kin (q111, q112). Retaining a good relation was described as a factor in the decision-making (‘in order not to lose good relations with the patient, it happens that I prescribe an antibiotic’, PL_GP_01); however, HCPs also reported that refraining from treatment is usually accepted by patients and relatives, when there is a good relation with the family, and when the decision is well-communicated (q113–115).
Context and organisation of care
Continuity of care and care out-of-hours
Continuity of care, including many factors related to out-of-hours care, was described to have a major impact on antibiotic prescribing decisions for UTIs. Being familiar with the patient and the ability to follow-up on the patient were reported as two vital factors; their absence was described to lead to more antibiotic prescribing. These factors were noted especially relevant in situations out-of-hours and during holidays, with part-time work, staff changes or locum physicians (q66, q116–119). HCPs described finding it difficult to wait and monitor if they lack the option to follow-up themselves. Some indicated not to trust their colleagues to make the right decisions, or to avoid burdening colleagues with more work (q120, q121). Furthermore, the inability to follow-up on patients at home without a good support system was repeatedly described as a reason to prescribe antibiotics (q122, q123). In Norway, several physicians described staying available for their nursing home team in the weekend to prevent the out-of-hours service to take action (q124). Problems with quality or access to documentation, difficult logistics and communication channels impede interprofessional communication and are often worsened out-of-hours. To circumvent these problems, the use of delayed antibiotic prescriptions was described, with specific instructions given to the patient and/or nursing staff (q125–128).
Time and attention
Workload was reported to affect prescribing decisions for UTI. In some cases, the expected antibiotic prescription may be prepared by a GP assistant or nurse using a UTI checklist in the GP practice. The final decision by the GP may be hastily made, e.g. during the coffee break (q92, q129–131). Furthermore, physicians reported not to visit their patients often enough due to workload and organisation of care. Patients and ICGs also voiced this wish to see the physician more often; especially immobile patients for whom visiting the GP practice is difficult (q132–135). Several HCPs described a watchful waiting approach as requiring more effort than an antibiotic prescription. This can be because the monitoring itself requires more work, or because non-prescribing takes more time to explain (q114, q136–139). Physicians expressed difficulties in finding time for such a conversation and may prescribe instead (‘Sometimes I am just not going to explain it all, I think I will lose this battle and I don’t have time for this, and then I give a prescription of which I secretly know it is unnecessary’, NL_GP_02). At the same time, physicians stressed that these treatment decisions for frail older adults deserve time due to the complexity (q140). While most noted that high workload leads to more antibiotic prescribing, a Swedish GP, working in a nursing home with relatively high continuity of care, described to opt for a watchful waiting approach in case of limited time (q141).
Availability of antibiotics
Availability of antibiotics and access to antibiotics were described as factors in antibiotic prescribing decisions. In Poland, furazidine is available over the counter. Patients and nurses reported to initiate the use themselves, leading to no or a delayed decision by the GP (‘I take it myself. If it didn’t help, then I go to a doctor’, PL_P_03) (q142, q143). Also, the type of care contract in Poland (public or private) was described to affect the availability of antibiotics and thus the prescribing decision. In Sweden on the other hand, a GP described being able to delay prescribing as antibiotics are always available at the nursing home (q144, q145). Furthermore, depending on the clinical situation, the use of intravenous antibiotics for upper UTIs was described in Norwegian nursing homes (q146).
Antibiotic stewardship
HCPs described antibiotic stewardship practices to affect antibiotic prescribing decisions, although the term ‘steward-ship’ was not named. Some physicians reported to strive to prescribe as little antibiotics as possible, as a personal goal or as a goal for their practice. Training and interventions were reported to be of influence, and changes in antibiotic prescribing practices in past years were described. In Norway and Sweden, specific examples regarding UTIs in older adults were given (‘At that time […] we ran around with that urine dipstick in our hand and then you got antibiotic treatment without really any questions about it. So I do see a difference, I do’, NO_NS_01) (q147–151). Multidisciplinary education was described to have impact through increased collective understanding and feeling of responsibility within the team (q152). Limiting the ability to initiate urine testing by nursing assistants was mentioned several times as a potentially effective intervention to decrease unnecessary prescriptions. However, challenges were described in changing practice, and not all interventions were described as effective (q153–157). Antibiotic stewardship practices were not discussed in interviews with patients and caregivers.
