Abstract
The regulation of glycometabolism homeostasis is vital to maintain health and development of animal and humans; however, the molecular mechanisms by which organisms regulate the glucose metabolism homeostasis from a feeding state switching to a non-feeding state are not fully understood. Using the holometabolous lepidopteran insect Helicoverpa armigera, cotton bollworm, as a model, we revealed that the steroid hormone 20-hydroxyecdysone (20E) upregulated the expression of transcription factor Krüppel-like factor (identified as Klf15) to promote macroautophagy/autophagy, apoptosis and gluconeogenesis during metamorphosis. 20E via its nuclear receptor EcR upregulated Klf15 transcription in the fat body during metamorphosis. Knockdown of Klf15 using RNA interference delayed pupation and repressed autophagy and apoptosis of larval fat body during metamorphosis. KLF15 promoted autophagic flux and transiting to apoptosis. KLF15 bound to the KLF binding site (KLF bs) in the promoter of Atg8 (autophagy-related gene 8/LC3) to upregulate Atg8 expression. Knockdown Atg8 reduced free fatty acids (FFAs), glycerol, free amino acids (FAAs) and glucose levels. However, knockdown of Klf15 accumulated FFAs, glycerol, and FAAs. Glycolysis was switched to gluconeogenesis, trehalose and glycogen synthesis were changed to degradation during metamorphosis, which were accompanied by the variation of the related genes expression. KLF15 upregulated phosphoenolpyruvate carboxykinase (Pepck) expression by binding to KLF bs in the Pepck promoter for gluconeogenesis, which utilised FFAs, glycerol, and FAAs directly or indirectly to increase glucose in the hemolymph. Taken together, 20E via KLF15 integrated autophagy and gluconeogenesis by promoting autophagy-related and gluconeogenesis-related genes expression.
Author summary
Glucose is the direct substrate for energy production in animal and humans. Autophagy and gluconeogenesis are known to help organisms maintaining energy substrates; however, the mechanism of integration of autophagy and gluconeogenesis is unclear. Holometabolous insects stop feeding during metamorphosis under steroid hormone 20-hydroxyecdysone (20E) regulation, providing a good model for the study. Using lepidopteran insect Helicoverpa armigera, cotton bollworm, as a model, we revealed that Krüppel-like factor 15 (KLF15) integrated autophagy and gluconeogenesis to maintain glucose homeostasis under 20E regulation. 20E increased Klf15 expression, and KLF15 in turn promoted autophagy-related and gluconeogenesis-related genes expression during metamorphosis. Autophagy and apoptosis of the fat body provided substrates for gluconeogenesis. This work clarified the important functions and mechanisms of KLF15 in autophagy and glycometabolism reprogramming for glucose homeostasis after feeding stop during insect metamorphosis.
Introduction
Glucose is an essential energy substance in animals and humans, which directly or indirectly produces energy for metabolic processes. Some tissues, such as the brain and red blood cells in mammals, rely on glucose to meet their energy requirements. Glycometabolism homeostasis maintains the blood glucose levels. Glucose is digested from food in normal feeding conditions and can be used directly in vivo through glycolysis to produce pyruvic acid (PA) and adenosine triphosphate (ATP). The circulating glucose is also produced from glycogenolysis under the catalysis of glycogen phosphorylase (GP) [1], and gluconeogenesis, the process of producing glucose from non-carbohydrate precursors, such as lactic acid (LA), glycerol, PA and free amino acids (FAAs) [2, 3]. Glycogen storage is depleted in 10 h during fasting [4], and gluconeogenesis becomes the predominant and almost exclusive glucose production process during fasting. Consequently, glycometabolism must be reprogrammed during fasting. Autophagy is also the important mechanism for supplying energy substrates during fasting and nutrients insufficient, however, the integration of autophagy and gluconeogenesis is unclear.
Krüppel-like factors (KLFs) are transcriptional regulators with three C2H2 zinc finger motifs at their carboxyl terminus, allowing them to bind to GC-rich DNA with a consensus binding site of CACCC (KLF bs) [5, 6]. KLFs respond to nuclear receptors of steroid and thyroid hormones to promote or repress downstream genes transcription [7]. KLFs play roles to extend lifespan in an autophagy-dependent manner in Caenorhabditis elegans [8]. KLFs actively play a role in the metabolic regulation. KLF 3 is essential for fat metabolism in C. elegans [9]. In mice, KLF15 is an important transcription factor involved in glycometabolism, especially in the regulation of gluconeogenesis [10]. The hepatic abundance of KLF15 increases during fasting and promotes the transcription of genes encoding the key enzymes of gluconeogenesis, i.e., glucose-6-phosphatase (G6p) and phosphoenolpyruvate carboxykinase (Pepck), to strengthen the occurrence of gluconeogenesis [11]. KLF15 also increases the expression of genes encoding amino acids catabolic enzymes in the liver to provide abundant substrates for gluconeogenesis [12, 13]. KLF15 suppresses cell growth in lung adenocarcinoma [14]. However, the role and mechanism of KLF15 regulating autophagy are unclear.
Insect metamorphic development presents good model for the study of autophagy and gluconeogenesis. When holometabolous insects stop feeding and transfer from larvae to adults, the energy for larvae survival and adult tissue development is derived from stored materials. Larval tissues are degraded by programmed cell death (PCD), including autophagy [15] and apoptosis [16]. As the homologous tissue of mammalian liver, the insect fat body is the primary tissue for sensing various hormones, nutrient storage [17], and glycometabolism [18]. Lipid droplets (LD) are the primary lipid storage organelles in the fat body cells, and triglycerides are the primary lipid storage components [19]. The insect molting hormone, steroid hormone 20-hydroxyecdysone (20E), is the principal regulator of metamorphosis [20, 21]. The 20E titer continues to rise during larvae-pupae transition [22]. 20E binds its nuclear receptor (EcR) and form a transcription complex with ultraspiracle isoform 1 (USP1) to bind with ecdysone response element (EcRE) [23] to initiate downstream genes expression that promotes metamorphosis [24]. High concentrations of 20E promotes the transformation of tissues from autophagy to apoptosis [25]. Glucose levels in the hemolymph increase after feeding stop during the larva-to-adult transition [26], and larval tissues are degraded by autophagy and apoptosis. Despite extensive research in 20E’s regulatory roles in metamorphosis, the mechanism by which 20E regulates gluconeogenesis and its relationship with larval fat body PCD, including autophagy and apoptosis remain unknown.
In the present study, we used the holometabolous insect, Helicoverpa armigera, cotton bollworm, as a model to reveal the mechanisms of glycometabolism reprogramming and fat body PCD under 20E regulation. We revealed that 20E, via KLF15, promoted larval fat body degradation by autophagy and apoptosis to produce substrates for gluconeogenesis during insect metamorphosis.
Results
Screen of KLFs involved in gluconeogenesis
By BLAST (Basic Local Alignment Search Tool), we screened five KLFs in the H. armigera genome using human KLFs as a guide, which are named as Krüppel-like factor luna (Luna), Dendritic arbor reduction protein (Dar), Klf8, Klf9 and Klf16. KLF9 was renamed as KLF15 because it was not clustered with Homo sapiens KLF9 (S1 Fig), but was clustered with Drosophila melanogaster KLF15 and has sequence similarity with D. melanogaster and H. sapiens KLF15 (S2 Fig). Klf15 expression was higher in the fat body at the sixth instar 96 h (6th-96 h) compared to 6th-24 h, the expression of Luna and Klf16 in the fat body were also significantly higher at the 6th-96 h (S3A–S3E Fig). To identify KLF involved in gluconeogenesis, we knocked down Luna, Klf15, and Klf16 and measured glucose levels in the hemolymph (S3F Fig). Glucose levels in the hemolymph were significantly reduced after knocked down Klf15 and Klf16 (S3G Fig), and dsKlf15 significantly decreased the expression of Pepck in the fat body, whereas dsKlf16 did not (S3H Fig). Thus, KLF15 was chosen for our further study in this work.
Klf15 was highly expressed in the fat body during metamorphosis under 20E regulation
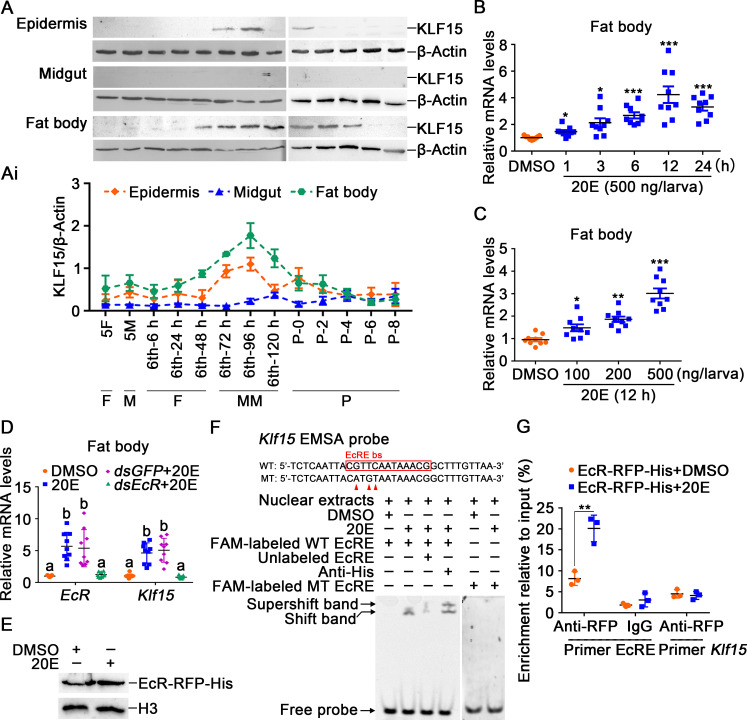
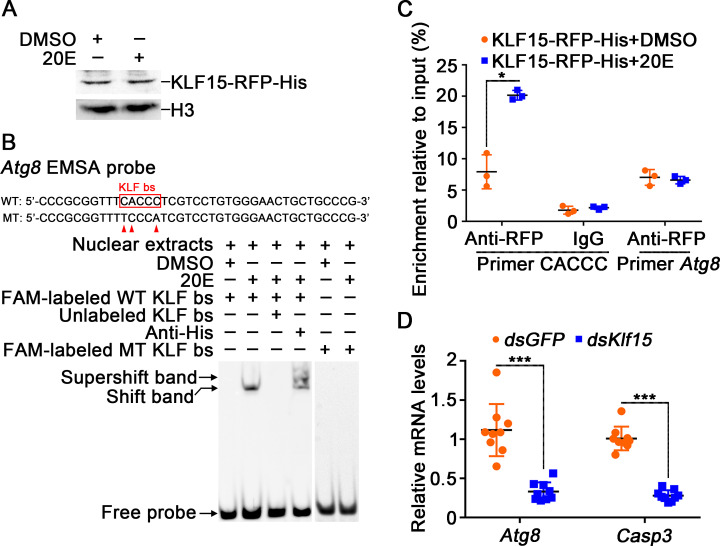
The expression profiles of KLF15 during metamorphosis was examined to analyze its function in development. KLF15 levels increased in the epidermis and fat body during metamorphosis (Fig 1A and 1Ai), indicating that KLF15 plays an important role during metamorphosis. To understand the regulation of 20E on KLF15, 20E was injected into the larval hemocoel. Klf15 expression was increased in a time and 20E-concentration dependent manner (Fig 1B and 1C), indicating that 20E promotes Klf15 expression. To confirm 20E upregulating Klf15 expression, we knocked down 20E nuclear receptor EcR in the fat body. Results showed that the expression of Klf15 was decreased after knockdown of EcR (Fig 1D). Four EcRE that could be combined with EcR in the Klf15 promoter were further predicted by reported EcRE consensus sequences and JASPAR transcription factor database [27, 28] (S4 Fig). Following successfully expressing EcR-RFP-His in H. armigera epidermal cell line (HaEpi) (Fig 1E), we found that the EcRE with the sequence 5’-CGTTCAATAAACG-3’ was able to be combined by the overexpressed EcR-RFP-His in the nuclear extracts after stimulation with 20E using EMSA, and the antibody against His tag produced a supper shift band. However, after point mutations were introduced into the EcRE motif according to the method [29, 30], whether 20E or dimethyl sulfoxide (DMSO) was added, the probe was not able to be combined by nuclear proteins (Fig 1F). A chromatin immunoprecipitation (ChIP) assay showed that EcR-RFP-His bound more EcRE in the Klf15 promoter under 20E treatment than it did in the DMSO treatment control (Fig 1G), which confirmed that 20E upregulated Klf15 expression through its nuclear receptor EcR.
Fig 1. 20E upregulated the expression of Klf15.
