Abstract
Lysergic acid diethylamide (LSD) is a prototypical serotonergic psychedelic drug and the subject of many clinical investigations. In recent years, a range of lysergamides has emerged with the production of some being inspired by the existing scientific literature. Others, for example various 1-acyl substituted lysergamides, did not exist before their appearance as research chemicals. 1-Cylopropanoyl-LSD (1CP-LSD) has recently emerged as a new addition to the group of lysergamide-based designer drugs and is believed to be psychoactive in humans. In this investigation, 1CP-LSD was subjected to detailed analytical characterizations including various mass spectrometry (MS) platforms, gas and liquid chromatography, nuclear magnetic resonance spectroscopy, solid phase and GC condensed phase infrared spectroscopy. Analysis by GC–MS also revealed the detection of artificially induced degradation products. Incubation of 1CP-LSD with human serum led to the formation of LSD, indicating that it may act as a prodrug for LSD in vivo, similar to other 1-acyl substituted lysergamides. The analysis of blotters and pellets is also included. 1CP-LSD also induces the head-twitch response (HTR) in C57BL/6 J mice, indicating that it produces an LSD-like behavioural profile. 1CP-LSD induced the HTR with an ED50 = 430.0 nmol/kg which was comparable to 1P-LSD (ED50 = 349.6 nmol/kg) investigated previously. Clinical studies are required to determine the potency and profile of the effects produced by 1CP-LSD in humans.
Keywords: 5-HT2A receptor, LSD, lysergamides, new psychoactive substances, psychedelics
1 |. INTRODUCTION
Lysergic acid diethylamide (LSD, Figure 1) is a prototypical serotonergic hallucinogen drug capable of inducing powerful psychedelic effects in humans.1–3 A variety of alterations of the lysergamide scaffold has been explored, such as modification of the amide and N6-alkyl groups4–7 or the indole N1-nitrogen atom.8–11 Perhaps one of the better known examples is 1-acetyl-LSD (1A-LSD, ALD-52, Figure 1), an acylated LSD derivative with psychoactive effects thought to be equipotent to LSD.12–16 ALD-52 was originally synthesized at Sandoz in the 1950s but has reemerged in Europe as a new psychoactive substance (NPS).17 In recent years, a number of other 1-acylated lysergamides appeared on the NPS market (1P-LSD, 1P-ETH-LAD, and 1B-LSD, Figure 1) with seemingly no history in the scientific literature.18–21
FIGURE 1.
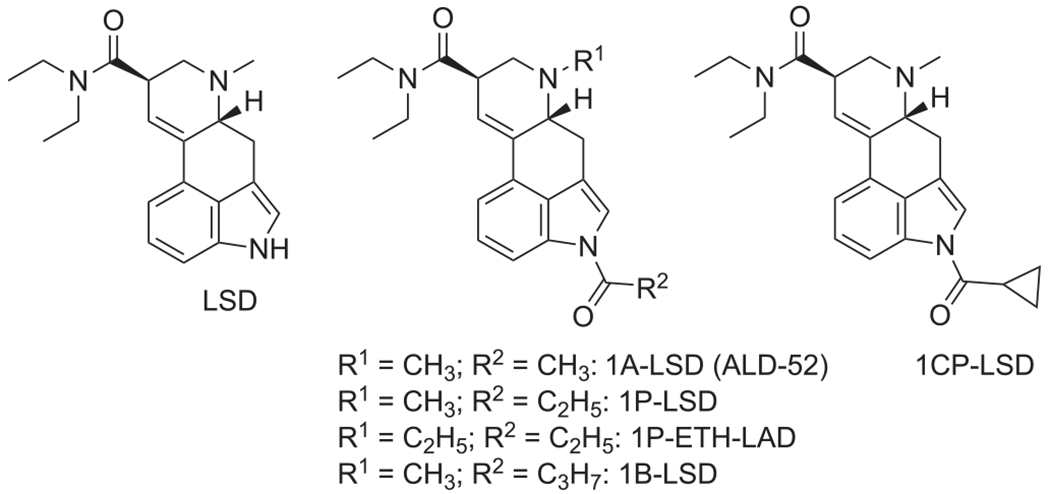
Chemical structures of lysergic acid diethylamide (LSD) and various 1-acyl analogs that emerged as research chemicals
It was shown recently that 1-acylated lysergamides likely serve as prodrugs to either LSD (1P-LSD, 1B-LSD, ALD-52)18,19,22–24 or N6-ethyl-nor-LSD (ETH-LAD) (from 1P-ETH-LAD),19,22 suggesting that the detection of the parent 1-acylated lysergamide in vivo following ingestion might be challenging.
Clinical research with newer lysergamides has yet to be carried out in detail but serotonergic hallucinogens such as LSD induce the head-twitch response (HTR) in mice and rats, which reflects 5-HT2A receptor activation (the primary target of hallucinogens in the CNS).25,26 In addition to having the ability to differentiate between hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists,27,28 the HTR assay also allows in vivo potencies to be compared in a manner that has cross-species translational relevance to humans.24,29 ALD-52, 1P-LSD, and 1B-LSD have been shown to elicit the HTR and it appears that this is the result of LSD formation in vivo.18,24 Compared with LSD, ALD-52, 1P-LSD, and 1B-LSD bind to the 5-HT2A receptor with lower affinity and act as very weak 5-HT2A partial agonists in a Ca2+flux assay.24
In this investigation, the analytical and behavioral characterization of 1-cyclopropanoyl-LSD (1CP-LSD, 1-(cyclopropanecarbonyl)-N,N-diethyl-6-methyl-9,10-didehydroergoline-8β-carboxamide, Figure 1) is described. 1CP-LSD has recently emerged as a new addition to the growing number of lysergamide-based designer drugs. It appears to be predominantly available in dosage forms such as blotters and pellets and purportedly produces psychedelic effects in humans. However, detailed analytical information appears to be unavailable and thus the analytical characterization included various mass spectrometry (MS) platforms, gas- and liquid chromatography (GC and LC), nuclear magnetic resonance spectroscopy (NMR), solid phase and GC-condensed-phase infrared spectroscopy (GC-sIR). To add a multidisciplinary dimension to this investigation, HTR experiments were included to determine whether 1CP-LSD produces LSD-like behavioural effects in C57BL/6 J mice. An initial exploration of the hydrolysis of 1CP-LSD to LSD in human serum has also been included, as well as an analysis of 1CP-LSD in blotter and pellet extracts.
2 |. EXPERIMENTAL
2.1 |. Materials
All chemicals used were of analytical and HPLC grade and obtained from Aldrich (Dorset, UK). DMSO-d6 (99.9% D) was from Sigma-Aldrich (Dorset, UK). 1-Cylopropanoyl-LSD hemitartrate (2:1) powder and free base (1CP-LSD) were supplied by Synex Synthetics BV, Maastricht, the Netherlands.
2.2 |. Instrumentation
2.2.1 |. Gas chromatography mass spectrometry
In this GC–MS method 1, samples were analyzed on an Agilent 6890 N gas chromatograph coupled to a 5975 inert MSD. A Restek Rxi-5Sil MS column (30 m × 0.25 mm × 0.25 μm; Thames Restek, High Wycombe, UK) was used in split mode (1:1) with helium carrier gas at a constant flow of 0.8 mL/min. The injection port and transfer line temperatures were set at 280°C and 290°C, respectively. The initial oven temperature was 200°C, held for 2 min, ramped at 25°C/min to 295°C, and held at 295°C for 45 min (run time 50.8 min). The ionization energy was set at 70 eV, the quadrupole at 150°C, the ion source at 230°C, and the mass range was set at m/z 40–800. The sample injection volume was 2 μL and a 200 μg/mL solution in acetonitrile (for the base) or acetonitrile/methanol (8:2 for the tartrate) was analyzed. Details for GC–MS methods 2 and 3 can be found in the Supporting Information.
2.2.2 |. Liquid chromatography electrospray ionization mass spectrometry
HPLC single quadrupole mass spectrometry (LC-Q-MS) analyses were carried out on an Agilent 1100 system using a Restek (Bellefonte, PA, USA) Allure PFPP column (5 μm, 50 mm × 2.1 mm). The aqueous mobile phase A consisted of 0.1% formic acid, whereas mobile phase B consisted of 0.1% formic acid in acetonitrile. The total run time was 25 min. The following gradient elution program was used: 0–2 min 2% B, followed by an increase to 60% within 15 min, then up to 80% within 20 min, and returning to 2% within 25 min. The Agilent LC–MSD settings were as follows: positive electrospray mode, capillary voltage 3500 V, drying gas (N2) 12 L/min at 350°C, nebulizer gas (N2) pressure 50 psi, scan mode m/z 70–500, fragmentor voltage value used for in-source collision-induced dissociation (CID) was 150 V. The mass spectrometer was tuned according to the manufacturer’s instructions using ESI Tuning Mix G2421A (Agilent Technologies). The sample was dissolved in acetonitrile/water (1:1, containing 0.1% formic acid) at a concentration of 10 μg/mL. The injection volume was 10 μL, the flow rate was 0.80 mL/min, and the column temperature was 30°C.