Discussion
Our qualitative analysis identified factors contributing to antibiotic prescribing decisions in frail older adults with suspected UTIs. We found that the decision-making is a complex multi-step process, influenced by the clinical situation of the patient, diagnostics, knowledge and attitudes, communication, and context and organisation of care. The present study confirms the known complexity of antibiotic prescribing decisions and provides more details specific to UTIs in older adults in different care settings across four European countries, including the perspectives of patients and ICGs.
Our findings confirm those of earlier studies and underscore the need for ASIs to target knowledge gaps, most notably regarding the role of non-specific symptoms and asymptomatic bacteriuria [14, 17, 19–22, 32]. Furthermore, we report an attitude to act: HCPs and patients often prefer to act instead of wait. Together, these factors result in over-alertness in signalling UTIs, in excessive use of urine tests, and subsequent antibiotic overuse. We identified multiple additional factors adding to the complexity of the prescribing decision, many of which have been previously reported [13–19, 21]. By visualising the decision-chain and modelling the influential factors in the prescribing decision, we aim to support HCPs in assessing their local situation and designing tailored interventions to facilitate appropriate antibiotic use for suspected UTIs.
Our interviews emphasise that good communication and continuity of care are essential to make appropriate decisions on suspected UTIs in frail older adults. This is jeopardised by the many intermediaries in the decision-chain; the physician’s prescribing decision is complicated by the dependence on information from nursing staff [15, 17–20]. In our interviews, this problem appeared most profound in the Netherlands where a GP assistant often further extends the decision-chain. This situation has been similarly described in Denmark [32]. A long chain may strengthen the attitude to act: once set in motion, it is difficult to wait and not prescribe antibiotics. Furthermore, good communication and organisation of care are often compromised by high workload and problems with continuity (e.g. staff changes) [9, 12–15, 33]. In our interviews, these challenges were present in all countries, and aggravated in out-of-hours situations. However, sufficient time and good teamwork were described to facilitate a watchful waiting approach. This was mostly reported in Norway, perhaps because physicians are employed by the nursing home itself. Generic interventions on continuity of care may thus prove valuable to improve appropriate antibiotic use.
The participation of four countries in our study led to increased diversity in our results. For example, we identified availability of antibiotics as a relevant factor mostly due to Polish interviews. In Poland, the use of furazidine (a nitrofuran derivative) without a prescription was described; antibiotic use without a prescription is an important target for antibiotic stewardship interventions worldwide [34]. Furthermore, whilst knowledge gaps on UTI-related symptoms and asymptomatic bacteriuria were described in all countries, there was much variation. In Poland and the Netherlands, the topic of asymptomatic bacteriuria was brought up only by a few HCPs, mostly physicians. Conversely, physicians and nursing staff in Norway and Sweden were more aware of these knowledge gaps and described changes in the care in recent years. This is presumably the result of more stringent guidelines and UTI-specific antibiotic stewardship activities by the Swedish Strama programme and the Norwegian RASK intervention [25–29, 35, 36]. These examples highlight the need for research across multiple countries and underscore the importance of tailoring interventions to the local setting.