A. The protein profiles of KLF15 in the epidermis, midgut, and fat body detected using western blotting after 12.5% SDS-PAGE. 5F: fifth instar feeding larvae; 5M: fifth instar molting larvae; 6th-6 h to 120 h: sixth instar larvae at different stages; P0 to P8: 0 to 8-day-old pupae. F: feeding; M: molting; MM: metamorphic molting; P: pupae. Ai. Quantification of KLF15 in A using Image J software. B. Time course of the Klf15 expression in the fat body after 20E (500 ng/larva) induction. DMSO was used as the control. C. The expression of Klf15 in the fat body under stimulation with different concentrations of 20E for 12 h. D. Knockdown of EcR in the fat body by dsEcR (3 μg/larva) followed by stimulation with 20E (500 ng/larva) for 12 h to detect the expression of Klf15. E. Nuclear proteins from EcR-RFP-His overexpressed cells were extracted for EMSA. F. EcRE on the Klf15 promoter bound to EcR detected by EMSA assay. WT and MT represent EcRE probe and EcRE mutant probe, respectively. G. ChIP assay showing 20E promoted Klf15 expression via EcR binding to EcRE and detected by qRT-PCR. Primer EcRE is the sequence containing EcRE. Primer Klf15, as non-EcRE control targeting to Klf15 open reading frame (ORF).
KLF15 promoted pupation and fat body autophagy and apoptosis
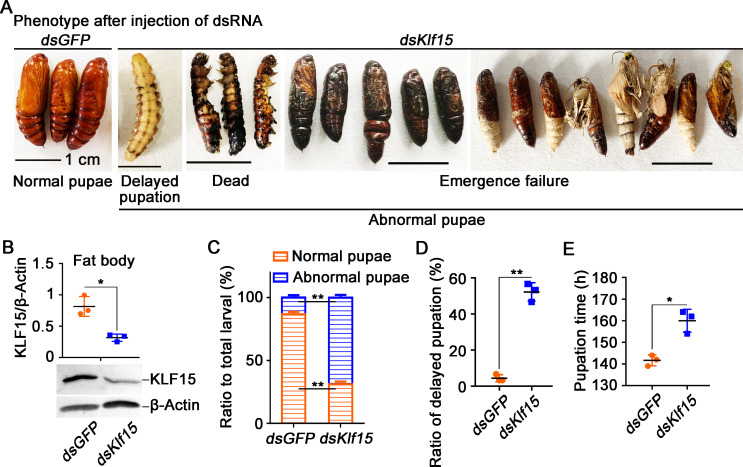
To determine the role of KLF15 in metamorphosis, we knocked down Klf15 by injecting dsKlf15 into the 6th-6 h larval hemocoel. The larvae showed delayed pupation and metamorphic failure after knockdown of Klf15 (Fig 2A). Klf15 was confirmed as being knocked down significantly in the fat body according to its protein levels (Fig 2B). After dsKlf15 injection, 68.9% larvae had abnormal pupation, including death in the pre-pupal stage and pupal stage, as well as failure to eclosion normally (Fig 2C), 52.2% larvae showed a significant delay in pupation for an average of 20 h, and then dead at pupal or adult stages (Fig 2D and 2E).
Fig 2. Knockdown of Klf15 delayed pupation.
A. Phenotypes after injection of Klf15 dsRNA from 6th-6 h larva to 72 h; the ruler represents 1 cm, dsGFP was used as the control. B. Western blotting validation of the interference efficiency in the fat body after the third dsRNA injection. C. Ratio of phenotypes, abnormal pupae include larvae that die during the prepupa stage, dead pupae and chimeric pupae that cannot normally emerge. D. The ratio of delayed pupation after the dsRNA injection. E. The time at which the larvae pupated after knockdown GFP and Klf15. The data were obtained in triplicate with thirty larvae each time.
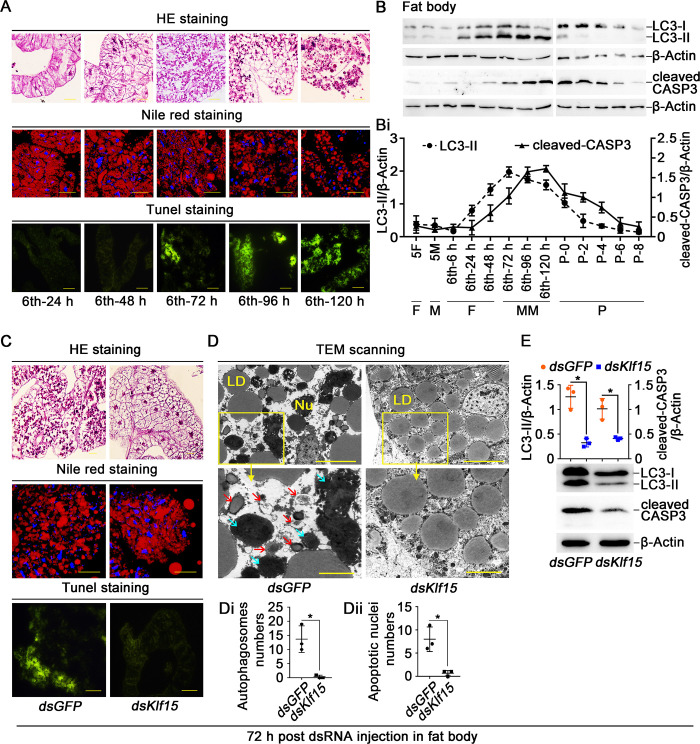
To address the reason of metamorphosis failure, we observed the fat body PCD after Klf15 knockdown. The fat body remained intact and consisted of soft flaky tissue during feeding (from 6th-24 h to 48 h). At the 6th-72 h, the fat body began to decompose and was severely dissociated at the 6th-120 h. Nile Red staining showed that the LD in the fat body cells were densely distributed during feeding period from 6th-24 h to 48 h and broken down to small droplets after entering the metamorphic phase. Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) staining revealed that fat body apoptosis occurred after larvae entered metamorphosis at the 6th-72 h and enhanced at the 6th-120 h (Fig 3A). The transformation of LC3-I (microtubule-associated protein 1 light chain 3, also known as autophagy-related 8, ATG8) to LC3-II (phosphatidyl ethanolamine form), which indicates autophagy [31], and cleaved-caspase 3 (CASP3), the indicator of apoptosis [32], were analyzed using western blotting to examine the autophagy and apoptosis in the fat body. LC3-II appeared at the 6th-24 h and lasted to 6th-120 h, and disappeared at pupal stage. The cleaved-CASP3 appeared later than LC3-II at the 6th-72 h and continued into pupal stage (Fig 3B and 3Bi), indicating the sequential occurrence of autophagy and apoptosis in the larval fat body during metamorphosis. However, after knockdown of Klf15, the fat body cells remained intact, LD were kept large and dense, and the TUNEL signal was not detected (Fig 3C). The transmission electron microscopy (TEM) showed that the number of typical autophagosomes, which had two or single-membraned and contained degenerating cytoplasmic organelles or degraded LD, and autophagic vacuoles [33], decreased in the fat body after Klf15 knockdown compared with the dsGFP control. Furthermore, the apoptotic nuclei that manifested as swelling, chromatin pyknosis and deepening of staining were repressed in the dsKlf15 group, compared to the cells in the dsGFP (Fig 3D). The statistical analysis validated the results (Fig 3Di and 3Dii). Western blotting further revealed that the formation of LC3-II and cleaved-CASP3 were decreased by Klf15 knockdown (Fig 3E). The data indicated that KLF15 promotes both autophagy and apoptosis in the fat body.
Fig 3. Knockdown of Klf15 repressed fat body degradation.
A. The morphological changes of fat body were assessed by HE staining, Nile red staining and TUNEL staining, the ruler represents 50 μm in the HE staining. Nile red stained for intracellular LD, the ruler represents 50 μm. The apoptotic cells in the fat body were determined by TUNEL staining assay, the ruler represents 100 μm. B. Western blotting detection of LC3-II and cleaved-CASP3 protein levels in the fat body using anti-LC3 and anti-CASP3 antibodies, with β-Actin as protein control after 15% SDS-PAGE. Bi. Quantification of the data in B. C. HE staining, Nile red staining and TUNEL staining showing fat body morphology after knockdown Klf15, observed after first injection dsRNA for 72 h. D. TEM observation after injection with dsKlf15 in the fat body. The bars in the wide field view represented 100 μm, in the small field view represented 40 μm. The red arrows indicated autophagosomes, the blue arrow represented the apoptotic nuclei. Nu: nucleus, LD: lipid droplets. Di. Counted the autophagosomes contained in three different sets of images. The area of each image is about 0.09 mm2. Dii. Counted the apoptotic nuclei of dsGFP and dsKlf15. E. After knockdown dsGFP and dsKlf15, western blotting detected LC3-II and cleaved-CASP3. 72 h post dsRNA injection in fat body: 72 h post dsRNA injection into hemocoel and observed the variation in the fat body.
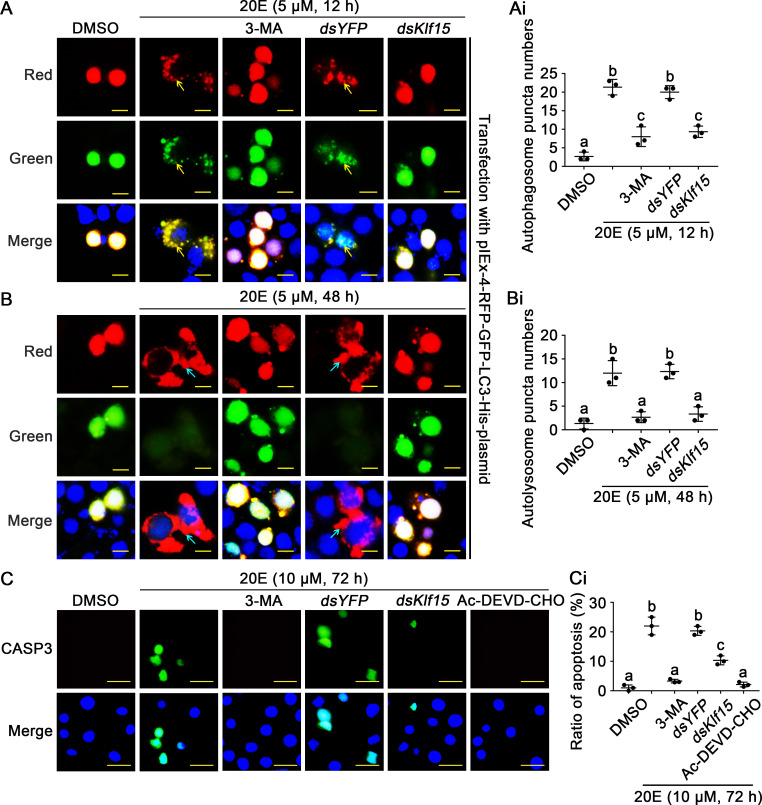
The role of KLF15 in 20E-mediated autophagic flux were further examined by overexpressing pIEx-4-RFP-GFP-LC3-His fluorescent double-labeled plasmid in HaEpi cells. The cells kept intact in DMSO solvent control. 20E induced puncta of autophagosome appearance in 12 h. The autophagy inhibitor 3-Methyladenine (3-MA) inhibited autophagy. dsYFP control did not change 20E-induced autophagy, however, knockdown of Klf15 blocked the formation of autophagosomes (Fig 4A and 4Ai). 48 h later, similar results were observed, in addition to the green fluorescence disappeared in autophagosomes (Fig 4B and 4Bi), showing the autophagic flux from autophagosome to autolysosome, and knockdown of Klf15 blocked these processes. Furthermore, knockdown of Klf15 also resulted in a significant decrease in CASP3 activity indicated by green fluorescence under 10 μM 20E stimulation for 72 h. Autophagy inhibitor 3-MA and apoptosis inhibitor Ac-DEVD-CHO also prevented 20E-induced apoptosis (Fig 4C and 4Ci), suggesting 20E-induced apoptosis relied on autophagy. The interference efficiency was showed (S5 Fig). Flow cytometry revealed that dsYFP plus 20E induced 13–19% cell apoptosis, whereas dsKlf15 plus 20E induced only 8–9% cell apoptosis (S6A and S6Ai Fig). The Cell Counting Kit-8 (CCK-8) assay was used to confirm the role of KLF15 in 20E-induced cell death. Results showed that 10 μM 20E significantly decreased cell viability, however, cell viability increased after Klf15 knockdown compared with dsYFP control (S6B Fig). All these data suggested that KLF15 was involved in 20E-induced autophagy and apoptosis.
Fig 4. KLF15 promoted autophagy and apoptosis as detected by autophagic flux and CASP3 activity.
A and B. The autophagic flux were detected in HaEpi cells when Klf15 was knocked down, dsYFP was used as the control. 3-MA (10 μM) is an autophagosome formation inhibitor; the yellow bars represented 20 μm. The yellow arrows represented autophagosome puncta. The blue arrows represented autolysosome puncta. Ai and Bi. Counted the number of autophagosome puncta and autolysosome puncta in successfully transfected with pIEx-GFP-RFP-LC3 cells. C. After knocked down Klf15 in HaEpi cells, examination of apoptosis by the addition of active CASP3, Ac-DEVD-CHO is a CASP3 inhibitor, the bars represented 50 μm. Ci. Quantification apoptotic cells in total from C.
