Abstract
Nanotechnology with artificial intelligence (AI) can metamorphose medicine to an extent that has never been achieved before. AI could be used in anesthesia to develop advanced clinical decision support tools based on machine learning, increasing efficiency, and accuracy. It is also potentially highly troublesome by creating insecurity among clinicians and allowing the transfer of expert domain knowledge to machines. Anesthesia is a complex medical specialty, and assuming AI can easily replace the expert as a clinically sound anesthetist is a very unrealistic expectation. This paper focuses on the association and opportunities for AI developments and deep learning with anesthesia. It reviews the current advances in AI tools and hardware technologies and outlines how these can be used in the field of anesthesia.
Keywords: Deep learning, machine learning, nanomedicine, personalized medicine
Introduction
Health care providers work in data-rich clinical environments. Every day, hundreds of terabytes of information are shared, and data recorded around the globe. There are unlimited data recorded from electronic health records to specialized data of genomics and physiological waveforms. The data are accessible to anesthetists or physicians in acute care settings.[1,2,3] Artificial intelligence (AI) may help the clinician to develop machine algorithms for decision making in hospital settings. It touches multiple facets of anesthesia care, along with perioperative and intensive care and pain management.
AI in Anesthesia
Anesthesiologists became multi-tasking and involved in processing any information like troubleshooting monitors, scribing detailed case records, and performing clinical procedures in the operating room.[4] Today, an anesthetist needs AI and machine learning to handle the wealth of information. AI in medicine has taken space for the last decade and is growing very fast. It may change the scenario of anesthesia practice, perioperative medicine in clinics, and interpretation of images. AI allows the anesthetist or clinician to work with computer scientists in computation, predictive analysis, and automation side by side. AI is already prevalent in daily life, starting with smartphones to houses or offices and even cars. Technological advancements and automation have dramatically helped the anesthetist to reduce the risk of complications associated with anesthesia care. AI can provide better patient safety and environment to the anesthetist.[5] Anesthesia Information and Management Systems (AIMS) is developed with integrated decision support and target-controlled infusion systems. The other paradigms are the superior model of anesthesia machines, closed-loop anesthesia delivery, and point of care ultrasound-guided procedures, which are good illustrations.[6,7]
A meta-analysis demonstrated that automatic administration of Propofol based on a bispectral index (BIS) seemed to be superior to anesthesiologists in terms of requirements for induction of anesthesia, rate of attainment of target anesthesia, and short wake-up time.[1] Additionally, automatic anesthesia using an AI system can significantly reduce delirium in arousal, suggesting the possibility of prognosis improvement.[6]
Computerized infusion administration systems with AI inbuilt systems can be used as an alternative. According to the study, the feedback Guideline Development Tool (GDT) system using the pulse contour algorithm is safe and can shorten postoperative complications and hospitalization.[8] Remifentanil administration based on the analgesia nociception index (ANI) and automatic administration of phenylephrine based on non-invasive blood pressure were also attempted.[9] Currently, these expert systems are attempting to automate sleep-analgesia and fluid administration and are likely to be applied in the clinic soon.[10]
In existing practice, a subject is well until he or she acquires signs or symptoms of a disease. However, the AI-managed system can start its work far before the appearance of signs or symptoms based on biochemical or physiological derangements or genetic and epigenetic alteration. Even AI can guide the clinician to start intervention before symptoms appear or need surgical intervention. AI advancement in these arenas of anesthesia is discussed below.[4,10]
History of AI
The initial effort in the history of AI was undertaken in 1950 by Bickford, who used electroencephalogram (EEG) signals to monitor and maintain the anesthesia depth. Later, the McSleepy AI machine was developed in 2008 based on a closed-loop feedback control system concept.[11] This system followed a hand-crafted rule and response algorithms around the setpoint to maintain anesthesia’s depth as shown in Figure 1. But this machine often failed to handle a complex situation. To handle such a drawback, AI utilized learning algorithms.[12] The automated anesthesia system is trained to perform a particular task by input-output mapping with an adaptive neural network under machine learning [Figure 2]. Further advancement under investigation is deep learning, in which an artificial neural network is developed to process information and get reasonable prediction [Figure 3].[7]
Figure 1.
