Abstract
Background
Emergence delirium can occur after general anesthesia in children. An intravenous infusion of alfentanil may reduce the incidence or severity of emergence delirium after sevoflurane anesthesia.
Objective
The study aimed to investigate the effects of alfentanil intravenous infusion on emergence delirium and other perioperative complications.
Method
This was a single-center, randomized, placebo-controlled, double-blind clinical trial. A total of 172 children undergoing ambulatory dental treatment were randomized into three groups. Alfentanil group Alf2 received 0.2 μg/kg/min of alfentanil for continuous infusion, alfentanil group Alf4 received 0.4 μg/kg/min alfentanil, and the saline group (group Sal) received a continuous infusion of normal saline, with the same volume as the two other groups, as a placebo. The incidence of emergence delirium (assessed by the Paediatric Anaesthesia Emergence Delirium [PAED] scale), hemodynamic parameters, and recovery characteristics were recorded during the recovery period. The Aono scale was also used to assess for emergence delirium. A WeChat applet was designed to facilitate a caregiver teleconsultation and to provide feedback on postoperative nausea and vomiting and any other complications after discharge.
Results
The incidence of emergence delirium in group Alf2 (22.9%) and group Alf4 (21.1%) was significantly lower than that observed in the Sal group (48.3%). The PAED scores in group Alf2 (6.4 ± 3.5) and group Alf4 (5.8 ± 3.8) were significantly lower than those for group Sal (9.6 ± 5.1) (p < 0.01). Ten children in the Alf4 group needed manual ventilatory assistance to maintain end-tidal carbon dioxide (ETCO2) < 55 mm; children in group Alf2 did not. There was no significant difference between the discharge time of groups Alf2 and Sal (31.2 ± 4.64 vs 30.5 ± 2.82 min; 0.659 [95% confidence interval {CI} −1.052 to 2.369], p = 0.643); the time to discharge of group Alf4 (35.16 ± 3.97 min) was significantly longer than that of groups Alf2 and Sal (p < 0.01). The incidence of nausea and vomiting was similar in the three groups. No other clinically relevant adverse events were observed.
Conclusions
Intravenous infusion of 0.2 μg/kg/min and 0.4 μg/kg/min alfentanil decreased the incidence of emergence delirium in the post-anesthesia care unit. The 0.2 μg/kg/min dose of alfentanil resulted in less respiratory depression and discharge delay than the 0.4 μg/kg/min alfentanil dose.
Trial registration
Chinese Clinical Trial Registry (ChiCTR2100043320)
Supplementary Information
The online version contains supplementary material available at 10.1007/s40272-022-00510-5.
Key Points
| Emergence delirium is a common complication after sevoflurane anesthesia in preschool children. |
| Intravenous infusion of 0.2 μg/kg/min and 0.4 μg/kg/min alfentanil reduced emergence delirium following ambulatory dental procedures. The lower dose of alfentanil resulted in less discharge delay than the higher dose. |
| The smartphone WeChat applet can be used for follow-up, and it also reduces physical contact during the coronavirus disease 2019 (COVID-19) pandemic. |
| Sevoflurane-alfentanil anesthesia in pediatric dental procedures does not increase the incidence of adverse events. |
Introduction
Preschool children (3–6 years) in need of dental care may require general anesthesia because of anxiety, fear, or physical avoidance and their inability to cooperate with treatment [1, 2]. However, ambulatory dental treatment for children under general anesthesia also has advantages, such as rapid recovery and fewer adverse events [3]. Sevoflurane is often used because of the rapid onset of anesthesia, hemodynamic stability, and lack of airway irritation [4, 5]. Compared with other adverse events, emergence delirium (ED) is a frequent complication of sevoflurane anesthesia, ranging from 20 to 80% [6]. Previous studies have revealed a 51.6% incidence of ED in pediatric oral treatment [7]. ED is characterized by a delirious effect with the child being hyperexcitable, disoriented, and crying inconsolably. ED is transient but places a child at risk for injury resulting from excessive flailing and writhing [8]. ED is a very stressful experience for the patient, families, caregivers, and clinicians [9–12]. Pharmacological agents such as propofol, 2-adrenoreceptor agonists, and fentanyl are known to reduce the incidence of ED [5, 9, 13–18]. Alfentanil is a synthetic, short-acting µ-opioid agonist associated with fewer adverse events, including less respiratory depression and postoperative nausea and vomiting (PONV), than fentanyl [19, 20]. Previous studies of alfentanil in reducing ED were inconclusive because of the small numbers of patients enrolled in the trials [21]. No study comparing an infusion of alfentanil to saline with respect to the incidence of ED after sevoflurane anesthesia has been done.
We aimed to determine if an infusion of alfentanil reduces the incidence of ED compared to sevoflurane alone. We conducted a single-center, randomized, placebo-controlled, double-blind clinical trial comparing the effect of alfentanil on the incidence of ED in preschool children undergoing sevoflurane-only anesthesia during ambulatory dental treatment.
Materials and Methods
Trial Design and Oversight
This study was a prospective, double-blind, randomized, controlled study conducted from March to August 2021 in accordance with the principles of the Declaration of Helsinki, and registered in the Chinese Clinical Trial Registry (ChiCTR2100043320). Written informed consent was obtained from the parents or guardians before the dental procedure.
