Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin disorder that affects a substantial number of children and has a significant negative impact on affected patients and their caregivers/families. Recent studies have led to significant evolutions in the understanding of AD pathogenesis, epidemiology, and treatment. The first point of contact for many patients with new-onset AD is usually with their primary care provider or pediatrician. This underscores the importance for pediatricians to understand the basic pathophysiology and current standards of care for AD. This article provides up-to-date information and reviews the basic principles of AD pathophysiology, diagnosis, and management. In addition, the article highlights recent advances in scientific research regarding the mechanisms involved in the pathogenesis of atopic dermatitis that have resulted in the discovery of novel therapeutic targets and the development of targeted biologic therapies with the potential to revolutionize AD therapy.
Key Points
| Major advances in AD research in the past decade have expanded our knowledge of the disease, supporting the concept of AD as an inflammatory skin disease with a systemic component. |
| AD places a substantial burden not only on affected children, but on their families and caregivers as well. Clinicians should make a holistic attempt to control the disease to mitigate this burden. |
| Novel targeted biologic therapies are being developed that target the underlying causes of AD, which may help define the future of AD therapy. |
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease that typically manifests in early childhood and may precede the development of other atopic disorders, including asthma, allergic rhinitis, and food allergies [1–3]. The onset of AD most commonly occurs between 3 and 6 months of age, with approximately 60% of children with AD presenting symptoms in the first 12 months [4]. Consensus guidelines indicate that AD is characterized by essential features such as pruritus and eczema (acute, subacute, or chronic), with eczema lesions displaying typical morphology or age-specific patterns and having a chronic or relapsing history [5]. The presence of these essential features in combination with an early age of onset, atopy, and xerosis support a diagnosis of AD [5].
The prevalence of AD varies widely across the globe due to regional, country-specific, age group, and data-capturing methodological differences, affecting 0.2% to 36% of the pediatric population (ages < 18 years) [2, 6, 7]. This high prevalence, coupled with high patient/caregiver burden and increased healthcare utilization, highlights the considerable public health burden associated with AD [8–10]. Furthermore, approximately 50% of patients with AD are treated in the primary care setting [3], with community-based primary care practices reporting a high prevalence of AD among their pediatric patients [11]. Therefore, pediatricians have a key role in the management of AD.
This review aims to highlight the recent breakthrough advances in research that have the potential to revolutionize AD therapy. In addition, the article will provide pediatricians with the most up-to-date epidemiology, pathophysiology, and treatment information on AD, and serve to inform clinicians and parents of age- and severity-specific treatment options for AD.
Epidemiology
The evaluation of AD prevalence is impacted by challenges inherent in the disease, including a lack of objective diagnostic tests, few widely accepted biomarkers, and a relapsing disease, leading to differing estimates across studies [8, 12]. However, several well-designed US caregiver-centered surveys have reported prevalence estimates of 11–13% for healthcare-diagnosed eczema, with notable variation between states (9–18%), which is in line with global data [2, 8, 11]. In the US primary care setting, a cross-sectional survey study reported an AD prevalence of 24% among pediatric patients aged 0–5 years, ranging from 15% of children aged < 1 year to 38% of children aged 4–5 years [11]. In this survey, an assessment of AD severity indicated that most patients presented with mild AD (58%) or moderate AD (39%), with only 3% of children having severe AD. Comorbidities that were more prevalent in the AD population than in the non-AD population included asthma (12 vs 4%; age-adjusted prevalence ratio, 3.0 [95% CI 1.8–4.9]; p < 0.001) and food allergy (8 vs 2%; 3.7 [1.5–9.2]; p = 0.005) [11]. Previous studies have also reported correlations between AD and several comorbidities, including asthma, allergic rhinitis, food allergy, and mental health disorders including attention deficit disorder/attention deficit hyperactivity disorder (ADD/ADHD), autism, and depression [13–17].
Pathophysiology
The pathophysiology of AD is complex and multifactorial, and there is increasing recognition of the heterogeneous nature of AD [18, 19]. Although the exact mechanisms of the pathogenesis are still unclear, major advances in AD research in the past decade have expanded our knowledge on the disease, supporting the concept of AD as an inflammatory skin disease with a systemic component.
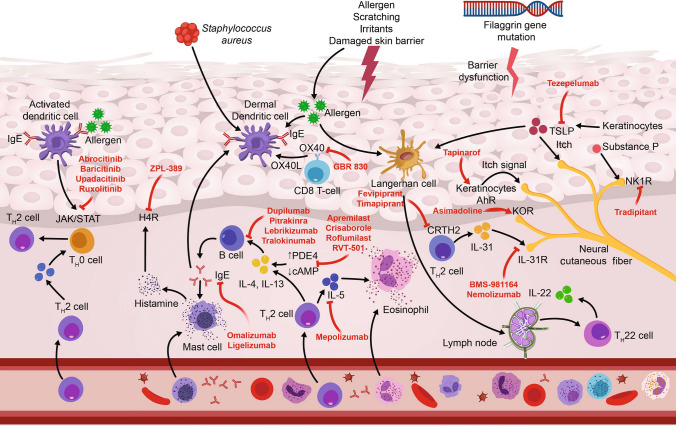
AD is precipitated by an interaction between environmental factors, disturbed skin barrier, the skin microbiome, and immune dysregulation among individuals with susceptibility genes for AD (Fig. 1) [20]. AD is a highly heritable disease, with phenotype-specific genes likely playing an important role alongside ‘generic’ atopy genes. Genome-wide association studies have identified loci correlated with autoimmune regulation, including genes associated with regulation of innate host defenses and T-cell function; these studies have also linked AD to other autoimmune or inflammatory diseases [18]. Linkage studies have also implicated loci containing clusters of genes associated with skin barrier dysfunction, such as the filaggrin (FLG) gene, which has the highest association with AD [18, 21].
Fig. 1.
Atopic pathogenesis and mechanisms of action of approved and emerging agents. Adapted with permission from Nguyen et al. [77]. AhR aryl hydrocarbon receptor, cAMP cyclic adenosine monophosphate, CRTH2 chemoattractant receptor homologs molecule expressed on TH2 cells, H4R histamine-4 receptor, IgE immunoglobulin-E, IL interleukin, JAK/STAT Janus kinase/signal transducer and activator of transcription, KOR κ-opioid receptor, NK1R neurokinin-1 receptor, PDE4 phosphodiesterase 4, R receptor, TSLP thymic stromal lymphopoietin
The etiology of AD appears to be driven by the reciprocal interaction between two biological pathways: skin epithelial function and innate/adaptive immune responses [22, 23]. Skin barrier dysfunction is a well-defined essential component in the pathogenesis of AD [21, 22]. In healthy skin, the epidermal barrier is a matrix of structural lipids and proteins that displays antimicrobial properties, functioning to maintain skin hydration and prevent penetration of allergens, and disruption to any one of these components contributes to AD [19]. The skin microbiome plays a key role in the skin’s innate immune response through the maintenance of immunological homeostasis and reduction of skin colonization by pathogenic bacteria [19, 24, 25]. Although numerous microbial species are involved in optimal function of the skin microbiome, Staphylococcus aureus has been found to colonize AD skin lesions in up to 90% of patients and can contribute to disease exacerbations or flare [24].
