Abstract
The Developmental Origins of Health and Disease (DOHaD) approach answers questions surrounding the early events suffered by the mother during reproductive stages that can either partially or permanently influence the developmental programming of children, predisposing them to be either healthy or exhibit negative health outcomes in adulthood. Globally, vulnerable populations tend to present high obesity rates, including among school-age children and women of reproductive age. In addition, adults suffer from high rates of diabetes, hypertension, cardiovascular, and other metabolic diseases. The increase in metabolic outcomes has been associated with the combination of maternal womb conditions and adult lifestyle–related factors such as malnutrition and obesity, smoking habits, and alcoholism. However, to date, “new environmental changes” have recently been considered negative factors of development, such as maternal sedentary lifestyle, lack of maternal attachment during lactation, overcrowding, smog, overurbanization, industrialization, noise pollution, and psychosocial stress experienced during the current SARS-CoV-2 pandemic. Therefore, it is important to recognize how all these factors impact offspring development during pregnancy and lactation, a period in which the subject cannot protect itself from these mechanisms. This review aims to introduce the importance of studying DOHaD, discuss classical programming studies, and address the importance of studying new emerging programming mechanisms, known as actual lifestyle factors, during pregnancy and lactation.
Keywords: Animal models, Gestation, Lactation, Maternal programming, Metabolism, New epigenetic factors
Introduction
Human epidemiological studies have focused on chronic degenerative diseases such as obesity and diabetes that have been carried out in recent decades. From these efforts, it is thought that early development is a critical stage characterized by an increased sensitivity to harmful environmental factors of both short- and medium-term consequences in the offspring [1, 2]. Experimental animal models have been used to confirm how negative changes in nutrition during pregnancy and/or lactation can be determinant in the offspring to induce greater susceptibility to chronic diseases from the early stages of their development and then accentuated, later during adult life. It is known that obesity can be established during the first 1000 days of life in offspring (the period from conception to 2 years old), with the first effects appearing by preschool stage [3, 4]. Worldwide, Mexico is considered the first country in children with overweight and obesity. It is well known that overweight persistence in the early-life population makes individuals prone to dyslipidemia, hypertension, diabetes, and other cardiovascular-related complications in adulthood. In 2012, the National Health and Nutrition Survey (ENSANUT) revealed severe nutrition-related problems, such as the low prevalence of exclusive breastfeeding, chronic malnutrition in children under 5 years old, anemia, overweight, and obesity. By 2016, it was confirmed that overweight and obesity were widely present in Mexican schools among adolescents and adults [5, 6]. In addition, in 2019, the ENSANUT 100 K showed that the high prevalence of obesity among the Mexican population did not differ by social status [6]. Therefore, it is important to analyze the environmental disadvantages that cause individuals to be more vulnerable to weight gain.
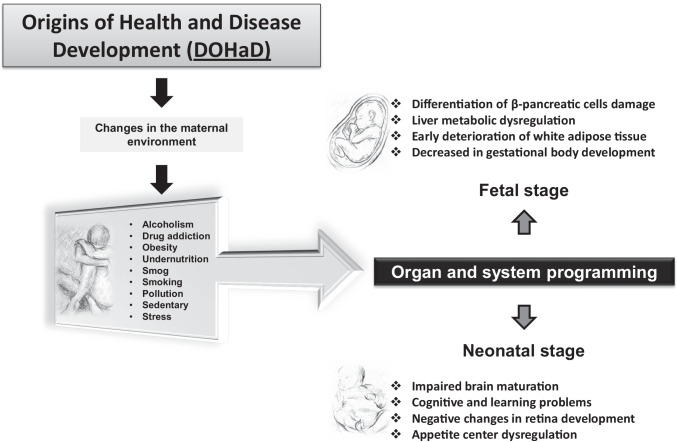
The Food and Agriculture Organization of the United Nations (FAO) revealed that between 2012 and 2016, obesity increased from 20.5 to 24.5 million people in the population of reproductive [7] age (18 years and older) in Mexico, standing in sixth place among 150 countries worldwide [8], thus clearly identifying an early predisposition for obesity in both sexes, with major concern for pregnant women. While it is correct to ascertain that the public health system does its best to serve this segment of the population in terms of service-oriented programs of nutritional counseling, there is no nutritional monitoring of maternal diets as a part of prenatal care in Mexico. However, specific campaigns, either clinical or training courses to guide or apply specific or comprehensive diet education, which are crucial to describe the basic macro- and micronutrients required during the early stages of pregnancy, are lacking [8–10]. In addition, although population studies have shown that the current generation is suffering not only from metabolic but also from behavioral and mental diseases through life stages [11, 12], the effects of lifestyle and climate change have been considered probable causes that negatively influence developmental programming; however, in the last decades, the increment of smog, industrialization, overcrowding, overurbanization, noise pollution, and psychosocial stress has led to the development of new programming factors as a result of different changes in the world [13, 14]. In turn, it is important to study the mechanisms that make organisms respond differently to the developmental processes of life. In recent decades, statistics have indicated that children not only are no longer physically active and suffer from overweight and obesity but also lack cultural identity and interest in school. Furthermore, prevalent broken family structures, which are often accompanied by no social coexistence, are leading children to develop aggressive behaviors, expressed as manifestations of depression and loneliness episodes [15, 16]. Therefore, it is important to highlight how fetal and neonatal development is a vital process that culminates in the mother’s womb and facilitates whether either positive or negative changes in the mother can directly impact offspring health. This, coupled with the presence of epigenetic factors that can also impact offspring by altering organism homeostasis, can explain the presence of multiple diseases in early and adult life, which are well known as the Developmental Origins of Health and Disease (DOHaD) (Fig. 1) [17–20]. Currently, obesity is considered one of the leading causes of death worldwide. Obesity is considered a multifactorial disease that prevails in all social strata, and is not specific to or determined by socioeconomic level, education level, religion, culture, or geographical region [21, 22].
Fig. 1.
Developmental Origins of Health and Disease (DOHaD)
Historical World Events to Negatively Programmed Human Health
Research studies have set their primary objectives to investigate disease distribution and health events in the human population while contributing to the discovery and characterization of the laws that govern or influence these conditions and have allowed us to understand the distribution of diseases and their epidemiological trajectories. Additionally, historical world events have allowed retrospective analysis that helps us determine the changes that occurred in a population’s pathophysiology. The DOHaD concepts recognize how the earliest events in human development are associated with permanent changes in structure and function to disease predisposition in adult life. Studies by Barker, published from 1986 until 2014, explain why obesity and chronic degenerative diseases are derived from these negative environmental changes in the early embryonic and periconceptional periods [18, 23–25]. These studies pioneered an understanding of how these changes could affect and evolve in humans when considering the events sustained by individuals throughout their existence, such as the Second World War, or by characterizing what happened in a population that suffered changes in its social or nearby environment [26–29]. The Barker theory describes and supports the events that occurred between 1911 and 1930 in Hertfordshire, UK, when mothers had their babies with the help of a midwife who reported gaining weight from birth to 1 year, finding a stronger correlation with the increment of birth weight and coronary heart disease in adult life; however, this correlation was neither the same in women and men nor between countries [30]. Additionally, in a Dutch famine, women exposed in early gestation to undernutrition during 1944 to 1945 had babies with a higher prevalence of coronary heart disease and metabolic problems and a greater predisposition to obesity in adult life, while mothers exposed to the same conditions but at midgestation were associated with lung disease and microalbuminuria. Finally, undernutrition occurring at the end of gestation was associated with less glucose tolerance in the affected populations [31]. Therefore, molecular and physiological approaches are necessary to fully understand the cellular complexities that drive diseases. However, ethical considerations in studies with human individuals impede us from fully understanding the phenotypic changes sustained by a particular person and how his or her health deteriorates in the short or long term.
Experimental Models of Research in Programming Development
Research using animal models allows us to understand disease mechanisms and their complex pathways at all biological or molecular levels. In addition, while some animal species have shorter lifespans than humans, animal models provide reproducible support inherent to the epidemiological events that have been reported thus far. In such a way, health authorities provide reliable and conclusive information to inform the population on social health problems. It is important that researchers control environmental variables in experimental design. In programming research, the literature recognizes more than 23 species in which DOHaD has been studied [18, 32]. The animals most often used to understand programming mechanisms are rodents [33–35], sheep [36–39], bovines [40–43], and nonhuman primates [44–46].
Research within this field aims to analyze how positive or negative factors in the maternal environment can significantly modify the establishment and development of offspring. In these studies, different types of treatments, such as a low-protein diet [47–49], a high-fat diet [50], a diet with modified sugar content [51], and interventions such as the use of pharmaceutical drugs [52] and nutritional supplements, have been tested in short- and long-term studies [53, 54], as well as in transgenerational designs. Differences in sex, age, and organ health conditions have also been considered [55, 56]. The use of animals as study models provides scientists with the opportunity to learn the mechanisms involved in health and disease at all biological levels, including molecular and cellular pathways and evolutive adaptations, and maintains control of the required environmental conditions. Due to the widespread presence of pollutants worldwide, the effects of environmental conditions and climate change have recently been considered in DOHaD research. However, studies that address the complex adaptation mechanisms occurring during maternal development and the effects on offspring phenotype are lacking.
Classical Maternal Diet Treatments in Programming Studies
Experimental designs within this field have been mostly directed to understand fetal metabolic programming due to changes in the maternal diet. Fetal life is distinguished as a stage of growth and maturation to establish specific structures and/or fetal physiological functions and encompasses key moments for early healthy development [57]. Under these sequential stages, genes trigger various developmental actions that must be properly synchronized and coordinated to activate reactions in the organism. Studies allow us to understand how diets and certain nutrient imbalances affect not only the mother’s health but also the offspring’s health programming and, in some cases, those of the elderly [58–60]. Different maternal dietary models have been used in these studies to verify this theory.
Protein Restriction Diet
Pregnancy
As previously mentioned, rats and mice are the most studied models in DOHaD. Research in rats has established clear relationships between the maternal environment and diseases related to metabolic syndrome during adult life in progeny [20]. In general, it has been shown that negative offspring programming due to maternal protein restriction manifests in different organs, such as the liver, pancreas, adipose tissue, kidney, and heart. Programming induces the early appearance of metabolic, cardiac, and hormonal alterations [47, 59, 61, 62]. Molecular metabolic pathways, such as lipid synthesis, have been established, and maternal–fetal protein restriction negatively dysregulates the expression and specific binding of proteins and receptors such as PPARα, CPT-1, SREBP-1, and FAS in the liver. Offspring may be predisposed to develop fatty liver, hepatic steatosis, cirrhosis, and liver cancer in adult life [63–66]. In addition, a maternal protein restriction diet also affects offspring glucose metabolism accompanied by insulin resistance in adulthood [67]. Nan Wang and cols. demonstrated in Sprague Dawley rats that maternal protein restriction affects not only glucose tolerance but also average islet size and decreases body weight. However, the results showed an increase in the expression of nutrient-responsive receptors and transporters such as T1R3, SGLT1, and GLUT2, indicating that maternal diet directly influences offspring metabolism [68]. Other studies within a maternal protein-restricted diet during pregnancy have shown not only negative impacts in fetal development and adult offspring but also negative adaptations in the maternal liver, with changes in desaturase and elongase gene expression that reduce the concentrations of long-chain polyunsaturated fatty acids (LC-PUFAs) and affect mammary gland development, differentiation, and proliferation [69]. In conclusion, there is evidence that small changes in maternal dietary protein intake modify liver and mammary gland maternal function, accompanied by negative changes in offspring [69].
Lactation
The lack of amino acids, proteins, and other nutrients in breast milk due to a low-protein diet affects body development and alters liver homeostasis in offspring, thus generating obesity in adult life [70–73]. Fat, an important nutrient during the lactation period, is affected by a low-protein diet regime. Studies in rodents under maternal protein restriction showed that milk has lower fat and arachidonic acid (AA) and docosahexaenoic acid (DHA), known as LC-PUFAs; these constitute the major LC-PUFAs in brain tissue and are essential structural components of the central nervous system [69]. Low content of AA and DHA due to fatty acid elongase and desaturase down-regulation is associated with abnormal prenatal and postnatal development of the retina and brain, causing the incorrect establishment and neural connection during fetal life that continue in lactation [48, 69, 74].
Hormones are another important component of milk [75]. During lactation, the mother must transfer hormones such as leptin, adiponectin, and ghrelin, to the baby to initiate various hormonal axes. Studies in rodents have shown that by 11 to 14 lactation days, an increase in leptin should occur in offspring serum. This increase is known to regulate offspring appetite. Furthermore, offspring from mothers under a low-protein diet regime during the lactation period showed lower leptin concentrations. After weaning, increased body weight, visceral adipose tissue, adipocyte hypertrophy, and insulin resistance could lead to obesity and the development of chronic degenerative diseases in adulthood [71]. Adiponectin has been found to play a critical role in obesity prevention during childhood by regulating lipid and carbohydrate metabolism [76, 77]. Moreover, it is well known that leptin and adiponectin are correlated with birth weight, body mass index, and adiposity [78, 79]. Studies performed in offspring from protein-restricted mothers have demonstrated a catch-up mechanism given that the neuroendocrine connections that regulate hunger, satiety, and maintenance for energy balance [80] were not well established during lactation [81–83]. Besides, many studies have shown that hormones in breast milk have an important role in the neonate and mother energy balance regulation [84]; human studies reported that higher body mass index was associated with high leptin and, insulin levels, and with lower ghrelin concentration in breast milk, which was due to the corresponding maternal hormone levels in serum. Therefore, maternal body mass index seemed to be an essential factor modulating hormone levels in breast milk. In addition, human and animal experimental model is constant to report the low concentration of ghrelin hormone in neonates and the direct regulation by maternal breast milk since it is found in baby’s plasma and milk [85]. Studies in rodents have shown positive correlations between ghrelin concentrations, individual age, weight, head circumference, and size of newborn during the first days of life [106, 107], suggesting the potential hormonal role in protecting the infant from the short-term acceleration of fatty deposit and the long-term obesity and diabetes [84–86]. A maternal protein restriction regime during lactation has been shown to produce changes in milk composition, modifying the supply of macro- and micronutrients and leading to the development of chronic degenerative diseases in offspring when reaching adult life [87].