Sites and Patients
In the Comfort Dental Center, the Affiliated Hospital of Stomatology, Chongqing Medical University, Chongqing, China, 180 patients were consecutively recruited into the study, aged 3–6 years old, with an American Society of Anesthesiologists (ASA) score of I. Treatments were limited to dental procedures, including caries, root canals, stainless steel crown placement, and tooth extractions. Exclusion criteria for the study were as follows: attention deficit hyperactivity disorder; fever, cough, or reactive respiratory diseases, such as asthma or upper respiratory tract infection; expected airway difficulties; hearing defects; neurological diseases; history of malignant hyperthermia; use of experimental drugs; or having liver or kidney disease. Children whose guardians could not use smartphones to fill out and submit questionnaires on the WeChat applet were not included in this study.
Randomization and Intervention
All children were randomly allocated to one of three groups using a computer-generated random number in envelopes provided by an anesthesiologist who did not participate in this research. The anesthesiologists and outcome assessors were blinded to the allocation. Alfentanil (alfentanil hydrochloride 1 mg:2 mL, SFDA No. 13S03021, Yichang Humanwell, Inc., Yichang, Hubei, CHN) was administered as a continuous infusion from the beginning to the end of the operation at two doses: group Alf2 received 0.2 μg/kg/min of alfentanil and group Alf4 received 0.4 μg/kg/min of alfentanil. The control group was given saline (group Sal) as a continuous infusion, in the same volume as the two other groups, as a placebo. The drugs used in this study were prepared by a nurse who was not involved in the anesthesia process. Researchers, attending anesthesiologists, pediatric dentists, resuscitation room nurses, children, and family members were blinded to the grouping. The study drugs, all uncolored, were given as an infusion from the start of the operation and then stopped when the procedure was completed. The dose of alfentanil administered in this study was approximately double that of the previous research, which still showed efficacy (more than 20 μg/kg and 40 μg/kg over the course of the procedure in this study versus 10 μg/kg and 20 μg/kg in the previous study by Kim et al. [22]).
Before the anesthesia, the children fasted for 6 h and were not given any water for 2 h. All medications were withheld for 24 h before anesthesia. A modified Yale Preoperative Anxiety Scale (mYPAS) score was recorded by the anesthetist before general anesthesia. After connecting the patient to monitoring devices, measurement of the child's vital signs (including blood pressure and continuous monitoring of pulse oximetry, respiratory rate, heart rate, EKG, and end-tidal carbon dioxide [ETCO2]) was conducted every 5 min.
All the children received a standardized anesthetic. Anesthesia was induced with 8% sevoflurane given with 100% oxygen (gas flow rate 5/min). A preshaped first-generation single-lumen laryngeal mask airway was chosen based on the patient's weight and then inserted. A bispectral index (BIS) monitoring device was placed, and the depth of anesthesia was maintained between 40 and 60. This usually required approximately 2.5–3.5% of sevoflurane administered with a 2 L/min mixture of air and oxygen. During anesthesia, spontaneous breathing was maintained. Non-invasive arterial blood pressure and heart rate measurements were recorded when entering the room (baseline), before the injection of the test drug, at the start of the operation, 5 min after the injection of the test drug, 1 h after the injection of the test drug, upon completion of the process, and before leaving the operating room. During the operation, the respiratory rate and ETCO2 were recorded.
The pediatric dentist performed infiltration of the surgical area with local anesthetic immediately when the treatment was completed. Children under 4 years old were given 2% lidocaine hydrochloride injection (5 mL:0.1 g, Southwest Pharmaceutical Co.; SFDA No. H50020038), with the total dose not exceeding 4 mg/kg. Children aged 4 years and older were given 4% articaine hydrochloride and epinephrine tartrate injection (1.7 mL:68 mg, Produits Dentaires Pierre Rolland; SFDA No. H20140732), with the maximum dose not exceeding 5 mg/kg.
No medications to manage ED were given after the operation was completed. An anesthesia mask was used to administer 5 L/min of pure oxygen for 5 min. Patients were observed for possible airway complications such as tongue retraction, breath-holding, and laryngospasm. Once unobstructed spontaneous breathing was monitored, the child was transferred for recovery to the post-anesthesia care unit (PACU), which was designed to provide a comfortable environment devoid of unnecessary stimulation.
Parents were allowed into the PACU to accompany the child and stayed with them until they were discharged from the hospital. After arriving in the PACU, a research anesthesiologist and research PACU nurse assessed the patient's level of ED and recovery. Agitation was initially treated with parental physical restraint and consolation to prevent self-harm. If ED persisted for more than 30 min or if the patient was at risk of self-harm, intravenous alfentanil (5 μg/kg) was given to manage the ED.
An original, in-house WeChat applet was developed to investigate the postoperative adverse events, and this was used by the children's guardians.
Outcomes Measures
Primary outcome: ED measurements were acquired every 5 min using Paediatric Anaesthesia Emergence Delirium (PAED) and the Aono's scores [9, 23]. ED was considered present when the PAED score was more significant than 10 or the Aono's score was ≥ 3.
Secondary outcomes: Postoperative pain was assessed with a modified Children's Hospital of Eastern Ontario Pain Scale (mCHEOPS). A modified Aldrete score [24] was obtained every 5 min to determine readiness for discharge from the hospital, with a score of 10 being required for release.
The following postoperative recovery indicators were recorded: (1) awakening time (first eye-opening/crying), (2) ED using the Aono's and PAED scores, (3) postoperative pain assessment using the mCHEOPS, and (4) time to discharge from the hospital using the modified Aldrete score.