Our study—as one of few—incorporated the perspectives of patients and ICGs. Our findings demonstrate a need to better involve them, inform them, and communicate with them in the decision-making process on UTIs. Earlier studies suggest that patients and ICGs are predominantly involved by exerting pressure to prescribe on HCPs [10, 15, 17, 19, 21]. We indeed found that HCPs perceive pressure; however, patients and ICGs demanding for antibiotics may represent a ‘loud minority’. Perhaps more importantly, we find that patients and ICGs do not always feel involved in the decision or may not even be informed or involved by HCPs at all. This was also reported by studies interviewing patients in the hospital-setting [20, 37–39]. This may be connected to the knowledge gaps and compliant attitude in many patients that we reported. These may signify a power imbalance between patients and HCPs, which is known to impair shared decision making [39, 40]. To empower patients and ICGs to take part in decision-making, interventions on patient education and communication are thus needed for them as well as for HCPs. Lastly, as we did not interview patients diagnosed with dementia, a further exploration of their views and those of their ICGs may be valuable to better tailor such interventions.
Strengths and limitations
We believe this study fills an important gap in antibiotic stewardship research for older adults. Our international multi-stakeholder approach, including patients and caregivers, increased the diversity and depth of our findings. We observed consistency of themes and factors across countries, indicating wider generalizability of our results. However, while we were able to describe variations in effects of factors between and within countries, our qualitative approach did not allow for detailed country-specific comparisons. Furthermore, although interpretation in context was extensively discussed for each country, the translation might have led to decreased understanding of factors within their local setting. Lastly, our results may not be generalizable to countries with a substantially different organisation of care for older adults.
A multidisciplinary and multifaceted approach
We recommend that ASIs have a multidisciplinary approach targeting all locally involved stakeholders and addressing each step in the decision-chain following suspected UTIs, i.e. signalling, diagnostics, and prescription. Furthermore, when developing ASIs, it is key to realise that factors are interconnected and may vary across settings. The older adult care setting is complex; implementation thus requires careful consideration of the local context [41, 42]. Multifaceted interventions, tailored to the specific setting may thus be more likely to succeed, as demonstrated previously [43, 44]. Within our ImpresU-consortium, we used these interviews to design and tailor an ASI with a multifaceted educational toolbox, using a participatory action research approach for local adaptation [24].
In conclusion, we showed that antibiotic prescribing decisions on UTIs in frail older adults are influenced by many factors and result from a complex process with patients, ICGs, nursing staff and physicians. Our model presents an overview of the factors at play and can be used when designing future ASIs.
Supplementary Material
Acknowledgements
We thank all patients, ICGs, physicians and nursing staff for their participation. Professor Kari Solbrække, University of Oslo, is acknowledged for valuable input in the initial work in Norway. Preliminary findings were presented at the online General Practice Research on Infections Network conference (24 September 2021). The study design and protocol were previously published [24].
Contributor Information
Esther A R Hartman, Department of Medicine for Older People, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, Amsterdam, the Netherlands; Amsterdam Public Health Research Institute, Aging & Later Life, Amsterdam, the Netherlands; Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands.
Wim G Groen, Department of Medicine for Older People, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, Amsterdam, the Netherlands; Amsterdam Public Health Research Institute, Aging & Later Life, Amsterdam, the Netherlands.
Silje Rebekka Heltveit-Olsen, The Antibiotic Centre for Primary Care, Department of General Practice, Institute of Health and Society, University of Oslo, Oslo, Norway.
Morten Lindbæk, The Antibiotic Centre for Primary Care, Department of General Practice, Institute of Health and Society, University of Oslo, Oslo, Norway.
Sigurd Høye, The Antibiotic Centre for Primary Care, Department of General Practice, Institute of Health and Society, University of Oslo, Oslo, Norway.
Pär-Daniel Sundvall, General Practice/Family Medicine, School of Public Health and Community Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Research, Education, Development & Innovation, Primary Health Care, Region Västra Götaland, Sweden.
Ingmarie Skoglund, General Practice/Family Medicine, School of Public Health and Community Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Research, Education, Development & Innovation, Primary Health Care, Region Västra Götaland, Sweden.
