Abstract
A strain of Rhodococcus designated MB1, which was capable of utilizing cocaine as a sole source of carbon and nitrogen for growth, was isolated from rhizosphere soil of the tropane alkaloid-producing plant Erythroxylum coca. A cocaine esterase was found to initiate degradation of cocaine, which was hydrolyzed to ecgonine methyl ester and benzoate; both of these esterolytic products were further metabolized by Rhodococcus sp. strain MB1. The structural gene encoding a cocaine esterase, designated cocE, was cloned from Rhodococcus sp. strain MB1 genomic libraries by screening recombinant strains of Rhodococcus erythropolis CW25 for growth on cocaine. The nucleotide sequence of cocE corresponded to an open reading frame of 1,724 bp that codes for a protein of 574 amino acids. The amino acid sequence of cocaine esterase has a region of similarity with the active serine consensus of X-prolyl dipeptidyl aminopeptidases, suggesting that the cocaine esterase is a serine esterase. The cocE coding sequence was subcloned into the pCFX1 expression plasmid and expressed in Escherichia coli. The recombinant cocaine esterase was purified to apparent homogeneity and was found to be monomeric, with an Mr of approximately 65,000. The apparent Km of the enzyme (mean ± standard deviation) for cocaine was measured as 1.33 ± 0.085 mM. These findings are of potential use in the development of a linked assay for the detection of illicit cocaine.
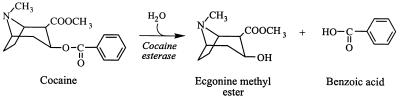
Tropane alkaloids, which possess a characteristic azabicylo[3,2,1] octane system, represent one of the most pharmacologically important groups among the alkaloids. In particular, the tropane alkaloids exhibit anticholinergic and anesthetic activities as well as parasympathetic inhibition. Cocaine is the most well known of the tropane alkaloids and is a powerful central nervous system stimulant and adrenergic blocking agent; its hydrochloride salt is also used as a local surface anesthetic in face, eye, nose, and throat operations. Cocaine is naturally located in the leaves of coca plants (Erythroxylum spp.) and can be up to 1% of the dry weight content (13). Cocaine is, however, a notorious drug of abuse and is considered to be one of the most powerfully addictive drugs Western society has ever had to confront. Illicit powder cocaine and crack cocaine are generally trafficked as solid particulate matter, and much effort is currently being directed at the development of sensors for drug detection. We envisaged that the isolation of microorganisms from the environment capable of metabolizing drugs, such as cocaine and heroin, as carbon sources for growth might provide a good source of enzymes which could be utilized as recognition components in biosensors for the detection of illicit drugs. Previous work in our laboratory has shown that the enzymes initiating heroin metabolism in bacteria could be successfully used in conjunction with bacterial luciferase for the detection of nanogram quantities of heroin (5, 12, 24). Work has subsequently been directed towards identifying appropriate enzymes active against cocaine. A cocaine esterase was previously identified in a strain of Pseudomonas maltophilia termed MB11L that was isolated from a drug processing plant (4). P. maltophilia MB11L was capable of utilizing cocaine as a sole source of carbon and energy for growth, and metabolism of cocaine was shown to be initiated by an esterase that hydrolyzed cocaine to benzoate and ecgonine methyl ester (Fig. 1). Unfortunately, the cocaine esterase was unsuitable for biosensor development, as the enzyme was observed in cell extracts either in an aggregated form or in association with membrane components, and, as such, it proved extremely difficult to purify.
FIG. 1.
Cocaine esterase reaction.
We report here the isolation of a strain of Rhodococcus that can utilize cocaine as a sole source of carbon and nitrogen for growth and the cloning, sequencing, and properties of a soluble cytosolic cocaine esterase.
MATERIALS AND METHODS
Organisms, plasmids, and growth conditions.
Rhodococcus sp. strain MB1 was isolated from soil samples collected from the rhizosphere of coca plants and on the basis of its ability to utilize cocaine as a sole source of carbon. The enrichment conditions and growth conditions were as described by Britt et al. (4). Escherichia coli JM109 was obtained from Promega (Southampton, Hampshire, United Kingdom [U.K.]) and was grown according to standard procedures (26). Epicurian Coli XL1-Blue MR was obtained from Stratagene (Cambridge, U.K.). Rhodococcus erythropolis CW25 and the E. coli-Rhodococcus shuttle vector pDA71 were kind gifts from E. Dabbs (University of the Witwatersrand, Johannesburg, South Africa). pCFX1 is an expression vector derived from pBluescript SK(+) by the insertion of the promoter region of pONR (9).
