Abstract
DNA viruses often persist in the body of their host, becoming latent and recurring many months or years later. By contrast, most RNA viruses cause acute infections that are cleared from the host as they lack the mechanisms to persist. However, it is becoming clear that viral RNA can persist after clinical recovery and elimination of detectable infectious virus. This persistence can either be asymptomatic or associated with late progressive disease or nonspecific lingering symptoms, such as may be the case following infection with Ebola or Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Why does viral RNA sometimes persist after recovery from an acute infection? Where does the RNA come from? And what are the consequences?
Most RNA viruses cause acute infections that are cleared from the host as they lack the mechanisms to persist; however, phenomena such as "long COVID" suggest that viral RNA can persist after clinical recovery and elimination of detectable infectious virus. This Unsolved Mystery article explores the meaning, origins and consequences of such persistent RNA.
Introduction
Viruses are obligate intracellular infectious agents that are maintained in a population by continuous transmission to new susceptible individuals. In the absence of a reservoir, such as an insect vector or animal population capable of facilitating transmission to humans, viruses require alternative strategies to remain within human populations (Fig 1). Herpesviruses (such as varicella, herpes simplex, or Epstein–Barr) are DNA viruses with an optimum strategy, because after the acute infection resolves and production of infectious virions ceases, they become latent and can reactivate (in the form of shingles, mucosal ulcers, or asymptomatic shedding) to produce infectious virions months, years or decades later to infect a new group of susceptible people [1–3]. Of the RNA viruses, some (such as hepatitis C virus (HCV) and human immunodeficiency virus (HIV)) can evade immune control and continuously produce infectious virions [4–6]. Because these viruses do not cause rapidly lethal disease and can be transmitted over a long period of time, transmission does not need to be efficient. However, most acute viral infections are caused by RNA viruses that produce disease for a relatively short period of time and are associated with recovery and immunity to reinfection (e.g., measles, rubella, polio, and hepatitis A viruses) [7]. For these acute RNA viral infections, infectious virions are produced only transiently, so transmission to new susceptible hosts during this time must be efficient. Because these viruses must find and infect susceptible people in the population during the acute phase of disease to avoid dying out, they may become targets for eradication [8].
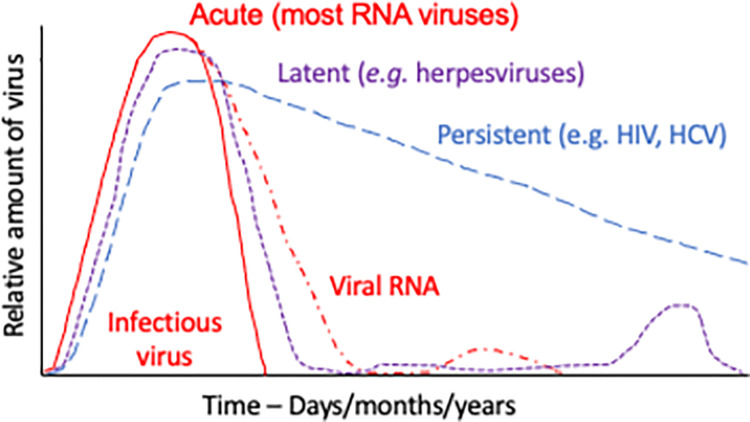
Fig 1. Patterns of virus production over time that maintain human viruses within the population.
Representative patterns are shown for RNA viruses often associated with persistent RNA that can cause late complications and occasionally reactivate (red), viruses that establish latency and reactivate (such as herpesviruses) (purple), and viruses not cleared by the immune response that continue to produce infectious virus (such as HIV and HCV) (blue). HCV, hepatitis C virus; HIV, human immunodeficiency virus.
However, it has become increasingly clear that recovery, elimination of infectious virus, and development of immunity to acute nonretroviral RNA viruses do not necessarily mean simultaneous elimination of the viral RNA [9–22]. The need to understand the pathophysiology of the prolonged symptoms that for many complicate recovery after infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)—so-called “long Coronavirus Disease (COVID)” or post-acute sequelae of COVID-19 (PASC)—has recently called attention to the potential role of RNA persistence in causing specific late complications, as well as in preventing complete recovery from acute infection [23–28]; consequences are also seen following other acute RNA virus infections (Table 1). But how and why does viral RNA persist, often without evidence of infectious virus, and what are the potential consequences of this persistence for human disease? These questions will form the basis of discussions in this Unsolved Mystery.
Table 1. Potential sites and consequences of RNA persistence after human infection with acute nonretroviral RNA viruses.
| Virus | Sites of RNA persistence | Cell type | Consequences | References |
|---|---|---|---|---|
| Picornavirus | ||||
| Rhinovirus | Respiratory tract | Epithelial cells? | Asthma | [29] |
| Enterovirus | Heart | Cardiac myocytes | Cardiomyopathy | [30] |
| Hepatitis A | Liver | Hepatocytes | Late hepatitis relapse | [7,15] |
| Polio | Brain and spinal cord | Motor neurons | Late progression of paralysis and fatigue | [19,31] |
| Alphavirus | ||||
| Chikungunya | Joints | Macrophages | Persistent joint pain | [10] |
| Ross River | Joints | Macrophages | Persistent joint pain | [32] |
| Sindbis | Joints | Macrophages? | Persistent joint pain | [33] |
| Flavivirus | ||||
| Zika | Testes | Sertoli cells | Late sexual transmission | [11,34] |
| Japanese encephalitis | Brain | Neurons | Encephalitis relapse and Parkinson-like disease | [35] |
| West Nile | Kidney? | Unknown | Kidney failure? | [36] |
| Tick-borne encephalitis | Brain | Neurons | Late progressive encephalitis | [37] |
| Coronavirus | ||||
| SARS-CoV-2 | Respiratory tract and intestine | Epithelial cells and macrophages? | Long COVID/PASC? | [18,38] |
| Arenavirus | ||||
| Lassa | Testes, kidney, and respiratory tract | Sertoli cells? | Epididymitis | [39,40] |
| Paramyxovirus | ||||
| Measles | Lymphoid tissue and brain | Lymphocytes, monocytes, and neurons | Life-long immunity; late progressive CNS disease (SSPE) | [41] |
| Respiratory syncytial | Respiratory tract | Epithelial cells and macrophages? | Chronic pulmonary disease | [42–44] |
| Filovirus | ||||
| Ebola | Testes, eye, and brain | Endothelial cells and macrophages | Late sexual transmission; recurrent/progressive uveitis and encephalitis; postviral syndrome | [45–49] |
| Marburg | Testes | Sertoli cells | Late sexual transmission | [50] |
CNS, central nervous system; COVID, Coronavirus Disease; PASC, post-acute sequelae of COVID-19; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; SSPE, subacute sclerosing panencephalitis.
Where does viral RNA persist?
The occurrence of long-term persistence of viral RNA has been known for decades, particularly in sites with specialized relationships to the immune system (so-called “immune-privileged” sites such as the brain, eyes, and testes), an early example being the identification of measles virus as the cause of subacute sclerosing panencephalitis (SSPE), a progressive fatal central nervous system (CNS) disease that becomes manifest many years after apparent recovery from the original acute measles virus infection [51–53]. More recently, late appearance of uveitis (Box 1) and recurrence of encephalomyelitis (Box 1) due to Ebola virus infection have emphasized the importance of RNA persistence in the eye, as well as the brain, and the potential for causing progressive disease [47 49]. Sexual transmission of Zika, Marburg, and Ebola viruses months to years after recovery from acute disease has also highlighted the importance of virus persistence in the testes for triggering new chains of transmission and transfer to new geographic regions [34,54–57].
Box 1. Definition of key terms used in this article
Adaptive immune response—production of virus-specific antibodies and T cells
Antigen—viral component, usually a protein, which stimulates production of virus-specific antibodies and T cells
Cardiomyopathy—dysfunction of the heart muscle
Cytolytic—causing death of a cell due to lysis
CpG—pairing of cytosine and guanosine in nucleic acid that is unusual in cellular RNA and DNA
Encephalomyelitis—inflammation of the brain and spinal cord that can be a response to viral infection
Immunocytochemical assays—methods for microscopically visualizing proteins, such as viral proteins, in cells using antibody to the protein
Innate immune mechanisms—intrinsic cellular responses to infection that usually occur rapidly and can often control pathogen replication and spread prior to induction of adaptive immune responses
MHC class I—polymorphic MHC; molecule that can bind viral peptides produced by infected cells, displaying them on the cell surface for presentation to virus-specific CD8 T cells that may be able to kill the infected cell
Peripheral blood mononuclear cells—lymphocytes and monocytes present in circulating blood that come primarily from bone marrow and lymphoid tissue and may infiltrate sites of infection
Ribonucleocapsid—viral RNA surrounded by nucleocapsid protein
Reverse transcriptase polymerase chain reaction (RT-PCR)—it is a method for converting RNA into a DNA copy for subsequent amplification using a thermostable DNA polymerase and primers specific for the gene of interest. The amplified product can be quantified or sequenced.