High Fat Diet
Pregnancy
Several studies have shown that dams exposed to a high-fat diet during gestation and lactation lead to offspring body weight gain and increased adipose index, hyperglycemia, and hepatic lipid composition at weaning compared with pups from control mothers who were fed a nutrient-balanced diet [50, 88–90].
Additionally, studies in pregnant rodents exposed to high-fat diets during pregnancy showed that the fetuses developed greater taste sensitivity to some flavors, such as sugar and fat. Offspring showed weight gain from the first days of extrauterine life and developed obesity and diabetes in adult life [91, 92]. It was also shown that the renin-angiotensin system found in white adipose tissue involved in the regulation of transcription factors and response proteins was altered in the fetus when changes in the maternal diet occurred. This type of maternal treatment negatively modifies adipose tissue homeostasis, which could trigger obesity and hypertension in adulthood [93]. Other research groups have demonstrated that maternal obesity negatively programs the development and proliferation of β cells in the fetus. Maternal obesity has been found to impair the regulation of transcription factors (Pax4, Nkx2.2, Pdx1, MafA, Nkx6.1, Pax6, and NeuroD1/Beta2), which modify synthesis and glucose metabolism in postnatal life, inducing tolerance and insulin resistance in adult offspring [94].
The same effects have been documented in nonhuman primates, showing that maternal obesity can alter liver metabolism in offspring [95, 96]. Additionally, studies in female baboons showed that overfeeding in the preweaning period improves the rate of adiposity index in offspring over fat cell hypertrophy [97, 98]. Meanwhile, in macaques, the maternal high-fat diet leads to lipotoxicity in the fetal liver and predisposes to nonalcoholic fatty liver disease in adult offspring [98, 99]. In addition, macaque offspring from mothers fed a high-fat diet before and during pregnancy showed smaller body weights in the first trimester but displayed catch-up growth and a higher adiposity index in the postnatal life, affecting not only body weight homeostasis but also stress response and cardiovascular abnormalities [100]. Another study in nonhuman primates showed that maternal obesity restricts fetal growth, induces fatty liver, and increases triglyceride levels compared to the control group. The offspring metabolic changes observed during early fetal life persisted in adulthood, with a two fold increase in body fat percentage [101].
Lactation
Several research groups have experimentally used maternal high-fat diet treatments due to their effectiveness in demonstrating the influence of the current lifestyle’s effect on the programming of obesity worldwide [102]. These studies consist of documenting the adverse effects found in offspring after weaning at different stages of their life. However, few studies have demonstrated the consequences on a mother’s health during lactation or on milk composition and how these outcomes would modify growth and maturation patterns in early offspring [48, 71, 91, 103–106]. A study under a maternal high-fat diet regimen found a higher percentage of total lipids in the mammary gland and liver [91]. Other studies demonstrated an increase in total fat content in milk, accompanied by a higher leptin concentration and increased adipose tissue and body weight in male offspring on day 21 of lactation [10, 91]. Furthermore, a high-fat diet consumed during lactation has been associated with a lower adiponectin concentration in offspring [107]. Moreover, female and male offspring with maternal obesity exposure showed increases in adiposity index and triglyceride, insulin, and glucose levels at 110 days of life. In primates, when the mother consumes a high-fat diet during lactation, the concentrations of eicosapentaenoic (EPA), DHA, and essential fatty acids in milk and protein diminish [108]. However, more studies are needed to determine if these negative effects persist or increase over the life course [92].
High-Carbohydrate Diet
Pregnancy
Higher carbohydrate consumption has also been studied. Pregnant mothers exposed to carbohydrate-rich beverages during the developmental period predispose their offspring, both female and male, to gain more weight in adult life. Additionally, females exposed to carbohydrate-rich beverages in gestation had increased body weight and alcohol intake, while males had increased locomotor activity by amphetamine action, as Bocarsly ME. reported [109]. Moreover, the combination of high fat and sugar intake (supplemental diet) during the developmental period increased body weight, and, fat, and concentrations of plasma leptin and glucose. However, when exposed to perinatal and postweaning supplemental diets, fasting glucose levels increased, and leptin resistance appeared in adult rats; the same treatment also sensitized the locomotor response to an acute injection of amphetamine, as Shavel U. and colleagues reported [110]. Accordingly, carbohydrate consumption increased the prevalence of obesity and drug abuse in adulthood in offspring [109, 111]. In addition, studies from Toop C. et al. demonstrated that perinatal exposure to high concentrations of fructose or sucrose during the critical window of development has deleterious metabolic consequences in offspring from young to adult life, mainly on adiposity index, free fatty acid plasma concentration, liver fat, and lipid composition. In conclusion, the carbohydrate type and the exposition period are both essential to establish the consequences of metabolic outcomes in the progeny [112].
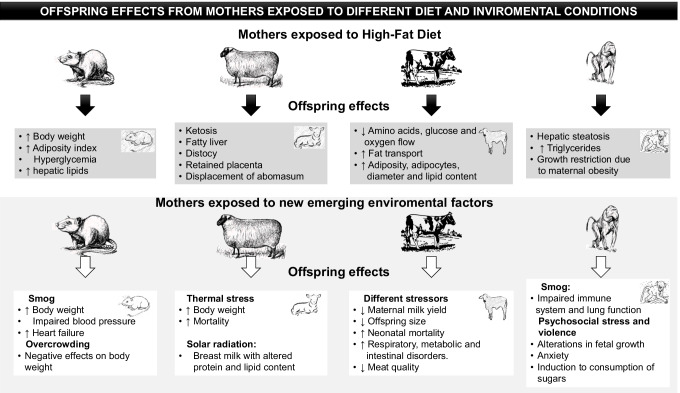
Finally, some additional programming experiments in other species have been studied. For example, the concept of developmental programming was resulted from human epidemiological data and animal experimental models [113] and that until then, it was unknown if it affected other species, such is the case of particular bovines, that are commonly used for meat production and by the food industry, wherein observed particular results due to the deleterious conditions that bovines have been exposed as a result to high demand for food by the human population, such as climate factors, space restrictions [114, 115], and husbandry practices during the last decade [116, 117]. Programming-related studies in these species have explored factors such as nutrient availability, environmental conditions (soil quality, climate and geographic area), heat-related stress, and female competition for resources. The results showed a decrease in maternal milk yield and a reduction in offspring size [118]. Furthermore, these factors negatively affect offspring by increasing neonatal mortality, respiratory, intestinal, and metabolic disorders, and notably, a reduction in meat quality [118]. Good body condition in dry cows is important to maintain lactation and milk production. However, if such cows are fed with an excess fat diet, then they are predisposed to lose body condition as they are at greater risk of metabolic problems after calving [119]. Bovine pregnant females can decrease food intake days before calving, and this is more frequent in obese females, thus predisposing them to mobilizing body reserves and metabolic diseases such as ketosis, fatty liver, distocy, and a reduction in dairy production. They are also at greater risk of vaginal lesions and hemorrhage at delivery, puerperal hypocalcemic paresis, ketosis, and displacement of the abomasum, postpartum immunosuppression, and retained placenta [120] (Fig. 2). Regarding programming, future research should focus on documenting the effect of food quality, especially meat from industrialized production which is associated with negative offspring outcomes, consumed by pregnant and lactating women.
Fig. 2.
Offspring effects from mothers exposed to different diets and environmental conditions
New Emerging (Lifestyle) Factors in Maternal Programming
To date, due to an emerging lifestyle, psychological and even climate-related factors can affect the maternal environment and therefore should be approached with scientific consideration as new emerging factors affecting maternal programming. People are facing new “normal living conditions,” and these emerging factors include a sedentary lifestyle, psychosocial stress due to work issues, overcrowding, smog, overurbanization, industrialization, and noise pollution [13, 14, 16, 71, 121–123], and these factors may either positively or negatively influence and impact offspring development. These factors are known to be determinants in generating permanent changes that can compromise fetal development and lead to further progression of chronic degenerative diseases in early and later life of offspring [59, 124]. Moreover, social environmental changes are a consequence of lifestyle decisions and poor government public policies. However, these decisions have a massive impact on individual health and are the most concerning factor during human reproductive events when a new individual is developing and cannot discern between positive and negative influences. These patterns become more disturbing when pregnant women cannot relocate to find other environmental, psychological, and social conditions. In this sense, there is a need to generate new protection systems as human species to increase resistance to these conditions. While it is difficult to predict and visualize the implications of many present and future diseases, but it is time to acknowledge and increase awareness that modern lifestyles are suboptimal. The following section will include different experimental models to review studies focusing on the new emerging factors that affect maternal programming during pregnancy and lactation.
Sedentary Lifestyle
Pregnancy
Sedentary behavior is present in at least 50% of the population and is an increasing risk factor for type 2 diabetes and developing cardiovascular disease [125]. The relationship between sedentary behavior and type 2 diabetes and impaired glucose tolerance is very high in women compared to men. However, sedentary behavior during pregnancy is poorly demonstrated, as Fazzi C. reported [126] but is directly tied to negative outcomes, not only in mothers but also in offspring. The results from 26 studies showed that pregnant women spent more than 50% of the time in sedentary behavior, and this phenomenon has been highly correlated with an increase in protein C reactive and LDL cholesterol in newborns. In addition, a larger abdominal circumference, hypertension, gestational diabetes, and depression have been observed [126]. In addition, the association between a sedentary lifestyle and a high-fat diet during pregnancy has been widely approached in experimental models [127]. Rats exposed to undernutrition during gestation resulted in offspring with low birth weight. At weaning, offspring were randomly divided into control or either fed hypercaloric diets. At 35, 145, and 420 postnatal days, the offspring from undernourished mothers showed less activity than those born to control mothers. Sedentary behavior was impaired by maternal programming and by postnatal hypercaloric nutrition. The results showed a predisposition to obesity due to prenatal eating behavior and sedentary lifestyle. This behavior was potentiated by postnatal hypercaloric nutrition, thus illustrating the impact of fetal programming, even in those rats where healthy diet behavior was introduced [127]. Therefore, it is necessary to begin recognizing maternal sedentary behavior as an important factor in DOHaD due to the adverse impacts on maternal and child outcomes to avoid metabolic and cardiovascular disorders in adult offspring. Furthermore, due to discrepancies in robustness for different protocols to evaluate sedentary lifestyle, it would be productive to standardize such measurements.
Maternal Stress
Pregnancy
Maternal stress during pregnancy induces irreversible damage to offspring development by compromising the direct neuronal connections that are commonly present between the mother and the fetus, thereby also affecting the immune system and hormone regulation, such as glucocorticoids and adrenaline, and predisposes the progeny to develop metabolic disorders in adulthood [128, 129].
Since the 1970s, human studies have been published about the effects of maternal psychosocial stress and its consequences on offspring. There are many sources of stress, such as experiencing social pressure, noise, marital conflicts, and death in the family, overcrowding, and poverty during pregnancy. The results showed that gestational stress modifies child weight at birth and delays development. Also, it has been associated with poor social interaction, an increase in depression and anxiety, [130–132], and changes in offspring behavior [132, 133] (Fig. 2).
To date, many experimental models have been developed to study stress during pregnancy and its consequences in offspring.
Lack of Maternal Attachment
Lactation
Although offspring are already fully formed during lactation, it has been well recognized that positive and negative maternal programming events occur throughout the lactation period, where the maturation of organs and systems mostly occurs. Therefore, the influence of the maternal environment can greatly affect offspring development. In addition, during lactation, the mother transfers macro- and micronutrients and other important molecules through milk that allow neonates to develop well [134, 135].
Experimental studies have demonstrated that mothers repressing licking, grooming, and normal social behaviors toward their progeny during the lactation period cause offspring to present neurological deficiencies in behavior and stress responses [136], similar to when a working woman is away from home for a long period, repressing herself from appropriately interacting with her baby. The lack of maternal attachment, is associated not only with behavioral-related issues in offspring but also endocrine, emotional, and cognitive responses to [137]. In addition, another study demonstrated that maternal isolation during the first days of life affects the normal development of children, causing alterations in reactivity and emotions at the neuroendocrine level that prevails in adulthood [136].
Transportation Stress
Pregnancy
Maternal Stress
In a study that subjected pregnant rats to traveling in aircraft for 90 min, the authors observed that transportation increased stress-related behaviors such as licking and grooming. After birth, dams were found to present lower body weight and were more susceptible to developing seizures by thermal shock. Interestingly, male offspring were smaller and prone to develop severe seizure attacks, showing that maternal stress is a significant factor that induces changes in fetal programming [138]. Restrain is another experimental model to study stress during pregnancy and lactation. Pregnant rats were kept in small boxes and exposed 45 min of light three times per day or were induced leg pain by electric shock for 1 week. The results showed that male offspring had lower levels of plasma ACTH versus corticosterone than restrained females and controls. Stressed males had lower testosterone levels, and females presented higher anxiety conduct [139]. In a fostering stress model, offspring were exchanged between mothers subjected to restraint stress during pregnancy and normal environmental pregnant mothers. Stressed mothers exhibited anxiety-related behavior, with an increase in retrieval rate, and a significant decrease in spent time interacting with their pups as well as licking duration compared with non-stressed mothers. Additionally, after 6 h of birth, pups were changed between mothers. Stressed foster mothers with non-stressed pups showed an increase in retrieval rate and lower spending time with the offspring and liking time compared with non-stressed foster mothers. When females from stressed mothers that reached maturity were mated, then the new stressed mothers had aggressive behavior increasing the pup’s attack compared with non-stressed mothers; also, stressed mothers had lower nest-building scores, and corticosterone levels were significantly higher in all stressed animals. However, no changes in neuronal morphology were found in cross-fostering mothers. These result showed that the programming behavior persists through generations [140].
Overcrowding
Pregnancy
Overcrowding stress
The effects of maternal overcrowding have been considered to play an essential role in progeny disease predisposition. It has been described that peripartum stress in rat females induces changes in the offspring. In gestate rats subjected to overcrowding, four non-related female rats were allocated to the same small box with a young pregnant rat from pregnancy day 4 until birth. The results showed that overcrowding induced stress behavior in gestate rats and a significant reduction in body weight gain during pregnancy. This phenomenon continued during the lactation period, and the authors observed the same effects in restrained rats that were isolated and exposed to 3 h of light per day. There were no differences in litter number or pup body weight. However, pups from stressed mothers presented less body weight gain, lower corticosterone levels, and increased anxiety behavior on lactating days than pups from non-stressed mothers [141].