Reagents.
Cocaine hydrochloride was purchased from Macfarlan Smith Ltd. (Edinburgh, Scotland, U.K.). Ecgonine methyl ester was synthesized from cocaine as described previously (17). Other reagents were of analytical or higher grade.
DNA manipulation.
All restriction enzymes were purchased from New England Biolabs (Hitchin, U.K.) and used according to the manufacturer's protocols. Southern blotting and cloning procedures were performed according to standard methods (26). Rhodococcal strains were transformed by electroporation and were rendered electrocompetent via the method used by Kesseler et al. (14). The transformations were conducted using a protocol adapted from those of Desomer et al. (7) and Andersen et al. (2). Cells were grown to exponential phase in a rich Luria-Bertani (LB) medium supplemented with 1.8% (wt/vol) sucrose and 1.5% (wt/vol) glycine. The cells were cooled on ice, harvested by centrifugation, and washed with ice-cold distilled H2O prior to resuspension in 30% (wt/vol) polyethylene glycol 8000, concentrating the cells 100-fold. Aliquots of cells (100 μl) were used directly in transformations with the addition of up to 1 μg of DNA, mixing, and incubation on ice for 10 min in 0.2-cm electroporation cuvettes. Cells were electroporated in a Gene Pulser (Bio-Rad Laboratories, Hercules, Calif.) with a capacitance of 25 μF, voltage of 2.5 kV, and resistance of 400 Ω to achieve a time constant of more than 7.5 ms. The cells were incubated at 30°C for 3 h in 1 ml of LB medium lacking antibiotic, which allowed phenotypic expression before plating onto LB or minimal medium plates containing 34 μg of chloramphenicol per ml to select for transformants containing pDA71.
Determination of 16S rDNA sequence.
Primers fD1 and rD1 were designed around the 5′ and 3′ ends of bacterial 16S ribosomal DNA (rDNA), respectively, to produce an approximately 1.5-kb fragment of 16S rDNA from bacteria by PCR amplification (29). The annealing temperature was 42°C. The primers had the following sequences: fD1, 5′-CCGAATTCGTCGACAACAGAGTTTGATCCTGGCTCAG-3′; rD1, 5′-CCCGGGATCCAAGCTTAAGGAGGTGATCCAGCC-3′. The 1.5-kb PCR product amplified from the isolate was gel purified and subjected to direct PCR sequencing using the primer fD1. The DNA sequence obtained was submitted to biological databases for comparison with other 16S rDNAs. The GenBank, EMBL, DDBJ, and PDB databases were searched using the BLASTN search on the National Center for Biological Information website (1).
Construction of genomic libraries.
To prepare genomic DNA fragments of approximately 5 to 10 kb, genomic DNA (100 μg) from Rhodococcus strain MB1 was subjected to partial digestion with Sau3AI and fractionated on a linear 10 to 40% sucrose density gradient. Fractions containing DNA fragments of the desired size were pooled and ligated into the E. coli-Rhodococcus shuttle vector pDA71. The plasmid DNA was transformed into the supercompetent E. coli (Epicurian Coli XL1-Blue MR) and into R. erythropolis CW25 by electroporation. In the case of both the E. coli and Rhodococcus libraries, colonies were washed off selective plates under sterile conditions using LB medium containing 15% glycerol (vol/vol) and stored at −80°C.
Cloning of cocE into an expression host.
cocE was subcloned by PCR. The primers used were as follows (the bases corresponding to introduced restriction sites NdeI and HindIII, respectively, are shown in bold): forward, CAG CGA AGG TCG GGA GCA TAT GGT GGA CGG G; reverse, TTT AAG CTT CAG CGT CAG CCA GGC GCG GCT GC. PCR was performed with BioTaq polymerase. The annealing temperature was 67°C. The product was digested with NdeI and HindIII and ligated into pCFX1 cut with the same enzymes to yield pCOC2.
Protein sequence comparisons.
Protein sequence comparisons were performed using the software package MACAW (version 2.05; National Center for Biotechnology Information, National Library of Medicine, Bethesda, Md.) and the BLOSUM62 matrix (11).
Reverse-phase HPLC for analysis of the breakdown of cocaine.