Uveitis—inflammation of the uvea, which is the middle vascular layer of the eye
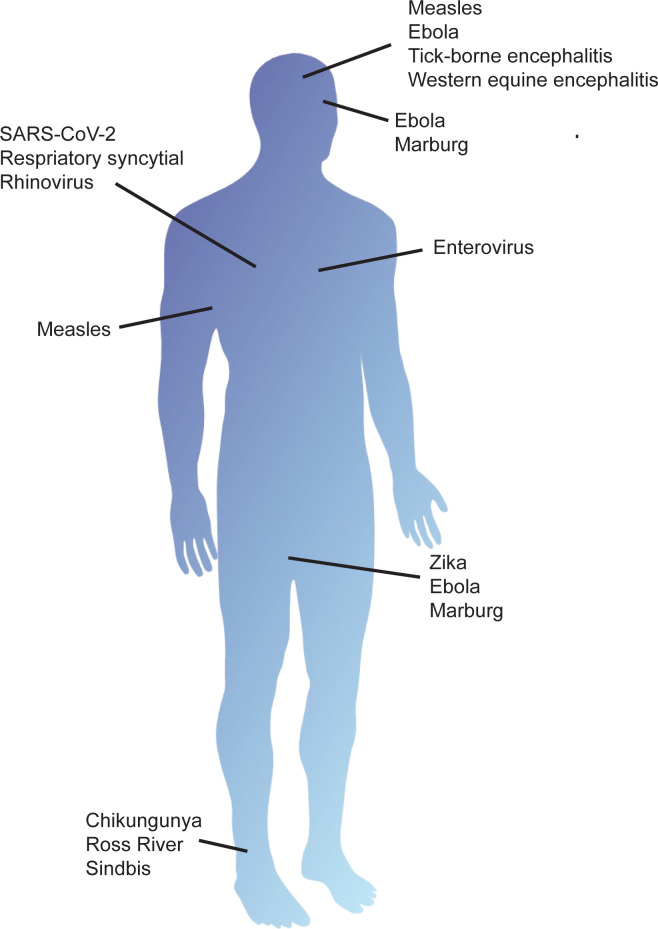
However, viral RNA persistence is not restricted to sites classically considered immune privileged, but can also occur in other sites including blood, lymphoid tissue, joints, respiratory tract, gastrointestinal tissues, and kidney, with a variety of known and unknown consequences [12,13,14,58–61] (Table 1, Fig 2). Organ-specific problems include chronic joint pain after infection with alphaviruses such as chikungunya, Ross River, and Sindbis that acutely cause rash and arthritis [10,32,62], cardiomyopathy (Box 1) after enterovirus infection [30], asymptomatic shedding of respiratory viruses [63], and chronic pulmonary disease associated with respiratory syncytial virus (RSV) and rhinovirus persistence [29,42,43]. Consequences may also include more nonspecific postviral syndromes such as PASC, post-Ebola, and post-polio syndromes, characterized by symptoms including fatigue, headache, muscle pain, and joint pain [23,31,64].
Fig 2. Sites of RNA persistence following infection.
Tissues in which RNA viruses persist after infection include the nervous system, eyes, joints, lymph nodes, heart, respiratory tract, and testes. SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2.
Viral RNA persistence in the absence of culturable virus is typically detected in RNA extracted from secretions, blood, or tissue samples. For long-lived cells such as neurons or cardiac myocytes, this RNA is presumed to come from the originally infected surviving cells present in those samples. However, few studies have attempted to identify or characterize the cellular source of the RNA detected, and clearance from some tissues may be more effective than from others. For example, after recovery of experimentally infected nonhuman primates from acute Ebola and Marburg filovirus infections, viral RNA is no longer detectable in primary sites of replication such as the liver but can often be found in the eyes and testes, where macrophages and Sertoli cells, respectively, remain RNA positive [45,46,50]. Tissue macrophages are also the sites of alphavirus RNA persistence in joints and Zika virus persistence in lymphoid tissues [10,12,65]. Prolonged detection of viral RNA in respiratory secretions, stool, sweat, conjunctival fluid, and urine likely comes from infected epithelial cells and is common even though these cells are relatively short lived and continuously replaced [11,18,36,58,61,66–68]. In measles virus infections, epithelial cells in multiple tissues, lymphocytes and monocytes in blood, and lymphoid tissue are prominent sites of infection [69,70]. Infectious virus is cleared during induction of the adaptive immune response and can no longer be cultured from any site shortly after resolution of the rash. However, viral RNA remains detectable in peripheral blood mononuclear cells (Box 1), respiratory secretions, and urine for weeks to months, and even longer in lymphoid tissue [14,41,61,68,71]. Little is known about the nature of the viral RNA that is detected in measles or other acute RNA viral infections or whether cells with viral RNA are the originally infected cells that survived acute infection and avoided immune elimination or newly infected cells through continued cell-to-cell transfer of viral RNA.
Detection of infectious virus is inherently less sensitive than detection of viral RNA and may be influenced by the presence of neutralizing antibody in the sample. Cocultivation of cells from tissues or secretions with susceptible cells is required to recover viruses such as measles but may not have been attempted for studies reporting the presence of viral RNA. Therefore, lack of detection of infectious virus may be due in part to differences in sensitivity and availability of the assays used. Development of techniques that can more easily identify the presence of assembled virions capable of initiating infection would provide increased understanding of the clearance and persistence of RNA viruses.
What form of viral RNA persists in the absence of infectious virus?
Because infectious virus cannot be recovered and RNA is susceptible to degradation, it is often assumed that what is detected by reverse transcriptase polymerase chain reaction (RT-PCR; Box 1) is fragmented or degraded viral RNA [25]. However, several studies have shown the long-term presence of full-length RNA capable of resuming productive replication if immune control is relaxed [16,21,72–74]. Unexpected late transmission of Ebola, Marburg and Zika viruses attest to the presence of persistent full-length genomic RNA after apparent resolution of these infections [57,75–77].
For picornaviruses, positive-strand RNAs are detectable for longer than negative-strand RNAs, and for coronaviruses, genomic RNAs are detectable for longer than the subgenomic RNAs that are produced during active virus replication [78,79]. However, these differences may reflect the relative abundance of these RNAs, and for alphaviruses, subgenomic RNA, which is more abundant than genomic RNA, is often detectable for longer.
For Borna disease virus that replicates in the nucleus, persistently infected cells retain genomic RNA in aggregates of viral ribonucleoproteins tethered to host chromosomes with host nuclear proteins that are maintained in daughter cells through the cell cycle [80,81]. However, most RNA viruses replicate in the cytoplasm, and, therefore, this is the likely site for RNA to persist, although reverse transcription by cellular enzymes has been postulated as a mechanism of persistence for nonretroviral RNA viruses as endogenous viral elements [82,83]. In the cytoplasm, ribonucleocapsid structures (Box 1) may protect the RNA of negative-strand viruses, while association with membranous structures may protect the RNA of positive-strand viruses, but this hypothesis requires further investigation.
How do RNA viruses evade the immune system to persist?
Innate immune mechanisms (Box 1) can control intracellular virus replication and target viral RNA for degradation, but adaptive immune responses are required for complete clearance of infected cells. Many intrinsic cellular antiviral mechanisms detect features of viral RNAs that are distinct from cellular RNAs, such as CpG content (Box 1), 5′ triphosphate, cap structure, and double-stranded RNA [84,85]. Recognition by innate sensors can target viral RNA for degradation or cause the inhibition of translation and replication and can activate pathways that result in the production of the signaling molecule interferon (IFN). Synthesis of IFN-stimulated antiviral proteins can further decrease virus replication and RNA synthesis [86]. Therefore, viral pathogens have often evolved RNA sequences and structures that circumvent induction of innate immune responses to promote virus replication and intracellular survival. However, adaptive immune responses consisting of virus-specific antibody and T cells are still induced.
Complete clearance of virus and virus-infected cells requires both prevention of virus spread to new cells and elimination of previously infected cells, either through virus-induced or immune-mediated cell death. Although viruses frequently lyse cells in tissue culture, primary cells and cells infected in vivo are often resistant to induction of cell death. These cells activate intrinsic cellular pathways that promote survival and combine with both host and viral strategies to downregulate replication and prevent lethal damage to the infected cell [87] (Fig 3). Persistence can evolve in the infected host through rapid mutation and selection of less lytic viral variants. This evolutionary process is facilitated by the error prone RNA-directed RNA polymerases that characterize RNA viruses [88,89] and by editing enzymes in the host cell [90,91]. In addition, early treatment with antibody may promote persistent infection [92,93].
