Abstract
Background
Non-pharmaceutical interventions (NPIs) used to limit SARS-CoV-2 transmission vary in their feasibility, appropriateness and effectiveness in different contexts. In Bangladesh a national lockdown implemented in March 2020 exacerbated poverty and was untenable long-term. A resurgence in 2021 warranted renewed NPIs. We sought to identify NPIs that were feasible in this context and explore potential synergies between interventions.
Methods
We developed an SEIR model for Dhaka District, parameterised from literature values and calibrated to data from Bangladesh. We discussed scenarios and parameterisations with policymakers with the aid of an interactive app. These discussions guided modelling of lockdown and two post-lockdown measures considered feasible to deliver; symptoms-based household quarantining and compulsory mask-wearing. We compared NPI scenarios on deaths, hospitalisations relative to capacity, working days lost, and cost-effectiveness.
Results
Lockdowns alone were predicted to delay the first epidemic peak but could not prevent overwhelming of the health service and were costly in lost working days. Impacts of post-lockdown interventions depended heavily on compliance. Assuming 80% compliance, symptoms-based household quarantining alone could not prevent hospitalisations exceeding capacity, whilst mask-wearing prevented overwhelming health services and was cost-effective given masks of high filtration efficiency. Combining masks with quarantine increased their impact. Recalibration to surging cases in 2021 suggested potential for a further wave in 2021, dependent on uncertainties in case reporting and immunity.
Conclusions
Masks and symptoms-based household quarantining synergistically prevent transmission, and are cost-effective in Bangladesh. Our interactive app was valuable in supporting decision-making, with mask-wearing being mandated early, and community teams being deployed to support quarantining across Dhaka. These measures likely contributed to averting the worst public health impacts, but delivering an effective response with consistent compliance across the population has been challenging. In the event of a further resurgence, concurrent messaging to increase compliance with both mask-wearing and quarantine is recommended.
Keywords: Coronavirus disease 2019 (COVID-19), Non-pharmaceutical interventions (NPIs), Low- and middle- income countries (LMICS), Cost-effectiveness, Facemasks
1. Introduction
During the early stages of the COVID-19 pandemic, countries around the world turned to a range of non-pharmaceutical interventions (NPIs) to limit transmission. These measures included improved hygiene practices, social distancing, contact tracing, travel restrictions, quarantines, shielding of the vulnerable, lockdowns of differing severity, and facemasks. For a variety of reasons, some of these measures may be less effective or more difficult to maintain in low- and middle-income countries (LMICs). Vaccines came into play in 2021, but rollout has been slow in LMICs. With negligible vaccination coverage and only NPIs to mitigate impacts, LMICs have faced subsequent epidemic waves of faster-spreading variants with possibly little protective immunity from prior infections (Cele et al., 2021, Planas et al., 2021).
Lockdowns can effectively control COVID-19 transmission (Flaxman et al., 2020). However, they also exacerbate poverty (Amewu et al., 2020, Andam et al., 2020) and risk food security, whilst poor adherence limits their effectiveness. Social distancing may be impractical in densely populated areas (Anwar et al., 2020, Chowdhury et al., 2020, Gupta et al., 2020), and in urban slums and refugee camps, where cramped conditions and poor healthcare access co-occur (Ahmed et al., 2020, Truelove et al., 2020). Shielding the most vulnerable, is also challenging in multi-generational households (Hodgins and Saad, 2020, Lloyd-Sherlock et al., 2020; United Nations, Department of Economic and Social Affairs, 2019a). Contact tracing is limited by testing capabilities (facilities, trained personnel, consumables, reagents and biosafety) (Anwar et al., 2020, Homaira et al., 2020, Rahaman et al., 2020) and information management capacity. Moreover, in settings where healthcare resources are already stretched, there is limited ability to temporarily increase capacity to deal with sudden increases in patient volume (Torres-Rueda et al., 2020). The impact of COVID-19, however, may be mitigated in LMICs by the relatively younger populations with fewer underlying risk factors, meaning that a smaller proportion of cases are likely to be severe relative to high-income countries (HICs) (Clark et al., 2020, Gupta et al., 2020, Hodgins and Saad, 2020). More generally, the wider social and economic consequences of NPIs may trade off against their impact on controlling disease, and the structure and underlying health of populations may impact the shape of this trade-off (Reidpath et al., 2020). Hence, there was an urgent need for the development and implementation of contextually appropriate interventions that take into account the population that they target (Hodgins and Saad, 2020).
During the pandemic, epidemiological models have received increased attention from governments and the public alike, and have influenced policy decisions worldwide (McBryde et al., 2020). However, a translational gap persists between policymakers and the scientists working on these models. Early in the pandemic, this gap was exacerbated by uncertainties about the biology and transmission of SARS-CoV-2, and continually compounded by limited understanding of and changes in people’s behaviour. As a consequence, there was often simultaneously both overconfidence in, and mistrust of, models. Ideally, policymakers, scientists and communities should work together to develop and implement locally appropriate interventions. By empowering decision makers to better understand the mechanisms underpinning the timescales and magnitude over which interventions lead to impact, as well as the uncertainties and social and behavioural factors that affect their efficacy, co-created models can inform short- and longer-term policies.
Cases of COVID-19 were first confirmed in Bangladesh on the 8th March 2020, and NPIs were subsequently introduced, beginning with postponements to mass gatherings, followed by international travel restrictions, and culminating in a national lockdown (announced as a ‘general holiday’) from 26th March (Ahmed et al., 2020, Anwar et al., 2020). The lockdown had swift economic repercussions: around 60% of households lost their main income source, the majority of previously vulnerable non-poor households slipped below the poverty line, and food security declined (Rahman et al., 2020). The nationwide lockdown was quickly recognized as untenable long-term (ultimately ending on 1st June 2020).
With input from policymakers on the feasibility of implementation, we developed an SEIR model to compare post-lockdown NPIs on: (1) their ability to reduce deaths and hospitalisations; and (2) both their cost-effectiveness to the health provider and their cost in terms of working days lost (one of many societal costs of the pandemic and associated control efforts). In particular, we sought to identify potential synergies between different measures. We also explored the consequences of long scale-up periods or delays in implementing interventions, and designed an associated interactive app within which policymakers could explore the NPI scenarios to gain an understanding of how the model worked and how uncertainties may influence outcomes. Finally, we examined scenarios following resurgence of cases and a renewed lockdown in March 2021.
2. Methods
2.1. Model description
We developed a deterministic SEIR model comprising a set of ordinary differential equations (ODEs) to describe SARS-CoV-2 transmission in Dhaka District, the most densely populated district in Bangladesh. Dhaka District includes rural areas in addition to the city area itself, but, as case data were resolved to district level, we consider the full district population, rather than restricting analyses to the city population.