Samples were separated by reverse-phase high-pressure liquid chromatography (HPLC) using a model 1050 component system with a multiple wavelength detector (Hewlett-Packard, Waldbronn, Germany) on a Techsphere 5 ODS column (0.46 by 25 cm, 5-μm particles) (HPLC Technology Co. Ltd., Macclesfield, Cheshire, U.K.). The mobile phase consisted of a gradient system comprising an organic phase of methanol and an aqueous phase which contained 1% (wt/vol) glacial acetic acid–50 mM pentane sulfonic acid, adjusted to pH 6.9 with ammonia (d = 0.88). Compounds were detected at 228 nm. After application of the sample, the gradient was started with 3% organic phase, which was increased linearly to 84% from 3 to 6 min, where it was held for a further 8 min before being decreased to 3% between 14 and 20 min.
TLC for analysis of the breakdown of cocaine.
Thin-layer chromatography (TLC) of cocaine and its metabolites was performed using 200-μm polyester plates precoated with UV-absorbing silica gel (Macherey-Nagel, Düren, Germany). The samples (10 μl) were developed with a mobile phase of ethylacetate-methanol-ammonia (13:7:1 [vol/vol/vol]; adapted from the method of Mira et al. [20]). Compounds were detected on the basis of their UV absorbance at 254 nm and color formation when sprayed with Ludy Tenger reagent (21). Relative migration distances of compounds detected in samples were compared with those of authentic cocaine, benzoate, benzoylecgonine, and ecgonine methyl ester standards.
Purification of cocaine esterase from recombinant E. coli.
E. coli (JM109) carrying pCOC2 was grown until stationary phase on SOB medium (26) containing 100 μg of ampicillin per ml and 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 37°C. Cells were pelleted by centrifugation at 10,000 × g and resuspended in 100 mM Tris-HCl at pH 7.5. A cell extract was prepared by sonication of the cells on ice (12 bursts of 15 s at an amplitude of 12 μm), followed by centrifugation of the extract at 20,000 × g for 15 min before ultracentrifugation at 100,000 × g for 60 min. Ammonium sulfate was slowly added to the extract on ice, to a final concentration of 1.25 M. Precipitated protein was removed by centrifugation at 12,000 × g, and the supernatant, which contained the cocaine esterase activity, was further purified by hydrophobic-interaction chromatography. A phenyl-Sepharose CL-4B column (100-ml bed volume; Amersham Pharmacia Biotech Ltd., St. Albans, Hertfordshire, U.K.) was run at 2 ml/min and equilibrated with 1.25 M ammonium sulfate–100 mM Tris-HCl at pH 7.5 before the protein solution was applied. Protein was eluted by decreasing the ammonium sulfate concentration from 0.63 to 0 M over 300 ml, followed by addition of 200 ml of 100 mM Tris-HCl at pH 7.5. Activity-containing fractions from the column were pooled and dialyzed against two 5-liter volumes of 100 mM Tris-HCl at pH 7.5. The dialyzed protein was applied to a Fast Flow Q Sepharose column run at 1 ml/min (80-ml bed volume; Amersham Pharmacia Biotech Ltd.). The column had been previously equilibrated with 100 mM Tris-HCl at pH 7.5 before the protein was applied. A 500-ml NaCl gradient from 0 to 0.6 M was used to elute bound protein. Fractions that contained cocaine esterase activity were pooled and were dialyzed against two 5-liter volumes of 5 mM KH2PO4 at pH 7.5. The protein was then loaded onto a Bio-Gel HT hydroxylapatite column run at 0.5 ml/min (12-ml bed volume; Bio-Rad Laboratories). The column had been preequilibrated with 5 mM KH2PO4 at pH 7.5. After the sample was loaded, the phosphate concentration was increased to 120 mM over 60 ml, during which time the majority of the bound protein was eluted. Activity-containing fractions from the column were pooled and dialyzed against two 2-liter volumes of 100 mM Tris-HCl buffer–100 mM NaCl at pH 7.5. The dialyzed protein was loaded onto a prepacked Resource Q column (6-ml column bed volume; Amersham Pharmacia Biotech Ltd.) at a flow rate of 1 ml/min. The column had been preequilibrated with 100 mM Tris-HCl buffer–100 mM NaCl at pH 7.5. A linear sodium chloride gradient from 250 to 400 mM over 132 ml was used to elute the esterase. Fractions containing the cocaine esterase were then pooled.
Estimation of the native molecular weight of the cocaine esterase.
The native molecular weight of the purified cocaine esterase was determined by gel filtration chromatography using a prepacked Superose 6 HR column (Amersham Pharmacia Biotech Ltd.). The column was equilibrated with 100 mM Tris-HCl–100 mM NaCl at pH 7.5 and calibrated using a low-molecular-weight calibration kit (Amersham Pharmacia Biotech Ltd.).
