Abstract
WNT signalling comprises a diverse spectrum of receptor-mediated pathways activated by a large family of WNT ligands and influencing fundamental biological processes. WNT signalling includes the β-catenin canonical pathway and the non-canonical pathways, namely the planar cell polarity and the calcium-dependent pathways. Advances over the past decade have linked non-canonical WNT signalling with key mechanisms of atherosclerosis, including oxidative stress, endothelial dysfunction, macrophage activation and vascular smooth muscle cell phenotype regulation. In addition, non-canonical WNT signalling is involved in crucial aspects of myocardial biology, from fibrosis to hypertrophy and oxidative stress. Importantly, non-canonical WNT signalling activation has complex effects in adipose tissue in the context of obesity, thereby potentially linking metabolic and vascular diseases. Tissue-specific targeting of non-canonical WNT signalling might be associated with substantial risks of off-target tumorigenesis, challenging its therapeutic potential. However, novel technologies, such as monoclonal antibodies, recombinant decoy receptors, tissue-specific gene silencing with small interfering RNAs and gene editing with CRISPR–Cas9, might enable more efficient therapeutic targeting of WNT signalling in the cardiovascular system. In this Review, we summarize the components of non-canonical WNT signalling, their links with the main mechanisms of atherosclerosis, heart failure and arrhythmias, and the rationale for targeting individual components of non-canonical WNT signalling for the treatment of cardiovascular disease.
Subject terms: Cardiovascular diseases, Obesity, Pathogenesis
In this Review, the authors discuss the roles of non-canonical WNT signalling in cardiovascular disease. They provide an overview of non-canonical WNT signalling, describe its links to the pathogenesis of atherosclerosis, heart failure and arrhythmias, and explore the clinical potential of targeting individual components of non-canonical WNT signalling in cardiovascular disease.
Key points
Cardiovascular disease is a major cause of morbidity and mortality worldwide, prompting the need for a better understanding of the underlying pathogenic mechanisms.
Non-canonical WNT signalling involves an evolutionarily conserved and ubiquitous range of pathways affecting fundamental processes such as inflammation, metabolism, cell motility, oxidative stress and homeostasis.
Non-canonical WNT signalling is a promising target in vascular disease, influencing vascular oxidative stress, endothelial dysfunction, inflammation, vascular smooth muscle cell phenotypes and cellular insulin resistance, all of which can affect atherosclerosis progression and plaque stability.
Non-canonical WNT signalling has putative links to cardiac disease by influencing myocardial oxidative stress, inflammation, repair capacity, energetics and remodelling, including fibrotic or adipose infiltration of the myocardium, all of which can generate the substrate for contractile dysfunction and arrhythmogenic potential.
Non-canonical WNT ligands are secreted by adipose tissue and are upregulated in obesity, acting as an endocrine and paracrine link between obesity and cardiovascular complications via adipose tissue–cardiovascular system crosstalk.
Non-canonical WNT signalling can be therapeutically targeted at multiple levels and could involve several state-of-the-art technologies; however, more research in this area is required.
Introduction
Cardiovascular diseases, including atherosclerosis and myocardial disease, remain the leading cause of mortality worldwide1. Atherosclerosis is an inflammatory disease2–4 influenced by genetic and demographic risk factors5–7 and is associated with complex phenotypic changes in endothelial cells, vascular smooth muscle cells (VSMCs) and macrophages8,9. Myocardial disease is often associated with the presence of coronary atherosclerosis and involves processes such as myocardial oxidative stress10,11, fibrosis and remodelling12, which can lead to diseases such as heart failure and arrhythmias11,13. Despite substantial advances over the past decade, there is an unmet need to describe cardiovascular disease pathogenesis more accurately.
The WNT ligand family includes several secreted glycoproteins (19 in mammals) that activate an evolutionarily conserved spectrum of signalling pathways14,15. WNT signalling is implicated in embryogenesis and development via the regulation of cell motility and cell polarization. WNT signalling also regulates cell survival, growth and motility and has therefore been pathophysiologically linked with tumorigenesis14,16 and cellular metabolism17. WNT signalling can be classified into two main modes of signalling: the canonical pathway, which was described first and involves a reduction in β-catenin degradation followed by the induction of the expression of β-catenin target genes, and the non-canonical pathway, which involves β-catenin-independent mechanisms such as Ca2+ signalling and activation of small GTPases18. WNT ligands have varying degrees of selectivity towards the two pathways and can activate either of the two pathways depending on spatiotemporal parameters and receptor availability19. Certain WNT ligands, such as WNT5A and WNT11, seem to predominantly activate non-canonical WNT signalling15. Non-canonical WNT ligands have been implicated in multiple biological processes such as inflammation, cell motility and metabolism14. WNT5A and WNT11 have even been proposed as markers of respiratory distress syndrome severity in patients with coronavirus disease 2019 (COVID-19)20, which might be related to the capacity of these ligands to regulate cell migration, survival and apoptosis in lung injury. Additionally, non-canonical WNT signalling has emerged as a potential regulator of vascular disease pathogenesis via mechanistic links with inflammation, cell motility and oxidative stress21–23. Moreover, activation of non-canonical WNT signalling might be increased in obesity via the increased secretion of non-canonical WNT ligands, such as WNT5A, by adipose tissue15.
In this Review, we explore the complex roles of non-canonical WNT signalling in cardiovascular disease. First, we provide an overview of WNT signalling, followed by a comprehensive description of the role of non-canonical WNT signalling in cardiovascular disease pathogenesis. Finally, we explore the clinical potential of targeting non-canonical WNT signalling to treat atherosclerosis.
WNT pathways and molecular targets
The prototype WNT signalling, currently referred to as canonical WNT signalling, was first described in Drosophila, where it was shown to be involved in wing formation by signalling mediated via the glycoprotein product of the Wg gene24. This gene was later found to be homologous to the mammalian gene INT1; therefore, the nomenclature for this family of Wg/INT1 genes fused and became WNT1, and more WNT ligands have been described since14,25. In mammals, the WNT family of ligands consists of 19 glycoproteins (Table 1) that undergo a variety of post-translational modifications, including glycosylation and palmitoleic acid modifications15,25. As a result of these modifications, WNT ligands have moderate water solubility, which facilitates rapid protein–protein interactions and the propagation of paracrine signalling25.
Table 1.
Overview of WNT ligands, receptors and associated pathways
| Protein | Involvement in signalling pathway | |
|---|---|---|
| Canonical | Non-canonical | |
| WNT ligands | ||
| WNT1 | ++ | + |
| WNT2 | ++ | + |
| WNT2B | + | + |
| WNT3 | ++ | + |
| WNT3A | ++ | + |
| WNT4 | + | + |
| WNT5A | + | ++ |
| WNT5B | + | ++ |
| WNT6 | + | + |
| WNT7A | + | + |
| WNT7B | + | + |
| WNT8A | ++ | + |
| WNT8B | + | + |
| WNT9A | + | + |
| WNT9B | + | + |
| WNT10A | ++ | + |
| WNT10B | ++ | + |
| WNT11 | + | ++ |
| WNT16 | + | + |
| Receptors | ||
| FZD1 | ++ | + |
| FZD2 | + | ++ |
| FZD3 | + | + |
| FZD4 | + | + |
| FZD5 | + | + |
| FZD6 | + | ++ |
| FZD7 | + | + |
| FZD8 | + | + |
| FZD9 | + | ++ |
| FZD10 | + | ++ |
| LRP1, LRP5, LRP6 | ++ | + |
| ROR1 | + | ++ |
| ROR2 | + | ++ |
| RYK | + | ++ |
All ligands and WNT members can activate both pathways; assumed predominance of a given pathway is denoted as ++. FZD, Frizzled; LRP, LDL receptor-related protein; ROR, tyrosine-protein kinase transmembrane receptor ROR; RYK, tyrosine-protein kinase RYK.
WNT ligands are ubiquitously secreted molecules without exclusive tissue sources. Pathophysiologically important sources include adipose tissue15 and immune cells23. WNT ligands are also secreted by cardiovascular cells, including cardiomyocytes and the endothelium, although the baseline expression of WNT ligands in vascular cells is lower than in adipose tissue15,26. Nonetheless, the relative contribution of each tissue to the systemic WNT ligand pool has been poorly described. For example, WNT ligand release is a complex process involving post-translational modifications and vesicle secretion, and the upstream stimuli are unclear27. WNT ligands are often constitutively expressed and certain processes, such as inflammation and obesity, can upregulate their expression via mediators such as adiponectin28.
WNT signalling is initiated after the binding of extracellular, secreted WNT ligands to various membrane receptors, mainly the Frizzled (FZD) family of G protein-coupled, seven-transmembrane receptors14. The tyrosine-protein kinase transmembrane receptors ROR1 and ROR2 and the tyrosine-protein kinase RYK have also been shown to interact with WNT ligands but these interactions are less well characterized than WNT–FZD interactions29. The WNT ligand–receptor interaction triggers an incompletely characterized chain of molecular events involving downstream protein interactions that lead to transcriptional regulation14,25. To date, two main pathways downstream of the WNT–receptor interaction have been described: the canonical WNT pathway and the non-canonical WNT pathway29. The canonical pathway prevents the degradation of β-catenin, thereby allowing β-catenin-dependent transcriptional regulation to occur, which affects cell proliferation and survival30. The non-canonical pathway involves β-catenin-independent downstream signalling and can be broadly divided into the planar cell polarity (PCP) pathway and the Ca2+-dependent pathway29. WNT signalling is negatively regulated by the interaction of WNT ligands with secreted FZD-related proteins (SFRP1–SFRP5 in humans), which are structurally similar to FZD receptors and thus have affinity to WNT ligands and act as decoy receptors29.
Of note, WNT ligands and receptors comprise an extremely complex canvas of interrelated pathways, and classification into canonical and non-canonical pathways and ligands is virtual and schematic. In reality, most WNT ligands might be able to activate both canonical or non-canonical WNT signalling, with the primary effects on one of the pathways depending on context, spatiotemporal parameters and receptor availability19. Moreover, a negative interaction between the canonical and non-canonical pathways has been described, suggesting continuous crosstalk between the various pathways and ligands31,32.
Non-canonical WNT signalling
After the description of the canonical, β-catenin-dependent WNT signalling pathway, it became evident that certain WNT ligands exerted a wide range of biological effects in β-catenin-independent ways, namely the PCP and the Ca2+-dependent pathway33. These non-canonical pathways are not fully characterized; however, certain relevant downstream mediators and phenotypic consequences have been identified33 (Fig. 1).
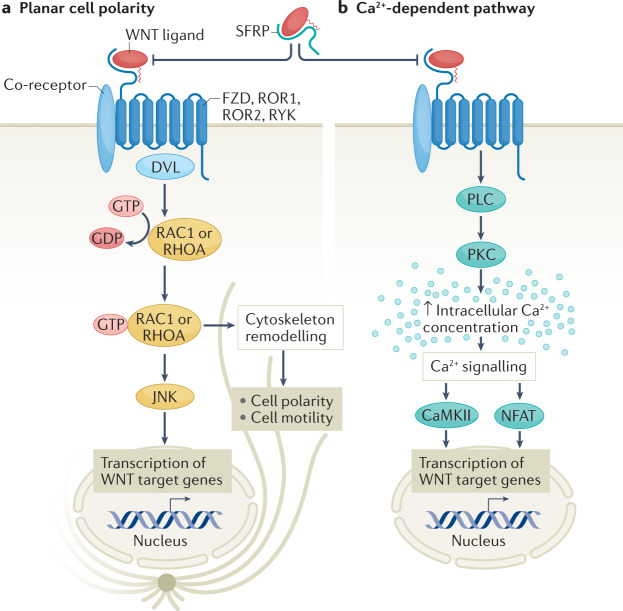
Fig. 1. Overview of non-canonical WNT signalling pathways.