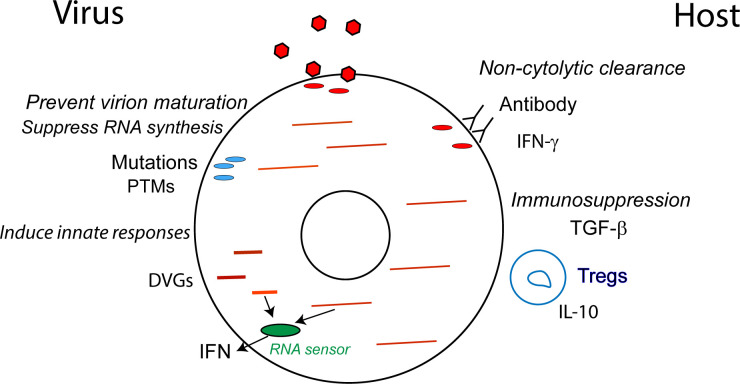
Fig 3. Mechanisms for suppressing production of infectious virions.
Several mechanisms exist whereby the virus and host can suppress the production of infectious virions to facilitate the survival of infected cells and viral RNA persistence. For example, the virus may acquire mutations that decrease virion assembly, induce innate responses, or decrease RNA synthesis, while the host employs antiviral immune responses that facilitate infected cell survival. DVG, defective viral genome; IFN, interferon; IL, interleukin; PTM, posttranslational modification; TGF, transforming growth factor.
Immune mechanisms for eliminating virus-infected cells that survive infection include cell killing by cytotoxic cells such as natural killer cells, which recognize a lack of major histocompatibility complex (MHC) class I expression (Box 1), and CD8+ T cells that recognize viral peptides expressed in the context of MHC class I molecules. In addition, binding of antibodies to the infected cell surface can direct cells toward antibody-mediated cytotoxicity or phagocytosis by immune cells [94–96]. Therefore, immune-mediated clearance requires recognition of the infected cell by immune effector cells, primarily through changes in surface expression of host or viral proteins. However, adaptive immune-mediated virus clearance is not always cytolytic (Box 1). For essential cells that are not easily replaced, such as neurons, noncytolytic control is advantageous for the host [97,98]. Antibodies that recognize alphavirus surface glycoproteins are required for clearance of infectious virions from the brains of infected mice and act by inducing antiviral signaling cascades that suppress production of viral RNA and infectious virions and inhibit virus release without harming the infected neurons [96,99–103]. Thus, the infected neuron survives with viral RNA still present. T cells can also employ noncytolytic mechanisms for cell type–specific clearance of infectious virus through local production of cytokines with antiviral activity such as IFN-γ [104–107]. T cell cytotoxicity may also be actively suppressed, particularly in immune-privileged sites, by expression of suppressive cytokines (e.g., TGF-β) and preferential recruitment of regulatory T cells [50,108]. Thus, the adaptive immune response can employ several noncytolytic mechanisms for clearance of infectious virus that allow survival of cells that still harbor viral RNA (Fig 3).
Strategies that avoid immune-mediated clearance of infected cells
To escape clearance, viruses must avoid both elimination by the immune response and killing of all infected cells, processes that are more likely to occur in some types of cells and tissues than in others. Avoiding immune-mediated clearance mechanisms requires the infected cell to become invisible to the immune system or unresponsive to cytolytic immune effectors by eliminating both surface expression of viral proteins and MHC presentation of viral peptides. Viruses infecting long-lived cells in immune-privileged tissues may be particularly likely to survive and retain persistent RNA after infection [11,19,21,50,109–113]. Several early studies of progressive tick-borne and western equine viral encephalitis conducted prior to the availability of sensitive methods for detecting viral RNA provided clinical and pathological evidence of RNA persistence and ongoing inflammation in the absence of infectious virus in the CNS [17,20,114–116]. As neurons (and likely other long-lived cells such as cardiac myocytes) mature and become fully differentiated, they acquire the ability to restrict virus replication and survive the stress of infection [117–119]. The mechanism(s) underlying differentiation-dependent susceptibility to virus infection have not been fully elucidated but likely involve both increased expression of innate factors that restrict virus replication and/or promote cell survival and decreased availability of factors required for virus replication in terminally differentiated cells [117,120].
Survival of infected cells is often accompanied by acquisition of viral mutations that foster persistence. For example, for viruses that are assembled and released from the cell surface, mutations that limit or prevent cell surface expression of viral proteins can prevent recognition by antibodies. In the measles virus-induced late disease SSPE, virion proteins required for particle assembly at the plasma membrane (hemagglutinin, fusion, and matrix) have acquired changes that prevent cell surface expression and virion assembly but promote cell-to-cell ribonucleoprotein transfer to uninfected cells, thereby allowing continued spread of viral RNA without producing infectious virions [121–124]. Similar mutations have been observed in the viral RNAs from persistent CNS infections due to mumps and mouse hepatitis viruses [113,125].
Persistence in cells that are replaced more frequently (e.g., endothelial cells, epithelial cells, lymphocytes, and monocytes) may continue for shorter periods of time. In lymphocytic choriomeningitis virus (LCMV) infection of cell fate reporter mice, noncytolytic clearance from hepatocytes is accompanied by continuous infection of new cells to maintain persistence [126]. Epithelial cells in the respiratory tract and elsewhere commonly permit rapid cell-to-cell transfer of viral nucleocapsids without release of virus from the cell surface that may foster persistence of detectable viral RNA long after infectious virions can be recovered [127]. It is not clear whether the observed slow decrease in levels of detectable viral RNA in peripheral blood mononuclear cells, urine, stool, and respiratory secretions (Fig 1) is due to turnover of these cells, RNA degradation, or eventual immune-mediated elimination [14].
Strategies that avoid killing of infected cells
Avoiding virus-induced cell death usually requires limiting virus replication [87,128]. A variety of mechanisms are employed by viruses to restrict replication. For example, several RNA viruses (e.g., Borna disease virus, LCMV, coxsackievirus, and hantavirus) undergo 5′-terminal trimming of the genome that both suppresses replication and prevents the activation of innate immune responses [129–132]. Ebola virus genomes from the eyes of infected humans and ferrets have acquired stop codons in the polymerase gene that would limit RNA synthesis [133,134], and phosphorylation of the paramyxovirus P protein represses viral replication late in infection and fosters persistence [135].
Replication may also be restricted through activation of IFN pathways and expression of IFN-stimulated genes encoding antiviral proteins. For example, production of defective viral genomes (DVGs), particularly so-called “copy-back” DVGs, by many RNA viruses leads to induction of innate immune responses that control virus replication and permit persistence [136]. Copy-back DVGs are generated when the viral polymerase becomes detached from the template genome and switches to another genome template to duplicate the terminal end. These shorter incomplete genomes have a replicative advantage over full-length genomes and can induce both IFN and pro-survival pathways to promote persistence [137,138]. For example, in lung infection with RSV, early production of DVGs activates RIG-I-like receptors to stimulate the activation of IRF3 and IRF1, leading to production of TNFα, IFNλ, and IFIT1, suppression of virus replication, and survival of persistently infected cells [136,139,140]. DVGs have been demonstrated in the testes during filovirus infection of nonhuman primates [141] and in the lungs of children with RSV infection [140].
What are the consequences of RNA persistence?
Viral RNA alone may stimulate innate immune responses and inflammation associated with IFN production to drive chronic inflammation [60]. However, viral RNA persistence without production of infectious virions is frequently accompanied by evidence of viral protein synthesis and T cell activation, indicating that viral RNA is being translated, if not replicated or assembled into culturable virus particles [10,142]. Viral protein can sometimes be detected by immunocytochemical assays [10,15,19,143] (Box 1), but such techniques are relatively insensitive compared with those for detecting RNA, and most often the evidence comes from ongoing or renewed stimulation of a local or systemic adaptive immune response [144]. For example, in mice that have recovered from acute rhabdovirus and influenza virus infections, passively transferred immune cells detect and are activated by persistent viral antigens [145,146] (Box 1). Although antigens may persist without ongoing translation of viral RNAs, longitudinal studies of measles and Ebola have identified recurrent waves of immune activation consistent with periodic increases in immune stimulation by viral proteins [71,147,148].
Consequences of chronic immune stimulation associated with persistent RNA are dependent on the site of persistence. For example, persistence of RNA in the CNS of mice that have recovered from acute alphavirus-induced encephalomyelitis is accompanied by detection of viral protein weeks after infection, and maintenance of B cells secreting antiviral antibodies and T cells producing IFN-γ for more than a year [100,149–152]. Likewise, oligodendrocytes surviving acute coronavirus infection with persistent RNA promote prolonged T cell residence and inflammation in the CNS [111,153]. This type of late CNS pathology may or may not be associated with progressive neurologic disease [17,115,154]. Persistence of alphavirus RNA in synovial tissues is linked to the prolonged inflammation and joint pain that many patients have after infection, and persistence of enteroviral RNA in the myocardium is associated with progressive cardiac dysfunction [10,30].