Egill Snaebjörnsson Arnljots, General Practice/Family Medicine, School of Public Health and Community Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Research, Education, Development & Innovation, Primary Health Care, Region Västra Götaland, Sweden.
Ronny Gunnarsson, General Practice/Family Medicine, School of Public Health and Community Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Research, Education, Development & Innovation, Primary Health Care, Region Västra Götaland, Sweden; Primary Health Care Clinic for Homeless People, Närhälsan, Region Västra Götaland, Sweden.
Anna Kowalczyk, Centre for Family and Community Medicine, the Faculty of Health Sciences, The Medical University of Lodz, Lodz, Poland.
Maciek Godycki-Cwirko, Centre for Family and Community Medicine, the Faculty of Health Sciences, The Medical University of Lodz, Lodz, Poland.
Katarzyna Kosiek, Family Doctors‘ Clinic, Lodz, Poland.
Tamara N Platteel, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands.
Alma C van de Pol, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands.
Theo J M Verheij, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands.
Annelie A Monnier, Department of Medicine for Older People, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, Amsterdam, the Netherlands; Amsterdam Public Health Research Institute, Aging & Later Life, Amsterdam, the Netherlands.
Cees M P M Hertogh, Department of Medicine for Older People, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, Amsterdam, the Netherlands; Amsterdam Public Health Research Institute, Aging & Later Life, Amsterdam, the Netherlands.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This work was supported by the Joint Programming Initiative on Antimicrobial Resistance (grant number JPIAMR_2017_P007), through national funding agencies: National Science Centre Poland (grant number UMO-2017/25/Z/NZ7/03024), ZonMw the Netherlands (grant number 549,003,002), the Research Council of Norway (grant number 284,253/H10) and the Swedish Research Council (grant number 2017–05975). The Healthcare Board, Region Västra Götaland (grant number VGFOUREG-855761) partially funded the Swedish part of the study.
References
- 1. van Buul LW, Veenhuizen RB, Achterberg WP et al. Antibiotic prescribing in Dutch nursing homes: how appropriate is it? J Am Med Dir Assoc 2015; 16: 229–37. [DOI] [PubMed] [Google Scholar]
- 2. Huttner A, Harbarth S, Carlet J et al. Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob Resist Infect Control 2013; 2: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holmes AH, Moore LS, Sundsfjord A et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016; 387: 176–87. [DOI] [PubMed] [Google Scholar]
- 4. Juthani-Mehta M, Quagliarello V, Perrelli E, Towle V, van Ness PH, Tinetti M. Clinical features to identify urinary tract infection in nursing home residents: a cohort study. J Am Geriatr Soc 2009; 57: 963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mayne S, Sundvall PD, Gunnarsson R. Confusion strongly associated with antibiotic prescribing due to suspected urinary tract infections in nursing homes. J Am Geriatr Soc 2018; 66: 274–81. [DOI] [PubMed] [Google Scholar]
- 6. van Buul LW, Vreeken HL, Bradley SF et al. The development of a decision tool for the empiric treatment of suspected urinary tract infection in frail older adults: a Delphi consensus procedure. J Am Med Dir Assoc 2018; 19: 757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nicolle LE, Gupta K, Bradley SF et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin Infect Dis 2019; 68: 1611–5. [DOI] [PubMed] [Google Scholar]
- 8. Charani E, Edwards R, Sevdalis N et al. Behavior change strategies to influence antimicrobial prescribing in acute care: a systematic review. Clin Infect Dis 2011; 53: 651–62. [DOI] [PubMed] [Google Scholar]
- 9. Colliers A, Coenen S, Remmen R, Philips H, Anthierens S. How do general practitioners and pharmacists experience antibiotic use in out-of-hours primary care? An exploratory qualitative interview study to inform a participatory action research project. BMJ Open 2018; 8: e023154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hulscher ME, van der Meer JW, Grol RP. Antibiotic use: how to improve it? Int J Med Microbiol 2010; 300: 351–6. [DOI] [PubMed] [Google Scholar]