Non-canonical WNT signalling pathways involve the planar cell polarity pathway (part a) and the Ca2+-dependent pathway (part b). Both pathways are initiated by binding of a WNT ligand to WNT receptors, such as Frizzled (FZD) receptors, the tyrosine-protein kinase transmembrane receptors ROR1 and ROR2, and tyrosine-protein kinase RYK, which belong to the family of G protein-coupled receptors (also known as seven-transmembrane receptors), with the potential contribution of various co-receptors. Binding of WNT ligands to secreted FZD-related proteins (SFRPs) blocks the WNT–receptor interaction. The downstream events involved in the planar cell polarity pathway are not well defined, but include Dishevelled (DVL) and lead to GTP-dependent activation of small GTPases, such as RAC1 and RHOA, which in turn activate JUN N-terminal kinase (JNK), and ultimately regulate cell polarity and motility and gene transcription. The Ca2+-dependent pathway involves activation of PLC and protein kinase C (PKC), which leads to increased intracellular Ca2+ concentration, triggering the activation of calcium/calmodulin-dependent protein kinase II (CaMKII) and the nuclear factor of activated T cells (NFAT) pathway, leading to transcriptional regulation.
The PCP pathway involves binding of a WNT ligand to FZD, ROR or RYK receptors33. This interaction leads to Dishevelled (DVL)-mediated GTP-dependent activation of small GTPases, such as RHOA and RAC1, although the exact intermediate events are not clear29. Activated RHOA and RAC1, in turn, stimulate the activation of JUN N-terminal kinase (JNK) via phosphorylation34. Given the involvement of RHOA and RAC1 in regulating cytoskeletal dynamics, changes in cell polarization and motility are the main phenotypes associated with activation of the PCP pathway33,35. In addition, WNT-mediated JNK activation has been linked with several other important pathophysiological processes such as inflammation and insulin resistance34,36. RAC1 can also influence oxidative stress via activation of NADPH oxidases15, indicating that oxidative stress can be regulated by the PCP pathway.
WNT ligands can also trigger β-catenin-independent effects via regulation of intracellular Ca2+ concentration33. Indeed, WNT signalling acts in synergy with phospholipase C to increase intracellular Ca2+ levels37, prompting activation of the Ca2+-sensitive kinases protein kinase C (PKC) and calcium/calmodulin-dependent protein kinase II (CaMKII)33. Ca2+-mediated WNT signalling activates the Ca2+-sensitive nuclear factor of activated T cells (NFAT) pathway33, which is important in immune response regulation38. Ca2+-dependent WNT signalling has mainly been explored in the context of embryonic development, and its clinical relevance in adults is less clear. However, considering the wide-ranging effects of its downstream targets PKC, CaMKII and NFAT, this mode of WNT signalling might contribute to several important biological processes35.
Importantly, non-canonical WNT signalling is negatively regulated by canonical WNT signalling. The extracellular domain of LDL receptor-related protein 6 (LRP6), a membrane co-receptor of the canonical WNT signalling pathway, has been shown to interact physically with WNT5A, acting as a decoy receptor for non-canonical WNT signals39. In vivo, Lrp6–/– mice have congenital defects, which are rescued by Wnt5a deletion39, suggesting that this LRP6-related phenotype is mediated by non-canonical WNT5A signalling. Similar defects were described in Xenopus embryos with knockdown of lrp5 or lrp6, which were reversed by knockdown of the non-canonical WNT ligands wnt5a and wnt11 (ref.39). WNT5A can activate or inhibit canonical WNT signalling in a spatiotemporal, tissue-specific manner40, which could be a result of spatially different receptor and related regulatory co-receptor profiles, similar to LRP5 and LRP6. Non-canonical WNT effects could therefore occur indirectly via changes in canonical WNT dynamics.
Non-canonical WNT signalling in vascular diseases
Non-canonical WNT signalling has been implicated in key contributory factors to atherosclerosis (Box 1), including oxidative stress, endothelial dysfunction, VSMC phenotypic switching and migration, and inflammation, as implied by observational association studies29 and mechanistic evidence15,23. Of note, WNT5A is the best-studied non-canonical WNT ligand in cardiovascular disease to date, followed by other ligands such as WNT11 (refs23,29). Therefore, most of the relevant studies used WNT5A as a representative ligand that primarily activates non-canonical WNT signalling in the cardiovascular system. Nevertheless, the downstream pathways described in these studies could be activated by any WNT ligand in the appropriate circumstances, and WNT5A itself can also activate canonical WNT signalling in vascular cells41,42.
Box 1 Fundamental mechanisms of atherosclerosis.
Atherosclerosis is a complex disease in terms of its underlying mechanisms. Disease initiation has long been thought to occur at endothelial sites that are subjected to dysregulated shear stress such as the coronary arteries and other sites prone to developing turbulent flow (such as vascular bifurcations)8. Pulsatile, turbulent shear stress results in endothelial dysfunction and disruption of endothelial barrier integrity177, resulting in the deposition of lipids, such LDL, in the subendothelial space8,178. LDL can then be oxidized as a result of various pro-oxidant stimuli such as local inflammation (secondary to local tissue damage)178. The oxidized LDL is internalized by macrophages, leading to macrophage activation and transformation into foam cells and further promoting inflammation and oxidative stress178. This vicious cycle establishes and promotes the formation and progression of atherosclerotic plaques.
Several pathogenic mechanisms are involved in promoting the atherogenic cycle, inducing further LDL oxidation, endothelial cell activation, macrophage recruitment and activation, and vascular smooth muscle cell phenotypic switch178,179, ultimately influencing atherosclerotic plaque characteristics and overall plaque burden180. Oxidative stress is one such mechanism, characterized by the overproduction of reactive oxygen species by enzymes such as NADPH oxidases and uncoupled endothelial nitric oxide synthase6. The overproduction of reactive oxygen species in turn leads to reduced nitric oxide bioavailability and endothelial dysfunction6. Local inflammation and cellular insulin resistance at the level of the vascular wall are additional mechanisms contributing to atherosclerotic plaque burden via modulation of vascular smooth muscle cell phenotype and promotion of lipid oxidation and plaque necrotic core remodelling5,159,180,181.
Observational data
One of the first pieces of conclusive evidence for a causal link between WNT signalling and atherosclerosis came from a study published in 2007 on the genetic basis of the risk of early coronary artery disease (CAD) in a family with a high prevalence of CAD43. Genome-wide linkage analysis followed by direct DNA sequencing of the annotated genes and in vitro functional assessments demonstrated a link between the missense mutation R611C in LRP6 and the early CAD phenotype43, thereby causally linking WNT signalling with vascular disease. Further research has explored the mechanisms underlying this link, particularly regarding non-canonical WNT signalling.
Observational data indicate that high levels of circulating WNT5A are associated with the presence of atherosclerosis and related diseases such as obesity and diabetes mellitus15,23,44. Work from our group has demonstrated that, in humans, CAD is associated with elevated WNT5A bioavailability in the plasma independently of demographic risk factors15. In addition, in patients who underwent two CT scans for coronary artery calcium score quantification, plasma WNT5A levels were positively associated with calcified plaque burden and new-onset calcification, independent of traditional risk factors15. Furthermore, WNT5A is locally expressed in mouse and human atherosclerotic plaques21. Levels of the non-canonical ligands WNT5A, WNT5B and WNT11 are also upregulated in human aortic calcified valves compared with non-calcified valves45. Given the link between these ligands and non-canonical activation of osteogenesis-related pathways in a variety of in vitro models46, non-canonical WNT might be hypothesized to contribute to valve or vascular wall calcification.
The aforementioned observational findings provide strong proof of concept for a link between non-canonical WNT signalling (mainly WNT5A) and atherosclerosis47 but observational results do not confirm causality. However, consistent with the observational findings, several experimental studies have revealed underlying cellular hubs through which non-canonical WNT signalling causally interacts with key mechanisms of atherosclerosis (Fig. 2).
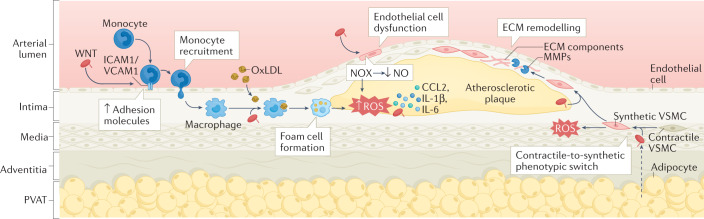
Fig. 2. Non-canonical WNT signalling in atherosclerosis.
Non-canonical WNT ligands, such as WNT5A, can enter the vascular wall from the circulation and are also released by vascular macrophages and the perivascular adipose tissue (PVAT). In endothelial cells, binding of non-canonical WNT ligands to their receptors leads to the upregulation of the expression of intracellular adhesion molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1), which promotes monocyte recruitment to the arterial intima. Non-canonical WNT signalling in endothelial cells also induces the activation of NADPH oxidases (NOX), which leads to increased production of reactive oxygen species (ROS), activation of pro-inflammatory redox signalling, reduced nitric oxide (NO) bioavailability and endothelial dysfunction. In monocytes and macrophages, non-canonical WNT signalling promotes pro-inflammatory activation, uptake of oxidized LDL (oxLDL) and foam cell formation, and production of pro-inflammatory cytokines such as C-C motif ligand 2 (CCL2), IL-1β and IL-6. In vascular smooth muscle cells (VSMCs), non-canonical WNT signalling induces a switch from a contractile to a synthetic phenotype and increases cell migration via ROS signalling, thereby promoting the migration of synthetic VSMCs into the atherosclerotic plaque, where they produce extracellular matrix (ECM) components and matrix metalloproteinases (MMPs). These mechanisms interact in complex ways to establish a vicious cycle, directly promoting atherogenesis and plaque instability.
Oxidative stress
WNT5A signalling increases the production of reactive oxygen species (ROS) in the human granulosa-like tumour cell line KGN, which was speculated to be related to the induction of inflammation considering that lipopolysaccharide stimulation had the same effect on ROS production in these cells36. Despite the incomplete mechanistic data, this observation supports the notion that WNT5A might directly increase ROS production. By contrast, WNT5A has been shown to protect against oxidative stress-induced VSMC apoptosis, although this effect was achieved with a supraphysiological concentration of WNT5A that cross-activated the canonical pathway42. Therefore, this finding should be regarded as being mediated by canonical WNT signalling rather than a specific WNT5A-mediated effect.
Work from our group was the first to directly explore the role of WNT5A in the regulation of vascular oxidative stress in the context of atherosclerosis15. After demonstrating the specificity of physiological WNT5A concentrations towards non-canonical signalling, we showed that WNT5A directly increased NADPH oxidase activity in vitro in arteries from patients with CAD15. This effect was mediated via increased GTP-dependent activation and membrane translocation of RAC1 (which is both a downstream target of the PCP pathway and a key subunit of the NADPH oxidase isoforms NOX1 and NOX2)15. This finding was further validated in vitro in WNT5A-treated primary VSMCs from patients with CAD as well as in aortic segments from mice overexpressing WNT5A15. Importantly, transcriptome analysis of WNT5A-treated human primary VSMCs revealed that WNT5A induces the differential expression of a large number of genes, and the expression profile of several of these genes was restored by administration of pegylated superoxide dismutase, suggesting that WNT5A is involved in redox-dependent transcriptional regulation in human vascular tissue15. Finally, we identified the ubiquitinating enzyme USP17 as a redox-sensitive downstream target of WNT5A that increases the GTP-dependent activation of RAC1 (ref.15). This study was the first to establish a causal role of WNT5A in increasing oxidative stress in human atherosclerosis and identifies USP17 and RAC1 as the key mediators of WNT5A-induced oxidative stress.
In contrast to our findings, other work suggests that WNT5A inhibits hydrogen peroxide (H2O2)-induced apoptosis in VSMCs via CCN family member 4 (also known as WISP1), an effect mediated through canonical WNT signalling42. This finding could suggest that WNT5A has an antioxidant function; however, WNT5A was used at a supraphysiological concentration in this study, which might mean that this canonical WNT signalling effect on H2O2-induced apoptosis might not be physiological. Conversely, H2O2 is a more stable type of ROS than superoxide and has subtler signalling roles42. As such, WNT5A-mediated signalling might theoretically stimulate the production of both superoxide (which could be detrimental when in excess) and H2O2 (which could provide a more prolonged signalling effect), hinting towards a delicate WNT5A-regulated redox balance, which warrants further investigation.
Given that the activation of endothelial nitric oxide synthase (eNOS) is partly mediated by CaMKII48 and that both PKC and CaMKII interact with NADPH oxidases49–51, it is highly plausible that non-canonical WNT Ca2+ signalling also regulates vascular oxidative stress and thereby contributes to atherosclerosis. However, this hypothesis remains to be studied.