2.2.3 |. High mass accuracy electrospray ionization mass spectrometry
Ultra high performance liquid chromatography single and tandem mass spectrometry (UHPLC-QTOF-MS/MS) data were obtained from an Agilent 6530B Accurate Mass QTOF LC–MS system coupled to an Agilent 1260 Infinity II UHPLC system (Agilent, Cheshire, UK). Mobile phases consisted of methanol (0.1% formic acid) and 0.1% formic acid in water. The samples were dissolved in methanol at a concentration of 1 μg/mL and a 5 μL injection volume was injected onto a stainless steel back pressure sample loop. The flow rate was isocratic, set to 50% methanol at 0.4 mL/min, the column controller was held at 25°C, and data were acquired for 3 min. The QTOF-MS data were acquired in positive mode scanning from m/z 100–1000 for MS and m/z 50–500 for MS/MS analysis. Ionization was achieved with an Agilent JetStream electrospray source and infused internal reference masses. QTOF parameters: gas temperature 325°C, drying gas 10 L/min, nebulizer gas 45 L/min, sheath gas temperature 400°C, sheath gas flow 11 L/min. Scan source parameters: VCap 3500, nozzle voltage 500 V, fragmentor 130, skimmer1 65, and octapole RF peak 750. Targeted MS/MS was obtained for m/z 392.2333 with a fixed 25 CeV collision energy. Internal reference ions at m/z 121.05087 and m/z 922.00980 were used for calibration purposes.
2.2.4 |. Infrared spectroscopy
The spectrometer used was a Nicolet 380 FT-IR with Smart Golden Gate Diamond ATR. The wavelength resolution was set to 4 cm−1. The IR spectra were collected in a range of 650–4000 cm−1 with 32 scans per spectrum. Solid phase IR spectra were recorded from the hemitartrate (2:1) salt.
2.2.5 |. Nuclear magnetic resonance spectroscopy
Samples were prepared in deuterated dimethyl sulfoxide (DMSO-d6) and 1H (600 MHz) and 13C DEPTQ (150 MHz) spectra were recorded on a Bruker AVANCE III 600 MHz NMR spectrometer. Spectra were referenced to residual solvent and assignments were supported by 1D and 2D experiments.
2.3 |. Animal pharmacology
Male C57BL/6 J mice (6–8 weeks old) were obtained from Jackson Laboratories (Bar Harbor, ME, USA) and housed up to four per cage on a reversed light-cycle (lights on at 1900 h, off at 0700 h). Food and water were provided ad libitum, except during behavioural testing. Testing was conducted between 1000 and 1830 h. The head-twitch response (HTR) was detected using a head mounted magnet and a magnetometer coil.28,30 After administration of vehicle or 1CP-LSD (0.1, 0.3, 1, or 3 mg/kg; n = 6/group), HTR was assessed immediately for 30 min. 1CP-LSD hemitartrate was dissolved in saline and administered i.p. (5 mL/kg).
2.4 |. Analysis
Head twitches were detected as described in previous experiments.28,30 Head twitch counts were analyzed using one-way analyses of variance (ANOVA), with time as a repeated measure (when appropriate). Post-hoc comparisons were made using Tukey’s Studentized range method. Significance was demonstrated by surpassing an α-level of 0.05. The ED50 values and 95% confidence intervals were calculated using nonlinear regression.
3 |. RESULTS AND DISCUSSION
3.1 |. Analytical features
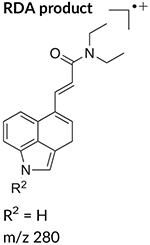
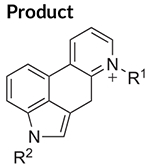
The electron ionization (EI) mass spectrum of 1CP-LSD is presented in Figure 2 and it can be seen that similar to other lysergamides, the molecular ion (m/z 391) was detected in high abundance. Fragments typically detected with structural templates based on the lysergamide nucleus include clusters at m/z 151–156, m/z 161–169, m/z 178–182, m/z 191–197, m/z 205–208, and m/z 219–221,18,31 and this was also the case here with 1CP-LSD. Fragments associated with the N,N-diethylamide component (e.g. m/z 100 and m/z 72) were also detected consistent with other N,N-diethylamide based analogs 1P-LSD,18 AL-LAD,32 ETH-LAD and 1P-ETH-LAD,19 and 1B-LSD20 Correspondingly, isomeric species, for example N-methyl-N-propyl-LSD (LAMPA) have also been shown to form similar m/z values.31 The EI mass spectra obtained previously from various 1-acyl substituted lysergamides also revealed that specific fragments related to N1-substitution might be detectable in the form of the corresponding acylium ions: m/z 43 (ALD-52),33 m/z 57 (1P-LSD),18 and m/z 71 followed by a neutral loss of CO yielding a propyl ion at m/z 43 (1B-LSD).20 In the case of 1CP-LSD, the corresponding acylium ion was detected at m/z 69 followed by a secondary loss of CO to give the m/z 41 species, possibly reflecting the presence of the cyclopropyl ion (Figure 2). Lysergamides have been shown to form characteristic fragments of low to moderate abundance thought to be formed by a retro-Diels-Alder (RDA) type mechanism. The diagnostic value derives from the fact that the proposed fragment contains the information related to the amide and N1-substituent. In the case of LSD, for example, the resulting RDA fragment is detectable at m/z 280, whereas 1P-LSD shifts the mass of the 1-acyl substituent to m/z 336.18 Correspondingly, the EI mass spectrum of 1CP-LSD displays this particular species at m/z 348. The mass difference between the molecular ion and the RDA fragment also provides information about the potential nature of the N6-substituent that, in the case of a methyl group (e.g. LSD, 1CP-LSD, and others), is represented by a neutral loss of 43 u. Table 1 provides a summary of RDA fragments formed via this particular mechanism in a number of N,N-diethylamide based lysergamides. Other lysergamides carrying the N6-methyl substituents but other amide groups, such as lysergic acid morpholide (LSM-775) or (2’S,4’S)-lysergic acid 2,4-dimethylazetidide (LSZ), show the equivalent neutral loss with RDA fragments being detected at m/z 29435 and m/z 292, respectively.32 Other more abundant fragments reflecting the impact of substitution both at the N6- and N1-atoms can be seen in the fragmentation thought to reflect a neutral loss of N,N-diethylformamide. In the case of 1CP-LSD, the resulting ion was detected at m/z 290 which, subsequently followed by a loss of a hydrogen radical, gave rise to m/z 289 (Figure 2). The presence of the cyclopropanoyl group led to a shift from m/z 221 as seen in the EI mass spectrum of LSD as summarized in Table 2 for a number of N,N-diethylamide based lysergamides. Suggested fragmentation pathways for 1CP-LSD are shown in Figure 3 and were based on and consistent with proposed patterns on related lysergamides reported previously.18–20,31,32,35
FIGURE 2.
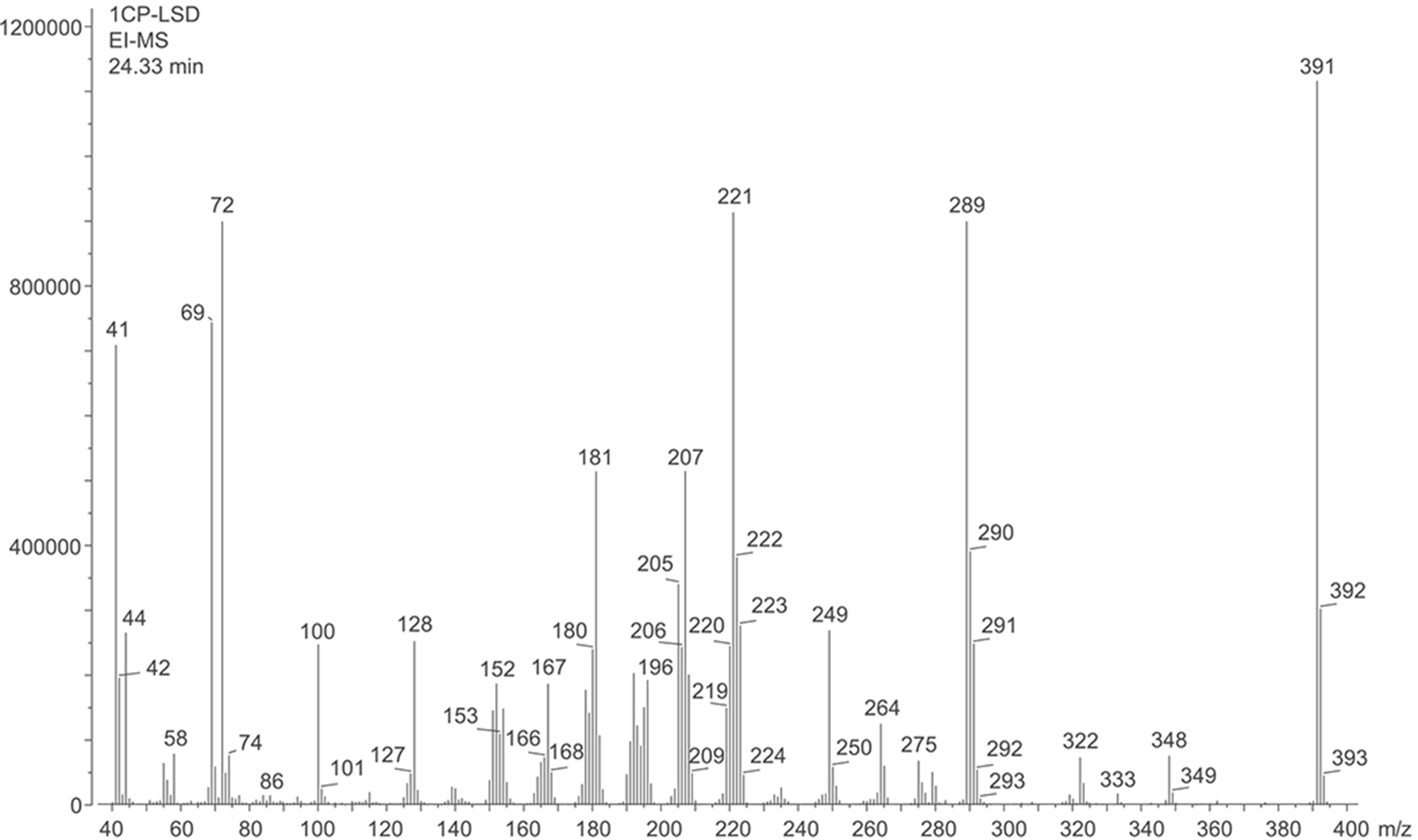
Electron ionization mass spectrum of 1CP-LSD
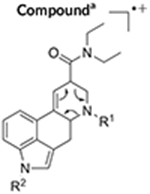
TABLE 1.
