Abstract
Serotonergic hallucinogens such as lysergic acid diethylamide (LSD) induce head twitches in rodents via 5-HT2A receptor activation. The goal of the present investigation was to determine whether a correlation exists between the potency of hallucinogens in the mouse head-twitch response (HTR) paradigm and their reported potencies in other species, specifically rats and humans. Dose-response experiments were conducted with phenylalkylamine and tryptamine hallucinogens in C57BL/6J mice, enlarging the available pool of HTR potency data to 40 total compounds. For agents where human data are available (n = 36), a strong positive correlation (r = 0.9448) was found between HTR potencies in mice and reported hallucinogenic potencies in humans. HTR potencies were also found to be correlated with published drug discrimination ED50 values for substitution in rats trained with either LSD (r = 0.9484, n = 16) or 2,5-dimethoxy-4-methylamphetamine (r = 0.9564, n = 21). All three of these behavioral effects (HTR in mice, hallucinogen discriminative stimulus effects in rats, and psychedelic effects in humans) have been linked to 5-HT2A receptor activation. We present evidence that hallucinogens induce these three effects with remarkably consistent potencies. In addition to having high construct validity, the HTR assay also appears to show significant predictive validity, confirming its translational relevance for predicting subjective potency of hallucinogens in humans. These findings support the use of the HTR paradigm as a preclinical model of hallucinogen psychopharmacology and in structure-activity relationship studies of hallucinogens. Future investigations with a larger number of test agents will evaluate whether the HTR assay can be used to predict the hallucinogenic potency of 5-HT2A agonists in humans.
Keywords: psychedelic; psilocybin; mescaline; N,N-dimethyltryptamine; DMT; NBOMe; DOM; behavioral model; head shake
INTRODUCTION
Serotonergic hallucinogens, such as psilocybin, (+)-lysergic acid diethylamide (LSD), and mescaline, remain an enigmatic class of agents. These compounds alter thought, perception, and mood without producing memory impairment, delirium, or addiction (Hollister 1968; Grinspoon and Bakalar 1979). Serotonergic hallucinogens can be divided into three main structural classes: tryptamines, ergolines, and phenylalkylamines. Tryptamine hallucinogens include psilocybin, N,N-dimethyltryptamine (DMT), N,N-diethyltryptamine (DET) and N,N-dipropyltryptamine (DPT). The prototypical hallucinogen LSD is the most important member of the tetracyclic ergoline class. The phenylalkylamine class can be subdivided into phenylethylamines, such as mescaline, 4-bromo-2,5-dimethoxyphenethylamine (2C-B) and 2,5-dimethoxy-4-iodophenethylamine (2C-I), and phenylisopropylamines (“amphetamines”), including 2,5-dimethoxy-4-methylamphetamine (DOM) and 4-bromo-2,5-dimethoxyamphetamine (DOB). In addition, the potency of phenylalkylamine hallucinogens has been found to increase markedly if an N-benzyl moiety is added (e.g., 25I-NBOMe) (Braden et al. 2006; Halberstadt and Geyer 2014) or if the ring-substituents are incorporated into rigid furan rings (e.g., bromo-Dragonfly, which is also known as DOB-DFLY) (Parker et al. 1998; Halberstadt et al. 2019b). Phenylalkylamine hallucinogens are relatively selective for the orthosteric binding site of 5-HT2 subtypes, including 5-HT2A, 5-HT2B, and 5-HT2C sites, whereas tryptamine and ergoline hallucinogens bind non-selectively to most 5-HT receptors (Nichols 2018). In recent years, an increasing number of studies have explored the potential therapeutic effects of serotonergic hallucinogens, with trials focusing on anxiety and depression (Grob et al. 2011; Griffiths et al. 2016; Ross et al. 2016), substance abuse (Johnson et al. 2014; Bogenschutz et al. 2015; Johnson et al. 2016), and obsessive-compulsive disorder (Moreno et al. 2006).
Given the likelihood that compounds from the hallucinogen class are therapeutically active, it is becoming more and more important to study the effects and mechanism of action of hallucinogens using preclinical models. Unfortunately, given the complexity, variety, and variability of the effects of hallucinogens in humans, it has been difficult to define animal behavioral tests for hallucinogenic activity (Glennon 1992; Halberstadt and Geyer 2010). Nevertheless, the use of animal models to study hallucinogen effects has enabled the evaluation of hypotheses regarding the neurochemical substrates of their action (reviewed: Fantegrossi et al. 2008a; Halberstadt 2015). The major contribution of 5-HT2A receptor agonism to the effects of hallucinogens was first demonstrated using animal behavioral models (Leysen et al. 1982; Glennon et al. 1984; Wing et al. 1990). Importantly, this mechanism is consistent with evidence from human studies using pharmacological antagonism (Vollenweider et al. 1998; Valle et al. 2016; Kraehenmann et al. 2017; Preller et al. 2017). Animal behavioral models used to study hallucinogens can be divided into two groups: (a) models that test for behavior(s) that are analogous to hallucinogen effects in humans (high face validity); and (b) models that test for a behavior that does not have a direct human counterpart but appear to involve the same pharmacological mechanism of action as the hallucinogenic effects in humans (high construct validity) (Halberstadt and Geyer 2010). Animal models of hallucinogen effects can be further classified depending on whether they produce qualitative or quantitative data. All animal hallucinogen models provide a qualitative assessment of activity (i.e., does a test compound mimic the action of serotonergic hallucinogens?). However, certain models can also be used to assess the relative potencies of hallucinogens in a manner that has considerable predictive validity with respect to their potency in humans. The availability of animal models to assess the in vivo potency of hallucinogens is especially important for studies of their structure-activity relationships (SAR).
Drug discrimination is a cross-species paradigm that is used to classify pharmacological agents based on perceived similarities in interoceptive stimulus effects. In this paradigm, animals are trained to press one of two levers after they receive a training drug, and must press the other lever after they receive saline (or another vehicle control). Once animals are trained to discriminate the training drug from vehicle, challenge experiments can be conducted with other drugs to evaluate whether their interoceptive stimulus effects are perceived as being similar to the cue produced by the training drug. Test agents that induce responding predominantly on the drug lever are said to fully substitute for the training drug. This assay has high pharmacological specificity and can distinguish compounds with different mechanisms of action. Many hallucinogens are capable of serving as discriminative stimuli in rats; their stimulus cues appear to be relatively uniform in nature because members of this drug class produce cross-generalization in drug discrimination studies (Glennon et al. 1983a; Winter et al. 2007; Nichols 2018). In addition to assessing whether test agents produce hallucinogen-like stimulus effects, drug discrimination studies can also be used to compare the relative potencies of hallucinogens. According to Glennon and colleagues, the potency of hallucinogens in the drug discrimination paradigm is significantly correlated with their potency in humans (Glennon et al. 1982a, 1983a, 1983 c). Hence, the drug discrimination paradigm has shown considerable utility in studies of hallucinogen SAR (Glennon et al. 1983a; Nichols 2018).
There are some potential drawbacks associated with the use of drug discrimination methodologies to study hallucinogens. Drug discrimination studies require the training drug to be administered repeatedly, which has limited relevance to typical hallucinogen use patterns in humans and can potentially result in neurochemical and behavioral adaptations (Benneyworth et al. 2008; Marona-Lewicka et al. 2011). Furthermore, drug discrimination studies cannot always reliably distinguish between hallucinogens and non-hallucinogenic 5-HT2A agonists such as lisuride (White and Appel 1982a; Glennon and Hauck 1985; Fiorella et al. 1995a). Some hallucinogens also reportedly have particularly steep dose-response functions in drug discrimination studies, with complete generalization occurring only within a narrow range of doses, making it difficult to detect full-substitution (Glennon et al. 1983a). The latter phenomenon may explain why hallucinogens sometimes fail to elicit full-substitution in rats trained to discriminate hallucinogens (Koerner and Appel 1982; Monte et al. 1997; Helsley et al. 1998; Fantegrossi et al. 2008b; Gatch et al. 2011, 2017). Responses can also be influenced by additional behavioral changes mediated by off-target pharmacological effects of a test compound, complicating analysis.
Another popular hallucinogen behavioral model is known as the head-twitch response (HTR). The HTR is a high-frequency paroxysmal head rotation that occurs in rats and mice after 5-HT2A receptor activation (Halberstadt et al. 2011; Canal and Morgan 2012). The HTR is commonly used as a behavioral proxy in rodents for human hallucinogen effects because it is one of only a few behaviors that can reliably distinguish hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists (Gonzalez-Maeso et al. 2007; Halberstadt and Geyer 2013). For example, LSD induces head twitches in mice and rats, whereas lisuride does not induce the behavior despite acting as a 5-HT2A agonist (Gonzalez-Maeso et al. 2007). Although the HTR is usually assessed by direct observation, we have developed an electronic assessment technique that can detect the behavior with high sensitivity and specificity (Halberstadt and Geyer 2013). These methods have shown great utility for studying novel hallucinogens (Halberstadt and Geyer 2014; Nichols et al. 2015; Brandt et al. 2016, 2017b, 2019; Halberstadt et al. 2016, 2017a, 2019a, 2019b, 2019c).
Given the potential complexities associated with the drug discrimination paradigm, there is a need for other animal behavioral models that can be used to study the SAR of serotonergic hallucinogens. It may be possible to use the HTR assay for this purpose, but it is not clear whether the paradigm can provide a reliable quantitative assessment of hallucinogen potency. Therefore, studies were conducted to evaluate whether a correlation exists between the potency of hallucinogens in the mouse HTR paradigm and reported potencies (behavioral and subjective) in other species, specifically rats and humans. One set of experiments tested whether a correlation exists between HTR potency in mice and reported hallucinogenic potency in humans. Additional studies were conducted to assess the relationship between ED50 values in HTR and drug discrimination studies. The results support the use of the HTR paradigm in studies investigating the SAR of hallucinogens; future work will be undertaken to test whether the generated linear regression models can be used to predict human potency based on HTR data
METHODS
Animals
Male C57BL/6J mice (6–8 weeks old) obtained from Jackson Laboratories (Bar Harbor, ME, USA) were housed in a vivarium at the University of California San Diego, an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-approved animal facility that meets all federal and state requirements for care and treatment of laboratory animals. Mice were housed up to four per cage in a climate-controlled room on a reverse-light cycle (lights on at 1900 h, off at 0700 h) and were provided with ad libitum access to food and water, except during behavioral testing. Testing was conducted between 1000 and 1800 h. All animal experiments were carried out in accordance with National Institute of Health guidelines and were approved by the University of California San Diego Institutional Animal Care and Use Committee.
Drugs
N,N-Diethyltryptamine (DET) fumarate, 2,5-dimethoxy-4-methylamphetamine (DOM) hydrochloride, N,N-dimethyltryptamine (DMT) fumarate, N,N-dipropyltryptamine (DPT) hydrochloride, 4-ethyl-2,5-dimethoxyamphetamine (DOET) hydrochloride, R-(-)-4-iodo-2,5-dimethoxyamphetamine (R-(-)-DOI) hydrochloride, 3,4-methylenedioxyamphetamine (MDA) hydrochloride, R-(-)-3,4-methylenedioxyamphetamine (R-(-)-MDA) hydrochloride, and 2,5-dimethoxy-4-nitroamphetamine (DON) hydrochloride were obtained from the National Institute on Drug Abuse (Rockville, MD, USA). 2,5-Dimethoxy-α-ethyl-4-methylphenylethylamine (α-Et-2C-D, ariadne) hydrochloride was obtained from Sigma Chemical Co. (St. Louis, MO, USA). 5-Methoxy-α-methyltryptamine hydrochloride was obtained from the NIMH Chemical Synthesis and Drug Supply Program (RTI International, Research Triangle Park, NC, USA). 4-Butyl-2,5-dimethoxyamphetamine (DOBU) freebase and N-(2-hydroxybenzyl)-4-iodo-2,5-dimethoxyphenethylamine (25I-NBOH) hydrochloride were obtained from Cayman Chemical (Ann Arbor, MI, USA). 4-Chloro-2,5-dimethoxyamphetamine (DOC) hydrochloride, N,N-diisopropyltyptamine (DIPT) hydrochloride, N-(2-methoxybenzyl)-2,5-dimethoxy-4-methylphenethylamine (25D-NBOMe) hydrochloride were available from previous studies conducted in our laboratories. The identity and analytical purity of the test substances were confirmed by mass spectrometry and nuclear magnetic resonance spectroscopy. Test substances were dissolved in isotonic saline and injected intraperitoneally (IP) at a volume of 5 mL/kg.