Lactation
Overcrowding has been studied as a new programming source in postnatal life in rats as living in small spaces leads to reduced body and organ development. An overcrowding experiment was conducted with three young male rats allocated to a small box (48 cm2 per rat for 27 days). At the end of the study, animals presented a lower body weight and a significant increase in corticosterone levels found in their fur. Additionally, their heart and kidney weights were lower, showing that overcrowding reduced young offspring development [142]. Another study showed the effects on immune function in offspring, as sensitivity and anxiety were found to increase accompanied by physiological and behavioral alterations and depressive symptoms, including changes in the circadian rhythm due to corticosterone and sleep abnormalities [130]. To date, overcrowding in urban areas has extensively increased to the point where small homes (almost 25 m2) are commonly shared between families of three to six people [143]. Recent studies have shown that overcrowding (three to five persons per bedroom) in low-income areas in Ecuador reduces the probability of teenage pregnancy in young women from 14 to 17 years old compared with no overcrowding areas. These findings greatly impact offspring programming because they induce stress that should be considered a covariate or “exposure” in human and animal DOHaD studies [144, 145].
Psychosocial Stress
Pregnancy
In pregnant sows, the effects of social stress were determinate when two multiparous and non-pregnant sows were introduced to the pen of a young pregnant sow. The results showed that pregnant animals adopted submissive behavior, presented slow weight gain, and increased the presence of injuries induced by other sows. Pregnant sows showed stress effects with a significant increase in cortisol levels. In addition, changes in the reproductive axis were observed in offspring [129, 146].
Primates have been considered the best experimental model to study psychosocial stress and violence, given that their brain structure is similar to that of humans. Gestational stress by the effects of psychosocial ambient modified corticotrophin (CRH) activity displayed alterations in the neuroendocrine system during fetal development in humans, however, in nonhuman primates, not only increases CRH activity but also intensifies the incidence of anxiogenic and depressive-like behavior [131]. Another study showed that when dams were injected with corticotrophins or adrenocorticotrophins, offspring showed deficiencies in stress control, similar to when a pregnant woman was exposed to work-related stress [147, 148].
Heat Stress
Pregnancy
Pregnant sheep have been commonly used as a research animal model because they possess important analogies in function and functional structure to the human placenta [149]. They deliver precocial young and fetal lambs with weights similar conditions to humans [150]. Studies have shown that during the first half of pregnancy, hypothermia is correlated with a smaller placenta size and diminished blood flow in the umbilical cord. Hence, lower contents of amino acids, glucose and oxygen [151, 152], and a greater transport of fat [153, 154] cause increases in adiposity, adipocyte diameter, and lipid content by the last pregnancy stage [155, 156]. In addition, when environmental changes occur, such as an increase in temperature, maternal heat stress can be present and has been associated with a reduction in food consumption as a thermoregulatory mechanism to reduce metabolic heat production [157, 158]. These effects have been reported to affect placental development, inducing uterine growth restriction followed by lower weight and a high mortality rate in offspring [153, 159]. Programming mechanisms due to climate change should be an important topic of research.
Lactation
In a study made by Yagil R. [160] in rodents, it was found that lactating mothers exposed to high ambient temperature for 8 h per day negatively affect rat alveolae development and induce adverse effects on milk production; the authors found that milk production was significantly reduced additionally had lower water, protein and potassium content; by the contrary, the fat content was significantly higher compared with the non-stressed group; interestingly, the pups had a significant reduction in milk consumption when they were compared with pups from no stressed mothers; these results suggested that heat stress has negative effects on offspring development [160].
Moreover, the deleterious effects of solar radiation on the protein and fat contents of sheep milk have been found to affect the coagulation properties and firmness of the clot, accompanied by a reduction in unsaturated fatty acid content [161]. While the quantities of lauric, myristic, and palmitic saturated fatty acids increased, a hypercholesterolemic effect on human health has been also reported [162]. In addition, sheep exposed to high temperatures have been found to develop neutrophilia and an increase in lipolytic and proteolytic enzymatic activities in milk (Fig. 2) [161, 163].
Smog Pollution and Overurbanization
Pregnancy
Air pollution has increased worldwide due to human activities [164, 165]. To date, few studies have focused on the effects of smog pollution on maternal offspring programming. In a study with pregnant rats, exposure to wood smoke pollution caused a decrease in the conducting airways of lungs. According to urban pollution studies, exposure to pollution could be associated with early childhood lower respiratory illness [166]. Another study in rats exposed to fine particulate matter (PM2.5) and gaseous pollutants during pregnancy showed behavioral deficits in male offspring at 21 days of life, including increased repetitive behavior, poor social interaction, and inability to differentiate social novelty [122]. Therefore, pollutant presence and concentration in air during pregnancy can directly affect offspring development.
Studies in mice chronically exposed to chemical particles derived from urban traffic have shown that smog plays an important role in regulating oxidative stress and microbiota diversity, which are also related to obesity [167]. In other studies, smog has been observed to affect dam reproduction by altering the placenta’s function and morphology [168–171] and its role in other adverse effects on the progeny from early life, such as weight gain, alterations in blood pressure, and increased risk of developing heart failure [170, 172]. Moreover, while searching for novel programming mechanisms, we found that early exposure to smog in primate mothers compromises the offspring’s immune system and lung function, which transgenerationally persists [108].
Lactation
The human lifestyles cause new epigenetic environmental factors to emerge [173]. Growing urbanization increases pollution, traffic congestion, and smog concentrations, thereby causing negative environmental changes and affecting emotional, physical, and psychological human health in both men and women of reproductive age. A study aimed at the toxicity of maternal exposure to ambient levels of 2.5 (ppm) on filial cardiovascular development in rats found that toxicity induced by homocysteine exacerbated structural abnormalities in filial cardiac tissue and was possibly associated with oxidative stress and reduced expression of the transcription factors GATA 4 and Nkx2-5, both involved in embryonic heart development and structural and functional abnormalities in the fetal heart [174]. Air pollution has recently been declared as a global emergency due to its association with many diseases in animals and humans [175]. However, the effects of air pollution on offspring programming have been poorly studied. Epidemiological analyses regarding air pollution and respiratory viral infections have shown a positive correlation between air levels (ppm) in certain urban areas and human mortality due to cardiovascular and respiratory conditions [176]. Mounting evidence has supported the association of smog and overurbanization with respiratory disease in offspring programming. It has also been shown that the respiratory syndrome caused by SARS-CoV-2 is difficult to control, not only because of the severity of the pathology but also due to the high agglomeration of air pollutants that facilitate its spread [175].
Noise Pollution
Pregnancy
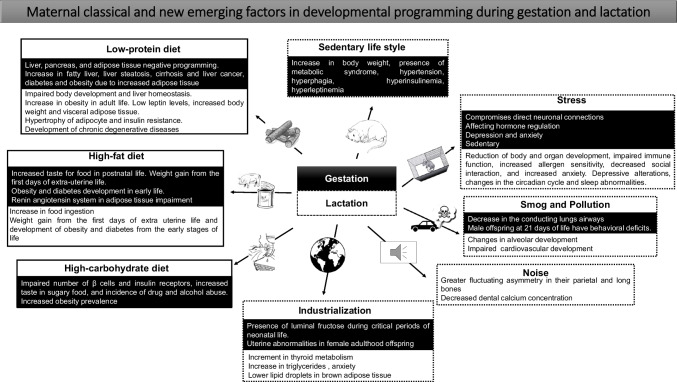
The human body reacts to noise pollution with a general stress response mechanism leading to neuroendocrine and cardiovascular alterations [121]. In a study on mice that examines the effect of noise during pregnancy, scientists found an increase in anxiety-like behavior, such as a reduction in the time spent exploring new objects, higher rates of resorbed embryos, and a reduction in litter size [177]. However, to better understand how noise pollution impacts maternal health in terms of metabolism, reproduction and placental–related issues, and offspring health from early life to adulthood, it is imperative to consider the development of basic experimental, clinical research and epidemiological-related research worldwide (Fig. 3, Table 1).
Fig. 3.
Maternal classical and new emerging factors in developmental programming during gestation and lactation
Table 1.
Critical evidence of current environmental negative changes
| Model | Reference | Environmental factor | Negative effect in the mother during gestation and lactation | Offspring developmental programming |
|---|---|---|---|---|
| Smoke exposure during gestation and lactation | [206, 207] | Smoke | Negative effect on maternal cardiovascular health during early pregnancy and early onset preeclampsia |
•Negative effects in birth weight •Fetal suffering •Affect cognitive development |
| Heavy metal exposure | [208] | Cadmium exposure by traffic | Higher concentrations of cadmium in cord blood |
•Lower fetus development •Low birth weight (less than 2,500 g) •Significantly negative correlation between cord blood cadmium concentrations and IQ development |
| Pollution exposure | [209, 210] | Traffic air pollutants |
Adverse reproductive outcomes Elevated risks for preeclampsia |
•Increased risk of preterm birth and very preterm birth induced by particulate less than 2.5-10 μm in air pollution measured at ambient stations |
| Pollution exposure | [123, 211–216] | Traffic-related air pollution exposure in pregnancy |
Mothers exposed to traffic during gestation, had babies with high risk to asthma in adult life Increase in oxidative stress and inflammation pathways in mothers at first-trimester |
•Asthma development •Children experiment domestic-violence by maternal stress •Damage in maternal metabolism markers by air pollution may cause adverse pregnancy and birth outcomes •Higher risk of small gestational age and reduce birth weight |
| Pollution exposure | [217] | Traffic air pollutants | Increased cadmium concentrations in breast milk on Saudi mothers from Riyadh and AI-Ehssa region | •The estimated weekly intakes of cadmium and mercury of breast-fed infants in this study were higher than the Provisional Tolerance Weekly Intake (PTWI) recommended by FAO/WHO |
| Pollution exposure | [218] | Heavy metal by smoke traffic | Increased mercury and lead in breast-milk | •Adverse effects on the central nervous system in infants |
| Pollution exposure | [219] | Perinatal exposure to traffic- related air pollution | Child's first year of life | •Development of allergies and atopy in offspring |
| Organic pollution | [220] |
Persistent organic pollutants POPs Gestation and lactation |
Changes in gene expression |
•Offspring show less cognitive knowledge alteration of the circadian cycle, increased in sedentary life •Changes of brain genes expression as Cd47, Il1α, Per1 and Clock among others |
| Exposure to toxic compounds | [221] |
Exposure to Fl− Gestation and lactation |
Increase I Fl− levels Oxide-redox imbalance |
•Reduction of the cognitive function •Increases in brain oxidative stress, NO2- and lipoperoxidation, and changes in gene expression in brain |
| Exposure to bisphenol | [222] |
Exposure to bisphenols Polybrominated and mixed halogenated dibenzo-p-dioxins and furans (PBDD/Fs) |
Increase of biphenols, diaoxine and furanes in maternal milk | •Low brain development and delay in development |
| Stress during gestation and lactation | [223,224] | Psychosocial stress during gestation |
Increased cortisol and glucocorticoid hormone |
•Offspring obesity, physical and mental health outcomes •Negative effects on neuroendocrine functions that are linked to food intake, obesity, and energy expenditure in both animals and humans |
| Maternal stress | [129,225–231] | Maternal psychosocial stress in pregnancy |
During early pregnancy resulted in pregnancy loss Mothers increases in serum levels pro-inflammatory cytokines in late pregnancy Increased cortisol, glucocorticoid hormone levels Negative effects in maternal physiology and immune function Increased placenta weight at birth Increased risk of pregnancy complications such as preeclampsia and premature labor |
•Negative effect on the telomere biology system development already present at birth, reflected by the LTL (leukocyte telomere length) •Increased anxiety behavior •Adverse birth outcomes |
| Work stress | [232–235] | Work-related psychologic stress during pregnancy | Increased risk of preeclampsia for women presented in high-stress jobs, compared to low stress job or non-working women | •Risk of preterm delivery, low birth weight infant |
| Post-natal stress | [236] | Return to work timing, job stress, and workplace support affected the lactation period |
Increased of fatigue levels and mental, physical symptoms, breast soreness Also, stressed mothers have an increase of cesarean section, orepisiotomy surgery compared with non- stressed mothers who had vaginal delivery |
•Negative effects in breastfeeding |
| Overcrowding during gestation and lactation | [237] | Overcrowding | Respiratory and digestive diseases due to the low quality of household materials |
•Low academic performance •Frequent accidents due to small space in the home |
| Poverty stress | [238] | Impoverished conditions during gestation | Negative effects on mother during gestation and lactation | •Health adverse effects |
| Noise exposure | [239,240] | Noise during gestation | Poor sleep quality, irritability, low neonatal weight, an induced nerve response occurs in the fetus that can lead to changes in blood pressure and increases in cardiovascular flow | •Stress, altered the ability to concentrate, sleep, performance, and altered psychological behaviors, hearing problems |
| High temperature exposure | [241] | High ambient temperature | Premature birth | •No determinate |
| Perinatal exposure to Polybrominated diphenyl ethers (PDBEs) | [242] |
Autism induced by PDBEs exposure gestation and lactation |
No determinate |
•Lack of social behavior •Reduction of dendrites number and size •Low brain development |
| Social stress | [243] |
Anxiety induced by Covid-19 pandemic in lactating women |
Women increased the risk to develop mild to severe depression or anxiety | •No determinate |
| Nutritional habits | [244] | Cafeteria diet in pregnancy and lactation | Mother depressive like behavior at 60 post natal day |
•Microstructural changes in the brain •Increased expression of GluR1, GluR2 synaptic markers •Less activity |
| Nutritional habits | [245] |
Lactating women subjects to different stressors |
Stress circumstances dictate food election during lactation only 37.4% of women consumed vegetables and fruits associated with less stress levels and regular BMI |
•No determinate |
| Depression | [246] |
Maternal depression lack of parental care during lactation |
Mother stress and anxiety behavior modification in future generations |
•Offspring had 18 down regulated brain genes •Changes were associated with stress dependent pathways, inflammation and behavior •Changes were detected in F2 generation |
Lactation
Longer-lasting sounds can affect animal health. Noise directly affects reproductive physiology and energy consumption. Pregnant female rats exposed to elevated environmental noise levels gave birth to pups with greater fluctuating asymmetry in the parietal zone, longer bones, and lower dental calcium concentrations [178]. Another study in minks showed that females exposed to elevated environmental noise levels killed their own offspring (Fig. 3, Table 1) [179].