- 11. Warreman EB, Lambregts MMC, Wouters RHP et al. Determinants of in-hospital antibiotic prescription behaviour: a systematic review and formation of a comprehensive framework. Clin Microbiol Infect 2019; 25: 538–45. [DOI] [PubMed] [Google Scholar]
- 12. Williams SJ, Halls AV, Tonkin-Crine S et al. General practitioner and nurse prescriber experiences of prescribing antibiotics for respiratory tract infections in UK primary care out-of-hours services (the UNITE study). J Antimicrob Chemother 2018; 73: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fleming A, Bradley C, Cullinan S, Byrne S. Antibiotic prescribing in long-term care facilities: a qualitative, multidisciplinary investigation. BMJ Open 2014; 4: e006442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pulia MS, Keller SC, Crnich CJ, Jump RLP, Yoshikawa TT. Antibiotic stewardship for older adults in ambulatory care settings: addressing an unmet challenge. J Am Geriatr Soc 2020; 68: 244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Buul LW, van der Steen JT, Doncker SM et al. Factors influencing antibiotic prescribing in long-term care facilities: a qualitative in-depth study. BMC Geriatr 2014; 14: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayward GN, Moore A, McKelvie S et al. Antibiotic prescribing for the older adult: beliefs and practices in primary care. J Antimicrob Chemother 2019; 74: 791–7. [DOI] [PubMed] [Google Scholar]
- 17. Walker S, McGeer A, Simor AE, Armstrong-Evans M, Loeb M. Why are antibiotics prescribed for asymptomatic bacteriuria in institutionalized elderly people? A qualitative study of physicians' and nurses' perceptions. CMAJ 2000; 163: 273–7. [PMC free article] [PubMed] [Google Scholar]
- 18. Schweizer AK, Hughes CM, Macauley DC, O’Neill C. Managing urinary tract infections in nursing homes: a qualitative assessment. Pharm World Sci 2005; 27: 159–65. [DOI] [PubMed] [Google Scholar]
- 19. Chambers A, MacFarlane S, Zvonar R et al. A recipe for antimicrobial stewardship success: using intervention mapping to develop a program to reduce antibiotic overuse in long-term care. Infect Control Hosp Epidemiol 2019; 40: 24–31. [DOI] [PubMed] [Google Scholar]
- 20. Saukko PM, Oppenheim BA, Cooper M, Rousham EK. Gaps in communication between different staff groups and older adult patients foster unnecessary antibiotic prescribing for urinary tract infections in hospitals: a qualitative translation approach. Antimicrob Resist Infect Control 2019; 8: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Kelly K, Phelps K, Regen EL et al. Why are we misdiagnosing urinary tract infection in older patients? A qualitative inquiry and roadmap for staff behaviour change in the emergency department. Eur Geriatr Med 2019; 10: 585–93. [DOI] [PubMed] [Google Scholar]
- 22. Jones LF, Cooper E, Joseph A et al. Development of an information leaflet and diagnostic flow chart to improve the management of urinary tract infections in older adults: a qualitative study using the theoretical domains framework. BJGP Open 2020; 4: bjgpopen20X101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Falcone M, Paul M, Yahav D et al. Antimicrobial consumption and impact of antimicrobial stewardship programmes in long-term care facilities. Clin Microbiol Infect 2019; 25: 562–9. [DOI] [PubMed] [Google Scholar]
- 24. Hartman EAR, Groen WG, Heltveit-Olsen SR et al. Multifaceted antibiotic stewardship intervention using a participatory-action-research approach to improve antibiotic prescribing for urinary tract infections in frail elderly (ImpresU): study protocol for a European qualitative study followed by a pragmatic cluster randomised controlled trial. BMJ Open 2021; 11: e052552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Läkemedelsbehandling av urinvägsinfektioner i öppenvård - behandlingsrekommendation. Swedish guideline. Läkemedelsverket, 2017; 21–36. [Google Scholar]
- 26. Richtlijn Urineweginfecties bij kwetsbare ouderen. Dutch guideline. Verenso, 2018. [Google Scholar]
- 27. Akselsen PEO, S . Urinveisinfeksjoner i sykehjem, versjon 2.2. Norwegian guideline Antibiotikasenteret, primærmedisin f, 2020.