To demonstrate the mechanism that KLF15 promoting autophagy and apoptosis, we analyzed the regulation of KLF15 on the transcription of the PCD-related genes. We did not find KLF bs in the Casp3 promoter, however, we found KLF bs 5′-CACCC-3′ in the promoter of Atg8 based on the KLF bs in humans [34, 35]. KLF15-RFP-His was thus overexpressed in cells and an EMSA demonstrated that the overexpressed KLF15-RFP-His in the nuclear extracts of HaEpi cells could bind to the CACCC containing probe designed from Atg8 promoter, after point mutation of KLF bs of Atg8 [10, 36], the probe was no longer bound by nuclear proteins (Fig 5A and 5B). ChIP experiment proved that KLF15-RFP-His bound the CACCC fragment of Atg8 under 20E induction (Fig 5C). Knockdown of Klf15 in larvae decreased Atg8 and Casp3 expression (Fig 5D). In addition to Atg8, knockdown of Klf15 also suppressed the expression of Atg4b, Atg12, and Atg14 (S7 Fig). All data suggested that 20E regulates autophagy and apoptosis via KLF15, which upregulating Atgs transcription.
Fig 5. KLF15 promoted autophagy through upregulating Atg8.
A. Nuclear proteins from KLF15-RFP-His overexpressed cells were extracted for EMSA. B. KLF bs on the Atg8 promoter bound to KLF15 detected by EMSA assay. WT and MT represent KLF bs probe and KLF bs mutant probe, respectively. C. ChIP assay showing 20E promoted Atg8 expression via KLF15 binding to KLF bs. Primer CACCC targeting KLF bs. Primer Atg8 targeting Atg8 ORF. D. Changes in the expression levels of Atg8 and Casp3 after Klf15 knockdown.
Autophagy and apoptosis produced substrates for gluconeogenesis
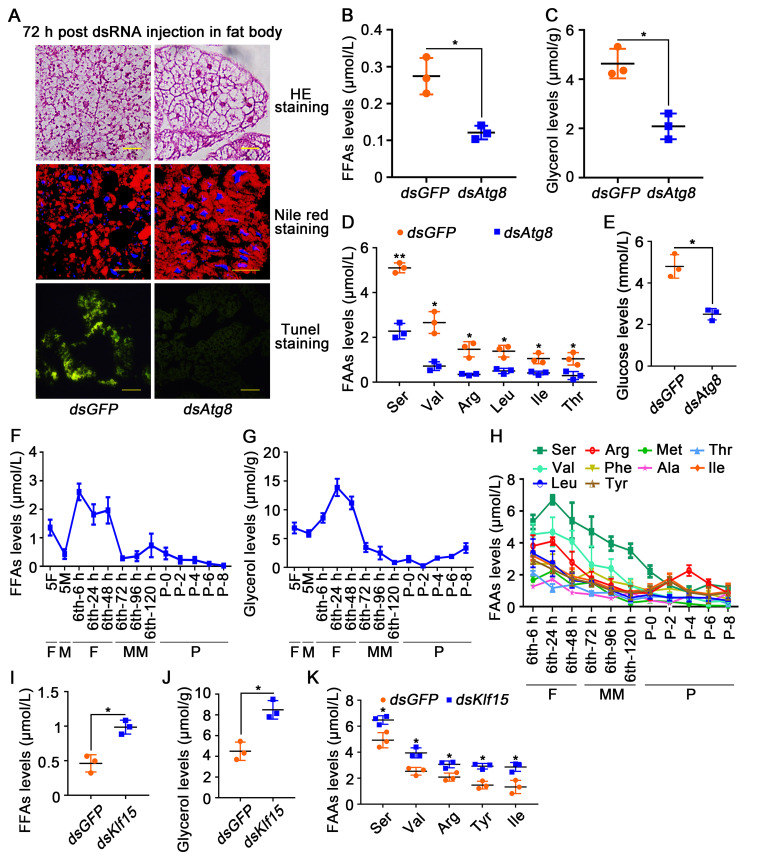
To determine the relationship between autophagy and gluconeogenesis, dsAtg8 was injected into the 6th-6 h larvae to block autophagy. Compared with dsGFP, the fat body was not dissociated, the LD was not broken, and the apoptosis was not observed after knockdown of dsAtg8 (Fig 6A). Moreover, the levels of FFAs and glycerol, the degraded products of fat body, were lower compared to dsGFP (Fig 6B and 6C). The levels of certain FAAs, including serine (Ser), valine (Val), arginine (Arg), leucine (Leu), isoleucine (Ile) and threonine (Thr), which have the potential to be metabolized to produce glucose via gluconeogenesis [36,37], exhibited significantly reduction after dsAtg8 knockdown (Fig 6D). The hemolymph glucose levels also decreased after knockdown of dsAtg8 (Fig 6E). Knockdown of Casp3 also inhibited fat body degradation and decreased glucose levels (S8 Fig). These results suggested that autophagy and apoptosis generate substrates for gluconeogenesis to produce glucose.
Fig 6. Autophagy presented substrates for gluconeogenesis.
A. HE staining, Nile red staining and TUNEL staining showing the morphology of fat body after dsGFP and dsAtg8 injection into hemocoel. B and C. Levels of FFAs in the hemolymph and glycerol in the fat body after knockdown of Atg8. D. FAAs levels in the hemolymph after knockdown of Atg8. E. Glucose levels in the hemolymph decreased after knockdown Atg8. F and G. FFAs levels in the hemolymph and glycerol levels in the fat body from 5th instar larvae to 8-day-old pupae. H. FAAs levels in the hemolymph. I and J. FFAs and glycerol levels after first injection dsKlf15 for 72 h. K. FAAs levels after knockdown of Klf15.
In addition, FFAs, glycerol and all FAAs levels were high during the feeding period from 6th-6 h to 48 h, but gradually decreased during metamorphosis (Fig 6F–6H), suggesting that FFAs, glycerol and FAAs were used for gluconeogenesis during metamorphosis. Knockdown of Klf15, FFAs (Fig 6I), glycerol (Fig 6J) and FAAs (Ser, Val, Arg, Tyr, Ile) (Fig 6K) were accumulated, indicating the role of KLF15 in the metabolism of these substrates in gluconeogenesis.
Glycometabolism was changed from glycolysis to gluconeogenesis during insect metamorphosis
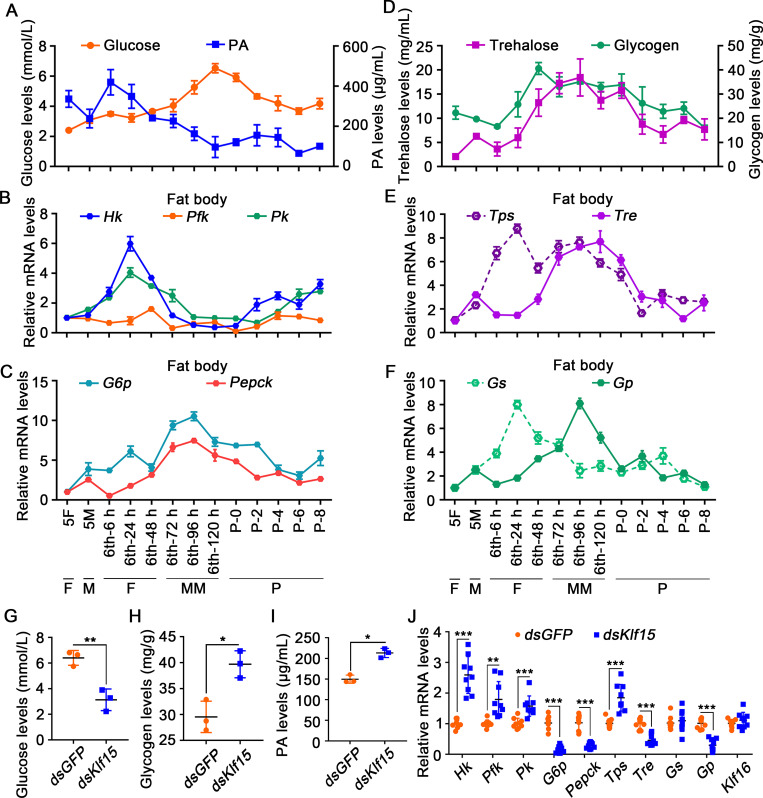
To assess changes in glycometabolism during development, we measured glucose and PA levels in the hemolymph. Glucose levels in larval hemolymph increased from 6th-72 h wandering stage and peaked at the 6th-120 h metamorphosis stage. PA levels were highest during the 6th-6 h to 24 h feeding stage and rapidly decreased after the 6th-48 h stage (Fig 7A), suggesting glucose not only could not be metabolized to PA by glycolysis, but also increased probably by gluconeogenesis after feeding cease.
Fig 7. Variation in the levels of metabolites and related genes expression after Klf15 was knocked down in larvae.
A. The levels of glucose and PA in the hemolymph. B. The expression profiles of Hk, Pfk, and Pk in the fat body. C. The expression profiles of G6p and Pepck in the fat body. D. Trehalose levels in the hemolymph and glycogen levels in the fat body. E. qRT-PCR showing the mRNA expression profiles of Tps and Tre in the fat body. F. The expression profiles of Gs and Gp in the fat body. G-I. Variation in the levels of metabolites after first injection dsKlf15 for 72 h. J. Changes in the expression levels of certain genes after Klf15 knockdown by injection of dsRNA, as detected using qRT-PCR.
Therefore, the expression levels of genes encoding key enzymes in glycolysis and gluconeogenesis were further examined to confirm the variation of glycolysis and gluconeogenesis. The mRNA encoding hexokinase (Hk), and pyruvate kinase (Pk), which are the key enzymes that catalyze irreversible reactions in the glycolysis pathway, were highly expressed at the 6th-24 h during the feeding period; however, their expression levels decreased during metamorphosis, with some increased after the mid-pupa stage (Fig 7B). In contrast, the mRNA levels of the key enzymes for gluconeogenesis, G6p and Pepck, were highly increased during metamorphosis (Fig 7C). These data confirmed the transformation of glycolysis to gluconeogenesis during metamorphosis, indicating that active glycolysis during the feeding period promotes glucose metabolism and an increase in PA levels, whereas during the metamorphosis phase, gluconeogenesis accelerates the utilization of PA and leads to an increase in glucose.
We also measured trehalose levels in the hemolymph and glycogen levels in the fat body to determine the source of high glucose levels during metamorphosis. We found trehalose increased from the 6th-48 h feeding stage and reached a peak at the 6th-96 h wandering stage, then decreased rapidly. Glycogen levels continued to rise during the feeding period from the 6th-24 h to 48 h, and then decreased after feeding stopped from the 6th-72 h larvae to pupae (Fig 7D), indicating that high levels of glucose may result from the degradation and no longer synthesis of trehalose and glycogen. To support the above hypothesis, the mRNA expression levels of trehalose-phosphate synthase (Tps), which synthesizes trehalose, and trehalase (Tre), which degrades trehalose, were examined in the fat body. Tps showed high expression during the feeding period at the 6th-24 h, and then declined during metamorphosis. By contrast, Tre showed low expression at the 6th-24 h feeding stage, but was highly expressed during metamorphosis (Fig 7E), supporting that glucose levels are increased during metamorphosis by decreasing trehalose synthesis and increasing its degradation simultaneously. Glycogen synthase (Gs) expression in the fat body increased from the 6th-6 h to 24 h feeding stage, and decreased during metamorphosis after feeding stopped. The expression levels of the gene encoding Gp, which degrades glycogen, increased at the 6th-96 h and decreased during the pupal stage (Fig 7F), indicating that a decrease in glycogen synthesis and an increase in glycogen degradation also contributed to the elevated glucose levels during metamorphosis. After Klf15 was knocked down, glucose levels dropped significantly (Fig 7G), trehalose without significant increased (S9 Fig), whereas the levels of glycogen accumulated (Fig 7H), and PA increased (Fig 7I).
The expression levels of genes involved in glycometabolism were examined to determine the mechanism by which KLF15 regulated glycometabolism reprogramming. After knocking down Klf15, the mRNA levels of G6p and Pepck for gluconeogenesis, Tre for trehalose degradation, and Gp for glycogen degradation were decreased. In contrast, the mRNA levels of Hk, Pfk and Pk for glycolysis, and Tps for trehalose synthesis were increased. Klf16 expression was detected to show that there was no off-target effect of Klf15 knockdown (Fig 7J). These data suggested that KLF15 promotes glycogen degradation and gluconeogenesis by upregulating the related genes expression.