Alcohol and Smoking
Pregnancy
Recent studies have shown that the offspring of primate mothers exposed to alcohol or cigarette consumption were negatively affected in fetal programming by an increase in hormonal levels crossing the placenta, resulting in early-life offspring anxiety [148, 180]. Stress conditions can also induce a high consumption of sugar, leading to metabolic deficiencies and obesity (Fig. 2) [148]. In this field, many authors suggest that primates are valuable as experimental models due to similarities in genome sequencing (close to 99%) when compared with humans. However, constraints due to special housing, nutritional requirements, maintenance costs, and ethics make primates a rarely used animal model [101, 148].
Industrialization
Pregnancy
Industrialization involves an extensive reorganization of national economies to manufacture a large number of products for human consumption, and the effects of all of industrial activities in offspring programming have been poorly studied. A recent review showed that the excessive intake of fructose, a common constituent of the Westernized diet due to low cost and production efficiencies, appears to be associated with adverse metabolic effects [181]. Accordingly, another study in rats showed that luminal fructose during critical periods of neonatal development has been reported to stimulate an early upregulation of GLUT-5 expression in the gut. The mechanism by which this upregulation occurs in young rats appears to involve c-fos and c-jun pathway activation, suggesting that postnatal fructose uptake abilities may be determined in early life [182]. Overall, excessive fructose intake is associated with a great number of metabolic disorders. More relevant, fructose intake during pregnancy impacts not only maternal metabolic parameters but also placenta and/or fetal development, influencing postnatal disease risk [181, 183–185]. Another important compound related to industrialization is bisphenol (BPA), which is an organic synthetic compound and a precursor to important plastics, primarily certain polycarbonates and epoxy resins that are used by humans. BPA is a common environmental pollutant that is ubiquitous in the natural environment and can affect human health [186]. Suvorov A. observed the effects of bisphenol exposure during pregnancy and lactation in 37 experimental studies in rodents. The results indicated an increase in uterine abnormalities in female adult offspring, such as changes in estradiol receptor expression, an increase in irregular circadian cycles, elongated cycles in mice, and an increase in infertility, and other conditions, concluding that the mechanism of uterine programming is still unknown. All of these changes founded by Suvorov A. in the 37 animal experimental studies can be related to environmental factors. However, by the moment, they did not report as substantial changes associated with developmental programming or DOHaD [187]. Other studies performed in Mongolian gerbil (Meriones unguiculatus), in which mothers were exposed to bisphenol during pregnancy and lactation, showed that bisphenol causes adverse effects on mammary gland tissue, inducing the development of carcinoma through the increase in TGFβ and metalloproteinases MMP2, MMP3, and MMP9 expression, making it obvious the deleterious effects of bisphenol exposure [188]. In addition, Yousef MM. [189] studied male albino rat offspring from mothers exposed to a low dose of bisphenol during gestation and lactation. The histological analysis showed testicular toxicity as changes in spermatogenic cells, spermatids, Sertoli cells, and Leydig cells. However, higher doses of maternal dose exposure markedly increased all testicular parameters, displaying hypo spermatogenesis and leading to infertility [189].
Industrialization also includes the increase in radiofrequency electromagnetic waves due to exposure to mobile phones, Wi-Fi, and a wide range of electronic devices that are regularly used in daily lives. Recent studies have focused on the effects of radiation due to global telecommunication systems during gestation and postnatal life. Rats were subjected to irradiation (1800 MHz) during pregnancy for 1 or 2 h per day for 1 to 2 weeks. The authors observed an increase in uterine hemorrhage and an increase in reabsorbed or dead fetuses after 2 weeks of exposure. At birth, exposed pups had an increase in body malformations and delay of growth, showing that the electromagnetic field causes detrimental effects in pre- and early postnatal life [190].
Lactation
As previously mentioned above, when studying the effects of industrialization, it is important to address the effects of bisphenol [186]. A recent study of rat offspring from mothers exposed to different bisphenol concentrations during gestation and lactation showed hypotriglyceridemia and hyperthyroxinemia in males and higher 25(OH)D levels in females in offspring early in life. In adult life, males showed lower visceral adiposity, females had smaller fat droplets in brown adipocytes, and changes were related to higher anxiety and locomotor activity in males. Females presented lower exploration behavior, showing that bisphenol exposure damaged the triacylglycerol hormone pathway and caused changes in offspring behavior. Both sexes of offspring presented less food intake [191]. Another study found negative effects on oxidative stress, metabolism disruption, and the liver antioxidant defense system in male offspring mice due to bisphenol exposure [186]. Recently, bisphenol exposure has been related to cognitive dysfunctions in rodents and humans. However, their mechanisms in brain damage are not yet clear. A study in rats exposed to 1 mg/kg/day bisphenol during gestation and lactation found a markedly decreased discrimination ability in pups (postnatal 21 days). Researchers concluded that bisphenol exposure disturbed the response properties of visual neurons for orientation and detection and caused a decrease in synaptic plasticity of pyramidal neurons in the primary visual cortex [192]. Accordingly, Wang Y. [193] demonstrated that higher bisphenol concentrations during pregnancy and lactation could impair the hippocampal function of male offspring by affecting hippocampal neuron growth and apoptosis.
Discussion
Approaches in scientific research within DOHaD have seen a change in priorities in study perspectives due to the emergence of factors that may negatively affect offspring health programming [194, 195]. Due to lifestyles in modern society, several factors stand out, such as eating food with little nutritional value, sedentary behavior, work-related stress, maternal attachment issues, overcrowding, and high pollution levels. In contrast, Delemarre-van de Waal HA. [196] reported that industrialization, physical activity, and socioeconomic status positively affected child growth when supported by a suitable food supply, sanitation services, and higher education level. Therefore, it is important to study the classical DOHaD approaches and consider the influence of these new factors [197–199]. Unfortunately, food shortages and excessive population growth have already led to the overexploitation of ecosystems and natural resources. Additionally, the need to supply food to the entire human population has led to the use of hormone supplements and transgenic biotechnology to reduce physical spaces to raise cattle for mass food production. In turn, the nutritional quality of food is affected, which can negatively affect the health of consumers [200, 201].
It is therefore a priority to question quality of life and health at the individual, familial, and social levels. Little is known about the real damage these negative factors cause to health. It is also imperative to investigate these consequences on women’s health at reproductive age and in the first 1000 days of offspring life to better understand how they affect programming [202, 203]. This stage includes maturation and differentiation processes and the full development of new individuals at incredible speed. New research should be directed with special priority on diseases of the highest incidence, such as obesity, metabolic syndrome, hypertension, cardiovascular diseases, diabetes, and cancer [204, 205].
Conclusion
Overall, studying DOHaD over time is important to understanding how epigenetic factors positively or negatively program the health of progeny. It is important to consider the new factors that now exist in women’s daily lives in the reproductive, pregnancy, and lactating stages. The prevalence of current diseases can be prevented in offspring if lifestyle-related decisions are consciously made based on health. These fundamental changes could be achieved through professional support from health centers, especially those fully oriented to obesity-related counseling, since we are now experiencing the worst epidemic of obesity in world history. However, we must never rule out that the manifestation of almost all current diseases has resulted from induced changes in the societies in which we all live. It is crucial to consider that the most important changes occur at home, and that critical external factors should be modified. This kind of consideration will help us to restore the natural order with respect to animal welfare, including natural spaces for species that have interacted with humans in several paths, and will likely continue in the foreseeable future.
Acknowledgements
The authors express their gratitude for the interinstitutional participation with Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubiran; [CICUAL-BRE-2011-20-22-1] and Servicio de Bioterio y Cirugía Experimental, Facultad Mexicana de Medicina, Universidad La Salle [FMM-SICUAL/002/2020], México City.
Data Availability
N/A.
Code Availability
N/A.
Declarations
Ethics Approval
N/A
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
All involved consented to publication.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Victoria Ramírez and Regina J. Bautista are considered co-first authors.’.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lane RH. Fetal programming, epigenetics, and adult onset disease. Clin Perinatol. 2014;41:815–831. doi: 10.1016/j.clp.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Zambrano E, Rodriguez-Gonzalez GL, Guzman C, Garcia-Becerra R, Boeck L, Diaz L, et al. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol. 2005;563:275–284. doi: 10.1113/jphysiol.2004.078543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayneris-Perxachs J, Swann JR. Metabolic phenotyping of malnutrition during the first 1000 days of life. Eur J Nutr. 2019;58:909–930. doi: 10.1007/s00394-018-1679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med. 2016;50:761–779. doi: 10.1016/j.amepre.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Romero-Martinez M, Shamah-Levy T, Vielma-Orozco E, Heredia-Hernandez O, Mojica-Cuevas J, Cuevas-Nasu L, et al. National Health and Nutrition Survey 2018–19: methodology and perspectives. Salud Publica Mex. 2019;61:917–23. doi: 10.21149/11095. [DOI] [PubMed] [Google Scholar]
- 6.Shamah-Levy T, Rivera-Dommarco J. Bertozzi S [Mexico’s National Health and Nutrition Survey 2018–19: analysis of its main results] Salud Publica Mex. 2020;62:614–617. doi: 10.21149/12280. [DOI] [PubMed] [Google Scholar]
- 7.FAO. 24 million people afflicted with obesity in Mexico - El ...https://www.eluniversal.com.mx ›. El universal 2018.
- 8.Dávila-Torres J G-IJ, Barrera-Cruz A. . . Panorama de la obesidad en México. Rev Med Inst Mex Seguro Soc. 2015. [PubMed]
- 9.Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33:673–689. doi: 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab. 2015;66(Suppl 2):7–12. doi: 10.1159/000375143. [DOI] [PubMed] [Google Scholar]
- 11.Friedman MA, Brownell KD. Psychological correlates of obesity: moving to the next research generation. Psychol Bull. 1995;117:3–20. doi: 10.1037/0033-2909.117.1.3. [DOI] [PubMed] [Google Scholar]
- 12.Orleans CT. Promoting the maintenance of health behavior change: recommendations for the next generation of research and practice. Health Psychol. 2000;19:76–83. doi: 10.1037/0278-6133.19.Suppl1.76. [DOI] [PubMed] [Google Scholar]
- 13.Bonea A DM, Shuttleworth S. . Anxious times: medicine and modernity in nineteenth-century Britain. University of Pittsburgh Press. 2019.
- 14.Sarkar C WCGJ. Healthy cities: public health through urban planning. Edward Elgar Publishing. 2014.
- 15.Huang H, Wan Mohamed Radzi CW, Salarzadeh Jenatabadi H. Family environment and childhood obesity: a new framework with structural equation modeling. Int J Environ Res Public Health. 2017;14. [DOI] [PMC free article] [PubMed]
- 16.KW WJC. Creative destruction, economic insecurity, stress, and epidemic obesity ajes_728 936..982. Am J Econ Sociol 69, 936–982. 2010;69.
- 17.Desai M, Jellyman JK, Ross MG. Epigenomics, gestational programming and risk of metabolic syndrome. Int J Obes (Lond) 2015;39:633–641. doi: 10.1038/ijo.2015.13. [DOI] [PubMed] [Google Scholar]
- 18.Olsen J. David Barker (1938–2013)–a giant in reproductive epidemiology. Acta Obstet Gynecol Scand. 2014;93:1077–1080. doi: 10.1111/aogs.12378. [DOI] [PubMed] [Google Scholar]
- 19.Ross MG, Desai M. Developmental programming of offspring obesity, adipogenesis, and appetite. Clin Obstet Gynecol. 2013;56:529–536. doi: 10.1097/GRF.0b013e318299c39d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zambrano E, Nathanielsz PW. Mechanisms by which maternal obesity programs offspring for obesity: evidence from animal studies. Nutr Rev. 2013;71(Suppl 1):S42–54. doi: 10.1111/nure.12068. [DOI] [PubMed] [Google Scholar]
- 21.Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Soc Sci Med. 2002;55:125–139. doi: 10.1016/S0277-9536(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 22.Leon PX, Morales A, Bautista CJ. LA DESNUTRICIÓN Y LA OBESIDAD EN AUMENTO: UN RETO MUNDIAL PARA LA ERRADICACIÓN. Revista de la Escuela de Medicina Dr José Sierra Flores Universidad del Noreste/Vol. 2020;34:2. [Google Scholar]
- 23.Barker DJ, Fall CH. Fetal and infant origins of cardiovascular disease. Arch Dis Child. 1993;68:797–799. doi: 10.1136/adc.68.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barker DJP, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in england and wales. The Lancet. 1986;327:1077–1081. doi: 10.1016/S0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 26.Henderson A, Schlaifer R. Mathematical programming. Better information for better decision-making. Harv Bus Rev. 1954;32:73–100. [Google Scholar]
- 27.Moritz KM, Singh RR, Probyn ME, Denton KM. Developmental programming of a reduced nephron endowment: more than just a baby’s birth weight. Am J Physiol Renal Physiol. 2009;296:F1–9. doi: 10.1152/ajprenal.00049.2008. [DOI] [PubMed] [Google Scholar]
- 28.Sussman S. A lifespan developmental-stage approach to tobacco and other drug abuse prevention. ISRN Addict. 2013;2013:745783. doi: 10.1155/2013/745783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaiserman A, Lushchak O. Developmental origins of type 2 diabetes: focus on epigenetics. Ageing Res Rev. 2019;55:100957. doi: 10.1016/j.arr.2019.100957. [DOI] [PubMed] [Google Scholar]
- 30.Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–S595. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 31.Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol. 2005;20:345–352. doi: 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Gyllenhammer LE, Entringer S, Buss C, Wadhwa PD. Developmental programming of mitochondrial biology: a conceptual framework and review. Proc Biol Sci. 2020;287:20192713. doi: 10.1098/rspb.2019.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raipuria M, Bahari H, Morris MJ. Effects of maternal diet and exercise during pregnancy on glucose metabolism in skeletal muscle and fat of weanling rats. PLoS ONE. 2015;10:e0120980. doi: 10.1371/journal.pone.0120980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saad MI, Abdelkhalek TM, Haiba MM, Saleh MM, Hanafi MY, Tawfik SH, et al. Maternal obesity and malnourishment exacerbate perinatal oxidative stress resulting in diabetogenic programming in F1 offspring. J Endocrinol Invest. 2016;39:643–655. doi: 10.1007/s40618-015-0413-5. [DOI] [PubMed] [Google Scholar]
- 35.Taylor PD, Samuelsson AM, Poston L. Maternal obesity and the developmental programming of hypertension: a role for leptin. Acta Physiol (Oxf) 2014;210:508–523. doi: 10.1111/apha.12223. [DOI] [PubMed] [Google Scholar]
- 36.Ghnenis AB, Odhiambo JF, McCormick RJ, Nathanielsz PW, Ford SP. Maternal obesity in the ewe increases cardiac ventricular expression of glucocorticoid receptors, proinflammatory cytokines and fibrosis in adult male offspring. PLoS ONE. 2017;12:e0189977. doi: 10.1371/journal.pone.0189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholas LM, Rattanatray L, MacLaughlin SM, Ozanne SE, Kleemann DO, Walker SK, et al. Differential effects of maternal obesity and weight loss in the periconceptional period on the epigenetic regulation of hepatic insulin-signaling pathways in the offspring. FASEB J. 2013;27:3786–3796. doi: 10.1096/fj.13-227918. [DOI] [PubMed] [Google Scholar]
- 38.Pankey CL, Walton MW, Odhiambo JF, Smith AM, Ghnenis AB, Nathanielsz PW, et al. Intergenerational impact of maternal overnutrition and obesity throughout pregnancy in sheep on metabolic syndrome in grandsons and granddaughters. Domest Anim Endocrinol. 2017;60:67–74. doi: 10.1016/j.domaniend.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Tuersunjiang N, Odhiambo JF, Shasa DR, Smith AM, Nathanielsz PW, Ford SP. Maternal obesity programs reduced leptin signaling in the pituitary and altered GH/IGF1 axis function leading to increased adiposity in adult sheep offspring. PLoS ONE. 2017;12:e0181795. doi: 10.1371/journal.pone.0181795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Astiz S, Gonzalez-Bulnes A, Sebastian F, Fargas O, Cano I, Cuesta P. Maternal aging affects life performance of progeny in a Holstein dairy cow model. J Dev Orig Health Dis. 2014;5:374–384. doi: 10.1017/S2040174414000361. [DOI] [PubMed] [Google Scholar]
- 41.Kamal MM, Van Eetvelde M, Bogaert H, Hostens M, Vandaele L, Shamsuddin M, et al. Environmental factors and dam characteristics associated with insulin sensitivity and insulin secretion in newborn Holstein calves. Animal. 2015;9:1490–1499. doi: 10.1017/S1751731115000701. [DOI] [PubMed] [Google Scholar]
- 42.Long NM, Vonnahme KA, Hess BW, Nathanielsz PW, Ford SP. Effects of early gestational undernutrition on fetal growth, organ development, and placentomal composition in the bovine. J Anim Sci. 2009;87:1950–1959. doi: 10.2527/jas.2008-1672. [DOI] [PubMed] [Google Scholar]
- 43.Eetvelde MV, Opsomer G. Prenatal programming of later performance in dairy cattle. Vlaams Diergeneeskundig Tijdschrift. 2020;89.