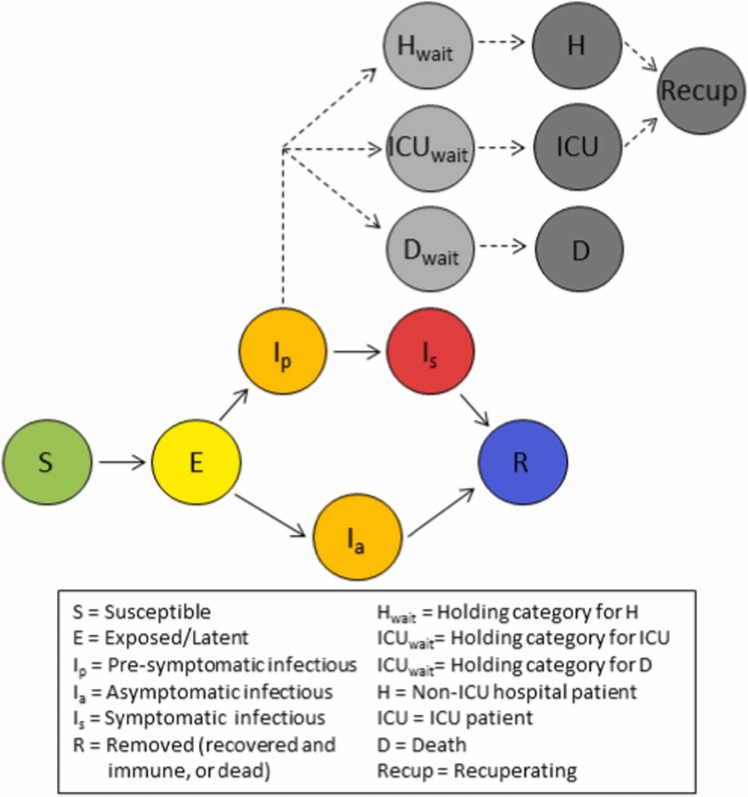
The model includes three infectious states, with latently infected individuals either becoming pre-symptomatically infectious () before progressing to symptomatic infection (), or becoming asymptomatically infectious () until their recovery ( Fig. 1). Susceptible individuals are exposed to SARS-CoV-2 according to transmission rates specific to each infectious state (, and ). These rates are based on: (1) asymptomatic individuals producing 65% of the secondary infections produced by pre-symptomatic-to-symptomatic individuals (Yi et al., 2020); (2) 35% of secondary infections from pre-symptomatic-to-symptomatic individuals happening in the pre-symptomatic period (Liu et al., 2020); and (3) the value of :
where , and are the mean durations (in days) of each infectious state (Byrne et al., 2020, Hu et al., 2020), and is the proportion of infections that are asymptomatic (Supplementary Table S4). Recovered individuals are considered to be immune. SEIR models have been widely used in the COVID-19 pandemic, though there has been some variation in the modelling of the infectious classes, with some using the same three classes described here (Davies et al., 2020b), while others do not break down the pre-symptomatic and symptomatic classes (Keeling et al., 2021) or use more complex infectious structures (Jayasundara et al., 2021).
Fig. 1.
Model schematic illustrating the movement of individuals between classes. Coloured circles indicate disease states that impact transmission. Grey circles describe health outcomes that are tracked but have no impact on disease transmission.
We track numbers of both general and Intensive Care Unit (ICU) beds required by COVID-19 patients, and deaths due to COVID-19 (Fig. 1). A proportion of individuals that leave the pre-symptomatic state join a holding category prior to progressing to general hospital beds, while a second proportion enters a holding category prior to entering ICU beds. Individuals remain in these two holding categories from the first appearance of symptoms until the point at which hospitalisation is required; on average 7 days (Liang et al., 2020, Linton et al., 2020). A third holding category retains a proportion of individuals from the first appearance of symptoms until death, on average 20.2 days later (Linton et al., 2020, Verity et al., 2020). While we assume no overlap between the groups in ICU versus general hospital beds, overlap between those that die and the hospitalized groups is both permitted and expected. On leaving hospital, after a mean of 5 days for general beds and 7 days for ICU (Rees et al., 2020), recovered individuals recuperate for an average of 3 weeks before resuming work, if employed (Halpin et al., 2020). The number of individuals in the seven health outcome states has no impact on transmission dynamics, and it is assumed that there is no impact of a lack of hospital beds on the COVID-19 death rate. This approach to modelling health outcomes outside of the main transmission model is similar to that described in several other COVID-19 models (Davies et al., 2020b, Keeling et al., 2021). Other models include hospitalised patients as infectious categories integrated into the progression from susceptible to removed (Aleta et al., 2020, Jayasundara et al., 2021, Kerr et al., 2021).
We use data on the age structure of the Dhaka population (Bangladesh Bureau of Statistics, 2011) (Supplementary Table S1) to inform parameters describing the risks of SARS-CoV-2 infections. Previously estimated age-specific risks of hospitalisation and death due to COVID-19 (Davies et al., 2020b), and of developing symptoms (Davies et al., 2020a) (Supplementary Table S2), were combined with the age distribution in Dhaka District, to estimate the overall proportions of (1) asymptomatic infections, , (2) symptomatic infections that lead to death, , and (3) symptomatic infections requiring hospitalisation, . Of those hospitalised we assume that the proportion requiring critical care in an ICU is (World Health Organisation (WHO), 2020a). We assumed no age-structure in contacts within our model.
The model was initially developed as an interactive epidemiological teaching tool (http://boydorr.gla.ac.uk/BGD_Covid-19/CEEDS/), to allow policymakers to explore the impact of interventions on health outcomes and working days lost. For speed and efficiency, and given the large population (Dhaka District population in 2020 was around 13.8 million (Bangladesh Bureau of Statistics, 2011; United Nations, Department of Economic and Social Affairs, 2019b)), the decision was made to make the model deterministic rather than stochastic. We minimise computational complexity by modelling transmission at the population level rather than at the level of the individual or household. However, to more accurately model household quarantining, we further subdivide the six disease states (Fig. 1) to track within-household transmission and account for household-level susceptible depletion (Supplement A).
The ODEs comprising the model are provided in Supplement A and the model parameter descriptions, values and sources, are listed in Supplementary Table S4. Analyses were implemented in R (R Core Team, 2021), with the ODEs numerically integrated using the package deSolve (Soetaert et al., 2010). Code can be accessed from our Github repository (https://github.com/boydorr/BGD_Covid-19/tree/main/BGD_NPI_model).
2.2. Interventions
We implemented three main NPIs – lockdown, household quarantining delivered through Community Support Teams (CSTs), and mask wearing – that were considered to be feasible by policymakers in Bangladesh.
We define a lockdown as a scenario where all except essential workplaces are closed, including educational facilities, and people are asked to stay home where possible and practice social distancing. For compliant individuals and those that are not essential workers, this intervention is assumed to reduce contacts, and therefore transmission, outside of the household by the proportion , while leaving within-household transmission unchanged. We achieve this by breaking down the transmission rates for the three infectious states into within- and between-household components using estimates of the SARS-CoV-2 household secondary attack rate, (Madewell et al., 2020), and the mean household size, (Bangladesh Bureau of Statistics, 2017). We considered a scale-up period for the lockdown, during which compliance increased linearly from zero to a maximum. Following scale-up, we assume compliance starts to decline sigmoidally towards a minimum. Full details of the lockdown compliance function are given in Supplement A (eq. (S.6)).
Under the household quarantine intervention, when a symptomatic individual occurs, that individual’s entire household is required to quarantine for 14 days. Those who develop symptoms can self-report to a national COVID-19 hotline or are identified by word-of-mouth, triggering a visit by CST, a volunteer workforce of community-based support workers trained by BRAC/FAO. The CST confirm that symptoms are consistent with COVID-19, provide information on how to limit spread, facilitate access to healthcare, and offer support to aid quarantine compliance, with additional follow-up during the quarantine period.