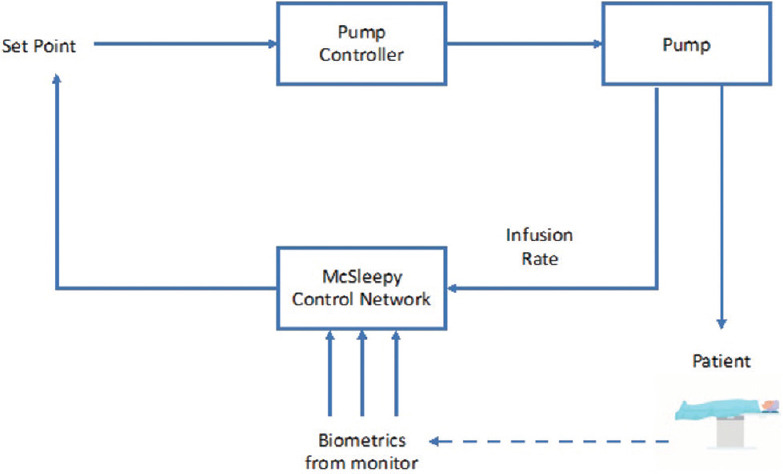
Schematic flow diagram of Mc Sleepy AI machine[7]
Figure 2.
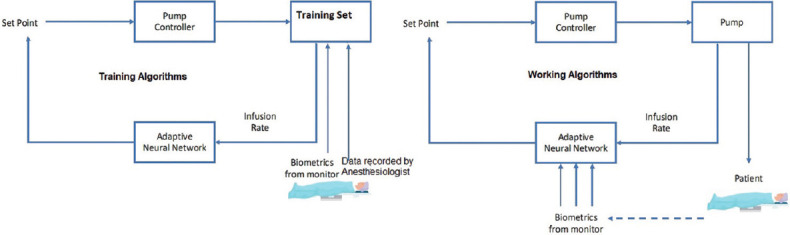
Flow diagram of automated anesthesia system with training and working[7]
Figure 3.
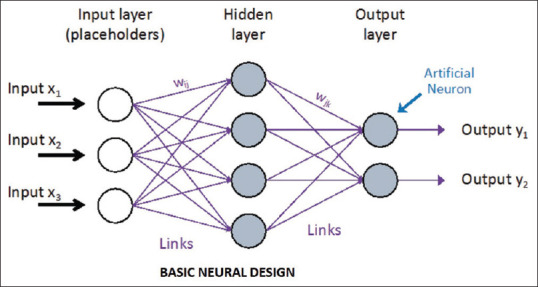
Flow diagram of an artificial neural network[7]
Machine learning in anesthesia
Machine learning is a significant component of AI. Machine learning is an algorithm for making decisions and problem-solving through analyzing data. The data are not only limited to numerical but also texts, images, audio, and video. There are various techniques in machine learning: Fuzzy logic, standard logic, neural networks, classical learning, deep learning, and Bayesian methods. Details of the techniques are beyond this manuscript’s scope, but brief information has been shared in Table 1.[13] Various works of literature are available which improve anesthesia monitoring with the help of AI. AI utilizes BIS and EEG to assess anesthesia depth with the use of the neural network. Like the variability of heart rate, the clinical variables are recorded to assess the approximate sedation level.[13,14] Natural language processing is for automated analysis of electronic health records. The same is currently being investigated to extract information to build structured databases and identify surgical candidates for assessing adverse events. Similarly, computer vision is used to automate the analysis of ultrasound images. Many other applications are mentioned in Table 2.[15]
Table 1.