Determining the importance of RNA persistence is of particular relevance for understanding the failure to fully recover from acute infections such as occurs after SARS-CoV-2 infection and Ebola virus disease. PASC afflicts 30% to 50% of those recovering from COVID-19 [23] and encompasses a variety of symptoms that affect different organ systems including fatigue, brain fog, muscle weakness, gastrointestinal distress, cough, and shortness of breath [26,155]. Infectious virions in blood (viremia) have not been documented, but viral RNA in blood (RNAemia) is found in those with more severe disease, suggesting systemic spread of infection, and is predictive of PASC [27,28]. Those with persistent symptoms at 3 months after acute disease are more likely to have increased levels of pro-inflammatory cytokines (e.g., TNF) and chemokines (e.g., IP-10 and MCP-1), as well as factors associated with vascular injury (e.g., VCAM-1 and ICAM-1) [156]. Prolongation of symptoms due to ongoing immune stimulation is suggested by identification of viral RNA and protein in a subset of monocytes [143]. The importance of persistent viral RNA relative to inflammation, autoimmunity, or reactivation of latent infection with other viruses (e.g., Epstein–Barr virus) in the pathogenesis of PASC remains to be determined, but PASC is likely to be more than one disease with multiple contributing factors [28]. Persistent RNA could continue to stimulate innate immune responses, but protein translation would be needed for continued activation of adaptive immune responses (Box 1).
Persistence and long-term immune stimulation in lymphoid tissue may also provide benefit to the host via prolonged stimulation and induction of durable immunity to reinfection [41,70]. Macaques infected with measles virus have persistent RNA in lymphocytes and myeloid cells for months after resolution of the acute rash disease. Pathologic examination of their lymph nodes shows a progressive increase in germinal centers with proliferating B cells accompanied by continued appearance of virus-specific peripheral follicular helper CD4+ T cells and antibody-secreting cells in circulation and affinity maturation of antiviral antibody [41]. This contrasts with the short-lived immunity induced by SARS-CoV-2 and many other respiratory viruses potentially due to a failure to establish the persistence of RNA in lymphoid tissue required for prolonged synthesis of viral antigens for immune stimulation [157–161].
Concluding remarks
Clinical recovery, elimination of detectable infectious virus, and development of immunity after infection with RNA viruses that cause acute infections do not necessarily result in complete elimination of the viral RNA. Both virus and host mechanisms can prevent production of infectious virions while allowing persistence of viral RNA in previously infected cells. Viral mechanisms include mutations in genes coding for proteins required for assembly or replication and evasion of the adaptive immune response. Host mechanisms include the use of noncytolytic clearance mechanisms that allow infected cells to survive and cell type–specific activation of innate immune responses that suppress virus replication in infected cells. How RNA is protected from degradation is unclear, but occasional late transmission and continuing stimulation of adaptive immune responses indicate persistence of genomic and translatable viral RNA.
Our understanding of the long-term consequences related to disease and durable immunity and the mechanisms of persistence will benefit from further investigation and development of appropriate animal models. Future studies will be needed to identify the types and locations of cells harboring viral RNA and the metabolic state of these cells compared with uninfected cells. In addition, a better understanding of the state of the viral RNA, how it is protected from degradation, the relative amounts of full-length and DVG or fragmented RNA, and the contribution of continued RNA synthesis to persistence will help to solve this mystery and inform potential interventions. Identification of the role of RNA persistence in late disease could be advanced with longitudinal studies that evaluate treatments that suppress RNA replication and examine their effects on RNA persistence and long-term outcomes.
Acknowledgments
Thanks go to the multiple members of my laboratory who have contributed to identification of the persistence of viral RNA and to addressing the questions raised by this observation.
Abbreviations
- CNS
central nervous system
- COVID
Coronavirus Disease
- DVG
defective viral genome
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IFN
interferon
- LCMV
lymphocytic choriomeningitis virus
- MHC
major histocompatibility complex
- PASC
post-acute sequelae of COVID-19
- RSV
respiratory syncytial virus
- RT-PCR
reverse transcriptase polymerase chain reaction
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- SSPE
subacute sclerosing panencephalitis
Funding Statement
Work from the author’s laboratory has been funded by the U.S. National Institutes of Health: R01 NS038932 (DEG), R01 NS87539 (DEG), R01 AI131228 (DEG), R21 AI095981 (DEG) and R01 AI153140 (DEG); and The Bill and Melinda Gates Foundation (DEG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Weller TH. Varicella and herpes zoster. Changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med. 1983;309(22):1362–8. doi: 10.1056/NEJM198312013092205 [DOI] [PubMed] [Google Scholar]
- 2.Worth AJ, Houldcroft CJ, Booth C. Severe Epstein-Barr virus infection in primary immunodeficiency and the normal host. Br J Haematol. 2016;175(4):559–76. doi: 10.1111/bjh.14339 [DOI] [PubMed] [Google Scholar]
- 3.Sacks SL, Griffiths PD, Corey L, Cohen C, Cunningham A, Dusheiko GM, et al. HSV shedding. Antivir Res. 2004;63(Suppl 1):S19–26. doi: 10.1016/j.antiviral.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 4.Anderson RM, Medley GF. Epidemiology of HIV infection and AIDS: incubation and infectious periods, survival and vertical transmission. AIDS. 1988;2(Suppl 1):S57–63. [PubMed] [Google Scholar]
- 5.Lyles RH, Munoz A, Yamashita TE, Bazmi H, Detels R, Rinaldo CR, et al. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J Infect Dis. 2000;181(3):872–80. doi: 10.1086/315339 [DOI] [PubMed] [Google Scholar]
- 6.Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284(4):450–6. doi: 10.1001/jama.284.4.450 [DOI] [PubMed] [Google Scholar]
- 7.Cuthbert JA. Hepatitis A: old and new. Clin Microbiol Rev. 2001;14(1):38–58. doi: 10.1128/CMR.14.1.38-58.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moss WJ, Strebel P. Biological feasibility of measles eradication. J Infect Dis. 2011;204(Suppl 1):S47–53. doi: 10.1093/infdis/jir065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yilmaz A, Marklund E, Andersson M, Nilsson S, Andersson LM, Lindh M, et al. Upper Respiratory Tract Levels of Severe Acute Respiratory Syndrome Coronavirus 2 RNA and Duration of Viral RNA Shedding Do Not Differ Between Patients With Mild and Severe/Critical Coronavirus Disease 2019. J Infect Dis. 2021;223(1):15–8. doi: 10.1093/infdis/jiaa632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010;184(10):5914–27. doi: 10.4049/jimmunol.0900255 [DOI] [PubMed] [Google Scholar]
- 11.Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, et al. Persistence of Zika Virus in Body Fluids—Final Report. N Engl J Med. 2018;379(13):1234–43. doi: 10.1056/NEJMoa1613108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch AJ, Smith JL, Haese NN, Broeckel RM, Parkins CJ, Kreklywich C, et al. Zika Virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017;13(3):e1006219. doi: 10.1371/journal.ppat.1006219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caviness K, Kuhn JH, Palacios G. Ebola virus persistence as a new focus in clinical research. Curr Opin Virol. 2017;23:43–8. doi: 10.1016/j.coviro.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 14.Lin WH, Kouyos RD, Adams RJ, Grenfell BT, Griffin DE. Prolonged persistence of measles virus RNA is characteristic of primary infection dynamics. Proc Natl Acad Sci U S A. 2012;109(37):14989–94. doi: 10.1073/pnas.1211138109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanford RE, Feng Z, Chavez D, Guerra B, Brasky KM, Zhou Y, et al. Acute hepatitis A virus infection is associated with a limited type I interferon response and persistence of intrahepatic viral RNA. Proc Natl Acad Sci U S A. 2011;108(27):11223–8. doi: 10.1073/pnas.1101939108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fragkoudis R, Dixon-Ballany CM, Zagrajek AK, Kedzierski L, Fazakerley JK. Following Acute Encephalitis, Semliki Forest Virus is Undetectable in the Brain by Infectivity Assays but Functional Virus RNA Capable of Generating Infectious Virus Persists for Life. Viruses. 2018;10(5). doi: 10.3390/v10050273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine B, Hardwick JM, Griffin DE. Persistence of alphaviruses in vertebrate hosts. Trends Microbiol. 1994;2(1):25–8. doi: 10.1016/0966-842x(94)90341-7 [DOI] [PubMed] [Google Scholar]