Head-twitch response studies
The HTR was assessed using a head-mounted magnet and a magnetometer detection coil (Halberstadt and Geyer 2013, 2014). Briefly, mice were anesthetized, a small incision was made in the scalp, and a small neodymium magnet was attached to the dorsal surface of the cranium using dental cement. Following a two-week recovery period, HTR experiments were carried out in a well-lit room with at least 7 days between sessions to avoid carryover effects. Test compounds were injected immediately prior to testing and then HTR activity was recorded in a glass cylinder surrounded by a magnetometer coil for 30 min. Coil voltage was low-pass filtered (2–10 kHz cutoff frequency), amplified, digitized (20 kHz sampling rate, 16-bit ADC resolution), and saved to disk using a Powerlab/8SP data acquisition system with LabChart software ver. 7.3.2 (ADInstruments, Colorado Springs, CO, USA), then filtered off-line (40–200 Hz band-pass). Head twitches were identified by trained personnel based on the following criteria: 1) sinusoidal wavelets; 2) evidence of at least three sequential head movements (usually exhibited as bipolar peaks) with frequency ≥ 40 Hz; 3) amplitude exceeding the level of background noise; 4) duration < 0.15 s; and 5) stable coil voltage immediately preceding and following each response.
After magnet implantation, mice were tested in multiple HTR experiments, for up to 4–5 months. Repeated administration of hallucinogens at weekly intervals does not produce tolerance in the HTR paradigm (Gewirtz and Marek 2000; Rangel-Barajas et al. 2014; Smith et al. 2014). We have confirmed that experimental results obtained using these procedures are stable and replicable over time, both within single cohorts of mice and across multiple independent cohorts (unpublished observations). Experiments were performed between-subjects, with pseudorandomized group assignments, which further reduces the likelihood of carryover effects.
Data analysis
Head-twitch counts were analyzed using one-way analyses of variance (ANOVA). Post hoc pairwise comparisons were performed using Dunnett’s test. Significance was demonstrated by surpassing an α-level of 0.05. Half maximal effective doses (ED50 values) and 95% confidence intervals (95% CI) for HTR dose-response experiments were calculated by nonlinear regression. Linear regression was used to assess the relationship between ED50 values in HTR experiments and potency measures in other species. Statistical and regression analyses were performed using Prism 7.00 (GraphPad Software, San Diego, CA, USA).
RESULTS
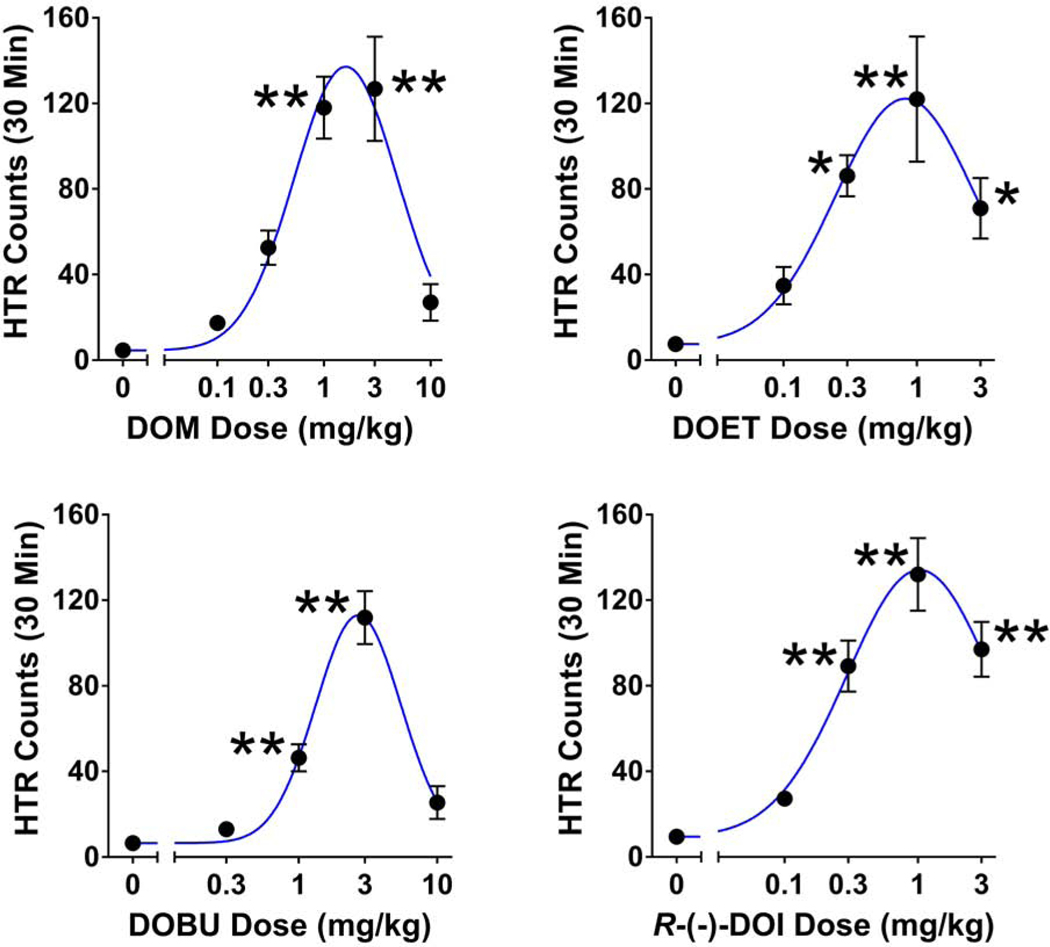
To date, we have tested a variety of hallucinogens in the HTR paradigm (Halberstadt and Geyer 2013, 2014; Brandt et al. 2017b; Klein et al. 2018; Halberstadt et al. 2019a, 2019b). Additional studies were conducted to increase the size of our existing data set. As shown in Table 1, hallucinogens from the phenylalkylamine (DOM, DOET, DOBU, DON, DOC, R-(-)-DOI, MDA, R-(-)-MDA, α-Et-2C-D, 25D-NBOMe, and 25I-NBOH) and tryptamine (DMT, DET, DPT, DIPT, and 5-MeO-AMT) structural classes were tested in male C57BL/6J mice and were found to induce head twitches. Similar to other hallucinogens (Fantegrossi et al. 2006, 2008b, 2010; Halberstadt et al. 2019a, 2019b), the responses observed in most of the experiments were non-monotonic with a marked response decrement occurring at high doses (see Figure 1).
Table 1.
Summary of the head twitch response (HTR) data from dose-response experiments.
| Compound | ANOVA | Time (min) | Dose (mg/kg) | N | HTR Counts (Mean ± SEM) | ED50 mg/kg (95% CI) | ED50 μmol/kg (95% CI) |
|---|---|---|---|---|---|---|---|
| DOMa | F(5,23)=17.1, p<0.0001 | 30 | 0 | 5 | 4.6 ± 0.9 | 0.43 (0.30–0.62) | 1.75 (1.22–2.52) |
| 0.1 | 5 | 17.4 ± 1.7 | |||||
| 0.3 | 5 | 52.6 ± 8.0 | |||||
| 1 | 5 | 118.0 ± 14.5 ** | |||||
| 3 | 5 | 126.8 ± 24.4 ** | |||||
| 10 | 4 | 27.0 ± 8.6 | |||||
|
| |||||||
| DOETa | F(4,22)=7.68, p=0.0005 | 30 | 0 | 6 | 7.5 ± 1.7 | 0.20 (0.11–0.36) | 0.77 (0.43–1.37) |
| 0.1 | 5 | 34.8 ± 8.7 | |||||
| 0.3 | 5 | 86.2 ± 9.6 * | |||||
| 1 | 6 | 122.0 ± 29.3 ** | |||||
| 3 | 5 | 71.0 ± 14.2 * | |||||
|
| |||||||
| DOBUc | F(4,26)=36.68, p<0.0001 | 30 | 0 | 7 | 6.6 ± 1.9 | 1.17 (0.99–1.38) | 4.64 (3.92–5.50) |
| 0.3 | 6 | 13.0 ± 2.5 | |||||
| 1 | 6 | 46.3 ± 6.3 ** | |||||
| 3 | 6 | 111.8 ± 12.4 ** | |||||
| 10 | 6 | 25.5 ± 7.7 | |||||
|
| |||||||
| DOCa | F(3,18)=23.92, p<0.0001 | 30 | 0 | 6 | 8.2 ± 1.4 | 0.32 (0.21–0.47) | 1.19 (0.80–1.77) |
| 0.3 | 5 | 64.0 ± 2.0 ** | |||||
| 1 | 6 | 125.3 ± 13.5 ** | |||||
| 3 | 5 | 89.8 ± 16.3 ** | |||||
|
| |||||||
| R-(-)-DOIa | F(4,23)=25.14, p<0.0001 | 30 | 0 | 7 | 9.4 ± 1.7 | 0.24 (0.17–0.33) | 0.66 (0.47–0.93) |
| 0.1 | 5 | 27.2 ± 3.1 | |||||
| 0.3 | 6 | 89.2 ± 12.0 ** | |||||
| 1 | 5 | 132.0 ± 17.0 ** | |||||
| 3 | 5 | 97.0 ± 12.8 ** | |||||
|
| |||||||
| DONa | F(4,20)=7.65, p=0.0007 | 30 | 0 | 5 | 6.4 ± 2.3 | 1.31 (0.70–2.43) | 4.72 (2.54–8.77) |
| 0.3 | 5 | 17.4 ± 3.4 | |||||
| 1 | 5 | 43.0 ± 1.2 | |||||
| 3 | 5 | 102.8 ± 6.9 ** | |||||
| 10 | 5 | 96.2 ± 34.9 ** | |||||
|
| |||||||
| MDAa | F(4,22)=3.53, p=0.0227 | 30 | 0 | 6 | 6.2 ± 1.4 | 1.46 (0.72–2.98) | 6.77 (3.33–13.8) |
| 0.3 | 5 | 6.6 ± 1.2 | |||||
| 1 | 5 | 9.6 ± 1.1 | |||||
| 3 | 6 | 16.7 ± 2.4 * | |||||
| 10 | 5 | 15.0 ± 5.1 | |||||
|
| |||||||
| R-(-)-MDAa | F(4,23)=8.11, P=0.0003 | 30 | 0 | 6 | 7.8 ± 1.4 | 1.45 (0.82–2.54) | 6.71 (3.82–11.8) |
| 0.3 | 5 | 8.6 ± 1.5 | |||||
| 1 | 6 | 15.5 ± 3.2 | |||||
| 3 | 6 | 30.0 ± 6.2 ** | |||||
| 10 | 5 | 27.6 ± 2.8 ** | |||||
|
| |||||||
| α-Et-2C-Da | F(4,23)=3.95, P=0.0139 | 30 | 0 | 5 | 9.4 ± 2.6 | 4.09 (2.70–6.22) | 15.8 (10.4–23.9) |
| 3 | 6 | 14.2 ± 4.1 | |||||
| 6 | 5 | 25.4 ± 4.9 * | |||||
| 12 | 6 | 26.0 ± 2.2 * | |||||
| 24 | 6 | 14.5 ± 4.0 | |||||
|
| |||||||
| 25D-NBOMea | F(5,28)=10.57, P<0.0001 | 30 | 0 | 6 | 6.2 ± 0.8 | 0.23 (0.12–0.43) | 0.64 (0.34–1.22) |
| 0.03 | 5 | 12.2 ± 2.9 | |||||
| 0.1 | 6 | 39.2 ± 7.5 | |||||
| 0.3 | 6 | 45.0 ± 12.5 | |||||
| 1 | 6 | 96.7 ± 17.2 ** | |||||
| 3 | 5 | 85.6 ± 18.9 ** | |||||
|
| |||||||
| 25I-NBOHa | F(5,24)=8.90, P<0.0001 | 30 | 0 | 5 | 4.4 ± 1.4 | 0.085 (0.055–0.132) | 0.19 (0.12–0.29) |
| 0.03 | 5 | 22.8 ± 3.1 | |||||
| 0.1 | 5 | 48.8 ± 4.7 * | |||||
| 0.3 | 5 | 95.6 ± 14.2 ** | |||||
| 1 | 5 | 64.0 ± 12.6 ** | |||||
| 3 | 5 | 44.4 ± 17.0 | |||||
|
| |||||||
| 5-MeO-AMTa | F(4,22)=10.65, p<0.0001 | 30 | 0 | 6 | 4.3 ± 0.8 | 0.53 (0.25–1.11) | 2.21 (1.06–4.60) |
| 0.1 | 5 | 9.4 ± 2.8 | |||||
| 0.3 | 5 | 11.0 ± 3.0 | |||||
| 1 | 6 | 45.0 ± 11.4 ** | |||||
| 3 | 5 | 50.4 ± 7.4 ** | |||||
|
| |||||||
| DMTb | F(4,21)=41.25, p<0.0001 | 30 | 0 | 6 | 6.5 ± 2.4 | 1.54 (1.08–2.19) | 5.05 (3.55–7.19) |
| 0.3 | 5 | 11.4 ± 1.8 | |||||
| 1 | 5 | 25.2 ± 3.5 * | |||||
| 3 | 5 | 65.6 ± 8.6 ** | |||||
| 10 | 5 | 76.4 ± 6.2 ** | |||||
|
| |||||||
| DETb | F(5,25)=6.20, p=0.0007 | 20 | 0 | 6 | 3.5 ± 0.5 | 2.28 (1.57–3.30) | 6.85 (4.72–9.93) |
| 0.625 | 5 | 4.0 ± 1.5 | |||||
| 1.25 | 5 | 4.6 ± 1.9 | |||||
| 2.5 | 5 | 13.0 ± 3.6 * | |||||
| 5 | 5 | 18.8 ± 3.0 ** | |||||
| 10 | 5 | 13.0 ± 3.7 * | |||||
|
| |||||||
| DPTa | F(4,20)=4.02, p=0.0150 | 20 | 0 | 5 | 4.4 ± 0.5 | 1.90 (1.14–3.19) | 6.78 (4.04–11.4) |
| 0.625 | 5 | 6.4 ± 1.5 | |||||
| 1.25 | 5 | 7.8 ± 2.7 | |||||
| 2.5 | 5 | 26.2 ± 7.1 * | |||||
| 5 | 5 | 24.8 ± 9.0 * | |||||
|
| |||||||
| DIPTa | F(4,22)=8.9, p=0.0002 | 30 | 0 | 6 | 6.0 ± 0.4 | 2.40 (1.67–3.44) | 8.54 (5.96–12.2) |
| 1.25 | 5 | 16.6 ± 4.3 | |||||
| 2.5 | 5 | 27.6 ± 7.1 | |||||
| 5 | 6 | 53.5 ± 5.3 ** | |||||
| 10 | 5 | 40.8 ± 12.0 ** | |||||
p < 0.05
p < 0.01, significant difference from the vehicle control group (Dunnett’s test).