Techniques and algorithms of artificial intelligence[10]
| Techniques and learning algorithms | Details |
|---|---|
| Fuzzy Logic | Standard logic for the concepts of true (a numerical value of 1.0) and false (a numerical value of 0.0). |
| Fuzzy logic allows for partial truth (i.e., a numerical value between 0.0 and 1.0). A comparison may be made to probability theory, where the probability of a statement being true is evaluated. | |
| A rule-based system primarily used in control systems, fuzzy logic approximates the presence of mild, moderate, and severe hypovolemia based on normalized values of the heart rate (HR), blood pressure, and pulse volume | |
| Classical Machine Learning | Analogous to independent variables in logistic regression. |
| Guide the algorithms in analyzing complex data such as patient demographics, vital signs, and aspects of their medical history, surgery type, and patient-controlled analgesia (PCA) doses. | |
| The algorithm that can be used to perform either classification (classification trees) or regression tasks (regression trees) to predict total PCA consumption. | |
| Neural Networks | It is made up of an input layer of neurons included in features that analyze the data. These at least single hidden layer of neurons performs mathematical operations on the input data and an output layer that gives algorithms to attain a particular aim (e.g., image recognition, data classification). |
| Depth of anesthesia monitoring and control of anesthesia delivery | |
| Deep Learning | A powerful tool with which to analyze massive datasets |
| It analyses all available data within the training set to determine the optimal output of the given task (e.g., object recognition from an image). | |
| Bayesian Methods | A frequentist approach to statistics is applied, wherein hypothesis testing occurs based on the frequency of events |
| Allows for both modeling of uncertainty and updating or learning repetitively as new data are made an available assessment of clinical tests. |
Table 2.
Uses of artificial intelligence in anesthesia practice.[10]
| Domain | Uses |
|---|---|
| Control of Anesthesia Delivery | Automated delivery of anesthesia by the machine based on the input of BIS and EEG |
| Forecasting of drug pharmacokinetics to further improve the control of Infusions of neuromuscular blockade or other related drugs | |
| Control of mechanical ventilation | |
| To automate weaning from mechanical ventilation | |
| Event Prediction | Predicts the hypnotic effect (as measured by BIS) of an induction bolus dose of Propofol |
| Prediction of return of consciousness after general anesthesia | |
| Neural networks have also been used to predict the rate of recovery from neuromuscular blockade | |
| Prediction of hypotensive episodes postinduction or during spinal anesthesia | |
| To automate the classification of ASA status | |
| To define difficult laryngoscopy findings | |
| To identify respiratory depression during conscious sedation | |
| To assist in decision making for the optimal method of anesthesia in pediatric surgery | |
| To predict hypotension in the ICU setting by arterial waveform | |
| To predict morbidity, weaning from ventilation, clinical deterioration, mortality, or readmission and in the ICU setting by machine learning | |
| To detect sepsis in the ICU setting | |
| Ultrasound Guidance | Differentiation of artery and vein with the help of convolution neural network |
| Identification of vertebral level for epidural catheter placement | |
| Pain Management | Prediction of opioid dosing |
| Assessment of pain from functional magnetic resonance imaging data | |
| Development of nociception level index based on machine learning analysis of photoplethysmograms and skin conductance waveforms | |
| Operating Room Logistics | Scheduling of operating room time |
| Tracking movements and actions of anesthesiologists | |
| Prediction of the duration of an operation based on the team, type of operation, and a patient’s relevant medical history |
Robotics in the operating room
Robotic systems have been discovered and are available in the market, which can perform tasks like peripheral and central venous access, endotracheal intubations, and assisting regional anesthesia administration. Two categories of robots accessible in the marketplace are manual and pharmacological robots. Manual robots are further sub-classified into Kepler intubation robots, video-laryngoscopy intubating robots, and endotracheal intubation robotic arms.[16] The Da Vinci surgical robot might be exploited for regional anesthesia. The Magellan robot can be used for peripheral nerve block. Mc Sleepy intravenous sedation machine has been advanced to inject intravenous anesthetic agents, narcotics, and muscle relaxants.[17] An additional machine, iControl-RP, has been recently built as a closed-loop system for an intravenous anesthetic delivery system. This machine is proficient at taking its own decisions concerning the administration of IV anesthetic agents like Propofol or remifentanil.[13] This machine has a facility of neuromonitoring based on EEG and hemodynamic monitoring based on the BIS monitor. No devices are available on the horizon that could replace the expert, but, it is challenging to run along with this AI-based machine.[14]