Retro-Diels-Alder (RDA) fragments detected in mass spectra of N,N-diethylamide based lysergamides under electron ionization conditions
|
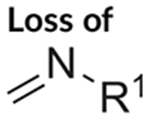
|
|
Reference |
|---|---|---|---|
| LSD | R1 = CH3 | R2 = H | 18,31 |
| M•+ = 323 | 43 u | m/z 280 | |
| R1 = CH3 | |||
| R2 = H | |||
|
| |||
| 1A-LSD (ALD-52) | R1 = CH3 | R2 = C(O)CH3 | 33,34 |
| M•+ = 365 | 43 u | m/z 322 | |
| R1 = CH3 | |||
| R2 = C(O)CH3 | |||
|
| |||
| 1P-LSD | R1 = CH3 | R2 = C(O)C2H5 | 18 |
| M•+ = 379 | 43 u | m/z 336 | |
| R1 = CH3 | |||
| R2 = C(O)C2H5 | |||
|
| |||
| AL-LAD | R1 = C3H5 | R2 = H | 32 |
| M•+ = 349 | 69 u | m/z 280 | |
| R1 = C3H5 (allyl) | |||
| R2 = H | |||
|
| |||
| ETH-LAD | R1 = C2H5 | R2 = H | 19 |
| M•+ = 337 | 57 u | m/z 280 | |
| R1 = C2H5 | |||
| R2 = H | |||
|
| |||
| 1P-ETH-LAD | R1 = C2H5 | R2 = C(O)C2H5 | 19 |
| M•+ = 393 | 57 u | m/z 336 | |
| R1 = C2H5 | |||
| R2 = C(O)C2H5 | |||
|
| |||
| 1B-LSD | R1 = CH3 | R2 = C(O)C3H7 | 20 |
| M•+ = 393 | 43 u | m/z 350 | |
| R1 = CH3 | |||
| R2 = C(O)C3H7 | |||
|
| |||
| 1CP-LSD | R1 = CH3 | R2 = C(O)C3H5 | This study |
| M•+ = 391 | 43 u | m/z 348 | |
| R1 = CH3 | |||
| R2 = C(O)C3H5 | |||
AL-LAD, N6-allyl-6-nor-LSD; ETH-LAD, N6-ethyl-6-nor-LSD.
TABLE 2.
Characteristic fragments detected in mass spectra of N,N-diethylamide based lysergamides under electron ionization conditions
|
|
Reference |
|---|---|---|
| LSD | R1 = CH3 | 18,31 |
| M•+ = 323 | R2 = H | |
| R1 = CH3 | m/z 221 | |
| R2 = H | ||
|
| ||
| 1A-LSD (ALD-52) | R1 = CH3 | 33,34 |
| M•+ = 365 | R2 = C(O)CH3 | |
| R1 = CH3 | m/z 263 | |
| R2 = C(O)CH3 | ||
|
| ||
| 1P-LSD | R1 = CH3 | 18 |
| M•+ = 379 | R2 = C(O)C2H5 | |
| R1 = CH3 | m/z 277 | |
| R2 = C(O)C2H5 | ||
|
| ||
| AL-LAD | R1 = C3H5 (allyl) | 32 |
| M•+ = 349 | R2 = H | |
| R1 = C3H5 (allyl) | m/z 247 | |
| R2 = H | ||
|
| ||
| ETH-LAD | R1 = C2H5 | 19 |
| M•+ = 337 | R2 = H | |
| R1 = C2H5 | m/z 235 | |
| R2 = H | ||
|
| ||
| 1P-ETH-LAD | R1 = C2H5 | 19 |
| M•+ = 393 | R2 = C(O)C2H5 | |
| R1 = C2H5 | m/z 291 | |
| R2 = C(O)C2H5 | ||
|
| ||
| 1B-LSD | R1 = CH3 | 20 |
| M•+ = 393 | R2 = C(O)C3H7 | |
| R1 = CH3 | m/z 291 | |
| R2 = C(O)C3H7 | ||
|
| ||
| 1CP-LSD | R1 = CH3 | This study |
| M•+ = 391 | R2 = C(O)C3H5 | |
| R1 = CH3 | m/z 289 | |
| R2 = C(O)C3H5 | ||
FIGURE 3.
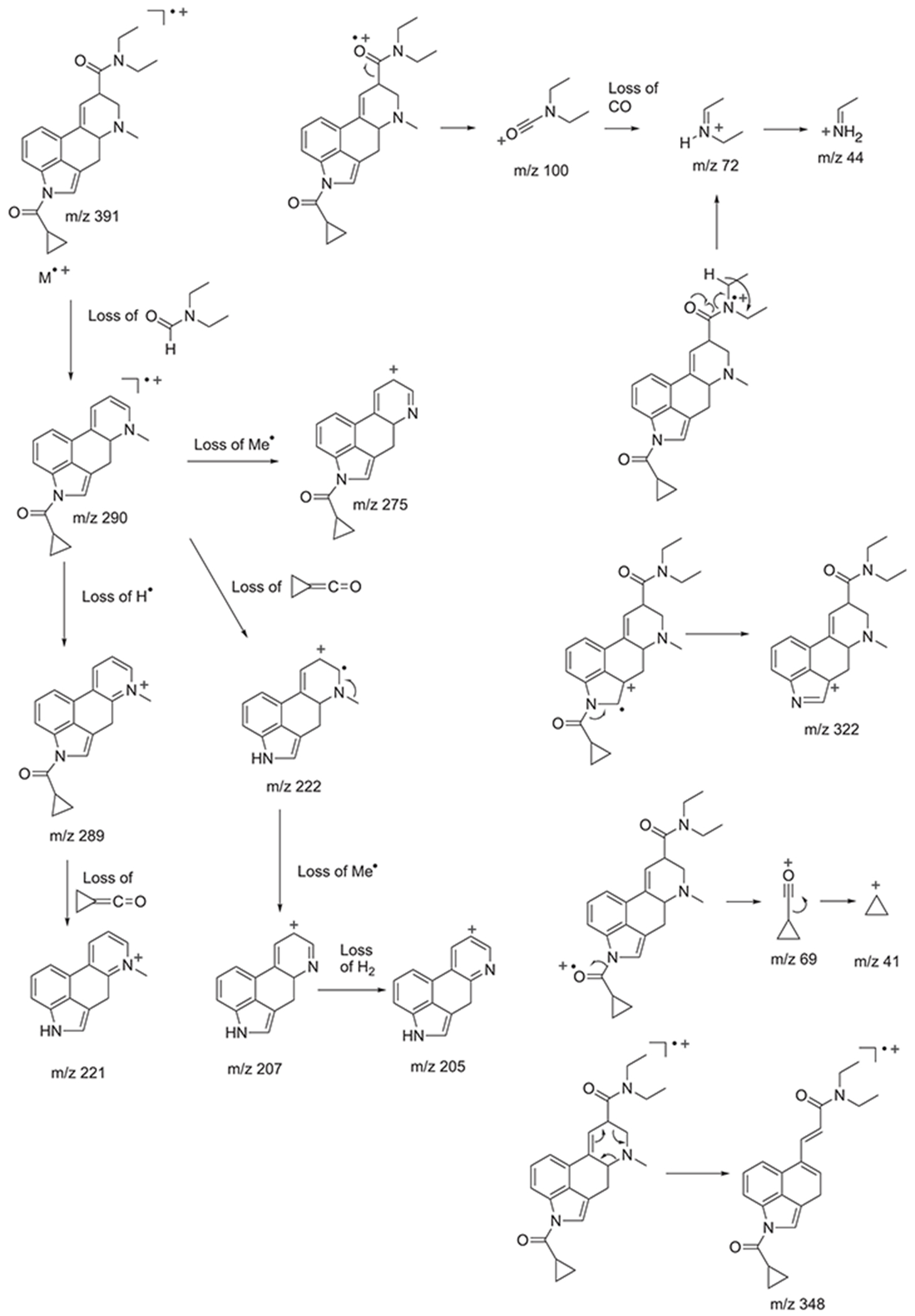
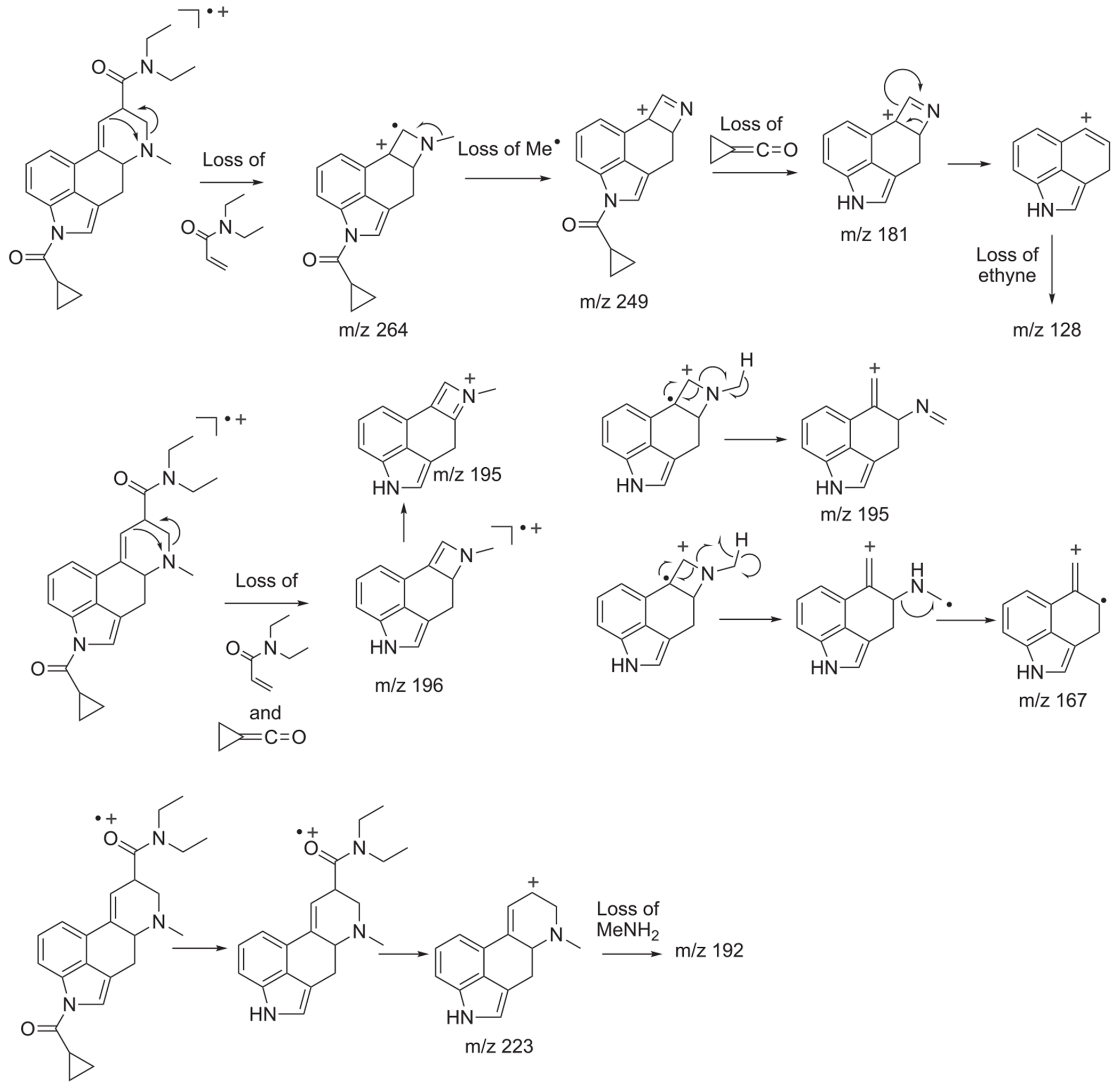
Proposed fragmentation pathways for 1CP-LSD following electron ionization
As shown in the Supporting Information, GC-EI-MS analysis also revealed the detection of GC-induced degradation products that were not detected under LC–MS conditions. Two of the three inspected mass spectra suggested the presence of a molecular ion at m/z 391 which indicated the potential presence of 1CP-LSD isomers, although the associated mass spectra indicated that the iso-form of 1CP-LSD ((5R,8S) instead of (5R,8R)) did not seem to be detected based on distinct differences. As shown in the Supporting Information, a small peak eluting before the 1CP-LSD peak at 22.71 min might have represented an imine formation as a consequence of ring D opening, whereas a later eluting isomer (labeled as 1CP-LSD isomer II) at 38.33 min might have also represented a ring opened compound carrying the N-methyl moiety. Proposed fragmentation pathways have been included as Supporting information. An interesting observation was that the mass spectrum of the late eluting isomer II did not appear to include m/z 348 (RDA fragment) and m/z 322 that were present in the EI mass spectrum of 1CP-LSD (Figure 2). Given that RDA fragments have also been described for a number of iso-forms (e.g. m/z 280 in iso-LSD and iso-LAMPA),36 it was considered plausible that this might have excluded the presence of the epimeric iso-1CP-LSD and it raised the question as to whether the absence of m/z 322 might have reflected a preferred radical cation delocalization to afford a thermodynamically stable naphthalene-type species (Supporting Information). A third degradant, eluting between 1CP-LSD and isomer II at 32.29 min revealed a molecular ion at m/z 373 and in conjunction with the mass spectra data recorded for this peak, its identity has been tentatively assigned to a