Hydrochloride salt
fumarate salt
freebase.
FIGURE 1.
Effect of 2,5-dimethoxy-4-methylamphetamine (DOM), 4-ethyl-2,5-dimethoxyamphetamine (DOET), 4-butyl-2,5-dimethoxyamphetamine (DOBU), and R-(-)-4-iodo-2,5-dimethoxyamphetamine (R-(-)-DOI) on the head twitch response (HTR). Data are presented as group means ± SEM for the entire 30-min test session. The individual data points for each compound are presented in Table S1. *p < 0.05, **p < 0.01, significant difference from the vehicle control group (Dunnett’s test).
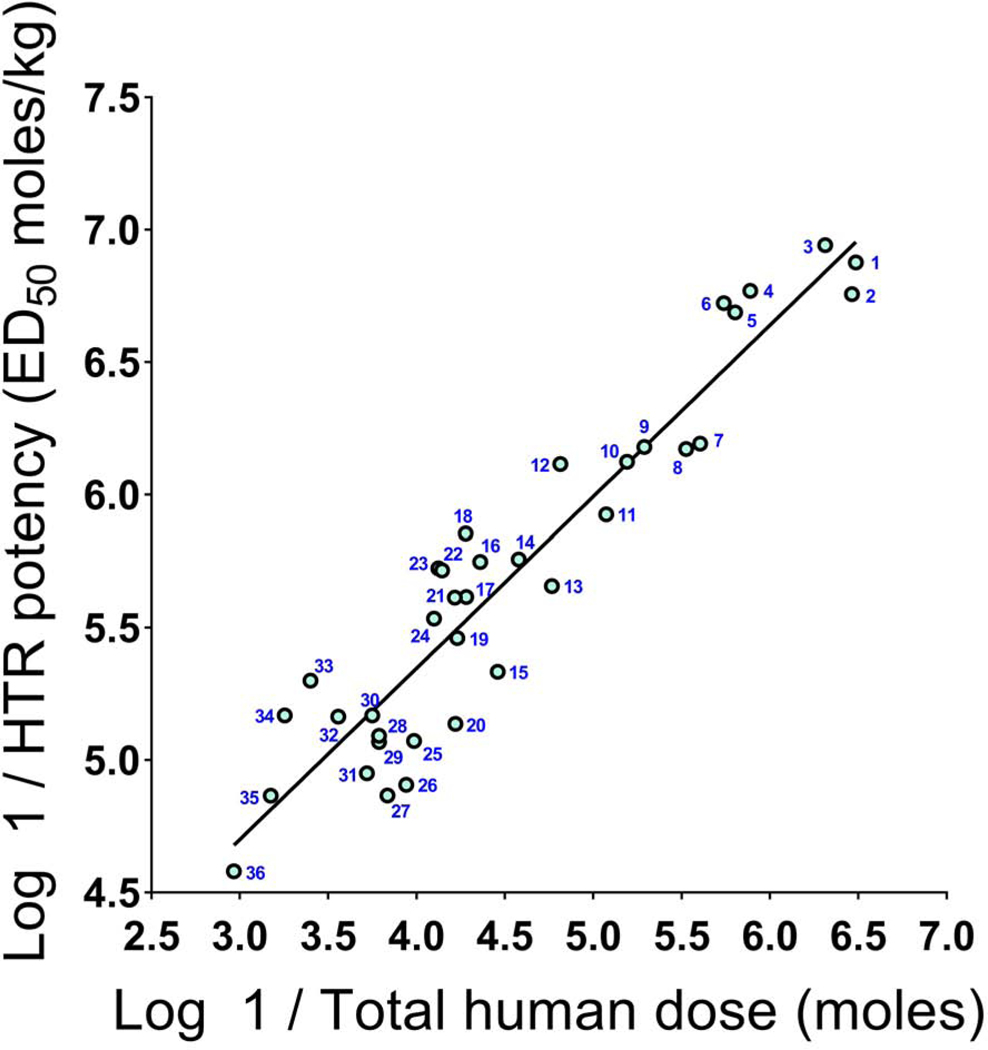
A linear regression analysis was performed to determine whether a relationship exists between the potency of hallucinogens in humans and their effects on head-twitch. In our HTR studies with serotonergic hallucinogens, potency is assessed by calculating the dose required to induce a half-maximal response (ED50), which facilitates potency comparisons between compounds. In addition to the HTR data in Table 1, relevant data for other compounds are available from our published and unpublished studies (see Table 2). The human potency data used in the analysis (Table 3) were collected from several sources, including clinical trials, results published by Dr. Alexander Shulgin and Dr. Daniel Trachsel, as well as from websites such as erowid.com and psychonautwiki.org. Human hallucinogenic potency data from those sources are typically reported as a range of effective doses; in those cases, the arithmetic mean of the dose range was calculated and then converted to a total dose in moles. For 36 hallucinogens, a statistically significant positive correlation was found between HTR-derived ED50 values and their potency in humans (r = 0.9448 [95% CI: 0.8937–0.9717], p < 0.0001; Figure 2). Importantly, the hallucinogens included in this analysis cover structurally distinct compounds and include a wide range of human potencies, spanning almost four orders of magnitude.
Table 2:
Summary of the head twitch response (HTR) data that were extracted from our previous publications.
| Compound | ED50 | Reference | |
|---|---|---|---|
| AL-LAD | 74.2 μg/kg | 174.9 nmol/kg | (Brandt et al. 2017b) |
| 2C-B | 0.72 mg/kg | 2.43 μmol/kg | (Halberstadt et al. 2019b) |
| 2C-B-FLY | 0.57 mg/kg | 1.79 μmol/kg | (Halberstadt et al. 2019b) |
| 3C-E | 2.36 mg/kg | 8.54 μmol/kg | (Halberstadt et al. 2019a) |
| 2C-I | 0.83 mg/kg | 2.42 μmol/kg | (Halberstadt and Geyer 2014) |
| 3C-P | 2.45 mg/kg | 8.47 μmol/kg | (Halberstadt et al. 2019a) |
| DOB | 0.23 mg/kg | 0.75 μmol/kg | (Halberstadt et al. 2019b) |
| DOB-DFLY | 68 μg/kg | 205 nmol/kg | (Halberstadt et al. 2019b) |
| DOB-FLY | 0.22 mg/kg | 0.67 μmol/kg | (Halberstadt et al. 2019b) |
| Escaline | 2.94 mg/kg | 11.2 μmol/kg | (Halberstadt et al. 2019a) |
| 4-HO-DET | 0.44 mg/kg | 1.89 μmol/kg | In preparation |
| 4-HO-DIPT | 1.03 mg/kg | 3.46 μmol/kg | In preparation |
| 4-HO-MIPT | 0.85 mg/kg | 2.92 μmol/kg | In preparation |
| 4-HO-MPT | 0.67 mg/kg | 1.92 μmol/kg | In preparation |
| 25I-NBOMe | 78 μg/kg | 0.17 μmol/kg | (Halberstadt and Geyer 2014) |
| LSD | 52.9 μg/kg | 132.8 nmol/kg | (Halberstadt and Geyer 2013) |
| SS-LSZ | 52 μg/kg | 114.2 nmol/kg | (Brandt et al. 2017b) |
| MEM | 3.75 mg/kg | 13.6 μmol/kg | (Halberstadt et al. 2019a) |
| 5-MeO-DALT | 2.25 mg/kg | 7.3 μmol/kg | (Klein et al. 2018) |
| Mescaline | 6.51 mg/kg | 26.3 μmol/kg | (Halberstadt et al. 2019a) |
| MIPLA | 136.4 μg/kg | 421.7 nmol/kg | (Halberstadt et al. 2019c) |
| Proscaline | 2.23 mg/kg | 8.09 μmol/kg | (Halberstadt et al. 2019a) |
| Psilocybin | 0.38 mg/kg | 1.40 μmol/kg | In preparation |
| TMA | 3.57 mg/kg | 13.6 μmol/kg | (Halberstadt et al. 2019a) |
| TMA-2 | 2.79 mg/kg | 12.4 μmol/kg | (Halberstadt et al. 2019a) |
Table 3.
Human potency data for selected hallucinogens.