- 18.Owusu D, Pomeroy MA, Lewis NM, Wadhwa A, Yousaf AR, Whitaker B, et al. Persistent SARS-CoV-2 RNA Shedding Without Evidence of Infectiousness: A Cohort Study of Individuals With COVID-19. J Infect Dis. 2021;224(8):1362–71. doi: 10.1093/infdis/jiab107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Destombes J, Couderc T, Thiesson D, Girard S, Wilt SG, Blondel B. Persistent poliovirus infection in mouse motoneurons. J Virol. 1997;71(2):1621–8. doi: 10.1128/JVI.71.2.1621-1628.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathur A, Arora KL, Rawat S, Chaturvedi UC. Persistence, latency and reactivation of Japanese encephalitis virus infection in mice. J Gen Virol. 1986;67(Pt 2):381–5. [DOI] [PubMed] [Google Scholar]
- 21.Appler KK, Brown AN, Stewart BS, Behr MJ, Demarest VL, Wong SJ, et al. Persistence of West Nile virus in the central nervous system and periphery of mice. PLoS ONE. 2010;5(5):e10649. doi: 10.1371/journal.pone.0010649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang B, Fan J, Huang J, Guo E, Fu Y, Liu S, et al. Clinical and molecular characteristics of COVID-19 patients with persistent SARS-CoV-2 infection. Nat Commun. 2021;12(1):3501. doi: 10.1038/s41467-021-23621-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw Open. 2021;4(10):e2128568. doi: 10.1001/jamanetworkopen.2021.28568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramakrishnan A, Zreloff J, Moore MA, Bergquist SH, Cellai M, Higdon J, et al. Prolonged Symptoms After COVID-19 Infection in Outpatients. Open Forum. Infect Dis. 2021;8(3):ofab060. doi: 10.1093/ofid/ofab060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramakrishnan RK, Kashour T, Hamid Q, Halwani R, Tleyjeh IM. Unraveling the Mystery Surrounding Post-Acute Sequelae of COVID-19. Front Immunol. 2021;12:686029. doi: 10.3389/fimmu.2021.686029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. 2021;12(1):6571. doi: 10.1038/s41467-021-26513-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ram-Mohan N, Kim D, Rogers AJ, Blish CA, Nadeau KC, Blomkalns AL, et al. Association Between SARS-CoV-2 RNAemia and Postacute Sequelae of COVID-19. Open Forum. Infect Dis. 2022;9(2):ofab646. doi: 10.1093/ofid/ofab646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022. doi: 10.1016/j.cell.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kling S, Donninger H, Williams Z, Vermeulen J, Weinberg E, Latiff K, et al. Persistence of rhinovirus RNA after asthma exacerbation in children. Clin Exp Allergy. 2005;35(5):672–8. doi: 10.1111/j.1365-2222.2005.02244.x [DOI] [PubMed] [Google Scholar]
- 30.Kuhl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, Poller W, et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112(13):1965–70. doi: 10.1161/CIRCULATIONAHA.105.548156 [DOI] [PubMed] [Google Scholar]
- 31.Li Hi Shing S, Chipika RH, Finegan E, Murray D, Hardiman O, Bede P. Post-polio Syndrome: More Than Just a Lower Motor Neuron Disease. Front Neurol. 2019;10(773). doi: 10.3389/fneur.2019.00773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soden M, Vasudevan H, Roberts B, Coelen R, Hamlin G, Vasudevan S, et al. Detection of viral ribonucleic acid and histologic analysis of inflamed synovium in Ross River virus infection. Arthritis Rheum. 2000;43(2):365–9. doi: [DOI] [PubMed] [Google Scholar]
- 33.Niklasson B, Espmark A, Lundstrom J. Occurrence of arthralgia and specific IgM antibodies three to four years after Ockelbo disease. J Infect Dis. 1988;157(4):832–5. doi: 10.1093/infdis/157.4.832 [DOI] [PubMed] [Google Scholar]
- 34.Russell K, Hills SL, Oster AM, Porse CC, Danyluk G, Cone M, et al. Male-to-Female Sexual Transmission of Zika Virus-United States, January-April 2016. Clin Infect Dis. 2017;64(2):211–3. doi: 10.1093/cid/ciw692 [DOI] [PubMed] [Google Scholar]
- 35.Pradhan S, Gupta RK, Singh MB, Mathur A. Biphasic illness pattern due to early relapse in Japanese-B virus encephalitis. J Neurol Sci. 2001;183(1):13–8. doi: 10.1016/s0022-510x(00)00453-6 [DOI] [PubMed] [Google Scholar]
- 36.Murray K, Walker C, Herrington E, Lewis JA, McCormick J, Beasley DW, et al. Persistent infection with West Nile virus years after initial infection. J Infect Dis. 2010;201(1):2–4. doi: 10.1086/648731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gritsun TS, Frolova TV, Zhankov AI, Armesto M, Turner SL, Frolova MP, et al. Characterization of a siberian virus isolated from a patient with progressive chronic tick-borne encephalitis. J Virol. 2003;77(1):25–36. doi: 10.1128/jvi.77.1.25-36.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das Adhikari U, Eng G, Farcasanu M, Avena LE, Choudhary MC, Triant VA, et al. Fecal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-Cov-2) RNA Is Associated With Decreased Coronavirus Disease 2019 (COVID-19) Survival. Clin Infect Dis. 2022;74(6):1081–4. doi: 10.1093/cid/ciab623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thielebein A, Ighodalo Y, Taju A, Olokor T, Omiunu R, Esumeh R, et al. Virus persistence after recovery from acute Lassa fever in Nigeria: a 2-year interim analysis of a prospective longitudinal cohort study. Lancet Microbe. 2022;3(1):e32–40. doi: 10.1016/S2666-5247(21)00178-6 [DOI] [PubMed] [Google Scholar]
- 40.Raabe VN, Kann G, Ribner BS, Morales A, Varkey JB, Mehta AK, et al. Favipiravir and Ribavirin Treatment of Epidemiologically Linked Cases of Lassa Fever. Clin Infect Dis. 2017;65(5):855–9. doi: 10.1093/cid/cix406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson AN, Lin WW, Shivakoti R, Putnam NE, Mangus LM, Adams RJ, et al. Association of persistent wild-type measles virus RNA with long-term humoral immunity in rhesus macaques. JCI. Insight. 2020. doi: 10.1172/jci.insight.134992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkinson TM, Donaldson GC, Johnston SL, Openshaw PJ, Wedzicha JA. Respiratory syncytial virus, airway inflammation, and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(8):871–6. doi: 10.1164/rccm.200509-1489OC [DOI] [PubMed] [Google Scholar]
- 43.Kokturk N, Bozdayi G, Yilmaz S, Dogan B, Gulbahar O, Rota S, et al. Detection of adenovirus and respiratory syncytial virus in patients with chronic obstructive pulmonary disease: Exacerbation versus stable condition. Mol Med Rep. 2015;12(2):3039–46. doi: 10.3892/mmr.2015.3681 [DOI] [PubMed] [Google Scholar]
- 44.Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–23. doi: 10.1164/ajrccm.164.9.2105011 [DOI] [PubMed] [Google Scholar]
- 45.Zeng X, Blancett CD, Koistinen KA, Schellhase CW, Bearss JJ, Radoshitzky SR, et al. Identification and pathological characterization of persistent asymptomatic Ebola virus infection in rhesus monkeys. Nat Microbiol. 2017;2:17113. doi: 10.1038/nmicrobiol.2017.113 [DOI] [PubMed] [Google Scholar]
- 46.Keita AK, Vidal N, Toure A, Diallo MSK, Magassouba N, Baize S, et al. A 40-Month Follow-Up of Ebola Virus Disease Survivors in Guinea (PostEbogui) Reveals Long-Term Detection of Ebola Viral Ribonucleic Acid in Semen and Breast Milk. Open Forum. Infect Dis. 2019;6(12):ofz482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varkey JB, Shantha JG, Crozier I, Kraft CS, Lyon GM, Mehta AK, et al. Persistence of Ebola Virus in Ocular Fluid during Convalescence. N Engl J Med. 2015;372(25):2423–7. doi: 10.1056/NEJMoa1500306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sow MS, Etard JF, Baize S, Magassouba N, Faye O, Msellati P, et al. New Evidence of Long-lasting Persistence of Ebola Virus Genetic Material in Semen of Survivors. J Infect Dis. 2016;214(10):1475–6. doi: 10.1093/infdis/jiw078 [DOI] [PubMed] [Google Scholar]
- 49.Jacobs M, Rodger A, Bell DJ, Bhagani S, Cropley I, Filipe A, et al. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet. 2016;388(10043):498–503. doi: 10.1016/S0140-6736(16)30386-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coffin KM, Liu J, Warren TK, Blancett CD, Kuehl KA, Nichols DK, et al. Persistent Marburg Virus Infection in the Testes of Nonhuman Primate Survivors. Cell Host Microbe. 2018;24(3):405–16 e3. doi: 10.1016/j.chom.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 51.Modlin JF, Jabbour JT, Witte JJ, Halsey NA. Epidemiologic studies of measles, measles vaccine, and subacute sclerosing panencephalitis. Pediatrics. 1977;59(4):505–12. [PubMed] [Google Scholar]
- 52.Connolly JH, Allen IV, Hurwitz LJ, Millar JH. Measles-virus antibody and antigen in subacute sclerosing panencephalitis. Lancet. 1967;1(7489):542–4. doi: 10.1016/s0140-6736(67)92117-4 [DOI] [PubMed] [Google Scholar]