Exploratory outcomes: A WeChat applet (electronic supplementary material Fig. 1) was written to collect information about potential adverse events related to alfentanil. These included, for example, nausea, emesis, pain, bleeding, and pruritus.
Statistical Analysis
We performed a preliminary study in 45 patients and found the incidence of ED was 20.0% in patients similar to those in group Alf2, 13.3% in group Alf4-like patients, and 46.7% in control patients. The control rate of ED was identical to that of another published study (51.6%). [7] Based on these numbers, we considered a 25% reduction in the incidence of ED to be clinically significant. Using an α error rate for control of the false-positive rate of 0.05 and power to detect a difference if one exists (to control the false-negative rate) of 80%, 55 patients per group were needed for this study (PASS 15.0, NCSS, USA). Anticipating dropouts and missing data, we planned to enroll 60 patients per group.
Categorical data were presented as frequencies and percentages. Statistical differences between groups were tested using the chi-square test or the Fisher exact test with the Bonferroni correction. Continuous variables were reported as mean and standard deviation (SD). One-way analysis of variance was used to compare normally distributed variables within each group. Levene's test was used to assess the homogeneity of the conflicts. The overall significance of the difference between groups was determined by analysis of variance, and the Tukey's honest significant difference (HSD) test was used to identify which groups were different. The Kruskall-Wallis test with Bonferroni's correction was used to test the statistical significance for data that were not non-normally distributed. P < 0.05 was considered to be statistically significant. All statistical analyses were performed using IBM SPSS Statistics software, version 26 (IBM Corp., Armonk, NY, USA).
Results
Patients
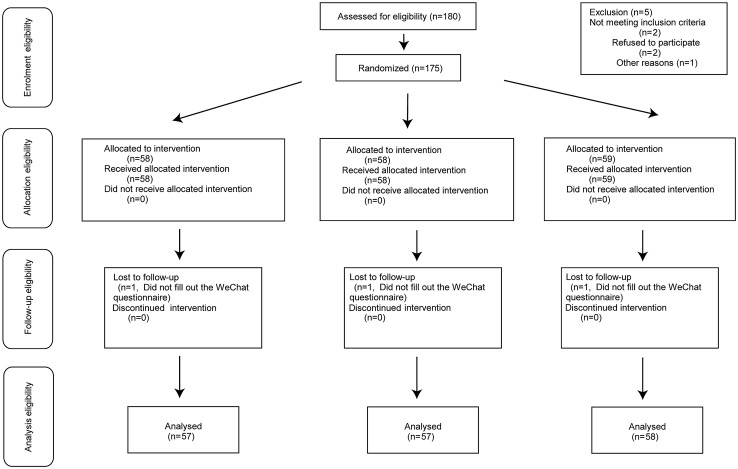
From March 2021 to August 2021, 180 children were enrolled in the study and randomly assigned to the treatment groups. Of these, five were not randomized, and two were lost to follow-up, leaving 172 children available for analysis (Fig.1). The baseline characteristics for the patients enrolled in the study are presented in Table 1. Their age, sex, weight, and height were not significantly different between the three groups after randomization.
Fig. 1.
Patient assignment to study group (randomized) and treatment protocols
Table 1.
Baseline demographic and clinical characteristics
| Group Alf2 (n = 57) |
Group Alf4 (n = 57) |
Group Sal (n = 58) |
P value | |
|---|---|---|---|---|
| Age (range) (years) | 4.5 (3–6) | 4.5 (3–6) | 4.4 (3–6) | 0.919 |
| Weight (kg) | 18.79 ± 3.34 | 17.94 ± 2.86 | 18.56 ± 3.17 | 0.324 |
| Height (cm) | 107.72 ± 8.97 | 107.53 ± 8.42 | 108.59 ± 7.29 | 0.644 |
| Male/female | 27/30 | 30/27 | 32/26 | 0.779 |
| mYPAS | 51.56 ± 21.53 | 52.49 ± 21.53 | 47.59 ± 23.75 | 0.457 |
| Duration of surgery (min) | 115 (90, 150) | 115.5 (90, 150) | 120 (90, 144) | 0.409 |
| Duration of anesthesia (min) | 131 (102, 165) | 130 (103, 165) | 135 (105, 160) | 0.496 |
Alf2 0.2 μg/kg/min alfentanil, Alf4 0.4 μg/kg/min alfentanil, mYPAS modified Yale Preoperative Anxiety Scale, Sal saline
Primary Outcome
Alfentanil reduced postoperative ED scores compared with saline control (Table 2). There were no significant differences between group Alf2 (6.4 ± 3.5) and group Alf4 (5.8 ± 3.8) PAED scores (mean difference 0.579 [95% confidence interval {CI} −1.279 to 2.437], p = 0.742), and scores were significantly lower than those for the saline control patients (9.6 ± 5.1) (mean difference 3.218 [95% CI 1.368–5.068], p < 0.01, and mean difference 3.797 [95% CI 1.947–5.647], p < 0.01, respectively) (Fig. 2). The proportion of patients experiencing ED was lower in the alfentanil-treated groups as measured by the PAED scale: group Alf2 22.9% and group Alf4 21.1% versus group Sal 48.3% (0.317 [95% CI 0.142–0.708], p = 0.004, and 0.286 [95% CI 0.126–0.647], p = 0.002, respectively). Similar results were obtained when ED incidence was measured by the Aono’s scale: group Alf2 22.8% (0.317 [95% CI 0.142–0.708]), group Alf4 22.8% (0.317 [95% CI 0.142–0.708]) versus group Sal 48.3% (Fig. 3). The Aono’s scale is as follows: 1 = calm; 2 = easily consoled state; 3 = moderate agitation; 4 = severe agitation.