| # | Compound | Human hallucinogenic potency (dose range in mg) | Arithmetic mean of the dose range | Salt form | Routea | Reference | |
|---|---|---|---|---|---|---|---|
| 1 | LSD | 0.06 – 0.2 | 0.13 mg | 326 nmol | Hemitartrate | PO | (Shulgin and Shulgin 1997) |
| 2 | AL-LAD | 0.08 – 0.16 | 0.12 mg | 343 nmol | Hemitartrate | PO | (Shulgin and Shulgin 1997) |
| 3 | SS-LSZ | 0.1 – 0.3 | 0.2 mg | 487 nmol | Hemitartrate | PO | Erowid.com “common” |
| 4 | 25I-NBOMe | 0.5 – 0.7 | 0.6 mg | 1.29 μmol | Hydrochloride | SL or BUC | Psychonautwiki.org “common” |
| 5 | DOB-DFLY | 0.2 – 0.8 | 0.5 mg | 1.51 μmol | Hydrochloride | PO | (Trachsel et al. 2013) |
| 6 | 25I-NBOH | 0.5 – 0.9 | 0.7 mg | 1.83 μmol | Hydrochloride | SL or BUC | Psychonautwiki.org “common” |
| y | 25D-NBOMe | 0.8 – 1 | 0.9 mg | 2.49 μmol | Hydrochloride | SL or BUC | Psychonautwiki.org “common” |
| s | DOB-FLY | 1 | 2.99 μmol | Hydrochloride | PO | (Shulgin et al. 2011) | |
| 9 | £-(-)-DOI | 1 – 2.3 | 1.65 mg | 5.14 μmol | Hydrochloride | PO | (Shulgin and Shulgin 1991) |
| 10 | DOB | 1 – 3 | 2 mg | 6.44 μmol | Hydrochloride | PO | (Shulgin and Shulgin 1991) |
| 11 | DOC | 1.5 – 3 | 2.25 mg | 8.45 μmol | Hydrochloride | PO | (Shulgin and Shulgin 1991) |
| 12 | DOET | 2 – 6 | 4 mg | 15.4 μmol | Hydrochloride | PO | (Shulgin and Shulgin 1991) |
| 13 | 5-MeO-AMT | 2.5 – 4.5 | 3.5 mg | 17.1 μmol | Freebase | PO | (Shulgin and Shulgin 1997) |
| 14 | DOM | 3 – 10 | 6.5 mg | 26.5 μmol | Hydrochloride | PO | (Shulgin and Shulgin 1991) |
| 15 | DOBU | 10 | 34.7 μmol | Hydrochloride | PO | (Braun et al. 1978) | |
| 16 | 2C-B-FLY | 10 – 18 | 14 mg | 43.7 μmol | Hydrochloride | PO | Psychonautwiki.org “common” |
| 17 | 2C-I | 14 – 22 | 18 mg | 52.4 μmol | Hydrochloride | PO | (Shulgin and Shulgin 1991) |
| 18 | Psilocybin | 10 – 20 | 15 mg | 52.8 μmol | Freebase | PO | (Shulgin and Shulgin 1997) |
| 19 | 4-HO-DIPT | 15 – 20 | 17.5 mg | 59.0 μmol | Hydrochloride | PO | (Shulgin and Shulgin 1997) |
| 20 | 5-MeO-DALT | 12 – 25 | 18.5 mg | 60.3 μmol | Hydrochloride | PO | Erowid.com “common” |
| 21 | 2C-B | 12 – 24 | 18 mg | 60.7 μmol | Hydrochloride | PO | (Shulgin and Shulgin 1991) |
| 22 | 4-HO-MPT | 20 – 30 | 25 mg | 71.8 μmol | Fumarate | PO | Psychonautwiki.org“common” |
| 23 | 4-HO-DET | 10 – 25 | 17.5 mg | 75.3 μmol | Freebase | PO | (Shulgin and Shulgin 1997) |
| 24 | 4-HO-MIPT | 12 – 25 | 18.5 mg | 79.6 μmol | Freebase | PO | (Shulgin and Shulgin 1997) |
| 25 | 3C-P | 20 – 40 | 30 mg | 104 μmol | Hydrochloride | PO | Tripsit.me “common” |
| 26 | TMA-2 | 20 – 40 | 30 mg | 115 μmol | Hydrochloride | PO | (Shulgin and Shulgin 1991) |
| 27 | MEM | 20 – 50 | 35 mg | 146 μmol | Hydrochloride | PO | (Shulgin and Shulgin 1991) |
| 28 | Proscaline | 30 – 60 | 45 mg | 163 μmol | Hydrochloride | PO | (Shulgin and Shulgin 1991) |
| 29 | 3C-E | 30 – 60 | 45 mg | 163 μmol | Hydrochloride | PO | (Shulgin and Shulgin 1991) |
| 30 | DPT | 30 – 70 | 50 mg | 178 μmol | Freebase | IM | (Faillace et al. 1967; Soskin et al. 1973) |
| 31 | Escaline | 40 – 60 | 50 mg | 191 μmol | Hydrochloride | PO | (Shulgin and Shulgin 1991) |
| 32 | DET | 50 – 70 | 60 mg | 277 μmol | Freebase | IM | (Boszormenyi et al. 1959; Szara et al. 1966) |
| 33 | DMT | 50 – 100 | 75 mg | 398 μmol | Freebase | IM | (Szara 1957; Gillin et al. 1976) |
| 34 | MDA | 80 – 160 | 120 mg | 556 μmol | Hydrochloride | PO | (Shulgin and Shulgin 1991) |
| 35 | TMA | 100 – 250 | 175 mg | 669 μmol | Hydrochloride | PO | (Shulgin and Shulgin 1991) |
| 36 | Mescaline | 178 – 356 | 267 mg | 1.08 mmol | Hydrochloride | PO | (Shulgin and Shulgin 1991) |
BUC, buccal; IM, intramuscular; PO, orally; SL, sublingually.
FIGURE 2.
Hallucinogen potencies in humans and mice are robustly correlated (r = 0.9448). The numbers correspond to the agents in Table 3. Potencies in humans are plotted as log 1/total hallucinogenic dose (in moles); potencies in the mouse head-twitch response assay are plotted as log 1/ED50 (in moles/kg).
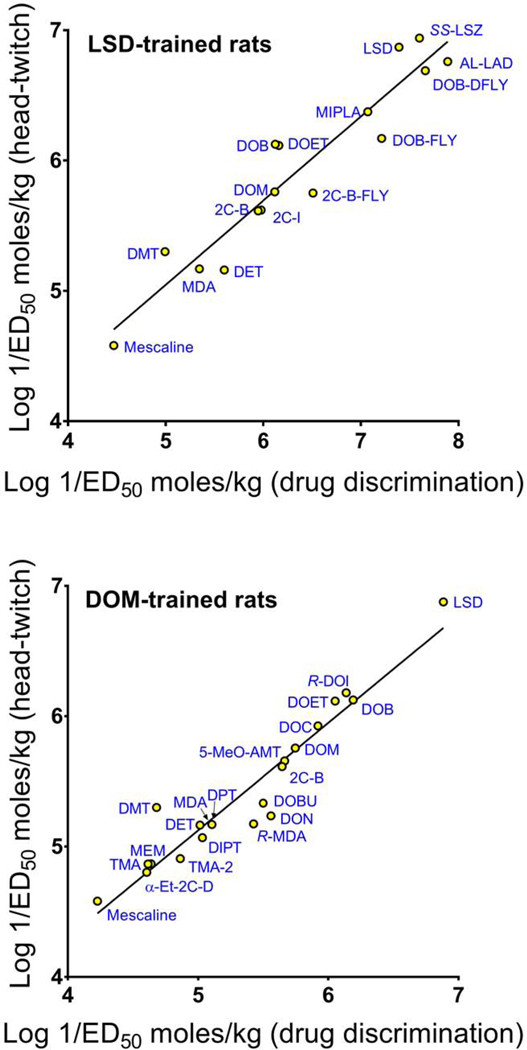
An additional series of experiments evaluated whether HTR-derived ED50 values are correlated with reported ED50 values from rat drug discrimination studies. The rat drug discrimination literature contains an extensive dataset of stimulus generalization ED50 values for hallucinogens. One regression analysis was performed using data from rat drug discrimination studies conducted by Nichols and colleagues, who used 0.08 mg/kg LSD as the training drug for the evaluation of the 16 test drugs in Table 4. Although typically a single ED50 value was available for each compound, multiple values were occasionally reported in the literature; in those cases, the arithmetic mean was calculated. The linear regression analysis confirmed that ED50 values from HTR studies are robustly and significantly correlated (r = 0.9484 [95% CI: 0.8542–0.9823], n = 16, p < 0.0001) with drug discrimination ED50 values from LSD-trained rats (Figure 3).
Table 4.
Median effective doses (ED50) for hallucinogens in rats trained to discriminate 0.08 mg/kg (+)- lysergic acid diethylamide (LSD) from saline.
| Drug | ED50 (μmol/kg) | Reference |
|---|---|---|
| AL-LAD | 0.013 | (Hoffman and Nichols 1985) |
| 2C-B | 1.13 | (Juncosa et al. 2013) |
| 2C-B-FLY | 0.31 | (Monte et al. 1996) |
| 2C-I | 1.04 | (Nichols et al. 1994) |
| DOB | 1.06 | (Chambers et al. 2003) |
| 1.12 | (Nichols et al. 1994) | |
| (average = 1.09) | ||
| DOB-DFLY | 0.022 | (Parker et al. 1998) |
| DOB-FLY | 0.061 | (Monte et al. 1996) |
| DOET | 0.69 | (Parker 1998) |
| DET | 2.53 | (Blair et al. 2000) |
| DMT | 10.2 | (Blair et al. 1999) |
| DOM | 0.61 | (Oberlender et al. 1995) |
| 0.89 | (Nichols et al. 1991) | |
| (average = 0.75) | ||
| LSD | 0.0275 | (Oberlender et al. 1992) |
| 0.037 | (Blair et al. 1999) | |
| 0.040 | (Monte et al. 1996) | |
| 0.045 | (Nichols et al. 2002) | |
| 0.046 | (Hoffman and Nichols 1985) | |
| 0.048 | (Monte et al. 1995) | |
| (average = 0.0406) | ||
| SS-LSZ | 0.025 | (Nichols et al. 2002) |
| MDA | 4.52 | (Parker 1998) |
| Mescaline | 34 | (McLean et al. 2006) |
| MIPLA | 0.085 | (Huang et al. 1994) |
FIGURE 3.
Hallucinogen potencies in mice and rats are robustly correlated. (TOP) Hallucinogen potencies in the mouse head-twitch response (HTR) assay are correlated (r = 0.9484) with published ED50 values from rat drug discrimination studies using 0.08 mg/kg LSD as the training drug. (BOTTOM) Hallucinogen potencies in the mouse HTR assay are correlated (r = 0.9564) with published ED50 values from rat drug discrimination studies using 1 mg/kg DOM as the training drug. The drug discrimination data are taken from Tables 4 and 5, respectively. Potencies in mice and rats are plotted as log 1/ED50 (in moles/kg).
Hallucinogens exhibit varying degrees of receptor selectivity and those pharmacological differences can influence their interoceptive stimulus effects, meaning that certain training drugs may produce idiosyncratic results. In contrast to LSD, which produces a compound discriminative stimulus in rats, the stimulus effects of DOM are less complex (Fiorella et al. 1995b). Although strong evidence supports the hypothesis that 5-HT2A activation is an essential component of the stimulus cues evoked by DOM and LSD (Glennon et al. 1983 d, 1984), dopamine D2 receptors may play a secondary role in the response to LSD (Appel et al. 1982a, 1982b; Meert et al. 1989; Marona-Lewicka et al. 2005). Because the HTR induced by hallucinogens appears to be solely mediated by 5-HT2A activation (Schreiber et al. 1995; Halberstadt et al. 2011) — similar to the discriminative stimulus effects of DOM and in contrast to the LSD stimulus cue — we also evaluated the relationship between ED50 values in the HTR paradigm and in rats trained to discriminate DOM. This analysis was performed using drug discrimination data published by Glennon and colleagues (Table 5), who trained rats to discriminate 1.0 mg/kg DOM from vehicle (Glennon et al. 1983a, 1988), resulting in relevant data for 21 compounds. Similar to the results from LSD-trained rats, HTR ED50 values are robustly and significantly correlated (r = 0.9564 [95% CI: 0.8937–0.9825], n = 21, p < 0.0001) with drug discrimination ED50 values from rats trained to discriminate DOM (Figure 3).
Table 5:
Median effective doses (ED50) for hallucinogens in rats trained to discriminate 1 mg/kg 2,5-dimethoxy-4-methylamphetamine (DOM) from saline.
| Drug | ED50 (mg/kg) | ED50 (μmol/kg) | Reference |
|---|---|---|---|
| 2C-B | 0.67 | 2.26 | (Glennon et al. 1988) |
| DET | 2.45 | 9.69 | (Glennon et al. 1983c) |
| DIPT | 2.60 | 9.26 | (Glennon et al. 1983c) |
| DMT | 5.80 | 20.8 | (Glennon et al. 1983b) |
| DPT | 2.20 | 7.83 | (Glennon et al. 1983c) |
| DOB | 0.20 | 0.64 | (Glennon et al. 1982a) |
| DOBU | 0.91 | 3.16 | (Glennon et al. 1982b) |
| DOC | 1.2 | (Glennon 1989) | |
| DOET | 0.23 | 0.89 | (Glennon et al. 1982a) |
| R-(-)-DOI | 0.26 | 0.73 | (Glennon et al. 1982a) |
| DOM | 0.44 | 1.79 | (Young et al. 1980) |
| DON | 0.76 | 2.75 | (Glennon et al. 1982a) |
| α-Et-2C-D | 6.44 | 24.8 | (Glennon et al. 1983b) |
| LSD | 0.052 | 0.13 | (Glennon et al. 1983c) |
| MDA | 1.68 | 7.79 | (Glennon et al. 1982c) |
| R-(-)-MDA | 0.81 | 3.76 | (Glennon et al. 1982c) |
| MEM | 6.33 | 23.0 | (Glennon et al. 1982a) |
| 5-MeO-AMT | 0.52 | 2.16 | (Glennon et al. 1983a) |
| Mescaline | 14.64 | 59.1 | (Glennon and Young 1982) |
| TMA | 6.34 | 24.2 | (Glennon and Young 1982) |
| TMA-2 | 3.59 | 13.7 | (Glennon et al. 1982a) |
In addition to the correlation between HTR- and drug-discrimination-derived ED50 values, we noted that some of the potency estimates from head-twitch and drug discrimination studies are nearly identical. For example, LSD induces head twitches in mice with an ED50 of 52.9 μg/kg (132.8 nmol/kg) (Halberstadt and Geyer 2013), whereas LSD reportedly substitutes in DOM-trained rats with an ED50 of 52 μg/kg (130.5 nmol/kg) (Glennon et al. 1983c). Likewise, the potency of DOM in the HTR experiment from Table 1 (ED50 = 1.75 (μmol/kg; Table 1) is virtually identical with the potency in the drug discrimination experiment summarized in Table 5 (ED50 = 1.79 μmol/kg; Young et al. 1980). To quantify the degree of similarity between drug discrimination and head-twitch data, normalized difference scores were calculated using the formula:
where ED50 HTR is the median effective molar dose for head-twitch and ED50 DD is the median effective molar dose for drug discrimination. For the 21 compounds tested in DOM-trained rats, the average normalized difference score was 31.6 ± 5.7% (mean ± SEM; range: 1.8% to 78.6%). For the 16 compounds tested in LSD-trained rats, the average normalized difference score was 330.2 ± 94.4% (range: 22.7% to 1,215.4%). Although the congruence was clearly higher when DOM was used as the training drug compared to LSD, it is difficult to compare the two data sets directly because their composition is heterogeneous. To address this confound, the analyses were repeated using only the subset of compounds that were present in both data sets (2C-B, DET, DMT, DOB, DOET, DOM, LSD, MDA, and mescaline); for those nine compounds, the difference scores were lower in DOM-trained rats (27.3 ± 9.4%) compared to LSD-trained rats (99.8 ± 25.7%; t16 = 2.608, p = 0.019). We also examined whether the three potency estimates for those nine compounds are correlated using linear regression; the HTR data are correlated with the drug discrimination data obtained using LSD (r = 0.9544 [95% CI: 0.7926–0.9906]) and DOM (r = 0.975 [95% CI: 0.8819–0.9949]) as training drugs. As expected, the ED50 values from LSD- and DOM-trained rats are highly correlated (r = 0.9722 [95% CI: 0.8692–0.9943]). Notably, however, the strength of the correlation between the HTR and drug discrimination data is just as strong as the correlation between the two sets of drug discrimination data. Because these analyses included a relatively small number of compounds, the results may not generalize to other members of the hallucinogen drug class. However, many of the compounds are prototypical hallucinogens (i.e., those from which many other hallucinogens are structurally related), increasing the likelihood that the relationship will hold true for other compounds. In summary, the results demonstrate that HTR studies in mice and drug discrimination studies in rats yield remarkably equivalent estimates of hallucinogen potency.