Endothelial dysfunction
Although canonical WNT signalling has been linked to endothelial cell survival and angiogenesis52, the role of non-canonical WNT signalling in endothelial cells has been explored only during the past decade. A study in primary endothelial cells from patients with diabetes revealed that WNT5A signalling impairs AKT phosphorylation, eNOS activity, nitric oxide (NO) bioavailability and, ultimately, endothelial function in vitro, mediated by non-canonical JNK-mediated signalling53. Consistent with this finding, our group demonstrated that WNT5A-mediated signalling directly impairs NO bioavailability and endothelial function, evidenced by ex vivo endothelium-dependent vasorelaxation of WNT5A-incubated artery samples from patients with CAD15. This reduced NO bioavailability was secondary to NADPH oxidase activation by WNT5A, as explained in previous sections, which led to oxidative depletion of tetrahydrobiopterin15. The absence of tetrahydrobiopterin resulted in eNOS uncoupling and production of superoxide instead of NO15.
These findings provide proof of the causal role of WNT5A in propagating a dysfunctional endothelial phenotype in humans. However, the underlying mechanisms for this role have not been fully explored. In addition, as mentioned in the previous section, Ca2+-dependent WNT signalling potentially affects both eNOS coupling and activity via CaMKII and PKC48,54–56 and could, therefore, be hypothesized to contribute to endothelial dysfunction, which warrants further investigation.
VSMC function and phenotype
Cell motility, migration and polarity are the best-studied phenotypes regulated by non-canonical WNT signalling, which has been demonstrated in a variety of embryonic development models and studies of tumours or cell types such as erythrocytes23,57–59. Canonical WNT signalling has been linked to VSMC biology, particularly cell migration42,60,61. However, evidence also suggests roles for non-canonical signalling in VSMCs.
After the demonstration of a link between the LPR6 R611C variant and CAD, further research has shed light on the underlying mechanisms with the use of VSMC in vitro assays and transgenic mouse models62. Indeed, loss of normal LRP6 activity as a result of the R611C variant is associated with increased non-canonical WNT signalling and is evidenced by increased RHOA and JNK activity62. The activation of non-canonical WNT signalling resulted in the activation of serine/threonine-protein kinase NLK followed by phosphorylation and subsequent ubiquitylation and degradation of transcription factor 7-like 2, a transcription factor that is associated with canonical WNT signalling62. These signalling events were associated with a phenotypic switch in VSMCs from a contractile to a synthetic phenotype, arterial media thickening and CAD promotion in mice62. Exogenous treatment with the canonical ligand WNT3A reversed the effects of the LRP6-R611C variant62. This finding is an elegant example of the crosstalk between the canonical and non-canonical pathways, with reciprocal effects on VSMC phenotypes.
Our group has shown that WNT5A induces redox-sensitive migration of human primary VSMCs without affecting their proliferation15. This effect could be partly mediated by RAC1 activation, but transcriptome analysis showed that WNT5A-mediated signalling also affected the expression of multiple genes related to migration in primary human VSMCs15. In addition, WNT5A can inhibit oxidative stress-induced apoptosis of VSMCs via the induction of WISP1 (ref.42). However, this effect was shown to be mediated by β-catenin signalling42 and might not be relevant in vivo given that WNT5A, at physiological concentrations, is a selective ligand for non-canonical WNT signalling. Another non-canonical WNT ligand, WNT4, has been shown to stimulate VSMC proliferation, whereas its downregulation attenuates intima–media thickening in mice63.
Beyond its effects on VSMC migration, non-canonical WNT signalling has been shown to induce a phenotypic switch in human primary VSMCs characterized by the loss of expression of the contractile markers aortic smooth muscle actin (also known as α2-SMA) and transgrelin, and an increased expression of matrix metalloproteinase 9 (MMP9)15. MMP9 has been shown to induce destabilization of atherosclerotic plaques in mouse models of atherosclerosis and in human genetics studies64–67. Taken together, these findings suggest that non-canonical WNT signalling might promote an ‘aggressive’ phenotype in VSMCs, characterized by an increased propensity for migration, the switch to a less differentiated phenotype and the upregulation of potentially plaque-destabilizing MMPs. WNT5A has been reported to be among the WNT ligands expressed by VSMCs isolated from artery samples from patients undergoing coronary artery bypass graft surgery, which might be related to neointima formation68.
Non-canonical WNT signalling can also influence atherosclerotic plaque calcification by inducing changes in VSMC biology. WNT5A was found to stimulate the expression of genes related to chondrogenesis in both mouse and human VSMCs69. WNT5A-mediated gene expression was inhibited by peroxisome proliferator-activated receptor-γ (PPARγ) signalling via SFRP2, suggesting that WNT5A promotes vascular calcification69. In addition, a correlation has been observed between the WNT5A–ROR2 pathway and the degree of VSMC calcification in vitro70. Canonical WNT signalling has also been shown to regulate vascular calcification in mouse aorta via bone morphogenetic protein 2 signalling46. Interestingly, co-expression of the non-canonical WNT receptor FZD10 and the canonical co-receptors LRP5 or LRP6 in VSMCs leads to cross-inhibitory signals between the two pathways71. By contrast, LPR5 or LRP6 loss of function increased non-canonical WNT signalling and inhibited canonical β-catenin signalling, which was associated with a shift towards osteochondrogenic programming and calcification in VSMCs71. These findings suggest a competition between the canonical and non-canonical pathways with regard to VSMC calcification, with LRP5 and LRP6 at the core.
Non-canonical WNT signalling can affect intracellular cholesterol accumulation. In an Apoe–/– mouse model of atherosclerosis, in vivo adenovirus-mediated delivery to aortic tissues of small interfering RNA targeting Wnt5a reduced atherosclerotic plaque lipid content without affecting blood lipid levels36. WNT5A has also been suggested to facilitate foam cell formation23. By contrast, another in vitro study indicated that WNT5A reduces intracellular cholesterol in VSMCs treated with oxidized LDL mediated by stimulating the expression of ATP-binding cassette transporter 1 (ABCA1)72, which is involved in reverse cholesterol transport. Overall, non-canonical WNT signalling seems to be involved in lipid handling in atherosclerotic plaque cells, but the exact effects and the mechanisms involved are unclear.
Non-canonical WNT signalling might also affect neoangiogenesis. In a rat model of ischaemic myocardial injury, peri-infarct injection of conditioned medium from WNT11-overexpressing mesenchymal stem cells improved cardiac function and reduced infarct size, which was shown to involve non-canonical JNK–PKC signalling73. By contrast, mutations in Wnt5a or Wnt11 in myeloid cells were associated with increased angiogenesis in mouse retinas mediated by downstream suppression of Flt1 expression74, which encodes an inhibitor of vascular endothelial growth factor (VEGF). These findings suggest potentially complex roles for non-canonical WNT signalling in the regulation of angiogenesis.
Inflammation
Extensive evidence links non-canonical WNT signalling to inflammation and inflammatory conditions such as rheumatoid arthritis23,75, psoriasis75–77 and atherosclerosis23. Indeed, non-canonical WNT signalling influences key mechanisms of vascular inflammation via multiple effects on vascular wall cells, thereby contributing to atherosclerosis23.
WNT5A (but not WNT3A, a canonical WNT ligand) was shown to increase the expression of genes encoding pro-inflammatory factors, including cyclooxygenase 2, IL-6, IL-1α, Toll-like receptor 4, granulocyte colony-stimulating factor, granulocyte–macrophage colony-stimulating factor (GM-CSF), CC-chemokine ligand 2 (CCL2) and CCL8, in human aortic endothelial cells in vitro via non-canonical Ca2+–PKC signalling22. These expression changes were associated with increased permeability of the endothelial cell monolayer22. WNT5A also activates pro-inflammatory nuclear factor-κB (NF-κB) signalling in endothelial cells22,36. Interestingly, activation of the Ca2+–NFAT pathway, a downstream target of non-canonical WNT signalling, has been shown to induce a pro-inflammatory phenotype in human coronary artery endothelial cells in vitro that is characterized by an increased expression of pro-inflammatory molecules78. However, a direct link between non-canonical WNT signalling and NFAT signalling in endothelial cells has not yet been documented. By contrast, cardiac-specific overexpression of WNT11 in mice attenuated the inflammatory response to myocardial infarction and facilitated recovery after myocardial infarction in vivo79. This finding suggests an anti-inflammatory role for non-canonical WNT signalling in this context.
WNT5A can be secreted by circulating monocytes59 and is involved in innate responses in monocytes and macrophages as evidenced by the upregulation of WNT5A gene expression in pathogen-activated macrophages and during monocyte differentiation into macrophages in response to GM-CSF and IL-4 treatment in vitro80–82. Importantly, pro-inflammatory and pro-oxidant signals, such as oxidized LDL, which are present in atherosclerotic plaques, upregulate the production of macrophage-derived WNT5A83. WNT5A in turn stimulates the secretion of pro-inflammatory cytokines (such as IL-1β, IL-6 and IL-8) from macrophages via Ca2+–CaMKII signalling and facilitates foam cell formation21,84,85. Evidence suggests that WNT5A promotes transforming growth factor-β (TGFβ)-mediated macrophage polarization, as demonstrated in the context of kidney fibrosis86. Extensive research in oncology suggests that WNT5A has a wide range of effects on macrophage activation such as in establishing an NF-κB autocrine loop87,88. However, these WNT5A effects have not been fully investigated in atherosclerosis.
Several observational and mechanistic studies in in vivo models of atherosclerosis have further supported a role for non-canonical WNT signalling in promoting a vascular pro-inflammatory phenotype. In an Apoe–/– mouse model of atherosclerosis, Wnt5a knockdown inhibited lipid accumulation and inflammation in atherosclerotic plaques, which was suggested to involve ROR2 non-canonical WNT signalling and downstream nuclear translocation of NF-κB89. Furthermore, circulating WNT5A levels are increased in a variety of inflammatory processes, such as sepsis, as shown in experimental models, further supporting a connection between non-canonical WNT signalling and inflammation23,75. Despite the available strong evidence, further research is required to elucidate the full spectrum of the effects of non-canonical WNT ligands on cytokine production, inflammatory cell recruitment and activation, and overall lipid-driven and cytokine-driven vascular inflammatory responses.
Cellular insulin resistance
Observational data show that increased bioavailability of WNT5A in the circulation and high WNT5A expression in adipose tissue are both associated with systemic insulin resistance (defined as glucose intolerance) as first demonstrated by a team led by Walsh, who were pioneers in the study of the metabolic and cardiovascular implications of non-canonical WNT signalling44,90–92. This association could be indirectly linked to atherosclerosis via the detrimental effects of hyperglycaemia on vascular function6. However, evidence suggests that WNT5A is associated with molecular insulin resistance in the vasculature, defined as abnormal vascular insulin signalling34,53. Indeed, ex vivo insulin-mediated vasorelaxation of arterioles from visceral adipose tissue isolated from individuals with obesity was impaired compared with that of arterioles from subcutaneous adipose tissue from the same individuals34. JNK activation and Wnt5a expression was increased in visceral adipose tissue compared with subcutaneous fat34. Furthermore, in endothelial cells isolated from adipose tissue, treatment with recombinant WNT5A stimulated JNK activation and induced insulin resistance, demonstrated by a reduction in downstream AKT and eNOS phosphorylation34. Consistently, the capacity of WNT5A to abolish insulin-induced AKT and eNOS phosphorylation and NO production has been replicated in studies using primary endothelial cells from patients with diabetes53.
Given the well-described link between JNK activity and molecular insulin resistance93, the link between non-canonical WNT signalling, particularly the PCP pathway, and induction of vascular insulin resistance is not surprising. Inflammatory factors, such as NF-κB and tumour necrosis factor, which are induced by non-canonical WNT signalling, can also interfere with molecular insulin signalling94–97, providing indirect links between non-canonical WNT signalling and vascular insulin resistance. Finally, Ca2+–PKC non-canonical WNT signalling might also contribute to vascular insulin resistance given that certain PKC isoforms directly induce cellular insulin resistance6.