Incorporating household quarantine into models can be achieved far more naturally in the realm of agent-based models, where individuals can be explicitly assigned to households (Aleta et al., 2020, Ferguson et al., 2020). Here, similarly to Keeling et al., (2021), we approximate household quarantine by sub-dividing the disease states into additional categories, but with some changes to allow inclusion of a pre-symptomatic infectious class. We also remove the assumption that only the first individual in a household transmits. Modelling household quarantine requires us to identify both those individuals that trigger quarantining (the first symptomatic individuals within households), and other individuals in their households who also undergo quarantine. We subdivide the disease states to identify those not currently in an infected household, those in a non-quarantined infected household, and those in a quarantined household. When a susceptible individual in an uninfected household becomes latently infected (through between-household transmission), other individuals also move from uninfected household categories into categories that identify them as being in a non-quarantined infected household, making them vulnerable to both within-household and between-household transmission. We track the group of individuals who were the first infected within a household separately to those who subsequently became infected, allowing us to observe when household index cases become symptomatic. A proportion of these symptomatic index cases comply with quarantine, taking individuals from non-quarantined infected households with them. We also trigger quarantines when within-household cases resulting from index asymptomatic cases become symptomatic. The equations generating these dynamics are described in Supplement A. Infectious individuals within quarantined households are assumed to not cause any between-household transmission. As with lockdown, household quarantining with CST support has defined start and end times, and the proportion of households that are compliant increases linearly through a scale-up period. Following the scale-up period, compliance remains at a constant maximum (see Supplement A).
Finally, we considered compulsory mask-wearing outside of the household. Within the model, masks are assumed to block a proportion, , of between-household transmission from compliant individuals, while also blocking a proportion, , of transmission to compliant individuals, where , i.e. masks protect others from the wearer, and the wearer from others, to different degrees, with protection provided to the wearer never greater than that provided to others (Howard et al., 2020, Stutt et al., 2020). We assume a linear scale-up period for mask use, after which compliance remains constant (Supplement A). We do not consider a decline in compliance over time for household quarantine and masks, since, unlike lockdown, these measures do not cause long-term loss of income, and should be possible to maintain with continued promotion. We recognise, however, that erosion of compliance may occur in reality as a result of, for example, reduced perception of risk.
We model working days lost due to both illness and interventions. Based on the 2011 census (Bangladesh Bureau of Statistics, 2011), we assume 52% of the population is formally employed, working five days a week. We assume that both symptomatic and recuperating individuals are unable to work, and that deaths result in loss of all subsequent working days through 2020. During lockdown, those workers that are both compliant and not essential workers lose their working days. Those in quarantined households do not work, and, since household quarantine is based on symptoms, we assume that, in addition to those quarantined due to COVID-19, a proportion of households affected by non-COVID-19 influenza-like illnesses also undergo quarantine. Based on the 2011 census, 23% of the population is unemployed, but works within the household, e.g. with caring responsibilities (Bangladesh Bureau of Statistics, 2011). When these individuals are hospitalised or die, we assume another (possibly formally employed) household member replaces them, leading to further loss of working days. Finally we assume that, in response to each death, a number of grieving individuals (taken to be ) do not work for a week. Details of the working days lost calculation are provided in eqs. S.16–17 in Supplement A.
2.3. Scenario comparison
For each intervention, and combination of interventions, we fully explored with policymakers in real-time the impact of different timings of implementation, scale-up periods, and levels of compliance. Here, we describe just 15 combinatorial scenarios for 2020, including a baseline with no interventions and unmitigated SARS-CoV-2 spread.
The first of four lockdown-only scenarios aimed to replicate the lockdown as implemented, from 26th March until 1st June 2020. The other three lockdown-only scenarios involved extending the lockdown by 1, 2 and 3 months. Despite their impracticality, these extended lockdowns allow comparison with more feasible scenarios. Google community mobility data (Google, 2021) were used to parameterise the function describing changes in lockdown compliance with time (see Supplement B for details). The estimated parameters indicate that the lockdown did not have an initial scale-up, with compliance being very high (93%) from the first day. However, the estimated compliance declined rapidly thereafter and was only 42% by the lockdown’s end (Supplementary Fig. S2D). The impact of lockdown on between-household transmission of compliant individuals was estimated during model calibration (see below).
We also examined scenarios where lockdown, as implemented in Bangladesh, was followed by either CST interventions, compulsory mask-wearing, or both; beginning a week before lockdown ended and continuing through 2020 with a 7-day scale-up period and 80% peak compliance with each intervention. The ability of masks both to protect others from transmission from the wearer and to protect the wearer from transmission from others depends on several variables, including the material used, the construction (e.g. layers of material), and the quality of fit (Aydin et al., 2020, Davies et al., 2013, Howard et al., 2020). Therefore, for mask-wearing we consider scenarios where there is low, medium, and high protection of others from the wearer (), and where the protection provided to the wearer is zero, half that provided to others, or equal to that provided to others (), giving nine mask-wearing scenarios.
For the 2020 time horizon we compared the total: (1) hospitalisations, (2) deaths, (3) percentage of patient days exceeding hospital capacity (the sum of patients in excess of hospital beds each day, divided by the summed total patients over all days), (4) working days lost, (5) cost of implementing interventions and of healthcare for COVID-19 patients, (6) cost per death averted (relative to no intervention), and (7) the percentage return (in terms of healthcare savings) on investment (%ROI) in interventions. For estimating total cost we include healthcare provision for hospitalised COVID-19 patients, media campaigns, training and deployment of CSTs, and mask distribution, as detailed in Table 1. Note that these costs include only the direct monetary costs to health provider, and not any of the wider economic or societal costs of the pandemic and interventions to, for example, businesses, employment, trade, etc.; many of which may persist long after initial pandemic waves. For each scenario, we used the total cost to estimate the cost per death averted. This was calculated by subtracting the cost of the baseline scenario from that of the focal scenario, then dividing by the reduction in deaths from the baseline. The %ROI for each scenario was calculated by subtracting the total cost of each scenario from the baseline, and dividing by the intervention implementation costs (i.e. the sum of all costs other than hospital care).
Table 1.
Direct costs associated with healthcare and implementation of interventions, derived from ongoing practices in Bangladesh.
| Item | Description of activities | Costs (USD) |
|---|---|---|
| Hospital care | Provision of hospital beds and associated care (mechanical ventilation, oxygen etc.) for severely ill COVID-19 patients. | $649/week/ICU patient; $266/week/non-ICU patient. |
| Community Support Team (CST) package | The CST package covers:
|
$466/volunteer trained and equipped; $8,470 one-time cost for telemedicine training for a subset of volunteers; $151 allowance/month/volunteer; $253 allowance/month/area manager; $447 allowance/month/ coordinator; $8,962/month advertising cost. |
| Masks | An average of 5 re-usable cloth masks was provided to each household in Dhaka District. Mask wearing was promoted through a public awareness campaign. | $1/mask; $8,962/month advertising cost. |
| Lockdown | Public awareness campaign to encourage people to remain at home and practice social distancing. | $8,962/month advertising cost. |
Given the heavy societal and economic costs of lockdowns, they are typically viewed as temporary measures to buy time for implementation of other less restrictive measures (e.g. mask wearing) or more targeted measures (e.g. household quarantines and contact tracing). To explore the impact of preparedness prior to lockdown ending, we examined the sensitivity of model outcomes (hospitalisations, deaths, working days lost, and exceeded hospital capacity) to the scale-up period and start date of post-lockdown interventions. Modelling lockdown as implemented in Bangladesh, we looked at scenarios where lockdown was followed by household quarantining only, mask-wearing only (assuming mask protectiveness parameters and ), or both. We varied the start date of the post-lockdown intervention(s) from 30 days pre- to 30 days post-lockdown ending, while keeping the scale-up period constant at 7 days, and vice versa varying the scale-up period (zero to 30 days), while keeping the start constant (7 days prior to the lockdown end).