KLF promoted Pepck expression to produce glucose for metamorphosis
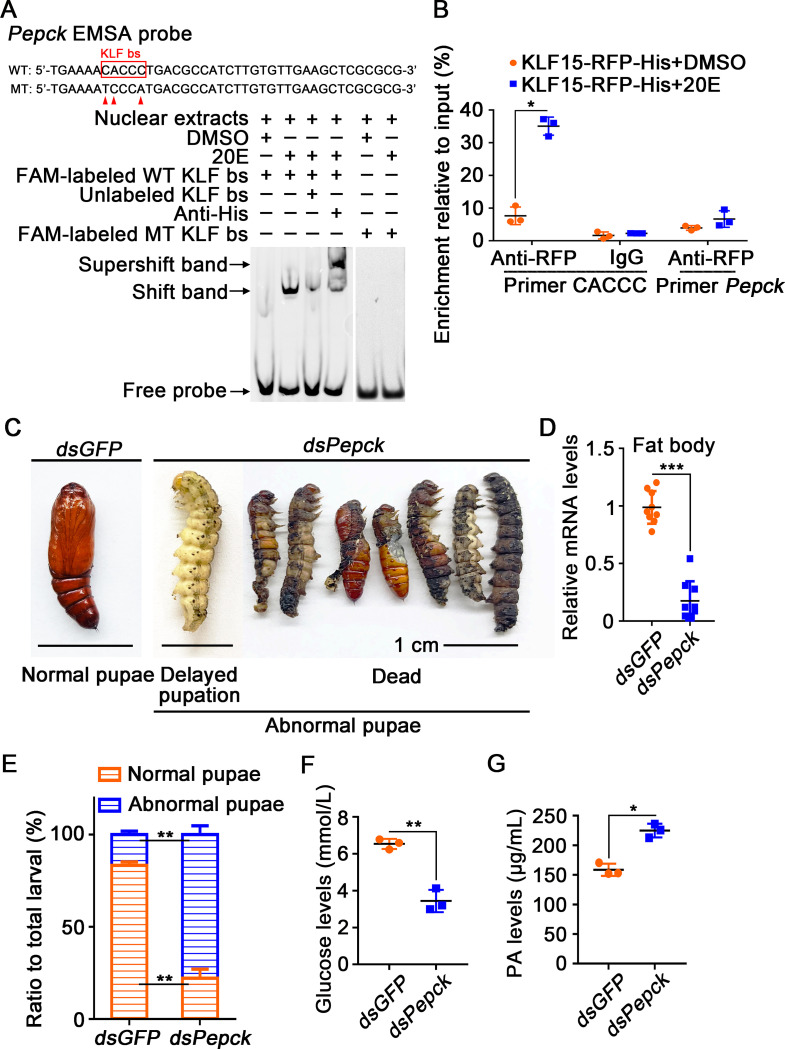
PEPCK is the first rate-limiting enzyme in the gluconeogenesis pathway, catalyzing the synthesis of phosphoenolpyruvate from oxaloacetate. The activity of PEPCK is primarily regulated at the transcription level, and the gene’s expression directly affects gluconeogenesis rate [37]. We found KLF bs in the promoter region of Pepck and designed an EMSA probe containing KLF bs to determine regulation of KLF15 to Pepck transcription. The formation of a normal shift band was observed after incubating the labeled probe with 20E-induced nuclear proteins that containing overexpressed KLF15-RFP-His, and could be competitively decreased by the unlabeled probe. Meanwhile, a supershift band formed when the antibody against His-tag, which identified the overexpressed KLF15-RFP-His, was added to the reaction mixture. The point mutation of KLF bs inhibited the binding of the probe to nuclear proteins (Fig 8A). ChIP experiment proved that 20E promoted the binding of KLF15 to CACCC motif of Pepck to increase the expression of Pepck (Fig 8B). These data revealed that KLF15 promotes gluconeogenesis by upregulating the Pepck transcription.
Fig 8. Knockdown of Pepck delayed pupation and decreased hemolymph glucose levels.
A. KLF bs on the Pepck promoter bound to KLF15 detected by EMSA assay. B. ChIP assay showing 20E promoted Pepck expression via KLF15 binding to KLF bs. C. Phenotypes after injection of dsPepck and dsGFP from 6th-6 h to 72 h; the ruler represents 1 cm. D. qRT-PCR validation of the interference efficiency in the fat body after the third dsRNA injection. E. Ratio of phenotypes. F. Glucose levels in the hemolymph decreased after knockdown Pepck. G. PA levels in the hemolymph increased after knockdown Pepck.
To directly demonstrate that gluconeogenesis promotes glucose accumulation, we knocked down Pepck and found that the larvae failed metamorphosis (Fig 8C–8E). The glucose levels in the hemolymph were decreased significantly (Fig 8F), and PA appeared to accumulate (Fig 8G), confirming the role of PEPCK in gluconeogenesis and insect metamorphosis.
pIEx-4-RFP-His and pIEx-4-KLF15-RFP-His were overexpressed in HaEpi cells separately (S10A and S10B Fig), and superimposed 20E stimulation. Compared with RFP-His, overexpression of KLF15-RFP-His significantly promoted Atg8, Casp3 and gluconeogenesis-related genes, G6p and Pepck, transcription (S10C Fig).
Discussion
The regulatory mechanism of glycometabolism homeostasis is fascinating and mysterious. The present study revealed that the insect molting hormone 20E via KLF15 regulates glycometabolism reprogramming from glycolysis to gluconeogenesis and promotes fat body autophagy and apoptosis to supply substrates for gluconeogenesis during metamorphosis.
20E reprogrammed glycometabolism from glycolysis to gluconeogenesis
Various hormones regulate glycometabolism. In humans, insulin maintains blood glucose levels via promoting the uptake of glucose from blood, utilizing glucose by glycolysis, increasing the synthesis and storage of glycogen in tissue cells [38], accelerating the aerobic oxidation of glucose, and inhibiting gluconeogenesis by limiting the release of substrates from storage, such as adipose tissue. Insulin like peptides (ILPs) play similar roles in insects via insulin/insulin-like growth factor (IGF) signaling (IIS) [39]. In feeding conditions, insulin promotes glucose uptake into cells via glucose transporter 4 (GLUT4) [40]. In the clinical treatment of human diseases, steroid hormones, such as glucocorticoids, repress insulin function and increase blood glucose levels. The insect hormone 20E represses larval feeding by competing for binding with the dopamine receptor (DopEcR) [41], and antagonizes IIS to elevate hemolymph glucose levels by repressing the expression and phosphorylation of the receptor during metamorphosis [26]. The glucose levels increased to a peak before pupation and then decreased after the mid-pupa period, which appeared similar to the curve of 20E titer during metamorphosis [22]. In the present study, we showed that 20E represses glycolysis and the synthesis of glycogen and trehalose by repressing the related genes expression to block the metabolic export of glucose in the hemolymph. On the other hand, 20E promotes the degradation of glycogen, trehalose and gluconeogenesis, which increases the metabolic import of glucose in the hemolymph. The levels of PA also continued to decrease after the larvae stop feeding. The decrease in PA is due to 20E counteracting insulin to suppress glycolysis during metamorphosis, as well as a 20E-mediated increase in gluconeogenesis. Taken together, 20E regulates the reprogramming of glycometabolism from glycolysis to gluconeogenesis to increase glucose during metamorphosis.
Transcriptional regulation links changes in a cell’s internal status to how it perceives and reacts to external stimuli [42, 43]. Variations in transcriptional regulation, such as transcription factors, chromatin modifications, or transcription factor co-regulators, alter cellular signaling pathways and initiate metabolic reprogramming to satisfy the needs of fast cell proliferation, differentiation and death [44–46]. In metabolic pathways, there are a variety of enzymes encoded by distinct genes, and their promoters may have different transcription factor binding sites, resulting in diverse transcriptional regulation and thus altering the metabolic process. In mammals, KLF15 is closely related to metabolism and highly expressed in the fasting period, thus playing an important role in gluconeogenesis [11]. KLF15 promotes the expression of a number of enzymes involved in AAs catabolism, including alanine aminotransferase 1 (ALT1), proline dehydrogenase (PRODH), and tryptophan 2, 3-dioxygenase (TDO2) to provide carbon substrates for the formation of nutrients [12, 13]. The gene encoding fasting-induced acyl-CoA synthetase short chain family member 1 (ACSS1) and other fasting-induced genes in skeletal muscle are mainly regulated by KLF15 [6]. The Klf15 gene is a direct transcriptional target of the glucocorticoid receptor (GR), participating in glucocorticoid-regulated physiological processes in mammals [7]. However, how KLF15 regulates glucose metabolism reprogramming and related mechanisms have not been reported. Our research showed that Klf15 is highly expressed by 20E regulation. 20E, via KLF15, increases the expression of a set of genes to promote the degradation of glycogen and trehalose, fat body autophagy and apoptosis and gluconeogenesis.
20E, via KLF15, promotes genes expression for autophagy
Autophagy is a common mechanism for energy supply in eukaryotic cells during nutrient shortage. It degrades and recycles self-protein, organelles or invading pathogens in cells through the lysosome pathway [47]. Autophagy is closely related to nutritional status. When cell nutrition is lacking, cells immediately initiate autophagy to maintain the balance of the AA pool in the cytoplasm. Human KLF15 inhibits the expression of cell cycle regulatory protein, cyclin D and E, and cyclin-dependent kinase 2 (CDK2), to inhibit glomerular mesangial cells entering the S phase in the cell cycle, thereby inhibiting cell proliferation [48]. KLF3 in C. elegans and KLF4 in H. sapiens promote autophagy, and KLF3 promotes Atg2, Atg9 and Atg16 expression [9]. We found a KLF bs in the Atg8 promoter, proved that KLF15 directly promoted Atg8 transcription. We also found other Atgs (Atg3, Atg4b, Atg7, Atg12 and Atg14) had the KLF bs in their promoters and knockdown of Klf15 inhibited the expression of Atg4b, Atg12, and Atg14. KLF15 also increased the expression of the genes involve in glycogen and trehalose degradation, gluconeogenesis and apoptosis. Therefore, KLF15 in H. armigera is a transcription factor that promotes the occurrence of autophagy and apoptosis. KLFs have distinct domains at amino-terminal regions, which determine their transcriptional properties [49]. While KLF bs are same, KLFs may be expressed in different tissues; on the other hand, they may compete for the same binding site [50, 51]. Besides KLF15, the H. armigera genome contains other KLFs, which need to be study on their functions.
Fat body autophagy and apoptosis contribute substrates for gluconeogenesis
Autophagy is closely related to apoptosis [52, 53]. A lack of nutrients causes autophagy, which is followed by apoptosis in mouse neuronal cells. The application of the autophagy inhibitor 3-MA inhibits the release of cytochrome C and the activation of caspase [54]. During the development of holometabolous insects, most autophagy-related genes are expressed before apoptosis-related genes. High titer 20E increases intracellular calcium ion levels, allowing ATG5 to be cleaved, activating CASP3 and promoting apoptosis [25]. 20E induces CTSD (cathepsin D) maturation through autophagy, which promoting CASP3 cleavage and apoptosis in the midgut [22].
The primary nutrients in LD are triglycerides, and the products of triglyceride decomposition are FFAs and glycerol [55, 56]. Glycerol is converted directly into metabolic intermediates to produce glucose, while FFAs are converted into acetyl-CoA via the β-oxidation pathway to enter the TCA cycle for energy supply. Under starvation conditions, the conversion of glycogenic AAs produced by hepatic autophagy into glucose via gluconeogenesis has been considered a potential metabolic contributor in mice [57, 58]. We found autophagy promotes the continuous breakdown of LD as metamorphosis develops after larvae stop feeding, and knockdown of Atg8 resulting in a decrease of FFAs, glycerol, FAAs and glucose, suggesting autophagy produces various substrates for gluconeogenesis. However, the gluconeogenesis promotes the consumption of FFAs, glycerol, FAAs to produce glucose directly or indirectly, therefore FAAs, FFAs and glycerol cannot be accumulated during metamorphosis.
KLF15 can directly and positively regulate Atg8 and Pepck expression, therefore, autophagy and gluconeogenesis were both inhibited after Klf15 knockdown. However, the variation of metabolites FFAs, glycerol and FAAs were opposite after knockdown of Atg8 and Klf15, that knockdown of Atg8 decreased FFAs, glycerol and FAAs, but knockdown of Klf15 increased these metabolites. This is due to knockdown of Atg8 only blocked autophagy and therefore blocked the coming of the substrates, and the gluconeogenesis was still performed by PEPCK under KLF15 regulation. Whereas, knockdown of Klf15 blocked not only autophagy and the coming of the substrates, but also blocked gluconeogenesis and the consumption of the substrates by repressing Pepck, thus FFAs, glycerol and FAAs were accumulated after balance of the metabolism network in vivo.