Adipose tissue secretome
Adipose tissue is a dynamic organ that interacts with the vascular wall in paracrine and endocrine manners via the secretion of biologically active molecules5,98,99. Metabolic diseases, such as visceral obesity and diabetes, are associated with a pro-inflammatory phenotype of visceral adipose tissue, which secretes molecules that can directly reach the vascular wall via the bloodstream and exert biological effects100,101. Epicardial adipose tissue is a multifaceted, dynamic marker of cardiometabolic disease that is affected by pleiotropic pharmacological therapies and influences the heart in multiple paracrine ways98,102–105. Perivascular adipose tissue (PVAT) can exert paracrine effects on the vasculature owing to its close proximity to the vascular wall and can also act as a receiver of biological signals from the vascular wall, thereby establishing bidirectional crosstalk with the vasculature106. PVAT senses signals of adjacent coronary inflammation, changing its phenotype and lipid content107. This phenomenon can be captured by an imaging biomarker of coronary inflammation derived from coronary CT angiography, which has been standardized and is used in clinical practice107–110.
Our group has shown that WNT5A is the predominant WNT ligand expressed by PVAT surrounding internal mammary arteries in patients undergoing coronary artery bypass graft surgery15. WNT5A expression in PVAT is upregulated in the context of obesity and is independently associated with the activity of NADPH oxidases in the underlying vessels15. Furthermore, human primary VSMCs co-cultured with human adipocytes with WNT5A knockdown produced less NADPH oxidase-derived superoxide than VSMCs co-cultured with control adipocytes15. This finding confirms the concept that adipocyte-derived WNT5A might have paracrine pro-oxidant effects on vascular cells in humans. These findings are in agreement with a study reporting elevated levels of WNT5A and reduced levels of SFRP5 in the plasma of patients with peripheral occlusive arterial disease than in the plasma of healthy individuals111. Therefore, non-canonical WNT signalling, based on findings from the paradigm ligand WNT5A, is a novel paracrine link between obesity and vascular disease pathogenesis (Fig. 3).
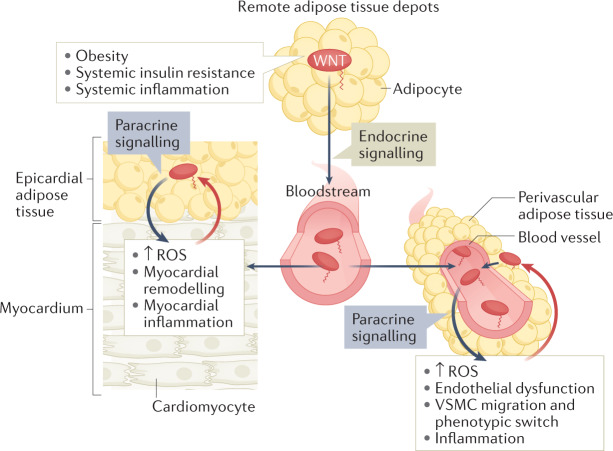
Fig. 3. Non-canonical WNT signalling and bidirectional interactions between adipose tissue depots and the cardiovascular system.
Obesity, systemic insulin resistance and systemic inflammation induce systemic upregulation of non-canonical WNT ligands, partly via upregulation of WNT secretion from adipose tissue depots. WNT ligands can reach the heart and blood vessels via the systemic circulation and from adjacent adipose tissue depots such as epicardial adipose tissue and perivascular adipose tissue, respectively. Regardless of the source, WNT ligands exert multiple effects on the cardiovascular system, including the induction of reactive oxygen species (ROS), myocardial remodelling, endothelial dysfunction, vascular smooth muscle cell (VSMC) migration and phenotypic switch, and inflammation. These changes in turn stimulate the release of signals that influence WNT expression (red arrows) in epicardial adipose tissue and perivascular adipose tissue, thereby establishing potential paracrine interaction loops between the cardiovascular system and adipose tissues.
Obesity and diabetes mellitus
Non-canonical WNT signalling has multiple broad roles related to the pathogenesis of metabolic diseases such as obesity and diabetes. These roles include multifaceted effects on adipose tissue, liver and pancreas, ranging from the regulation of energy storage and handling to adipogenesis and apoptosis91,112,113. Work by Fuster, Walsh and colleagues strongly suggests a causal association between obesity and increased bioavailability of WNT5A in the circulation and visceral adipose tissue in animal models and humans15,90,91,114. Furthermore, WNT5A overexpression in mouse myeloid cells augmented adipose tissue inflammation in vitro, and WNT5A directly induced JNK signalling and molecular insulin resistance in adipocytes of visceral adipose tissue in obese mice91. All these effects might influence obesity-related adipose tissue function and, indirectly, cardiovascular biology via regulation of the adipose tissue secretome.
Non-canonical WNT signalling influences adipose tissue biology in the context of obesity and diabetes in terms of adipose tissue volume, distribution, and secretome and, therefore, also influences the interactions between adipose tissue and the cardiovascular system. Non-canonical WNT signalling, mediated by WNT10B and WNT5A, is believed to have an anti-adipogenic effect via FZD and LRP receptors leading to reduced adipocyte size and also regulates the adipose tissue secretome and overall insulin sensitivity115. Non-canonical WNT signalling also induces adipose tissue inflammation independently of adipose tissue expansion91. In summary, non-canonical WNT signalling can contribute to the formation of small adipocytes, with low lipid content and increased inflammation, which are all hallmarks of visceral adipose tissue. We must note that WNT ligands create a complicated, cross-interacting canvas in adipose tissue, which makes deciphering the integrated effects extremely challenging and prone to inaccuracies115.
Non-canonical WNT signalling in cardiac diseases
Cardiac diseases, such as heart failure and arrhythmias, are caused by various pathogenic mechanisms such as arrhythmogenesis116, dysregulated cardiac biomechanics117, structural remodelling118, abnormal energetics119,120 and oxidative stress121. Observational evidence suggests that non-canonical WNT signalling is linked to cardiac diseases. For example, circulating levels of WNT5A were elevated in patients with heart failure compared with individuals without heart failure and were associated with haemodynamic markers of heart failure such as ejection fraction and filling pressures122. In a cohort of patients with dilated cardiomyopathy, higher plasma WNT5A levels were associated with increased right ventricular filling pressures and decreased right ventricular ejection fraction, and WNT5A expression was elevated in the right ventricle compared with the left ventricle123. Importantly, several experimental studies suggest that non-canonical WNT signalling is a causal regulator of cardiac disease (Fig. 4).
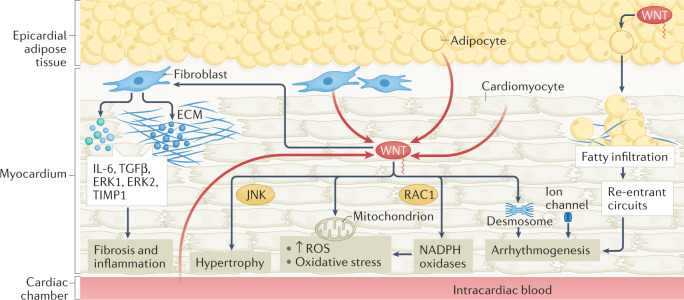
Fig. 4. Non-canonical WNT signalling in myocardial disease.
Summary of established and putative mechanisms linking non-canonical WNT signalling and myocardial disease. In the myocardium, sources of non-canonical WNT ligands, such as WNT5A, include cardiomyocytes, the microcirculation, blood in the cardiac cavities and the adjacent epicardial adipose tissue (red arrows). Non-canonical WNT signalling can stimulate cardiac fibroblasts, inducing the upregulation of expression of IL-6, transforming growth factor-β (TGFβ), ERK1 and ERK2, and tissue inhibitor of metalloproteinase 1 (TIMP1), thereby potentially promoting fibrosis and inflammation. Non-canonical WNT signalling can induce cardiac hypertrophy via JUN N-terminal kinase (JNK) activation. Non-canonical WNT signalling can promote oxidative stress through activation of NADPH oxidases mediated by the small GTPase RAC1. WNT might be linked to mitochondrial biology, regulating mitochondrial aggregation and the production of mitochondrial reactive oxygen species (ROS), although the direction of this interaction is unclear. WNT can also regulate desmosome and ion channel function, potentially promoting arrhythmogenesis. WNT secreted by epicardial adipose tissue can cause adipose tissue expansion and myocardial fatty infiltration, facilitating the development of re-entrant circuits and arrhythmias such as atrial fibrillation. ECM, extracellular matrix.
Arrhythmogenesis
Arrhythmias are caused by abnormal electrical impulse generation or conduction116,124. Abnormal impulses arise from increased automaticity, early afterdepolarizations causing slow action potential repolarization, or late diastolic depolarizations caused by sarcoplasmic reticulum Ca2+ leakage124. Abnormal conduction includes accessory pathways and re-entry circuits (often caused by a structural substrate such as regional fibrosis)124. Triggers include myocardial ischaemia, electrolyte disorders, medications and genetic channelopathies116.
A large number of experimental studies have linked canonical WNT signalling to arrhythmogenic conditions such as arrhythmogenic cardiomyopathy, which is not surprising given the crucial role of β-catenin in the regulation of desmosomal intercellular junctions125. Furthermore, canonical WNT signalling regulates myocardial fibrosis, as shown in mouse models, thereby interfering with the formation of re-entry substrates such as in atrial fibrillation126,127. Both canonical β-catenin WNT signalling and non-canonical RHO-mediated WNT signalling have been associated with the pathophysiology of arrhythmogenic right ventricular cardiomyopathy (ARVC) in in silico models, which showed that the inactivation of the aforementioned pathways is linked with increased PPARγ expression and ARVC pathogenesis128.
By contrast, the mechanistic role of non-canonical WNT signalling in human arrhythmogenesis has not been adequately explored. In a study in patients with rheumatic valve disease undergoing valve surgery, the presence of atrial fibrillation was associated with elevated expression of the transcription factor SNAIL1 and several WNT ligands, including the non-canonical WNT ligands WNT5A and WNT11, in the right atrium129. WNT5A has been associated with the upregulation of SNAIL1 protein levels in melanoma cells via non-canonical PKC activation, which was associated with epithelial-to-mesenchymal transition (EMT) and metastasis potential130. Furthermore, WNT5A has been linked to TGFβ-dependent fibrosis and EMT in a variety of organs such as the liver126,131. Atrial fibrillation is tightly linked to processes such as EMT and fibrosis132. Therefore, these findings might imply a causal role for non-canonical WNT signalling in arrhythmogenesis by facilitating the development of re-entrant circuits.
Cardiac remodelling
Cardiac remodelling involves structural changes caused by myocardial wall stress (as a result of, for example, hypertension or valvular disease) and inflammation (such as after ischaemic myocardial injury)118. At the cellular level, remodelling is caused by cardiomyocyte hypertrophy and collagen deposition in the extracellular matrix caused by pro-inflammatory and redox signalling and metabolic signals118,121,133. These changes can induce arrhythmogenic substrates or impair contractile efficiency120.
Non-canonical WNT signalling might contribute to cardiac fibrosis. For example, WNT5A was shown to stimulate human primary cardiac fibroblasts in vitro, inducing the production of IL-6 and tissue inhibitor of metalloproteinase 1 (TIMP1) and the activation of ERK1/ERK2 signalling122. This finding suggests that WNT5A can potentially promote inflammation and fibrosis in vivo. WNT5A also induces a reduction in glycogen synthase kinase 3β (GSK3β) levels in human cardiac fibroblasts in vitro, which promoted fibrosis partly via transactivation of TGFβ, although this effect seemed to be predominantly mediated by canonical WNT signalling134. In human ventricular cardiomyocytes, activation of non-canonical, Ca2+-dependent WNT signalling stimulates the activation of the NFAT–calcineurin pathway123, which has been shown to contribute to cardiac fibrosis in experimental models.
Non-canonical WNT signalling has been shown to stimulate cardiomyocyte hypertrophy126. More specifically, WNT5A activated PCP signalling mediated by Dapper 1 and led to downstream activation of JNK to promote hypertrophy in human cardiomyocytes in vitro, as assessed by microscopy and cardiomyocyte surface area135. Interestingly, in a mouse model of left ventricular hypertrophy induced by aortic constriction, both neutrophil depletion and myeloid-specific knockdown of Wnt5a reduced neutrophil infiltration in the myocardium and cardiac hypertrophy136. This finding suggests that WNT5A might also regulate cardiac hypertrophy in indirect ways involving neutrophils and local inflammatory responses in the heart.
Fatty infiltration of the myocardium is found in a spectrum of myocardial disease phenotypes ranging from arrhythmogenesis to contractile dysfunction137. Although suppression of canonical WNT signalling has been linked to fatty infiltration of the myocardium, for example, in animal models of ARVC138, limited evidence exists on non-canonical WNT signalling and fatty remodelling of the myocardium. Non-canonical WNT signalling has been implicated in fatty infiltration in other organs139,140 such as in a mouse model of non-alcoholic fatty liver disease, which was rescued by canonical WNT3A signalling141.