- 44.Maloyan A, Muralimanoharan S, Huffman S, Cox LA, Nathanielsz PW, Myatt L, et al. Identification and comparative analyses of myocardial miRNAs involved in the fetal response to maternal obesity. Physiol Genomics. 2013;45:889–900. doi: 10.1152/physiolgenomics.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nathanielsz PW, Yan J, Green R, Nijland M, Miller JW, Wu G, et al. Maternal obesity disrupts the methionine cycle in baboon pregnancy. Physiol Rep. 2015;3. [DOI] [PMC free article] [PubMed]
- 46.Puppala S, Li C, Glenn JP, Saxena R, Gawrieh S, Quinn A, et al. Primate fetal hepatic responses to maternal obesity: epigenetic signalling pathways and lipid accumulation. J Physiol. 2018;596:5823–5837. doi: 10.1113/JP275422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zambrano E, Bautista CJ, Deás M, Martínez-Samayoa PM, González-Zamorano M, Ledesma H, et al. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol. 2006;571:221–230. doi: 10.1113/jphysiol.2005.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bautista CJ, Rodriguez-Gonzalez GL, Torres N, Hernandez-Pando R, Ramirez V, Rodriguez-Cruz M, et al. Protein restriction in the rat negatively impacts long-chain polyunsaturated fatty acid composition and mammary gland development at the end of gestation. Arch Med Res. 2013;44:429–436. doi: 10.1016/j.arcmed.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Zambrano E, Guzman C, Rodriguez-Gonzalez GL, Durand-Carbajal M, Nathanielsz PW. Fetal programming of sexual development and reproductive function. Mol Cell Endocrinol. 2014;382:538–549. doi: 10.1016/j.mce.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Vega CC, Reyes-Castro LA, Bautista CJ, Larrea F, Nathanielsz PW, Zambrano E. Exercise in obese female rats has beneficial effects on maternal and male and female offspring metabolism. Int J Obes (Lond) 2015;39:712–719. doi: 10.1038/ijo.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diaz-Aguila Y, Castelan F, Cuevas E, Zambrano E, Martinez-Gomez M, Munoz A, et al. Consumption of sucrose from infancy increases the visceral fat accumulation, concentration of triglycerides, insulin and leptin, and generates abnormalities in the adrenal gland. Anat Sci Int. 2016;91:151–162. doi: 10.1007/s12565-015-0279-9. [DOI] [PubMed] [Google Scholar]
- 52.Padmanabhan V, Salvetti NR, Matiller V, Ortega HH. Developmental programming: prenatal steroid excess disrupts key members of intraovarian steroidogenic pathway in sheep. Endocrinology. 2014;155:3649–3660. doi: 10.1210/en.2014-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chong MF, Ong YL, Calder PC, Colega M, Wong JX, Tan CS, et al. Long-chain polyunsaturated fatty acid status during pregnancy and maternal mental health in pregnancy and the postpartum period: results from the GUSTO study. J Clin Psychiatry. 2015;76:e848–e856. doi: 10.4088/JCP.14m09191. [DOI] [PubMed] [Google Scholar]
- 54.Steenweg-de Graaff JC, Tiemeier H, Basten MG, Rijlaarsdam J, Demmelmair H, Koletzko B, et al. Maternal LC-PUFA status during pregnancy and child problem behavior: the Generation R Study. Pediatr Res. 2015;77:489–497. doi: 10.1038/pr.2014.204. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez-Gonzalez GL, Vigueras-Villasenor RM, Millan S, Moran N, Trejo R, Nathanielsz PW, et al. Maternal protein restriction in pregnancy and/or lactation affects seminiferous tubule organization in male rat offspring. J Dev Orig Health Dis. 2012;3:321–326. doi: 10.1017/S2040174412000360. [DOI] [PubMed] [Google Scholar]
- 56.Zambrano E, Reyes-Castro LA, Nathanielsz PW. Aging, glucocorticoids and developmental programming. Age (Dordr) 2015;37:9774. doi: 10.1007/s11357-015-9774-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segovia SA, Vickers MH, Gray C, Reynolds CM. Maternal obesity, inflammation, and developmental programming. Biomed Res Int. 2014;2014:418975. doi: 10.1155/2014/418975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khorram O, Chuang TD, Pearce WJ. Long-term effects of maternal undernutrition on offspring carotid artery remodeling: role of miR-29c. J Dev Orig Health Dis. 2015;6:342–349. doi: 10.1017/S2040174415001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Léonhardt M, Lesage J, Croix D, Dutriez-Casteloot I, Beauvillain JC, Dupouy JP. Effects of perinatal maternal food restriction on pituitary-gonadal axis and plasma leptin level in rat pup at birth and weaning and on timing of puberty. Biol Reprod. 2003;68:390–400. doi: 10.1095/biolreprod.102.003269. [DOI] [PubMed] [Google Scholar]
- 60.Symonds ME, Budge H, Stephenson T, Gardner DS. Experimental evidence for long-term programming effects of early diet. Adv Exp Med Biol. 2005;569:24–32. doi: 10.1007/1-4020-3535-7_4. [DOI] [PubMed] [Google Scholar]
- 61.Roeder LM, Chow BF. Maternal undernutrition and its long-term effects on the offspring. Am J Clin Nutr. 1972;25:812–821. doi: 10.1093/ajcn/25.8.812. [DOI] [PubMed] [Google Scholar]
- 62.Zambrano E, Rodríguez-González GL, Guzmán C, García-Becerra R, Boeck L, Díaz L, et al. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol. 2005;563:275–284. doi: 10.1113/jphysiol.2004.078543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Da Costa NM, Visoni SB, Dos Santos IL, Barja-Fidalgo TC, Ribeiro-Pinto LF. Maternal protein restriction during lactation modulated the expression and activity of rat offspring hepatic CYP1A1, CYP1A2, CYP2B1, CYP2B2, and CYP2E1 during development. Braz J Med Biol Res. 2016;49:e5238. doi: 10.1590/1414-431x20165238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moraes C, Rebelato HJ, Amaral ME, Resende TM, Silva EV, Esquisatto MA, et al. Effect of maternal protein restriction on liver metabolism in rat offspring. J Physiol Sci. 2014;64:347–355. doi: 10.1007/s12576-014-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qasem RJ, Li J, Tang HM, Browne V, Mendez-Garcia C, Yablonski E, et al. Decreased liver triglyceride content in adult rats exposed to protein restriction during gestation and lactation: role of hepatic triglyceride utilization. Clin Exp Pharmacol Physiol. 2015;42:380–388. doi: 10.1111/1440-1681.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng J, Xiao X, Zhang Q, Yu M, Xu J, Wang Z. Maternal protein restriction induces early-onset glucose intolerance and alters hepatic genes expression in the peroxisome proliferator-activated receptor pathway in offspring. J Diabetes Investig. 2015;6:269–279. doi: 10.1111/jdi.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim J, Choi A, Kwon YH. Maternal protein restriction altered insulin resistance and inflammation-associated gene expression in adipose tissue of young adult mouse offspring in response to a high-fat diet. Nutrients. 2020;12. [DOI] [PMC free article] [PubMed]
- 68.Wang N, Lv B, Guan L, Qiao H, Sun B, Luo X, et al. Maternal low protein exposure alters glucose tolerance and intestinal nutrient-responsive receptors and transporters expression of rat offspring. Life Sci. 2020;243:117216. doi: 10.1016/j.lfs.2019.117216. [DOI] [PubMed] [Google Scholar]
- 69.Torres N, Bautista CJ, Tovar AR, Ordáz G, Rodríguez-Cruz M, Ortiz V, et al. Protein restriction during pregnancy affects maternal liver lipid metabolism and fetal brain lipid composition in the rat. Am J Physiol Endocrinol Metab. 2010;298:E270–E277. doi: 10.1152/ajpendo.00437.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alheiros-Lira MC, Araújo LL, Trindade NG, da Silva EM, Cavalcante TC, de Santana MG, et al. Short- and long-term effects of a maternal low-energy diet ad libitum during gestation and/or lactation on physiological parameters of mothers and male offspring. Eur J Nutr. 2015;54:793–802. doi: 10.1007/s00394-014-0758-0. [DOI] [PubMed] [Google Scholar]
- 71.Bautista CJ, Boeck L, Larrea F, Nathanielsz PW, Zambrano E. Effects of a maternal low protein isocaloric diet on milk leptin and progeny serum leptin concentration and appetitive behavior in the first 21 days of neonatal life in the rat. Pediatr Res. 2008;63:358–363. doi: 10.1203/01.pdr.0000304938.78998.21. [DOI] [PubMed] [Google Scholar]
- 72.Ronayne De Ferrer PA, Sambucetti ME. Casein to whey protein ratio in rat and human milks: effects of maternal protein intake. J Dairy Sci. 1993;76:1645–53. doi: 10.3168/jds.S0022-0302(93)77498-6. [DOI] [PubMed] [Google Scholar]
- 73.de Sousa SM, Braz GRF, Freitas CM, de Santana DF, Sellitti DF, Fernandes MP, et al. Oxidative injuries induced by maternal low-protein diet in female brainstem. Nutr Neurosci. 2018;21:580–588. doi: 10.1080/1028415X.2017.1325974. [DOI] [PubMed] [Google Scholar]
- 74.Martin Agnoux A, Antignac JP, Boquien CY, David A, Desnots E, Ferchaud-Roucher V, et al. Perinatal protein restriction affects milk free amino acid and fatty acid profile in lactating rats: potential role on pup growth and metabolic status. J Nutr Biochem. 2015;26:784–795. doi: 10.1016/j.jnutbio.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 75.Lee S, Kelleher SL. Biological underpinnings of breastfeeding challenges: the role of genetics, diet, and environment on lactation physiology. Am J Physiol Endocrinol Metab. 2016;311:E405–E422. doi: 10.1152/ajpendo.00495.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gil-Campos M, Cañete R, Gil A. Hormones regulating lipid metabolism and plasma lipids in childhood obesity. Int J Obes Relat Metab Disord. 2004;28(Suppl 3):S75–80. doi: 10.1038/sj.ijo.0802806. [DOI] [PubMed] [Google Scholar]
- 77.Gil-Campos M, Cañete RR, Gil A. Adiponectin, the missing link in insulin resistance and obesity. Clin Nutr. 2004;23:963–974. doi: 10.1016/j.clnu.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 78.Brunner S, Schmid D, Zang K, Much D, Knoeferl B, Kratzsch J, et al. Breast milk leptin and adiponectin in relation to infant body composition up to 2 years. Pediatr Obes. 2015;10:67–73. doi: 10.1111/j.2047-6310.2014.222.x. [DOI] [PubMed] [Google Scholar]
- 79.Dundar NO, Anal O, Dundar B, Ozkan H, Caliskan S, Büyükgebiz A. Longitudinal investigation of the relationship between breast milk leptin levels and growth in breast-fed infants. J Pediatr Endocrinol Metab. 2005;18:181–187. doi: 10.1515/JPEM.2005.18.2.181. [DOI] [PubMed] [Google Scholar]
- 80.Huang LL, Yang F. Xiong F [Association of leptin, adiponectin, and ghrelin in breast milk with the growth of infants with exclusive breastfeeding] Zhongguo Dang Dai Er Ke Za Zhi. 2018;20:91–96. doi: 10.7499/j.issn.1008-8830.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dulloo AG. Thrifty energy metabolism in catch-up growth trajectories to insulin and leptin resistance. Best Pract Res Clin Endocrinol Metab. 2008;22:155–171. doi: 10.1016/j.beem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Remmers F, Delemarre-van de Waal HA. Developmental programming of energy balance and its hypothalamic regulation. Endocr Rev. 2011;32:272–311. doi: 10.1210/er.2009-0028. [DOI] [PubMed] [Google Scholar]
- 83.Zhu S, Eclarinal J, Baker MS, Li G, Waterland RA. Developmental programming of energy balance regulation: is physical activity more ‘programmable’ than food intake? Proc Nutr Soc. 2016;75:73–77. doi: 10.1017/S0029665115004127. [DOI] [PubMed] [Google Scholar]
- 84.Savino F, Liguori SA. Update on breast milk hormones: leptin, ghrelin and adiponectin. Clin Nutr. 2008;27:42–47. doi: 10.1016/j.clnu.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 85.Yu X, Rong SS, Sun X, Ding G, Wan W, Zou L, et al. Associations of breast milk adiponectin, leptin, insulin and ghrelin with maternal characteristics and early infant growth: a longitudinal study. Br J Nutr. 2018;120:1380–1387. doi: 10.1017/S0007114518002933. [DOI] [PubMed] [Google Scholar]
- 86.Kon IY, Shilina NM, Gmoshinskaya MV, Ivanushkina TA. The study of breast milk IGF-1, leptin, ghrelin and adiponectin levels as possible reasons of high weight gain in breast-fed infants. Ann Nutr Metab. 2014;65:317–323. doi: 10.1159/000367998. [DOI] [PubMed] [Google Scholar]
- 87.Reyes-Castro LA, Rodriguez JS, Charco R, Bautista CJ, Larrea F, Nathanielsz PW, et al. Maternal protein restriction in the rat during pregnancy and/or lactation alters cognitive and anxiety behaviors of female offspring. Int J Dev Neurosci. 2012;30:39–45. doi: 10.1016/j.ijdevneu.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 88.Buison A, Lu H, Guo F, Catherine Jen KL. High-fat feeding of different fats during pregnancy and lactation in rats: effects on maternal metabolism, pregnancy outcome, milk and tissue fatty acid profiles. Nutr Res. 1997;17:1541–1554. doi: 10.1016/S0271-5317(97)00150-4. [DOI] [Google Scholar]
- 89.Guo F, Jen KL. High-fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiol Behav. 1995;57:681–686. doi: 10.1016/0031-9384(94)00342-4. [DOI] [PubMed] [Google Scholar]
- 90.Guo F ZSJK. High-fat feeding during pregnancy affects pregnancy outcome in rats. FASEB JOURNA. 1992;6.