Recent developments in AD research have provided evidence supporting the contribution of immune mechanisms to AD pathogenesis. In particular, a number of immune biomarkers of inflammatory mediators have been identified, including T helper (Th)2, Th22, interleukin (IL)-4, IL-13, IL-31, Th1, and Th17 [26]. The onset of acute AD is characterized predominantly by the activation of Th2 and Th22 pathways, which are subsequently intensified in chronic disease, along with upregulation of the Th1 pathway [26, 27]. In the pediatric setting, studies of new-onset pediatric AD demonstrated strong immune activation of Th2, Th9, and Th17 in skin lesions, as well as increased levels of Th2 and Th17 markers in the blood of pediatric patients with AD [28–30]. Consequently, the current understanding of pediatric AD has become one wherein Th2 signaling remains the main driver; however, Th17 signaling is more pronounced when compared with adults with AD [26, 28]. This Th17-skewed molecular profile more closely resembles immune signaling observed in patients with psoriasis rather than adult AD, and this difference may indicate a further need for different therapeutic approaches to pediatric and adult AD [28].
Multiple studies have underscored the importance of Th2 cytokines in the pathogenesis of AD, namely IL-4, IL-13 and IL-31, the Th22 cytokine IL-22, and the epithelial-derived cytokine thymic stromal lymphopoietin [18, 21, 27]. Phosphodiesterase 4 (PDE4), an isoenzyme that regulates cytokine production, has also been identified in immune and inflammatory cells (i.e., T cells, B cells, monocytes, macrophages, neutrophils, etc.), suggesting that PDE4 may play a role in controlling AD-associated inflammation [21, 31]. Indeed, selective inhibition of PDE4 was associated with reductions in several proinflammatory mediators, including tumor necrosis factor, IL-2, IL-5, IL-8, IL-12, and IL-22 [32]. The Janus kinase (JAK) pathway is a signal transduction pathway for several cytokines and growth factors and is thought to be involved in the inflammatory immune responses in AD, primarily through enhancement of the Th2 cell differentiation [21, 33]. Recently, a natural killer (NK) cell deficiency was also observed in the blood of AD patients, and this may be associated with enhanced Th2-driven skin inflammation [34]. Taken together, elucidation of the proinflammatory immune mechanisms that drive AD pathogenesis has important implications, resulting in the identification of novel therapeutic targets that can inform future advances in AD therapy.
Burden of Atopic Dermatitis (AD)
The hallmark symptom of AD is pruritus, which is responsible for most of the negative impact on quality of life experienced by patients with the disease [3]. Accordingly, the treatment of acute AD prioritizes the mitigation of this pruritus [3]. Beyond itch, children with AD experience a range of comorbidities related to the disease, including a higher risk for skin infections due to AD-associated skin barrier defects, cutaneous dysbiosis, and innate immune suppression via type 2 inflammatory signaling [35]. Children with AD also exhibit increased healthcare utilization, including healthcare provider visits, relative to children of the same age without AD, and this healthcare use increase rises in tandem with AD severity [1, 36]. Furthermore, AD is associated with a significant psychosocial burden that impacts patients, their caregivers, and wider society that can be traced back to the significant pruritus experienced by these patients [3, 37]. In particular, sleep disturbances characterized by difficulty with both sleep initiation and sleep maintenance appear to have a notable impact, even among patients in clinical remission, potentially resulting in daytime drowsiness and resultant poor academic performance at school [37–39]. In addition, the literature suggests that the magnitude of effects on sleep increases as AD severity increases [1]. In a longitudinal cohort study following 13,988 children in the United Kingdom from the ages of 2 to 16 years, it was found that children with any form of AD, even mild or inactive AD, had statistically significantly increased odds of poor sleep quality, and that children with active AD were almost 50% more likely to encounter increased disturbances to their sleep throughout childhood [40]. Similarly, a questionnaire-based survey of 91,642 children in the United States between the ages of 0 and 17 years found that children with AD had a higher chance of experiencing four or more nights of impaired sleep per year [1, 41]. This effect was even more pronounced in children with severe AD than in those with mild or moderate AD [1, 41].
Sleep disturbances due to pruritus have also been identified as a major cause of stress within the family unit and consequent sleep deprivation affects all family members [3, 37, 39]. Loss of 1–2 h of sleep per night has been shown to lead to suboptimal performance in the workplace and a reduction in coping skills at work and home [37]. Self-reported sleep measures from a longitudinal study in the United Kingdom of 11,649 mothers and their children with and without AD indicated that mothers of children with AD were approximately 40% more likely to report each of the following: difficulty falling asleep, insufficient sleep, and daytime exhaustion [42].
Pediatric patients with AD may present with a number of behavioral problems, potentially exacerbated by impaired sleep quality, that can have multifarious negative impacts on their social and intellectual development [37]. Cross-sectional data from 19 US population-based surveys has demonstrated that children with AD have an increased likelihood of being diagnosed with ADD/ADHD [14]. AD has also been associated with school absenteeism [43–45], and children may feel socially isolated by their peers as a result of bullying and teasing due to the physical appearance of their AD lesions; this can negatively affect a child’s self-perception and self-esteem [46]. The International Study on Life with Atopic Eczema revealed large impacts on self-esteem in children with AD, with 27% of children with AD indicating that they’d been bullied due to their AD and 36% reporting feeling less self-confident because of their AD [47]. In focus sessions where expert clinicians interviewed the parents of 26 American children with AD, more than half of parents reported that their children with AD were avoided by other children and adults [48]. Although the impact of AD on stigmatization/discrimination has not been well studied, children with lesions on visible sites of the skin may be at higher risk of discrimination [49].
Adolescents with AD may also be more likely to experience symptoms of depression and psychological distress [50, 51]. In South Korea, surveys have reported that patients with AD are significantly more likely to experience in suicidal ideation and suicide attempts, leading researchers to speculate that the exacerbating effects of AD on negative psychological states can create a vicious cycle, especially in adolescents, who are already more vulnerable to suicidality on average [50, 51]. An increasingly large data set in pediatric patients with AD corroborates this negative impact of AD with regard to depression and anxiety, both of which appear to be largely driven by pruritus, sleep disturbances, and social stigmatization due to visible skin lesions [52]. Quality of life and disease severity are also linked in pediatric patients with AD, with long-term effects on childhood behavior and development [53]. Findings from a cross-sectional study in Brazil reported a moderate positive correlation between disease severity, as assessed by the SCORing Atopic Dermatitis (SCORAD) scale, and quality of life, as assessed by the Children’s Dermatology Life Quality Index (CDLQI), in patients aged 5–16 years receiving care at a pediatric dermatology clinic [53].