DISCUSSION
The goal of the present investigation was to assess the reliability and cross-species translatability of hallucinogen potency estimates generated using the HTR paradigm. While head-twitch data have traditionally been used in a qualitative manner (i.e., to assess whether compounds produce LSD-like behavioral effects), these studies evaluated whether the HTR assay can also be used to generate quantitative measures of hallucinogen activity. Given those goals, we first conducted 16 dose-response experiments to enlarge the available pool of HTR data to 40 total compounds. Compounds were selected based on the availability of relevant data in rats and humans, as well as by the desire to maximize the range of potencies and structural diversity. These studies confirmed that hallucinogens from multiple structural classes reliably induce head twitches in mice. Next, linear regression was used to evaluate the relationship between median effective doses in the HTR paradigm and potencies in other species including humans. Notably, a strong positive correlation was found between HTR potency in mice and reported hallucinogenic potency in humans. Head-twitch potencies were also found to be correlated with drug discrimination-derived ED50 values for substitution in rats trained with either LSD or DOM. All three of the behavioral effects included in the present investigation (HTR in mice, hallucinogen discriminative stimulus effects in rats, and psychedelic effects in humans) have been linked to 5-HT2A activation (Halberstadt 2015; Nichols 2016). Incredibly, hallucinogens induce those three behaviors with remarkably consistent relative potencies. The shared role of 5-HT2A activation in HTR in mice, hallucinogenic drug discrimination in rats and hallucinogenic effects in humans, support strong construct validity of these experimental paradigms. Likewise, the predictive validity of the HTR in mice and drug discrimination are supported by the high correlation between potencies in these paradigms with reported human potencies of hallucinogens. Based on these results, mouse HTR studies with hallucinogens clearly have cross-species translational relevance. Although head twitches are thought to have limited value as a model of hallucinogenesis (i.e., poor face validity) (Canal and Morgan 2012), these findings support the use of the HTR paradigm for predicting potencies of compounds to induce hallucinogenic effects in humans and in SAR studies of hallucinogens. Future investigations will more thoroughly test the utility of HTR studies to understand the pharmacological interactions underlying the effects of hallucinogens in humans.
These studies evaluated whether the HTR assay can provide a reliable assessment of hallucinogen potency in humans. As shown in Figure 2, the positive linear correlation between human hallucinogenic potency and HTR ED50 values is extremely robust using linear regression (r2 = 0.8927). According to a previous study (Glennon et al. 1982a), there is a similar relationship between human potency and drug discrimination-derived ED50 values for 14 amphetamine hallucinogens (r2 = 0.93). Although the coefficient of determination was slightly higher for drug discrimination compared to HTR, it is difficult to compare the results directly because the HTR analysis included compounds from multiple structural classes whereas the study by Glennon et al. (1982a) looked only at amphetamine hallucinogens. To simplify comparison of the relationship between human potency and ED50 values derived from head twitch and drug discrimination, we repeated the analyses using the compounds that are shared between Tables 3 and 4 (AL-LAD, DOB-DFLY, 2C-B, 2C-B-FLY, 2C-I, DET, DMT, DOB, DOB-FLY, DOET, DOM, LSD, SS-LSZ, MDA, and mescaline). For those 15 compounds, the coefficient of determination was higher for HTR (r2 = 0.9602) than for drug discrimination (r2 = 0.9064; Figure S1). In conclusion, the HTR assay provides a quantitative assessment of hallucinogen potency that closely parallels results obtained in humans and in drug discrimination studies. Finding that ED50 values for head-twitch are robustly correlated with other measures of activity in humans and rats supports the predictive validity of this paradigm to assess potency relationships between hallucinogens in SAR studies.
The fact that HTR studies can quantify hallucinogen potency with about the same sensitivity as drug discrimination is rather remarkable because it can be challenging to estimate the potency of hallucinogens based on HTR data. The location of the top and bottom of the dose-response curve in HTR studies is not fixed, which can complicate curve fitting using a regression model. Mice emit head twitches spontaneously (Dursun and Handley 1996) and the baseline rate shows some variability; likewise, the peak HTR rate depends on the particular compound being tested and may be linked to 5-HT2A agonist efficacy (Vickers et al. 2001). In addition, dose-response curves in the HTR assay are non-monotonic, with ascending and descending phases. There is evidence that the two phases of the curve may be mediated by different receptors, with 5-HT2A activation driving the ascending phase and 5-HT2C activation driving the descending phase (Fantegrossi et al. 2010). Nevertheless, despite these complexities, HTR potencies were robustly correlated with activity in rats and humans.
Certain hallucinogens induced head twitches in mice and DOM-like stimulus effects in rats with potencies that are nearly identical. Although we expected to find a close relationship between hallucinogen potencies in HTR and drug discrimination, the high degree of similarity between potencies in the two paradigms is remarkable. The potency equivalence, however, may simply reflect the fact that the HTR peaks within the dosage range that is optimal for drug discrimination training. Training doses for drug discrimination must be carefully selected; doses that are too low may not be discriminable, whereas high doses may impair task performance. For LSD, typical training doses range from 80 μg/kg to 120 μg/kg; lower doses are discriminated poorly and higher doses produce considerable behavioral disruption (Cameron and Appel 1973; White and Appel 1982b). Likewise, common doses for DOM training include 1.0 mg/kg (Young et al. 1980) and 1.5 mg/kg (Silverman and Ho 1980). By coincidence, the HTR induced by LSD and DOM peaks at about 100 μg/kg and 1 mg/kg, respectively. Increasing or reducing the training dose in drug discrimination shifts the generalization curve rightward or leftward, respectively (Stolerman et al. 2011), so the degree of potency equivalence observed in our analyses is probably a consequence of the specific doses used for training in the drug discrimination studies.
Evidence is accumulating that serotonergic hallucinogens from multiple structural classes reliably induce head twitches. Although 2C-I and 2C-B failed to induce the HTR in one study (Moya et al. 2007), those agents were active in follow-up investigations (Halberstadt and Geyer 2014; Elmore et al. 2018; Halberstadt et al. 2019b). Nevertheless, although hundreds of hallucinogens have been discovered to date (Shulgin and Shulgin 1991, 1997; Trachsel et al. 2013), only a small subset have been evaluated in the HTR paradigm. As far as we are aware, these are the first mouse HTR studies conducted with DOET, DOBU, DON, DOC, α-Et-2C-D, MDA, R-(-)-MDA, 25D-NBOMe, 25I-NBOH, and DET. Some of the other compounds listed in Table 1 were tested previously by other groups; the present experiments serve to validate the earlier findings. For example, R-(-)-DOI (Darmani et al. 1990; Fantegrossi et al. 2010), DOM (Gonzalez-Maeso et al. 2007), DMT (Corne and Pickering 1967; Gonzalez-Maeso et al. 2007; Carbonaro et al. 2015), DPT (Fantegrossi et al. 2008b), DIPT (Smith et al. 2014; Carbonaro et al. 2015), and 5-MeO-AMT (May et al. 2006; Abiero et al. 2019) have been previously shown to induce head twitches in mice. Overall, the results of these studies confirm that 40 hallucinogens from multiple structural classes induce head twitches in mice, demonstrating the high predictive validity of this model.
One complexity associated with the use of the HTR assay to study hallucinogens is that this behavioral paradigm is potentially susceptible to false-positive responses. For example, rolipram, [Met5]enkephalin, and icilin induce head twitches through non-5-HT2A receptor-dependent mechanisms (Drust et al. 1981; Przegalinski et al. 1981). Indirect 5-HT2A agonists such as fenfluramine, p-chloroamphetamine (PCA), and 5-hydroxytryptophan (5-HTP) induce head twitches in rodents (Corne et al. 1963; Singleton and Marsden 1981; Darmani 1998) but do not act as hallucinogens in humans (van Praag et al. 1971; Brauer et al. 1996; Turner et al. 2006), However, overdoses of compounds that increase serotonin (5-HT) release can result in 5-HT syndrome, which sometimes includes hallucinations (Birmes et al. 2003; Evans and Sebastian 2007). Nevertheless, it is highly unlikely that any of the compounds tested in the present studies are acting as false-positives in the HTR assay. All of the compounds act as hallucinogens in humans and the vast majority produce DOM- and/or LSD-like stimulus effects in rats. In addition, virtually all of the compounds have moderate to high binding affinity for the 5-HT2A receptor and act as agonists in functional assays; compounds capable of activating the 5-HT2A receptor directly are highly unlikely to produce false-positive responses in the HTR assay.
Some of the compounds in Table 1 deserve comment because they were not included in the human dose correlation due to uncertainties regarding their effective dose range. DIPT is reportedly active at 25–50 mg (Shulgin and Carter 1980) or 25–100 mg (Shulgin and Shulgin 1991). However, at those doses, DIPT distorts auditory perception and does not produce psychedelic effects. Higher doses of DIPT produce more typical LSD-like reactions, but the effective dose range for those effects is not well defined. Likewise, only limited data has been reported regarding the activity of DON in humans. Although DON is reportedly active at oral doses of 4.5 mg (Gomez-Jeria et al. 1986), not much else is known about its effective dose range, so it was not included in the human potency analysis.
Our studies confirm that a-Et-2C-D (also known as BL-3912, 4C-D, or ariadne), the α-ethyl homologue of DOM, induces head twitches in mice. Although α-Et-2C-D does not act as a psychedelic drug at p.o. doses up to 32 mg (Shulgin and Shulgin 1991), subjects administered higher doses (up to 270 mg) reportedly experienced “euphoria and other LSD-like effects” (Winter 1980). Consistent with our results, α-Et-2C-D produces full substitution in rats trained to discriminate DOM (Glennon et al. 1983b) and LSD (Winter 1980). However, because the available information on human doses are limited, we chose not to include it in the correlation analyses and linear regression between human hallucinogenic potency and HTR. Although α-Et-2C-D did not induce head or body shaking in cats (Rusterholz et al. 1978), the dose used (1 mg/kg IP) was probably too low to produce a behavioral response. Indeed, we did not observe a significant increase in HTR counts unless mice were treated with 6 mg/kg IP.
