Abstract
Objective
The COVID-19 pandemic continues, and the death toll continues to surge. This systematic review and meta-analysis aimed to determine the efficacy of therapeutic plasma exchange (TPE) on mortality in patients with COVID-19.
Methods
A systematic search was made of PubMed, Embase, Cochrane Library, and clinicaltrials.gov, without language restrictions. Controlled clinical trials on treatment of COVID-19 with TPE, compared with standard of care, were reviewed. Studies were pooled according to risk ratios (RRs) and weighted mean differences, with 95% confidence intervals (CIs).
Results
A total of six trials (enrolling 343 participants) met the inclusion criteria. Therapeutic plasma exchange showed significant effect on mortality (RR 0.41, 95% CI 0.24 to 0.69; P = 0.0008).
Conclusion
TPE significantly reduced mortality in hospitalized patients with moderate-to-critical COVID-19. Plasma exchange therapy should be considered for patients with COVID-19.
Keywords: Mortality, Plasma exchange, COVID-19, Meta-analysis
Introduction
The COVID-19 pandemic is the worst in more than 100 years, causing numerous infections and deaths worldwide. Despite the use of multiple drugs with different mechanisms, mortality from COVID-19 remains high, especially in critically ill patients with acute respiratory distress syndrome (ARDS), sepsis, and associated cytokine release syndrome (CRS) (Cegolon et al., 2022; Cegolon et al., 2020; Memish et al., 2021). Therapeutic plasma exchange (TPE) is a safe and effective method for treating various diseases by removing pathological substances and replenishing the deficient plasma components (Cegolon et al., 2022; Fernández-Zarzoso et al., 2019). Several controlled clinical trials have evaluated the effects of TPE in severely ill patients with COVID-19, with varying results.
Therefore, this study aimed to perform a systematic review and meta-analysis of controlled trials to determine the efficacy of TPE on mortality in patients with COVID-19.
Methods
Data sources and search strategy
This systematic review and meta-analysis was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (Moher et al., 2009). The protocol was previously registered in April 2022 in the International Prospective Register of Systematic Reviews database (Review register: CRD42022325020). PubMed, Embase, Cochrane Library, and clinicaltrials.gov were searched for studies up to April 2022.
Study selection
To be eligible for inclusion in the meta-analysis, studies had to meet the following criteria: (a) inclusion of hospitalized patients with COVID-19 aged 18 years or older; (b) polymerase chain reaction positive for SARS-CoV-2; and (c) use of a controlled design to make a comparison of standard of care (SOC) plus TPE with SOC. The search strings used for the databases were (“COVID-19” OR “SARS-CoV-2” OR “SARS-CoV-19” OR “novel coronavirus 2019” OR “novel coronavirus pneumonia”) AND (“plasma exchange” OR “therapeutic plasma exchange” OR “plasmapheresis” OR “TPE”). The reference lists of relevant review articles were also screened to identify studies that might have been missed in this search. No language restrictions were applied to our study selection process.
Data extraction and quality assessment
Two reviewers independently screened articles according to the inclusion criteria. The reviewers compared selected studies, and differences were resolved by consensus. Data tables were used to collect all relevant data from texts, tables, and figures of each included trial, including author, year of publication or last update posted, severity of the disease, patient number and age, body mass index, co-morbidities, Acute Physiology and Chronic Health Evaluation II score, Sequential Organ Failure Assessment score, and outcomes such as mortality, length of intensive care unit (ICU) stay, and duration of invasive mechanical ventilation (IMV). Study quality was assessed using the Detsky Quality Assessment Scale (Detsky et al., 1992; Qin et al., 2022; Shang et al., 2022). This is a 20-point scale for studies with statistically significant results and a 21-point scale for studies without statistically significant results.