It is important to recognize the negative impact on decreased quality of life of the parents/caregivers and siblings of children and adolescents with AD; this effect is also correlated with disease severity, with greater severity linked with increased family disruption [37, 53]. In a cross-sectional study of children aged < 16 years with AD, caregivers’ mental and physical health were negatively affected by children’s health-related quality of life [54]. A recent online caregiver-centered survey assessing the burden of AD on children and families noted frequent sleep disturbances, exhaustion, worry, and social isolation related to their child’s AD [55]. Even as the impact of pediatric AD on caregivers and family units as a whole have been documented, effects on patients’ siblings have not been well studied. However, there is evidence that siblings of patients with chronic physical disorders are more likely to internalize their problems, experience emotional stress, and have decreases in school attendance and impaired academic performance [56, 57]. When considering the evidence in the literature, it is clear that the impact of AD is wide-ranging, spanning sleep disturbances, lifestyle changes, treatment issues, social disruptions, school performance, time lost from work, family activities, and financial and mental strain [37]. Therefore, the impact of pediatric AD extends beyond the pathological nature of the disease, highlighting the need to consider the psychosocial aspects of AD for both patients and their families.
Diagnosis of Pediatric AD
Diagnosis of AD relies on clinical presentation because there is currently no reliable biomarker. The diagnostic criteria for AD have evolved, with the seminal diagnostic criteria developed by Hanifin and Rajka [58] modified in 1994 by the UK Working Party [59] and again in 2003 by the American Academy of Dermatology (AAD) in an attempt to generate and refine a diagnostic tool that is suited for clinical practice [60, 61]. The AAD guidelines indicate that a clinical diagnosis should be based on historical features, morphology and distribution of skin lesions, and associated clinical signs [5]. Criteria to be considered in the diagnosis of patients with AD include essential features such as pruritus and eczema; important features that support a diagnosis of AD, such as early age of onset, atopy, and xerosis; and associated features that are suggestive of a diagnosis of AD, such as atypical vascular responses, keratosis pilaris/pityriasis alba/hyperlinear palms/ichthyosis, ocular/periorbital changes, other regional findings, and perifollicular accentuation/lichenification/prurigo lesions [5].
It is important to note that the differential diagnosis for AD can be extensive given the heterogenous nature of the disease. AAD guidelines indicate that a diagnosis of AD is dependent on the exclusion of conditions, such as impetigo, scabies, seborrheic dermatitis, contact dermatitis, ichthyoses, cutaneous T-cell lymphoma, psoriasis, photosensitivity dermatoses, immune deficiency diseases, and erythroderma of other causes [5]. Table 1 provides a more extensive list of potential differential diagnoses of AD, with impetigo, molluscum contagiosum, tinea corporis, and viral exanthems being most common in children [62]. The exclusion of conditions like impetigo and molluscum contagiosum can be particularly difficult, as these conditions may occur concomitantly with, as well as exacerbate, a patient’s AD [5].
Table 1.
Differential diagnosis of atopic dermatitis. Modified with permission from Frazier and Bhardwaj [62]
| Diagnosis | Age | Morphology | Etiology | Other features |
|---|---|---|---|---|
| Impetigo | Common in children | Honey-colored crusts | Bacterial | Very contagious |
| Molluscum contagiosum | Common in children | Skin-colored or erythematous papules | Viral | May induce eczematous responses |
| Tinea corporis | Common in children | Erythematous, annular patches with raised scaly borders | Fungal | May occur in addition to Tinea capitis |
| Viral exanthems | Common in children | Diffuse erythematous macules and papules | Viral | Resolves after illness |
| Contact dermatitis | All ages | Erythematous vesicles, papules, or plaques | Hypersensitivity reaction | Rash occurs at site of exposure |
| Scabies | All ages | Intertriginous, erythematous papules with serpiginous burrows | Parasitic | Intense itching at night |
| Seborrheic dermatitis | All ages | Yellow, greasy scales | Unknown | Scalp and face distribution |
| Urticaria | All ages | Erythematous papules or plaques | Immune-mediated | Individual lesions usually resolve within 24 hours |
| Cutaneous T-cell lymphoma | Rare in children | Erythematous, dry patches | Unknown | Rash develops slowly |
| Dermatitis herpetiformis | Rare in children | Symmetrical vesicles and papules | Autoimmune | Association with gluten sensitivity |
| Lichen simplex chronicus | Rare in young children | Well-circumscribed, thick scales | Chronic scratching | Association with stress and anxiety |
| Nummular eczema | All ages | Annular erythematous patches | Variable | Patches can last weeks to months |
| Psoriasis | Rare in children | Erythematous patches with silvery scale | Immune-mediated | Can have nail involvement |
Identification and assessment of disease severity may be useful for a differential diagnosis of AD, to monitor disease progression, and to evaluate treatment outcomes. Measurement of disease severity or intensity can be characterized by numerous severity indices, including body surface area, Investigator’s Global Assessment (such as the V-IGA, a validated global assessment score), Eczema Area and Severity Index (EASI), SCORAD, and Patient-Oriented Eczema Measure (POEM) (Table 2) [63–65]. Recently, a consensus statement for the global, multi-professional Harmonising Outcome Measures for Eczema (HOME) initiative indicated that patient-reported symptoms were a top-prioritized domain, recommending use of the POEM index [66], a clinically validated patient-derived measure of seven symptoms on a 5-point scale, for evaluating severity of atopic dermatitis, and an updated version of SCORAD known as the patient-oriented SCORAD index [67]. By utilizing these tools, pediatricians can more accurately track the course of their patients’ disease and tailor their treatment regimens accordingly.
Table 2.