Myocardial energetics
Under physiological conditions, fatty acid oxidation is the major source of ATP in the heart, followed by glycolysis and lactate metabolism120. Different myocardial diseases are associated with different metabolic profiles but most eventually lead to ATP depletion, reducing contractile efficiency and altering a wide range of downstream signalling events142. Several types of myocardial dysfunction are characterized by a shift towards glycolysis and ketone body oxidation to meet the increasing energy demands of the failing heart under stress143. Medications such as sodium–glucose cotransporter 2 inhibitors might exert some of their beneficial effects by regulating ketone body metabolism, glycolysis substrate inflow and fatty acid oxidation144, which highlights the concept that metabolic pathway balance and fuel shifts might be crucial for modifying myocardial performance143.
Evidence suggests that WNT signalling mediates metabolic pathway reprogramming in a variety of cell types although this action mainly involves canonical signalling145,146. Non-canonical WNT signalling attenuates mitochondrial aggregation in the HEK93 cell line147 and protects mitochondria from fission–fusion alterations and prevents mitochondrial loss in neurons148, both via PKC and regulation of intracellular Ca2+ dynamics. Overexpression of the non-canonical WNT ligand WNT11 preserves mitochondrial membrane potential and protects cardiomyocytes against hypoxia in vitro via mechanisms involving insulin-like growth factor 1 and VEGF149. Non-canonical WNT signalling also accelerates glucose oxidative metabolism in the liver, which leads to steatosis via de novo lipogenesis150. Although these findings provide putative links to myocardial metabolism, the role of non-canonical WNT signalling in myocardial energetics has not been directly explored.
Oxidative stress
Oxidative stress has a key pathophysiological role in myocardial diseases such as in ischaemia–reperfusion injury after myocardial infarction151 and through redox signalling in heart failure and arrhythmia152. Oxidative stress results from ischaemia–reperfusion injury153, inflammation-mediated stimulation of NADPH oxidases121,154 and disturbed mitochondrial energetics120,155. Myocardial ROS, in turn, can induce hypertrophy, apoptosis, autophagy, metabolic enzyme dysregulation and impaired contractility, thereby drastically contributing to myocardial disease11,121,153,155.
Ischaemia–reperfusion injury is characterized by sudden oxygen abundance in a stunned myocardium, in which free radicals are produced because of an imbalance between pro-oxidant and antioxidant enzymes151,153. The excess free radicals induces cardiomyocyte death via multiple intracellular pathways, including proteolysis, caspase activation and mitochondrial regulation151,153. Co-expression of AKT1 and the non-canonical ligand WNT11 stimulates the proliferation and differentiation of mesenchymal stem cells into cardiomyocytes and attenuates hypoxia–reperfusion injury156. By contrast, a study in mice showed that blockade of non-canonical WNT signalling attenuates myocardial ischaemia–reperfusion injury157. NADPH oxidases have been implicated in atrial fibrillation and heart failure via a multitude of redox-sensitive intracellular transcription pathways154,158. Non-canonical WNT signalling is linked to NADPH oxidases via RAC1, as shown in VSMCs in vitro15. Whether these findings are relevant to the human myocardium remains to be proven.
Targeting non-canonical WNT signalling
The studies discussed above indicate a strong mechanistic link between non-canonical WNT signalling and cardiovascular disease. Therefore, successful targeting of this pathway could have multiple beneficial effects in patients, especially in the context of obesity, in which the bioavailability of the principal non-canonical WNT ligand, WNT5A, is increased15,23. Interestingly, the downstream mediators of non-canonical WNT signalling (PLC–CaMKII, RAC1, RHOA and JNK) are convergence points for multiple non-WNT pathways such as the catecholamine and renin–angiotensin–aldosterone pathways159–161. The exact contribution of WNT ligands to cardiovascular pathophysiology in this context is unknown. However, targeting WNT signalling might offer an advantage over targeting downstream molecules, which would also influence the outputs of multiple other, non-WNT pathways.
Non-canonical WNT signalling is a conserved pathway affecting virtually all cell types and governing fundamental biological processes18,162. Consequently, its targeting is prone to non-specific, off-target adverse effects (with tumorigenesis being the most important) and is challenging when embryonic development is relevant (for example, in pregnant women)18,162. The interconnected network of WNT ligands and receptors further complicates targeting a single ligand18,162.
So far, early attempts have mainly focused on targeting the canonical WNT signalling pathway, especially in the context of cancer18,162,163. By contrast, targeting of non-canonical WNT signalling has not been explored. Advances in biotechnology, gene editing and drug delivery, coupled with a better understanding of the downstream mediators of non-canonical WNT signalling, might help towards this end.
Targeting strategies
Targeting non-canonical WNT signalling might be theoretically achieved with the use of chemical inhibitors of, for example, SFRPs or RAC1 (refs15,18). The use of monoclonal antibodies, antisense oligonucleotides (ASOs) and gene-editing methods (such as CRISPR–Cas9) can allow more efficient targeting of non-canonical WNT signalling than previously thought164–167. More efficient drug delivery to target tissues might also be achieved with the use of nanoparticles164,168,169. The main challenge of using monoclonal antibodies is their systemic, whole-body delivery, posing the risk of substantial adverse effects resulting from systemic WNT inhibition in tissues outside of the cardiovascular system. This issue might be bypassed by using tissue-specific targeting of WNT elements with tools such as ASOs and CRISPR–Cas9 editing. However, these strategies are not clinically feasible at present and require extensive validation and optimization. Nanoparticle technology might be used to guide drugs and vectors to sites of interest via systemic or local administration of nanoparticles with affinity to particular molecules such as endothelial markers (for example, vascular cell adhesion molecules). This approach, in combination with molecular techniques (CRISPR–Cas9 or ASOs), could increase local efficacy and tissue specificity of WNT targeting, as demonstrated with the use of macrophage-specific, promoter-driven plasmids contained in lipid nanoparticles to target inflammatory cells such as macrophages170. The characteristics of these strategies are summarized in Fig. 5.
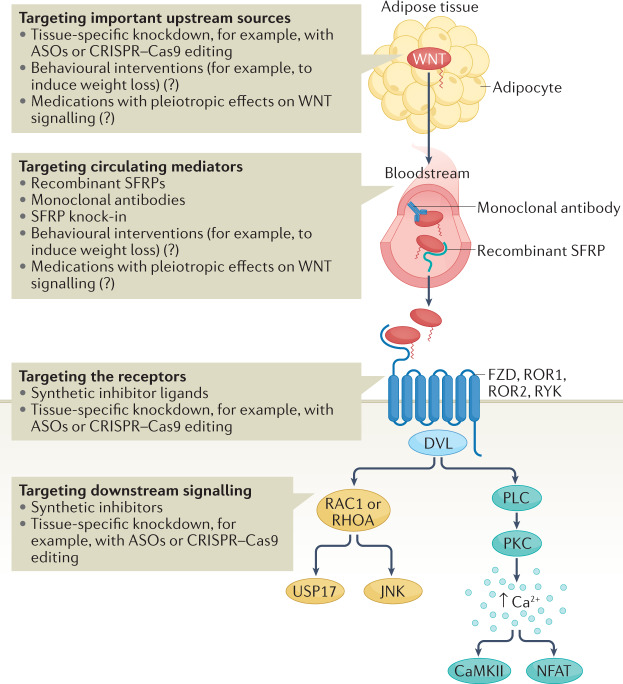
Fig. 5. Potential strategies for therapeutic targeting of non-canonical WNT signalling.
Modulating WNT effects can be achieved by targeting the main sources of WNT ligands, such as adipose tissue, with the use of tissue-specific knockdown technologies, for example, antisense oligonucleotides (ASOs) and CRISPR–Cas9 methods. However, these approaches require considerable research before their eventual translation into the clinic. The balance between different circulating WNT ligands can be targeted by using recombinant decoy receptors of WNT ligands (such as secreted frizzled-related proteins (SFRPs)) or monoclonal antibodies against WNT ligands. Overall, targeting of WNT signalling systemically might be associated with off-target adverse effects. WNT signalling can also be targeted by blocking or decreasing WNT receptors, with the added theoretical benefit of tissue specificity when using knockdown methods. Finally, downstream signalling could be targeted by developing chemical inhibitors or knockdown technologies. Targeting individual downstream molecules instead of the WNT ligands would provide a theoretical benefit of more specifically targeting individual subphenotypes caused by WNT signalling activation. Given that obesity is associated with increased WNT bioavailability, behavioural interventions, such as interventions to induce weight loss, and the use of medications with known metabolic effects might have pleiotropic effects on WNT signalling although the effect of these approaches on WNT signalling is unclear. CaMKII, Calcium/calmodulin-dependent protein kinase II; DVL, Dishevelled; FZD, Frizzled; JNK, JUN N-terminal kinase; NFAT, nuclear factor of activated T cells; PKC, protein kinase C; ROR, tyrosine-protein kinase transmembrane receptor ROR; RYK, tyrosine-protein kinase RYK.
Potential targets
Non-canonical WNT signalling comprises a complex network of receptors and downstream molecules. Therefore, one could, in theory, interfere with non-canonical WNT signalling at different levels by using the techniques mentioned in the previous section. Importantly, the canonical and non-canonical pathways cross-regulate each other; therefore, any intervention on non-canonical WNT signalling is expected to also have effects on canonical WNT signalling162,171.
WNT ligands and SFRP inhibitors
Targeting non-canonical WNT ligands would be the most obvious way to inhibit all downstream signalling. However, simultaneous targeting of all non-canonical WNT ligands would not be feasible. WNT5A is a paradigm non-canonical WNT ligand, having been the focus of most research on non-canonical WNT signalling; therefore, WNT5A would seem to be an appropriate therapeutic target in this context15,63. Systemic WNT5A targeting (for example, with monoclonal antibodies or exogenously administered recombinant forms of SFRPs) might be the most obvious way forward, but this approach would pose the aforementioned risks of global inhibition. Considering the pathophysiological mechanisms evaluated in the previous sections, tissue-specific (for example, adipose tissue-specific) targeting would be more efficient, although still not feasible at present.
WNT receptors
Downregulation of WNT receptors, such as FZD2 and FZD5, could markedly attenuate non-canonical WNT signalling. This strategy could be important given that FZD2 and FZD5 have been shown to be upregulated in internal mammary arteries from individuals with obesity, suggesting a higher sensitivity to WNT ligands15. Downregulation of WNT receptors could be achieved by ASO-mediated silencing or CRISPR–Cas9 gene editing, in which the use of tissue-specific promoters would be ideal. However, as mentioned, these strategies remain hypothetical at present.
Downstream signal transduction molecules
Non-canonical WNT signalling converges on a number of downstream molecules, including RAC1, JNK, CaMKII and PKC as well as USP17, a newly described WNT5A target15,52. Targeting of these downstream targets might reverse the detrimental effects of non-canonical WNT signalling. However, many non-WNT pathways also converge on these downstream molecules, which would make their targeting prone to non-specific effects.
Studies in mouse models of RAC1 depletion have reported beneficial effects, such as reduced endoplasmic reticulum stress and reduced cardiac oxidative stress172. In addition, several allosteric inhibitors of RAC1 have been developed. Several JNK inhibitors have been used in animal models of diseases such as neurodegeneration173 and in a phase Ib clinical trial in patients with pulmonary fibrosis174, supporting the clinical relevance of targeting JNK. Similarly, CaMKII inhibition in vivo is associated with decreased atherosclerotic plaque burden in Apoe–/– mice175. Administration of the CaMKII inhibitor KN93 in a mouse model of heart failure induced by pressure overload had beneficial effects176. PKC inhibition in vivo is more challenging given the large number of PKC isoforms6. Finally, USP17 is a newly identified link between WNT signalling, RAC1 activation and downstream redox signalling, which might warrant further investigation as a potential therapeutic target15.