- 91.Bautista CJ, Montaño S, Ramirez V, Morales A, Nathanielsz PW, Bobadilla NA, et al. Changes in milk composition in obese rats consuming a high-fat diet. Br J Nutr. 2016;115:538–546. doi: 10.1017/S0007114515004547. [DOI] [PubMed] [Google Scholar]
- 92.Lomas-Soria C, Reyes-Castro LA, Rodríguez-González GL, Ibáñez CA, Bautista CJ, Cox LA, et al. Maternal obesity has sex-dependent effects on insulin, glucose and lipid metabolism and the liver transcriptome in young adult rat offspring. J Physiol. 2018;596:4611–4628. doi: 10.1113/JP276372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guberman C, Jellyman JK, Han G, Ross MG, Desai M. Maternal high-fat diet programs rat offspring hypertension and activates the adipose renin-angiotensin system. Am J Obstet Gynecol. 2013;209(262):e1–8. doi: 10.1016/j.ajog.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu H, Liu Y, Wang H, Xu X. High-fat diet induced insulin resistance in pregnant rats through pancreatic pax6 signaling pathway. Int J Clin Exp Pathol. 2015;8:5196–5202. [PMC free article] [PubMed] [Google Scholar]
- 95.Choi J, Li C, McDonald TJ, Comuzzie A, Mattern V, Nathanielsz PW. Emergence of insulin resistance in juvenile baboon offspring of mothers exposed to moderate maternal nutrient reduction. Am J Physiol Regul Integr Comp Physiol. 2011;301:R757–R762. doi: 10.1152/ajpregu.00051.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suter M, Bocock P, Showalter L, Hu M, Shope C, McKnight R, et al. Epigenomics: maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates. Faseb j. 2011;25:714–726. doi: 10.1096/fj.10-172080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lewis DS, Bertrand HA, McMahan CA, McGill HC, Jr, Carey KD, Masoro EJ. Preweaning food intake influences the adiposity of young adult baboons. J Clin Invest. 1986;78:899–905. doi: 10.1172/JCI112678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li M, Sloboda DM, Vickers MH. Maternal obesity and developmental programming of metabolic disorders in offspring: evidence from animal models. Exp Diabetes Res. 2011;2011:592408. doi: 10.1155/2011/592408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bruns CM, Kemnitz JW. Sex hormones, insulin sensitivity, and diabetes mellitus. Ilar j. 2004;45:160–169. doi: 10.1093/ilar.45.2.160. [DOI] [PubMed] [Google Scholar]
- 100.Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology. 2010;151:1622–1632. doi: 10.1210/en.2009-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Williams L, Seki Y, Vuguin PM, Charron MJ. Animal models of in utero exposure to a high fat diet: a review. Biochim Biophys Acta. 2014;1842:507–519. doi: 10.1016/j.bbadis.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bautista CJ. Zambrano E [Biology and biochemical aspects of long-chains polyunsaturated fatty acid during gestation] Rev Invest Clin. 2010;62:267–275. [PubMed] [Google Scholar]
- 104.Mendes-da-Silva C, Giriko C, Mennitti LV, Hosoume LF, Souto Tdos S, Silva AV. Maternal high-fat diet during pregnancy or lactation changes the somatic and neurological development of the offspring. Arq Neuropsiquiatr. 2014;72:136–144. doi: 10.1590/0004-282X20130220. [DOI] [PubMed] [Google Scholar]
- 105.Morales A F-GO, de Jesús Bautista R , Bautista CJ,. LACTANCIA MATERNA: Una gota blanca de oportunidades. Revista de la Escuela de Medicina Dr José Sierra Flores Universidad del Noreste. 2018;32.
- 106.Ramirez V, Montaño FA, Bautista RJ, Mummidi S, Alvarenga JC, Bautista CJ. Lactation plays a fundamental role in developmental programming. endocr metab immune disord drug targets. 2020. [DOI] [PubMed]
- 107.Gregoraszczuk E, Slupecka M, Wolinski J, Hejmej A, Bilinska B, Fiedor E, et al. Maternal high-fat diet during pregnancy and lactation had gender difference effect on adiponectin in rat offspring. J Physiol Pharmacol. 2016;67:543–553. [PubMed] [Google Scholar]
- 108.Miller LA. Are adverse health effects from air pollution exposure passed on from mother to child? wildfire exposure to rhesus monkeys final report prepared for the california air resources board and the California Environmental Protection Agency 2019.
- 109.Bocarsly ME, Barson JR, Hauca JM, Hoebel BG, Leibowitz SF, Avena NM. Effects of perinatal exposure to palatable diets on body weight and sensitivity to drugs of abuse in rats. Physiol Behav. 2012;107:568–575. doi: 10.1016/j.physbeh.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shalev U, Tylor A, Schuster K, Frate C, Tobin S, Woodside B. Long-term physiological and behavioral effects of exposure to a highly palatable diet during the perinatal and post-weaning periods. Physiol Behav. 2010;101:494–502. doi: 10.1016/j.physbeh.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 111.Gold MS, Avena NM. Animal models lead the way to further understanding food addiction as well as providing evidence that drugs used successfully in addictions can be successful in treating overeating. Biol Psychiatry. 2013;74:e11. doi: 10.1016/j.biopsych.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Toop CR, Muhlhausler BS, O'Dea K, Gentili S. Impact of perinatal exposure to sucrose or high fructose corn syrup (HFCS-55) on adiposity and hepatic lipid composition in rat offspring. J Physiol. 2017;595:4379–4398. doi: 10.1113/JP274066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Funston RN, Mulliniks JT. Developmental programming in livestock production. Vet Clin: Food Anim Pract. 2019;35:xiii–xiv. doi: 10.1016/j.cvfa.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 114.Lebarbenchon C, Gauthier-Clerc M, Thomas F. Parasitological consequences of overcrowding in protected areas. EcoHealth. 2006;3:303–307. doi: 10.1007/s10393-006-0067-z. [DOI] [Google Scholar]
- 115.Scott ME. The impact of infection and disease on animal populations: implications for conservation biology. Conserv Biol. 1988;2:40–56. doi: 10.1111/j.1523-1739.1988.tb00334.x. [DOI] [Google Scholar]
- 116.Bouzid M, Kintz E, Hunter PR. Risk factors for Cryptosporidium infection in low and middle income countries: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12:e0006553. doi: 10.1371/journal.pntd.0006553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Greger M. The human/animal interface: emergence and resurgence of zoonotic infectious diseases. Crit Rev Microbiol. 2007;33:243–299. doi: 10.1080/10408410701647594. [DOI] [PubMed] [Google Scholar]
- 118.Góngora A, Hernández A. LA REPRODUCCIÓN DE LA VACA SE AFECTA POR LAS ALTAS TEMPERATURAS AMBIENTALES. Revista UDCA Actualidad & Divulgación Científica. 2010;13:163–173. [Google Scholar]
- 119.Lopez FJ. Relación entre condición corporal y eficiencia reproductiva en vacas holstein. Biotecnología en el Sector Agropecuario y Agroindustrial. 2006;4:77–86. [Google Scholar]
- 120.Redrovan Passato DC GFJ, Durán Aguillón LE.. IDENTIFICACIÓN DE FACTORES DE RIESGO PARA LA PRESENTACIÓN DE CETOSIS SUBCLÍNICA EN GANADO BOVINO LECHERO. Revista Científica Ecuatoriana. 2020;7.
- 121.Dzhambov AM, Dimitrova DD, Dimitrakova ED. Noise exposure during pregnancy, birth outcomes and fetal development: meta-analyses using quality effects model. Folia Med (Plovdiv) 2014;56:204–214. doi: 10.2478/folmed-2014-0030. [DOI] [PubMed] [Google Scholar]
- 122.Emam B, Shahsavani A, Khodagholi F, Zarandi SM, Hopke PK, Hadei M, et al. Effects of PM(2.5) and gases exposure during prenatal and early-life on autism-like phenotypes in male rat offspring. Part Fibre Toxicol. 2020;17:8. [DOI] [PMC free article] [PubMed]
- 123.Gehring U, Wijga AH, Fischer P, de Jongste JC, Kerkhof M, Koppelman GH, et al. Traffic-related air pollution, preterm birth and term birth weight in the PIAMA birth cohort study. Environ Res. 2011;111:125–135. doi: 10.1016/j.envres.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 124.Férézou-Viala J, Roy AF, Sérougne C, Gripois D, Parquet M, Bailleux V, et al. Long-term consequences of maternal high-fat feeding on hypothalamic leptin sensitivity and diet-induced obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1056–R1062. doi: 10.1152/ajpregu.00117.2007. [DOI] [PubMed] [Google Scholar]
- 125.de Rezende LF, Rodrigues Lopes M, Rey-Lopez JP, Matsudo VK, Luiz OC. Sedentary behavior and health outcomes: an overview of systematic reviews. PLoS ONE. 2014;9:e105620. doi: 10.1371/journal.pone.0105620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fazzi C, Saunders DH, Linton K, Norman JE, Reynolds RM. Sedentary behaviours during pregnancy: a systematic review. Int J Behav Nutr Phys Act. 2017;14:32. doi: 10.1186/s12966-017-0485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vickers MH, Breier BH, McCarthy D, Gluckman PD. Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. Am J Physiol Regul Integr Comp Physiol. 2003;285:R271–R273. doi: 10.1152/ajpregu.00051.2003. [DOI] [PubMed] [Google Scholar]
- 128.Weinstock M, Fride E, Hertzberg R. Prenatal stress effects on functional development of the offspring. Prog Brain Res. 1988;73:319–331. doi: 10.1016/S0079-6123(08)60513-0. [DOI] [PubMed] [Google Scholar]
- 129.Brunton PJ. Effects of maternal exposure to social stress during pregnancy: consequences for mother and offspring. Reproduction. 2013;146:R175–R189. doi: 10.1530/REP-13-0258. [DOI] [PubMed] [Google Scholar]
- 130.Weinstock M. Effects of maternal stress on development and behaviour in rat offspring. Stress. 2001;4:157–167. doi: 10.3109/10253890109035015. [DOI] [PubMed] [Google Scholar]
- 131.Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 132.Edlow AG. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat Diagn. 2017;37:95–110. doi: 10.1002/pd.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Richetto J, Riva MA. Prenatal maternal factors in the development of cognitive impairments in the offspring. J Reprod Immunol. 2014;104–105:20–25. doi: 10.1016/j.jri.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 134.Haschke F, Grathwohl D, Haiden N. Metabolic programming: effects of early nutrition on growth, metabolism and body composition. Nestle Nutr Inst Workshop Ser. 2016;86:87–95. doi: 10.1159/000442728. [DOI] [PubMed] [Google Scholar]
- 135.Keen CL, Lönnerdal B, Clegg M, Hurley LS. Developmental changes in composition of rat milk: trace elements, minerals, protein, carbohydrate and fat. J Nutr. 1981;111:226–236. doi: 10.1093/jn/111.2.226. [DOI] [PubMed] [Google Scholar]
- 136.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/S0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 138.Moriyama C, Galic MA, Mychasiuk R, Pittman QJ, Perrot TS, Currie RW, et al. Prenatal transport stress, postnatal maternal behavior, and offspring sex differentially affect seizure susceptibility in young rats. Epilepsy Behav. 2013;29:19–27. doi: 10.1016/j.yebeh.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 139.Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- 140.Del Cerro MC, Perez-Laso C, Ortega E, Martin JL, Gomez F, Perez-Izquierdo MA, et al. Maternal care counteracts behavioral effects of prenatal environmental stress in female rats. Behav Brain Res. 2010;208:593–602. doi: 10.1016/j.bbr.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 141.Hillerer KM, Reber SO, Neumann ID, Slattery DA. Exposure to chronic pregnancy stress reverses peripartum-associated adaptations: implications for postpartum anxiety and mood disorders. Endocrinology. 2011;152:3930–3940. doi: 10.1210/en.2011-1091. [DOI] [PubMed] [Google Scholar]
- 142.Uarquin DG, Meyer JS, Cardenas FP, Rojas MJ. Effect of overcrowding on hair corticosterone concentrations in juvenile male Wistar rats. J Am Assoc Lab Anim Sci. 2016;55:749–755. [PMC free article] [PubMed] [Google Scholar]
- 143.Niu G, Zhao G. Living condition among China’s rural–urban migrants: recent dynamics and the inland–coastal differential. Hous Stud. 2018;33:476–493. doi: 10.1080/02673037.2017.1351924. [DOI] [Google Scholar]
- 144.Grandjean P, Barouki R, Bellinger DC, Casteleyn L, Chadwick LH, Cordier S, et al. Life-long implications of developmental exposure to environmental stressors: new perspectives. Endocrinology. 2015;156:3408–3415. doi: 10.1210/en.2015-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Díaz-Sánchez JP, Lanchimba C, Obaco M. The association between overcrowded households and adolescent pregnancy. Sexuality Research and Social Policy. 2020;18:555–563. doi: 10.1007/s13178-020-00480-8. [DOI] [Google Scholar]
- 146.Ashworth CJ, Hogg CO, Hoeks CW, Donald RD, Duncan WC, Lawrence AB, et al. Pre-natal social stress and post-natal pain affect the developing pig reproductive axis. Reproduction. 2011;142:907–914. doi: 10.1530/REP-11-0280. [DOI] [PubMed] [Google Scholar]
- 147.Schneider ML, Coe CL, Lubach GR. Endocrine activation mimics the adverse effects of prenatal stress on the neuromotor development of the infant primate. Dev Psychobiol. 1992;25:427–439. doi: 10.1002/dev.420250604. [DOI] [PubMed] [Google Scholar]
- 148.Schneider ML MC. Prenatal stress and offspring development in nonhuman primates. In: Tremblay RE, Boivin M, Peters RDeV, eds Glover V, topic ed Encyclopedia on Early Childhood Development [online]. 2011.