KLF15 expressed in the fat body and epidermis during metamorphosis. RNAi in insect may produce a systematic effect in various tissues, and the metabolites may be communicated in tissues. However, the fat body is the center of insect metabolism, which stores energy substrates during feeding and produces energy substrates by autophagy and apoptosis during non-feeding or nutrients shortage. Epidermis has not been reported occur autophagy and apoptosis as those in the midgut and fat body, in addition to local cells, which may occur autophagy and apoptosis [22, 59]. Therefore, KLF15 plays key roles in the fat body to integrate autophagy and gluconeogenesis to maintain glucose homeostasis under 20E regulation.
Previous study addressed that high glucose levels during metamorphosis of H. armigera is due to 20E represses insulin receptor expression and phosphorylation [39], it is similar to the type 2 diabetes (T2D) in humans [60], which means that glucose cannot be absorbed into the cells, therefore increasing hemolymph glucose levels. Here, we found glycolysis was changed to gluconeogenesis during metamorphosis by 20E regulation, which also increases hemolymph glucose. However, the glucose levels decreased after insect entering the pupal stage, suggesting the high levels glucose stored during metamorphosis may become the main energy to be used to support the pupal development. Therefore, the glucose levels are well regulated in physiological regions during insect development.
Conclusions
20E, via KLF15, promoted autophagy, apoptosis, and gluconeogenesis. Autophagy and apoptosis supplied substrates for gluconeogenesis. Glycolysis was changed to gluconeogenesis from feeding to non-feeding during metamorphosis. The elevation of hemolymph glucose levels was the result of blocking of glycolysis, enhancing of degradation of glycogen and trehalose, and increased gluconeogenesis (Fig 9).
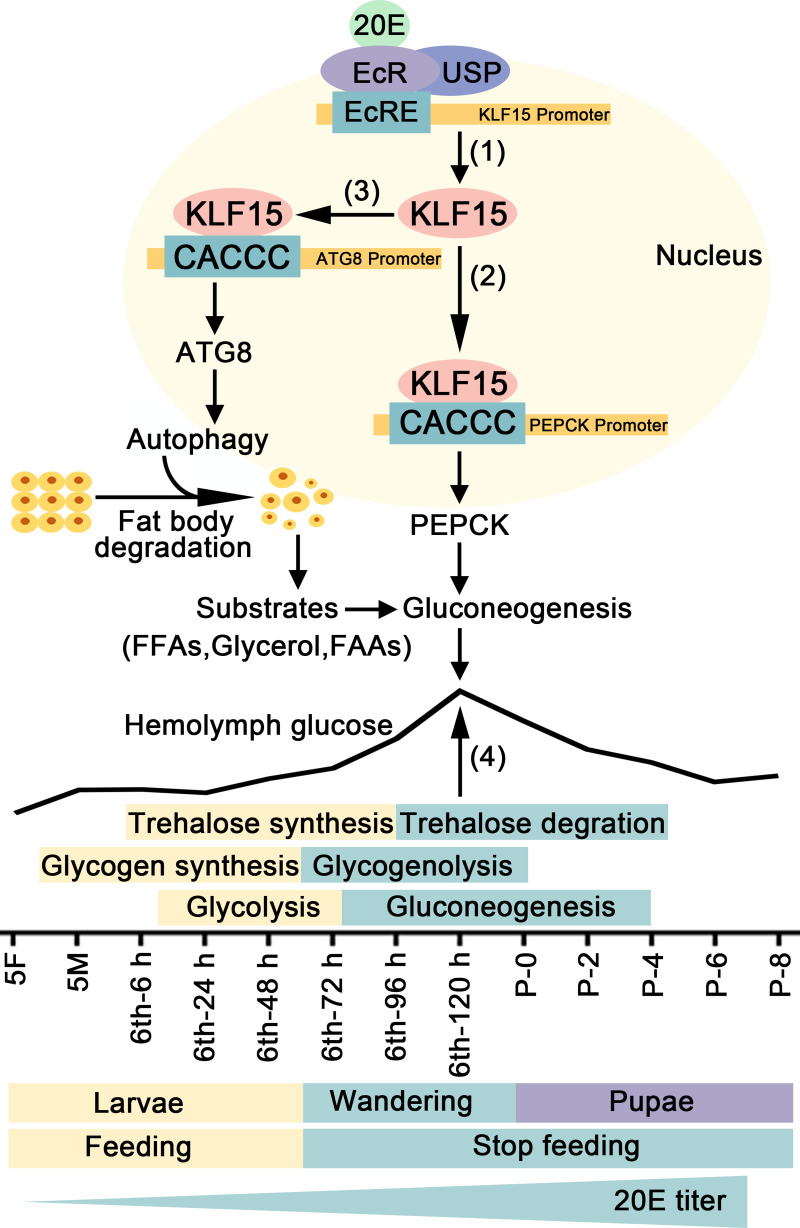
Fig 9. A diagram illustrating 20E regulates glycometabolism reprogramming, autophagy, and apoptosis via KLF15.
20E promotes the expression of Klf15 via nuclear receptors (1). By binding to the KLF bs, KLF15 promotes the transcription of Pepck, a crucial enzyme in gluconeogenesis (2). KLF15 increases the transcription of the autophagy-related gene Atg8 to induce autophagy (3), thereby providing substrates for gluconeogenesis. The increase of glucose during metamorphosis is due to trehalose and glycogen degradation, glycolysis inhibition, and gluconeogenesis (4).
Materials and methods
Ethics statement
The antibody preparation in rabbit was in accordance with protocols approved by the Animal Care & Welfare Committee at Shandong University School of Life Sciences (SYDWLL-2021-54).
Experimental animals
H. armigera were reared on an artificial diet in our laboratory at 27 ± 1°C with 60–70% humidity under a light dark schedule of 14 h:10 h. The larvae were fed with artificial feed composed of soy flour, wheat germ, multivitamins, sucrose, and inorganic salts [61].
Preparation of rabbit polyclonal antibody against KLF15
A fragment of Klf15 (nucleotide sequence 1 bp to 564 bp, amino acids 1 to 188) was inserted into plasmid pET-30a. The recombinant plasmid was sequenced and expressed in Escherichia coli strain Rosetta (DE3). Recombinant KLF15 protein was formed in inclusion body and was purified after denaturation and refolding. Rabbits were immunized with the purified antigen to obtain rabbit polyclonal antibodies (anti-KLF15 antibodies); the molecular weight of KLF15 is 30 kDa (S11 Fig).
Western blotting
We selected larvae or pupae at different ages, cut open the body along the midline and rinsed the epidermis, midgut, and fat body separately in PBS, placed the tissues in 400 μL Tris-HCl buffer (pH 7.5, 40 mM) with 4 μL phenylmethylsulfonyl fluoride (PMSF) on ice and ground them fully. The samples were centrifuged at 10000 × g for 15 min, and the supernatant was retained and incubated in a 100°C water bath for 15 min. Protein (20 μg) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a nitrocellulose membrane. The membrane was incubated in blocking solution prepared with TBST (0.02% tween in TBS) for 1 h at room temperature. The primary antibodies were added into the blocking buffer and incubated overnight at 4°C. The polyclonal antibodies against H. armigera LC3 and KLF15 were produced in our laboratory and were diluted in 5% skim milk at 1:100. The antibodies against H. armigera β-actin and cleaved-CASP3 were diluted in 5% skim milk at 1:500–1:5000. After being washed two times with TBST for 10 min each time, the membrane was incubated with the diluted secondary antibodies (ZB-2301/ZB-2308, ZSGB-BIO, Beijing, China) labeled with horseradish peroxidase/alkaline phosphatase (1:7000 in the blocking buffer) for 2 h at room temperature. The membrane was washed twice with TBST for ten min each time, followed by TBS (pH 7.5, 150 mM NaCl, 10 mM Tris-HCl) for five min once. The membrane was immersed in High-sig ECL Western Blotting Substrate (180–501, Tanon Science & Technology, Shanghai, China) and the immunoreactive protein bands were visualized using the ECL luminescence method. The protein bands were detected using a 5200 Chemiluminescence Imaging System (Tanon Science & Technology). The immunoreactive protein bands were analyzed using Image J software (NIH, Bethesda, MN, USA).
Hormone stimulation of Klf15
20E (16145, Cayman Chemical, Ann Arbor, USA) was diluted to 10 mg/mL with DMSO for storage. When conducting experiments, 20E was diluted 100-fold with sterile phosphate buffered saline (PBS, pH 7.4, 10 mM Na2HPO4, 1.8 mM KH2PO4, 140 mM NaCl, and 2.7 mM KCl). We selected three 6th-6 h larvae and injected them with 20E at 100, 200, and 500 ng, respectively; cultured them for 1, 3, 6, 12, and 24 h; and total RNA was extracted for qRT-PCR. The control groups were treated with the same amount of DMSO and processing time.
qRT-PCR
Total RNA was extracted from the larvae and pupae using the Trizol reagent (O10820, TransGen Biotech, Beijing, China) and then detected the RNA concentration, quantified 2 μg RNA transcribed to single stranded cDNA using a cDNA synthesis kit (G492, Abm, Richmond, Canada). qRT-PCR was performed in a final volume of 10 μL, containing 5 μL of TransStart Tip Green qPCR Supermix (291829AX, Aidlab, Beijing, China), 1 μL of cDNA (1:7 dilution), and 2 μL each of the forward and reverse primers (1 μM) (sequences showed in S1 Table), the β-actin gene (Actb) was used as the internal reference. Data were analyzed by the formula R = 2– (ΔCt sample–ΔCt control), representing the relative transcription levels, ΔCt is the difference between the cycle threshold (Ct) of the genes and the average Actb transcript levels in the experimental or control sample.
EMSA
Nuclear proteins were extracted from HaEpi cells expressing EcR and KLF15 using a nuclear extract kit (R0050, Solarbio, Shanghai, China) according to the manufacturer’ s protocol. After 6 h induction with DMSO or 20E (5 μM), nuclear proteins were extracted. Fluorophore 6-carboxy-fluorescein (FAM)-labeled probes (sense 5’- TCTCAATTACGTTCAATAAACGGCTTTGTTAA-3’ and antisense 5’- TTAACAAAGCCGTTTATTGAACGTAATTGAGA-3’) used in EMSA from the EcRE fragment of Klf15, (sense 5’-TCTCAATTACATGTAATAAACGGCTTTGTTAA-3’ and antisense 5’-TTAACAAAGCCGTTTATTACATGTAATTGAGA-3’) were mutation probe; (sense 5’-CCCGCGGTTTCACCCTCGTCCTGTGGGAACTGCTGCCCG-3’ and antisense 5’-CGGGCAGCAGTTCCCACAGGACGAGGGTGAAACCGCGGG-3’) used in EMSA from the KLF bs of Atg8 and (sense 5’-TGAAAACACCCTGACGCCATCTTGTGTTGAAGCTCGCGCG-3’ and antisense 5’- CGCGCGAGCTTCAACACAAGATGGCGTCAGGGTGTTTTCA-3’) used in EMSA from the KLF bs of Pepck, (sense 5’-CCCGCGGTTTTCCCATCGTCCTGTGGGAACTGCTGCCCG-3’ and antisense 5’- CGGGCAGCAGTTCCCACAGGACGATGGGAAAACCGCGGG-3’), (sense 5’- TGAAAATCCCATGACGCCATCTTGTGTTGAAGCTCGCGCG-3’ and antisense 5’- CGCGCGAGCTTCAACACAAGATGGCGTCATGGGATTTTCA-3’) were mutation probe (produced by the Sangon Company). The probes were dissolved in the annealing buffer (pH 7.5, 10 mM Tris, 50 mM NaCI, 1 mM EDTA). The mixture was heated at 95°C for 10 min and slowly cooled to room temperature. About 15 μg of extracted protein in binding buffer (pH 7.5, 10 mM TrisHCl, 1 mM MgCl2, 40 mM KCl, 0.1 mg/ml BSA, 5% glycerol) was incubated with 200 fmol of FAM-labeled probes at room temperature for 15 min [62, 63]. An antibody for His (1.5 μg) was added to the binding reaction in the supershift experiment. A 50-fold amount of unlabeled probe was added for competition experiments. The protein-DNA samples were then run on a 6% polyacrylamide gel at 80 V for 70 min in 0.5 × TBE buffer (NA0036, LEAGENE, Beijing, China). Tanon Gel Imager System was used for imaging and data analysis.
ChIP
We used 5 μg EcR-RFP-His transfected into HaEpi cells for 72 h (the number of cells is about 8×105−10×105), which were then treated with 5 μM 20E for 6 h to detect the binding of EcR in the promoter of Klf15. For KLF bs of Atg8 and Pepck detection, 5 μg KLF15-RFP-His were transfected into HaEpi cells for 72 h, and then treated with 5 μM 20E for 6 h. The cells were treated according to the instructions of the ChIP assay kit (P2078, Beyotime Biotechnology, Shanghai, China). Anti-His antibody was used to detect EcR-RFP-His and KLF15-RFP-His, and IgG was used as a negative control.