pyridine derivative (Supporting Information). When subjecting 1CP-LSD to analysis on a separate system (GC–MS method 2, Supporting Information), both the m/z 373 and isomer II species were also detected, which confirmed that this might have been a phenomenon induced during sample injection. On the other hand, analysis by GC–MS on a third system (Supporting Information) only revealed the detection of one peak for 1CP-LSD. It was unknown whether this reflected poorer sensitivity or more inert conditions but it also indicated that the extent of GC-induced degradation that might occur during analysis might differ between the various conditions involved. In order to test whether heat-induced degradation products were also detectable under LC–MS conditions, a 1 mg sample (base and salt) was heated in a glass vial (purged with nitrogen and plugged with glass wool) at 280°C for 3 min and subjected to analysis by LC–MS. Although it was unclear whether this would mimic the exact conditions found in a GC liner, various peaks were detected that represented m/z 392 and m/z 374 (tentative pyridine derivative) species (Supporting Information). Whether the proposed imine degradant encountered during GC–MS analysis remained detectable under the acidic conditions used during LC–MS analysis was unknown.
The infrared spectrum obtained from ATR-FTIR analysis of the hemitartrate salt is shown in Figure 4. The signals linked to the carbonyl groups (1681 and 1623 cm−1) were comparable to those reported for 1P-LSD (1704 and 1638 cm−1),18 1P-ETH-LAD (1704 and 1640 cm−1),19 and 1B-LSD (1702 and 1639 cm−1)20 that were recorded using GC-sIR conditions. The GC-sIR spectra obtained from 1CP-LSD and the two degradation products (tentative pyridine derivative at m/z 373 and isomer II) that were subjected to analysis by GC–MS method 2 are provided as Supporting Information. The two carbonyl stretches of 1CP-LSD were detected at 1689 and 1637 cm−1, whereas these were observed to coalesce in the cases of isomer II and the m/z 373 degradant.
FIGURE 4.
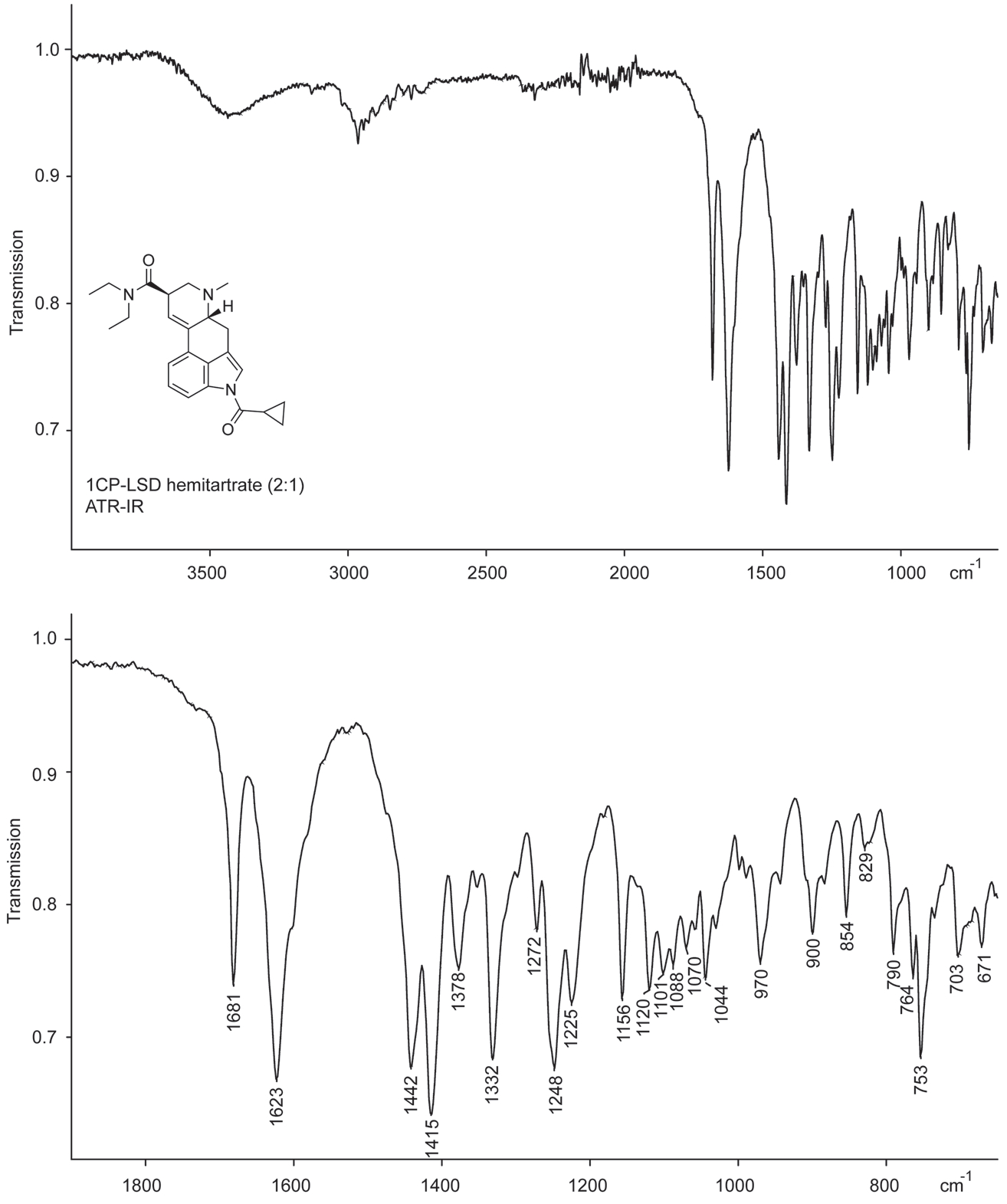
ATR-FTIR of 1CP-LSD hemitartrate. Top: Entire scan range. Bottom: Partial scan range
The electrospray ionization (ESI) QTOF-MS/MS and ESI single quadrupole mass spectra recorded for 1CP-LSD are presented in Figure 5 followed by proposed formations of product ions summarized in Figure 6 which were based on and consistent with those reported for other lysergamides previously.18–20,32,35 Examples included the detection of product ions typically detected for LSD based lysergamides resulting in ions at m/z 128, m/z 100, and m/z 74 (e.g. 1P-LSD,18 ETH-LAD, 1P-ETH-LAD,19 and 1B-LSD20) under comparable conditions. Another characteristic feature observed for LSD derived lysergamides included a loss of what might have been attributed to N,N-diethylformamide which in the case of 1CP-LSD might have led to m/z 291 with high abundance (Figure 5). Similarly, cleavage of neutral N,N-diethylformamide in other LSD based lysergamides was observed previously as mirrored in the formation of m/z 279 (1P-LSD18) and m/z 293 (1P-ETH-LAD19) and 1B-LSD20), respectively. Another relatively abundant product ion observed in the spectrum of 1CP-LSD was detected at m/z 349 whereby the mass difference of 43 u might have reflected a neutral loss of N-methylmethanimine (Figure 6), thus, providing potential diagnostic information related to a LSD-based substance. For example, the ion corresponding to the loss of 43 u was therefore found at m/z 337 in 1P-LSD,18 m/z 293 in LSZ,32 m/z 295 in LSM-775,35 and m/z 351 in 1B-LSD.20 Substituents other than methyl located at the N6-position were therefore observed to display a neutral loss represented by a corresponding mass shift. In other words, both ETH-LAD and 1P-ETH-LAD displayed a loss of 57 u (N-ethylmethanimine) (resulting in m/z 281 and m/z 337),19 whereas the N6-allyl-6-nor-LSD analog AL-LAD showed a neutral loss of N-allylmethanimine (69 u) with detection of the product ion at m/z 281.32 The spectrum of 1CP-LSD recorded from in-source dissociation CID (Figure 5) was also comparable to one recorded following analysis by triple quadrupole tandem mass spectrometry (Supporting Information).