Enzyme assays.
Cocaine esterase activity was measured by quantifying the amount of benzoate produced from cocaine by reverse-phase HPLC. The standard assay conditions were 100 mM Tris-HCl at pH 7.5, with 10 mM cocaine in a total volume of 100 μl incubated at 30°C. All assay results were compared with those from control assays containing no extract and extract boiled for 5 min to denature the enzyme. For a given cocaine concentration, five assays were set up and incubated at 30°C and stopped at 1-min intervals by the addition of 100 μl of 1 M HCl. The specific activity was expressed in units of cocaine esterase per milligram of total protein, where 1 U of cocaine esterase was defined as the amount required to produce 1 μmol of benzoate per min under these conditions. Assays involving atropine were performed under the same conditions with samples taken after 15, 30, 90, and 180 min. Km and Vmax were determined using 0.24 μg (44 U/mg) of cocaine esterase and varying the cocaine concentration between 0.15 and 30 mM. Samples were assayed over a period of 5 min. Kinetic equations were fitted to data using the GraFit 3 software package (Erithacus Software Limited, Middlesex, U.K.).
Nucleotide sequence accession number.
The nucleotide sequence data reported have been submitted to GenBank and assigned the accession number AF173165.
RESULTS AND DISCUSSION
Isolation of Rhodococcus sp. strain MB1.
As cocaine is a natural compound, we considered it likely that microorganisms that had evolved the ability to metabolize cocaine as a carbon source would exist in close proximity to cocaine-producing coca plants. We therefore carried out selective enrichments with soil samples taken from the rhizosphere of coca plants in defined liquid medium, which resulted in the isolation of a bacterium, designated strain MB1, that could utilize cocaine as the sole carbon and nitrogen source for growth. Analysis of the culture broth by TLC and HPLC showed the disappearance of cocaine and the transient accumulation of ecgonine methyl ester and benzoate (data not shown); both of these metabolites supported growth of the bacterium when supplied in minimal medium as a sole carbon source, while ecgonine methyl ester could also be utilized as a source of nitrogen.
Strain MB1 was found to be a gram-positive, rod-forming bacterium. A partial (500-bp) 16S rDNA sequence was obtained for strain MB1, and comparative phylogenetic analysis of the sequences revealed that strain MB1 clustered closely with species entirely from the mycolic acid-containing nocardioform actinomycetes. The highest scoring match was to the 16S rDNA of Rhodococcus equi strain DSM 20307T (accession number X80614), to which the DNA from MB1 displayed 95.8% sequence similarity. On the basis of the phylogenetic analysis, organism MB1 was identified as a species of the genus Rhodococcus.
Cocaine esterase.
Extracts from cells grown on 5 mM cocaine were found to possess a cytosolic cocaine esterase with a specific activity of 0.15 U/mg that hydrolyzed cocaine to benzoate and ecgonine methyl ester (Fig. 1). This activity was found to be inducible in Rhodococcus sp. strain MB1, since no cocaine esterase activity was observed in cells grown on 15 mM succinate as a sole source of carbon.
Cloning and sequencing of cocE.
In order to obtain significant quantities of biomass for enzyme purification and characterization studies, prohibitive quantities of cocaine were required as a growth substrate; therefore, it was decided to clone the gene encoding the cocaine esterase activity and express it in E. coli. The cloning strategy was to construct genomic libraries of MB1 using the E. coli-Rhodococcus shuttle vector pDA71. The libraries were then transformed into a rhodococcal host strain, R. erythropolis CW25, which is capable of growth on benzoate but not on cocaine. Expression of a cocaine esterase gene was selected for by screening recombinant strains of R. erythropolis CW25 for growth on cocaine as the sole carbon source. This procedure yielded eight clones that were capable of slowly hydrolyzing cocaine to benzoate and ecgonine methyl ester; the benzoate was further metabolized as a growth substrate by the clones. All the cocaine-degrading clones contained an identical 2.7-kb fragment in the recombinant plasmid designated pCOC1. Sequencing of the inserted fragment indicated the presence of an open reading frame encoding a polypeptide of 574 amino acids from which the theoretical molecular mass of the protein was calculated to be 62,128 Da. Cell extracts of R. erythropolis CW25 as well as extracts prepared from E. coli JM109 harboring the construct pCOC1 displayed low levels of cocaine esterase activity (4.8 × 10−3 and 1.5 × 10−3 U/mg, respectively). This evidence, taken together with the sequence analysis (see below), supports the suggestion that cocE encoded the cocaine esterase.