Severity scales for assessment of atopic dermatitis (AD)
(modified from Lara-Corrales et al., 2019 [61])
| Diagnostic index | Description of assessment | Benefits | Limitations |
|---|---|---|---|
| EASI | Validated scale utilizing 7-point assessment of disease extent in 4 defined body regions, with severity of 4 clinical signs on a 4-point scale, to a maximum score of 72 |
Evaluates both extent of eczema and lesion severity Good to fair intra-evaluator reliability |
Less sensitive in patients with very low %BSA Inter-evaluator variability lies in the dimension of induration/papulation Patient-reported symptoms are not evaluated |
| SCORAD [65] | Clinically validated scale assesses disease extent based on the rule of 9s, intensity based on 6 clinical signs rated on a 4-point scale, plus patient-reported pruritus and sleep loss | Evaluates both BSA and lesion severity | Measures such as pruritus and sleep loss can be influenced by factors other than AD |
| PO-SCORAD [99] | Patient self-assessment scale developed based on SCORAD criteria, but optimized for use by patients and their families by the inclusion of images and other assessment support |
Evaluates both BSA and lesion severity Patients can monitor AD symptoms between clinical consultations |
Measures such as pruritus and sleep loss can be influenced by factors other than AD |
| BSA | Disease extent assessed as a percentage of total BSA |
Difficult to evaluate in patients with less severe lesions, especially in the presence of xerosis Does not assess lesion severity |
|
| IGA [63] | 4 or 5-point scale of global disease severity based on morphological appearance of lesions, e.g., V-IGA, a validated global assessment score | Facilitates easy, rapid assessment of AD | No standardized ISGA, with variable definitions and implementations in clinical studies |
| POEM [66, 100] | Validated score assessing 7 symptoms over the preceding 7 days using a 5-point scale system (to maximum of 35) |
Specifically developed for AD Scores are correlated with disease severity Appropriate to assess clinical trial populations Easily utilized in routine clinical practice Increasingly used in AD studies and clinical trials |
High utility in clinical studies but low utility in a dermatology clinic |
AD atopic dermatitis, BSA body surface area, EASI Eczema Area and Severity Index, IGA Investigator’s Global Assessment, ISGA Investigator’s Static Global Assessment, POEM Patient-Oriented Eczema Measure, PO-SCORAD Patient-Oriented SCORing Atopic Dermatitis, SCORAD SCORing Atopic Dermatitis
Management of Pediatric AD
A US survey of children presenting to primary care practice indicated that most patients are employing skin care practices that may be detrimental to the skin barrier, highlighting the need for recommendations to guide appropriate treatment practices [11]. A range of treatments are recommended for AD, including emollients (e.g., creams, lotions, ointments), topical corticosteroids (e.g., hydrocortisone, triamcinolone acetonide), topical calcineurin inhibitors (tacrolimus, pimecrolimus), PDE4 inhibitors (crisaborole 2%), systemic oral and injectable treatments, and phototherapy.
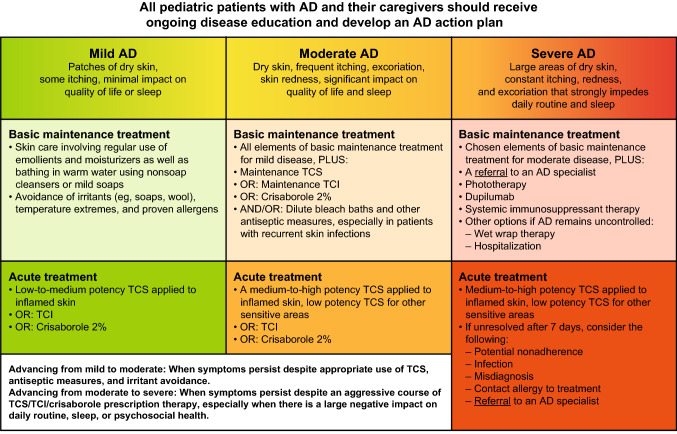
AD is typically treated as a single entity despite its heterogeneous nature [68], with numerous guidelines recommending stepwise therapy initiated according to disease severity [60, 68–73]. Current practice has evolved away from an emphasis solely on controlling acute flares to a more holistic, long-term approach that focuses on comprehensive disease management via the combination of baseline maintenance treatments in tandem with intermittent use of topical corticosteroids (TCS), topical calcineurin inhibitors (TCI), or a PDE4 inhibitor (crisaborole 2%) as needed for more acute manifestations of AD. Figure 2 summarizes a proposed approach to the management of pediatric AD patients. Prior to escalating or stepping up therapy, patients should undergo assessment for nonadherence, potential comorbidities, and to confirm that the increased level of symptoms warranting a step up in therapy is related to AD [71]. Basic management of AD comprises two important nonpharmacologic interventions that should be implemented in all patients at all levels of disease severity: (i) skin care involving regular emollient use and regular (daily or every other day) bathing in warm water using non soap cleansers or mild soaps, and (ii) avoidance of irritants (e.g., soaps, wool), temperature extremes, and proven allergens [71, 74]. Treatment of acute flares with topical therapies, including corticosteroids, calcineurin inhibitors, and PDE4 inhibitors (crisaborole 2%) can be administered as needed in pediatric patients for maintenance therapy and the prevention of flares [69–71, 74]. Notably, the treatment options for infant patients with AD are more limited. Fluocinolone acetonide, fluticasone propionate, desonide, and hydrocortisone butyrate are the only topical corticosteroids that are FDA-approved in infants (≥ 3 months old) [75, 76]. Crisaborole is the only FDA-approved topical PDE4 inhibitor for pediatric AD, and a recent study demonstrated crisaborole’s safety and efficacy in infants aged ≥ 3 to < 24 months with mild-to-moderate AD [70, 77, 78]. TCIs are not approved by the US Food and Drug Administration (FDA) for use in children < 24 months of age [79, 80].
Fig. 2.
Comprehensive long-term approach to the management of atopic dermatitis in children. Adapted with permission from Boguniewicz et al. [71]. AD atopic dermatitis, TCI topical calcineurin inhibitor, TCS topical corticosteroids
Patients with mild-to-moderate AD who remain symptomatic despite optimal basic management and use of low-to-medium–potency topical therapies are candidates for stepping up therapy [71]. Treatment options in these patients include adding a TCS or TCI or crisaborole into their basic maintenance treatment routine.
Approximately one-third of pediatric patients have moderate-to-severe AD, which may not be adequately managed with topical therapy/phototherapy—thereby necessitating a step-up in therapy that includes the initiation of systemic therapy [81, 82]. Current guidelines recommend systemic therapy for pediatric patients with AD who have inadequate disease control despite appropriate topical therapy and/or phototherapy [69–71, 74]. Despite conventional systemic immunomodulators being recommended for the management of moderate-to-severe AD, only a few are licensed for this indication (i.e., systemic corticosteroids in the United States). The findings from a pivotal phase III trial resulted in FDA approval of the targeted biologic therapy, dupilumab, in children aged 6–11 years old with moderate-to-severe, inadequately controlled eczema [77, 83]. Methotrexate, along with cyclosporine, mycophenolate mofetil, and azathioprine, can also be considered as off-label treatments for pediatric patients with severe disease, although data supporting their use are sparse [69–71, 81]. There is limited evidence supporting the prophylactic use of systemic antibiotics in the AD setting, and improvement in outcomes with the addition of topical or oral antibiotics to topical steroid and emollient treatment in children with eczema has not been shown [84]. However, antibiotics are customarily used for the treatment of clinically evident secondary bacterial infections, and many experts have seen success using oral antibiotics to treat infected eczema [71, 72, 81]. There is also little data supporting the use of oral antihistamines in the treatment of AD, though nonsedating antihistamines may be considered in the presence of urticaria and sedating antihistamines may be considered in patients with sleep disturbance secondary to pruritus [5, 62, 72].