Data synthesis and statistical analysis
Meta-analyses were conducted where applicable; otherwise, outcomes were presented in narrative form. Data were analyzed using the RevMan Version 5.4.1 (The Cochrane Collaboration). Next, risk ratios (RRs) for discontinuous outcomes and weighted mean differences (WMDs) for continuous outcomes, with corresponding 95% confidence intervals (CIs), were computed for individual trials. Chi-squared and Higgins I2 tests were used to assess heterogeneity among included trials. If significant heterogeneity (P ≤ 0.10 for Chi-squared test results or I2 ≥ 50%) was obtained, we used a random-effects model, otherwise, a fixed-effects model was used. A P-value < 0.05 was considered to indicate statistical significance.
To assess the robustness of the results, meta-regression analyses (STATA 12.0) were carried out for sensitivity analysis (Shang et al., 2021) to test the influence of potential effect modifiers such as randomization, sample size, sex, co-morbidities, and Detsky quality score. Begg's rank correlation test (Shang et al., 2022) (STATA 12.0) was used to assess the presence of publication bias in included articles for each outcome.
Results
Study selection and characteristics
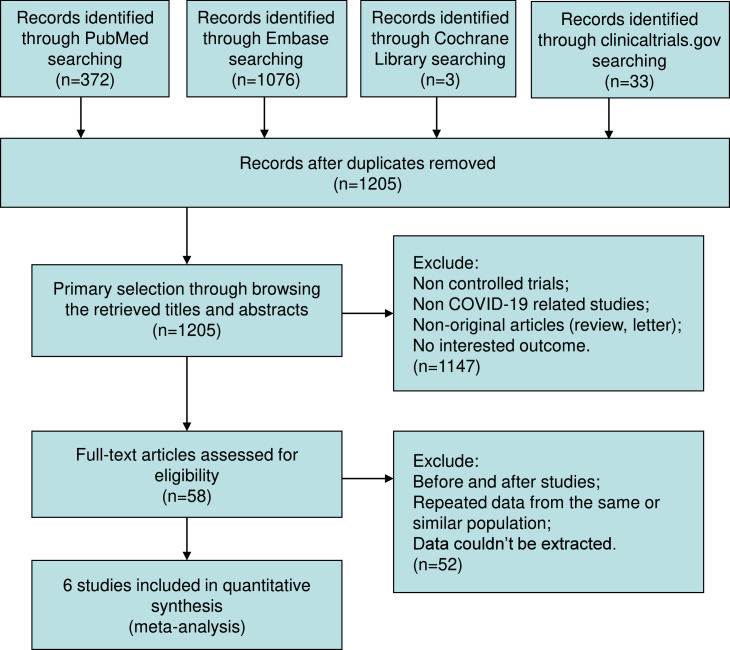
Of 1484 trials recognized by the initial search, 58 were retrieved for more detailed assessment, and six trials (Cegolon et al., 2022; Faqihi et al., 2021; Gucyetmez et al., 2020; Kamran et al., 2021; Khamis et al., 2020; Novacescu et al., 2022) were included in this meta-analysis (Figure 1 ). Baseline characteristics of trials included in this meta-analysis are listed in Table 1 . A total of 343 patients were included: 173 were assigned to the TPE groups and 170 to the control groups.
Figure 1.
Flow chart for selection of studies.
aConsider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers).
bIf automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.
From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71.
Table 1.
Baseline characteristics of trials included in meta-analysis.