RNA interference (RNAi) in larvae
Long double-stranded RNA (dsRNA) can be used in insects and is broken down into several smaller dsRNA in vivo to degrade the transcript of the targeted gene [64]. Using the target sequence as a template, an RNAi kit (AM1626, Thermo Fisher Scientific, Waltham, MA, USA) was used to synthesize dsRNA according to the instructions of the manufacturer. For RNAi knockdown of genes in larvae, 30 larvae at the 6th-6 h were injected with 3 μg of dsRNA into the hemocoel, three times at 24 h apart. The control larvae were treated with the same amount of dsGFP. The interference efficiency, key enzymes of the gluconeogenesis pathway, hemolymph glucose levels, and the morphology of the fat body were determined after first injection dsRNA for 72 h. Each RNAi was performed independently three times, using a total ninety insects.
Fat body morphology observation
Took fresh fat body and embed it in paraffin (Servicebio, Wuhan, China). The paraffin-embedded fat body were cut into 4 μm pieces and fixed on a glass slide before being dried at 60°C for 2 h and stained according to the YF488 TUNEL assay apoptosis detection instructions (T6013, US EVERBRIGHT INC, Suzhou, China). Servicebio company performed the HE staining, Nile red staining and TEM images (S12 Fig). We used an Olympus BX51 fluorescence microscope (Olympus Optical Co., Tokyo, Japan) to observe the fat body morphology and chose the appropriate magnification to take photos and statistics.
Cell culture
The HaEpi cell line was established from the epidermis of 5th instar larvae in our laboratory [65]. Cells were developed in cell culture flasks loosely attached monolayer and maintained at 27°C. 5 mL Grace’s medium (11300–043, Gibco, USA) and 10% heat-inactivated fetal bovine serum (FBS) (16140063, Gibco, USA) were added to each culture flask and subcultured once a week. We transferred the cells with medium to a 6 or 24-well plate, added 20E for different time stimulation, and used equal diluted DMSO as a control when monitoring autophagic flux, CASP3 activity and flow cytometry. A six-well plate contains approximately 8×105−10×105 cells, while a 24-well plate contains approximately 2×105−3×105 cells. We observed the cells using an Olympus BX51 fluorescence microscope and chose the appropriate magnification to take photos and statistics.
Autophagy detection
LC3-I and LC3-II were detected in a western blot assay with polyclonal rabbit antibody against H. armigera LC3. RFP-GFP-LC3-His was overexpressed in HaEpi cells using pIEx-4-RFP-GFP-LC3-His fluorescent double-labeled plasmid to determine the autophagic flux by 20E regulation according to our previous study [25]. As GFP fluorescence is sensitive to an acidic pH, it can be compared with red fluorescence to monitor the occurrence of autophagy [66]. We set different time gradients to observe the occurrence of 20E regulated autophagy. After 5 μM 20E induction for 1 h, both green fluorescence and red fluorescence were seen in cells, which were similar to the control group treated with DMSO. However, after 12 h of stimulation with 20E, some small puncta were visible in the cells, which indicated that autophagosomes were forming. When the stimulation time reached 24 h, the puncta still existed, but the green light began to dim. After 48 h of stimulation, only red fluorescence was visible, green fluorescence was quenched (S13A Fig), indicating the formation of acidic autolysosomes and 20E promoted the transition from autophagy to apoptosis. The autophagic flux was determined by counting the number of autophagosome and autolysosome puncta in successfully transfected with pIEx-GFP-RFP-LC3 cells from three different pictures.
Apoptosis detection
The rabbit polyclonal anti-cleaved-CASP3 antibody was used to detect the active CASP3 in a western blot assay. The SuperView 488 caspase 3 assay kit (S6007S, US EVERBRIGHT INC, Suzhou, China) was used to detect CASP3 activity in HaEpi cells by immunocytochemistry. The treatment of cells with 10 μM 20E for 24–48 h did not promote cell apoptosis, but the amount of CASP3 green fluorescence significantly increased after the treatment with 10 μM 20E for 72 h, indicating 20E promotes apoptosis in a long-term treatment (S13B Fig). The YF488-Annexin V and propidium iodide (PI) apoptosis kit (Y6002, US EVERBRIGHT INC, Suzhou, China) and flow cytometry were used to detect apoptosis. YF488-Annexin V is Annexin V labeled with the green fluorescent probe YF488, which binds to phosphatidylserine on the cell membrane’s outside to label apoptotic cells. PI is a DNA binding dye that emits red fluorescence in late-stage or necrotic apoptotic cells.
CCK-8 detected cell viability
CCK-8 assay was used to measure cell viability [67, 68]. CCK-8 cell viability assay kit (C6005M, US EVERBRIGHT INC, Suzhou, China) contains WST-8, which is reduced by dehydrogenase in the mitochondria to a highly water-soluble orange-yellow formazan product in the presence of electron carrier. The depth of the dye is proportional to the number of living cells. After adjusting the cell density to 4 ×104 cells/mL, a 100 μL cell suspension was put to a 96-well plate, followed by 10 μL CCK-8, and incubated for 2–3 h in an incubator. When the color steadied, the absorbance value at 450 nm was determined.
FFAs determination
Removed 50 μL of hemolymph from larvae and pupae at various developmental stages and mixed with phenylthiourea (PTU) to prevent hemolymph melanization, added reagents as directed (BC0590, Solarbio, Shanghai, China), and measured absorbance at a wavelength of 550 nm by spectrophotometry. The standard curve was created by diluting the standard to various concentrations, and the FFA levels were estimated using the standard curve.
Glycerol determination
Weighed the fat body at various development stages and rinsed with PBS, added 10 μL of lysis solution per 1 mg of tissue in proportion and ground it quickly on ice (F005-2-1, Njjcbio, Nanjing, China). After incubating samples at 70°C for 10 min to inactivate lipase, they were centrifuged and the supernatant was used to detect at 620 nm using a spectrophotometer. A standard curve was established based on the concentration and absorbance value of the standard substance: y = 0.0002 × x+0.1022, x: glycerol content (μmol/L); y: absorbance. Divided the value by the mass fraction to obtain the glycerol levels (μmol/g).
FAAs determination
Hemolymph (25 μL) was quickly collected into a 1.5 mL tube containing 1.5 μL 1 mM PTU and 25 μL anticoagulant (450 mM NaCl, 10 mM KCI, 10 mM EDTA, 10 mM HEPES). After centrifuging the samples at 1000 × g for 10 min, 30 μL of the supernatant was removed and mixed with 300 μL of 20% trichloroacetic acid. After thorough mixing, the samples were centrifuged at 8000 × g for 10 min, the supernatant was removed, filtered through a 0.22 μm filter, and injected into L-8900 Amino Acid Analyzer (Hitachi Limited, Tokyo, Japan) to detect different FAAs levels.
Hemolymph glucose determination
Hemolymph (30 μL) was collected from at least three insects into tubes containing 1.5 μL of 1 mM PTU and centrifuged at 1000 × g for 3 min to remove blood cells. 10 μL of serum was added with 1 mL of glucose extract from glucose determination kit (ml076789, Mlbio, Shanghai, China), incubated at 37°C for 10 min in the dark, and then the absorbance was measured at a wavelength of 505 nm by spectrophotometry. The glucose levels of the samples were calculated according to the formula: Sample tube absorbance/calibration tube absorbance × calibration solution concentration (5.5 mmol/L).
PA determination
From selected larvae or pupae, the fourth pair of appendages were cut off, 45 μL of hemolymph was collected into a tube containing 2.5 μL 1 mM PTU, added with 200 μL of extract buffer, and quickly ground on ice. After standing for 30 min at room temperature, the samples were centrifuged at 8000 × g for 10 min at room temperature. 75 μL supernatant was added with reagent one and two in order, following the instructions of the PA determination kit (BC2205, Solarbio, Shanghai, China). The absorbance was measured at 520 nm using spectrophotometry immediately after the sample was mixed. A standard curve was established based on the concentration and absorbance value of the standard substance: y = 52.439 × x-6.6884; x: absorbance; y: sodium PA levels (μg/mL). The value of sodium PA was obtained from the standard curve and then multiplied by 10 to obtain the levels of PA in vivo.
Trehalose determination
Hemolymph (50 μL) was quickly collected from more than three larvae or pupae from the fourth pair of appendages into a 1.5 mL centrifuge tube containing 2.5 μL 1 mM PTU and centrifuged at 1000 × g for 3 min. The supernatant was retained and added with 450 μL of extract in trehalose determination kit (BC0335, Solarbio, Shanghai, China). After standing at room temperature for 45 min, the samples were centrifuged at 8000 × g for 10 min. The supernatant (250 μL) were removed and mixed with the working solution in the kit. After heating the samples at 95°C for 10 min and allowing them to cool naturally, the absorbance at 620 nm was measured using spectrophotometry. A standard curve was established based on the absorbance value of the standard: y = 5.6955 × x+0.0908; x: trehalose content (mg/g); y: absorbance. The trehalose value was obtained from the standard curve and multiplied by 10 to calculate the in vivo trehalose levels.
Glycogen determination
The fat body (0.1 g) was extracted at different development stages and rinsed with PBS, added with extract buffer from a glycogen determination kit (ml016886, Mlbio, Shanghai, China), incubated in water bath at 100°C for 20 min, and shaken it every 5 min to fully mix. After the tissue samples were completely cooled, other reagents were added according to the instructions of the kit. The absorbance of each sample was detected at 620 nm using a spectrophotometer, and the glycogen levels were calculated as: Glycogen (mg/g) = tested tube absorbance value/standard tube absorbance value × standard tube content × dilution factor/0.11.
The antibodies used in this study
The rabbit polyclonal anti-cleaved-CASP3 antibody was provided by WanLei biological company (WL01992, Wanlei, Shenyang, China). Monoclonal anti-ACTB antibody (AC026, ABclonal Technology, Wuhan, China) and monoclonal anti-His antibody (AE003, ABclonal Technology, Wuhan, China) were obtained from ABclonal. The polyclonal rabbit antibodies against H. armigera LC3 and KLF15 were prepared in our laboratory using recombinantly expressed protein in E. coli according to previous description [69].
Bioinformatic analysis
The protein sequences were analyzed using the MEGA 7 software to construct a phylogenetic tree. The DNAMAN software (Lynnon Biosoft, San Ramon, CA, USA) was used to generate the multiple sequence alignment. The reading frames of genes were analyzed at the NCBI (https://www.ncbi.nlm.nih.gov/). The protein structural domains were analyzed using SMART (http://smart.embl-heidelberg.de/). The EcRE motif was predicted by JASPAR transcription factor database (http://jaspar.genereg.net/).
Statistical analysis
We used Excel and GraphPad 7 software (GraphPad Inc., La Jolla, CA, USA) to analyze the data and generate the figures. All metabolites were measured from at least three larvae or pupae, and the experiments were repeated three times. The paired data were analyzed statistically using Student’s t-test, asterisks represent significant differences (* p < 0.05, ** p < 0.01, *** p < 0.001). In the figures, the bars represent the mean ± standard deviation (SD) for three independent biological experiments. The comparison between multiple sets of data was analysis of variance (ANOVA). The different lowercase letters showed significant differences (p < 0.05).
Supporting information
There are five Klfs in the genome, Luna, LOC110376548 (XP_021190718.1, XP_021190719.1, XP_021190721.1); Dar, LOC110374594 (XP_021188035.1, XP_021188036.1, XP_021188037.1); Klf8, LOC110378525 (XP_021193494.1); Klf9, LOC110384292 (XP_021201196.1); Klf16, LOC110373997 (XP_021187184.1). H. armigera: Helicoverpa armigera; B. mori: Bombyx mori; H. sapiens: Homo sapiens; D. melanogaster: Drosophila melanogaster; C. elegans: Caenorhabditis elegans.
(TIF)
A. Phylogenetic tree analysis of KLFs from H. armigera and D. melanogaster. B. Sequence alignment of H. sapiens KLF9 and KLF15, D. melanogaster KLF15, and H. armigera KLF15 to name the targeted gene correctly. The area marked in the red box is the structural domain.
(TIF)
A-E. Reverse transcription polymerase chain reaction (RT-PCR) screened highly expressed Klfs in the fat body. E: epidermis; M: midgut; F: fat body. F. The RNAi efficiency of Luna, Klf15 and Klf16 in the fat body. G. Glucose levels were detected in the hemolymph after knockdown Luna, Klf15 and Klf16. H. Detected the expression of G6p and Pepck after Klf15, Klf16 knockdown by qRT-PCR.
(TIF)
A. Alignment of the EcRE sites in the promoter of Klf15 predicted by JASPAR transcription factor database. B. Genomic amplification of sequences containing EcRE in the Klf15 promoter and incubated with EcR nuclear proteins for EMSA to screen the EcRE site that bind to EcR.
(TIF)
A. qRT-PCR validation of the interference efficiency of Klf15 in HaEpi cells. B. GFP expression was detected after YFP was knocked down.