It is important to note that patients with severe disease should be referred to an AD specialist [60, 71]; indications for referral include those for whom diagnosis is not confirmed, who have poor disease control with appropriate first-line treatments, who have severe or recurrent skin infections (viral or bacterial), who are possible candidates for systemic therapy or phototherapy, who have significant allergies such that they could benefit from allergy evaluation and/or specific immunotherapy (i.e., immunoglobulin E > 150 IU/mL), or who have significant AD-related psychosocial problems [62, 85].
Any comprehensive management plan should also incorporate ongoing disease education and an AD action plan [69–71, 74, 86]. Ongoing parental education is particularly important with regard to the phenomenon of steroid phobia, which may result in poor treatment adherence if not addressed and minimized [71]. The development of an action plan is also crucial for the long-term care of patients with pediatric AD, as the current treatment paradigm necessitates the stepping up of therapies in response to acute or poorly controlled disease [71]. Any action plan should be malleable in response to factors including patient satisfaction and treatment tolerability to optimize results for each patient [74]. In the context of AD, early and aggressive treatment response to worsening signs and symptoms can yield significant benefits with regard to health outcomes, and an action plan with predetermined responses and prevention strategies can help increase adherence, decrease treatment failure, and minimize the long-term effects of AD in the pediatric population [71, 74].
As our understanding of the mechanisms involved in the pathogenesis of AD has evolved with advances in AD research, novel biologic therapeutic targets have been identified that have the potential to change the treatment paradigm in patients with AD. Targeted therapies provide treatment options that are more effective and less toxic, compared with broad immunomodulatory approaches. Dupilumab, available as a subcutaneous injection, is a first-in-class dual inhibitor of IL-4 and IL-13 signaling that has been approved for use in children aged 6–11 years old with moderate-to-severe, inadequately controlled eczema [77, 83], and has demonstrated efficacy in adolescents aged 12–17 years with refractory moderate-to-severe AD, providing rapid improvements in disease severity. Dupilumab pediatric studies have included patients 6 months of age and older. However, adverse effects, such as ocular complications (e.g., conjunctivitis) or joint pain should be carefully monitored in the pediatric population. A number of novel systemic agents in development for moderate-to-severe AD may be promising treatment options in the pediatric setting, including oral JAK inhibitors (i.e., abrocitinib, baricitinib, and upadacitinib) and injectable anti–IL-13 antibodies (i.e., tralokinumab and lebrikizumab). These systemic agent classes have shown promise in clinical studies in adults [77, 87–90], and are also in development for pediatric use [86]. Abrocitinib, a next-generation selective JAK 1 inhibitor that received breakthrough therapy designation in February 2018, has been investigated in multiple phase III studies investigating its use in adults and adolescents aged > 12 years for the treatment of moderate-to-severe atopic dermatitis, and has been approved in the United States, European Union, Japan, and in other countries [91, 92]. A phase III extension study for abrocitinib is still underway (ClinicalTrials.gov identifier: NCT03422822) [77]. Upadacitinib, a reversible, selective JAK1 inhibitor, has completed phase III trials in atopic dermatitis [88], has been approved for the treatment of atopic dermatitis in the UK, European Union, and Japan [93, 94], and is under regulatory review in the United States. Baricitinib has been recently approved in adults by the European Medicines Agency [95, 96]. Topical ruxolitinib, a topical JAK1/JAK2 inhibitor, has also undergone clinical trials for AD in both children aged 12–17 years as well as adults and has been approved by the FDA for treatment of AD [77]. The anti–IL-31Rα monoclonal antibody nemolizumab is specifically being developed to treat pruritus and is currently being investigated in children aged 6–17 years and has shown promising results regarding a reduction in pruritus [77].
Role of Telemedicine in AD
The current global pandemic of COVID-19 has highlighted the need for strong and robust telemedicine to deliver optimal patient care to patients with AD. Web-based consultation in children with AD has been shown to be equally effective in terms of patient self-management, health outcomes, and costs when compared with traditional delivery of care [97, 98]. However, given the technological advances of the past decade and the rapid innovation driven by COVID-19, more studies are needed to more fully determine how to integrate telemedicine into current treatment paradigms.
Summary
In the primary care setting, pediatricians play an important role in the management of AD, treating patients with mild-to-moderate disease themselves, referring those with moderate-to-severe disease for specialist care, and providing ongoing maintenance care after initial treatment by AD specialists. As a result, it is critical that pediatricians have a sound knowledge of AD pathophysiology, diagnosis, basic skin care, and management recommendations for mild-to-moderate disease. As our understanding of AD has grown to recognize the systemic components of this disease and the scope of its lifelong impact, it is incumbent upon treatment providers to shift their thinking away from merely addressing episodic, acute flares in their patients and work toward a more comprehensive paradigm of controlling the disease in all its manifestations. Given the substantial burden that AD places on affected children and their families/caregivers, a holistic approach to assessing the disease should be considered by the pediatrician, taking into account both the physical nature of AD and its impact on the psychosocial wellbeing and quality of life of the child and his/her family. Finally, recent advances in research and breakthroughs in science have vastly improved our understanding of AD, particularly the crucial role that inflammatory immune responses play in the pathogenesis of this disease. As a result, novel targeted systemic therapies are being developed that target the underlying causes of AD, which have the potential to change the future of AD therapy.
Acknowledgements
Editorial and medical writing support, under the guidance of the authors, was provided by Robert Schoen, PharmD, and Christopher Goodwin, PhD, of ApotheCom (San Francisco, CA, USA), and was funded by Pfizer Inc., in accordance with Good Publication Practice guidelines (Ann Intern Med. 2015;163:461–464).
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Funding
Pfizer Inc.
Conflict of interest
LFE has served as a scientific adviser, consultant, and/or clinical study investigator for Pfizer Inc., AbbVie, Almirall, Amgen, Asana Biosciences, Cutanea, Dermavant, Dermira, Dr. Reddy’s Laboratory, DS Biopharma, Eli Lilly, Forté Pharma, Galderma, Glenmark, Incyte, LEO Pharma, Matrisys Bioscience, Novan, Novartis, Ortho Dermatologics/Valeant, Sanofi Regeneron, Sanofi Genzyme, TopMD, UCB, and Verrica. SS has served as a scientific adviser, consultant, and/or clinical study investigator for Pfizer Inc. SF, AC, and AOB are employees of and own stock in Pfizer, Inc. LAS has served as a scientific advisor, consultant, lecturer and/or on advisory boards for Pfizer, Mustela, CeraVe, Sanofi-Regeneron, Hoth, Alphyn, TopMD, and Verrica.