| Study | Year | Quality Score | Randomization | WHO classification | Sort of plasma | Hospital location | Admission time | n |
|---|---|---|---|---|---|---|---|---|
| Cegolon | 2022 | 14 | Non-randomized | Severe | Standard | Tehran, Iran | March 4 to May 20, 2020 | 43 30 |
| Faqihi | 2021 | 19 | Randomized | Critical | Standard | Riyadh, Saudi Arabia | July 1 to October 1, 2020 | 43 44 |
| Gucyetmez | 2020 | 10 | Non-randomized | Severe-to-critical | NR | Istanbul, Turkey | March 10 to May 10, 2020 | 12 12 |
| Kamran | 2021 | 12 | Non-randomized | Moderate-to-critical | Standard | Rawalpindi, Pakistan | April 1 to July 31, 2020 | 45 45 |
| Khamis | 2020 | 10 | Non-randomized | Severe-to-critical | Standard | Muscat, Oman | April 17 to May 11, 2020 | 11 20 |
| Novacescu | 2022 | 12 | Non-randomized | Severe-to-critical | Standarda | Timisoara, Romania | August 8, 2020 to January 9, 2021 | 19 19 |
| Age, years (SD) | Male, % | BMI, kg/m2 (SD) | Co-morbidities, % | Diabetes, % | Hyper-tension, % | APACHE II score (SD) | SOFA score (SD) | |
| NR | 67 | NR | 42 | 26 | 37 | NR | NR | |
| NR | 40 | NR | 53 | 43 | 50 | NR | NR | |
| 48 (33-63) b | 83.7 | 27 (22-32) b | 51.2 | 46 | 86 | 23 (21-25) b | 10 (8-13) b | |
| 49 (33-63) b | 81.8 | 26 (20-33) b | 43.2 | 42 | 84 | 22 (21-23) b | 9 (6-12) b | |
| 61 (14) | 67 | 28.5 (6.1) | NR | NR | NR | 17 (3.3) | 6 (2) | |
| 64 (17) | 67 | 25.0 (6.6) | NR | NR | NR | 17.5 (5.6) | 6 (2) | |
| 60 (32-73) b | 100 | NR | 53.3 | 24 | 20 | NR | NR | |
| 60 (37-75) b | 100 | NR | 53.3 | 24 | 20 | NR | NR | |
| 50 (10) | 100 | NR | NR | 73 | 55 | NR | 6 (3-9) b | |
| 51 (17) | 85 | NR | NR | 35 | 30 | NR | 3 (2-6) b | |
| 58.7 (7.8) | 68.4 | 29.8 (5.0) | 95 | NR | NR | 5.7 (3.2) | NR | |
| 62 (12) | 57.9 | 27.2 (6.9) | 89 | NR | NR | 7.6 (6.5) | NR |
Abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; BMI = body mass index; NR = not reported; SD = standard deviation; SOFA = Sequential Organ Function Assessment; WHO = World Health Organization.
Followed by 500ml convalescent plasma transfusion
Values are median and interquartile range.
Mortality
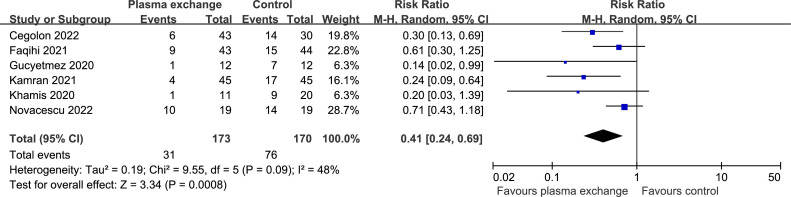
Data on mortality were available from six controlled trials (343 patients). Compared with the SOC, the mortality was significantly lower in the TPE groups (RR 0.41, 95% CI 0.24 to 0.69; P = 0.0008 [Figure 2 ]), with a rate of 17.92% versus 44.71%. There was significant heterogeneity (I2 = 48%; P = 0.09). Begg's test (P = 0.091) did not show evidence of publication bias. Results did not change significantly after excluding two trials (Gucyetmez et al., 2020; Khamis et al., 2020) with lower quality scores (RR 0.46, 95% CI 0.27 to 0.80; P = 0.005).
Figure 2.
Forest plot assessing the efficacy of therapeutic plasma exchange on mortality.