Conclusions
Non-canonical WNT signalling, acting via receptor-mediated pathways and through activation of second messengers, such as RAC1, JNK, Ca2+-mediated CaMKII and PKC, is causally linked to vascular and myocardial disease in animal and human experimental models. In particular, non-canonical WNT signalling induces vascular oxidative stress via NADPH oxidase activation, promotes endothelial dysfunction and insulin resistance via JNK signalling and oxidative eNOS uncoupling, increases vascular inflammation in endothelial cells and macrophages, and triggers a VSMC contractile-to-synthetic phenotypic switch that might promote atherosclerotic plaque instability. Importantly, non-canonical WNT signalling is upregulated in adipose tissue (including PVAT) in obesity, exerting paracrine and endocrine vascular effects. Non-canonical WNT signalling has putative links to a wide spectrum of cardiac disease phenotypes via regulation of myocardial metabolism, fibrosis, and adipogenesis pathways and of redox signalling mediators such as NADPH oxidases and NF-κΒ, potentially contributing to myocardial remodelling, arrhythmogenic substrate formation and contractile dysfunction.
Non-canonical WNT signalling is therefore a multifaceted causal mediator of atherosclerosis and a link between obesity and vascular disease. Advances in biotechnology have opened up new potential approaches to modulate non-canonical WNT signalling through targeting specific tissues, proteins or genes related to WNT ligands, receptors or the downstream signalling network, all of which warrant further investigation.
Acknowledgements
C.A. acknowledges support from the British Heart Foundation (CH/F/21/90009, TG/19/2/34831 and RG/F/21/110040), theOxford BHF Centre of Research Excellence (RE/18/3/34214) and the Oxford NIHR Biomedical Research Centre.
Author contributions
I.A. and M.P. researched data for the article. I.A. and C.A. contributed to the discussion of content, and I.A. wrote the manuscript. All the authors reviewed and/or edited the manuscript before submission.
Peer review
Peer review information
Nature Reviews Cardiology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Competing interests
C.A. is founder, shareholder and director of Caristo Diagnostics, a CT image analysis company. The other authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Manemann SM, et al. Recent trends in cardiovascular disease deaths: a state specific perspective. BMC Public Health. 2021;21:1031. doi: 10.1186/s12889-021-11072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson K. The heart of immunology: immune mechanisms in cardiovascular medicine. Cardiovasc. Res. 2021;117:e166–e168. doi: 10.1093/cvr/cvab314. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 2021;117:2525–2536. doi: 10.1093/cvr/cvab303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fredman G, MacNamara KC. Atherosclerosis is a major human killer and non-resolving inflammation is a prime suspect. Cardiovasc. Res. 2021;117:2563–2574. doi: 10.1093/cvr/cvab309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akoumianakis I, Antoniades C. The interplay between adipose tissue and the cardiovascular system: is fat always bad? Cardiovasc. Res. 2017;113:999–1008. doi: 10.1093/cvr/cvx111. [DOI] [PubMed] [Google Scholar]
- 6.Akoumianakis I, Antoniades C. Impaired vascular redox signaling in the vascular complications of obesity and diabetes mellitus. Antioxid. Redox Signal. 2019;30:333–353. doi: 10.1089/ars.2017.7421. [DOI] [PubMed] [Google Scholar]
- 7.Wang JC, Bennett M. Aging and atherosclerosis. Circ. Res. 2012;111:245–259. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- 8.Ross R. Atherosclerosis — an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 9.Libby P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu L, et al. NOX1 mediates metabolic heart disease in mice and is upregulated in monocytes of humans with diastolic dysfunction. Cardiovasc. Res. 2021 doi: 10.1093/cvr/cvab349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H2181–H2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 12.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: the fibroblast awakens. Circ. Res. 2016;118:1021–1040. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazbanov IV, Ten Tusscher KHWJ, Panfilov AV. Effects of heterogeneous diffuse fibrosis on arrhythmia dynamics and mechanism. Sci. Rep. 2016;6:20835. doi: 10.1038/srep20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 15.Akoumianakis I, et al. Adipose tissue–derived WNT5A regulates vascular redox signaling in obesity via USP17/RAC1-mediated activation of NADPH oxidases. Sci. Transl Med. 2019;11:eaav5055. doi: 10.1126/scitranslmed.aav5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laudes M. Role of WNT signalling in the determination of human mesenchymal stem cells into preadipocytes. J. Mol. Endocrinol. 2011;46:R65–R72. doi: 10.1530/JME-10-0169. [DOI] [PubMed] [Google Scholar]
- 17.Sethi JK, Vidal-puig A. Wnt signalling and the control of cellular metabolism. Biochem. J. 2015;427:1–17. doi: 10.1042/BJ20091866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmerman ZF, Moon RT, Chien AJ. Targeting wnt pathways in disease. Cold Spring Harb. Perspect. Biol. 2012;4:a008086. doi: 10.1101/cshperspect.a008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dijksterhuis JP, Petersen J, Schulte G. WNT/Frizzled signalling: receptor-ligand selectivity with focus on FZD-G protein signalling and its physiological relevance: IUPHAR Review 3. Br. J. Pharmacol. 2014;171:1195–1209. doi: 10.1111/bph.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi EY, et al. Wnt5a and Wnt11 as acute respiratory distress syndrome biomarkers for severe acute respiratory syndrome coronavirus 2 patients. Eur. Respir. J. 2020;56:2001531. doi: 10.1183/13993003.01531-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christman MA, et al. Wnt5a is expressed in murine and human atherosclerotic lesions. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H2864–H2870. doi: 10.1152/ajpheart.00982.2007. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, et al. Wnt5a induces endothelial inflammation via beta-catenin-independent signaling. J. Immunol. 2010;185:1274–1282. doi: 10.4049/jimmunol.1000181. [DOI] [PubMed] [Google Scholar]
- 23.Bhatt PM, Malgor R. Wnt5a: a player in the pathogenesis of atherosclerosis and other inflammatory disorders. Atherosclerosis. 2014;237:155–162. doi: 10.1016/j.atherosclerosis.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 25.Wiese KE, Nusse R, van Amerongen R. Wnt signalling: conquering complexity. Development. 2018;145:dev165902. doi: 10.1242/dev.165902. [DOI] [PubMed] [Google Scholar]
- 26.Foulquier S, et al. WNT signaling in cardiac and vascular disease. Pharmacol. Rev. 2018;70:68–141. doi: 10.1124/pr.117.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikels AJ, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006;25:7461–7468. doi: 10.1038/sj.onc.1210053. [DOI] [PubMed] [Google Scholar]
- 28.Wada N, et al. Selective modulation of Wnt ligands and their receptors in adipose tissue by chronic hyperadiponectinemia. PLoS ONE. 2013;8:e67712. doi: 10.1371/journal.pone.0067712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marinou K, Christodoulides C, Antoniades C, Koutsilieris M. Wnt signaling in cardiovascular physiology. Trends Endocrinol. Metab. 2012;23:628–636. doi: 10.1016/j.tem.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Wook-Jin C, Bothwell ALM. Canonical and non-canonical Wnt signaling in immune cells. Trends Immunol. 2018;39:830–847. doi: 10.1016/j.it.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baksh D, Boland GM, Tuan RS. Cross-talk between Wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation. J. Cell. Biochem. 2007;101:1109–1124. doi: 10.1002/jcb.21097. [DOI] [PubMed] [Google Scholar]
- 33.James RG, Conrad WH, Moon RT. β-Catenin-independent Wnt pathways: signals, core proteins, and effectors. Methods Mol. Biol. 2008;468:131–144. doi: 10.1007/978-1-59745-249-6_10. [DOI] [PubMed] [Google Scholar]
- 34.Farb MG, et al. WNT5A-JNK regulation of vascular insulin resistance in human obesity. Vasc. Med. 2016;21:489–496. doi: 10.1177/1358863X16666693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semenov MV, Habas R, MacDonald BT, He X. SnapShot: noncanonical wnt signaling pathways. Cell. 2007;131:1378. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y, et al. Wnt5a promotes inflammatory responses via nuclear factor κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways in human dental pulp cells. J. Biol. Chem. 2014;289:21028–21039. doi: 10.1074/jbc.M113.546523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- 38.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109:S67–S79. doi: 10.1016/S0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 39.Vitezslav Bryja ERA, et al. The extracellular domain of lrp5/6 inhibits noncanonical wnt signaling in vivo. Mol. Biol. Cell. 2009;20:924–936. doi: 10.1091/mbc.e08-07-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Amerongen R, Fuerer C, Mizutani M, Nusse R. Wnt5a can both activate and repress Wnt/β-catenin signaling during mouse embryonic development. Dev. Biol. 2012;369:101–114. doi: 10.1016/j.ydbio.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu H-D, et al. Wnt5a mediated canonical Wnt signaling pathway activation in orthodontic tooth movement: possible role in the tension force-induced bone formation. J. Mol. Histol. 2016;47:455–466. doi: 10.1007/s10735-016-9687-y. [DOI] [PubMed] [Google Scholar]
- 42.Mill C, et al. Wnt5a-induced Wnt1-inducible secreted protein-1 suppresses vascular smooth muscle cell apoptosis induced by oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2014;34:2449–2456. doi: 10.1161/ATVBAHA.114.303922. [DOI] [PubMed] [Google Scholar]
- 43.Mani A, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu YC, et al. Circulating secreted frizzled-related protein 5 (Sfrp5) and wingless-type MMTV integration site family member 5a (Wnt5a) levels in patients with type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2013;29:551–556. doi: 10.1002/dmrr.2426. [DOI] [PubMed] [Google Scholar]
- 45.Albanese I, et al. Role of noncanonical wnt signaling pathway in human aortic valve calcification. Arterioscler. Thromb. Vasc. Biol. 2017;37:543–552. doi: 10.1161/ATVBAHA.116.308394. [DOI] [PubMed] [Google Scholar]
- 46.Albanese I, Khan K, Barratt B, Al-Kindi H, Schwertani A. Atherosclerotic calcification: Wnt is the hint. J. Am. Heart Assoc. 2018;7:e007356. doi: 10.1161/JAHA.117.007356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badimon L, Borrell-Pages M. Wnt signaling in the vessel wall. Curr. Opin. Hematol. 2017;24:230–239. doi: 10.1097/MOH.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 48.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dubiella U, et al. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl Acad. Sci. USA. 2013;110:8744–8749. doi: 10.1073/pnas.1221294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cook-Mills JM, et al. Calcium mobilization and Rac1 activation are required for VCAM-1 (vascular cell adhesion molecule-1) stimulation of NADPH oxidase activity. Biochem. J. 2004;378:539–547. doi: 10.1042/bj20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fontayne A, Dang PM-C, Gougerot-Pocidalo M-A, El Benna J. Phosphorylation of p47phox sites by PKC α, βΙΙ, δ, and ζ: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- 52.Reis M, Liebner S. Wnt signaling in the vasculature. Exp. Cell Res. 2013;319:1317–1323. doi: 10.1016/j.yexcr.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 53.Bretón-Romero R, et al. Endothelial dysfunction in human diabetes is mediated by Wnt5a-JNK signaling. Arterioscler. Thromb. Vasc. Biol. 2016;36:561–569. doi: 10.1161/ATVBAHA.115.306578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shiraishi H, et al. cGMP inhibits GTP cyclohydrolase I activity and biosynthesis of tetrahydrobiopterin in human umbilical vein endothelial cells. J. Pharmacol. Sci. 2003;93:265–271. doi: 10.1254/jphs.93.265. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi S, Mendelsohn ME. Synergistic activation of endothelial nitric-oxide synthase (eNOS) by HSP90 and Akt. J. Biol. Chem. 2003;278:30821–30827. doi: 10.1074/jbc.M304471200. [DOI] [PubMed] [Google Scholar]