2.4. Model calibration
We calibrated two parameters, and (the proportion by which lockdown reduces between-household transmission from compliant people), against daily COVID-19 death data in Bangladesh (European Centre for Disease Prevention and Control, 2020). We chose to calibrate against reported deaths rather than cases, since death data are less affected by testing and reporting capacity. To obtain a time-series of deaths in Dhaka District, we extracted the proportion of district-resolved case totals for Dhaka District from Bangladesh’s COVID-19 dashboard ("Coronavirus COVID-19 Dashboard, 2020,” 2020) and assumed this proportion remained constant. The first three cases in Bangladesh were confirmed on 8th March 2020, but it is thought likely that infection began circulating undetected prior to this, with genomic data indicating an introduction in mid-February and at least eight introduced cases prior to the ban on international travel (Cowley et al., 2021). We therefore initialise the model with eight infectious cases on 15th February 2020. The value of was optimised to minimise the sum of squared differences between modelled and reported cumulative deaths on each day during the period from first detection (8th March) to the start of lockdown (26th March). Following this optimisation of , the value of was similarly estimated by minimizing the sum of squared differences between modelled deaths and data during the period the lockdown was in operation.
We recalibrated the model to data from the rapid resurgence beginning in March 2021 to learn about the likely at that time, when Beta (B.1.351) increased in frequency to become the dominant virus variant (Saha et al., 2021a). These data were curated from a range of sources, including both data aggregating websites and national government health departments (Dong et al., 2020), and were accessed using the ‘coronavirus’ package in R (Krispin and Byrnes, 2021). In March 2021, levels of mask-wearing and compliance with household quarantine were low, and there was no lockdown in place. As cases increased, a loose lockdown was introduced on 5th April, with stronger restrictions applied on 14th April. The lockdown was maintained until 23rd May, with some relaxing of restrictions over this period. We modelled this with a lockdown from 5th April to 23rd May, with a 9-day scale-up period, maximum compliance of 80%, and the same rate of declining compliance as in 2020 (Supplementary Fig. S1C). We also assume that from 5th April to 23rd May 2021 there are low levels of both mask-wearing (with and ) and household quarantine, with a scale-up period of 9 days to reach a compliance of 20%. Some loose social distancing measures were maintained beyond 23rd May, but the vast majority of workplaces were open by this point, so we do not consider these to constitute a continuation of lockdown.
There was considerable uncertainty around the number of infectious and immune individuals in the population at the time of the March 2021 resurgence, so we calibrated the model under a range of initialisation scenarios in terms of immunity levels and circulating cases on 1st March. A cross-sectional study from late 2020 to early 2021 in slum and slum-adjacent areas of Dhaka indicated 71% seroprevalence (icddrb, 2021), while a study in mid-2020 indicated a similar 74% seroprevalence in slums, but only 45% seroprevalence overall in Dhaka city (icddrb, 2020). Given that antibodies from previous COVID-19 infections are less protective against the Beta variant (Planas et al., 2021), the immunity to this variant in March 2021 may have been considerably lower than the seroprevalence. We, therefore, considered initialisation scenarios of 20%, 40% and 60% immunity when estimating .
At the end of the initial lockdown period in mid-2020, reported cases in Bangladesh were 6% of those estimated by our model (Supplementary Fig. S2C), and we assume that this 6% case detection still holds in March 2021. We further assume that prevalent infectious individuals were about eight times estimated incident daily cases given the infectious period duration. Dividing by two, assuming that these individuals are on average halfway through their infectious period, we get an effective number of 19,086 infectious individuals for initialization, and assume an equivalent number of incubating individuals. We also considered numbers of initial infections 50% higher and lower than this estimate to allow for error.
In the results, we refer to 2021 estimates obtained under the assumptions of 19,086 circulating cases and a moderate 40% immunity as our ‘best guess’ estimates, since, while error-prone, these assumptions best reflect our limited knowledge of the situation at that time. We took two approaches to optimisation of in the 2021 resurgence, minimizing: (1) the sum of squared differences between modelled and reported cumulative deaths on each day over the period from 1st March-5th April 2021 (the same approach taken for 2020 calibration), and (2) the difference in timing of the peaks in reported cases and modelled symptomatic cases (with the constraint that modelled cumulative deaths from 1st March until 20th May had to be at least as high as reported deaths over the same period). By matching peak timing, rather than death data, we sought to reveal any potential under-reporting of COVID-19 related deaths. This second optimisation approach could not be used in the 2020 calibration, since to fit and separately, we had to fit using only data that fell before the lockdown start date, prior to the peak in cases.
2.5. Sensitivity analyses
There is considerable uncertainty in the parameters governing SARS-CoV-2 transmission and health impacts. We therefore undertook one-way sensitivity analyses across parameter value ranges for , the duration of disease states and health outcome stages, the introduction date, the household secondary attack rate, the ratios of asymptomatic to symptomatic transmission, and of pre- versus symptomatic transmission identified as plausible from the literature (see Supplementary Table S4 for the parameter ranges considered and their sources). The impact of changes in each of these parameters on the main model outputs was assessed individually, while keeping other parameter values fixed. We ran this analysis over scenarios of: (1) no interventions; (2) lockdown as implemented; (3) lockdown plus household quarantining; and (4) lockdown plus compulsory mask wearing (with and ).
3. Results
3.1. Model calibration
We estimated that for transmission of SARS-CoV-2 in Dhaka in 2020, and that the lockdown reduced between-household transmission () by 76% for compliant individuals. The match between modelled deaths and data during the early stages of the epidemic is illustrated in Supplementary Fig. S2A-B. Output from the calibrated model indicated that around 6% of total cases were recorded during the period from SARS-CoV-2 introduction to the end of the first lockdown (Supplementary Fig. S2C).
Our estimates of in 2021 (associated with the second wave caused by the Beta variant) varied based on the initialisation scenario, with values increasing with lower initial circulating cases and higher levels of immunity in the population (Supplementary Table S5). The approach used to optimise also impacted the estimates, with optimisation based on matching to deaths in the early stages of the resurgence leading to lower values (range of 2.13–6.78) compared to peak matching (range of 4.18–8.52). Our best guess initialisation scenario (19,086 initial infections and 40% prior immunity), led to an estimate of 3.46 when matching to deaths and 5.58 when matching to peak timing. Death detection estimates were in the range 32–119% (matching to deaths), or 11–31% (peak matching). The peak in symptomatic cases obtained by matching to deaths was always slightly later than the peak in reported cases, though never by more than four days. When calibrated to the trajectory of deaths, the model predicted that while NPIs in April-May 2021 initially controlled the resurgence, another peak would occur in the following months following their relaxation (Supplementary Fig. S3C). In contrast, calibration by matching peak timing leads to the prediction that the resurgence would sufficiently spread through the remaining susceptible population to prevent further waves (Supplementary Fig. S3D). Subsequent data showed that a third larger COVID‐19 wave caused by the Delta variant did in fact occur, with deaths rising again from late May/early June. The best visual match to the timing and size of both the second and third waves is given by fitting to death data, with an initial infectious of 19,086 and initial immunity of 20–40%. Many of the other fits to the death data were considerably poorer, likely because the assumptions of initial numbers of infectious and immune individuals were too far from reality.