Length of ICU stay
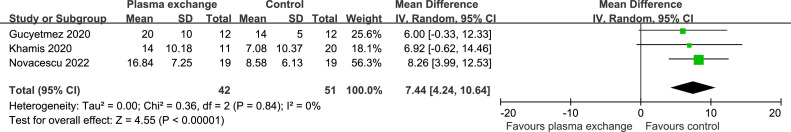
Data on length of ICU stay were available from three trials (93 patients). The length of ICU stay was significantly shorter in the SOC groups (WMD 7.44 days, 95% CI 4.24 to 10.64 days; P < 0.00001 [Figure 3 ]). There was no significant heterogeneity (I2 = 0%; P = 0.84). Begg's test (P = 0.602) did not show evidence of publication bias.
Figure 3.
Forest plot assessing the efficacy of therapeutic plasma exchange on length of ICU stay.
ICU = intensive care unit.
Duration of IMV
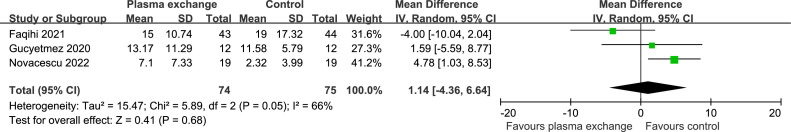
Data on the duration of IMV were extracted from three studies (149 patients). There was no statistically significant difference in the duration of IMV between the two groups (WMD 1.14 days, 95% CI -4.36 to 6.64 days; P = 0.68 [Figure 4 ]). There was significant heterogeneity (I2 = 66%; P = 0.05). Begg's test (P = 0.602) did not show evidence of publication bias.
Figure 4.
Forest plot assessing the efficacy of therapeutic plasma exchange on duration of invasive mechanical ventilation.
Sensitivity analysis
Our results were mostly confirmed when potential effect modifiers were introduced as covariates in the meta-regression analysis. In this analysis, no significant impact was found on either mortality, length of ICU stay, or duration of IMV (Table 2 ).
Table 2.
Potential effect modifier with change in tau2 and statistical significance for each outcome.
| Change in tau2 | P-value | |
|---|---|---|
| Mortality | ||
| Randomization | -0.81 | 0.461 |
| Detsky quality score | 0.91 | 0.415 |
| Sample size | -0.04 | 0.973 |
| Men | -0.54 | 0.618 |
| Co-morbidities | 1.11 | 0.384 |
| Length of intensive care unit stay | ||
| Detsky quality score | 1.14 | 0.459 |
| Sample size | 0.93 | 0.524 |
| Men | -0.88 | 0.541 |
| Duration of invasive mechanical ventilation | ||
| Randomization | 1.80 | 0.323 |
| Detsky quality score | -1.08 | 0.477 |
| Sample size | -1.08 | 0.477 |
| Men | -2.59 | 0.234 |
Discussion
This meta-analysis is designed specifically to evaluate the efficacy of TPE in hospitalized patients with COVID-19. Based on the present results, we observed that TPE significantly reduced mortality. The length of ICU stay was significantly shorter in the SOC groups, possibly because of the premature death of patients in the SOC groups (Novacescu et al., 2022). In one included trial (Novacescu et al., 2022), seven (36.8%) patients in the SOC group died before day 7, resulting in a significant reduction in ICU stay and IMV duration. Therefore, TPE may avoid premature death in critically ill patients, thereby prolonging ICU stay.
COVID-19 is caused by SARS-CoV-2 and has caused a global pandemic. Although most patients have mild symptoms, mortality remains high in critically ill patients, which may be largely attributable to an overactive immune response rather than the viral infection itself (Beraud et al., 2022). This excessive inflammatory response, commonly referred to as cytokine storm syndrome or CRS, can lead to lung damage, ARDS, multiple organ dysfunction syndrome (MODS), sepsis, and ultimately death (Huang et al., 2020; Ye et al., 2020; Yuki et al., 2020). Healthcare systems in most countries are overwhelmed as the global COVID-19 surge continues. Therefore, there is an urgent need for safe and effective therapeutic approaches to inhibit excessive cytokine release, halt disease progression, and reduce mortality.