- 56.Murthy S, et al. Endothelial CaMKII as a regulator of eNOS activity and NO-mediated vasoreactivity. PLoS ONE. 2017;12:e0186311. doi: 10.1371/journal.pone.0186311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gómez-Orte E, Sáenz-Narciso B, Moreno S, Cabello J. Multiple functions of the noncanonical Wnt pathway. Trends Genet. 2013;29:545–553. doi: 10.1016/j.tig.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Masckauchán TNH, et al. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol. Biol. Cell. 2006;17:5163–5172. doi: 10.1091/mbc.e06-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siman-Tov R, et al. Circulating Wnt ligands activate the wnt signaling pathway in mature erythrocytes. Arterioscler. Thromb. Vasc. Biol. 2021;41:E243–E264. doi: 10.1161/ATVBAHA.120.315413. [DOI] [PubMed] [Google Scholar]
- 60.Hulin-Curtis S, Williams H, Wadey KS, Sala-Newby GB, George SJ. Targeting Wnt/β-catenin activated cells with dominant-negative N-cadherin to reduce neointima formation. Mol. Ther. Methods Clin. Dev. 2017;5:191–199. doi: 10.1016/j.omtm.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown BA, et al. Aging differentially modulates the Wnt pro-survival signalling pathways in vascular smooth muscle cells. Aging Cell. 2019;18:e12844. doi: 10.1111/acel.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Srivastava R, et al. Impaired LRP6-TCF7L2 activity enhances smooth muscle cell plasticity and causes coronary artery disease. Cell Rep. 2015;13:746–759. doi: 10.1016/j.celrep.2015.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsaousi A, Mill C, George SJ. The Wnt pathways in vascular disease. Curr. Opin. Lipidol. 2011;22:350–357. doi: 10.1097/MOL.0b013e32834aa701. [DOI] [PubMed] [Google Scholar]
- 64.San José G, et al. Insulin-induced NADPH oxidase activation promotes proliferation and matrix metalloproteinase activation in monocytes/macrophages. Free Radic. Biol. Med. 2009;46:1058–1067. doi: 10.1016/j.freeradbiomed.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Kong Y-Z, et al. Macrophage migration inhibitory factor induces MMP-9 expression: implications for destabilization of human atherosclerotic plaques. Atherosclerosis. 2005;178:207–215. doi: 10.1016/j.atherosclerosis.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 66.Fiotti N, et al. MMP-9 microsatellite polymorphism and susceptibility to carotid arteries atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006;26:1330–1336. doi: 10.1161/01.ATV.0000219233.31702.c9. [DOI] [PubMed] [Google Scholar]
- 67.Fiotti N, et al. MMP-9 microsatellite polymorphism: association with the progression of intima-media thickening and constrictive remodeling of carotid atherosclerotic plaques. Atherosclerosis. 2005;182:287–292. doi: 10.1016/j.atherosclerosis.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 68.Pandey S, Chandravati Wnt signaling cascade in restenosis: a potential therapeutic target of public health relevance in a North American cohort of Nebraska State. Mol. Biol. Rep. 2014;41:4549–4554. doi: 10.1007/s11033-014-3325-0. [DOI] [PubMed] [Google Scholar]
- 69.Woldt E, et al. The nuclear hormone receptor PPARγ counteracts vascular calcification by inhibiting Wnt5a signalling in vascular smooth muscle cells. Nat. Commun. 2012;3:1077. doi: 10.1038/ncomms2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xin H, Xin F, Zhou S, Guan S. The Wnt5a/Ror2 pathway is associated with determination of the differentiation fate of bone marrow mesenchymal stem cells in vascular calcification. Int. J. Mol. Med. 2013;31:583–588. doi: 10.3892/ijmm.2013.1242. [DOI] [PubMed] [Google Scholar]
- 71.Cheng S-L, et al. Vascular smooth muscle LRP6 limits arteriosclerotic calcification in diabetic LDLR-/- mice by restraining noncanonical Wnt signals. Circ. Res. 2015;117:142–156. doi: 10.1161/CIRCRESAHA.117.306712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qin L, et al. The novel role and underlying mechanism of Wnt5a in regulating cellular cholesterol accumulation. Clin. Exp. Pharmacol. Physiol. 2014;41:671–678. doi: 10.1111/1440-1681.12258. [DOI] [PubMed] [Google Scholar]
- 73.Wang J, et al. WNT11-conditioned medium promotes angiogenesis through the activation of non-canonical WNT-PKC-JNK signaling pathway. Genes. 2020;11:1277. doi: 10.3390/genes11111277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stefater JA, et al. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. 2011;474:511–515. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pashirzad M, et al. Role of Wnt5a in the pathogenesis of inflammatory diseases. J. Cell. Physiol. 2017;232:1611–1616. doi: 10.1002/jcp.25687. [DOI] [PubMed] [Google Scholar]
- 76.Tian F, Mauro TM, Li Z. The pathological role of Wnt5a in psoriasis and psoriatic arthritis. J. Cell. Mol. Med. 2019;23:5876–5883. doi: 10.1111/jcmm.14531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fiechter RH, et al. IL-12p40/IL-23p40 blockade with ustekinumab decreases the synovial inflammatory infiltrate through modulation of multiple signaling pathways including MAPK-ERK and Wnt. Front. Immunol. 2021;12:611656. doi: 10.3389/fimmu.2021.611656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, et al. The role of Ca2+/NFAT in dysfunction and inflammation of human coronary endothelial cells induced by sera from patients with Kawasaki disease. Sci. Rep. 2020;10:4706. doi: 10.1038/s41598-020-61667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morishita Y, et al. Wnt11 gene therapy with adeno-associated virus 9 improves recovery from myocardial infarction by modulating the inflammatory response. Sci. Rep. 2016;6:21705. doi: 10.1038/srep21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chaussabel D, et al. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood. 2003;102:672–681. doi: 10.1182/blood-2002-10-3232. [DOI] [PubMed] [Google Scholar]
- 81.Nau GJ, et al. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl Acad. Sci. USA. 2002;99:1503–1508. doi: 10.1073/pnas.022649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lehtonen A, et al. Gene expression profiling during differentiation of human monocytes to macrophages or dendritic cells. J. Leukoc. Biol. 2007;82:710–720. doi: 10.1189/jlb.0307194. [DOI] [PubMed] [Google Scholar]
- 83.Shao Y, et al. Biological functions of macrophage-derived Wnt5a, and its roles in human diseases. Oncotarget. 2016;7:67674–67684. doi: 10.18632/oncotarget.11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ackers I, et al. Blocking Wnt5a signaling decreases CD36 expression and foam cell formation in atherosclerosis. Cardiovasc. Pathol. 2018;34:1–8. doi: 10.1016/j.carpath.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 85.Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler. Thromb. Vasc. Biol. 2008;28:504–510. doi: 10.1161/ATVBAHA.107.157438. [DOI] [PubMed] [Google Scholar]
- 86.Feng Y, et al. The signaling protein Wnt5a promotes TGFβ1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz. J. Biol. Chem. 2018;293:19290–19302. doi: 10.1074/jbc.RA118.005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Naskar D, et al. Wnt5a–Rac1–NF-κB homeostatic circuitry sustains innate immune functions in macrophages. J. Immunol. 2014;192:4386–4397. doi: 10.4049/jimmunol.1302817. [DOI] [PubMed] [Google Scholar]
- 88.Barbero G, et al. An autocrine Wnt5a loop promotes NF-κB pathway activation and cytokine/chemokine secretion in melanoma. Cells. 2019;8:1060. doi: 10.3390/cells8091060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang C-J, et al. Wnt5a/Ror2 pathway contributes to the regulation of cholesterol homeostasis and inflammatory response in atherosclerosis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865:158547. doi: 10.1016/j.bbalip.2019.158547. [DOI] [PubMed] [Google Scholar]
- 90.Ouchi N, et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329:454–457. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fuster JJ, et al. Noncanonical Wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes. 2015;64:1235–1248. doi: 10.2337/db14-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghila L, et al. Chronically elevated exogenous glucose elicits antipodal effects on the proteome signature of differentiating human IPSC-derived pancreatic progenitors. Int. J. Mol. Sci. 2021;22:3698. doi: 10.3390/ijms22073698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 94.Hotamisligil GS, et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 95.da Costa RM, et al. TNF-α induces vascular insulin resistance via positive modulation of PTEN and decreased Akt/eNOS/NO signaling in high fat diet-fed mice. Cardiovasc. Diabetol. 2016;15:119. doi: 10.1186/s12933-016-0443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hotamisligil G. Mechanisms of TNF-α-induced insulin resistance. Exp. Clin. Endocrinol. Diabetes. 2009;107:119–125. doi: 10.1055/s-0029-1212086. [DOI] [PubMed] [Google Scholar]
- 97.Shoelson SE, Lee J, Yuan M. Inflammation and the IKKβ/IκB/NF-κB axis in obesity- and diet-induced insulin resistance. Int. J. Obes. 2003;27:S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- 98.Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol. 2015;11:363–371. doi: 10.1038/nrendo.2015.58. [DOI] [PubMed] [Google Scholar]
- 99.Oikonomou EK, Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat. Rev. Cardiol. 2019;16:83–99. doi: 10.1038/s41569-018-0097-6. [DOI] [PubMed] [Google Scholar]
- 100.Van Gaal LF. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 101.Camarena V, et al. Novel atherogenic pathways from the differential transcriptome analysis of diabetic epicardial adipose tissue. Nutr. Metab. Cardiovasc. Dis. 2017;27:739–750. doi: 10.1016/j.numecd.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Antonopoulos AS, et al. Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR-γ/Adiponectin Signalling. Circ. Res. 2016;118:842–855. doi: 10.1161/CIRCRESAHA.115.307856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iacobellis G, Baroni MG. Cardiovascular risk reduction throughout GLP-1 receptor agonist and SGLT2 inhibitor modulation of epicardial fat. J. Endocrinol. Invest. 2021;45:489–495. doi: 10.1007/s40618-021-01687-1. [DOI] [PubMed] [Google Scholar]
- 104.Malavazos AE, Goldberger JJ, Iacobellis G. Does epicardial fat contribute to COVID-19 myocardial inflammation? Eur. Heart J. 2020;41:2333–2333. doi: 10.1093/eurheartj/ehaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Villasante Fricke AC, Iacobellis G. Epicardial adipose tissue: clinical biomarker of cardio-metabolic risk. Int. J. Mol. Sci. 2019;20:5989. doi: 10.3390/ijms20235989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Akoumianakis I, Tarun A, Antoniades C. Perivascular adipose tissue as a regulator of vascular disease pathogenesis: identifying novel therapeutic targets. Br. J. Pharmacol. 2016;174:3411–3424. doi: 10.1111/bph.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Antonopoulos AS, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl Med. 2017;9:eaal2658. doi: 10.1126/scitranslmed.aal2658. [DOI] [PubMed] [Google Scholar]
- 108.Oikonomou EK, et al. Standardized measurement of coronary inflammation using cardiovascular computed tomography: integration in clinical care as a prognostic medical device. Cardiovasc. Res. 2021;117:2677–2689. doi: 10.1093/cvr/cvab286. [DOI] [PubMed] [Google Scholar]
- 109.Oikonomou EK, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392:929–939. doi: 10.1016/S0140-6736(18)31114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kotanidis CP, Antoniades C. Perivascular fat imaging by computed tomography (CT): a virtual guide. Br. J. Pharmacol. 2021;178:4270–4290. doi: 10.1111/bph.15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang B, et al. Sfrp5/Wnt5a and leptin/adiponectin levels in the serum and the periarterial adipose tissue of patients with peripheral arterial occlusive disease. Clin. Biochem. 2021;87:46–51. doi: 10.1016/j.clinbiochem.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 112.Catalán V, et al. Activation of noncanonical wnt signaling through WNT5A in visceral adipose tissue of obese subjects is related to inflammation. J. Clin. Endocrinol. Metab. 2014;99:E1407-17. doi: 10.1210/jc.2014-1191. [DOI] [PubMed] [Google Scholar]