Table 2.
Incidence of emergence agitation
| Group Alf2 (n = 57) |
Group Alf4 (n = 57) |
Group Sal (n = 58) |
|
|---|---|---|---|
| PAED score | 6.4 (3.5)† | 5.8 (3.8)† | 9.6 (5.1)† |
| PAED score vs group Sal (95% CI) | 3.218 (1.368–5.068) | 3.797 (1.947–5.647) | – |
| P value | < 0.01 | < 0.01 | – |
| PAED > 10 | 13 (22.9%)* | 12 (21.1%)* | 28 (48.3%)* |
| PAED vs group Sal (95% CI) | 0.317 (0.142–0.708) | 0.286 (0.126–0.647) | – |
| P value | < 0.01 | < 0.01 | – |
| Aono’s score ≥ 3 | 13 (22.8%)‡ | 13 (22.8%)‡ | 28 (48.3%)‡ |
| Aono’s score vs group Sal (95% CI) | 0.317 (0.142–0.708) | 0.317 (0.142–0.708) | |
| P value | < 0.01 | < 0.01 | – |
Alf2 0.2 μg/kg/min alfentanil, Alf4 0.4 μg/kg/min alfentanil, CI confidence interval, PAED Paediatric Anaesthesia Emergence Delirium, Sal saline
*The incidence of emergence agitation in Group Sal was significantly higher than other two groups (P < 0.05)
†There were no significant differences between Groups Alf2 and Alf4, the PAED score of GroupAlf2 and Alf4 was significantly lower than that of Groups Sal (P < 0.05)
‡The aono’s scores in Group Sal was significantly higher than other two groups (P < 0.05)
Fig. 2.
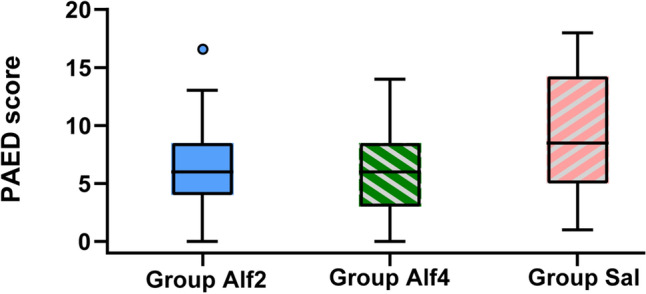
Distributions of the PAED score. Alf2 0.2 μg/kg/min alfentanil, Alf4 0.4 μg/kg/min alfentanil, Sal saline, PAED Paediatric Anaesthesia Emergence Delirium
Fig. 3.
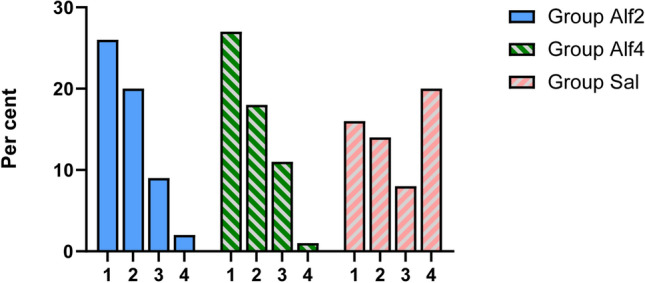
Distributions of Aono's scale scores
Secondary Outcomes
As measured by the mCHEOPS scores, postoperative pain was also reduced by alfentanil. Pain scores in group Alf2 (2.4 ± 1.5) and group Alf4 (2.1 ± 1.6) were similar (mean difference 0.386 [95% CI −0.425 to 1.197], p = 0.5) and were significantly lower than those in group Sal (4.1 ± 2.3) (mean difference −1.665 [95% CI −2.472 to −0.858], p < 0.01, and mean difference −2.051 [95% CI −2.858 to −1.244], p < 0.01, respectively) (Fig. 4).
Fig. 4.
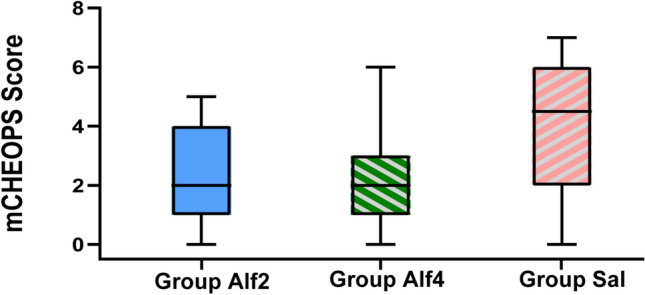
Distributions of the mCHEOPS score. Alf2 0.2 μg/kg/min alfentanil, Alf4 0.4 μg/kg/min alfentanil, mCHEOPS modified Children's Hospital of Eastern Ontario Pain Scale, Sal saline
Figure 5 shows the average blood pressure and heart rate trend before and after medication. Five minutes after injection of the study drug, heart rate of the Alf4 group at time T2–5 was reduced compared with the Sal group (− 4.683 [95% CI − 8.789 to − 0.577], p = 0.021, − 12.310 [95% CI − 16.624 to − 7.997], p < 0.01, −12.420 [95% CI − 16.016 to − 8.824], p < 0.01, and − 5.145 [95% CI − 8.373 to − 1.916], p < 0.01, respectively). Heart rate of the Alf2 group at T5 time was reduced compared with that of the Sal group (− 4.285 [95% CI − 7.513 to − 1.057], p = 0.006). Compared with the Alf2 group, the Alf4 group had a lower heart rate and mean arterial pressure during the injection study T3 and T4 periods (p < 0.01). None of the patients required treatment for decreased heart rate or blood pressure. T1 was baseline, before administration of alfentanil/saline; T2 was 5 min after administration of alfentanil/saline; T3 was 1 h after administration of alfentanil/saline; T4 was the end of surgery; and T5 was discharge from the operating room.