FIGURE 5.
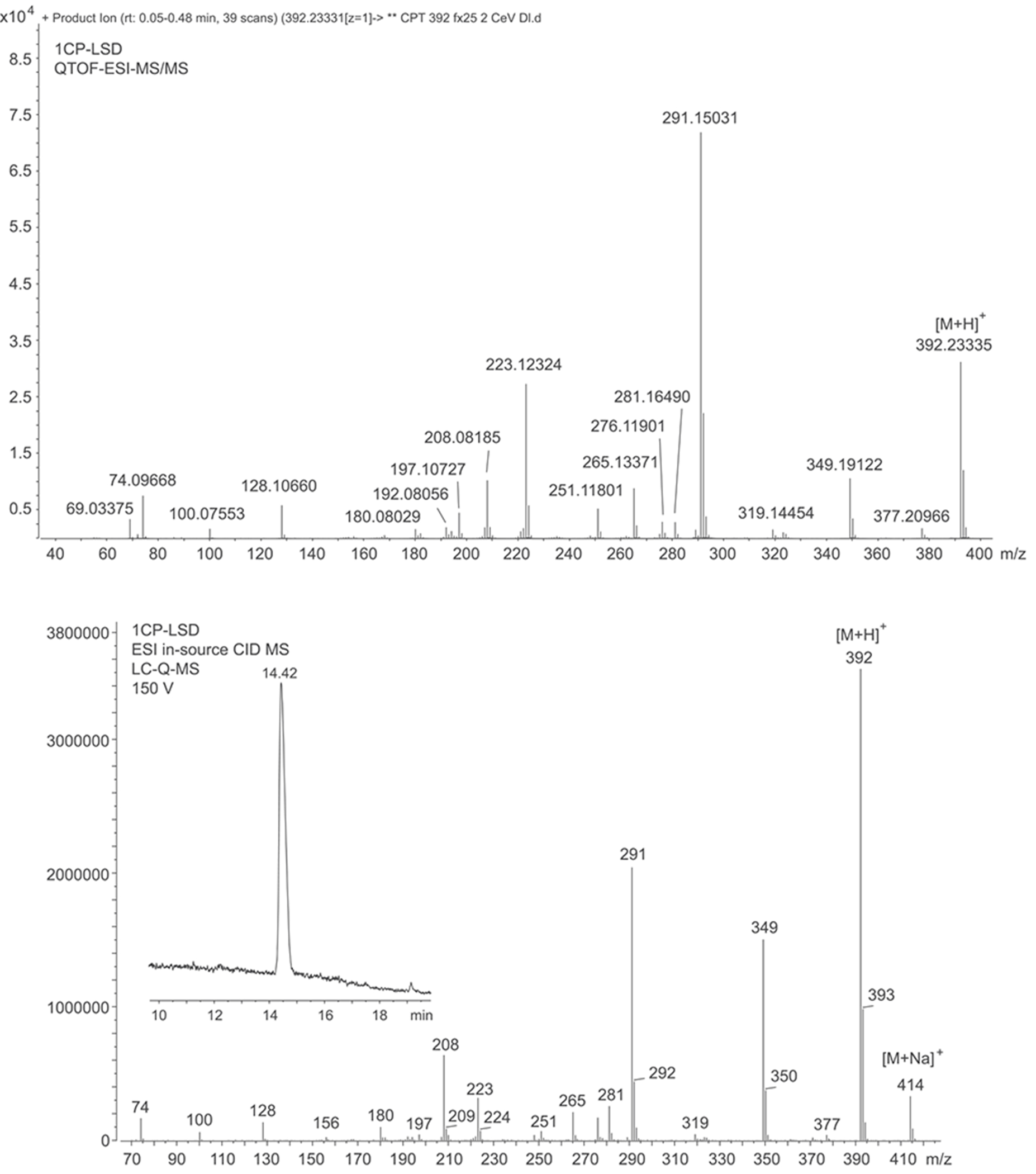
Electrospray ionization (ESI) quadrupole-time-of-flight tandem mass spectrum (top) and ESI single quadrupole mass spectrum of 1CP-LSD involving in-source collision-induced-dissociation (bottom). The ion observed at m/z 208.0819 appeared to be a mixture of two ions detected at m/z 208.0764 and m/z 208.0974 (Supporting Information.)
FIGURE 6.