3.2. Impacts of scenarios on health outcomes and working days lost
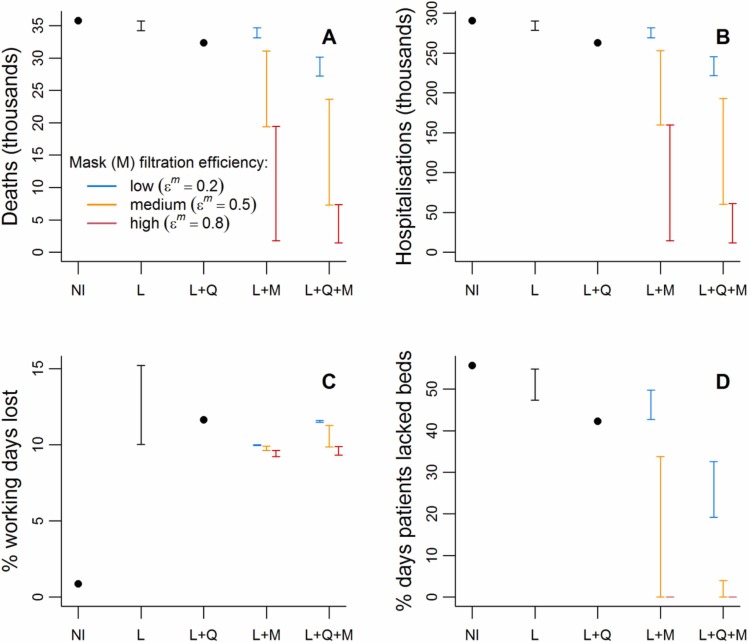
In the absence of interventions we predicted that COVID-19 patients in 2020 would peak at 45,664 in late May ( Fig. 2A), greatly exceeding hospital bed capacity (estimated to be 10,947 in Dhaka District (World Health Organisation (WHO), 2020b); Supplementary Table S4). Under this scenario, hospital beds would have been unavailable for at least 55% of patient days ( Fig. 3D), without accounting for non-COVID-19 bed needs. Assuming unmitigated transmission, the epidemic would have likely led to around 13.2 million cases (i.e. most of the Dhaka District population; Supplementary Table S4) and 35,765 deaths (Fig. 3A; Supplementary Figs. S3A & S4A). Although high relative to some of the mitigated scenarios, these deaths represent 0.26% of the population, and would be expected to lead to a loss of < 1% of total working days in 2020 (Fig. 3C).
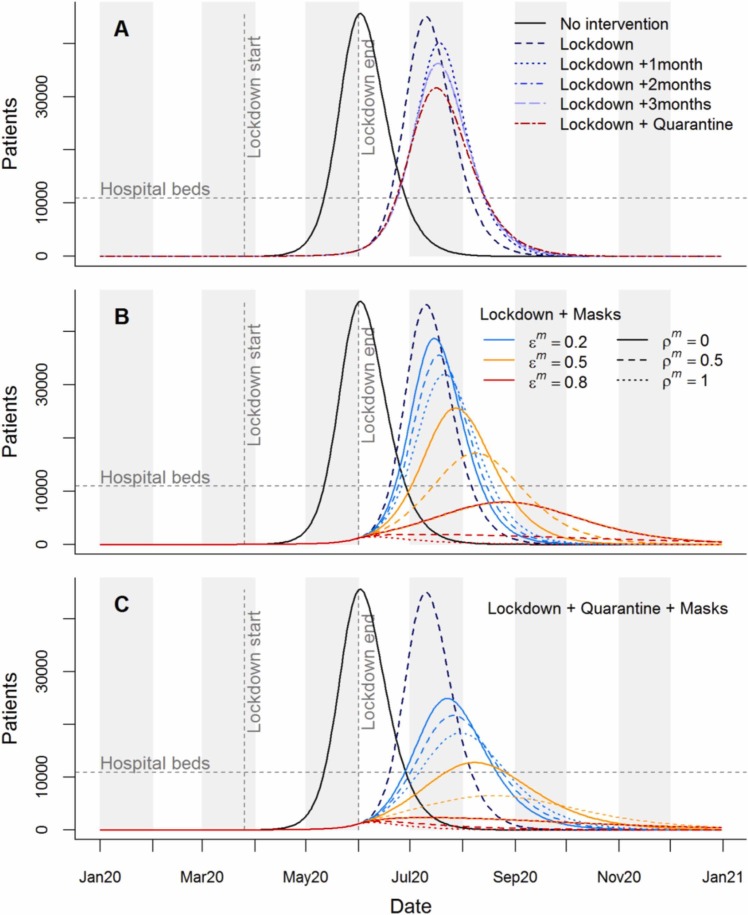
Fig. 2.
Time series of hospital patients for intervention scenarios in 2020. Horizontal dashed lines indicate hospital bed capacity in Dhaka District. Vertical lines indicate the start and end points of lockdown as implemented in Bangladesh. (A) Hospital patients in the absence of interventions, for the implemented lockdown plus extensions of up to 3 months, and for lockdown followed by household quarantine with community support teams. (B) Lockdown as implemented followed by compulsory mask wearing, considering nine mask effectiveness scenarios; describes the proportion reduction in outward emissions by mask wearers, while describes the proportion protection to mask wearers from others’ emissions. (C) Combined impacts of the lockdown, household quarantine, and masks of different effectiveness.
Fig. 3.
Summaries of health outcomes and working days lost for a range of interventions during 2020. NI=no intervention, L=lockdown, Q=household quarantine with community support teams, M=compulsory mask wearing. The black bars for the lockdown (L) intervention describe the range of each outcome over the scenarios where lockdown is as implemented in Bangladesh and where this lockdown is extended by up to three months. For interventions involving mask-wearing (M), the three coloured bars describe scenarios with different values of , the proportional reduction in outward emissions by mask wearers. These bars range over the scenarios where masks provide no protection to the wearer to where they provide proportional protection to the wearer from others’ emissions that is equal to .
We forecast that the first lockdown as implemented in Bangladesh would delay the initial epidemic (Fig. 2A and Supplementary Fig. S3A), with minor impacts on deaths and hospitalised cases (Fig. 3), while increasing working days lost to 10%. Extensions were predicted to further delay and slightly widen the first peak (Fig. 2A and Supplementary Fig. S3A), though extensions beyond two months had no added impact on the epidemic peak and outcomes. Even with lockdown extensions, hospitalisations were still expected to outstrip capacity (Fig. 2A), with only modest reductions in deaths (Fig. 3). Working days lost were predicted to increase by 1.6–1.9% points with each extra month of lockdown.
We found that introducing household quarantining following lockdown led to the epidemic peak being later, lower and wider than with lockdown alone (Fig. 2A and Supplementary Fig. S3A). Hospital capacity was still exceeded, but this intervention was more effective in reducing hospitalisations than any of the lockdown-only scenarios. The additional working days lost by introducing and maintaining household quarantines throughout 2020 were similar to those lost by a one-month lockdown extension.