Role of AI computers in pre-operative assessment clinics
In day-to-day practice, the computer programmed for recording the patient’s information is essential to maintain the records of the patient’s history, findings of physical examination, and laboratory studies. The AI computers add another hand, which help analyze the data for diagnosis and to monitor the treatment regimen for a high percentage of success. IBM’s Watson computer is well designed to analyze more than 10 million data from patients or evidence reports from medical journals.[18] This machine cannot be superior to the doctor as projected, but it dramatically improves delivering service by the clinician. The AI machines can provide input of new patients in a flow-chart, and it can guide the physician to ask a specific question to reach the diagnosis. Once the diagnosis is confirmed, then the AI machine can follow the already established algorithm for treatment.[19]
Role of AI computer in image analysis and diagnostic laboratory
The AI computers have the capability for deep learning from the previous database and pattern analysis. The same is applicable for image analysis in medicine. The AI computers are well trained to assess the array of pixels and analyze data from digital radiograph, a computerized scan of tomography, magnetic resonance, or ultrasonography. An AI computer that masters in deep learning may not be able to provide an exact diagnosis but directs toward the possible diagnosis; for example, it can analyze a biopsy of a skin lesion with greater than 99% chance of being diagnostically correct from microscopic images or a radiologic scan with more than a 99% chance of accuracy.[17,20]
Role of AI in tele-anesthesia
The concept of tele-anesthesia could allow distant pre-operative assessment of the patients’ fitness for anesthesia or execute anesthetic tasks. It would regulate anesthesia delivery in a far-off location with automated anesthesia drug delivery systems maintaining a sufficient depth of anesthesia.[21] The demography and global healthcare bill have been pushed further for this change. This century is an eyewitness of exponential growth and advances in technology occurring at an unparalleled scale.
Telemonitoring is one step ahead of telemedicine. The automated critical care system (ACCS) is under development in the United States. This system may be used to maintain intravenous anesthesia for a critically injured person during transportation from a remote location. It works autonomously or assists in the mode for the physician.[22]
Critical care of the COVID-19 patients using AI
The AI algorithms could be used to predict if a subject has a higher risk of health crisis based on clinical signs and symptoms of COVID 19. A predicting tool via a data mining approach could be developed to estimate the viral load. The viral spread could be tracked at hot-spots and epicenters based on the input of data from the Arogya Setu app, social media, phone contacts, and traffic mapping. AI could be utilized to develop the tool for personalized medicine and tracking the vaccination on a subject basis. AI-based monitoring of mildly symptomatic patients will reduce the burden of tertiary care hospitals and treatment costs. Machine or deep learning could be used to predict the vulnerability or resistance of patients based on the immunological status and predisposing factors. A study has shown that AI-based monitoring of critically ill patients is possible with a deep learning system. The deep learning system needs history, physical examination, paraclinical data, chest CT (Computed Tomography) scan, along with RT-PCR tests from deep nasotracheal samples. An AI machine has the capacity to guide a clinician by predicting the risk and need for mechanical ventilation and potential regimen for COVID 19. AI and related mechanisms could enable us to make more efficient decisions. This starts from risk stratification, diagnostic data, prognosis and survival trends, risk classification, risk prediction, and finally, survival prediction; all of them would be appropriate for COVID19 patients.[23] Figure 4 summarizes the role of AI in the care of COVID-19 patients.
Figure 4.