Although there was some variability in the data, the strength of the correlation in Figure 2 is notable given how difficult it is to accurately quantify the potency of hallucinogens in humans. As noted above, effective or typical dosage ranges are usually provided, with the lower value representing the minimum dose that produces clearly defined effects and the upper value representing the maximally active level. These dose ranges, however, are really rough approximations. Furthermore, there is frequently considerable variability in human potency data due to the subjective nature of the assessment and the inherent methodological weaknesses associated with non-institutional studies of hallucinogens. Another confounding variable is the route of administration; most of the human potency data used in the present study was based on the oral route of administration, although in several cases p.o. activity is poor and thus alternate routes are used (including sublingual administration and parenteral injections). Despite this limitation, the correlation between HTR and human subjective potency in the present study was high. The strength of the correlation is also notable because the analysis was performed using a mixture of phenylalkylamine, tryptamine, and ergoline hallucinogens. In contrast to phenylalkylamine hallucinogens, which are relatively selective for 5-HT2 sites, tryptamine and ergoline hallucinogens also produce behavioral effects via 5-HT1A and potentially other receptors (Halberstadt and Geyer 2011). In one clinical study, pretreatment with the mixed 5-HT1A/1B/β-adrenergic antagonist pindolol was reported to markedly potentiate the subjective effects of DMT (Strassman 1996). Since DMT has low affinity for β-adrenergic receptors but nM affinity at 5-HT1A (Deliganis et al. 1991; Ray 2010), these results can be interpreted to suggest that interactions with the 5-HT1A receptor dampen the action of DMT at the 5-HT2A receptor. Indeed, according to our recent studies, the ergoline derivative lysergic acid morpholide (LSM-775) does not induce head twitches unless 5-HT1A receptors are blocked by pretreatment with the antagonist WAY-100,635 (Brandt et al. 2018). We have also shown that 5-HT1A receptor interactions influence the potency of N,N-diallyltryptamines in the HTR paradigm (Klein et al. 2018). Although the extent to which the 5-HT1A receptor influences the in vivo psychopharmacology of hallucinogens is still not entirely clear, these interactions appear to play a similar role in humans and mice given the robust correlation between hallucinogen effects in these species.
There are reportedly significant strain differences in the response of mice to 5-HT2A receptor agonists. The magnitude of the HTR induced by DOI is known to vary across different strains of mice (Weiss et al. 2003; Canal et al. 2010; Rangel-Barajas et al. 2014). For example, the number of head twitches induced by DOI is dramatically larger in DBA/2J mice compared to C57BL/6J mice (Canal et al. 2010). Likewise, C57BL, DBA, ICR, C3H and ddY mice exhibit varying degrees of sensitivity to tryptamine-induced head twitches (Sugimoto et al. 1986; Yamada et al. 1987). Because of these differences, the extent to which our findings with C57BL/6J mice are relevant to other strains is not clear. Future studies will examine whether HTR potencies in other mouse lines are also correlated with activity in humans and rats.
One unanswered question is whether a linear regression model based on HTR data could be used to estimate the potency of novel 5-HT2A agonists in humans. Given the robust relationship between potency in the HTR assay and activity in humans, follow-up studies are necessary to test the predictive validity of HTR data. Although the present studies were conducted using a structurally diverse series of hallucinogens, it cannot be excluded that the use of a more structurally homogeneous group of hallucinogens would have reduced the variance and increased the coefficient of determination in our regression models. Although interactions with 5-HT2A receptors are known to play an important role in determining the behavioral potency of hallucinogens (Glennon et al. 1984; Luethi and Liechti 2018), factors such as distribution, intrinsic clearance, metabolism, and off-target interactions will also influence their behavioral potency. Although the latter factors are not necessarily uniform within a particular chemical scaffold, they are likely to vary to a much greater extent across different structural classes of hallucinogens. Therefore, although it may be possible to develop a single regression model to predict the potency of hallucinogens in humans based on their activity in mice, it may be more effective to develop discrete models for tryptamines, ergolines, and phenylalkylamines. Although additional HTR data is required to develop suitable regression models, the present studies provide clear evidence supporting the feasibility of this approach.
In summary, these studies confirm that the potency of hallucinogens in the HTR paradigm are highly correlated with their activity in rats and humans. Based on these findings, it may be possible to use HTR assay in mice as a preclinical model of the pharmacological interactions underlying the effects of hallucinogens in humans. It must be emphasized, however, that the HTR assay is not actually modeling the psychedelic or subjective effects of hallucinogens. Despite the robust evidence collected in these experiments, follow-up studies are necessary to address many unanswered questions regarding the pharmacology of the HTR and its utility as a behavioral model. For example, although 5-HT2A receptor activation appears to mediate the HTR induced by hallucinogens (Schreiber et al. 1995; Gonzalez-Maeso et al. 2007; Halberstadt et al. 2011), there is also evidence that the expression of head-twitch behavior is modulated by activation of 5-HT1A (Darmani et al. 1990; Klein et al. 2018) and 5-HT2C receptors (Vickers et al. 2001; Canal et al. 2010; Fantegrossi et al. 2010; Canal et al. 2013). It is also not clear why certain compounds — most notably lisuride — fail to induce the HTR in mice despite acting as 5-HT2A receptor agonists. Although lisuride appears to be a functionally-selective agonist and may not be capable of activating the specific 5-HT2A-coupled signaling pathways that drive HTR expression (Gonzalez-Maeso et al. 2007), other factors may also be involved (Marona-Lewicka et al. 2002; Cussac et al. 2008). Finally, given the increasing focus on the therapeutic efficacy of hallucinogens in humans, it is intriguing to note that these compounds promote neural plasticity and neurogenesis in rodents (Jones et al. 2009; Lima da Cruz et al. 2018; Ly et al. 2018) and produce positive effects in preclinical models relevant to anxiety, depression, and post-traumatic stress disorder (Catlow et al. 2013; Buchborn et al. 2014; Cameron et al. 2018; Cameron et al. 2019). It would be interesting to determine whether correlations also exist between those effects of hallucinogens and their activity in the HTR paradigm. Although additional studies are clearly warranted, the present findings clearly show that the mouse HTR assay can be used to generate data that are relevant and translatable to other species.
Supplementary Material
FIGURE S1. Comparison of the relationship between human hallucinogenic potencies and ED50 values from rats and mice. (LEFTPANEL) Relationship between potencies in humans and mouse head-twitch response (HTR) studies. (RIGHTPANEL) Relationship between potencies in humans and published rat drug discrimination studies using 0.08 mg/kg LSD as the training drug (RIGHTPANEL). Data for AL-LAD, DOB-DFLY, 2C-B, 2C-B-FLY, 2C-I, DET, DMT, DOB, DOB-FLY, DOET, DOM, LSD, LSZ, MDA, and mescaline are shown. The drug discrimination data are taken from Table 4. Potencies in humans are plotted as log 1/total hallucinogenic dose (in moles); potencies in mice and rats are plotted as log 1/ED50 (in moles/kg).
Serotonergic hallucinogen induce the head-twitch response (HTR) in mice
HTR potencies in mice are correlated with hallucinogenic potencies in humans
Hallucinogen potencies in HTR are also correlated with rat drug discrimination data
Hallucinogen potencies in the HTR assay have significant predictive validity
ACKNOWLEDGEMENTS
Supported by awards from the National Institute on Drug Abuse (5R01 DA041336) and the National Institute of Mental Health (5T32 MH018399). Some of the test substances were donated by Mark A. Geyer, Ph.D. Test substances were also donated by the NIDA Drug Supply Program and by the NIMH Chemical Synthesis and Drug Supply Program.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abiero A, Botanas CJ, Sayson LV, Custodio RJ, de la Pena JB, Kim M, Lee HJ, Seo JW, Ryu IS, Chang CM, Yang JS, Lee YS, Jang CG, Kim HJ, Cheong JH (2019) 5-Methoxy-alpha-methyltryptamine (5-MeO-AMT), a tryptamine derivative, induces head-twitch responses in mice through the activation of serotonin receptor 2a in the prefrontal cortex. Behav Brain Res 359: 828–835. [DOI] [PubMed] [Google Scholar]
- Appel JB, White FJ, Holohean AM (1982a) Analyzing mechanism(s) of hallucinogenic drug action with drug discrimination procedures. Neurosci Biobehav Rev 6: 529–36. [DOI] [PubMed] [Google Scholar]
- Appel JB, White FJ, West WB, Holohean AM (1982b) Discriminative stimulus properties of ergot alkaloids. In: Colpaert FC, Slangen JL (eds) Drug Discrimination: Applications in CNS Pharmacology. Elsevier Biomedical Press, Amsterdam, pp 49–67 [Google Scholar]
- Benneyworth MA, Smith RL, Sanders-Bush E (2008) Chronic phenethylamine hallucinogen treatment alters behavioral sensitivity to a metabotropic glutamate 2/3 receptor agonist. Neuropsychopharmacology 33: 2206–16. [DOI] [PubMed] [Google Scholar]
- Birmes P, Coppin D, Schmitt L, Lauque D (2003) Serotonin syndrome: a brief review. CMAJ 168: 1439–42. [PMC free article] [PubMed] [Google Scholar]
- Blair JB, Kurrasch-Orbaugh D, Marona-Lewicka D, Cumbay MG, Watts VJ, Barker EL, Nichols DE (2000) Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines. J Med Chem 43: 4701–10. [DOI] [PubMed] [Google Scholar]
- Blair JB, Marona-Lewicka D, Kanthasamy A, Lucaites VL, Nelson DL, Nichols DE (1999) Thieno[3,2-b]- and thieno[2,3-b]pyrrole bioisosteric analogues of the hallucinogen and serotonin agonist N,N-dimethyltryptamine. J Med Chem 42: 1106–11. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PC, Strassman RJ (2015) Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol 29: 289–99. [DOI] [PubMed] [Google Scholar]
- Boszormenyi Z, Der P, Nagy T (1959) Observations on the psychotogenic effect of N-N diethyltryptamine, a new tryptamine derivative. J Ment Sci 105: 171–181. [DOI] [PubMed] [Google Scholar]
- Braden MR, Parrish JC, Naylor JC, Nichols DE (2006) Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol Pharmacol 70: 1956–64. [DOI] [PubMed] [Google Scholar]
- Brandt SD, Kavanagh PV, Twamley B, Westphal F, Elliott SP, Wallach J, Stratford A, Klein LM, McCorvy JD, Nichols DE, Halberstadt AL (2017a) Return of the lysergamides. Part IV: Analytical and pharmacological characterization of lysergic acid morpholide (LSM- 775). Drug Test Anal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, Kavanagh PV, Twamley B, Westphal F, Elliott SP, Wallach J, Stratford A, Klein LM, McCorvy JD, Nichols DE, Halberstadt AL (2018) Return of the lysergamides. Part IV: Analytical and pharmacological characterization of lysergic acid morpholide (LSM- 775). Drug Test Anal 10: 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, Kavanagh PV, Westphal F, Elliott SP, Wallach J, Colestock T, Burrow TE, Chapman SJ, Stratford A, Nichols DE, Halberstadt AL (2017b) Return of the lysergamides. Part II: Analytical and behavioural characterization of N6 -allyl-6-norlysergic acid diethylamide (AL-LAD) and (2'S,4'S)-lysergic acid 2,4-dimethylazetidide (LSZ). Drug Test Anal 9: 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, Kavanagh PV, Westphal F, Stratford A, Elliott SP, Dowling G, Wallach J, Halberstadt AL (2019) Return of the lysergamides. Part V: Analytical and behavioural characterization of 1-butanoyl-d-lysergic acid diethylamide (1B-LSD). Drug Test Anal 11: 1122–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, Kavanagh PV, Westphal F, Stratford A, Elliott SP, Hoang K, Wallach J, Halberstadt AL (2016) Return of the lysergamides. Part I: Analytical and behavioural characterization of 1-propionyl-d-lysergic acid diethylamide (1P-LSD). Drug Test Anal 8: 891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer LH, Johanson CE, Schuster CR, Rothman RB, de Wit H (1996) Evaluation of phentermine and fenfluramine, alone and in combination, in normal, healthy volunteers. Neuropsychopharmacology 14: 233–41. [DOI] [PubMed] [Google Scholar]
- Braun U, Braun G, Jacob P, 3rd, Nichols DE, Shulgin AT (1978) Mescaline analogs: substitutions at the 4-position. NIDA Res Monogr: 27–37. [PubMed] [Google Scholar]