Author contributions
All authors made substantial contributions to the conception or design of the work, revised it critically for important intellectual content, approved the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24(5):476–486. doi: 10.1111/pai.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8–16. doi: 10.1159/000370220. [DOI] [PubMed] [Google Scholar]
- 3.Yang EJ, Sekhon S, Sanchez IM, Beck KM, Bhutani T. Recent developments in atopic dermatitis. Pediatrics. 2018;142(4). 10.1542/peds.2018-1102. [DOI] [PubMed]
- 4.Kay J, Gawkrodger DJ, Mortimer MJ, Jaron AG. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30(1):35–39. doi: 10.1016/S0190-9622(94)70004-4. [DOI] [PubMed] [Google Scholar]
- 5.Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–351. doi: 10.1016/j.jaad.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Ramette A, Jurca M, Goutaki M, Beardsmore CS, Kuehni CE. Association between breastfeeding and eczema during childhood and adolescence: a cohort study. PLoS One. 2017;12(9):e0185066. doi: 10.1371/journal.pone.0185066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bylund S, von Kobyletzki LB, Svalstedt M, Svensson A. Prevalence and incidence of atopic dermatitis: a systematic review. Acta Derm Venereol. 2020;100(12):adv00160. doi: 10.2340/00015555-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35(3):283–289. doi: 10.1016/j.det.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Investig Dermatol. 2017;137(1):26–30. doi: 10.1016/j.jid.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Chung J, Simpson EL. The socioeconomics of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122(4):360–366. doi: 10.1016/j.anai.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Al-Naqeeb J, Danner S, Fagnan LJ, et al. The burden of childhood atopic dermatitis in the primary care setting: a report from the Meta-LARC Consortium. J Am Board Fam Med. 2019;32(2):191–200. doi: 10.3122/jabfm.2019.02.180225. [DOI] [PubMed] [Google Scholar]
- 12.Abuabara K, Margolis DJ, Langan SM. The long-term course of atopic dermatitis. Dermatol Clin. 2017;35(3):291–297. doi: 10.1016/j.det.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bantz SK, Zhu Z, Zheng T. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol. 2014 doi: 10.4172/2155-9899.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strom MA, Fishbein AB, Paller AS, Silverberg JI. Association between atopic dermatitis and attention deficit hyperactivity disorder in US children and adults. Br J Dermatol. 2016;175(5):920–929. doi: 10.1111/bjd.14697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131(2):428–433. doi: 10.1016/j.jaci.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Kobyletzki LB, Bornehag CG, Hasselgren M, Larsson M, Lindström CB, Svensson Å. Eczema in early childhood is strongly associated with the development of asthma and rhinitis in a prospective cohort. BMC Dermatol. 2012;12:11. doi: 10.1186/1471-5945-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsakok T, Marrs T, Mohsin M, et al. Does atopic dermatitis cause food allergy? A systematic review. J Allergy Clin Immunol. 2016;137(4):1071–1078. doi: 10.1016/j.jaci.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 18.Novak N, Bieber T, Leung DY. Immune mechanisms leading to atopic dermatitis. J Allergy Clin Immunol. 2003;112(6 Suppl):S128–S139. doi: 10.1016/j.jaci.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Malik K, Heitmiller KD, Czarnowicki T. An update on the pathophysiology of atopic dermatitis. Dermatol Clin. 2017;35(3):317–326. doi: 10.1016/j.det.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Wollina U. Microbiome in atopic dermatitis. Clin Cosmet Investig Dermatol. 2017;10:51–56. doi: 10.2147/CCID.S130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rerknimitr P, Otsuka A, Nakashima C, Kabashima K. The etiopathogenesis of atopic dermatitis: barrier disruption, immunological derangement, and pruritus. Inflamm Regen. 2017;37:14. doi: 10.1186/s41232-017-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bin L, Leung DY. Genetic and epigenetic studies of atopic dermatitis. Allergy Asthma Clin Immunol. 2016;12:52. doi: 10.1186/s13223-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cork MJ, Danby SG, Ogg GS. Atopic dermatitis epidemiology and unmet need in the United Kingdom. J Dermatol Treat. 2019 doi: 10.1080/09546634.2019.1655137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powers CE, McShane DB, Gilligan PH, Burkhart CN, Morrell DS. Microbiome and pediatric atopic dermatitis. J Dermatol. 2015;42(12):1137–1142. doi: 10.1111/1346-8138.13072. [DOI] [PubMed] [Google Scholar]
- 25.Blicharz L, Rudnicka L, Samochocki Z. Staphylococcus aureus: an underestimated factor in the pathogenesis of atopic dermatitis? Postep Dermatol Alergol. 2019;36(1):11–17. doi: 10.5114/ada.2019.82821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renert-Yuval Y, Thyssen JP, Bissonnette R, et al. Biomarkers in atopic dermatitis-a review on behalf of the International Eczema Council. J Allergy Clin Immunol. 2021;147(4):1174–90.e1. doi: 10.1016/j.jaci.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansouri Y, Guttman-Yassky E. Immune pathways in atopic dermatitis, and definition of biomarkers through broad and targeted therapeutics. J Clin Med. 2015;4(5):858–873. doi: 10.3390/jcm4050858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esaki H, Brunner PM, Renert-Yuval Y, et al. Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J Allergy Clin Immunol. 2016;138(6):1639–1651. doi: 10.1016/j.jaci.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Brunner PM, Israel A, Zhang N, et al. Early-onset pediatric atopic dermatitis is characterized by TH2/TH17/TH22-centered inflammation and lipid alterations. J Allergy Clin Immunol. 2018;141(6):2094–2106. doi: 10.1016/j.jaci.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 30.Brunner PM, He H, Pavel AB, et al. The blood proteomic signature of early-onset pediatric atopic dermatitis shows systemic inflammation and is distinct from adult long-standing disease. J Am Acad Dermatol. 2019;81(2):510–519. doi: 10.1016/j.jaad.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 31.Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28(1):3–10. doi: 10.1111/exd.13808. [DOI] [PubMed] [Google Scholar]
- 32.Samrao A, Berry TM, Goreshi R, Simpson EL. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148(8):890–897. doi: 10.1001/archdermatol.2012.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2(3):e24137. doi: 10.4161/jkst.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mack MR, Brestoff JR, Berrien-Elliott MM, et al. Blood natural killer cell deficiency reveals an immunotherapy strategy for atopic dermatitis. Sci Transl Med. 2020 doi: 10.1126/scitranslmed.aay1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang V, Boguniewicz J, Boguniewicz M, Ong PY. The infectious complications of atopic dermatitis. Ann Allergy Asthma Immunol. 2021;126(1):3–12. doi: 10.1016/j.anai.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hebert AA, Stingl G, Ho LK, et al. Patient impact and economic burden of mild-to-moderate atopic dermatitis. Curr Med Res Opin. 2018;34(12):2177–2185. doi: 10.1080/03007995.2018.1498329. [DOI] [PubMed] [Google Scholar]
- 37.Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB, Jr, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22(3):192–199. doi: 10.1111/j.1525-1470.2005.22303.x. [DOI] [PubMed] [Google Scholar]
- 38.Reuveni H, Chapnick G, Tal A, Tarasiuk A. Sleep fragmentation in children with atopic dermatitis. Arch Pediatr Adolesc Med. 1999;153(3):249–253. doi: 10.1001/archpedi.153.3.249. [DOI] [PubMed] [Google Scholar]