The impact of introducing compulsory mask-wearing following lockdown varied based on the effectiveness of the masks used (Figs. 2B and 3). When , i.e. low filtration efficiency, only relatively small reductions to the epidemic peak were predicted, with the decline increasing with mask effectiveness in terms of PPE (indicated by increasing ; Fig. 2B). Masks with high filtration efficiency (), or medium filtration efficiency combined with high PPE efficiency (, ), flattened the peak sufficiently to keep patient numbers below hospital capacity (Figs. 2B and 3D). Effective masks also led to substantial drops in deaths and hospitalisations, while slightly reducing working days lost relative to the lockdown-only scenario (Fig. 3).
Combining mask-wearing with household quarantine led to greater predicted reductions in the epidemic peak, and in both deaths and hospitalisations, than either intervention alone (Figs. 2C and 3 A-B). In fact, the reductions were typically larger than the sums of the individual effects of these interventions, indicating synergy. Percentages of working days lost increase as mask quality decreases, ranging from 9.3% to 11.6%. A combined strategy of masks and quarantine following lockdown was strategized for implementation in Dhaka District. In the end, mask provision was not directly funded despite recommendations, but mask-wearing was promoted; by late 2020 mask use had largely declined to normal levels. The data-based estimates of cumulative deaths in Dhaka District take a path similar to those modelled by combining quarantining with masks of high filtration effectiveness () and medium to high PPE abilities ( of 0.5 or 1.0).
3.3. Scenario costs
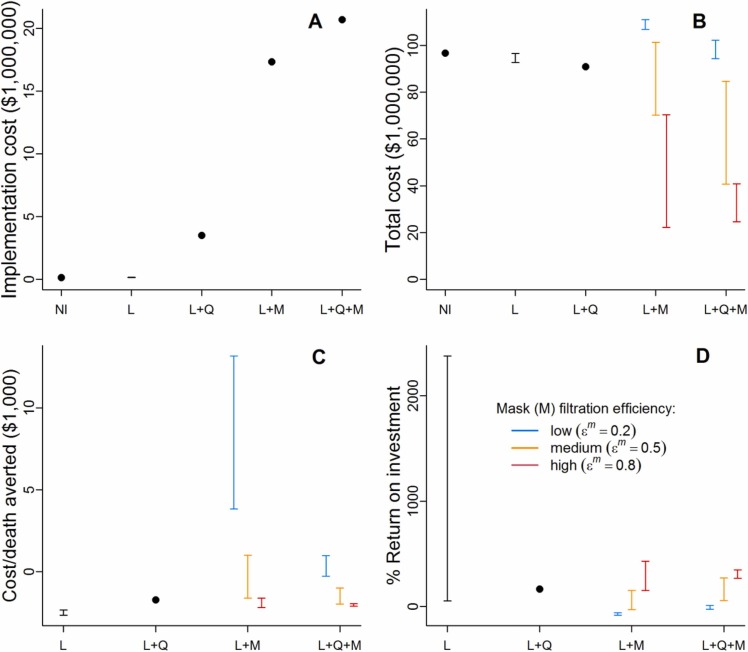
Total costs of the scenarios explored ranged from $22.2–111.0 million ( Fig. 4B). Despite requiring no direct intervention costs, the baseline scenario is among the most expensive due to the healthcare costs incurred by hospitalisations. Lockdown as implemented was predicted to be similar in cost to the baseline (unmitigated transmission) with slight cost reductions for lockdown extensions. Incorporating household quarantine led to lower costs than lockdowns. Combining lockdown with low effectiveness masks led to the highest costs due to the expense of mask distribution, offset by only small reductions in healthcare costs. Masks of high effectiveness, however, led to substantial cost reductions, which generally increased when masks and household quarantine were modelled together.
Fig. 4.
Costs of intervention scenarios. NI=no intervention, L=lockdown, Q=household quarantine with community support teams, M=compulsory mask wearing. The black bars for the lockdown (L) intervention describe the range of each cost outcome over the scenarios where lockdown is as implemented in Bangladesh and where this lockdown is extended by up to three months. For interventions involving mask-wearing (M), the three coloured bars describe scenarios with different values of , the proportional reduction in outward emissions by mask wearers. These bars range over the scenarios where masks provide no protection to the wearer to where they provide proportional protection to the wearer from others’ emissions that is equal to . (A) Implementation costs of each intervention scenario (i.e. total cost minus the hospital care cost). Note that the costs of the mask-wearing scenarios are assumed to be the same regardless of mask effectiveness. (B) Total cost of each intervention scenario based on costings in Table 1. (C) Cost of each death averted relative to the baseline (NI) scenario. (D) Percentage return on investment in interventions in terms of healthcare savings.
Since most scenarios had a lower total cost than the baseline, the cost per death averted is negative except in some of the lower effectiveness mask scenarios (Fig. 4C). Lockdowns, and lockdowns combined with household quarantine and/or high effectiveness masks all provide similar savings per death averted in the $1,616-$2,700 range.
The %ROI was generally positive, again with some exceptions where lower effectiveness masks were used (Fig. 4D). By far the highest %ROIs were given by extending lockdown by 2–3 months. This is because, despite relatively small reductions in hospitalisation costs (Fig. 3B), the only implementation cost to lockdown is advertising (Table 1), and this cost is an order of magnitude smaller than the implementation costs of quarantine with CSTs, and two orders of magnitude smaller than the implementation costs of mask-wearing (due to the high cost of providing masks to every household) (Fig. 4A). It should be remembered that while the %ROI for extended lockdowns is high, the return itself is relatively small and total costs are among the highest as a result (Fig. 4B). Lockdown plus quarantining gave a %ROI of 168%. The %ROI of all scenarios involving masks increased with the effectiveness of the masks in blocking transmission. Interventions involving high filtration efficiency masks all had a %ROI that was similar to or better than the lockdown plus quarantine scenario.
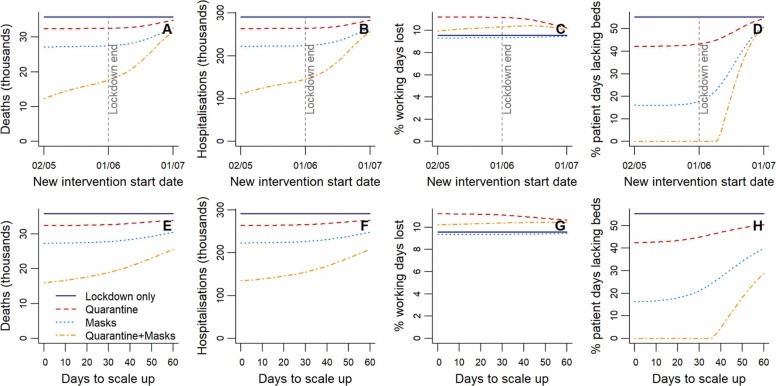
3.4. Intervention timing and scale-up
Starting either household quarantining or compulsory mask-wearing prior to the end of lockdown had little projected impact on health outcomes or the percentage of working days lost ( Fig. 5A-D). When masks and quarantining are combined, however, total deaths and hospitalisations are seen to decline in response to moving their start dates further before the end of lockdown. This difference occurs because the combined early start date of the two interventions pushes the epidemic peak later (into 2021) than when considering either individually. Continuing interventions and calculating outcomes over both 2020 and 2021, removes this apparent improvement in outcomes with start dates prior to lockdown’s end (Supplementary Fig. S6A-D). As the start date of quarantining and mask-wearing is delayed beyond the lockdown end date, their health benefits decline, with all outcomes approaching those of the lockdown-only scenario. Similar consequences to delaying intervention start dates result from lengthening the scale-up period (Fig. 5E-H).
Fig. 5.