- Buchborn T, Schroder H, Hollt V, Grecksch G (2014) Repeated lysergic acid diethylamide in an animal model of depression: Normalisation of learning behaviour and hippocampal serotonin 5-HT2 signalling. J Psychopharmacol 28: 545–52. [DOI] [PubMed] [Google Scholar]
- Cameron LP, Benson CJ, DeFelice BC, Fiehn O, Olson DE (2019) Chronic, Intermittent Microdoses of the Psychedelic N,N-Dimethyltryptamine (DMT) Produce Positive Effects on Mood and Anxiety in Rodents. ACS Chem Neurosci 10: 3261–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron LP, Benson CJ, Dunlap LE, Olson DE (2018) Effects of N, N-Dimethyltryptamine on Rat Behaviors Relevant to Anxiety and Depression. ACS Chem Neurosci 9: 1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron OG, Appel JB (1973) A behavioral and pharmacological analysis of some discriminable properties of d-LSD in rats. Psychopharmacologia 33: 117–34. [DOI] [PubMed] [Google Scholar]
- Canal CE, Booth RG, Morgan D (2013) Support for 5-HT2C receptor functional selectivity in vivo utilizing structurally diverse, selective 5-HT2C receptor ligands and the 2,5-dimethoxy-4-iodoamphetamine elicited head-twitch response model. Neuropharmacology 70: 112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Morgan D (2012) Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal 4: 556–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC (2010) The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology (Berl) 209: 163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro TM, Eshleman AJ, Forster MJ, Cheng K, Rice KC, Gatch MB (2015) The role of 5-HT2A, 5-HT 2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N,N-dimethyltryptamine and N,N-diisopropyltryptamine in rats and mice. Psychopharmacology (Berl) 232: 275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlow BJ, Song S, Paredes DA, Kirstein CL, Sanchez-Ramos J (2013) Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp Brain Res 228: 481–91. [DOI] [PubMed] [Google Scholar]
- Chambers JJ, Parrish JC, Jensen NH, Kurrasch-Orbaugh DM, Marona-Lewicka D, Nichols DE (2003) Synthesis and pharmacological characterization of a series of geometrically constrained 5-HT(2A/2C) receptor ligands. J Med Chem 46: 3526–35. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW (1967) A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia 11: 65–78. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW, Warner BT (1963) A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Br J Pharmacol Chemother 20: 106–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussac D, Boutet-Robinet E, Ailhaud MC, Newman-Tancredi A, Martel JC, Danty N, Rauly- Lestienne I (2008) Agonist-directed trafficking of signalling at serotonin 5-HT2A, 5-HT2B and 5-HT2C-VSV receptors mediated Gq/11 activation and calcium mobilisation in CHO cells. Eur J Pharmacol 594: 32–8. [DOI] [PubMed] [Google Scholar]
- Darmani NA (1998) Cocaine and selective monoamine uptake blockers (sertraline, nisoxetine, and GBR 12935) prevent the d-fenfluramine-induced head-twitch response in mice. Pharmacol Biochem Behav 60: 83–90. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA (1990) Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav 36: 901–6. [DOI] [PubMed] [Google Scholar]
- Deliganis AV, Pierce PA, Peroutka SJ (1991) Differential interactions of dimethyltryptamine (DMT) with 5-HT1A and 5-HT2 receptors. Biochem Pharmacol 41: 1739–44. [DOI] [PubMed] [Google Scholar]
- Drust EG, Sloviter RS, Conner JD (1981) Methionine enkephalin-induced shaking behavior in rats: dissociation from brain serotonin mechanisms. Neuropharmacology 20: 473–5. [DOI] [PubMed] [Google Scholar]
- Dursun SM, Handley SL (1996) Similarities in the pharmacology of spontaneous and DOI-induced head-shakes suggest 5HT2A receptors are active under physiological conditions. Psychopharmacology (Berl) 128: 198–205. [DOI] [PubMed] [Google Scholar]
- Elmore JS, Decker AM, Sulima A, Rice KC, Partilla JS, Blough BE, Baumann MH (2018) Comparative neuropharmacology of N-(2-methoxybenzyl)-2,5-dimethoxyphenethylamine (NBOMe) hallucinogens and their 2C counterparts in male rats. Neuropharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CE, Sebastian J (2007) Serotonin syndrome. Emerg Med J 24: e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faillace LA, Vourlekis A, Szara S (1967) Clinical evaluation of some hallucinogenic tryptamine derivatives. J Nerv Ment Dis 145: 306–13. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH (2006) Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav 83: 122–9. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ (2008a) The behavioral pharmacology of hallucinogens. Biochem Pharmacol 75: 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Reissig CJ, Katz EB, Yarosh HL, Rice KC, Winter JC (2008b) Hallucinogen-like effects of N,N-dipropyltryptamine (DPT): possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol Biochem Behav 88: 358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH (2010) Interaction of 5-HT2A and 5-HT2C receptors in R(-)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J Pharmacol Exp Ther 335: 728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC (1995a) Role of 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. II: Reassessment of LSD false positives. Psychopharmacology (Berl) 121: 357–63. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC (1995b) The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. I: Antagonist correlation analysis. Psychopharmacology (Berl) 121: 347–56. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ (2017) Locomotor and discriminative stimulus effects of four novel hallucinogens in rodents. Behav Pharmacol 28: 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ, Janowsky A, Eshleman AJ (2011) Abuse liability profile of three substituted tryptamines. J Pharmacol Exp Ther 338: 280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ (2000) Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology 23: 569–76. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Kaplan J, Stillman R, Wyatt RJ (1976) The psychedelic model of schizophrenia: the case of N,N-dimethyltryptamine. Am J Psychiatry 133: 203–8. [DOI] [PubMed] [Google Scholar]
- Glennon RA (1989) Stimulus properties of hallucinogenic phenalkylamines and related designer drugs: formulation of structure-activity relationships. NIDA Res Monogr 94: 43–67. [PubMed] [Google Scholar]
- Glennon RA (1992) Animal models for assessing hallucinogenic agents. In: Boulton AA, Baker GB, Wu PH (eds) Animal Models of Drug Addiction (Neuromethods, Vol. 24). Humana Press, Totowa, N.J., pp 345–381 [Google Scholar]
- Glennon RA, Hauck AE (1985) Mechanistic studies on DOM as a discriminative stimulus. Pharmacol Biochem Behav 23: 937–41. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Rosecrans JA, Young R (1983a) Drug-induced discrimination: a description of the paradigm and a review of its specific application to the study of hallucinogenic agents. Med Res Rev 3: 289–340. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, Lyon RA (1988) A preliminary investigation of the psychoactive agent 4-bromo-2,5-dimethoxyphenethylamine: a potential drug of abuse. Pharmacol Biochem Behav 30: 597–601. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD (1984) Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci 35: 2505–11. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R (1982) Comparison of behavioral properties of di- and tri-methoxyphenylisopropylamines. Pharmacol Biochem Behav 17: 603–7. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Benington F, Morin RD (1982a) Behavioral and serotonin receptor properties of 4-substituted derivatives of the hallucinogen 1-(2,5-dimethoxyphenyl)-2-aminopropane. J Med Chem 25: 1163–8. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Jacyno JM (1983b) Indolealkylamine and phenalkylamine hallucinogens. Effect of alpha-methyl and N-methyl substituents on behavioral activity. Biochem Pharmacol 32: 1267–73. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Jacyno JM, Slusher M, Rosecrans JA (1983c) DOM-stimulus generalization to LSD and other hallucinogenic indolealkylamines. Eur J Pharmacol 86: 453–9. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Rosecrans JA (1982b) A comparison of the behavioral effects of DOM homologs. Pharmacol Biochem Behav 16: 557–9. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Rosecrans JA (1983d) Antagonism of the effects of the hallucinogen DOM and the purported 5-HT agonist quipazine by 5-HT2 antagonists. Eur J Pharmacol 91: 189–96. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Rosecrans JA, Anderson GM (1982c) Discriminative stimulus properties of MDA analogs. Biol Psychiatry 17: 807–14. [PubMed] [Google Scholar]
- Gomez-Jeria JS, Cassels BK, Clavijo R, Vargas V, Quintana R, Saavededra-Aguilar JC (1986) Spectroscopic characterization of a new hallucinogen: 1-(2,5-dimethoxy-4-nitrophenyl)-2-aminopropane (DON). Microgram 19: 153–161. [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53: 439–52. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, Cosimano MP, Klinedinst MA(2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized doubleblind trial. J Psychopharmacol 30: 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspoon L, Bakalar J (1979) Psychedelic drugs reconsidered. Basic Books, New York [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, Greer GR (2011) Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry 68: 71–8. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL (2015) Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav Brain Res 277: 99–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Chatha M, Chapman SJ, Brandt SD (2019a) Comparison of the behavioral effects of mescaline analogs using the head twitch response in mice. J Psychopharmacol 33: 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Chatha M, Stratford A, Grill M, Brandt SD (2019b) Comparison of the behavioral responses induced by phenylalkylamine hallucinogens and their tetrahydrobenzodifuran (“FLY”) and benzodifuran (“DragonFLY”) analogs. Neuropharmacology 144: 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA (2010) Hallucinogens. In: Koob G, Thompson RM, Le Moal M (eds) Encyclopedia of Behavioral Neuroscience, Volume 2. Academic Press, London, pp 12–20 [Google Scholar]
- Halberstadt AL, Geyer MA (2011) Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 61: 364–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA (2013) Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology (Berl) 227: 727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA (2014) Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology 77: 200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Klein LM, Chatha M, Valenzuela LB, Stratford A, Wallach J, Nichols DE, Brandt SD (2019c) Pharmacological characterization of the LSD analog N-ethyl-N-cyclopropyl lysergamide (ECPLA). Psychopharmacology (Berl) 236: 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Koedood L, Powell SB, Geyer MA (2011) Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J Psychopharmacol 25: 1548–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Sindhunata IS, Scheffers K, Flynn AD, Sharp RF, Geyer MA, Young JW (2016) Effect of 5-HT2A and 5-HT2C receptors on temporal discrimination by mice. Neuropharmacology 107: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsley S, Fiorella D, Rabin RA, Winter JC (1998) A comparison of N,N-dimethyltryptamine, harmaline, and selected congeners in rats trained with LSD as a discriminative stimulus. Prog Neuropsychopharmacol Biol Psychiatry 22: 649–63. [DOI] [PubMed] [Google Scholar]
- Hoffman AJ, Nichols DE (1985) Synthesis and LSD-like discriminative stimulus properties in a series of N(6)-alkyl norlysergic acid N,N-diethylamide derivatives. J Med Chem 28: 1252–5. [DOI] [PubMed] [Google Scholar]
- Hollister LE (1968) Chemical psychoses: LSD and related drugs. Charles C. Thomas, Springfiled, IL [Google Scholar]
- Huang X, Marona-Lewicka D, Pfaff RC, Nichols DE (1994) Drug discrimination and receptor binding studies of N-isopropyl lysergamide derivatives. Pharmacol Biochem Behav 47: 667–73. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR (2014) Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol 28: 983–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Griffiths RR (2016) Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Srivastava DP, Allen JA, Strachan RT, Roth BL, Penzes P (2009) Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc Natl Acad Sci U S A 106: 19575–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncosa JI Jr., Hansen M, Bonner LA, Cueva JP, Maglathlin R, McCorvy JD, Marona-Lewicka D, Lill MA, Nichols DE (2013) Extensive rigid analogue design maps the binding conformation of potent N-benzylphenethylamine 5-HT2A serotonin receptor agonist ligands. ACS Chem Neurosci 4: 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein LM, Cozzi NV, Daley PF, Brandt SD, Halberstadt AL (2018) Receptor binding profiles and behavioral pharmacology of ring-substituted N,N-diallyltryptamine analogs. Neuropharmacology 142: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner J, Appel JB (1982) Psilocybin as a discriminative stimulus: lack of specificity in an animal behavior model for 'hallucinogens'. Psychopharmacology (Berl) 76: 130–5. [DOI] [PubMed] [Google Scholar]