Fig. 5.
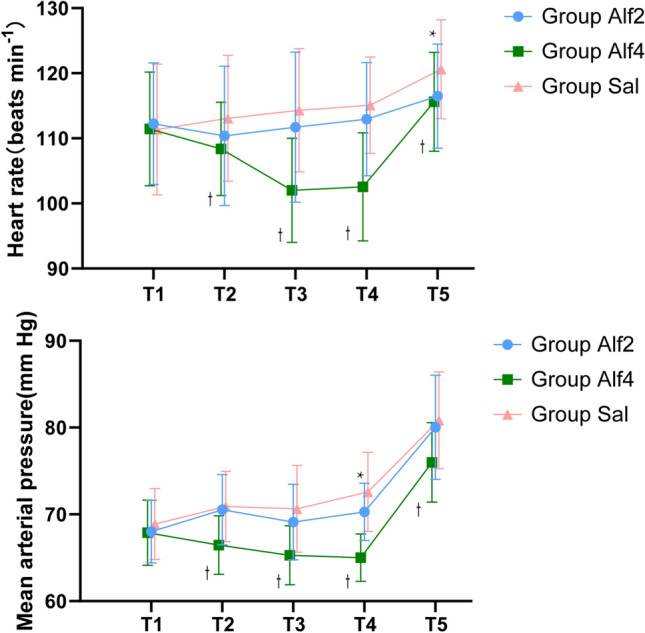
Hemodynamic changes during the anesthesia. Data are expressed as mean (SD). * P < 0.05 compared with group Sal. †P < 0.05 compared with group Alf4. Alf4 0.4 μg/kg/min alfentanil, Sal saline
Respiratory outcomes are shown in Fig. 6. Five minutes after injection of the study drug, the respiratory rate of the Alf4 group was reduced at T2–4 time points as compared with the Sal group (p < 0.01). The respiratory rate of the Alf2 group at the T3–4 time points was significantly different than that of the Sal group (p < 0.01). During the T3 and T4 time points, the respiratory frequency was significantly reduced in the Alf4 group as compared with that of the Sal group. Five minutes after the study drug was injected, the ETCO2 of the Alf2 and Alf4 groups increased significantly at the T3–4 time points (p < 0.01) relative to control. The ETCO2 of the Alf4 group was significantly increased during the injection of the study drug at the T3 and T4 time points compared with controls (p < 0.01). However, ten children in the Alf4 group needed manual ventilatory assistance to maintain ETCO2 < 55 mm; children in group Alf2 did not require such intervention.
Fig. 6.
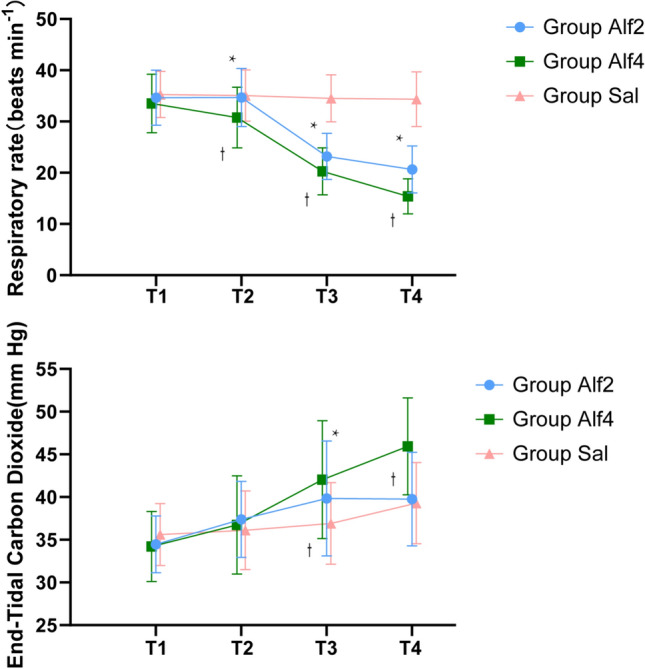
Respiratory parameters change during the anesthesia. Data are expressed as mean (SD). * P < 0.05 compared with group C (Bonferroni corrected). † P < 0.05 compared with group A4 (Bonferroni corrected)
Time for awakening of group Alf2 and group Sal was comparable (0.913 [95% CI − 0.697 to 2.524], p = 0.375), which was significantly shorter than that of group Alf4 (− 4.456 [95% CI − 6.074 to − 2.838], p < 0.01, and − 5.370 [95% CI − 6.980 to − 3.759], p < 0.01, respectively) (min) (Table 3). There was no significant difference between the discharge time of group Alf2 and group Sal (31.2 ± 4.64 vs 30.5 ± 2.82, 0.659 [95% CI − 1.052 to 2.369], p = 0.643) (min); the time to discharge of group Alf4 (35.16 ± 3.97 min) was significantly longer than that of group Alf2 and group Sal (p < 0.01) (Table 3).
Table 3.