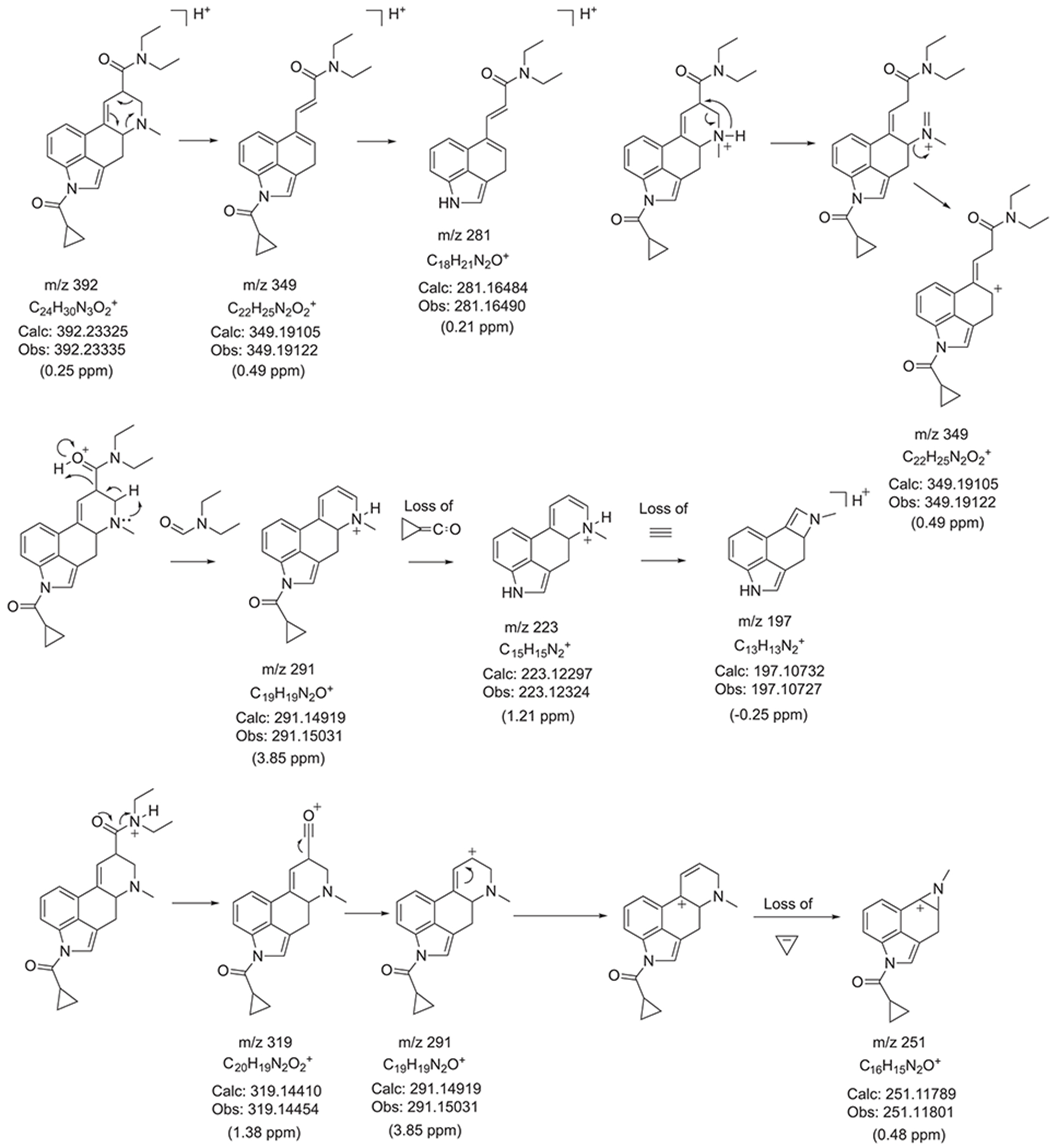
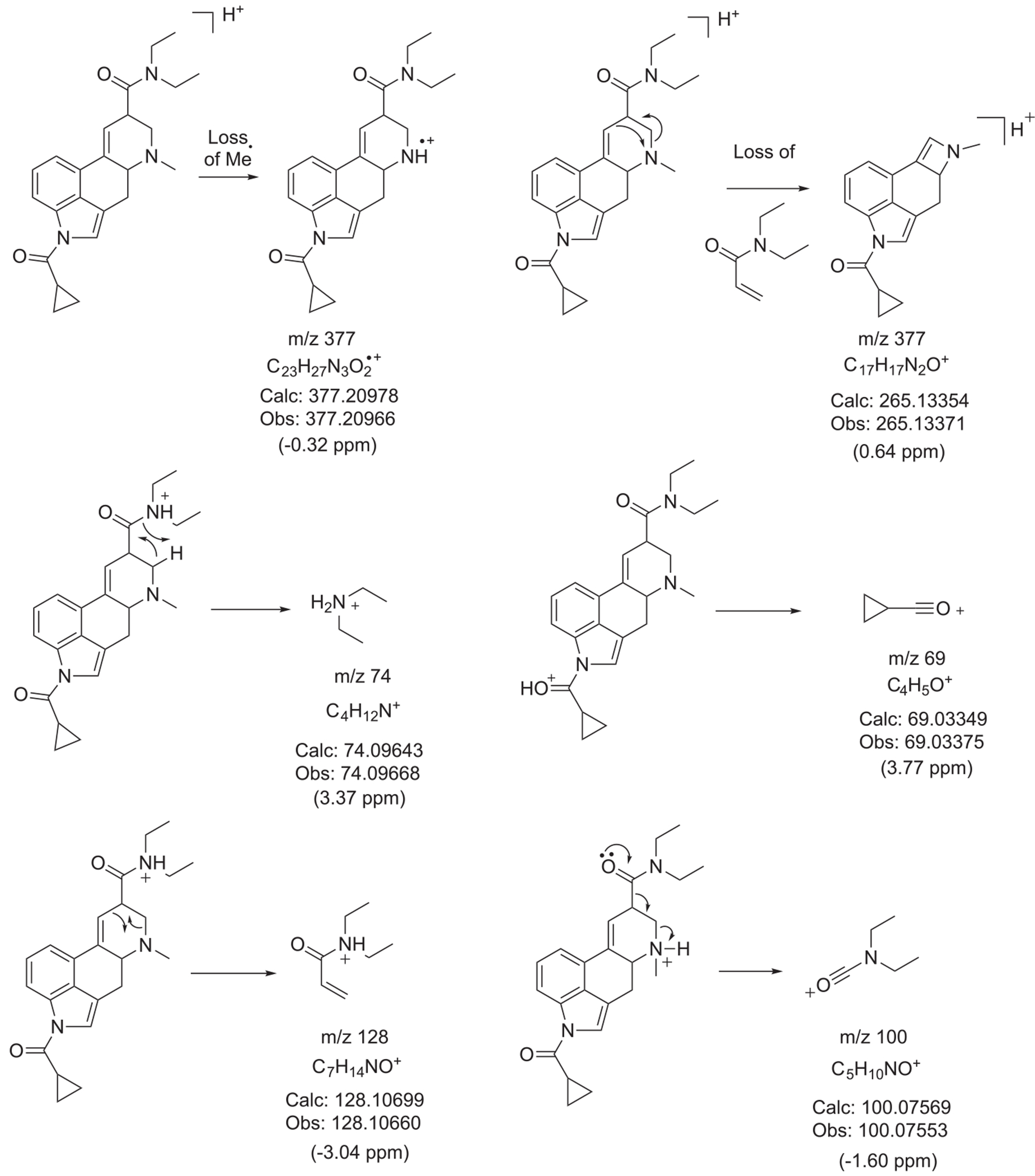
Proposed formation of product ions following ESI-QTOF-MS/MS analysis
Inspection of the QTOF tandem mass spectrum in Figure 5 suggested the m/z 208 ion to be detected at m/z 208.0819 which was surprising since previous investigations on a number of lysergamides were frequently showing a species consistent with C14H10NO+ (m/z 208.0757).18–20,32,35 The molecular formulae derived for the m/z 208.0819 ion also did not appear to be consistent with anything meaningful which gave rise to the hypothesis that it might have arisen from an averaged mass obtained by two separate m/z 208 ions. As shown in the Supporting Information, a repeated analysis in profile mode indeed confirmed the detection of two distinct ions at m/z 208.0764 (C14H10NO+, delta 3.36 ppm) and m/z 208.0974 (, m/z 208.0995, delta −10.09 ppm). Whilst the detection of m/z 208.0764 was consistent with a fragment reported previously, the detection of m/z 208.0974 might have been consistent with the N-demethylated 1CP-LSD (m/z 377) followed by loss of the acyl group (Supporting Information). Interestingly, it was also observed that the formation of both ions seemed to depend on the collision energies (CEs) involved. The m/z 208.0764 appeared to be more prominent at lower CEs whereas the m/z 208.0974 ion began to emerge at higher CE values (Supporting Information). In a previous investigation involving the characterization of the lysergamide AL-LAD, the m/z 208 detected was also consistent with that might have reflected the N6-dealkylated ion32 similar to the fragment observed in this investigation.
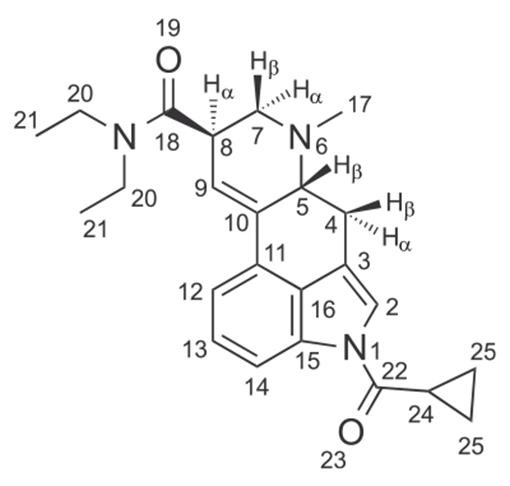
The nuclear magnetic resonance (NMR) spectroscopy data for 1CP-LSD are summarized in Table 3 (full spectra, along with chemical shift assignments for 1CP-LSD freebase are shown in the Supporting Information). The assignments were supported by HSQC, HMBC, and COSY experiments and were based on and consistent with those reported previously on related lysergamides.18–20,32,35 In comparison with the spectral data reported for the related 1B-LSD previously,20 some distinct differences could be observed that reflected the differences between the 1-butanoyl- (1B-LSD) and 1-cyclopropanoyl-(1CP-LSD) groups. For example, the two methylene units -N(CO) (CH2)2− present in 1B-LSD were absent in the proton NMR spectra of 1CP-LSD. Instead, the methanetriyl unit reflecting the -N(CO)(CH)-component present in 1CP-LSD was observed to partially coalesce with the triplet assigned to H-7β in the spectrum (Table 3). A spectral comparison between 1B-LSD and 1CP-LSD can be found in the Supporting Information section. Correspondingly, the carbon NMR spectrum of 1CP-LSD also revealed the absence of the two methylene units, whereas the methanetriyl carbon of the -N(CO)(CH)- part resonated at a relatively low chemical shift between the two amide methyl groups (Table 3). In 1CP-LSD, the two methylene units belonging to the cyclopropane ring resonated more upfield than the terminal methyl group of the 1-butanoyl substituent (Supporting Information). For completeness, the 1D and 2D NMR spectra of 1CP-LSD freebase recorded in CDCl3 have also been provided as Supporting Information.
TABLE 3.