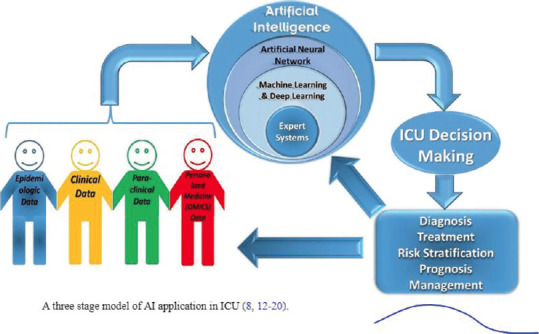
Role of AI in the care of COVID-19 patients[23]
Role of Nanotechnology
Nanotechnology, coupled with AI, has the extraordinary capacity to monitor the human body at the molecular level. Nanotechnology is used in medicine for drug delivery, in vivo imaging, and nanotherapeutics. More than 100 FDA (Food and Drug Administration) approved nanomolecules are already in use, and thrice of that are undertrials. These nanomolecules and nanodevices are designed to interact with high specificity with subcellular or molecular levels in the body.[24] Nanoparticles, along with drug molecules, can target specific cells or tissues to delay drug metabolism and clearance and improve the drug’s bioavailability. Their submicron size permits them to pass through endothelial fenestrae and penetrate the tissues at the capillary level. In the coming days, it is possible to induce local anesthesia by colloidal suspension containing millions of active analgesics instilled on the patient’s mucosa, and the nanorobots can guide these molecules deep into the tissue, even in the bone or teeth canal.[25] In post-surgical pain, anesthetists may use new molecular targets, such as sodium channel blockers (Nav 1.3, Nav 1.7, and Nav 1.8); potassium channel openers in sensory neurons; N-type calcium channels (Cav 2.2) blockers; P2X4 and P2X7 receptor antagonists in microglia; vanilloid receptor-1 antagonists; and the cannabinoid-2 receptor agonists. Introducing nanocarriers inside the halothane molecule can increase its benefits as an anesthetic in the lungs and cardiovascular system and prevent liver exposure. Anesthetic agents, such as Propofol, can improve anesthesia quality using nanomedicine.[26]
The innovations are undertrial to replace the functions of the entire cells like red blood cells by respirocytes. They have an excellent design that acts as a tiny container storing the gases at high pressure. It could bind with oxygen and carbon dioxide like normal erythrocytes. These respirocytes may carry blood glucose as an abundant energy source for collecting, compressing, storing, and releasing the gases and powering the nearby cells.[27]
Role of genomic medicine
The concept of personalized medicine was introduced to overcome the inter-individual differences in the pharmacogenetics of a given drug. Pharmacogenomics has been able to exert a considerable influence on anesthetic and surgical outcomes, thereby decreasing perioperative anesthesia morbidity.[28] Dexmedetomidine may produce variable vasoconstrictor response in individuals with a homozygous genetic variation at the AR-alpha-2BD allele.[29] Individuals with a variant of the SLCO1B1 gene are susceptible to statins-induced rhabdomyolysis. The subjects with mutations of NMDA (N-methyl-D-aspartate) and GABAA (Gamma-aminobutyric acid) receptors show the resistance to hypnotic effects of nitrous oxide or sedative and hypnotic effects of Propofol and etomidate.[30,31,32,33,34]
Genetic variations may sway the activity of hepatic enzymes CYP2D6, which impact many pharmacological activities during the perioperative period. Gene expression in enhanced CYP2D6 activity may induce conversion of codeine to morphine resulting in a greater incidence of opioid-related side effects or vice versa. The enhanced metabolic activity of CYP2D6 ensues in increased metabolic turnover of 5HT3 antagonists like ondansetron and a consequent higher incidence of PONV (Postoperative nausea and vomiting).[32] Genetic variations in the m-receptors are associated with the G variant of the OPRM1304 receptor, leading to an exaggerated response of intrathecal fentanyl administration. Genetic variations in the hepatic enzyme CYP3A4 result in variable response to routinely-used drugs such as benzodiazepines, opioids, steroids, and 5HT3 antagonists.[35]