Sensitivity to the start date and scale-up period of quarantine or mask wearing following lockdown. (A-D) Changes in health outcomes, working days lost and excess hospital demand over 2020 when the start date of interventions (household quarantine, masks, or quarantine and masks) following lockdown is adjusted relative to the lockdown end date. The days taken to scale up interventions to their full effectiveness is held constant at seven days. (E-H) Changes in the same outcomes when the time in days taken to scale up post-lockdown interventions is varied. The start date of the post-lockdown interventions is held constant at seven days prior to the lockdown end date.
3.5. Sensitivity analyses
was among the top three most influential parameters for all five outcome measures reported under all four baseline scenarios (Supplementary Figs. S7-10). The duration of symptoms was among the top three most influential parameters in determining working days lost (since symptomatic people are unable to work) over all baselines, and, predictably, the mean lengths of stay in general hospital and ICU beds were highly influential for the percentage of patient days that lacked beds. For all baselines, other than that with no interventions, the introduction date of the disease ranked in the top three most important parameters for all outcomes except the percentage of patient days lacking beds. Some parameters only gained importance under specific baseline scenarios (for example, the level of asymptomatic transmission becomes important under symptoms-based household quarantine); for more details see Supplement E.
4. Discussion
We modelled interventions for controlling COVID-19 transmission in Dhaka District, Bangladesh, comparing their: (1) ability to prevent both deaths and overwhelming of the health system; and (2) percentage working days lost and health provider costs. We found that under expected compliance, lockdowns alone, regardless of duration, were both costly and unable to keep cases below hospital capacity, while preventing only a small proportion of deaths, confirming that they are not an appropriate long-term measure in this context (Amewu et al., 2020, Andam et al., 2020, Rahman et al., 2020). Additional quarantining of households with symptomatic individuals similarly was not predicted to prevent hospitalisations exceeding capacity. Modelling compulsory mask-wearing after the lockdown produced outcomes that varied widely depending on the effectiveness of masks in blocking transmission. Low-filtration efficiency masks had limited impacts and were not cost-effective, whereas high-filtration efficiency masks substantially reduced deaths, protected the healthcare system and were cost-effective. Perhaps our most important finding was that combining mask-wearing and household quarantine synergistically led to further reductions in deaths and excess hospitalisations, and was cost-effective when masks were of high filtration efficiency. For this reason we recommend a combination of these measures to provide the best levels of control. Simulations also suggest that early introductions of post-lockdown measures (i.e. prior to the lockdown’s end) would have had negligible additional impact over the full course of the epidemic (though they may have improved outcomes in the short-term). However, gaps between lockdown ending and post-lockdown interventions (or long periods of scale-up) quickly dilute their impacts.
The inability of symptomatic household quarantining alone to prevent hospitalisations exceeding capacity is unsurprising. Even in HICs with greater healthcare capacity, lower estimates, and a lower proportion of asymptomatic transmission, other modelling studies have suggested that symptoms-based household quarantine would still allow overwhelming of health systems (Aleta et al., 2020, Ferguson et al., 2020). Additional tracing and quarantine of non-household contacts of symptomatic individuals can improve the effectiveness of quarantine measures (Aleta et al., 2020). However, capacity for extensive contact tracing is limited in high-density resource-constrained settings like Dhaka. The finding that combining symptomatic household quarantining with mask mandates (using mid- to high-filtration efficiency masks) leads to effective control was crucial at the time, since both these NPIs were considered feasible. Given that household quarantining is only triggered by a symptomatic case, and we estimate that most cases in Bangladesh are asymptomatic, a large proportion of infectious households are likely missed by this measure, which will also have only limited impact on pre-symptomatic transmission. Since masks reduce transmission for all infections, irrespective of symptoms, masks work synergistically with household quarantine. While we consider scenarios where masks block only 20% of transmission, we note that this is a worst case scenario; experimental work suggests that our mid- to high-quality mask scenarios (blocking 50–80% of transmission) are more likely (Aydin et al., 2020, Davies et al., 2013). Observational (Abaluck et al., 2021, Hong et al., 2020, Wang et al., 2020) and other modelling (Stutt et al., 2020) studies provide further evidence for the effectiveness of masks in blocking transmission.
In reality, achieving the modelled effectiveness of household quarantining and mask-wearing depends on high compliance (we assumed 80% compliance in our main results). A survey in Israel suggested that quarantining compliance is likely to be highly dependent on compensation for loss of work (Bodas and Peleg, 2020). However, such compensation may be unachievable in many LMICs. Reducing insecurity experienced by households under quarantine in other ways, for example by food provisioning and healthcare access, may help mitigate income loss and boost compliance amongst poorer households. In Bangladesh, CSTs are already playing this role in supporting quarantining households, and in LMICs more broadly, community health workers are likely to prove invaluable in using their established trust to encourage compliance with NPIs (Ballard et al., 2020).
While the total costs of many of the interventions explored were high, most provided savings per death averted and a positive %ROI because of the high contribution of healthcare to overall costs. Note that these returns occur despite the relatively young population in Bangladesh, which leads to a low percentage of cases being hospitalized (2.2%). Furthermore, the costs in working days of the post-lockdown NPIs considered were small when compared with the initial lockdown costs and potential extensions. These findings are in line with other work showing that the costs of unmitigated transmission exceed those of implementing NPIs (Thunström et al., 2020, Torres-Rueda et al., 2020). The costs we explore here are based on crude assumptions for mask purchase and costs of training and rollout of CSTs in Dhaka where there is already a large community volunteer workforce that can be mobilized. However, we do not include food packages or additional support for vulnerable communities that may increase NPI effectiveness but also costs. A further limitation to the cost analysis conducted here is that we only consider direct costs to the health provider in providing care to COVID-19 patients and in implementing interventions; we do not consider potential costs to businesses (some of which may not survive prolonged lockdowns) and individuals within the population, except via the indirect measure of estimated working days lost. Furthermore, working days lost are only one of many societal costs of the pandemic and associated interventions; impacts on education, mental health, healthcare for non-COVID-19 patients, etc., are likely to be significant, but are not considered in this study.
Throughout the development of our model and interactive app, we incorporated suggestions from policymakers on questions that most urgently needed answers, and on NPIs under consideration and thought feasible to implement. This co-development allowed the investigation of scenarios appropriate to the local context that addressed pressing policy concerns (McBryde et al., 2020). The app (boydorr.gla.ac.uk/BGD_Covid-19/CEEDS), which allows a user-friendly exploration and visualisation of how scenarios impact health outcomes and costs, proved to be an effective tool to support discussions with policymakers, as the timing and combination of interventions, along with uncertainties in parameters, including compliance, could be explored on the fly. This proved to be crucial in understanding the economic and health trade-offs involved, as well as helping to demystify the model itself and the ensuing epidemic.