1H and 13C DEPTQ NMR data for 1CP-LSD hemitartrate (2:1) in DMSO-d6 at 600/150 MHz
| ||
|---|---|---|
| No. | 13C [δ/ppm] | 1H [δ/ppm] |
| 1 | – | – |
| 2 | 120.09 | 7.89 (d, J = 1.6 Hz, 1H) |
| 3 | 116.25 | – |
| 4 | 26.02 | 2.51–2.48 (m, 4α-H) *coalescing with DMSO 3.52 (dd, J = 15.3, 5.5 Hz, 4β-H, 1 H) |
| 5 | 61.75 | 3.15–3.13 (m, H-5β, 1H) |
| 6 | – | – |
| 7 | 55.24 | 3.04 (dd, J = 11.1, 5.0 Hz, H-7α, 1H) 2.66 (t, J = 9.8 Hz, H-7β, 1H) *partially coalescing with H-24 |
| 8 | 38.83 | 3.85–3.82 (m, H-8α, 1H) |
| 9 | 121.85 | 6.36 (s, 1H) |
| 10 | 133.34 | – |
| 11 | 127.73 | – |
| 12 | 116.66 | 7.36 (d, J = 7.5 Hz, 1H) |
| 13 | 125.92 | 7.30 (t, J = 7.8 Hz, 1H) |
| 14 | 114.92 | 8.01 (d, J = 8.0 Hz, 1H) |
| 15 | 133.22 | – |
| 16 | 127.61 | – |
| 17 | 43.04 | 2.53 (s, 3H) |
| 18 | 170.30 | – |
| 19 | – | – |
| 20 | 41.59 | 3.45 (q, J = 7.1 Hz, 2H) |
| 20 | 39.56 | 3.32 (AB qq, J20,20 = 13.8, J20,21 = 7.0 Hz, 2 H) |
| 21 | 14.82 | 1.19 (t, J = 7.1 Hz, 3H) |
| 21 | 13.06 | 1.12–1.03 (m, 3H) *coalescing with H-25 |
| 22 | 172.20 | – |
| 23 | – | – |
| 24 | 13.23 | 2.65–2.61 (m, 1H) *partially coalescing with H-7β) |
| 25 | 9.36, 9.34 | 1.13–1.05 (m, 4H) *coalescing with H-21 |
| TAa | 173.20 | – |
| TAa | 72.06 | 4.27 (s, ~1.4 H)b |
TA, tartaric acid.
Integration of the TA singlet after three separate analyses gave 1CP-LSD: TA integration ratios of 1.00:1.00, 1.00:0.98, and 1.00:1.39, respectively (Supporting Information).
When 1P-LSD was first investigated following its emergence as a designer drug, it was found that the incubation with human serum led to its hydrolysis to LSD which suggested that it might serve as a prodrug in vivo.18 Similarly, incubation of 1P-ETH-LAD was found to result in the formation of ETH-LAD.19 It has since been confirmed that the 1-acyl substituted lysergamides ALD-52, 1P-LSD, 1P-ETH-LAD, and 1B-LSD were indeed transformed into LSD and ETH-LAD, respectively.22,24 These findings were also consistent with the data reported in an intoxication where the ingestion of 1P-LSD in blotter form did not result in its detection in either urine or serum but LSD instead.23 In the present investigation, an incubation of 1CP-LSD with human serum also revealed the formation of LSD when the incubation mixture was analyzed by LC-ESI single quadrupole MS. As summarized in the Supporting Information section (analyses performed in triplicate but only one representative example shown), it was found that the signal response related to the hydrolysis product increased over time when analyzed at 0, 1, 2, 3, 5, 7, and 24 h. These results showed that 1CP-LSD was hydrolyzed to LSD in serum but that conversion was not complete within the first 24 h. However, 1CP-LSD is likely to be hydrolyzed at a much faster rate in vivo because humans express carboxyesterase in red blood cell membranes but not in serum.37,38 Indeed, 1P-LSD has been shown to undergo relatively rapid hydrolysis in vivo. In rats, high levels of LSD are found in the plasma 15 min after subcutaneous administration of ALD-52 and 1P-LSD, indicating they likely undergo rapid hydrolysis.24 Likewise, in a human self-administration study, 1P-LSD was completely converted to LSD after about 4 h (submitted for publication).
In a follow-up experiment in the present investigation, the extent of LSD formation arising from incubation with human serum with 1CP-LSD and 1P-LSD was compared. Interestingly, when the LSD signal responses and the peak area ratios (LSD/test drug) were compared, it was observed that the 1P-LSD appeared to hydrolyze more readily when compared with 1CP-LSD (Supporting Information). It is important to note that these preliminary results should be assessed in further in vivo studies. At this stage it is unclear whether the nature of the 1-acyl substituent leads to differences in pharmacokinetic parameters including the formation of LSD in vivo.
Finally, blotter papers (labeled to contain 100 μg 1CP-LSD free-base) and pellets (labeled to contain 150 μg 1CP-LSD hemitartrate) available from various Internet retailers were subjected to extraction and analysis by LC-ESI-Q-MS. As shown in the Supporting Information section, the analysis of two blotter extracts revealed the detection of 120 and 112 μg 1CP-LSD (freebase), whereas the analysis of two pellet extracts uncovered the detection of 116 and 125 μg 1CP-LSD hemitartrate (97 and 105 μg freebase equivalence). A qualitative analysis of the extracts by NMR confirmed the presence of the freebase and salt in those two samples (not shown). Lower amounts of 1CP-LSD than expected were detected in the two test pellets and one potential reason might also include difficulties in complete extraction from the microcrystalline matrix of the pellets.
3.2 |. Head-twitch response
Head-twitch response (HTR) studies were conducted in C57BL/6J mice to determine whether 1CP-LSD produces LSD-like behavioural effects in vivo. The HTR was assessed for 30 min following administration of 1CP-LSD. As shown in Figure 7A, there was a dose-dependent increase in HTR counts after administration of 1CP-LSD (F (4,25) = 36.66, P < 0.0001). 1CP-LSD induced the HTR with an ED50 = 204.6 (95% CI 150.6–278.0) μg/kg, which is equivalent to 430.0 (316.5–584.3) nmol/kg after correcting the molecular weight based on the proton NMR data (see Table 3 and Supporting Information). The potency was comparable to 1P-LSD (ED50 = 349.6 nmol/kg) investigated previously, whereas the potency was only 31% compared with LSD (ED50 = 132.8 nmol/kg).24 The response to 1CP-LSD was also analyzed in 2-min time blocks; the effect of 1CP-LSD was time-dependent (drug × time: F(56,350) = 2.32, P < 0.0001). As shown in Figure 7B, after administration of 1 and 3 mg/kg 1CP-LSD, the maximal response occurred during the 10–12 min time block. Based on those results, 1CP-LSD appears to act as a 5-HT2A receptor agonist in mice. Although the receptor pharmacology of 1CP-LSD was not investigated, other 1-acyl-substituted LSD derivatives act as very weak partial agonists at the 5-HT2A receptor.24 ALD-52, 1P-LSD, and 1B-LSD are believed to act as prodrugs for LSD. Based on the fact that 1CP-LSD is hydrolyzed to LSD after incubation with human serum (see the Supporting Information), 1CP-LSD also likely serves as a prodrug for LSD, although biotransformation and receptor pharmacology studies are ultimately required to conclusively understand the mechanism of action of 1CP-LSD. The concept of prodrugs, although not necessarily new in the world of certain medicines, is becoming increasingly common within the context of hallucinogens an other classes of NPS.39
FIGURE 7.
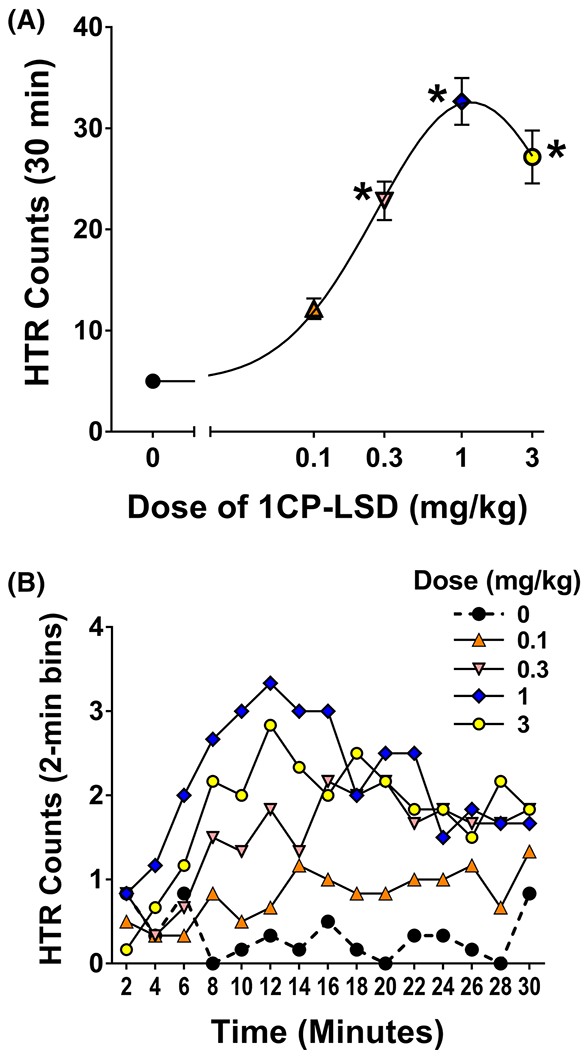
(A) Dose-dependent increase in head twitch response counts after administration of 1CP-LSD (*P < 0.001, significant difference vs. vehicle control (Tukey’s test)). (B) after administration of 1 and 3 mg/kg 1CP-LSD, the maximal response occurred during the 10–12 min time block [Colour figure can be viewed at wileyonlinelibrary.com]
4 |. CONCLUSION
1-Cylopropanoyl-LSD (1CP-LSD) has recently emerged as a new addition to the growing number of lysergamide-based designer drugs. It appears to have been absent from the scientific literature, which triggered the present investigation to disseminate key analytical data. The observation that 1CP-LSD hydrolyzed into LSD after incubation with human serum suggests that it may serve as a prodrug in vivo, similar to other 1-acyl substituted lysergamides. The results obtained using the HTR paradigm indicate that 1CP-LSD produces LSD-like behavioural effects, potentially because it is metabolized to LSD. Clinical studies are necessary to understand the activity and effects of 1CP-LSD in humans and to determine its potential for non-medical use.