The low rate of growth on cocaine of R. erythropolis CW25 transformants harboring pCOC1 can, certainly in part, be explained by the fact that only 23 bp of the native Rhodococcus sp. strain MB1 DNA upstream of the cocE start codon was cloned. The regions controlling transcription and protein translation in Rhodococcus are not well characterized, and the position at which transcription is initiated in Rhodococcus genes whose promoter regions have been identified varies considerably. For example, the mRNA transcript of the nitA (nitrilase) gene from Rhodococcus rhodochrous J1 is initiated only 26 bases upstream of the ATG start site (15), while the transcription initiation site of the cmr (chloramphenicol resistance) gene from Rhodococcus fascians NCPPB 1675 was determined to be 279 bases upstream of the start codon (8). In the other rhodococcal genes whose transcriptional regulation has been investigated, transcription was shown to be initiated at distances of 46 bases (16), 78 bases (22), and 168 bases (10) upstream of the translational start site, and promoter regions further upstream of these sites were shown to be essential for gene expression.
A region to which ribosomes are capable of binding to initiate translation in the rhodococcal host harboring cocE appears to be located within a few bases upstream of the ATG start codon (5′GGGAG3′); however, it is likely that transcription is initiated from fortuitous regions of the EcoRI endonuclease gene in pCOC1 that the Rhodococcus transcription machinery is capable of recognizing. Since there appears to be little homology between the Rhodococcus promoter regions identified to date, such regions could not be identified with confidence. However, regions of the EcoRI sequence upstream of cocE in pCOC1 with homology to known E. coli −10 and −35 promoter regions were detected in the upstream EcoRI DNA (data not shown) that may be responsible for the low levels of expression in E. coli JM109.
Sequence comparisons of CocE with other related sequences.
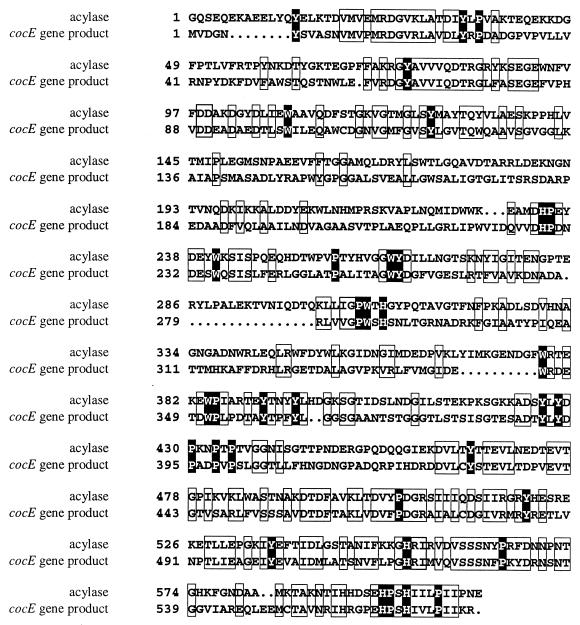
The deduced amino acid sequence was used for homology searches with a range of biological databases, and it was found that CocE was related to a number of prokaryotic enzymes. The closest match was to a glutaryl 7-aminocephalosporanic acid acylase enzyme from Bacillus laterosporus J1, which converts glutaryl 7-aminocephalosporanic acid to 7-aminocephalosporanic acid through the cleavage of an acyl linkage (3). The two proteins exhibit 37.2% sequence identity and 46.7% sequence similarity. Their similarity is illustrated by an alignment of the amino acid sequences (Fig. 2). The other high-scoring matches on the protein level were three hypothetical proteins of unknown function from Mycobacterium and five matches to X-prolyl dipeptidyl aminopeptidase enzymes from Lactococcus lactis and Lactobacillus spp. (Fig. 3), but the homology to these protein sequences was over a significantly smaller region (approximately 60 residues [18, 19, 23, 27, 30]). On average, a sequence identity of 39% and a sequence similarity of 50% with the dipeptidyl aminopeptidases were displayed over this region. The X-prolyl dipeptidyl aminopeptidases have amidase and esterase activities in addition to peptidase activities (29). These enzymes are serine proteases, and the consensus sequence surrounding the active-site serine has been identified as G-X-S-Y-X-G, where X is a nonconserved amino acid (6). The sequence of CocE shows conservation of sequence with the X-prolyl dipeptidyl aminopeptidases from L. lactis and Lactobacillus spp. around the active-site serine, suggesting that cocE encodes a serine esterase (Fig. 3).