Comparison of time for awakening and time to discharge duration the PACU among the three groups
| Group Alf2 (n = 42) |
Group Alf4 (n = 42) |
Group Sal (n = 42) |
|
|---|---|---|---|
| Time for awakening (mins) | 10.98 ± 3.43* | 15.44 ± 3.40* | 10.07 ± 4.07* |
| Time to discharge (mins) | 31.19 ± 4.64† | 35.16 ± 3.97† | 30.53 ± 2.82† |
Alf2 0.2 μg/kg/min alfentanil, Alf4 0.4 μg/kg/min alfentanil, PACU post-anesthesia care unit, Sal saline
*The time for awakening of Groups Alf2 and S was comparable and significantly shorter than that of Group Alf4 (P < 0.01)
†There were no significant differences between Groups Alf2 and Sal, the time to discharge of Group Alf4 was significantly longer than that of Groups Alf2 and Sal (P < 0.01)
Exploratory Outcomes
There was no significant difference in the incidence of PONV between the three groups, with two patients in group Alf2, two patients in group Sal, and six patients in group Alf4 experiencing nausea (3.5% and 3.5% vs 10.5%; 0.464 [95% CI 0.110–1.952], p = 0.285, and 0.464 [95% CI 0.110–1.952], p = 0.285, respectively). One patient in group Alf2, one patient in group Sal, and three patients in group Alf4 experienced vomiting (1.75% and 1.75% vs 5.26%; 0.321 [95% CI − 0.032 to 3.186], p = 0.309, and 0.321 [95% CI − 0.032 to 3.186], p = 0.309, respectively). No other clinically relevant adverse events were observed (Table 4).
Table 4.
Postoperative adverse effects were collected from the smartphone WeChat applet
| Group Alf2 (n = 57) |
Group Alf4 (n = 57) |
Group Sal (n = 58) |
P value | |
|---|---|---|---|---|
| Nausea | 2 | 6 | 2 | 0.269 |
| Vomiting | 1 | 3 | 1 | 0.432 |
| Intestinal bloating | 0 | 0 | 0 | – |
| Constipation | 0 | 0 | 0 | – |
| Pruritus | 0 | 0 | 0 | – |
| Headache | 0 | 0 | 0 | – |
| Others | 0 | 0 | 0 | – |
Alf2 0.2 μg/kg/min alfentanil, Alf4 0.4 μg/kg/min alfentanil, Sal saline
Discussion
Continuous infusion of alfentanil 0.2 μg/kg/min and 0.4 μg/kg/min in conjunction with sevoflurane anesthesia significantly reduced postoperative ED. The lower alfentanil dose, 0.2 μg/kg/min, resulted in a shorter facility stay after surgery. Alfentanil also reduced the incidence of ED after general anesthesia in children and did not increase the incidence of any other complications.
Previous studies reported the effects of different doses of alfentanil, including 5, 10, and 20 μg/kg on ED during the recovery period. These studies used alfentanil along with sevoflurane inhalation anesthesia but also employed other auxiliary drugs such as midazolam and nitrous oxide [22, 25]. Many drugs, including benzodiazepines and opioids, can affect children's behavior after general anesthesia. These adjuvant drugs affect sevoflurane-associated ED and will interfere with the evaluation and recording of postoperative adverse events. Therefore, studies on the effects of sevoflurane alone on restlessness during recovery are rare.
In the current study, a balance between respiratory depression, pain control, and reduction of complications was sought. All patients received local anesthesia after the dental procedures. Due to the reduced impact of pain, the average duration of ED in our study was less than 30 min, precluding the need for further treatment of ED. Although we ruled out postoperative pain, the incidence of ED in the control group in this study was 48.3%. This is higher than the 13–27% incidence previously observed in the pediatric dental surgery population [16, 26]. Our study population comprised preschool-age patients undergoing sevoflurane anesthesia who were undergoing ambulatory procedures; this could significantly influence PAED results [27] because preschool age has been certified to be one of the risk factors for ED [28].
Alfentanil is a short-acting opioid analgesic and has significant clinical advantages during outpatient anesthesia. Alfentanil can reduce intraoperative respiratory depression and muscle stiffness. A previous study revealed that µ-opioid agonists effectively prevented the emergence of delirium [21]. Compared with fentanyl, alfentanil, as a µ-opioid receptor agonist, has a shorter half-life and faster recovery time [29]. An intranasal dose of 10 µg/kg alfentanil in addition to oral midazolam did not decrease sevoflurane-induced ED in children undergoing urological surgery [25].
The maximal plasma concentration with intranasal alfentanil is reached within 9 min, and its bioavailability is 64.7% compared to intravenous administration [30]. The incomplete bioavailability of nasal alfentanil may explain why it did not prevent the ED. However, Kim et al. [22] reported that the intravenous administration of 10 µg/kg alfentanil after induction of anesthesia could prevent ED in pediatric patients undergoing adenotonsillectomy without delaying the recovery time or causing significant hypotension. This might be because of the reduced bioavailability of nasal alfentanil. In our preliminary experiments, a single bolus of 20 μg/kg alfentanil 10 min before the end of surgery resulted in transient respiratory depression. Previous studies of alfentanil used mechanical ventilation to maintain oxygenation in children [22, 25, 31]. In the current study, we used an approach that preserved spontaneous breathing.
The developed online follow-up tool aimed to provide follow-up treatment suggestions and improve service quality for children and their families, while recording adverse events, getting immediate feedback, maintaining medical services, and reducing physical contact during the coronavirus disease 2019 (COVID-19) pandemic. The tool resulted in high satisfaction rates on both sides.