- Kraehenmann R, Pokorny D, Vollenweider L, Preller KH, Pokorny T, Seifritz E, Vollenweider FX (2017) Dreamlike effects of LSD on waking imagery in humans depend on serotonin 2A receptor activation. Psychopharmacology (Berl). [DOI] [PubMed] [Google Scholar]
- Leysen JE, Niemegeers CJ, Van Nueten JM, Laduron PM (1982) [3H]Ketanserin (R 41 468), a selective 3H-ligand for serotonin2 receptor binding sites. Binding properties, brain distribution, and functional role. Mol Pharmacol 21: 301–14. [PubMed] [Google Scholar]
- Lima da Cruz RV, Moulin TC, Petiz LL, Leao RN (2018) A Single Dose of 5-MeO-DMT Stimulates Cell Proliferation, Neuronal Survivability, Morphological and Functional Changes in Adult Mice Ventral Dentate Gyrus. Front Mol Neurosci 11: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luethi D, Liechti ME (2018) Monoamine Transporter and Receptor Interaction Profiles in Vitro Predict Reported Human Doses of Novel Psychoactive Stimulants and Psychedelics. Int J Neuropsychopharmacol 21: 926–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, Burbach KF, Soltanzadeh Zarandi S, Sood A, Paddy MR, Duim WC, Dennis MY, McAllister AK, Ori-McKenney KM, Gray JA, Olson DE (2018) Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep 23: 3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marona-Lewicka D, Kurrasch-Orbaugh DM, Selken JR, Cumbay MG, Lisnicchia JG, Nichols DE (2002) Re-evaluation of lisuride pharmacology: 5-hydroxytryptamine1A receptor-mediated behavioral effects overlap its other properties in rats. Psychopharmacology (Berl) 164: 93–107. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols CD, Nichols DE (2011) An animal model of schizophrenia based on chronic LSD administration: old idea, new results. Neuropharmacology 61: 503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marona-Lewicka D, Thisted RA, Nichols DE (2005) Distinct temporal phases in the behavioral pharmacology of LSD: dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology (Berl) 180: 427–35. [DOI] [PubMed] [Google Scholar]
- May JA, Dantanarayana AP, Zinke PW, McLaughlin MA, Sharif NA (2006) 1-((S)-2-aminopropyl)-1H-indazol-6-ol: a potent peripherally acting 5-HT2 receptor agonist with ocular hypotensive activity. J Med Chem 49: 318–28. [DOI] [PubMed] [Google Scholar]
- McLean TH, Chambers JJ, Parrish JC, Braden MR, Marona-Lewicka D, Kurrasch-Orbaugh D, Nichols DE (2006) C-(4,5,6-trimethoxyindan-1-yl)methanamine: a mescaline analogue designed using a homology model of the 5-HT2A receptor. J Med Chem 49: 4269–74. [DOI] [PubMed] [Google Scholar]
- Meert TF, de Haes P, Janssen PA (1989) Risperidone (R 64 766), a potent and complete LSD antagonist in drug discrimination by rats. Psychopharmacology (Berl) 97: 206–12. [DOI] [PubMed] [Google Scholar]
- Monte AP, Marona-Lewicka D, Kanthasamy A, Sanders-Bush E, Nichols DE (1995) Stereoselective LSD-like activity in a series of d-lysergic acid amides of (R)- and (S)-2-aminoalkanes. J Med Chem 38: 958–66. [DOI] [PubMed] [Google Scholar]
- Monte AP, Marona-Lewicka D, Parker MA, Wainscott DB, Nelson DL, Nichols DE (1996) Dihydrobenzofuran analogues of hallucinogens. 3. Models of 4-substituted (2,5-dimethoxyphenyl)alkylamine derivatives with rigidified methoxy groups. J Med Chem 39: 2953–61. [DOI] [PubMed] [Google Scholar]
- Monte AP, Waldman SR, Marona-Lewicka D, Wainscott DB, Nelson DL, Sanders-Bush E, Nichols DE (1997) Dihydrobenzofuran analogues of hallucinogens. 4. Mescaline derivatives. J Med Chem 40: 2997–3008. [DOI] [PubMed] [Google Scholar]
- Moreno FA, Wiegand CB, Taitano EK, Delgado PL(2006) Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry 67: 1735–40. [DOI] [PubMed] [Google Scholar]
- Moya PR, Berg KA, Gutierrez-Hernandez MA, Saez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP (2007) Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Pharmacol Exp Ther 321: 1054–61. [DOI] [PubMed] [Google Scholar]
- Nichols DE (2016) Psychedelics. Pharmacol Rev 68: 264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE (2018) Chemistry and Structure-Activity Relationships of Psychedelics. Curr Top Behav Neurosci 36: 1–43. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Frescas S, Marona-Lewicka D, Huang X, Roth BL, Gudelsky GA, Nash JF (1994) 1-(2,5-Dimethoxy-4-(trifluoromethyl)phenyl)-2-aminopropane: a potent serotonin 5-HT2A/2C agonist. J Med Chem 37: 4346–51. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM (2002) Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD). J Med Chem 45: 4344–9. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Sassano MF, Halberstadt AL, Klein LM, Brandt SD, Elliott SP, Fiedler WJ (2015) N-Benzyl-5-methoxytryptamines as Potent Serotonin 5-HT2 Receptor Family Agonists and Comparison with a Series of Phenethylamine Analogues. ACS Chem Neurosci 6: 1165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE, Snyder SE, Oberlender R, Johnson MP, Huang XM (1991) 2,3-Dihydrobenzofuran analogues of hallucinogenic phenethylamines. J Med Chem 34: 276–81. [DOI] [PubMed] [Google Scholar]
- Oberlender R, Pfaff RC, Johnson MP, Huang XM, Nichols DE (1992) Stereoselective LSD-like activity in d-lysergic acid amides of (R)- and (S)-2-aminobutane. J Med Chem 35: 203–11. [DOI] [PubMed] [Google Scholar]
- Oberlender R, Ramachandran PV, Johnson MP, Huang X, Nichols DE (1995) Effect of a chiral 4-alkyl substituent in hallucinogenic amphetamines. J Med Chem 38: 3593–601. [DOI] [PubMed] [Google Scholar]
- Parker MA (1998) Studies of perceptiotropic phenethylamines: Determinants of affinity for the 5-HT2A-receptor. Perdue University, West Lafayette, IN [Google Scholar]
- Parker MA, Marona-Lewicka D, Lucaites VL, Nelson DL, Nichols DE (1998) A novel (benzodifuranyl)aminoalkane with extremely potent activity at the 5-HT2A receptor. J Med Chem 41: 5148–9. [DOI] [PubMed] [Google Scholar]
- Preller KH, Herdener M, Pokorny T, Planzer A, Kraehenmann R, Stampfli P, Liechti ME, Seifritz E, Vollenweider FX (2017) The Fabric of Meaning and Subjective Effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr Biol 27: 451–457. [DOI] [PubMed] [Google Scholar]
- Przegalinski E, Bigajska K, Lewandowska A (1981) The influence of rolipram on the central serotoninergic system. Pharmacopsychiatria 14: 162–6. [DOI] [PubMed] [Google Scholar]
- Rangel-Barajas C, Malik M, Vangveravong S, Mach RH, Luedtke RR (2014) Pharmacological modulation of abnormal involuntary DOI-induced head twitch response in male DBA/2J mice: I. Effects of D2/D3 and D2 dopamine receptor selective compounds. Neuropharmacology 83: 18–27. [DOI] [PubMed] [Google Scholar]
- Ray TS (2010) Psychedelics and the human receptorome. PLoS One 5: e9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, Mennenga SE, Belser A, Kalliontzi K, Babb J, Su Z, Corby P, Schmidt BL (2016) Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol 30: 1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusterholz DB, Spratt JL, Long JP, Kelly TF (1978) Serotonergic and dopaminergic involvement in the mechanism of action of R-(-)-2,5-dimethoxy-4-bromoamphetamine (DOB) in cats. Life Sci 23: 1499–506. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ (1995) (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther 273: 101–12. [PubMed] [Google Scholar]
- Shulgin AT, Carter MF (1980) N, N-Diisopropyltryptamine (DIPT) and 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DIPT). Two orally active tryptamine analogs with CNS activity. Commun Psychopharmacol 4: 363–9. [PubMed] [Google Scholar]
- Shulgin AT, Manning T, Daley PF (2011) The Shulgin Index, Vol. 1, Psychedelic Phenethylamines and Related Compounds. Transform Press, Berkeley, CA [Google Scholar]
- Shulgin AT, Shulgin A (1991) PiHKAL: A Chemical Love Story. Transform Press, Berkeley, CA [Google Scholar]
- Shulgin AT, Shulgin A (1997) TIHKAL: the Continuation. Transform Press, Berkeley [Google Scholar]
- Silverman PB, Ho BT (1980) The discriminative stimulus properties of 2,5-dimethoxy-4-methylamphetamine (DOM): differentiation from amphetamine. Psychopharmacology (Berl) 68: 209–15. [DOI] [PubMed] [Google Scholar]
- Singleton C, Marsden CA (1981) Circadian variation in the head twitch response produced by 5-methoxy-N1,N1-dimethyltryptamine and p-chloroamphetamine in the mouse. Psychopharmacology (Berl) 74: 173–6. [DOI] [PubMed] [Google Scholar]
- Smith DA, Bailey JM, Williams D, Fantegrossi WE (2014) Tolerance and cross-tolerance to head twitch behavior elicited by phenethylamine- and tryptamine-derived hallucinogens in mice. J Pharmacol Exp Ther 351: 485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soskin RA, Grof S, Richards WA (1973) Low doses of Dipropyltryptamine in psychotherapy. Arch Gen Psychiatry 28: 817–21. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Childs E, Ford MM, Grant KA (2011) Role of training dose in drug discrimination: a review. Behav Pharmacol 22: 415–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman RJ (1996) Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res 73: 121–4. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Yamada J, Horisaka K (1986) Strain differences in the behaviour induced by tryptamine in five strains of mice. Neuropharmacology 25: 1289–91. [DOI] [PubMed] [Google Scholar]
- Szara S (1957) The comparison of the psychotic effect of tryptamine derivatives with the effects of mescaline and LSD-25 in selfexperiments. In: Garattini S, Ghetti V (eds) Psychotropic Drugs. Elsevier, Amsterdam, pp 460–467 [Google Scholar]
- Szara S, Rockland LH, Rosenthal D, Handlon JH (1966) Psychological effects and metabolism of N,N-diethyltryptamine in man. Arch Gen Psychiatry 15: 320–9. [DOI] [PubMed] [Google Scholar]
- Trachsel D, Lehmann D, Enzensperger C (2013) Phenethylamine: Von der Struktur zur Funktion. Nachtschatten, Solothurn [Google Scholar]
- Turner EH, Loftis JM, Blackwell AD (2006) Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol Ther 109: 325–38. [DOI] [PubMed] [Google Scholar]
- Valle M, Maqueda AE, Rabella M, Rodriguez-Pujadas A, Antonijoan RM, Romero S, Alonso JF, Mananas MA, Barker S, Friedlander P, Feilding A, Riba J (2016) Inhibition of alpha oscillations through serotonin-2A receptor activation underlies the visual effects of ayahuasca in humans. Eur Neuropsychopharmacol 26: 1161–75. [DOI] [PubMed] [Google Scholar]
- van Praag HM, Schut T, Bosma E, van den Bergh R (1971) A comparative study of the therapeutic effects of sone 4-chlorinated amphetamine derivatives in depressive patients. Psychopharmacologia 20: 66–76. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, Kennett GA (2001) Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists. Pharmacol Biochem Behav 69: 643–52. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9: 3897–902. [DOI] [PubMed] [Google Scholar]
- Weiss KC, Kim DY, Pawson CT, Cordes SP (2003) A genetic screen for mouse mutations with defects in serotonin responsiveness. Brain Res Mol Brain Res 115: 162–72. [DOI] [PubMed] [Google Scholar]
- White FJ, Appel JB (1982a) Lysergic acid diethylamide (LSD) and lisuride: differentiation of their neuropharmacological actions. Science 216: 535–7. [DOI] [PubMed] [Google Scholar]
- White FJ, Appel JB (1982b) Training dose as a factor in LSD-saline discrimination. Psychopharmacology (Berl) 76: 20–5. [DOI] [PubMed] [Google Scholar]
- Wing LL, Tapson GS, Geyer MA (1990) 5HT-2 mediation of acute behavioral effects of hallucinogens in rats. Psychopharmacology (Berl) 100: 417–25. [DOI] [PubMed] [Google Scholar]
- Winter JC (1980) Effects of the phenethylamine derivatives, BL-3912, fenfluramine, and Sch-12679, in rats trained with LSD as a discriminative stimulus. Psychopharmacology (Berl) 68: 159–62. [DOI] [PubMed] [Google Scholar]
- Winter JC, Rice KC, Amorosi DJ, Rabin RA (2007) Psilocybin-induced stimulus control in the rat. Pharmacol Biochem Behav 87: 472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Sugimoto Y, Horisaka K (1987) Pharmacological analysis of the variation in behavioural responses to tryptamine in five strains of mice. Eur J Pharmacol 140: 323–30. [DOI] [PubMed] [Google Scholar]
- Young R, Glennon RA, Rosecrans JA (1980) Discriminative stimulus properties of the hallucinogenic agent DOM. Commun Psychopharmacol 4: 501–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1. Comparison of the relationship between human hallucinogenic potencies and ED50 values from rats and mice. (LEFTPANEL) Relationship between potencies in humans and mouse head-twitch response (HTR) studies. (RIGHTPANEL) Relationship between potencies in humans and published rat drug discrimination studies using 0.08 mg/kg LSD as the training drug (RIGHTPANEL). Data for AL-LAD, DOB-DFLY, 2C-B, 2C-B-FLY, 2C-I, DET, DMT, DOB, DOB-FLY, DOET, DOM, LSD, LSZ, MDA, and mescaline are shown. The drug discrimination data are taken from Table 4. Potencies in humans are plotted as log 1/total hallucinogenic dose (in moles); potencies in mice and rats are plotted as log 1/ED50 (in moles/kg).