FIG. 2.
Alignment of protein encoded by cocE with glutaryl 7-aminocephalosporanic acid acylase. The deduced amino acid sequence of the putative cocaine esterase encoded by cocE was aligned with the amino acid sequence of the glutaryl 7-aminocephalosporanic acid acylase from B. laterosporus J1 (3). The alignment was constructed using the software package MACAW (version 2.05; National Center for Biotechnology Information, National Library of Medicine) and the BLOSUM62 matrix. Dots indicate gaps introduced to maximize the alignment. Pairwise scores from 0 to 33% are not shaded, scores from 33 to 67% are boxed, and scores from 67 to 100% are shaded black, where 100% is the maximum score for the BLOSUM62 matrix.
FIG. 3.
Alignment of cocaine esterase with X-prolyl dipeptidyl aminopeptidases from lactic acid bacteria. The amino acid sequence of a region of the cocaine esterase encoded by cocE (cocE) was aligned with the homologous regions of five cloned X-prolyl dipeptidyl aminopeptidases from Lactobacillus and Lactococcus spp. (labeled by accession no.) (6, 18, 19, 23, 27, 29, 30). The alignment was constructed using the software package MACAW (version 2.05; National Center for Biotechnology Information, National Library of Medicine) and the BLOSUM62 matrix. Mean scores from 0 to 33% are not shaded, mean scores from 33 to 67% are boxed, and mean scores from 67 to 100% are shaded black, where 100% is the maximum mean score for the BLOSUM62 matrix. The G-X-S-Y-X-G consensus sequence surrounding the active-site serine (*) in the three known X-prolyl dipeptidyl aminopeptidases (mammalian, yeast, and bacterial) is indicated (X is a nonconserved amino acid).
Expression of cocE in E. coli.
High levels of expression of cocE were achieved by subcloning the cocE coding region, obtained by PCR, into pCFX1 to yield pCOC2. Cell extracts of recombinant E. coli were found to contain cocaine esterase activity with a specific activity of 0.13 U/mg of protein.
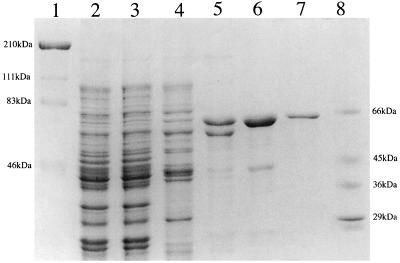
In order to characterize the recombinant cocaine esterase, the enzyme was purified 350-fold to homogeneity, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 4); it possessed a specific activity of 44 U/mg (Table 1). The native molecular mass of the purified esterase was determined by gel filtration to be approximately 65,000 Da. This value is consistent with the subunit molecular weight of the cocaine esterase, as determined by SDS-PAGE and through translation of cocE, which suggests that active cocaine esterase exists as a monomer. The apparent Km (mean ± standard deviation) of the enzyme was found to be 1.33 ± 0.085 mM. The cocaine esterase displayed low levels of activity (2.1 U/mg) with 20 mM atropine, a structurally related tropane alkaloid. Interestingly, bacterial atropine esterases do not display any activity against cocaine (25), while an esterolytic activity from a strain of P. maltophilia that was isolated from industrial waste liquors was shown to display activity against cocaine but not atropine (4). The N-terminal sequence of the first 11 amino acid residues of the purified protein was determined, and it was found to match the deduced amino acid sequence of cocaine esterase (CocE).
FIG. 4.
SDS-PAGE analysis of the purification of cocaine esterase from recombinant E. coli. Lanes 1 and 8, high- and low-range molecular mass markers; lane 2, cell extract from E. coli JM109/pCOC2; lane 3, product after ammonium sulfate fractionation; lanes 4 to 6, cocaine esterase activity obtained after hydrophobic-interaction chromatography, anion-exchange chromatography, and hydroxylapatite chromatography, respectively; lane 7, purified cocaine esterase (2 μg).
TABLE 1.