- 28. Holecki M, Hryniewicz W, Imiela J, Klinger M, Pawlik K, Wanke-Ryt M. Rekomendacje diagnostyki, terapii i profilaktyki zakażeń układu moczowego u dorosłych. Narodowy Program Ochrony Antybiotyków na lata 2011–2015: Narodowy Instytut Leków, Warszawa, 2015. [Google Scholar]
- 29. Van Buul LW, Monnier AA, Sundvall PD et al. Antibiotic stewardship in european nursing homes: experiences from the Netherlands, Norway, Poland, and Sweden. J Am Med Dir Assoc 2020; 21: 34–40.e1. [DOI] [PubMed] [Google Scholar]
- 30. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. International J Qual Health Care 2007; 19: 349–57. [DOI] [PubMed] [Google Scholar]
- 31. Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013; 13: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arnold SH, Olesen JA, Jensen JN, Bjerrum L, Holm A, Kousgaard MB. Development of a tailored, complex intervention for clinical reflection and communication about suspected urinary tract infections in nursing home residents. Antibiotics (Basel) 2020; 9: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sundvall PD, Skoglund I, Hess-Wargbaner M, Åhrén C. Rational antibiotic prescribing in primary care: qualitative study of opportunities and obstacles. BJGP Open 2020; 4: bjgpopen20X101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Machowska A, Lundborg CS. Drivers of irrational use of antibiotics in Europe. Int J Environ Res Public Health 2018; 16: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. The Swedish strategic programme against antibiotic resistance . https://strama.se/contact/?lang=en (March 2022). [DOI] [PMC free article] [PubMed]
- 36. NORM/NORM-VET . Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway, 2020. ISSN:1502-2307 (print) / 1890-9965 (electronic); 42–3.
- 37. Rawson TM, Moore LS, Hernandez B et al. Patient engagement with infection management in secondary care: a qualitative investigation of current experiences. BMJ Open 2016; 6: e011040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saukko PM, Rousham EK. Diagnosis between chaos and control: affect and hospital clinicians' and older adult patients' narratives of urinary tract infections. Front Sociol 2020; 5: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zanichelli V, Monnier AA, Tebano G et al. Views and experiences with regard to antibiotic use of hospitalized patients in five European countries: a qualitative descriptive study. Clin Microbiol Infect 2019; 25: 249.e7–12. [DOI] [PubMed] [Google Scholar]
- 40. Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns 2014; 94: 291–309. [DOI] [PubMed] [Google Scholar]
- 41. Peryer G, Kelly S, Blake J et al. Contextual factors influencing complex intervention research processes in care homes: a systematic review and framework synthesis. Age Ageing 2022; 51: 1–16. 10.1093/ageing/afac014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chambers A, Chen C, Brown KA et al. Virtual learning collaboratives to improve urine culturing and antibiotic prescribing in long-term care: controlled before-and-after study. BMJ Qual Saf 2022; 31: 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arnold SH, Nygaard Jensen J, Bjerrum L et al. Effectiveness of a tailored intervention to reduce antibiotics for urinary tract infections in nursing home residents: a cluster, randomised controlled trial. Lancet Infect Dis 2021; 21: 1549–56. [DOI] [PubMed] [Google Scholar]
- 44. Pasay DK, Guirguis MS, Shkrobot RC et al. Antimicrobial stewardship in rural nursing homes: impact of interprofessional education and clinical decision tool implementation on urinary tract infection treatment in a cluster randomized trial. Infect Control Hosp Epidemiol 2019; 40: 432–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.