- 149.Barry JS, Anthony RV. The pregnant sheep as a model for human pregnancy. Theriogenology. 2008;69:55–67. doi: 10.1016/j.theriogenology.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Carter AM. Animal models of human placentation–a review. Placenta. 2007;28(Suppl A):S41–7. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 151.Rees S, Mallard C, Breen S, Stringer M, Cock M, Harding R. Fetal brain injury following prolonged hypoxemia and placental insufficiency: a review. Comp Biochem Physiol A: Mol Integr Physiol. 1998;119:653–660. doi: 10.1016/S1095-6433(98)01001-0. [DOI] [PubMed] [Google Scholar]
- 152.Regnault TR, Galan HL, Parker TA, Anthony RV. Placental development in normal and compromised pregnancies– a review. Placenta. 2002;23(Suppl A):S119–29. doi: 10.1053/plac.2002.0792. [DOI] [PubMed] [Google Scholar]
- 153.Wallace JM, Bourke DA, Aitken RP, Palmer RM, Da Silva P, Cruickshank MA. Relationship between nutritionally-mediated placental growth restriction and fetal growth, body composition and endocrine status during late gestation in adolescent sheep. Placenta. 2000;21:100–108. doi: 10.1053/plac.1999.0440. [DOI] [PubMed] [Google Scholar]
- 154.Wallace JM, Bhattacharya S, Campbell DM, Horgan GW. Inter-pregnancy weight change impacts placental weight and is associated with the risk of adverse pregnancy outcomes in the second pregnancy. BMC Pregnancy Childbirth. 2014;14:40. doi: 10.1186/1471-2393-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Long NM, Rule DC, Zhu MJ, Nathanielsz PW, Ford SP. Maternal obesity upregulates fatty acid and glucose transporters and increases expression of enzymes mediating fatty acid biosynthesis in fetal adipose tissue depots. J Anim Sci. 2012;90:2201–2210. doi: 10.2527/jas.2011-4343. [DOI] [PubMed] [Google Scholar]
- 156.Long NM, Rule DC, Tuersunjiang N, Nathanielsz PW, Ford SP. Maternal obesity in sheep increases fatty acid synthesis, upregulates nutrient transporters, and increases adiposity in adult male offspring after a feeding challenge. PLoS ONE. 2015;10:e0122152. doi: 10.1371/journal.pone.0122152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Krishnan G, Bagath M, Pragna MKVP, Vidya MK, Aleena J, Archana PR, et al. Mitigation of the heat stress impact in livestock reproduction. Theriogenology2017.
- 158.Salama AAK, Caja G, Hamzaoui S, Badaoui B, Castro-Costa A, Façanha DAE, et al. Different levels of response to heat stress in dairy goats. Small Rumin Res. 2014;121:73–79. doi: 10.1016/j.smallrumres.2013.11.021. [DOI] [Google Scholar]
- 159.Vonnahme KA, Hess BW, Nijland MJ, Nathanielsz PW, Ford SP. Placentomal differentiation may compensate for maternal nutrient restriction in ewes adapted to harsh range conditions. J Anim Sci. 2006;84:3451–3459. doi: 10.2527/jas.2006-132. [DOI] [PubMed] [Google Scholar]
- 160.Yagil R, Etzion Z, Berlyne GM. Changes in rat milk quantity and quality due to variations in litter size and high ambient temperature. Lab Anim Sci. 1976;26:33–37. [PubMed] [Google Scholar]
- 161.Sevi A, Annicchiarico G, Albenzio M, Taibi L, Muscio A, Dell’Aquila S. Effects of solar radiation and feeding time on behavior, immune response and production of lactating ewes under high ambient temperature. J Dairy Sci. 2001;84:629–640. doi: 10.3168/jds.S0022-0302(01)74518-3. [DOI] [PubMed] [Google Scholar]
- 162.Gowane GR, Gadekar YP, Prakash V, Kadam V, Chopra A, Prince LLL. Climate change impact on sheep production: growth, milk, wool, and meat. 2017.
- 163.Sevi A, Caroprese M. Impact of heat stress on milk production, immunity and udder health in sheep: a critical review. Small Rumin Res. 2012;107:1–7. doi: 10.1016/j.smallrumres.2012.07.012. [DOI] [Google Scholar]
- 164.Babatola SS. Global burden of diseases attributable to air pollution. J Public Health Afr. 2018;9:813. doi: 10.4081/jphia.2018.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Carlson CJ, Codeço CT, Brauer M, Evengård B, Cai W, de la Fuente J, et al. Climate and health: an evolving relationship. Med. 2021;2:344–347. doi: 10.1016/j.medj.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 166.Salinas P, Veuthey C, Bruna N, Bongiorno A, Romero I. Impact of Maternal Exposure to Wood Smoke Pollution on Fetal Lung Morphology in a Rat Model. Int J Morphol. 2020;38:1250–1257. doi: 10.4067/S0717-95022020000501250. [DOI] [Google Scholar]
- 167.Wong TY. Smog induces oxidative stress and microbiota disruption. J Food Drug Anal. 2017;25:235–244. doi: 10.1016/j.jfda.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rahmalia A, Giorgis-Allemand L, Lepeule J, Philippat C, Galineau J, Hulin A, et al. Pregnancy exposure to atmospheric pollutants and placental weight: an approach relying on a dispersion model. Environ Int. 2012;48:47–55. doi: 10.1016/j.envint.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 169.van den Hooven EH, Pierik FH, de Kluizenaar Y, Hofman A, van Ratingen SW, Zandveld PY, et al. Air pollution exposure and markers of placental growth and function: the generation R study. Environ Health Perspect. 2012;120:1753–1759. doi: 10.1289/ehp.1204918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Veras MM, Damaceno-Rodrigues NR, Caldini EG, Maciel Ribeiro AA, Mayhew TM, Saldiva PH, et al. Particulate urban air pollution affects the functional morphology of mouse placenta. Biol Reprod. 2008;79:578–584. doi: 10.1095/biolreprod.108.069591. [DOI] [PubMed] [Google Scholar]
- 171.Veras MM, Damaceno-Rodrigues NR, Guimaraes Silva RM, Scoriza JN, Saldiva PH, Caldini EG, et al. Chronic exposure to fine particulate matter emitted by traffic affects reproductive and fetal outcomes in mice. Environ Res. 2009;109:536–543. doi: 10.1016/j.envres.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 172.Weldy CS, Liu Y, Liggitt HD, Chin MT. In utero exposure to diesel exhaust air pollution promotes adverse intrauterine conditions, resulting in weight gain, altered blood pressure, and increased susceptibility to heart failure in adult mice. PLoS ONE. 2014;9:e88582. doi: 10.1371/journal.pone.0088582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kenney M, Müller R. Of rats and women: narratives of motherhood in environmental epigenetics. BioSocieties. 2017;12.
- 174.Chen H, Chen X, Hong X, Liu C, Huang H, Wang Q, et al. Maternal exposure to ambient PM(2.5) exaggerates fetal cardiovascular maldevelopment induced by homocysteine in rats. Environ Toxicol. 2017;32:877–89. doi: 10.1002/tox.22287. [DOI] [PubMed] [Google Scholar]
- 175.Martelletti L, Martelletti P. Air Pollution and the Novel COVID-19 disease: a putative disease risk factor. SN Compr Clin Med. 2020:1–5. [DOI] [PMC free article] [PubMed]
- 176.Ciencewicki J, Jaspers I. Air pollution and respiratory viral infection. Inhal Toxicol. 2007;19:1135–1146. doi: 10.1080/08958370701665434. [DOI] [PubMed] [Google Scholar]
- 177.Jafari Z, Faraji J, Mirza Agha B, Metz GAS, Kolb BE, Mohajerani MH. The adverse effects of auditory stress on mouse uterus receptivity and behaviour. Sci Rep. 2017;7:4720. doi: 10.1038/s41598-017-04943-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kight CR, Swaddle JP. How and why environmental noise impacts animals: an integrative, mechanistic review. Ecol Lett. 2011;14:1052–1061. doi: 10.1111/j.1461-0248.2011.01664.x. [DOI] [PubMed] [Google Scholar]
- 179.Broucek J. Effects of noise on performance, stress, and behaviour of animals: a review. Slovak J Anim Sci. 2014;47:111–123. [Google Scholar]
- 180.Schneider ML, Moore CF, Kraemer GW. Moderate alcohol during pregnancy: learning and behavior in adolescent rhesus monkeys. Alcohol Clin Exp Res. 2001;25:1383–1392. doi: 10.1111/j.1530-0277.2001.tb02362.x. [DOI] [PubMed] [Google Scholar]
- 181.Regnault TR, Gentili S, Sarr O, Toop CR, Sloboda DM. Fructose, pregnancy and later life impacts. Clin Exp Pharmacol Physiol. 2013;40:824–837. doi: 10.1111/1440-1681.12162. [DOI] [PubMed] [Google Scholar]
- 182.Jiang L, Ferraris RP. Developmental reprogramming of rat GLUT-5 requires de novo mRNA and protein synthesis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G113–G120. doi: 10.1152/ajpgi.2001.280.1.G113. [DOI] [PubMed] [Google Scholar]
- 183.Astbury S, Song A, Zhou M, Nielsen B, Hoedl A, Willing BP, et al. High fructose intake during pregnancy in rats influences the maternal microbiome and gut development in the offspring. Front Genet. 2018;9:203. doi: 10.3389/fgene.2018.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mizuno G, Munetsuna E, Yamada H, Yamazaki M, Ando Y, Hattori Y, et al. Maternal fructose consumption downregulates hippocampal catalase expression via DNA methylation in rat offspring. Nutr Res. 2021;92:40–48. doi: 10.1016/j.nutres.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 185.Rodríguez L, Panadero MI, Roglans N, Otero P, Alvarez-Millán JJ, Laguna JC, et al. Fructose during pregnancy affects maternal and fetal leptin signaling. J Nutr Biochem. 2013;24:1709–1716. doi: 10.1016/j.jnutbio.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 186.Meng Z, Tian S, Yan J, Jia M, Yan S, Li R, et al. Effects of perinatal exposure to BPA, BPF and BPAF on liver function in male mouse offspring involving in oxidative damage and metabolic disorder. Environ Pollut. 2019;247:935–943. doi: 10.1016/j.envpol.2019.01.116. [DOI] [PubMed] [Google Scholar]
- 187.Suvorov A, Waxman DJ. Early programing of uterine tissue by bisphenol A: critical evaluation of evidence from animal exposure studies. Reprod Toxicol. 2015;57:59–72. doi: 10.1016/j.reprotox.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ruiz TFR, Colleta SJ, Leonel ECR, Taboga SR. Mammary carcinoma in aged gerbil mothers after endocrine disruption in pregnancy and lactation. Endocr Relat Cancer. 2021;28:715–730. doi: 10.1530/ERC-21-0198. [DOI] [PubMed] [Google Scholar]
- 189.Abd Elhady ASA, Autifi MA, El Gizawy ME, Yousef MM. Effect of bisphenol A on the testis of offspring of white albino rats during pregnancy and lactation: (light and electron microscopic study) Egypt J Hosp Med. 2021;84:1920–1931. doi: 10.21608/ejhm.2021.178609. [DOI] [Google Scholar]
- 190.Alchalabi ASH, Aklilu E, Aziz AR, Malek F, Ronald SH, Khan MA. Different periods of intrauterine exposure to electromagnetic field: influence on female rats' fertility, prenatal and postnatal development. Asian Pacific Journal of Reproduction. 2016;5:14–23. doi: 10.1016/j.apjr.2015.12.003. [DOI] [Google Scholar]
- 191.da Silva BS, Pietrobon CB, Bertasso IM, Lopes BP, Carvalho JC, Peixoto-Silva N, et al. Short and long-term effects of bisphenol S (BPS) exposure during pregnancy and lactation on plasma lipids, hormones, and behavior in rats. Environ Pollut. 2019;250:312–322. doi: 10.1016/j.envpol.2019.03.100. [DOI] [PubMed] [Google Scholar]
- 192.Hu F, Zhang L, Li T, Wang H, Liang W, Zhou Y. Bisphenol-A exposure during gestation and lactation causes visual perception deficits in rat pups following a decrease in interleukin 1β expression in the primary visual cortex. Neuroscience. 2020;434:148–160. doi: 10.1016/j.neuroscience.2020.03.035. [DOI] [PubMed] [Google Scholar]
- 193.Wang Y, Du X, Wang D, Wang J, Du J. Effects of bisphenol A exposure during pregnancy and lactation on hippocampal function in newborn rats. Int J Med Sci. 2020;17:1751–1762. doi: 10.7150/ijms.47300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Costa LG, Cole TB, Dao K, Chang YC, Garrick JM. Developmental impact of air pollution on brain function. Neurochem Int. 2019;131:104580. doi: 10.1016/j.neuint.2019.104580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kim D, Chen Z, Zhou LF, Huang SX. Air pollutants and early origins of respiratory diseases. Chronic Dis Transl Med. 2018;4:75–94. doi: 10.1016/j.cdtm.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Delemarre-van de Waal HA. Environmental factors influencing growth and pubertal development. Environ Health Perspect. 1993;101(Suppl 2):39–44. doi: 10.1289/ehp.93101s239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bautista CJ, Reyes-Castro LA, Bautista RJ, Ramirez V, Elias-López AL, Hernández-Pando R, et al. Different protein sources in the maternal diet of the rat during gestation and lactation affect milk composition and male offspring development during adulthood. Reprod Sci. 2021;28:2481–2494. doi: 10.1007/s43032-021-00492-8. [DOI] [PubMed] [Google Scholar]
- 198.Pereira SP, Tavares LC, Duarte AI, Baldeiras I, Cunha-Oliveira T, Martins JD, et al. Sex-dependent vulnerability of fetal nonhuman primate cardiac mitochondria to moderate maternal nutrient reduction. Clin Sci (Lond) 2021;135:1103–1126. doi: 10.1042/CS20201339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zambrano E, Lomas-Soria C, Nathanielsz PW. Rodent studies of developmental programming and ageing mechanisms: special issue: in utero and early life programming of ageing and disease. Eur J Clin Invest. 2021;51:e13631. doi: 10.1111/eci.13631. [DOI] [PubMed] [Google Scholar]
- 200.Dinh H, Nguyen B, Morimoto J, Lundback I, Kumar SS, Ponton F. Transgenerational effects of parental diet on offspring development and disease resistance in flies. Frontiers in Ecology and Evolution. 2021;9.