- 53.Baczko K, Liebert UG, Billeter M, Cattaneo R, Budka H, ter Meulen V. Expression of defective measles virus genes in brain tissues of patients with subacute sclerosing panencephalitis. J Virol. 1986;59(2):472–8. doi: 10.1128/JVI.59.2.472-478.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diallo B, Sissoko D, Loman NJ, Bah HA, Bah H, Worrell MC, et al. Resurgence of Ebola Virus Disease in Guinea Linked to a Survivor With Virus Persistence in Seminal Fluid for More Than 500 Days. Clin Infect Dis. 2016;63(10):1353–6. doi: 10.1093/cid/ciw601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mate SE, Kugelman JR, Nyenswah TG, Ladner JT, Wiley MR, Cordier-Lassalle T, et al. Molecular Evidence of Sexual Transmission of Ebola Virus. N Engl J Med. 2015;373(25):2448–54. doi: 10.1056/NEJMoa1509773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schindell BG, Webb AL, Kindrachuk J. Persistence and Sexual Transmission of Filoviruses. Viruses. 2018;10(12). doi: 10.3390/v10120683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turmel JM, Abgueguen P, Hubert B, Vandamme YM, Maquart M, Le Guillou-Guillemette H, et al. Late sexual transmission of Zika virus related to persistence in the semen. Lancet. 2016;387(10037):2501. doi: 10.1016/S0140-6736(16)30775-9 [DOI] [PubMed] [Google Scholar]
- 58.Chughtai AA, Barnes M, Macintyre CR. Persistence of Ebola virus in various body fluids during convalescence: evidence and implications for disease transmission and control. Epidemiol Infect. 2016;144(8):1652–60. doi: 10.1017/S0950268816000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuno G. Persistence of arboviruses and antiviral antibodies in vertebrate hosts: its occurrence and impacts. Rev Med Virol. 2001;11(3):165–90. doi: 10.1002/rmv.314 [DOI] [PubMed] [Google Scholar]
- 60.McCarthy MK, Morrison TE. Persistent RNA virus infections: do PAMPS drive chronic disease? Curr Opin Virol. 2017;23:8–15. doi: 10.1016/j.coviro.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riddell MA, Moss WJ, Hauer D, Monze M, Griffin DE. Slow clearance of measles virus RNA after acute infection. J Clin Virol. 2007;39(4):312–7. doi: 10.1016/j.jcv.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 62.Adouchief S, Smura T, Sane J, Vapalahti O, Kurkela S. Sindbis virus as a human pathogen-epidemiology, clinical picture and pathogenesis. Rev Med Virol. 2016. doi: 10.1002/rmv.1876 [DOI] [PubMed] [Google Scholar]
- 63.Shaman J, Morita H, Birger R, Boyle M, Comito D, Lane B, et al. Asymptomatic Summertime Shedding of Respiratory Viruses. J Infect Dis. 2018;217(7):1074–7. doi: 10.1093/infdis/jix685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scott JT, Sesay FR, Massaquoi TA, Idriss BR, Sahr F, Semple MG. Post-Ebola Syndrome, Sierra Leone. Emerg Infect Dis. 2016;22(4):641–6. doi: 10.3201/eid2204.151302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest. 2010;120(3):894–906. doi: 10.1172/JCI40104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giannella M, Alonso M. Garcia de Viedma D, Lopez Roa P, Catalan P, Padilla B, et al. Prolonged viral shedding in pandemic influenza A(H1N1): clinical significance and viral load analysis in hospitalized patients. Clin Microbiol Infect. 2011;17(8):1160–5. doi: 10.1111/j.1469-0691.2010.03399.x [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Guo Q, Yan Z, Zhou D, Zhang W, Zhou S, et al. Factors Associated With Prolonged Viral Shedding in Patients With Avian Influenza A(H7N9) Virus Infection. J Infect Dis. 2018;217(11):1708–17. doi: 10.1093/infdis/jiy115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Permar SR, Moss WJ, Ryon JJ, Monze M, Cutts F, Quinn TC, et al. Prolonged measles virus shedding in human immunodeficiency virus-infected children, detected by reverse transcriptase-polymerase chain reaction. J Infect Dis. 2001;183(4):532–8. doi: 10.1086/318533 [DOI] [PubMed] [Google Scholar]
- 69.Laksono BM, de Vries RD, Verburgh RJ, Visser EG, de Jong A, Fraaij PLA, et al. Studies into the mechanism of measles-associated immune suppression during a measles outbreak in the Netherlands. Nat Commun. 2018;9(1):4944. doi: 10.1038/s41467-018-07515-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin WW, Moran E, Adams RJ, Sievers RE, Hauer D, Godin S, et al. A durable protective immune response to wild-type measles virus infection of macaques is due to viral replication and spread in lymphoid tissues. Sci Transl Med. 2020;12(537). doi: 10.1126/scitranslmed.aax7799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nelson AN, Putnam N, Hauer D, Baxter VK, Adams RJ, Griffin DE. Evolution of T Cell Responses during Measles Virus Infection and RNA Clearance. Sci Rep. 2017;7(1):11474. doi: 10.1038/s41598-017-10965-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levine B, Griffin DE. Persistence of viral RNA in mouse brains after recovery from acute alphavirus encephalitis. J Virol. 1992;66(11):6429–35. doi: 10.1128/JVI.66.11.6429-6435.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miller KD, Matullo CM, Milora KA, Williams RM, O’Regan KJ, Rall GF. Immune-Mediated Control of a Dormant Neurotropic RNA Virus Infection. J Virol. 2019;93(18). doi: 10.1128/JVI.00241-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mathur A, Kulshreshtha R, Chaturvedi UC. Induction of secondary immune response by reactivated Japanese encephalitis virus in latently infected mice. Immunology. 1987;60(4):481–4. [PMC free article] [PubMed] [Google Scholar]
- 75.Heeney JL. Ebola: Hidden reservoirs. Nature. 2015;527(7579):453–5. doi: 10.1038/527453a [DOI] [PubMed] [Google Scholar]
- 76.Harrower J, Kiedrzynski T, Baker S, Upton A, Rahnama F, Sherwood J, et al. Sexual Transmission of Zika Virus and Persistence in Semen, New Zealand, 2016. Emerg Infect Dis. 2016;22(10):1855–7. doi: 10.3201/eid2210.160951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keita AK, Koundouno FR, Faye M, Dux A, Hinzmann J, Diallo H, et al. Resurgence of Ebola virus in 2021 in Guinea suggests a new paradigm for outbreaks. Nature. 2021;597(7877):539–43. doi: 10.1038/s41586-021-03901-9 [DOI] [PubMed] [Google Scholar]
- 78.Dimcheff DE, Valesano AL, Rumfelt KE, Fitzsimmons WJ, Blair C, Mirabelli C, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Total and Subgenomic RNA Viral Load in Hospitalized Patients. J Infect Dis. 2021;224(8):1287–93. doi: 10.1093/infdis/jiab215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Santos Bravo M, Nicolas D, Berengua C, Fernandez M, Hurtado JC, Tortajada M, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Normalized Viral Loads and Subgenomic RNA Detection as Tools for Improving Clinical Decision Making and Work Reincorporation. J Infect Dis. 2021;224(8):1325–32. doi: 10.1093/infdis/jiab394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsumoto Y, Hayashi Y, Omori H, Honda T, Daito T, Horie M, et al. Bornavirus closely associates and segregates with host chromosomes to ensure persistent intranuclear infection. Cell Host Microbe. 2012;11(5):492–503. doi: 10.1016/j.chom.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 81.Garcia BCB, Horie M, Kojima S, Makino A, Tomonaga K. BUD23-TRMT112 interacts with the L protein of Borna disease virus and mediates the chromosomal tethering of viral ribonucleoproteins. Microbiol Immunol. 2021;65(11):492–504. doi: 10.1111/1348-0421.12934 [DOI] [PubMed] [Google Scholar]
- 82.Klenerman P, Hengartner H, Zinkernagel RM. A non-retroviral RNA virus persists in DNA form. Nature. 1997;390(6657):298–301. doi: 10.1038/36876 [DOI] [PubMed] [Google Scholar]
- 83.Aiewsakun P, Katzourakis A. Endogenous viruses: Connecting recent and ancient viral evolution. Virology. 2015;479–480:26–37. doi: 10.1016/j.virol.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 84.Fensterl V, Sen GC. Interferon-induced Ifit proteins: their role in viral pathogenesis. J Virol. 2015;89(5):2462–8. doi: 10.1128/JVI.02744-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takata MA, Goncalves-Carneiro D, Zang TM, Soll SJ, York A, Blanco-Melo D, et al. CG dinucleotide suppression enables antiviral defence targeting non-self RNA. Nature. 2017;550(7674):124–7. doi: 10.1038/nature24039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schoggins JW. Interferon-Stimulated Genes: What Do They All Do? Annu Rev Virol. 2019;6(1):567–84. doi: 10.1146/annurev-virology-092818-015756 [DOI] [PubMed] [Google Scholar]