The current study has several limitations. This trial only examined the effects of alfentanil in children undergoing dental procedures. Although pain after the dental procedures performed in this study should be minimal due to the administration of local anesthetics, pain can occur for various other reasons (intubation, intravenous discomfort, etc.). It can confound the evaluation of ED. The control group had significantly higher pain scores, which may have contributed to the increased incidence of ED, and could explain, in part, the efficacy of alfentanil. Second, we only tested the effectiveness of two doses of intravenous alfentanil. Further studies should be conducted to verify whether other doses or different administration strategies for alfentanil, such as target-controlled alfentanil infusion, will result in better outcomes.
Conclusion
In conclusion, intravenous infusion of 0.2 μg/kg/min and 0.4 μg/kg/min alfentanil decreased the incidence of ED in the PACU. The 0.2 μg/kg/min dose of alfentanil resulted in less respiratory depression and discharge delay than the 0.4 μg/kg/min alfentanil dose. Implementation of teleconsultation for postoperative complications into a clinical routine could help maintain medical care during the COVID-19 pandemic.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
This study was supported by the following funds: (1) joint project of Chongqing Health Commission and Science and Technology Bureau Chongqing, China (Grant No. 2020MSXM098; 2021MSXM310) and (2) CSA Clinical Research Fund (CSA-A2021-05).
Conflict of interest
All authors report no declarations of interests.
Ethical approval
This study has been approved by the Ethics Committee of Chongqing Medical University. The study is in accordance with the principles of the Declaration of Helsinki and was registered in the Chinese Clinical Trial Registry (ChiCTR2100043320). Written informed consent was obtained from the parents or guardians before the dental procedure.
Informed consent
Informed consent was obtained from all individual participants included in the study prior to any study-mandated procedure.
Consent for publication
Not applicable.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Author contributions
Substantial contributions to the conception or design of the work: Cong Yu and Nan Zhao. Statistics: Jie Zeng. Planning, conduct, and reporting of the work: Lin Fan, Chao Zhang, YuJia Wu, Xin Wang, and Feng Gao. Drafting the manuscript: Nan Zhao. Critical revision of the article: All declared authors. Final approval of the version published: All declared authors. Agreement to be accountable that all aspects of the work were appropriately investigated and resolved: All declared authors.
References
- 1.Affairs AAOPDCOC. Policy on the use of deep sedation and general anesthesia in the pediatric dental office. Pediatric dentistry. 2008;30(7 Suppl):66-7. [PubMed]
- 2.Savanheimo N, Vehkalahti MM. Five-year follow-up of children receiving comprehensive dental care under general anesthesia. BMC Oral Health. 2014;14(1):1–8. doi: 10.1186/1472-6831-14-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell RL, Shetty NS, Shetty KS, Pope HL, Campbell JR. Pediatric dental surgery under general anesthesia: uncooperative children. Anesth Prog. 2018;65(4):225–230. doi: 10.2344/anpr-65-03-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao Z, Gui Jin H, Cong Y. The effect of general anesthesia for ambulatory dental treatment on children in Chongqing, Southwest China. Pediatr Anesth. 2017;27(1):98–105. doi: 10.1111/pan.12983. [DOI] [PubMed] [Google Scholar]
- 5.Costi D, Cyna AM, Ahmed S, Stephens K, Strickland P, Ellwood J, et al. Effects of sevoflurane versus other general anaesthesia on emergence agitation in children. Cochrane Database Syst Rev. 2014(9):CD007084. [DOI] [PMC free article] [PubMed]
- 6.Vlajkovic GP, Sindjelic RP. Emergence delirium in children: many questions, few answers. Anesth Analg. 2007;104(1):84–91. doi: 10.1213/01.ane.0000250914.91881.a8. [DOI] [PubMed] [Google Scholar]
- 7.Jooma Z, Perrie H, Scribante J, Kleyenstuber T. Emergence delirium in children undergoing dental surgery under general anesthesia. Pediatr Anesth. 2020;30(9):1020–1026. doi: 10.1111/pan.13937. [DOI] [PubMed] [Google Scholar]
- 8.Bajwa SA, Costi D, Cyna AM. A comparison of emergence delirium scales following general anesthesia in children. Pediatr Anesth. 2010;20(8):704–711. doi: 10.1111/j.1460-9592.2010.03328.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim M-S, Moon B-E, Kim H, Lee J-R. Comparison of propofol and fentanyl administered at the end of anaesthesia for prevention of emergence agitation after sevoflurane anaesthesia in children. Br J Anaesth. 2013;110(2):274–280. doi: 10.1093/bja/aes382. [DOI] [PubMed] [Google Scholar]
- 10.Nair S, Wolf A. Emergence delirium after paediatric anaesthesia: new strategies in avoidance and treatment. BJA Educ. 2018;18(1):30–33. doi: 10.1016/j.bjae.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason K. Paediatric emergence delirium: a comprehensive review and interpretation of the literature. BJA: Br J Anaesthesia. 2017;118(3):335-43. [DOI] [PubMed]