- 39.Drucker AM. Atopic dermatitis: burden of illness, quality of life, and associated complications. Allergy Asthma Proc. 2017;38(1):3–8. doi: 10.2500/aap.2017.38.4005. [DOI] [PubMed] [Google Scholar]
- 40.Ramirez FD, Chen S, Langan SM, et al. Association of atopic dermatitis with sleep quality in children. JAMA Pediatr. 2019;173(5):e190025. doi: 10.1001/jamapediatrics.2019.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tracy A, Bhatti S, Eichenfield LF. Update on pediatric atopic dermatitis. Cutis. 2020;106(3):143–146. doi: 10.12788/cutis.0077. [DOI] [PubMed] [Google Scholar]
- 42.Ramirez FD, Chen S, Langan SM, et al. Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019;155(5):556–563. doi: 10.1001/jamadermatol.2018.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bridgman AC, Eshtiaghi P, Cresswell-Melville A, Ramien M, Drucker AM. The burden of moderate to severe atopic dermatitis in Canadian children: a cross-sectional survey. J Cutan Med Surg. 2018;22(4):443–444. doi: 10.1177/1203475418761859. [DOI] [PubMed] [Google Scholar]
- 44.Wan J, Margolis DJ, Mitra N, Hoffstad OJ, Takeshita J. Racial and ethnic differences in atopic dermatitis-related school absences among US children. JAMA Dermatol. 2019;155(8):973–975. doi: 10.1001/jamadermatol.2019.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim SY, Kim MS, Park B, Kim JH, Choi HG. Allergic rhinitis, atopic dermatitis, and asthma are associated with differences in school performance among Korean adolescents. PLoS One. 2017;12(2):e0171394. doi: 10.1371/journal.pone.0171394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magin P, Adams J, Heading G, Pond D, Smith W. Experiences of appearance-related teasing and bullying in skin diseases and their psychological sequelae: results of a qualitative study. Scand J Caring Sci. 2008;22(3):430–436. doi: 10.1111/j.1471-6712.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 47.Zuberbier T, Orlow SJ, Paller AS, et al. Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol. 2006;118(1):226–232. doi: 10.1016/j.jaci.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 48.Chamlin SL, Frieden IJ, Williams ML, Chren MM. Effects of atopic dermatitis on young American children and their families. Pediatrics. 2004;114(3):607–611. doi: 10.1542/peds.2004-0374. [DOI] [PubMed] [Google Scholar]
- 49.Chernyshov PV. Stigmatization and self-perception in children with atopic dermatitis. Clin Cosmet Investig Dermatol. 2016;9:159–166. doi: 10.2147/ccid.S91263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee S, Shin A. Association of atopic dermatitis with depressive symptoms and suicidal behaviors among adolescents in Korea: the 2013 Korean Youth Risk Behavior Survey. BMC Psychiatry. 2017;17(1):3. doi: 10.1186/s12888-016-1160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kyung Y, Choi MH, Jeon YJ, et al. Association of atopic dermatitis with suicide risk among 788,411 adolescents: a Korean cross-sectional study. Ann Allergy Asthma Immunol. 2020;125(1):55–64. doi: 10.1016/j.anai.2020.03.023. [DOI] [PubMed] [Google Scholar]
- 52.Ronnstad ATM, Halling-Overgaard AS, Hamann CR, Skov L, Egeberg A, Thyssen JP. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;79(3):448–56.e30. doi: 10.1016/j.jaad.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 53.Campos ALB, Araújo FM, Santos M, Santos A, Pires CAA. Impact of atopic dermatitis on the quality of life of pediatric patients and their guardians. Rev Paul Pediatr. 2017;35(1):5–10. doi: 10.1590/1984-0462/;2017;35;1;00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu X, van Galen LS, Koh MJA, et al. Factors influencing quality of life in children with atopic dermatitis and their caregivers: a cross-sectional study. Sci Rep. 2019;9(1):15990. doi: 10.1038/s41598-019-51129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Capozza K, Gadd H, Kelley K, Russell S, Shi V, Schwartz A. Insights from caregivers on the impact of pediatric atopic dermatitis on families: “I’m tired, overwhelmed, and feel like i’m failing as a mother.’. Dermatitis. 2020;31(3):223–227. doi: 10.1097/der.0000000000000582. [DOI] [PubMed] [Google Scholar]
- 56.Gan LL, Lum A, Wakefield CE, Nandakumar B, Fardell JE. School experiences of siblings of children with chronic illness: a systematic literature review. J Pediatr Nurs. 2017;33:23–32. doi: 10.1016/j.pedn.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 57.Vermaes IP, van Susante AM, van Bakel HJ. Psychological functioning of siblings in families of children with chronic health conditions: a meta-analysis. J Pediatr Psychol. 2012;37(2):166–184. doi: 10.1093/jpepsy/jsr081. [DOI] [PubMed] [Google Scholar]
- 58.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;60(92):44–47. [Google Scholar]
- 59.Williams HC, Burney PG, Strachan D, Hay RJ. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. II. Observer variation of clinical diagnosis and signs of atopic dermatitis. Br J Dermatol. 1994;131(3):397–405. doi: 10.1111/j.1365-2133.1994.tb08531.x. [DOI] [PubMed] [Google Scholar]
- 60.Eichenfield LF, Ahluwalia J, Waldman A, Borok J, Udkoff J, Boguniewicz M. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49–S57. doi: 10.1016/j.jaci.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 61.Lara-Corrales I, Bergman JN, Landells I, Ramien ML, Lansang P. Approach to the assessment and management of pediatric patients with atopic dermatitis: a consensus document. Section I: overview of pediatric atopic dermatitis. J Cutan Med Surg. 2019;23(5_suppl):3s–11s. doi: 10.1177/1203475419882049. [DOI] [PubMed] [Google Scholar]
- 62.Frazier W, Bhardwaj N. Atopic dermatitis: diagnosis and treatment. Am Fam Physician. 2020;101(10):590–598. [PubMed] [Google Scholar]
- 63.Futamura M, Leshem YA, Thomas KS, Nankervis H, Williams HC, Simpson EL. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol. 2016;74(2):288–294. doi: 10.1016/j.jaad.2015.09.062. [DOI] [PubMed] [Google Scholar]
- 64.Leshem YA, Hajar T, Hanifin JM, Simpson EL. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172(5):1353–1357. doi: 10.1111/bjd.13662. [DOI] [PubMed] [Google Scholar]
- 65.Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195(1):10–19. doi: 10.1159/000245677. [DOI] [PubMed] [Google Scholar]
- 66.Charman CR, Venn AJ, Ravenscroft JC, Williams HC. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br J Dermatol. 2013;169(6):1326–1332. doi: 10.1111/bjd.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leshem YA, Chalmers JR, Apfelbacher C, et al. Measuring atopic eczema symptoms in clinical practice: the first consensus statement from the Harmonising Outcome Measures for Eczema in clinical practice initiative. J Am Acad Dermatol. 2020;82(5):1181–1186. doi: 10.1016/j.jaad.2019.12.055. [DOI] [PubMed] [Google Scholar]
- 68.Feldman SR, Cox LS, Strowd LC, et al. The challenge of managing atopic dermatitis in the United States. Am Health Drug Benefits. 2019;12(2):83–93. [PMC free article] [PubMed] [Google Scholar]
- 69.Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657–682. doi: 10.1111/jdv.14891. [DOI] [PubMed] [Google Scholar]
- 70.Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850–878. doi: 10.1111/jdv.14888. [DOI] [PubMed] [Google Scholar]
- 71.Boguniewicz M, Fonacier L, Guttman-Yassky E, Ong PY, Silverberg J, Farrar JR. Atopic dermatitis yardstick: Practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120(1):10–22.e2. doi: 10.1016/j.anai.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 72.National Institute for Heath and Care Excellence. Atopic eczema in under 12s. National Institute for Heath and Care Excellence, London, UK. 2013. https://www.nice.org.uk/guidance/QS44. Accessed 27 Aug 2020.