Future direction
AI is contributing to healthcare improvement, but it leads to a paradigm shift in medicine. At the same time, the anesthesia workforce should also align themselves with these changes.[13] It needs continuous motivation and devotion to walk parallel with the AI system. AI algorithms are still not competent enough to surpass human performance. But AI has a rapid ability to learn and correct errors. With the advancement of technologies and quick learning, AI can minimize system error, which would be more user-friendly and without human errors. Such advancement would be a boon for ICU settings and pain clinics.[13]
The genomic medicine could be self-programmed with the help of the micro-biome for personalized or précised anesthetic needs.[29] Within a few decades, technologies will flourish in the market. These efforts are continuously going on to reduce the global burden of disease or financial burden. Entrepreneurs assume that healthcare is a multi-billion-dollar business and utilize these avenues of a lucrative business. The AI machine developers are trying to amalgamate electronic health information statistics from large patient populations, increasing computing power, and artificial intelligence devices, with the eventual aim of cutting down healthcare expenses while increasing efficacy.[35]
Limitation and ethical issues
We have a very high expectation with AI. It has its limitations, like the predicting algorithms of the machine would not support further action of intervention in a clinical setting. The AI machine’s prediction is based on the training data, which would not help specific situations. A single machine would not solve all clinical problems. The user needs adequate training for a good outcome; otherwise, it may lead to a worse outcome. There is criticism of AI because of the inability to establish a causal relationship, and it may not be more than a black box. Some reports have shown inadequate transparency and trust about AI machines during clinical decisions. The specific patient population for clinical trials can lead to various types of biases, including bias in the treatment decision when applied to real-world care. This can significantly affect the types of predictions made by AI.
In brief, technological advancement can widen the safety margin and reduce human error to a minimum. The quality of anesthesia practice will achieve its highest level. Automation will reduce human effort, but it may also decrease the human workforce. There will be competition between the human workforce and the robotics workforce, which may reduce job opportunities—one of the major concerns in the anesthetist community.
Thus, anesthesiologists should shoulder with data scientists and other healthcare providers to develop systemic error-free AI algorithms. The anesthesiologists would be team leaders in implementing AI and nanotechnology for safe clinical practice and continuing the innovations in safe anesthesia practice with AI and robotics.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hemmerling TM, Terrasini N. Robotic anesthesia:Not the realm of science fiction any more. Curr Opin Anesthesiol. 2012;25:736–42. doi: 10.1097/ACO.0b013e328359aa9f. [DOI] [PubMed] [Google Scholar]
- 2.Murthy BP, Molinari N-AM, LeBlanc TT, Vagi SJ, Avchen RN. Progress in public health emergency preparedness—United States, 2001–2016. Am J Public Health. 2017;107:S180–5. doi: 10.2105/AJPH.2017.304038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickson B. How artificial intelligence is revolutionizing healthcare [Internet]. TNW |Tnw. 2017. [cited 2021 Jun 3]. Available from: https://thenextweb.com/news/artificial-intelligence-revolutionizing-healthcare .