- 87.Randall RE, Griffin DE. Within host RNA virus persistence: mechanisms and consequences. Curr Opin Virol. 2017;23:35–42. doi: 10.1016/j.coviro.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vignuzzi M, Stone JK, Arnold JJ, Cameron CE, Andino R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature. 2006;439(7074):344–8. doi: 10.1038/nature04388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wolf YI, Kazlauskas D, Iranzo J, Lucia-Sanz A, Kuhn JH, Krupovic M, et al. Origins and Evolution of the Global RNA Virome. mBio. 2018;9(6). doi: 10.1128/mBio.02329-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Piontkivska H, Wales-McGrath B, Miyamoto M, Wayne ML. ADAR Editing in Viruses: An Evolutionary Force to Reckon with. Genome Biol Evol. 2021;13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cattaneo R, Schmid A, Eschle D, Baczko K, ter Meulen V, Billeter MA. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988;55(2):255–65. doi: 10.1016/0092-8674(88)90048-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu J, Trefry JC, Babka AM, Schellhase CW, Coffin KM, Williams JA, et al. Ebola virus persistence and disease recrudescence in the brains of antibody-treated nonhuman primate survivors. Sci Transl Med. 2022;14(631):eabi5229. doi: 10.1126/scitranslmed.abi5229 [DOI] [PubMed] [Google Scholar]
- 93.Rammohan KW, McFarland HF, McFarlin DE. Subacute sclerosing panencephalitis after passive immunization and natural measles infection: role of antibody in persistence of measles virus. Neurology. 1982;32(4):390–4. doi: 10.1212/wnl.32.4.390 [DOI] [PubMed] [Google Scholar]
- 94.Cheng HD, Dowell KG, Bailey-Kellogg C, Goods BA, Love JC, Ferrari G, et al. Diverse antiviral IgG effector activities are predicted by unique biophysical antibody features. Retrovirology. 2021;18(1):35. doi: 10.1186/s12977-021-00579-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2018;18(1):46–61. doi: 10.1038/nri.2017.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin J, Galaz-Montoya JG, Sherman MB, Sun SY, Goldsmith CS, O’Toole ET, et al. Neutralizing Antibodies Inhibit Chikungunya Virus Budding at the Plasma Membrane. Cell Host Microbe. 2018;24(3):417–28 e5. doi: 10.1016/j.chom.2018.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Griffin DE, Metcalf T. Clearance of virus infection from the CNS. Curr Opin Virol. 2011;1(3):216–21. doi: 10.1016/j.coviro.2011.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bartlett ML, Griffin DE. Acute RNA Viral Encephalomyelitis and the Role of Antibodies in the Central Nervous System. Viruses. 2020;12(9). doi: 10.3390/v12090988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Levine B, Hardwick JM, Trapp BD, Crawford TO, Bollinger RC, Griffin DE. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254(5033):856–60. doi: 10.1126/science.1658936 [DOI] [PubMed] [Google Scholar]
- 100.Nilaratanakul V, Chen J, Tran O, Baxter VK, Troisi EM, Yeh JX, et al. Germ Line IgM Is Sufficient, but Not Required, for Antibody-Mediated Alphavirus Clearance from the Central Nervous System. J Virol. 2018;92(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nilaratanakul V, Hauer DA, Griffin DE. Visualization of cell-type dependent effects of anti-E2 antibody and interferon-gamma treatments on localization and expression of Broccoli aptamer-tagged alphavirus RNAs. Sci Rep. 2020;10(1):5259. doi: 10.1038/s41598-020-61015-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yeh JX, Schultz KLW, Calvert V, Petricoin EF, Griffin DE. The NF-kappaB/leukemia inhibitory factor/STAT3 signaling pathway in antibody-mediated suppression of Sindbis virus replication in neurons. Proc Natl Acad Sci U S A. 2020;117(46):29035–45. doi: 10.1073/pnas.2016691117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Williamson LE, Reeder KM, Bailey K, Tran MH, Roy V, Fouch ME, et al. Therapeutic alphavirus cross-reactive E1 human antibodies inhibit viral egress. Cell. 2021;184(17):4430–46 e22. doi: 10.1016/j.cell.2021.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hausmann J, Pagenstecher A, Baur K, Richter K, Rziha HJ, Staeheli P. CD8 T cells require gamma interferon to clear borna disease virus from the brain and prevent immune system-mediated neuronal damage. J Virol. 2005;79(21):13509–18. doi: 10.1128/JVI.79.21.13509-13518.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Burdeinick-Kerr R, Govindarajan D, Griffin DE. Noncytolytic clearance of sindbis virus infection from neurons by gamma interferon is dependent on Jak/STAT signaling. J Virol. 2009;83(8):3429–35. doi: 10.1128/JVI.02381-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Binder GK, Griffin DE. Immune-mediated clearance of virus from the central nervous system. Microbes Infect. 2003;5(5):439–48. doi: 10.1016/s1286-4579(03)00047-9 [DOI] [PubMed] [Google Scholar]
- 107.Patterson CE, Lawrence DM, Echols LA, Rall GF. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J Virol. 2002;76(9):4497–506. doi: 10.1128/jvi.76.9.4497-4506.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reuter D, Sparwasser T, Hunig T, Schneider-Schaulies J. Foxp3+ regulatory T cells control persistence of viral CNS infection. PLoS ONE. 2012;7(3):e33989. doi: 10.1371/journal.pone.0033989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Atkinson B, Thorburn F, Petridou C, Bailey D, Hewson R, Simpson AJ, et al. Presence and Persistence of Zika Virus RNA in Semen, United Kingdom, 2016. Emerg Infect Dis. 2017;23(4):611–5. doi: 10.3201/eid2304.161692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thorson AE, Deen GF, Bernstein KT, Liu WJ, Yamba F, Habib N, et al. Persistence of Ebola virus in semen among Ebola virus disease survivors in Sierra Leone: A cohort study of frequency, duration, and risk factors. PLoS Med. 2021;18(2):e1003273. doi: 10.1371/journal.pmed.1003273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pan R, Zhang Q, Anthony SM, Zhou Y, Zou X, Cassell M, et al. Oligodendrocytes that survive acute coronavirus infection induce prolonged inflammatory responses in the CNS. Proc Natl Acad Sci U S A. 2020;117(27):15902–10. doi: 10.1073/pnas.2003432117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kaur G, Wright K, Verma S, Haynes A, Dufour JM. The Good, the Bad and the Ugly of Testicular Immune Regulation: A Delicate Balance Between Immune Function and Immune Privilege. Adv Exp Med Biol. 2021;1288:21–47. doi: 10.1007/978-3-030-77779-1_2 [DOI] [PubMed] [Google Scholar]
- 113.Adami C, Pooley J, Glomb J, Stecker E, Fazal F, Fleming JO, et al. Evolution of mouse hepatitis virus (MHV) during chronic infection: quasispecies nature of the persisting MHV RNA. Virology. 1995;209(2):337–46. doi: 10.1006/viro.1995.1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Noran HH, Baker AB. Western Equine Encephalitis—the Pathogenesis of the Pathological Lesions. J Neuropath Exp Neur. 1945;4(3):269–76. [Google Scholar]
- 115.Frolova MP, Pogodina VV. Persistence of tick-borne encephalitis virus in monkeys. VI. Pathomorphology of chronic infection in central nervous system. Acta Virol. 1984;28(3):232–9. [PubMed] [Google Scholar]
- 116.Pogodina VV, Frolova MP, Malenko GV, Fokina GI, Levina LS, Mamonenko LL, et al. Persistence of tick-borne encephalitis virus in monkeys. I. Features of experimental infection. Acta Virol. 1981;25(6):337–43. [PubMed] [Google Scholar]
- 117.Schultz KL, Vernon PS, Griffin DE. Differentiation of neurons restricts Arbovirus replication and increases expression of the alpha isoform of IRF-7. J Virol. 2015;89(1):48–60. doi: 10.1128/JVI.02394-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vernon PS, Griffin DE. Characterization of an in vitro model of alphavirus infection of immature and mature neurons. J Virol. 2005;79(6):3438–47. doi: 10.1128/JVI.79.6.3438-3447.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lewis J, Wesselingh SL, Griffin DE, Hardwick JM. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol. 1996;70(3):1828–35. doi: 10.1128/JVI.70.3.1828-1835.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Griffin DE. Why are neurons susceptible to Zika virus? Science. 2017;357(6346):33–4. doi: 10.1126/science.aan8626 [DOI] [PubMed] [Google Scholar]
- 121.Poelaert KCK, Williams RM, Matullo CM, Rall GF. Noncanonical Transmission of a Measles Virus Vaccine Strain from Neurons to Astrocytes. mBio. 2021;12(2). doi: 10.1128/mBio.00288-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pfaller CK, George CX, Samuel CE. Adenosine Deaminases Acting on RNA (ADARs) and Viral Infections. Annu Rev Virol. 2021;8(1):239–64. doi: 10.1146/annurev-virology-091919-065320 [DOI] [PubMed] [Google Scholar]
- 123.Cathomen T, Naim HY, Cattaneo R. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J Virol. 1998;72(2):1224–34. doi: 10.1128/JVI.72.2.1224-1234.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mathieu C, Bovier FT, Ferren M, Lieberman NAP, Predella C, Lalande A, et al. Molecular Features of the Measles Virus Viral Fusion Complex That Favor Infection and Spread in the Brain. mBio. 2021:e0079921. doi: 10.1128/mBio.00799-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morfopoulou S, Mee ET, Connaughton SM, Brown JR, Gilmour K, Chong WK, et al. Deep sequencing reveals persistence of cell-associated mumps vaccine virus in chronic encephalitis. Acta Neuropathol. 2017;133(1):139–47. doi: 10.1007/s00401-016-1629-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Reuther P, Martin K, Kreutzfeldt M, Ciancaglini M, Geier F, Calabrese D, et al. Persistent RNA virus infection is short-lived at the single-cell level but leaves transcriptomic footprints. J Exp Med. 2021;218(10). doi: 10.1084/jem.20210408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cifuentes-Munoz N, Dutch RE, Cattaneo R. Direct cell-to-cell transmission of respiratory viruses: The fast lanes. PLoS Pathog. 2018;14(6):e1007015. doi: 10.1371/journal.ppat.1007015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Girard S, Gosselin AS, Pelletier I, Colbere-Garapin F, Couderc T, Blondel B. Restriction of poliovirus RNA replication in persistently infected nerve cells. J Gen Virol. 2002;83(Pt 5):1087–93. doi: 10.1099/0022-1317-83-5-1087 [DOI] [PubMed] [Google Scholar]