Supplementary Material
ACKNOWLEDGEMENTS
The behavioural studies were supported by an award from the National Institute on Drug Abuse (NIDA) (R01 DA041336). The analytical work was supported by the project ADEBAR plus, which is cofunded by the Internal Security Fund of the European Union (grant no.: IZ25-5793-2019-33, www.projekt-adebar.eu). GC–MS, solid IR, GC-IR und LC–MS (linear ion trap) data were provided to the EMCDDA’s European Database on New Drugs (EDND2). The authors would like to thank Dr. Wolfgang Dreiseitel (Hessian State Bureau of Criminal Investigation, Wiesbaden, Germany) for support with mass spectral measurements. SDB gratefully acknowledges the support of Stephen J. Chapman (Isomer Design, Toronto, Canada).
FUNDING INFORMATION
Behavioural studies supported by National Institute on Drug Abuse (NIDA) (R01 DA041336). Analytical work supported by project ADEBAR plus, co-funded by the Internal Security Fund of the European Union (grant no.: IZ25-5793-2019-33, www.projekt-adebar.eu).
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Hintzen A, Passie T. The Pharmacology of LSD. A Critical Review. Oxford: Oxford University Press/Beckley Foundation Press;2010. [Google Scholar]
- 2.Preller KH, Vollenweider FX. Phenomenology, structure, and dynamic of psychedelic states. Curr Top Behav Neurosci. 2018;18:221–256. [DOI] [PubMed] [Google Scholar]
- 3.Nichols DE. Dark classics in chemical neuroscience: lysergic acid diethylamide (LSD). ACS Chem Neurosci. 2018;9(10):2331–2343. [DOI] [PubMed] [Google Scholar]
- 4.Pfaff RC, Huang X, Marona-Lewicka D, Oberlender R, Nichols DE. Lysergamides revisited. In: Lin GC, Glennon RA, eds. Hallucinogens: An Update. NIDA Research Monograph 146. Rockville, MD: National Institute on Drug Abuse; 1994:52–73. [PubMed] [Google Scholar]
- 5.Hofmann A Die Mutterkornalkaloide: vom Mutterkorn zum LSD - Die Chemie der Mutterkornalkaloide. Solothurn, Switzerland: Nachtschatten Verlag; 2000. [Google Scholar]
- 6.Nichols DE. Chemistry and structure-activity relationships of psychedelics. Curr Top Behav Neurosci. 2017;36:1–43. [DOI] [PubMed] [Google Scholar]
- 7.Halberstadt AL, Klein LM, Chatha M, et al. Pharmacological characterization of the LSD analog N-ethyl-N-cyclopropyl lysergamide (ECPLA). Psychopharmacology (Berl). 2018;236(2):799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoll A, Hofmann A, Troxler F. Lysergic acid derivatives acylated at the indol nitrogen. US2810723. Newark, NJ: Saul & Co.; 1957. [Google Scholar]
- 9.Troxler F, Hofmann A. Substitutionen am Ringsystem der Lysergsäure I. Substitutionen am Indol-Stickstoff. 43. Mitteilung über Mutterkornalkaloide. Helv Chim Acta. 1957;40(6):1706–1720. [Google Scholar]
- 10.Troxler F, Hofmann A. Substitutionen am Ringsystem der Lysergsäure II. Alkylierung. 44. Mitteilung über Mutterkornalkaloide. Helv Chim Acta. 1957;40(6):1721–1732. [Google Scholar]
- 11.Troxler F, Hofmann A. Substitutionen am Ringsystem der Lysergsäure. III. Halogenierung. 45. Mitteilung über Mutterkornalkaloide. Helv Chim Acta. 1957;40(7):2160–2170. [Google Scholar]
- 12.Rothlin E Pharmacology of lysergic acid diethylamide and some of its related compounds. J Pharm Pharmacol. 1957;9(1):569–587. [DOI] [PubMed] [Google Scholar]
- 13.Rothlin E Lysergic acid diethylamide and related substances. Ann N Y Acad Sci. 1957;66(3):668–676. [DOI] [PubMed] [Google Scholar]
- 14.Abramson HA. Lysergic acid diethylamide (LSD-25). XXIX. Response index as a measure of threshold activity of psychotropic drugs in man. J Psychol. 1959;48:65–78. [Google Scholar]
- 15.Isbell H, Miner EJ, Logan CR. Relationships of psychotomimetic to anti-serotonin potencies of congeners of lysergic acid diethylamide (LSD-25). Psychopharmacologia. 1959;1:20–28. [DOI] [PubMed] [Google Scholar]
- 16.Malitz S, Wilkens B, Roehrig WC, Hoch PH. A clinical comparison of three related hallucinogens. Psychiatry Q. 1960;34:333–345. [DOI] [PubMed] [Google Scholar]
- 17.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). EMCDDA-Europol 2016 annual report on the implementation of council decision 2005/387/JHA. EMCDDA, Lisbon, 2017. Available at: http://www.emcdda.europa.eu/system/files/publications/4724/TDAN17001ENN_PDFWEB.pdf [Accessed 15 December 2019]. [Google Scholar]
- 18.Brandt SD, Kavanagh PV, Westphal F, et al. Return of the lysergamides. Part I: analytical and behavioural characterization of 1-propionyl-d-lysergic acid diethylamide (1P-LSD). Drug Test Anal. 2016;8(9):891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandt SD, Kavanagh PV, Westphal F, et al. Return of the lysergamides. Part III: analytical characterization of N6-ethyl-6-norlysergic acid diethylamide (ETH-LAD) and 1-propionyl ETH-LAD (1P-ETH-LAD). Drug Test Anal. 2017;9(10):1641–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandt SD, Kavanagh PV, Westphal F, et al. Return of the lysergamides. Part V: analytical and behavioural characterization of 1-butanoyl-d-lysergic acid diethylamide (1B-LSD). Drug Test Anal. 2019;11(8):1122–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsochatzis E, Lopes AJ, Reniero F, Holland M, Åberg J, Guillou C. Identification of 1-butyl-lysergic acid diethylamide (1B-LSD) in seized blotter paper using an integrated workflow of analytical techniques and chemo-informatics. Molecules. 2020;25(3):E712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagmann L, Richter LHJ, Kehl T, et al. In vitro metabolic fate of nine LSD-based new psychoactive substances and their analytical detectability in different urinary screening procedures. Anal Bioanal Chem. 2019;411(19):4751–4763. [DOI] [PubMed] [Google Scholar]
- 23.Grumann C, Henkel K, Stratford A, et al. Validation of an LC-MS/MS method for the quantitative analysis of 1P-LSD and its tentative metabolite LSD in fortified urine and serum samples including stability tests for 1P-LSD under different storage conditions. J Pharm Biomed Anal. 2019;174:270–276. [DOI] [PubMed] [Google Scholar]
- 24.Halberstadt AL, Chatha M, Klein AK, et al. Pharmacological and biotransformation studies of 1-acyl-substituted derivatives of d-lysergic acid diethylamide (LSD). Neuropharmacology. 2019;107856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halberstadt AL, Geyer MA. Effect of hallucinogens on unconditioned behavior. Curr Top Behav Neurosci. 2018;36:159–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canal CE, Morgan D. Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal. 2012;4(7–8):556–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González-Maeso J, Weisstaub NV, Zhou M, et al. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53(3):439–452. [DOI] [PubMed] [Google Scholar]
- 28.Halberstadt AL, Geyer MA. Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology (Berl). 2013;227(4):727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halberstadt AL, Chatha M, Klein AK, Wallach J, Brandt SD. Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology. 2020;167:107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halberstadt AL, Geyer MA. Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology. 2014;77:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westphal F, Junge T. Massenspektrometrische Unterscheidung von LSD, LAMPA und anderen LSD-Isobaren. Toxichem Krimtech. 2014;81(3):129–135. [Google Scholar]
- 32.Brandt SD, Kavanagh PV, Westphal F, et al. Return of the lysergamides. Part II: analytical and behavioural characterization of N6-allyl-6-norlysergic acid diethylamide (AL-LAD) and (2’S,4’S)-lysergic acid 2,4-dimethylazetidide (LSZ). Drug Test Anal. 2017;9(1):38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roesner P Designer drugs – mass spectra database. DigiLab software GmbH, Altenholz, Germany. Available at: https://www.designer-drugs.de/ [Accessed 22.12.2019]. 2019. [Google Scholar]
- 34.Brandt SD, Kavanagh PV, Twamley B, et al. Return of the lysergamides. Part IV: analytical and pharmacological characterization of lysergic acid morpholide (LSM-775). Drug Test Anal. 2018;10(2):310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark CC. Differentiation of lysergic acid diethylamide (LSD) from N-methyl-N-propyl and N-butyl amides of lysergic acid. J Forensic Sci. 1989;34(3):532–546. [Google Scholar]
- 36.Li B, Sedlacek M, Manoharan I, et al. Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem Pharmacol. 2005;70(11):1673–1684. [DOI] [PubMed] [Google Scholar]
- 37.Elliott SP, Holdbrook T, Brandt SD. Prodrugs of new psychoactive substances (NPS): a new challenge. J Forensic Sci. 2020. 10.1111/1556-4029.14268 [DOI] [PubMed] [Google Scholar]
- 38.Minagawa T, Kohno Y, Suwa T, Tsuji A Species differences in hydrolysis of isocarbacyclin methyl ester (TEI-9090) by blood esterases. Biochem Pharmacol. 1995;49(10):1361–1365. [DOI] [PubMed] [Google Scholar]
- 39.Bellman SW. Mass spectral identification of some hallucinogenic drugs. J Assoc Off Anal Chem. 1968;51(1):164–175. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