Purification of cocaine esterase activity
| Purification step | Amt of protein (mg) | Sp act (U/mg) | Total activity (U) | Purification (fold) | Recovery (%) |
|---|---|---|---|---|---|
| Cell extract | 4,884 | 0.124 | 605.6 | 1.00 | 100 |
| Ammonium sulfate | 4,091 | 0.144 | 589.1 | 1.16 | 97.3 |
| Phenyl-Sepharose | 1,034 | 0.329 | 340.7 | 2.66 | 56.3 |
| Q Sepharose | 80.4 | 2.543 | 204.5 | 20.5 | 33.8 |
| Hydroxylapatite | 12.9 | 12.13 | 156.7 | 97.9 | 25.9 |
| Resource Q | 2.75 | 43.92 | 120.8 | 354 | 20.0 |
Finally, the discovery of this soluble cocaine esterase is of particular interest for its use in a potential illicit-drug sensor. Enzymatic detection of illicit heroin has already been described (12). In conjunction with a suitable NADP+-dependent dehydrogenase active against ecgonine methyl ester, the cocaine esterase could enable simultaneous detection of heroin and cocaine, using established bioluminescence technology (12).
ACKNOWLEDGMENTS
This work was supported by the Leverhulme Trust and the BBSRC.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Meyers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Andersen S J, Quan S, Gowan B, Dabbs E R. Monooxygenase-like sequence of a Rhodococcus equi gene conferring increased resistance to rifampin by inactivating this antibiotic. Antimicrob Agents Chemother. 1997;41:218–221. doi: 10.1128/aac.41.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aramori I, Fukagawa M, Tsumura M, Iwami M, Ono H, Kojo H, Kohsaka M, Ueda Y, Imanaka H. Cloning and nucleotide sequencing of a novel 7β-(4-carboxybutanamido)cephalosporanic acid acylase gene of Bacillus laterosporus and its expression in Escherichia coli and Bacillus subtilis. J Bacteriol. 1991;173:7848–7855. doi: 10.1128/jb.173.24.7848-7855.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britt A J, Bruce N C, Lowe C R. Identification of a cocaine esterase in a strain of Pseudomonas maltophilia. J Bacteriol. 1992;174:2087–2094. doi: 10.1128/jb.174.7.2087-2094.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron G W W, Jordan K N, Holt P-J, Baker P B, Lowe C R, Bruce N C. Identification of a heroin esterase in Rhodococcus sp. strain H1. Appl Environ Microbiol. 1994;60:3881–3883. doi: 10.1128/aem.60.10.3881-3883.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chich J F, Chapot-Chartier M P, Ribadeau-Dumas B, Gripon J C. Identification of the active-site serine of the X-prolyl dipeptidyl aminopeptidase from Lactococcus lactis. FEBS Lett. 1992;314:139–142. doi: 10.1016/0014-5793(92)80960-o. [DOI] [PubMed] [Google Scholar]
- 7.Desomer J, Dhaese P, Van Montagu M. Transformation of Rhodococcus fascians by high-voltage electroporation and development of R. fascians cloning vectors. Appl Environ Microbiol. 1990;56:2818–2825. doi: 10.1128/aem.56.9.2818-2825.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desomer J, Vereecke D, Crespi M, van Montagu M. The plasmid-encoded chloramphenicol-resistance protein of Rhodococcus fascians is homologous to the transmembrane tetracycline efflux proteins. Mol Microbiol. 1992;6:2377–2385. doi: 10.1111/j.1365-2958.1992.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 9.French C E, Nicklin S, Bruce N C. Sequence and properties of pentaerythritol tetranitrate reductase from Enterobacter cloacae PB2. J Bacteriol. 1996;178:6623–6627. doi: 10.1128/jb.178.22.6623-6627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grzeszik C, Lubbers M, Reh M, Schlegel H G. Genes encoding the NAD-reducing hydrogenase of Rhodococcus opacus MR11. Microbiology. 1997;143:1271–1286. doi: 10.1099/00221287-143-4-1271. [DOI] [PubMed] [Google Scholar]
- 11.Heikoff S, Heikoff J G. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci USA. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt P J, Bruce N C, Lowe C R. Bioluminescent assay for heroin and its metabolites. Anal Chem. 1996;68:1877–1882. doi: 10.1021/ac951207r. [DOI] [PubMed] [Google Scholar]
- 13.Johnson E L. Alkaloid content in Erythroxylum coca tissue during reproductive development. Phytochemistry. 1996;42:35–38. [Google Scholar]