- 129.Schneider U, Martin A, Schwemmle M, Staeheli P. Genome trimming by Borna disease viruses: viral replication control or escape from cellular surveillance? Cell Mol Life Sci. 2007;64(9):1038–42. doi: 10.1007/s00018-007-6545-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim KS, Tracy S, Tapprich W, Bailey J, Lee CK, Kim K, et al. 5’-Terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negative-strand viral RNA. J Virol. 2005;79(11):7024–41. doi: 10.1128/JVI.79.11.7024-7041.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meyer BJ, Southern PJ. A novel type of defective viral genome suggests a unique strategy to establish and maintain persistent lymphocytic choriomeningitis virus infections. J Virol. 1997;71(9):6757–64. doi: 10.1128/JVI.71.9.6757-6764.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Meyer BJ, Schmaljohn CS. Persistent hantavirus infections: characteristics and mechanisms. Trends Microbiol. 2000;8(2):61–7. doi: 10.1016/s0966-842x(99)01658-3 [DOI] [PubMed] [Google Scholar]
- 133.Dong X, Munoz-Basagoiti J, Rickett NY, Pollakis G, Paxton WA, Gunther S, et al. Variation around the dominant viral genome sequence contributes to viral load and outcome in patients with Ebola virus disease. Genome Biol. 2020;21(1):238. doi: 10.1186/s13059-020-02148-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Watson RJ, Tree J, Fotheringham SA, Hall Y, Dong X, Steeds K, et al. Dose-dependent response to infection with Ebola virus in the ferret model and evidence of viral evolution in the eye. J Virol. 2021:JVI0083321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Young DF, Wignall-Fleming EB, Busse DC, Pickin MJ, Hankinson J, Randall EM, et al. The switch between acute and persistent paramyxovirus infection caused by single amino acid substitutions in the RNA polymerase P subunit. PLoS Pathog. 2019;15(2):e1007561. doi: 10.1371/journal.ppat.1007561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vignuzzi M, Lopez CB. Defective viral genomes are key drivers of the virus-host interaction. Nat Microbiol. 2019;4(7):1075–87. doi: 10.1038/s41564-019-0465-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu J, Sun Y, Li Y, Ruthel G, Weiss SR, Raj A, et al. Replication defective viral genomes exploit a cellular pro-survival mechanism to establish paramyxovirus persistence. Nat Commun. 2017;8(1):799. doi: 10.1038/s41467-017-00909-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Killip MJ, Young DF, Gatherer D, Ross CS, Short JA, Davison AJ, et al. Deep sequencing analysis of defective genomes of parainfluenza virus 5 and their role in interferon induction. J Virol. 2013;87(9):4798–807. doi: 10.1128/JVI.03383-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sun Y, Jain D, Koziol-White CJ, Genoyer E, Gilbert M, Tapia K, et al. Immunostimulatory Defective Viral Genomes from Respiratory Syncytial Virus Promote a Strong Innate Antiviral Response during Infection in Mice and Humans. PLoS Pathog. 2015;11(9):e1005122. doi: 10.1371/journal.ppat.1005122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Felt SA, Sun Y, Jozwik A, Paras A, Habibi MS, Nickle D, et al. Detection of respiratory syncytial virus defective genomes in nasal secretions is associated with distinct clinical outcomes. Nat Microbiol. 2021;6(5):672–81. doi: 10.1038/s41564-021-00882-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Johnson RI, Boczkowska B, Alfson K, Weary T, Menzie H, Delgado J, et al. Identification and Characterization of Defective Viral Genomes in Ebola Virus-Infected Rhesus Macaques. J Virol. 2021;95(17):e0071421. doi: 10.1128/JVI.00714-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.LaVergne SM, Sakabe S, Kanneh L, Momoh M, Al-Hassan F, Yilah M, et al. Ebola-Specific CD8+ and CD4+ T-Cell Responses in Sierra Leonean Ebola Virus Survivors With or Without Post-Ebola Sequelae. J Infect Dis. 2020;222(9):1488–97. doi: 10.1093/infdis/jiaa268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Patterson BK, Francisco EB, Yogendra R, Long E, Pise A, Rodrigues H, et al. Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection. Front Immunol. 2021;12:746021. doi: 10.3389/fimmu.2021.746021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhao J, Zhao J, Perlman S. De novo recruitment of antigen-experienced and naive T cells contributes to the long-term maintenance of antiviral T cell populations in the persistently infected central nervous system. J Immunol. 2009;183(8):5163–70. doi: 10.4049/jimmunol.0902164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Turner DL, Cauley LS, Khanna KM, Lefrancois L. Persistent antigen presentation after acute vesicular stomatitis virus infection. J Virol. 2007;81(4):2039–46. doi: 10.1128/JVI.02167-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zammit DJ, Turner DL, Klonowski KD, Lefrancois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24(4):439–49. doi: 10.1016/j.immuni.2006.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Adaken C, Scott JT, Sharma R, Gopal R, Dicks S, Niazi S, et al. Ebola virus antibody decay-stimulation in a high proportion of survivors. Nature. 2021;590(7846):468–72. doi: 10.1038/s41586-020-03146-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pan CH, Valsamakis A, Colella T, Nair N, Adams RJ, Polack FP, et al. Modulation of disease, T cell responses, and measles virus clearance in monkeys vaccinated with H-encoding alphavirus replicon particles. Proc Natl Acad Sci U S A. 2005;102(33):11581–8. doi: 10.1073/pnas.0504592102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tyor WR, Wesselingh S, Levine B, Griffin DE. Long term intraparenchymal Ig secretion after acute viral encephalitis in mice. J Immunol. 1992;149(12):4016–20. [PubMed] [Google Scholar]
- 150.Metcalf TU, Griffin DE. Alphavirus-induced encephalomyelitis: antibody-secreting cells and viral clearance from the nervous system. J Virol. 2011;85(21):11490–501. doi: 10.1128/JVI.05379-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Metcalf TU, Baxter VK, Nilaratanakul V, Griffin DE. Recruitment and retention of B cells in the central nervous system in response to alphavirus encephalomyelitis. J Virol. 2013;87(5):2420–9. doi: 10.1128/JVI.01769-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Baxter VK, Griffin DE. Interferon-Gamma Modulation of the Local T Cell Response to Alphavirus Encephalomyelitis. Viruses. 2020;12(1). doi: 10.3390/v12010113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Marten NW, Stohlman SA, Bergmann CC. Role of viral persistence in retaining CD8(+) T cells within the central nervous system. J Virol. 2000;74(17):7903–10. doi: 10.1128/jvi.74.17.7903-7910.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Noran HHB A.B. Sequels of equine encephalomyelitis. Arch Neurol Psych. 1943;49:398–413. [Google Scholar]
- 155.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–64. doi: 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 156.Zhou M, Yin Z, Xu J, Wang S, Liao T, Wang K, et al. Inflammatory Profiles and Clinical Features of Coronavirus 2019 Survivors 3 Months After Discharge in Wuhan, China. J Infect Dis. 2021;224(9):1473–88. doi: 10.1093/infdis/jiab181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163(4):693–8. doi: 10.1093/infdis/163.4.693 [DOI] [PubMed] [Google Scholar]
- 158.Falsey AR, Singh HK, Walsh EE. Serum antibody decay in adults following natural respiratory syncytial virus infection. J Med Virol. 2006;78(11):1493–7. doi: 10.1002/jmv.20724 [DOI] [PubMed] [Google Scholar]
- 159.Wu LP, Wang NC, Chang YH, Tian XY, Na DY, Zhang LY, et al. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13(10):1562–4. doi: 10.3201/eid1310.070576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Alshukairi AN, Khalid I, Ahmed WA, Dada AM, Bayumi DT, Malic LS, et al. Antibody Response and Disease Severity in Healthcare Worker MERS Survivors. Emerg Infect Dis. 2016;22(6). doi: 10.3201/eid2206.160010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cromer D, Juno JA, Khoury D, Reynaldi A, Wheatley AK, Kent SJ, et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol. 2021;21(6):395–404. doi: 10.1038/s41577-021-00550-x [DOI] [PMC free article] [PubMed] [Google Scholar]