- 201.Monaghan P. Early growth conditions, phenotypic development and environmental change. Philos Trans R Soc Lond B Biol Sci. 2008;363:1635–1645. doi: 10.1098/rstb.2007.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hu J, Aris IM, Lin PD, Rifas-Shiman SL, Perng W, Woo Baidal JA, et al. Longitudinal associations of modifiable risk factors in the first 1000 days with weight status and metabolic risk in early adolescence. Am J Clin Nutr. 2020;113:113–122. doi: 10.1093/ajcn/nqaa297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Matvienko-Sikar K, Cooney J, Flannery C, Murphy J, Khashan A, Huizink A. Maternal stress in the first 1000 days and risk of childhood obesity: a systematic review. J Reprod Infant Psychol. 2021;39:180–204. doi: 10.1080/02646838.2020.1724917. [DOI] [PubMed] [Google Scholar]
- 204.Fragkou PC, Karaviti D, Zemlin M, Skevaki C. Impact of early life nutrition on children’s immune system and noncommunicable diseases through its effects on the bacterial microbiome, virome and mycobiome. Front Immunol. 2021;12:644269. doi: 10.3389/fimmu.2021.644269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.van Zyl C, van Wyk C. Exploring factors that could potentially have affected the first 1000 days of absent learners in South Africa: a qualitative study. Int J Environ Res Public Health. 2021;18. [DOI] [PMC free article] [PubMed]
- 206.Assibey-Mensah V, Glantz JC, Hopke PK, Jusko TA, Thevenet-Morrison K, Chalupa D, et al. Wintertime wood smoke, traffic particle pollution, and preeclampsia. Hypertension. 2020;75:851–858. doi: 10.1161/HYPERTENSIONAHA.119.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Harris MH, Gold DR, Rifas-Shiman SL, Melly SJ, Zanobetti A, Coull BA, et al. Prenatal and childhood traffic-related pollution exposure and childhood cognition in the project viva cohort (Massachusetts, USA) Environ Health Perspect. 2015;123:1072–1078. doi: 10.1289/ehp.1408803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tian LL, Zhao YC, Wang XC, Gu JL, Sun ZJ, Zhang YL, et al. Effects of gestational cadmium exposure on pregnancy outcome and development in the offspring at age 4.5 years. Biol Trace Elem Res. 2009;132:51–9. doi: 10.1007/s12011-009-8391-0. [DOI] [PubMed] [Google Scholar]
- 209.Wu J, Ren C, Delfino RJ, Chung J, Wilhelm M, Ritz B. Association between local traffic-generated air pollution and preeclampsia and preterm delivery in the south coast air basin of California. Environ Health Perspect. 2009;117:1773–1779. doi: 10.1289/ehp.0800334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wu J, Wilhelm M, Chung J, Ritz B. Comparing exposure assessment methods for traffic-related air pollution in an adverse pregnancy outcome study. Environ Res. 2011;111:685–692. doi: 10.1016/j.envres.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Brauer M, Lencar C, Tamburic L, Koehoorn M, Demers P, Karr C. A cohort study of traffic-related air pollution impacts on birth outcomes. Environ Health Perspect. 2008;116:680–686. doi: 10.1289/ehp.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Clougherty JE, Levy JI, Kubzansky LD, Ryan PB, Suglia SF, Canner MJ, et al. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ Health Perspect. 2007;115:1140–1146. doi: 10.1289/ehp.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fleisch AF, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, et al. Prenatal exposure to traffic pollution: associations with reduced fetal growth and rapid infant weight gain. Epidemiology. 2015;26:43–50. doi: 10.1097/EDE.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Slama R, Morgenstern V, Cyrys J, Zutavern A, Herbarth O, Wichmann HE, et al. Traffic-related atmospheric pollutants levels during pregnancy and offspring's term birth weight: a study relying on a land-use regression exposure model. Environ Health Perspect. 2007;115:1283–1292. doi: 10.1289/ehp.10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Stieb DM, Chen L, Hystad P, Beckerman BS, Jerrett M, Tjepkema M, et al. A national study of the association between traffic-related air pollution and adverse pregnancy outcomes in Canada, 1999–2008. Environ Res. 2016;148:513–526. doi: 10.1016/j.envres.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 216.Yan Q, Liew Z, Uppal K, Cui X, Ling C, Heck JE, et al. Maternal serum metabolome and traffic-related air pollution exposure in pregnancy. Environ Int. 2019;130:104872. doi: 10.1016/j.envint.2019.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Al-Saleh I, Shinwari N, Mashhour A. Heavy metal concentrations in the breast milk of Saudi women. Biol Trace Elem Res. 2003;96:21–37. doi: 10.1385/BTER:96:1-3:21. [DOI] [PubMed] [Google Scholar]
- 218.Dorea JG. Mercury and lead during breast-feeding. Br J Nutr. 2004;92:21–40. doi: 10.1079/BJN20041163. [DOI] [PubMed] [Google Scholar]
- 219.Sbihi H, Allen RW, Becker A, Brook JR, Mandhane P, Scott JA, et al. Perinatal exposure to traffic-related air pollution and atopy at 1 year of age in a multi-center Canadian birth cohort study. Environ Health Perspect. 2015;123:902–908. doi: 10.1289/ehp.1408700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Myhre O, Zimmer KE, Hudecova AM, Hansen KEA, Khezri A, Berntsen HF, et al. Maternal exposure to a human based mixture of persistent organic pollutants (POPs) affect gene expression related to brain function in mice offspring hippocampus. Chemosphere. 2021;276:130123. doi: 10.1016/j.chemosphere.2021.130123. [DOI] [PubMed] [Google Scholar]
- 221.Ferreira MKM, Aragão WAB, Bittencourt LO, Puty B, Dionizio A, Souza MPC, et al. Fluoride exposure during pregnancy and lactation triggers oxidative stress and molecular changes in hippocampus of offspring rats. Ecotoxicol Environ Saf. 2021;208:111437. doi: 10.1016/j.ecoenv.2020.111437. [DOI] [PubMed] [Google Scholar]
- 222.Bruce-Vanderpuije P, Megson D, Jones GR, Jobst K, Reiner E, Clarke E, et al. Infant dietary exposure to dioxin-like polychlorinated biphenyls (dlPCBs), polybrominated and mixed halogenated dibenzo-p-dioxins and furans (PBDD/Fs and PXDD/Fs) in milk samples of lactating mothers in Accra. Ghana Chemosphere. 2021;263:128156. doi: 10.1016/j.chemosphere.2020.128156. [DOI] [PubMed] [Google Scholar]
- 223.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572:31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lamichhane N, Olsen NJ, Mortensen EL, Obel C, Heitmann BL, Händel MN. Associations between maternal stress during pregnancy and offspring obesity risk later in life-a systematic literature review. Obes Rev. 2020;21:e12951. doi: 10.1111/obr.12951. [DOI] [PubMed] [Google Scholar]
- 225.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 2007;21:343–350. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 226.Entringer S, Epel ES, Lin J, Buss C, Shahbaba B, Blackburn EH, et al. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am J Obstet Gynecol. 2013;208(134):e1–7. doi: 10.1016/j.ajog.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Loomans EM, van Dijk AE, Vrijkotte TG, van Eijsden M, Stronks K, Gemke RJ, et al. Psychosocial stress during pregnancy is related to adverse birth outcomes: results from a large multi-ethnic community-based birth cohort. Eur J Public Health. 2013;23:485–491. doi: 10.1093/eurpub/cks097. [DOI] [PubMed] [Google Scholar]
- 228.Rakers F, Rupprecht S, Dreiling M, Bergmeier C, Witte OW, Schwab M. Transfer of maternal psychosocial stress to the fetus. Neurosci Biobehav Rev. 2017. [DOI] [PubMed]
- 229.Tanpradit K, Kaewkiattikun K. The effect of perceived stress during pregnancy on preterm birth. Int J Womens Health. 2020;12:287–293. doi: 10.2147/IJWH.S239138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tegethoff M, Greene N, Olsen J, Meyer AH, Meinlschmidt G. Maternal psychosocial stress during pregnancy and placenta weight: evidence from a national cohort study. PLoS ONE. 2010;5:e14478. doi: 10.1371/journal.pone.0014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yu Y, Zhang S, Wang G, Hong X, Mallow EB, Walker SO, et al. The combined association of psychosocial stress and chronic hypertension with preeclampsia. Am J Obstet Gynecol. 2013;209:438.e1–.e12. doi: 10.1016/j.ajog.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Homer CJ, James SA, Siegel E. Work-related psychosocial stress and risk of preterm, low birthweight delivery. Am J Public Health. 1990;80:173–177. doi: 10.2105/AJPH.80.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Klonoff-Cohen HS, Cross JL, Pieper CF. Job stress and preeclampsia. Epidemiology. 1996;7:245–249. doi: 10.1097/00001648-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 234.Leather P, Beale D, Sullivan L. Noise, psychosocial stress and their interaction in the workplace. J Environ Psychol. 2003;23:213–222. doi: 10.1016/S0272-4944(02)00082-8. [DOI] [Google Scholar]
- 235.Lee BE, Ha M, Park H, Hong YC, Kim Y, Kim YJ, et al. Psychosocial work stress during pregnancy and birthweight. Paediatr Perinat Epidemiol. 2011;25:246–254. doi: 10.1111/j.1365-3016.2010.01177.x. [DOI] [PubMed] [Google Scholar]
- 236.McGovern P, Dowd B, Gjerdingen D, Dagher R, Ukestad L, McCaffrey D, et al. Mothers’ health and work-related factors at 11 weeks postpartum. Ann Fam Med. 2007;5:519–527. doi: 10.1370/afm.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gowin F. Hacinamiento y Consecuencias en México con imagenes. 2021.
- 238.Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 239.Martín Calero, de Villodres P, Hueso Montoro C, Pleguezuelos Navarro H, Balanza Galindo S, Merino Torres MA, Merino Torres JR. Calidad de vida relacionada con la salud en trabajadores del área medioambiental. Medicina y Seguridad del Trabajo. 2012;58:35–48. doi: 10.4321/S0465-546X2012000100005. [DOI] [Google Scholar]
- 240.Burgos Díez P, Ruiz Albi T, QueipoBurón D, Rescalvo Santiago F, Martínez León MM, Amo Merino Pd, et al. Calidad de vida relacionada con la salud en trabajadores sanitarios. Medicina y Seguridad del Trabajo. 2012;58:27–34. doi: 10.4321/S0465-546X2012000100004. [DOI] [Google Scholar]
- 241.Maria VCA. Exposición a temperaturas extremas y riesgo de parto pretérmino en Valencia. 2014.
- 242.Li Z, You M, Che X, Dai Y, Xu Y, Wang Y. Perinatal exposure to BDE-47 exacerbated autistic-like behaviors and impairments of dendritic development in a valproic acid-induced rat model of autism. Ecotoxicol Environ Saf. 2021;212:112000. doi: 10.1016/j.ecoenv.2021.112000. [DOI] [PubMed] [Google Scholar]
- 243.Mirzaei N, Jahanian Sadatmahalleh S, Bahri Khomami M, Moini A, Kazemnejad A. Sexual function, mental health, and quality of life under strain of COVID-19 pandemic in Iranian pregnant and lactating women: a comparative cross-sectional study. Health Qual Life Outcomes. 2021;19:66. doi: 10.1186/s12955-021-01720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Trujillo-Villarreal LA, Romero-Díaz VJ, Marino-Martínez IA, Fuentes-Mera L, Ponce-Camacho MA, Devenyi GA, et al. Maternal cafeteria diet exposure primes depression-like behavior in the offspring evoking lower brain volume related to changes in synaptic terminals and gliosis. Transl Psychiatry. 2021;11:53. doi: 10.1038/s41398-020-01157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kibr G. Food choice behaviors of lactating women: association with body mass index and fruits and vegetables intake in central Amhara Region. Ethiopia-An Observational Study J Nutr Metab. 2021;2021:6654659. doi: 10.1155/2021/6654659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Alyamani R, Nephew B, Murgatroyd C. Intergenerational changes in hippocampal transcription in an animal model of maternal depression. Eur J Neurosci. 2021. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A.
N/A.