- 4.Hemmerling TM, Arbeid E, Wehbe M, Cyr S, Taddei R, Zaouter C. Evaluation of a novel closed-loop total intravenous anesthesia drug delivery system:A randomized controlled trial. Br J Anaesth. 2013;110:1031–9. doi: 10.1093/bja/aet001. [DOI] [PubMed] [Google Scholar]
- 5.Hemmerling TM, Taddei R, Wehbe M, Zaouter C, Cyr S, Morse J. First robotic tracheal intubations in humans using the Kepler intubation system. Br J Anaesth. 2012;108:1011–6. doi: 10.1093/bja/aes034. [DOI] [PubMed] [Google Scholar]
- 6.Liu N, Chazot T, Hamada S, Landais A, Boichut N, Dussaussoy C, et al. Closed-loop coadministration of propofol and remifentanil guided by bispectral index:A randomized multicenter study. Anesth Analg. 2011;112:546–57. doi: 10.1213/ANE.0b013e318205680b. [DOI] [PubMed] [Google Scholar]
- 7.Mc Grath H, Flanagan C, Zeng L, Lei Y. Anaesthesia monitoring using artificial intelligence techniques. Int J Anesth Anesthesiol. 2019;6:098–103. doi:10.23937/2377-4630/1410098. [Google Scholar]
- 8.Dieleman JL, Templin T, Sadat N, Reidy P, Chapin A, Foreman K, et al. National spending on health by source for 184 countries between 2013 and 2040. Lancet. 2016;387:2521–35. doi: 10.1016/S0140-6736(16)30167-2. [DOI] [PubMed] [Google Scholar]
- 9.Sia ATH, Tan HS, Sng BL. Closed-loop double-vasopressor automated system to treat hypotension during spinal anaesthesia for caesarean section:A preliminary study. Anaesthesia. 2012;67:1348–55. doi: 10.1111/anae.12000. [DOI] [PubMed] [Google Scholar]
- 10.Meijler AP. Automation in Anesthesia —A Relief? A Systematic Approach to Computers in Patient Monitoring. Springer Science and Business Media. 2012:245. [Google Scholar]
- 11.Aframian A, Iranpour F, Cobb J. Artificial Intelligence in Healthcare. Elsevier; 2020. Medical devices and artificial intelligence; pp. 163–77. [Google Scholar]
- 12.Zaouter C, Joosten A, Rinehart J, Struys MM, Hemmerling TM. Autonomous systems in anesthesia:Where do we stand in 2020? A narrative review. Anesth Analg. 2020;130:1120–32. doi: 10.1213/ANE.0000000000004646. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto DA, Witkowski E, Gao L, Meireles O, Rosman G. Artificial intelligence in anesthesiology:Current techniques, clinical applications, and limitations. Anesthesiology. 2020;132:379–94. doi: 10.1097/ALN.0000000000002960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosow C, Manberg PJ. Bispectral index monitoring. Anesthesiol Clin North Am. 2001;19:947–66. doi: 10.1016/s0889-8537(01)80018-3. [DOI] [PubMed] [Google Scholar]
- 15.Penz JFE, Wilcox AB, Hurdle JF. Automated identification of adverse events related to central venous catheters. J Biomed Inform. 2007;40:174–82. doi: 10.1016/j.jbi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 16.McKendrick M, Yang S, McLeod GA. The use of artificial intelligence and robotics in regional anaesthesia. Anaesthesia. 2021;76:171–81. doi: 10.1111/anae.15274. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Gonzalez CG, Herranz-Alonso A, Escudero-Vilaplana V, Ais-Larisgoitia MA, Iglesias-Peinado I, Sanjurjo-Saez M. Robotic dispensing improves patient safety, inventory management, and staff satisfaction in an outpatient hospital pharmacy. J Eval Clin Pract. 2019;25:28–35. doi: 10.1111/jep.13014. [DOI] [PubMed] [Google Scholar]
- 18.Dattoli K, Hannon M, Wakefield C, Hickey IBM Watson Hard At Work:New Breakthroughs Transform Quality Care for Patients [Internet] IBM News Room. 2021. [cited 2021 Jun 3]. Available from: https://newsroom.ibm.com/2013-02-08-IBM-Watson-Hard-At-Work-New-Breakthroughs-Transform-Quality-Care-for-Patients .