Persistent mutations in SARS-CoV-2 may lead to resistance to vaccines and antiviral drugs, leading to treatment failure (Cegolon et al., 2022). Therefore, non-drug treatments such as TPE may be an option for COVID-19. For over a century, TPE has been extensively studied and used to treat a variety of serious illnesses (Fernández-Zarzoso et al., 2019), including ARDS (Cegolon et al., 2022), influenza (Patel et al., 2011), and sepsis-related MODS (Busund et al., 2002; Keith et al., 2020). TPE may interrupt the progression of CRS by removing increased cytokines and inflammatory mediators, thereby reducing fatal complications such as septic shock, pulmonary embolism, renal injury, or disseminated intravascular coagulation (Cegolon et al., 2022; Cegolon et al., 2020). TPE is effective and safe without developing drug resistance; therefore, it may play an important role in the treatment of COVID-19, especially in critically ill patients. Future large randomized controlled trials are needed to explore the specific regimen and efficacy of TPE and to determine how TPE affects the clinical course of COVID-19 and its mechanisms.
This study met most of the methodological criteria recommended for systematic reviews and meta-analyses (Liberati et al., 2009). However, some limitations need to be considered when interpreting the results of this study. First, some included trials had small sample sizes, which may have reduced the power of the results. Second, most included trials were non-randomized. Finally, this meta-analysis was not patient-level, so the results should be considered provisional.
Conclusions
The addition of TPE significantly reduced mortality in hospitalized patients with moderate-to-critical COVID-19. Plasma exchange therapy should be considered for COVID-19, especially in critically ill patients.
Conflict of interest
The authors have no competing interests to declare.
Acknowledgments
Funding
None.
Data availability statement
Extracted data are available on request to the corresponding author.
Ethical approval
Not applicable.
Contributors
All authors, led by DH, were involved in the concept and protocol design of the meta-analysis. JQ and GW screened the titles and abstracts and extracted data from the articles. JQ was primarily responsible for statistical analyses. DH was primarily involved in the interpretation of the quality data. All authors contributed to interpreting the results. GW and DH accessed and verified the data. All authors contributed to the writing of the article and approved its submission. DH was responsible for the decision to submit the article.
Footnotes
Registration number with PROSPERO: CRD42022325020
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.06.014.
Appendix. Supplementary materials
References
- Beraud M, Hashami SA, Lozano M, Bah A, Keith P. Role of therapeutic plasma exchange in the management of COVID-19-induced cytokine storm syndrome. Transfus Apher Sci. 2022 doi: 10.1016/j.transci.2022.103433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busund R, Koukline V, Utrobin U, Nedashkovsky E. Plasmapheresis in severe sepsis and septic shock: a prospective, randomised, controlled trial. Intensive Care Med. 2002;28:1434–1439. doi: 10.1007/s00134-002-1410-7. [DOI] [PubMed] [Google Scholar]
- Cegolon L, Einollahi B, Panahi Y, Imanizadeh S, Rezapour M, Javanbakht M, Nikpouraghdam M, Abolghasemi H, Mastrangelo G. On therapeutic plasma exchange against severe COVID-19-associated pneumonia: an observational clinical study. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.809823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cegolon L, Javanbakht M, Mastrangelo G. Nasal disinfection for the prevention and control of COVID-19: A scoping review on potential chemo-preventive agents. Int J Hyg Environ Health. 2020;230 doi: 10.1016/j.ijheh.2020.113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detsky AS, Naylor CD, O'Rourke K, McGeer AJ, L'Abbé KA. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol. 1992;45:255–265. doi: 10.1016/0895-4356(92)90085-2. [DOI] [PubMed] [Google Scholar]