- 113.Ackers I, Malgor R. Interrelationship of canonical and non-canonical Wnt signalling pathways in chronic metabolic diseases. Diabetes Vasc. Dis. Res. 2018;15:3–13. doi: 10.1177/1479164117738442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ. Res. 2016;118:1786–1807. doi: 10.1161/CIRCRESAHA.115.306885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Adipogenesis and WNT signalling. Trends Endocrinol. Metab. 2009;20:16–24. doi: 10.1016/j.tem.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tse G. Mechanisms of cardiac arrhythmias. J. Arrhythmia. 2016;32:75–81. doi: 10.1016/j.joa.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Voorhees AP, Han HC. Biomechanics of cardiac function. Compr. Physiol. 2015;5:1623–1644. doi: 10.1002/cphy.c140070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Azevedo PS, Polegato BF, Minicucci MF, Paiva SAR, Zornoff LAM. Cardiac remodeling: concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arquivos Bras. Cardiol. 2016;106:62–69. doi: 10.5935/abc.20160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lopez R, et al. Impaired myocardial energetics causes mechanical dysfunction in decompensated failing hearts. Function. 2020;1:zqaa018. doi: 10.1093/function/zqaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond atp production. Circ. Res. 2013;113:709–724. doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.D’Oria R, et al. The role of oxidative stress in cardiac disease: from physiological response to injury factor. Oxid. Med. Cell. Longev. 2020;2020:1–29. doi: 10.1155/2020/5732956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Abraityte A, et al. Wnt5a is elevated in heart failure and affects cardiac fibroblast function. J. Mol. Med. 2017;95:767–777. doi: 10.1007/s00109-017-1529-1. [DOI] [PubMed] [Google Scholar]
- 123.Abraityte A, et al. Wnt5a is associated with right ventricular dysfunction and adverse outcome in dilated cardiomyopathy. Sci. Rep. 2017;7:3490. doi: 10.1038/s41598-017-03625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dumotier BM. A straightforward guide to the basic science behind arrhythmogenesis. Heart. 2014;100:1907–1915. doi: 10.1136/heartjnl-2014-305647. [DOI] [PubMed] [Google Scholar]
- 125.Austin KM, et al. Molecular mechanisms of arrhythmogenic cardiomyopathy. Nat. Rev. Cardiol. 2019;16:519–537. doi: 10.1038/s41569-019-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dawson K, Aflaki M, Nattel S. Role of the Wnt-Frizzled system in cardiac pathophysiology: a rapidly developing, poorly understood area with enormous potential. J. Physiol. 2013;591:1409–1432. doi: 10.1113/jphysiol.2012.235382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lv X, et al. Overexpression of miR-27b-3p targeting Wnt3a regulates the signaling pathway of Wnt/β-catenin and attenuates atrial fibrosis in rats with atrial fibrillation. Oxid. Med. Cell. Longev. 2019;2019:5703764. doi: 10.1155/2019/5703764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Parrotta EI, et al. Deciphering the role of wnt and rho signaling pathway in IPSC-derived ARVC cardiomyocytes by in silico mathematical modeling. Int. J. Mol. Sci. 2021;22:2004. doi: 10.3390/ijms22042004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guo F, Yi X, Li M, Fu J, Li S. Snail1 is positively correlated with atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease. Exp. Ther. Med. 2017;14:4231–4237. doi: 10.3892/etm.2017.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dissanayake SK, et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J. Biol. Chem. 2007;282:17259–17271. doi: 10.1074/jbc.M700075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Beljaars L, Daliri S, Dijkhuizen C, Poelstra K, Gosens R. WNT-5A regulates TGF-β-related activities in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G219–G227. doi: 10.1152/ajpgi.00160.2016. [DOI] [PubMed] [Google Scholar]
- 132.Kato T, et al. Endothelial–mesenchymal transition in human atrial fibrillation. J. Cardiol. 2017;69:706–711. doi: 10.1016/j.jjcc.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 133.Hinderer S, Schenke-Layland K. Cardiac fibrosis–a short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev. 2019;146:77–82. doi: 10.1016/j.addr.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 134.Działo E, et al. WNT3a and WNT5a transported by exosomes activate WNT signaling pathways in human cardiac fibroblasts. Int. J. Mol. Sci. 2019;20:1436. doi: 10.3390/ijms20061436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hagenmueller M, et al. Dapper-1 is essential for Wnt5a induced cardiomyocyte hypertrophy by regulating the Wnt/PCP pathway. FEBS Lett. 2014;588:2230–2237. doi: 10.1016/j.febslet.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 136.Wang Y, et al. Wnt5a-mediated neutrophil recruitment has an obligatory role in pressure overload-induced cardiac dysfunction. Circulation. 2019;140:487–499. doi: 10.1161/CIRCULATIONAHA.118.038820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Anumonwo JMB, Herron T. Fatty infiltration of the myocardium and arrhythmogenesis: potential cellular and molecular mechanisms. Front. Physiol. 2018;9:2. doi: 10.3389/fphys.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lorenzon A, et al. Wnt/β-catenin pathway in arrhythmogenic cardiomyopathy. Oncotarget. 2017;8:60640–60655. doi: 10.18632/oncotarget.17457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Abou Ziki MD, Mani A. The interplay of canonical and noncanonical Wnt signaling in metabolic syndrome. Nutr. Res. 2019;70:18–25. doi: 10.1016/j.nutres.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang J, et al. Vibration and β-hydroxy-β-methylbutyrate treatment suppresses intramuscular fat infiltration and adipogenic differentiation in sarcopenic mice. J. Cachexia Sarcopenia Muscle. 2020;11:564–577. doi: 10.1002/jcsm.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang S, et al. Nonalcoholic fatty liver disease induced by noncanonical Wnt and its rescue by Wnt3a. FASEB J. 2015;29:3436–3445. doi: 10.1096/fj.15-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tuomainen T, Tavi P. The role of cardiac energy metabolism in cardiac hypertrophy and failure. Exp. Cell Res. 2017;360:12–18. doi: 10.1016/j.yexcr.2017.03.052. [DOI] [PubMed] [Google Scholar]
- 143.Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circ. Res. 2021;128:1487–1513. doi: 10.1161/CIRCRESAHA.121.318241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang X, et al. SGLT2 inhibitors break the vicious circle between heart failure and insulin resistance: targeting energy metabolism. Heart Fail. Rev. 2022;27:961–980. doi: 10.1007/s10741-021-10096-8. [DOI] [PubMed] [Google Scholar]
- 145.Moorer MC, Riddle RC. Regulation of osteoblast metabolism by Wnt signaling. Endocrinol. Metab. 2018;33:318–330. doi: 10.3803/EnM.2018.33.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mo Y, et al. The role of Wnt signaling pathway in tumor metabolic reprogramming. J. Cancer. 2019;10:3789–3797. doi: 10.7150/jca.31166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Serrat R, et al. The non-canonical Wnt/PKC pathway regulates mitochondrial dynamics through degradation of the arm-like domain-containing protein Alex3. PLoS ONE. 2013;8:e67773. doi: 10.1371/journal.pone.0067773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arrázola MS, Silva-Alvarez C, Inestrosa NC. How the Wnt signaling pathway protects from neurodegeneration: the mitochondrial scenario. Front. Cell. Neurosci. 2015;9:1–13. doi: 10.3389/fncel.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li H-X, Lin J, Jiang B, Yang X-J. Wnt11 preserves mitochondrial membrane potential and protects cardiomyocytes against hypoxia through paracrine signaling. J. Cell. Biochem. 2020;121:1144–1155. doi: 10.1002/jcb.29349. [DOI] [PubMed] [Google Scholar]
- 150.Zhu L, et al. Upregulation of non-canonical Wnt ligands and oxidative glucose metabolism in NASH induced by methionine-choline deficient diet. Trends Cell Mol. Biol. 2018;13:47–56. doi: 10.31300/TCMB.13.2018.47-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Heusch G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020;17:773–789. doi: 10.1038/s41569-020-0403-y. [DOI] [PubMed] [Google Scholar]
- 152.van der Pol A, van Gilst WH, Voors AA, van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur. J. Heart Fail. 2019;21:425–435. doi: 10.1002/ejhf.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kurian GA, Rajagopal R, Vedantham S, Rajesh M. The role of oxidative stress in myocardial ischemia and reperfusion injury and remodeling: revisited. Oxid. Med. Cell. Longev. 2016;2016:1656450. doi: 10.1155/2016/1656450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Iwata K, et al. Up-regulation of NOX1/NADPH oxidase following drug-induced myocardial injury promotes cardiac dysfunction and fibrosis. Free Radic. Biol. Med. 2018;120:277–288. doi: 10.1016/j.freeradbiomed.2018.03.053. [DOI] [PubMed] [Google Scholar]
- 155.Scolletta S, Biagioli B. Energetic myocardial metabolism and oxidative stress: let’s make them our friends in the fight against heart failure. Biomed. Pharmacother. 2010;64:203–207. doi: 10.1016/j.biopha.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 156.Chen B, et al. Co-expression of Akt1 and Wnt11 promotes the proliferation and cardiac differentiation of mesenchymal stem cells and attenuates hypoxia/reoxygenation-induced cardiomyocyte apoptosis. Biomed. Pharmacother. 2018;108:508–514. doi: 10.1016/j.biopha.2018.09.047. [DOI] [PubMed] [Google Scholar]
- 157.Nakamura K, et al. Secreted frizzled-related protein 5 diminishes cardiac inflammation and protects the heart from ischemia/reperfusion injury. J. Biol. Chem. 2016;291:2566–2575. doi: 10.1074/jbc.M115.693937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Matsushima S, Tsutsui H, Sadoshima J. Physiological and pathological functions of NADPH oxidases during myocardial ischemia-reperfusion. Trends Cardiovasc. Med. 2014;24:202–205. doi: 10.1016/j.tcm.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Akoumianakis I, et al. Insulin-induced vascular redox dysregulation in human atherosclerosis is ameliorated by dipeptidyl peptidase 4 inhibition. Sci. Transl Med. 2020;12:eaav8824. doi: 10.1126/scitranslmed.aav8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang P. CaMKII: the molecular villain that aggravates cardiovascular disease. Exp. Ther. Med. 2017;13:815–820. doi: 10.3892/etm.2017.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- 162.Blagodatski A, Poteryaev D, Katanaev VL. Targeting the Wnt pathways for therapies. Mol. Cell. Ther. 2014;2:28. doi: 10.1186/2052-8426-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 164.Naik, P., Marwah, M., Venkataraman, M., Rajawat, G. S. & Nagarsenker, M. Drug Delivery Approaches and Nanosystems. Volume 2: Drug Targeting Aspects of Nanotechnology (Apple Academic, 2017).
- 165.Zhang F, Wen Y, Guo X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum. Mol. Genet. 2014;23:R40–R46. doi: 10.1093/hmg/ddu125. [DOI] [PubMed] [Google Scholar]
- 166.Crooke ST. Molecular mechanisms of antisense oligonucleotides. Nucleic Acid. Ther. 2017;27:70–77. doi: 10.1089/nat.2016.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Reichert J. Monoclonal antibodies as innovative therapeutics. Curr. Pharm. Biotechnol. 2008;9:423–430. doi: 10.2174/138920108786786358. [DOI] [PubMed] [Google Scholar]
- 168.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 170.Luo Y-L, et al. Macrophage-specific in vivo gene editing using cationic lipid-assisted polymeric nanoparticles. ACS Nano. 2018;12:994–1005. doi: 10.1021/acsnano.7b07874. [DOI] [PubMed] [Google Scholar]
- 171.Schulte G, Bryja V. WNT signalling: mechanisms and therapeutic opportunities. Br. J. Pharmacol. 2017;174:4543–4546. doi: 10.1111/bph.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li J, et al. Deficiency of Rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes. 2010;59:2033–2042. doi: 10.2337/db09-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Porte B, Marguerit G, Thomasseau S, Paquet C, Hugon J. Dose-dependent neuroprotective effect of the JNK inhibitor Brimapitide in 5xFAD transgenic mice. Brain Res. 2020;1727:146587. doi: 10.1016/j.brainres.2019.146587. [DOI] [PubMed] [Google Scholar]
- 174.Greenberg S, et al. Evaluation of the JNK inhibitor, CC-90001, in a phase 1b pulmonary fibrosis trial. Eur. Respir. J. 2017;50:OA474. [Google Scholar]
- 175.Ebenebe O, Heather A, Erickson J. The role of CaMKII in atherosclerotic plaque calcification in the ApoE-null mouse. Heart Lung Circ. 2017;26:S65. doi: 10.1016/j.hlc.2017.06.051. [DOI] [Google Scholar]
- 176.He Q, Cheng J, Wang Y. Chronic CaMKII inhibition reverses cardiac function and cardiac reserve in HF mice. Life Sci. 2019;219:122–128. doi: 10.1016/j.lfs.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 177.Douglas G, et al. A key role for the novel coronary artery disease gene JCAD in atherosclerosis via shear stress mechanotransduction. Cardiovasc. Res. 2020;116:1863–1874. doi: 10.1093/cvr/cvz263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 179.Pirillo A, Bonacina F, Norata GD, Catapano AL. The interplay of lipids, lipoproteins, and immunity in atherosclerosis. Curr. Atheroscler. Rep. 2018;20:12. doi: 10.1007/s11883-018-0715-0. [DOI] [PubMed] [Google Scholar]
- 180.Silvestre-Roig C, et al. Atherosclerotic plaque destabilization. Circ. Res. 2014;114:214–226. doi: 10.1161/CIRCRESAHA.114.302355. [DOI] [PubMed] [Google Scholar]
- 181.Hansson GK. Inflammation, atherosclerosis and coronary artery disease. N. Engl. J. Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]