- 14.Kesseler M, Dabbs E R, Averhoff B, Gottschalk G. Studies on the isopropylbenzene 2,3-dioxygenase and the 3-isopropylcatechol 2,3-dioxygenase genes encoded by the linear plasmid of Rhodococcus erythropolis BD2. Microbiology. 1996;142:3241–3251. doi: 10.1099/13500872-142-11-3241. [DOI] [PubMed] [Google Scholar]
- 15.Komeda H, Hori Y, Kobayashi M, Shimizu S. Transcriptional regulation of the Rhodococcus rhodochrous J1 nitA gene encoding a nitrilase. Proc Natl Acad Sci USA. 1996;93:10572–10577. doi: 10.1073/pnas.93.20.10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M Z, Squires C H, Monticello D J, Childs J D. Genetic analysis of the dsz promoter and associated regulatory regions of Rhodococcus erythropolis IGTS8. J Bacteriol. 1996;178:6409–6418. doi: 10.1128/jb.178.22.6409-6418.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lister D L, Sproule R F, Britt A J, Lowe C R, Bruce N C. Degradation of cocaine by a mixed culture of Pseudomonas fluorescens MBER and Comamonas acidovorans MBLF. Appl Environ Microbiol. 1996;62:94–99. doi: 10.1128/aem.62.1.94-99.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayo B, Kok J, Venema K, Bockelmann W, Teuber M, Reinke H, Venema G. Molecular cloning and sequence analysis of the X-prolyl dipeptidyl aminopeptidase gene from Lactococcus lactis subsp. cremoris. Appl Environ Microbiol. 1991;57:38–44. doi: 10.1128/aem.57.1.38-44.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer-Barton E C, Klein J R, Imam M, Plapp R. Cloning and sequence-analysis of the X-prolyl dipeptidyl-aminopeptidase gene (Pepx) from Lactobacillus delbrueckii ssp. lactis DSM 7290. Appl Microbiol Biotechnol. 1993;40:82–89. doi: 10.1007/BF00170433. [DOI] [PubMed] [Google Scholar]
- 20.Mira A L, Pontani R B, Mule S J. Separation of cocaine, some of its metabolites and congeners on glass fibre sheets. J Chromatogr. 1973;81:167–169. doi: 10.1016/s0021-9673(01)82333-5. [DOI] [PubMed] [Google Scholar]
- 21.Moffat A C. Clarke's isolation and identification of drugs. 2nd ed. London, England: The Pharmaceutical Press; 1986. [Google Scholar]
- 22.Nagy I, Schoofs G, Compernolle F, Proost P, Vanderleyden J, De Mot R. Degradation of the thiocarbamate herbicide EPTC (S-ethyl dipropylcarbamothioate) and biosafening by Rhodococcus sp. strain NI86/21 involve an inducible cytochrome P-450 system and aldehyde dehydrogenase. J Bacteriol. 1995;177:676–687. doi: 10.1128/jb.177.3.676-687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nardi M, Chopin M-C, Chopin A, Cals M-M, Gripon J-C. Cloning and DNA sequence analysis of an X-prolyl dipeptidyl aminopeptidase gene from Lactococcus lactis subsp. lactis NCDO 763. Appl Environ Microbiol. 1991;57:45–50. doi: 10.1128/aem.57.1.45-50.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rathbone D A, Holt P J, Lowe C R, Bruce N C. The use of a novel recombinant heroin esterase in the development of an illicit drugs biosensor. Ann N Y Acad Sci. 1996;799:90–96. doi: 10.1111/j.1749-6632.1996.tb33184.x. [DOI] [PubMed] [Google Scholar]
- 25.Rorsch A, Berends F, Bartlema H C, Stevens W F. The isolation and properties of Pseudomonas strains growing on atropine and producing an atropine esterase. Proc K Ned Akad Wet Ser C. 1971;74:132–147. [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.Vesanto E, Savijoki K, Rantanen T, Steele J L, Palva A. An X-prolyl dipeptidyl aminopeptidase (Pepx) gene from Lactobacillus helveticus. Microbiology. 1995;141:3067–3075. doi: 10.1099/13500872-141-12-3067. [DOI] [PubMed] [Google Scholar]
- 28.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshpe-Besancon I, Gripon J C, Ribadeau-Dumas B. Xaa-Pro dipeptidyl aminopeptidase from Lactococcus lactis catalyzes kinetically controlled synthesis of peptide bonds involving proline. Biotechnol Appl Biochem. 1994;20:131–140. [PubMed] [Google Scholar]
- 30.Yüksel G U, Steele J L. DNA sequence analysis, expression, distribution, and physiological role of the Xaa-prolyl dipeptidyl aminopeptidase gene from Lactobacillus helveticus CNRZ32. Appl Microbiol Biotechnol. 1996;44:766–773. doi: 10.1007/BF00178616. [DOI] [PubMed] [Google Scholar]