- Faqihi F, Alharthy A, Abdulaziz S, Balhamar A, Alomari A, AlAseri Z, Tamim H, Alqahtani SA, Kutsogiannis DJ, Brindley PG, Karakitsos D, Memish ZA. Therapeutic plasma exchange in patients with life-threatening COVID-19: a randomised controlled clinical trial. Int J Antimicrob Agents. 2021;57 doi: 10.1016/j.ijantimicag.2021.106334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Zarzoso M, Gómez-Seguí I, de la Rubia J. Therapeutic plasma exchange: review of current indications. Transfus Apher Sci. 2019;58:247–253. doi: 10.1016/j.transci.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Gucyetmez B, Atalan HK, Sertdemir I, Cakir U, Telci L. COVID-19 Study Group. Therapeutic plasma exchange in patients with COVID-19 pneumonia in intensive care unit: a retrospective study. Crit Care. 2020;24:492. doi: 10.1186/s13054-020-03215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamran SM, Mirza ZE, Naseem A, Liaqat J, Fazal I, Alamgir W, Saeed F, Saleem S, Nisar S, Yousaf MA, Khan AZ, Hussain M, Azam R, Hussain M, Khan KA, Jamal Y, Iftikhar R. Therapeutic plasma exchange for coronavirus disease-2019 triggered cytokine release syndrome; a retrospective propensity matched control study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0244853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith PD, Wells AH, Hodges J, Fast SH, Adams A, Scott LK. The therapeutic efficacy of adjunct therapeutic plasma exchange for septic shock with multiple organ failure: a single-center experience. Crit Care. 2020;24:518. doi: 10.1186/s13054-020-03241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamis F, Al-Zakwani I, Al Hashmi S, Al Dowaiki S, Al Bahrani M, Pandak N, Al Khalili H, Memish Z. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int J Infect Dis. 2020;99:214–218. doi: 10.1016/j.ijid.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- Memish ZA, Faqihi F, Alharthy A, Alqahtani SA, Karakitsos D. Plasma exchange in the treatment of complex COVID-19-related critical illness: controversies and perspectives. Int J Antimicrob Agents. 2021;57 doi: 10.1016/j.ijantimicag.2020.106273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group PRISMA. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W264. [DOI] [PubMed] [Google Scholar]
- Novacescu AN, Duma G, Buzzi B, Baditoiu LM, Bedreag O, Papurica M, Sandesc D, Sorescu T, Vlad D, Licker M. Therapeutic plasma exchange followed by convalescent plasma transfusion in severe and critically ill COVID-19 patients: a single centre non-randomized controlled trial. Exp Ther Med. 2022;23:76. doi: 10.3892/etm.2021.10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P, Nandwani V, Vanchiere J, Conrad SA, Scott LK. Use of therapeutic plasma exchange as a rescue therapy in 2009 pH1N1 influenza A–an associated respiratory failure and hemodynamic shock. Pediatr Crit Care Med. 2011;12:e87–e89. doi: 10.1097/PCC.0b013e3181e2a569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Wang G, Han D. Benefits of LAMA in patients with asthma-COPD overlap: a systematic review and meta-analysis. Clin Immunol. 2022;237 doi: 10.1016/j.clim.2022.108986. [DOI] [PubMed] [Google Scholar]
- Shang W, Wang G, Wang Y, Han D. The safety of long-term use of inhaled corticosteroids in patients with asthma: a systematic review and meta-analysis. Clin Immunol. 2022;236 doi: 10.1016/j.clim.2022.108960. [DOI] [PubMed] [Google Scholar]
- Shang W, Zhang Y, Wang G, Han D. Benefits of continuous positive airway pressure on glycaemic control and insulin resistance in patients with type 2 diabetes and obstructive sleep apnoea: a meta-analysis. Diabetes Obes Metab. 2021;23:540–548. doi: 10.1111/dom.14247. [DOI] [PubMed] [Google Scholar]
- Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `cytokine Storm' in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Extracted data are available on request to the corresponding author.