(TIF)
A. Flow cytometry analysis of YF488-annexin V and PI staining after knocked down YFP and Klf15. R1, normal cells; R2, early apoptotic cells; R3, middle and late apoptotic cells; R4, necrotic cells. Ai. Statistical analysis for A. B. CCK-8 detected cell viability after knocked down YFP and Klf15.
(TIF)
(TIF)
A. The RNAi efficiency of Atg8 and Casp3 in the epidermis, midgut, and fat body assessed using qRT-PCR after the third injection of dsRNA. B. HE staining, Nile red staining and TUNEL staining showing the morphology of fat body after dsCasp3 injection. C. Glucose levels in the hemolymph decreased after knockdown Casp3.
(TIF)
(TIF)
A. RFP-His, KLF15-RFP-His detection by western blotting. B. RFP-His and KLF15-RFP-His fluorescent plasmid transfection efficiency detection. C. qRT-PCR showing the expression of Atg8, Casp3, G6p and Pepck after overexpression of RFP-His and KLF15-RFP-His in HaEpi cells.
(TIF)
A. 564 bp length KLF15 was overexpressed in E. coli via the pET-30a plasmid. The expression product was 32 kDa (20 kDa KLF15 plus 12 kDa his-tag). Lane 1, protein lysates of E. coli transformed with plasmid pET-30a-KLF15 before IPTG induction. Lane 2, protein lysates after IPTG (0.5 mM) induction overnight at 37°C. Lane 3, the supernatant of E. coli lysates. Lane 4, the precipitate of E. coli lysates. Lane 5, the purified KLF15 used for rabbit antibody preparation. B. Specificity detection of KLF15 antibody with epidermis of the 6th-96 h larvae. C-E. Specificity detection of β-Actin, LC3 and cleaved-CASP3 antibodies with fat body of the 6th-96 h larvae.
(TIF)
The area pointed by the red arrows includes autophagosomes contained degenerating cytoplasmic organelles or degraded lipid droplets, and autophagic vacuoles, the blue arrows represents the apoptotic nuclei. The bars represent 100 μm.
(TIF)
A. Transfection of RFP-GFP-LC3-His fluorescent double-labeled plasmid in HaEpi cells. 5 μM 20E to stimulate 1 h, 12 h, 24 h and 48 h, respectively, DMSO was used as the control. The yellow bars represented 20 μm. The yellow arrows represented autophagosome puncta, the blue arrow represented autolysosome puncta. B. Examination of apoptosis in HaEpi cells by the SuperView 488 caspase 3 assay kit. 10 μM 20E to stimulate 24 h, 48 h and 72 h, respectively. The yellow bars represented 100 μm.
(TIF)
(DOCX)
Acknowledgments
Thanks to Shandong University Public Technology Platform. Hai-Yan Sui and Zhao-Yuan Cui helped us with data processing and computer operation.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No. 31730083). https://www.nsfc.gov.cn/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Agius L. Role of glycogen phosphorylase in liver glycogen metabolism. Mol Aspects Med. 2015;46:34–45. doi: 10.1016/j.mam.2015.09.002 . [DOI] [PubMed] [Google Scholar]
- 2.Doorneweerd DD, Tan AW, Nuttall FQ. Liver phosphorylase kinase: characterization of two interconvertible forms and partial purification of phosphorylase kinase a. Mol Cell Biochem. 1982;47(1):45–53. doi: 10.1007/BF00241565 . [DOI] [PubMed] [Google Scholar]
- 3.Miyamoto T, Amrein H. Neuronal gluconeogenesis regulates systemic glucose homeostasis in Drosophila melanogaster. Curr Biol. 2019;29(8):1263–72 e5. doi: 10.1016/j.cub.2019.02.053 . [DOI] [PubMed] [Google Scholar]
- 4.Ekberg K, Landau BR, Wajngot A, Chandramouli V, Efendic S, Brunengraber H, et al. Contributions by kidney and liver to glucose production in the postabsorptive state and after 60 h of fasting. Diabetes. 1999;48(2):292–8. doi: 10.2337/diabetes.48.2.292 . [DOI] [PubMed] [Google Scholar]
- 5.Shields JM, Yang VW. Identification of the DNA sequence that interacts with the gut-enriched Krüppel-like factor. Nucleic Acids Res. 1998;26(3):796–802. doi: 10.1093/nar/26.3.796 ; PubMed Central PMCID: PMC147321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto J, Ikeda Y, Iguchi H, Fujino T, Tanaka T, Asaba H, et al. A Krüppel-like factor KLF15 contributes fasting-induced transcriptional activation of mitochondrial acetyl-CoA synthetase gene AceCS2. J Biol Chem. 2004;279(17):16954–62. doi: 10.1074/jbc.M312079200 . [DOI] [PubMed] [Google Scholar]
- 7.Knoedler JR, Denver RJ. Krüppel-like factors are effectors of nuclear receptor signaling. Gen Comp Endocrinol. 2014;203:49–59. doi: 10.1016/j.ygcen.2014.03.003 ; PubMed Central PMCID: PMC4339045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh PN, Zhou G, Yuan Y, Zhang R, Prosdocimo DA, Sangwung P, et al. A conserved KLF-autophagy pathway modulates nematode lifespan and mammalian age-associated vascular dysfunction. Nat Commun. 2017;8(1):914. doi: 10.1038/s41467-017-00899-5 ; PubMed Central PMCID: PMC5640649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Yang C, Brey C, Rodriguez M, Oksov Y, Gaugler R, et al. Mutation in Caenorhabditis elegans Krüppel-like factor, KLF-3 results in fat accumulation and alters fatty acid composition. Exp Cell Res. 2009;315(15):2568–80. doi: 10.1016/j.yexcr.2009.04.025 . [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi Y, Yahagi N, Aita Y, Murayama Y, Sawada Y, Piao X, et al. KLF15 enables rapid switching between lipogenesis and gluconeogenesis during fasting. Cell Rep. 2016;16(9):2373–86. doi: 10.1016/j.celrep.2016.07.069 ; PubMed Central PMCID: PMC5031553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teshigawara K, Ogawa W, Mori T, Matsuki Y, Watanabe E, Hiramatsu R, et al. Role of Krüppel-like factor 15 in PEPCK gene expression in the liver. Biochem Biophys Res Commun. 2005;327(3):920–6. doi: 10.1016/j.bbrc.2004.12.096 . [DOI] [PubMed] [Google Scholar]
- 12.Gray S, Wang B, Orihuela Y, Hong EG, Fisch S, Haldar S, et al. Regulation of gluconeogenesis by Krüppel-like factor 15. Cell Metab. 2007;5(4):305–12. doi: 10.1016/j.cmet.2007.03.002 ; PubMed Central PMCID: PMC1892530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takashima M, Ogawa W, Hayashi K, Inoue H, Kinoshita S, Okamoto Y, et al. Role of KLF15 in regulation of hepatic gluconeogenesis and metformin action. Diabetes. 2010;59(7):1608–15. doi: 10.2337/db09-1679 ; PubMed Central PMCID: PMC2889759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang XY, He MQ, Li JZ, Wang HY, Huang JA. KLF15 suppresses cell growth and predicts prognosis in lung adenocarcinoma. Biomed Pharmacother. 2018;106:672–7. WOS:000442600300080. doi: 10.1016/j.biopha.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 15.Li S, Yu X, Feng Q. Fat body biology in the last decade. Annu Rev Entomol. 2019;64:315–33. doi: 10.1146/annurev-ento-011118-112007 . [DOI] [PubMed] [Google Scholar]
- 16.Liu CY, Liu W, Zhao WL, Wang JX, Zhao XF. Upregulation of the expression of prodeath serine/threonine protein kinase for programmed cell death by steroid hormone 20-hydroxyecdysone. Apoptosis. 2013;18(2):171–87. doi: 10.1007/s10495-012-0784-4 . [DOI] [PubMed] [Google Scholar]
- 17.Arya R, White K. Cell death in development: Signaling pathways and core mechanisms. Semin Cell Dev Biol. 2015;39:12–9. doi: 10.1016/j.semcdb.2015.02.001 ; PubMed Central PMCID: PMC4410075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beenakkers AM, Van der Horst DJ, Van Marrewijk WJ. Insect lipids and lipoproteins, and their role in physiological processes. Prog Lipid Res. 1985;24(1):19–67. doi: 10.1016/0163-7827(85)90007-4 . [DOI] [PubMed] [Google Scholar]
- 19.Arrese EL, Soulages JL. Insect fat body: Energy, metabolism, and regulation. Annu Rev Entomol. 2010;55:207–25. doi: 10.1146/annurev-ento-112408-085356 ; PubMed Central PMCID: PMC3075550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pritchett TL, Tanner EA, McCall K. Cracking open cell death in the Drosophila ovary. Apoptosis. 2009;14(8):969–79. doi: 10.1007/s10495-009-0369-z ; PubMed Central PMCID: PMC2810646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CY, Simon CR, Woodard CT, Baehrecke EH. Genetic mechanism for the stage- and tissue-specific regulation of steroid triggered programmed cell death in Drosophila. Dev Biol. 2002;252(1):138–48. doi: 10.1006/dbio.2002.0838 . [DOI] [PubMed] [Google Scholar]
- 22.Di YQ, Han XL, Kang XL, Wang D, Chen CH, Wang JX, et al. Autophagy triggers CTSD (cathepsin D) maturation and localization inside cells to promote apoptosis. Autophagy. 2020:1–23. doi: 10.1080/15548627.2020.1752497 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CH, Pan J, Di YQ, Liu W, Hou L, Wang JX, et al. Protein kinase C delta phosphorylates ecdysone receptor B1 to promote gene expression and apoptosis under 20-hydroxyecdysone regulation. Proc Natl Acad Sci U S A. 2017;114(34):E7121–E30. doi: 10.1073/pnas.1704999114 ; PubMed Central PMCID: PMC5576805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riddiford LM. Hormone receptors and the regulation of insect metamorphosis. Receptor. 1993;3(3):203–9. WOS:A1993MQ22100009. [PubMed] [Google Scholar]
- 25.Li YB, Li XR, Yang T, Wang JX, Zhao XF. The steroid hormone 20-hydroxyecdysone promotes switching from autophagy to apoptosis by increasing intracellular calcium levels. Insect Biochem Molec. 2016;79:73–86. WOS:000389731500009. doi: 10.1016/j.ibmb.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 26.Li YL, Yao YX, Zhao YM, Di YQ, Zhao XF. The steroid hormone 20-hydroxyecdysone counteracts insulin signaling via insulin receptor dephosphorylation. J Biol Chem. 2021:100318. doi: 10.1016/j.jbc.2021.100318 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riddihough G, Pelham HRB. An ecdysone response element in the Drosophila Hsp27 promoter. Embo J. 1987;6(12):3729–34. WOS:A1987K948600024. doi: 10.1002/j.1460-2075.1987.tb02707.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antoniewski C, Laval M, Lepesant JA. Structural features critical to the activity of an ecdysone receptor-binding site. Insect Biochem Molec. 1993;23(1):105–14. WOS:A1993LA15200014. [DOI] [PubMed] [Google Scholar]
- 29.Cakouros D, Daish TJ, Kumar S. Ecdysone receptor directly binds the promoter of the Drosophila caspase dronc, regulating its expression in specific tissues. J Cell Biol. 2004;165(5):631–40. doi: 10.1083/jcb.200311057 ; PubMed Central PMCID: PMC2172386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shirai H, Kamimura M, Yamaguchi J, Imanishi S, Kojima T, Fujiwara H. Two adjacent cis-regulatory elements are required for ecdysone response of ecdysone receptor (EcR) B1 transcription. PLoS One. 2012;7(11):e49348. doi: 10.1371/journal.pone.0049348 ; PubMed Central PMCID: PMC3498158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330(6009):1344–8. WOS:000284902100034. doi: 10.1126/science.1193497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green DR, Llambi F. Cell death signaling. Csh Perspect Biol. 2015;7(12). WOS:000368537300001. doi: 10.1101/cshperspect.a006080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy. 2021;17(1):1–382. WOS:000636121800001. doi: 10.1080/15548627.2020.1797280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McConnell BB, Yang VW. Mammalian Krüppel-like factors in health and diseases. Physiol Rev. 2010;90(4):1337–81. WOS:000283216100003. doi: 10.1152/physrev.00058.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quadrini KJ, Bieker JJ. Krüppel-like zinc fingers bind to nuclear import proteins and are required for efficient nuclear localization of erythroid Krüppel-like factor. J Biol Chem. 2002;277(35):32243–52. WOS:000177718700118. doi: 10.1074/jbc.M205677200 [DOI] [PubMed] [Google Scholar]
- 36.Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, et al. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10(3):353–60. doi: 10.1038/ncb1698 . [DOI] [PubMed] [Google Scholar]
- 37.Nuttall FQ, Ngo A, Gannon MC. Regulation of hepatic glucose production and the role of gluconeogenesis in humans: Is the rate of gluconeogenesis constant? Diabetes Metab Res Rev. 2008;24(6):438–58. doi: 10.1002/dmrr.863 . [DOI] [PubMed] [Google Scholar]