- 12.Almenrader N, Galante D, Engelhardt T. Emergence agitation: is there a European consensus? Br J Anaesth. 2014;113(3):515–516. doi: 10.1093/bja/aeu281. [DOI] [PubMed] [Google Scholar]
- 13.Wang H-Y, Chen T-Y, Li D-J, Lin P-Y, Su K-P, Chiang M-H, et al. Association of pharmacological prophylaxis with the risk of pediatric emergence delirium after sevoflurane anesthesia: an updated network meta-analysis. J Clin Anesth. 2021;75:110488. doi: 10.1016/j.jclinane.2021.110488. [DOI] [PubMed] [Google Scholar]
- 14.Lee J-R, Kim M-S, Moon B-E, Kim H. Comparison of propofol and fentanyl for preventing emergence agitation in children. Br J Anaesth. 2013;111(1):121–122. doi: 10.1093/bja/aet182. [DOI] [PubMed] [Google Scholar]
- 15.Inomata S, Maeda T, Shimizu T, Satsumae T, Tanaka M. Effects of fentanyl infusion on tracheal intubation and emergence agitation in preschool children anaesthetized with sevoflurane. Br J Anaesth. 2010;105(3):361–367. doi: 10.1093/bja/aeq168. [DOI] [PubMed] [Google Scholar]
- 16.Kim N, Park JH, Lee JS, Choi T, Kim MS. Effects of intravenous fentanyl around the end of surgery on emergence agitation in children: systematic review and meta-analysis. Pediatr Anesth. 2017;27(9):885–892. doi: 10.1111/pan.13181. [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Kim J, Lee J, Song B, Koo B. Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br J Anaesth. 2013;111(2):222–228. doi: 10.1093/bja/aet056. [DOI] [PubMed] [Google Scholar]
- 18.Dahmani S, Stany I, Brasher C, Lejeune C, Bruneau B, Wood C, et al. Pharmacological prevention of sevoflurane-and desflurane-related emergence agitation in children: a meta-analysis of published studies. Br J Anaesth. 2010;104(2):216–223. doi: 10.1093/bja/aep376. [DOI] [PubMed] [Google Scholar]
- 19.Reitz JA. Alfentanil in anesthesia and analgesia. Drug Intell Clin Pharm. 1986;20(5):335–341. doi: 10.1177/106002808602000501. [DOI] [PubMed] [Google Scholar]
- 20.Langevin S, Lessard MR, Trépanier CA, Baribault J-P. Alfentanil causes less postoperative nausea and vomiting than equipotent doses of fentanyl or sufentanil in outpatients. J Am Soc Anesthesiol 1999;91(6):1666. [DOI] [PubMed]
- 21.Tan Y, Shi Y, Ding H, Kong X, Zhou H, Tian J. μ-Opioid agonists for preventing emergence agitation under sevoflurane anesthesia in children: a meta-analysis of randomized controlled trials. Pediatr Anesth. 2016;26(2):139–150. doi: 10.1111/pan.12815. [DOI] [PubMed] [Google Scholar]
- 22.Kim JY, Chang YJ, Lee JY, Park HY, Kwak HJ. Post-induction alfentanil reduces sevoflurane-associated emergence agitation in children undergoing an adenotonsillectomy. Acta Anaesthesiol Scand. 2009;53(5):678–681. doi: 10.1111/j.1399-6576.2009.01943.x. [DOI] [PubMed] [Google Scholar]
- 23.Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. J Am Soc Anesthesiol. 2004;100(5):1138–1145. doi: 10.1097/00000542-200405000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg. 1970;49(6):924–934. doi: 10.1213/00000539-197011000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Bilgen S, Köner Ö, Karacay S, Sancar NK, Kaspar EC, Sözübir S. Effect of ketamine versus alfentanil following midazolam in preventing emergence agitation in children after sevoflurane anaesthesia: a prospective randomized clinical trial. J Int Med Res. 2014;42(6):1262–1271. doi: 10.1177/0300060514543039. [DOI] [PubMed] [Google Scholar]
- 26.Beringer RM, Segar P, Pearson A, Greamspet M, Kilpatrick N. Observational study of perioperative behavior changes in children having teeth extracted under general anesthesia. Pediatr Anesth. 2014;24(5):499–504. doi: 10.1111/pan.12362. [DOI] [PubMed] [Google Scholar]
- 27.König MW, Varughese AM, Brennen KA, Barclay S, Shackleford TM, Samuels PJ, et al. Quality of recovery from two types of general anesthesia for ambulatory dental surgery in children: a double-blind, randomized trial. Pediatr Anesth. 2009;19(8):748–755. doi: 10.1111/j.1460-9592.2009.03054.x. [DOI] [PubMed] [Google Scholar]
- 28.Moore AD, Anghelescu DL. Emergence delirium in pediatric anesthesia. Pediatr Drugs. 2017;19(1):11–20. doi: 10.1007/s40272-016-0201-5. [DOI] [PubMed] [Google Scholar]
- 29.White PF, Coe V, Shafer A, Sung M-L. Comparison of alfentanil with fentanyl for outpatient anesthesia. Anesthesiol (Philadelphia). 1986;64(1):99–106. doi: 10.1097/00000542-198601000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Schwagmeier R, Boerger N, Meissner W, Striebel HW. Pharmacokinetics of intranasal alfentanil. J Clin Anesth. 1995;7(2):109–113. doi: 10.1016/0952-8180(94)00023-W. [DOI] [PubMed] [Google Scholar]
- 31.Choi YH, Kim KM, Lee SK, Kim YS, Kim SJ, Hwang WS, et al. Effects of remifentanil and remifentanil-alfentanil administration on emergence agitation after brief ophthalmic surgery in children. BMC Anesthesiol. 2015;16(1):1–7. doi: 10.1186/s12871-015-0163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.