- 73.Oberlin KE, Nanda S. Atopic dermatitis made easy: the Schachner ladder. Pediatr Dermatol. 2019;36(6):1017–1018. doi: 10.1111/pde.13862. [DOI] [PubMed] [Google Scholar]
- 74.Lansang P, Lara-Corrales I, Bergman JN, et al. Approach to the assessment and management of pediatric patients with atopic dermatitis: a consensus document. Section IV: consensus statements on the assessment and management of pediatric atopic dermatitis. J Cutan Med Surg. 2019;23(5_suppl):32s–s39. doi: 10.1177/1203475419882654. [DOI] [PubMed] [Google Scholar]
- 75.Huang A, Cho C, Leung DYM, Brar K. Atopic dermatitis: early treatment in children. Curr Treat Options Allergy. 2017;4(3):355–369. doi: 10.1007/s40521-017-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Derma-Smoothe. Prescribing information. Stanford: Hill Dermaceuticals, Inc.; 2007.
- 77.Nguyen HL, Anderson KR, Tollefson MM. New and emerging therapies for pediatric atopic dermatitis. Paediatr Drugs. 2019;21(4):239–260. doi: 10.1007/s40272-019-00342-w. [DOI] [PubMed] [Google Scholar]
- 78.Schlessinger J, Shepard JS, Gower R, et al. Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to < 24 months with mild-to-moderate atopic dermatitis: a phase IV open-label study (CrisADe CARE 1) Am J Clin Dermatol. 2020;21(2):275–284. doi: 10.1007/s40257-020-00510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elidel. Prescribing information. Valeant Pharmaceuticals North America; 2014.
- 80.Protopic. Prescribing information. Astellas Pharma Tech Co., Ltd.; 2016.
- 81.Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327–349. doi: 10.1016/j.jaad.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Drucker AM, Ellis AG, Bohdanowicz M, et al. Systemic immunomodulatory treatments for patients with atopic dermatitis: a systematic review and network meta-analysis. JAMA Dermatol. 2020 doi: 10.1001/jamadermatol.2020.0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83(5):1282–1293. doi: 10.1016/j.jaad.2020.06.054. [DOI] [PubMed] [Google Scholar]
- 84.Francis NA, Ridd MJ, Thomas-Jones E, et al. Oral and topical antibiotics for clinically infected eczema in children: a pragmatic randomized controlled trial in ambulatory care. Ann Fam Med. 2017;15(2):124–130. doi: 10.1370/afm.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mancuso JB, Lee SS, Paller AS, Ohya Y, Eichenfield LF. Management of severe atopic dermatitis in pediatric patients. J Allergy Clin Immunol Pract. 2021;9(4):1462–1471. doi: 10.1016/j.jaip.2021.02.017. [DOI] [PubMed] [Google Scholar]
- 86.Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71(6):1218–1233. doi: 10.1016/j.jaad.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155(12):1371–1379. doi: 10.1001/jamadermatol.2019.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guttman-Yassky E, Thaci D, Pangan AL, et al. Upadacitinib in adults with moderate-to-severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2019;145(3):877–884. doi: 10.1016/j.jaci.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 89.Wollenberg A, Howell MD, Guttman-Yassky E, et al. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol. 2019;143(1):135–141. doi: 10.1016/j.jaci.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 90.Simpson EL, Flohr C, Eichenfield LF, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE) J Am Acad Dermatol. 2018;78(5):863–71 e11. doi: 10.1016/j.jaad.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 91.Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863–873. doi: 10.1001/jamadermatol.2020.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–266. doi: 10.1016/s0140-6736(20)30732-7. [DOI] [PubMed] [Google Scholar]
- 93.European Medicines Agency. EMEA-001741-PIP04-17-M01. European Medicines Agency, Amsterdam, Netherlands. https://www.ema.europa.eu/en/medicines/human/paediatric-investigation-plans/emea-001741-pip04-17-m01. Accessed 06 Dec 2021.
- 94.Pharmaceuticals and Medical Devices Agency. List of Approved Products (New Drugs): FY 2021 (April 2021–November 2021). Pharmaceuticals and Medical Devices Agency, Tokyo, Japan. https://www.pmda.go.jp/files/000243812.pdf. Accessed 06 Dec 2021.
- 95.Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242–255. doi: 10.1111/bjd.18898. [DOI] [PubMed] [Google Scholar]
- 96.Olumiant. Summary of product characteristics. Utrecht: Eli Lilly Nederland B.V.; 2020.
- 97.Bergmo TS, Wangberg SC, Schopf TR, Solvoll T. Web-based consultations for parents of children with atopic dermatitis: results of a randomized controlled trial. Acta Paediatr. 2009;98(2):316–320. doi: 10.1111/j.1651-2227.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 98.Schopf TR, Bolle R, Solvoll T. The workload of web-based consultations with atopic eczema patients at home. BMC Res Notes. 2010;3:71. doi: 10.1186/1756-0500-3-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stalder JF, Barbarot S, Wollenberg A, et al. Patient-Oriented SCORAD (PO-SCORAD): a new self-assessment scale in atopic dermatitis validated in Europe. Allergy. 2011;66(8):1114–1121. doi: 10.1111/j.1398-9995.2011.02577.x. [DOI] [PubMed] [Google Scholar]
- 100.Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140(12):1513–1519. doi: 10.1001/archderm.140.12.1513. [DOI] [PubMed] [Google Scholar]