- 19.Diprose WK, Buist N, Hua N, Thurier Q, Shand G, Robinson R. Physician understanding, explainability, and trust in a hypothetical machine learning risk calculator. J Am Med Inform Assoc. 2020;27:592–600. doi: 10.1093/jamia/ocz229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kermany DS, Goldbaum M, Cai W, Valentim CC, Liang H, Baxter SL, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172:1122–31.e9. doi: 10.1016/j.cell.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Bridges KH, McSwain JR, Wilson PR. To infinity and beyond:The past, present, and future of tele-anesthesia. Anesth Analg. 2020;130:276–84. doi: 10.1213/ANE.0000000000004346. [DOI] [PubMed] [Google Scholar]
- 22.Hwang D, Chang JW, Benjafield AV, Crocker ME, Kelly C, Becker KA, et al. Effect of telemedicine education and telemonitoring on continuous positive airway pressure adherence. The Tele-OSA randomized trial. Am J Respir Crit Care Med. 2017;197:117–26. doi: 10.1164/rccm.201703-0582OC. [DOI] [PubMed] [Google Scholar]
- 23.Rahmatizadeh S, Valizadeh-Haghi S, Dabbagh A. The role of artificial intelligence in management of critical COVID-19 patients. J Cell Mol Anesth. 2020;5:16–22. [Google Scholar]
- 24.Freitas RA., Jr Exploratory design in medical nanotechnology:A mechanical artificial red cell. Artif Cells Blood Substit Immobil Biotechnol. 1998;26:411–30. doi: 10.3109/10731199809117682. [DOI] [PubMed] [Google Scholar]
- 25.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 26.Dabbagh A, Rajaei S. Halothane:Is there still any place for using the gas as an anesthetic? Hepat Mon. 2011;11:511–2. [PMC free article] [PubMed] [Google Scholar]
- 27.Webster NR, Galley HF. Malden, Mass, Oxford: Blackwell/BMJ; 2006. [Last accessed on 2021 Feb 21]. Anaesthesia Science. Available from: http://site.ebrary.com/id/10158918 . [Google Scholar]
- 28.Kaye AD, Mahakian T, Kaye AJ, Pham AA, Hart BM, Gennuso S, et al. Pharmacogenomics, precision medicine, and implications for anesthesia care. Best Pract Res Clin Anaesthesiol. 2018;32:61–81. doi: 10.1016/j.bpa.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Nash HA. In vivo genetics of anaesthetic action. Br J Anaesth. 2002;89:143–55. doi: 10.1093/bja/aef159. [DOI] [PubMed] [Google Scholar]
- 30.Sato Y, Kobayashi E, Murayama T, Mishina M, Seo N. Effect of N-methyl-d-aspartate receptor e1subunit gene disruption of the action of general anesthetic drugs in mice. Anesthesiology. 2005;102:557–61. doi: 10.1097/00000542-200503000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Linden AM, Sandu C, Aller MI, Vekovischeva OY, Rosenberg PH, Wisden W, et al. TASK-3 knockout mice exhibit exaggerated nocturnal activity, impairments in cognitive functions, and reduced sensitivity to inhalation anesthetics. J Pharmacol Exp Ther. 2007;323:924–34. doi: 10.1124/jpet.107.129544. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds DS, Rosahl TW, Cirone J, O’Meara GF, Haythornthwaite A, Newman RJ, et al. Sedation and anesthesia mediated by distinct GABAA receptor isoforms. J Neurosci. 2003;23:8608–17. doi: 10.1523/JNEUROSCI.23-24-08608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talke P, Stapelfeldt C, Lobo E, Brown R, Scheinin M, Snapir A. Alpha-2B adrenoceptor polymorphism and peripheral vasoconstriction. Pharmacogenet Genomics. 2005;15:357–63. doi: 10.1097/01213011-200505000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Search Collaborative Group. Link E, Parish S, Armitage J, Bowman L, Heath S, et al. SLCO1B1 variants and statin-induced myopathy—A genomewide study. N Engl J Med. 2008;359:789–99. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 35.Landau R, Kern C, Columb MO, Smiley RM, Blouin JL. Genetic variability of the μ-opioid receptor influences intrathecal fentanyl analgesia requirements in laboring women. Pain. 2008;139:5–14. doi: 10.1016/j.pain.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