- 38.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. WOS:000172676200060. doi: 10.1038/414799a [DOI] [PubMed] [Google Scholar]
- 39.Lin XY, Smagghe G. Roles of the insulin signaling pathway in insect development and organ growth. Peptides. 2019;122. WOS:000502028600010. doi: 10.1016/j.peptides.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 40.Gabbouj S, Ryhanen S, Marttinen M, Wittrahm R, Takalo M, Kemppainen S, et al. Altered insulin signaling in alzheimer’s disease brain—Special emphasis on PI3K-Akt pathway. Front Neurosci. 2019;13:629. doi: 10.3389/fnins.2019.00629 ; PubMed Central PMCID: PMC6591470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang XL, Zhang JY, Wang D, Zhao YM, Han XL, Wang JX, et al. The steroid hormone 20-hydroxyecdysone binds to dopamine receptor to repress lepidopteran insect feeding and promote pupation. PLoS Genet. 2019;15(8):e1008331. doi: 10.1371/journal.pgen.1008331 ; PubMed Central PMCID: PMC6693746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiviet DJ, Nghe P, Walker N, Boulineau S, Sunderlikova V, Tans SJ. Stochasticity of metabolism and growth at the single-cell level. Nature. 2014;514(7522):376–9. doi: 10.1038/nature13582 . [DOI] [PubMed] [Google Scholar]
- 43.Ortmayr K, Dubuis S, Zampieri M. Metabolic profiling of cancer cells reveals genome-wide crosstalk between transcriptional regulators and metabolism. Nat Commun. 2019;10(1):1841. doi: 10.1038/s41467-019-09695-9 ; PubMed Central PMCID: PMC6478870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuneva MO, Fan TW, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T, et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012;15(2):157–70. doi: 10.1016/j.cmet.2011.12.015 ; PubMed Central PMCID: PMC3282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17(4):351–9. doi: 10.1038/ncb3124 ; PubMed Central PMCID: PMC4939711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reznik E, Wang Q, La K, Schultz N, Sander C. Mitochondrial respiratory gene expression is suppressed in many cancers. Elife. 2017;6. doi: 10.7554/eLife.21592 ; PubMed Central PMCID: PMC5243113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–75. WOS:000253453600034. doi: 10.1038/nature06639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang LF, Lin WA, Chen JH. Krüppel-like factor 15: A potential therapeutic target for kidney disease. Int J Biol Sci. 2019;15(9):1955–61. WOS:000481578100015. doi: 10.7150/ijbs.34838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rane MJ, Zhao Y, Cai L. Krüppel-like factors (KLFs) in renal physiology and disease. EBioMedicine. 2019;40:743–50. doi: 10.1016/j.ebiom.2019.01.021 ; PubMed Central PMCID: PMC6414320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dang DT, Pevsner J, Yang VW. The biology of the mammalian Krüppel-like family of transcription factors. Int J Biochem Cell Biol. 2000;32(11–12):1103–21. doi: 10.1016/s1357-2725(00)00059-5 ; PubMed Central PMCID: PMC2754176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eaton SA, Funnell AP, Sue N, Nicholas H, Pearson RC, Crossley M. A network of Krüppel-like factors (Klfs). Klf8 is repressed by Klf3 and activated by Klf1 in vivo. J Biol Chem. 2008;283(40):26937–47. doi: 10.1074/jbc.M804831200 ; PubMed Central PMCID: PMC2556010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mukhopadhyay S, Panda PK, Sinha N, Das DN, Bhutia SK. Autophagy and apoptosis: Where do they meet? Apoptosis. 2014;19(4):555–66. doi: 10.1007/s10495-014-0967-2 . [DOI] [PubMed] [Google Scholar]
- 53.Wu H, Che X, Zheng Q, Wu A, Pan K, Shao A, et al. Caspases: a molecular switch node in the crosstalk between autophagy and apoptosis. Int J Biol Sci. 2014;10(9):1072–83. doi: 10.7150/ijbs.9719 ; PubMed Central PMCID: PMC4183927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xue L, Fletcher GC, Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci. 1999;14(3):180–98. doi: 10.1006/mcne.1999.0780 . [DOI] [PubMed] [Google Scholar]
- 55.Walther TC, Chung J, Farese RV Jr. Lipid droplet biogenesis. Annu Rev Cell Dev Biol. 2017;33:491–510. doi: 10.1146/annurev-cellbio-100616-060608 ; PubMed Central PMCID: PMC6986389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tauchi-Sato K, Ozeki S, Houjou T, Taguchi R, Fujimoto T. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J Biol Chem. 2002;277(46):44507–12. doi: 10.1074/jbc.M207712200 . [DOI] [PubMed] [Google Scholar]
- 57.Ezaki J, Matsumoto N, Takeda-Ezaki M, Komatsu M, Takahashi K, Hiraoka Y, et al. Liver autophagy contributes to the maintenance of blood glucose and amino acid levels. Autophagy. 2011;7(7):727–36. WOS:000292300600008. doi: 10.4161/auto.7.7.15371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mallette LE, Exton JH, Park. Effects of glucagon on amino acid transport and utilization in the perfused rat liver. J Biol Chem. 1969;244(20):5724–8. [PubMed] [Google Scholar]
- 59.Nardi JB, Bee CM, Wallace CL. Remodeling of the abdominal epithelial monolayer during the larva-pupa-adult transformation of Manduca. Dev Biol. 2018;438(1):10–22. doi: 10.1016/j.ydbio.2018.03.017 . [DOI] [PubMed] [Google Scholar]
- 60.Ashcroft FM, Rorsman P. Diabetes mellitus and the beta cell: The last ten years. Cell. 2012;148(6):1160–71. WOS:000301889500014. doi: 10.1016/j.cell.2012.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao XF, Wang JX, Wang YC. Purification and characterization of a cysteine proteinase from eggs of the cotton boll worm, Helicoverpa armigera. Insect Biochem Molec. 1998;28(4):259–64. WOS:000074824400007. [Google Scholar]
- 62.Wang K, Gao Y, Peng X, Yang G, Gao F, Li S, et al. Using FAM labeled DNA oligos to do RNA electrophoretic mobility shift assay. Mol Biol Rep. 2010;37(6):2871–5. doi: 10.1007/s11033-009-9841-7 . [DOI] [PubMed] [Google Scholar]
- 63.Zhang F, Li B, Dong H, Chen M, Yao S, Li J, et al. YdiV regulates Escherichia coli ferric uptake by manipulating the DNA-binding ability of Fur in a SlyD-dependent manner. Nucleic Acids Res. 2020;48(17):9571–88. doi: 10.1093/nar/gkaa696 ; PubMed Central PMCID: PMC7515728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101(1):25–33. doi: 10.1016/S0092-8674(00)80620-0 . [DOI] [PubMed] [Google Scholar]
- 65.Shao HL, Zheng WW, Liu PC, Wang Q, Wang JX, Zhao XF. Establishment of a new cell line from lepidopteran epidermis and hormonal regulation on the genes. PLoS One. 2008;3(9):e3127. doi: 10.1371/journal.pone.0003127 ; PubMed Central PMCID: PMC2518862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homolog of yeast Apg8p, is localized in autophagosome membranes after processing (vol 19, pg 5720, 2000). Embo J. 2003;22(17):4577–. WOS:000185147900023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hou Y, Zhang Q, Pang W, Hou L, Liang Y, Han X, et al. YTHDC1-mediated augmentation of miR-30d in repressing pancreatic tumorigenesis via attenuation of RUNX1-induced transcriptional activation of Warburg effect. Cell Death Differ. 2021;28(11):3105–24. doi: 10.1038/s41418-021-00804-0 ; PubMed Central PMCID: PMC8563797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H, Qin G, Zhang C, Yang H, Liu J, Hu H, et al. TRAIL promotes epithelial-to-mesenchymal transition by inducing PD-L1 expression in esophageal squamous cell carcinomas. J Exp Clin Cancer Res. 2021;40(1):209. doi: 10.1186/s13046-021-01972-0 ; PubMed Central PMCID: PMC8223376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu W, Cai MJ, Wang JX, Zhao XF. In a nongenomic action, steroid hormone 20-hydroxyecdysone induces phosphorylation of cyclin-dependent kinase 10 to promote gene transcription. Endocrinology. 2014;155(5):1738–50. doi: 10.1210/en.2013-2020 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
There are five Klfs in the genome, Luna, LOC110376548 (XP_021190718.1, XP_021190719.1, XP_021190721.1); Dar, LOC110374594 (XP_021188035.1, XP_021188036.1, XP_021188037.1); Klf8, LOC110378525 (XP_021193494.1); Klf9, LOC110384292 (XP_021201196.1); Klf16, LOC110373997 (XP_021187184.1). H. armigera: Helicoverpa armigera; B. mori: Bombyx mori; H. sapiens: Homo sapiens; D. melanogaster: Drosophila melanogaster; C. elegans: Caenorhabditis elegans.
(TIF)
A. Phylogenetic tree analysis of KLFs from H. armigera and D. melanogaster. B. Sequence alignment of H. sapiens KLF9 and KLF15, D. melanogaster KLF15, and H. armigera KLF15 to name the targeted gene correctly. The area marked in the red box is the structural domain.
(TIF)
A-E. Reverse transcription polymerase chain reaction (RT-PCR) screened highly expressed Klfs in the fat body. E: epidermis; M: midgut; F: fat body. F. The RNAi efficiency of Luna, Klf15 and Klf16 in the fat body. G. Glucose levels were detected in the hemolymph after knockdown Luna, Klf15 and Klf16. H. Detected the expression of G6p and Pepck after Klf15, Klf16 knockdown by qRT-PCR.
(TIF)
A. Alignment of the EcRE sites in the promoter of Klf15 predicted by JASPAR transcription factor database. B. Genomic amplification of sequences containing EcRE in the Klf15 promoter and incubated with EcR nuclear proteins for EMSA to screen the EcRE site that bind to EcR.
(TIF)
A. qRT-PCR validation of the interference efficiency of Klf15 in HaEpi cells. B. GFP expression was detected after YFP was knocked down.
(TIF)
A. Flow cytometry analysis of YF488-annexin V and PI staining after knocked down YFP and Klf15. R1, normal cells; R2, early apoptotic cells; R3, middle and late apoptotic cells; R4, necrotic cells. Ai. Statistical analysis for A. B. CCK-8 detected cell viability after knocked down YFP and Klf15.
(TIF)
(TIF)
A. The RNAi efficiency of Atg8 and Casp3 in the epidermis, midgut, and fat body assessed using qRT-PCR after the third injection of dsRNA. B. HE staining, Nile red staining and TUNEL staining showing the morphology of fat body after dsCasp3 injection. C. Glucose levels in the hemolymph decreased after knockdown Casp3.
(TIF)
(TIF)
A. RFP-His, KLF15-RFP-His detection by western blotting. B. RFP-His and KLF15-RFP-His fluorescent plasmid transfection efficiency detection. C. qRT-PCR showing the expression of Atg8, Casp3, G6p and Pepck after overexpression of RFP-His and KLF15-RFP-His in HaEpi cells.
(TIF)
A. 564 bp length KLF15 was overexpressed in E. coli via the pET-30a plasmid. The expression product was 32 kDa (20 kDa KLF15 plus 12 kDa his-tag). Lane 1, protein lysates of E. coli transformed with plasmid pET-30a-KLF15 before IPTG induction. Lane 2, protein lysates after IPTG (0.5 mM) induction overnight at 37°C. Lane 3, the supernatant of E. coli lysates. Lane 4, the precipitate of E. coli lysates. Lane 5, the purified KLF15 used for rabbit antibody preparation. B. Specificity detection of KLF15 antibody with epidermis of the 6th-96 h larvae. C-E. Specificity detection of β-Actin, LC3 and cleaved-CASP3 antibodies with fat body of the 6th-96 h larvae.
(TIF)
The area pointed by the red arrows includes autophagosomes contained degenerating cytoplasmic organelles or degraded lipid droplets, and autophagic vacuoles, the blue arrows represents the apoptotic nuclei. The bars represent 100 μm.
(TIF)
A. Transfection of RFP-GFP-LC3-His fluorescent double-labeled plasmid in HaEpi cells. 5 μM 20E to stimulate 1 h, 12 h, 24 h and 48 h, respectively, DMSO was used as the control. The yellow bars represented 20 μm. The yellow arrows represented autophagosome puncta, the blue arrow represented autolysosome puncta. B. Examination of apoptosis in HaEpi cells by the SuperView 488 caspase 3 assay kit. 10 μM 20E to stimulate 24 h, 48 h and 72 h, respectively. The yellow bars represented 100 μm.
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.