Our model has a number of limitations. First, to ensure it could run sufficiently quickly within the interactive app, stochasticity and individual variation in transmission was not incorporated (in contrast to a number of other models developed for COVID-19: Aleta et al., 2020; Davies et al., 2020b; Ferguson et al., 2020; Kerr et al., 2021). We expect only a minimal impact of these simplifications on disease trajectories and key results due to the large population size considered and rapid epidemic growth observed. However, they do limit the usefulness of the model for exploring elimination scenarios, since stochasticity and superspreading, which are inherent to SARS-CoV-2 transmission (Adam et al., 2020), become more influential under low levels of infection (Vespignani et al., 2020). Imported infections, which may similarly become important near elimination, were also not considered. Age-structured models have previously been proposed for studying COVID-19 (Davies et al., 2020b, Ferguson et al., 2020, Keeling et al., 2021). We, however, did not include age-structured transmission, which, though not entirely realistic, may be reasonable given the high degree of intergenerational mixing in Bangladesh (United Nations, Department of Economic and Social Affairs, 2019a) and some other LMICs (Hodgins and Saad, 2020). Like many other models (Davies et al., 2020b, Ferguson et al., 2020, Jayasundara et al., 2021, Keeling et al., 2021), we also do not consider exacerbated mortality when hospital capacity is exceeded, likely leading to underestimated mortality under those scenarios where this occurs, along with knock-on effects on working days lost and cost per death averted. This will not, however, impact the general ranking of scenarios by numbers of deaths, or outcomes relating to those scenarios where hospital capacity was not exceeded (which are the only intervention scenarios we recommended pursuing). Therefore, our main conclusions should be unaffected by this assumption. Parameters used for case fatality, and proportions of symptomatic and hospitalised infections, were based on age-dependent estimates from HICs (Davies et al., 2020b, Davies et al., 2020a) and the age-distribution in Dhaka District; an approach similar to that taken by Truelove et al. (2020). However, these HIC-derived parameters may be less accurate when applied in this setting, as the incidence among age classes of underlying conditions that increase COVID-19 risk likely differs in LMICs (Clark et al., 2020). We also note that our estimates of working days lost make the assumption that employed people cannot switch to working from home, possibly leading to overestimation.
More generally there remains considerable uncertainty around many of the model parameters. Our sensitivity analyses indicated that was, predictably, very influential in determining health outcomes, but our 2020 estimate is sensitive to the introduction date and the number of imported cases, which are both uncertain. In addition, prior to the lockdown in 2020, only 5 deaths due to COVID-19 had been recorded in Bangladesh, and given the inherent stochasticity in these events, tuning to data in this time window is unlikely to be very accurate. Finally, prior to the 2020 lockdown, some control measures, such as cancellations of large gatherings, had already been taken (Anwar et al., 2020), potentially lowering our estimate. Our estimates of in 2021 similarly assumed no interventions in the run-up to the 5th April lockdown, but low levels of mask-wearing and quarantine may have led to underestimation. The 2020 estimate for Dhaka lies within the 90% confidence interval estimated from a meta-analysis of pre-March 2020 estimates (Davies et al., 2020b). Many of our 2021 estimates exceed this confidence interval, but none are in excess of the confidence interval reported by Sanche et al. (2020). A possibly higher value for 2021 is also not unexpected, given the dominant variant at the time (Beta) was known to be more transmissible than variants prevalent in 2020.
Our model, like many others, assumed that recovered individuals remain immune through 2020. Although immunity to SARS-CoV-2 is not permanent (Iwasaki, 2021), this assumption appeared reasonable given effects of immunity loss were likely limited over this period. However, with resurging cases from March 2021 concomitant with relaxed NPIs and the emergence of the more transmissible Beta variant, the question of immunity became paramount (Saha et al., 2021a). The level of immunity in the population at the time of the resurgence was very uncertain; seroprevalence in Dhaka was estimated to be 45% (71% in slum areas) in mid-2020 (icddrb, 2020), but laboratory evidence suggests that prior COVID-19 infections elicit less protection to the Beta variant (Planas et al., 2021), making it unclear how this seroprevalence translated into effective immunity against this variant. These uncertainties translated into our estimates of in 2021, which varied widely (2.13–8.52) under different assumptions, but with a best guess of 3.46–5.58. The uncertainty in in turn led to uncertainty in predictions, with some suggesting a possible further wave later in 2021, while others precluded this. Ultimately a further wave did occur, but it is unclear whether this was possible due to sufficient susceptibles remaining after the earlier wave or due to immune escape resulting from the arrival of the Delta (B.1.617.2) variant, which rapidly became dominant during this later wave (Saha et al., 2021b).
We demonstrated the sensitivity of outcomes to the timing and scale-up of interventions, but human behavioural responses most dramatically impact outcomes. For these reasons, our model was primarily developed as a means to understand the potentially synergistic impact of interventions, rather than to accurately forecast dynamics subject to unpredictable changing human behaviours (perhaps leading to some of the inconsistencies between the model and data during the 2020 lockdown period; Supplementary Fig. S2B). We therefore considered compliance to interventions to be a crucial interactive element of our app to build understanding and guidance on policy. Within the app, we also modelled the degree to which the limited (but greatly increased) testing capacity would still under-detect circulating cases, given some degree of cognitive dissonance and the considerable uncertainty in pre- and asymptomatic transmission during the early months of the pandemic. Overall we found the interactive app to be effective for communicating epidemiological modelling outcomes to policymakers together with their caveats, and we recommend the use of such tools that can be tailored to other settings and interventions.
In summary, we found that two NPIs combined, masks and symptoms-based household quarantining, were capable of averting an anticipated public health crisis in Dhaka, while also being good value for money. These measures were to a large extent rolled out in Bangladesh in 2020, and appear to have contributed to limiting transmission, but the ensuing epidemic stretched the health system. In practice, compliance with these interventions and fidelity of their implementation was highly heterogeneous, with measures relaxing over the year as activity returned to levels approaching normalcy (although schools have remained closed until September 2021). The second and third waves of cases in 2021, apparently driven by the Beta and Delta variants respectively, have now largely subsided. However, potential for the arrival of further new variants and waning in immunity present a risk of further waves. Mask-wearing and symptoms-based quarantine may therefore still have an important role to play, and the vaccine rollout currently underway needs to be pursued at pace. Further work to introduce vaccination to our model and examine its interaction with NPIs is now in progress.
Funding
The Bill and Melinda Gates Foundation funded work by FAO and UoG (INV-022851), and UoG reports funding from Wellcome (207569/Z/17/Z).
CRediT authorship contribution statement
Elaine A Ferguson: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. Eric Brum: Conceptualization, Funding acquisition, Investigation, Project administration, Writing – review & editing. Anir Chowdhury: Investigation. Shayan Chowdhury: Investigation. Mikolaj Kundegorski: Data curation. Ayesha S Mahmud: Methodology, Writing – review & editing. Nabila Purno: Data curation, Investigation, Project administration. Ayesha Sania: Funding acquisition, Investigation. Rachel Steenson: Data curation, Software, Visualisation. Motahara Tasneem: Data curation, Project administration. Katie Hampson: Conceptualisation, Funding acquisition, Data curation, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
None.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.epidem.2022.100592.
Appendix A. Supplementary material
Supplementary material
.
Data availability
All data and code can be accessed via out Github repository (https://github.com/boydorr/BGD_Covid-19/ tree/main/ BGD_NPI_model). The interactive app is available at http://boydorr.gla.ac.uk/BGD_Covid-19/CEEDS/.
References
- Abaluck, J., Kwong, L.H., Styczynski, A., Haque, A., Kabir, A., Bates-jeffries, E., Crawford, E., Benjamin-Chung, J., Raihan, S., Rahman, S., Benhachmi, S., Zaman, N., Winch, P.J., Hossain, M., Reza, H.M., Jaber, A.A., Momen, S.G., Bani, F.L., Rahman, A., Huq, T.S., Luby, S.P., Mobarak, A.M., 2021. The Impact of Community Masking on COVID-19: A Cluster-Randomized Trial in Bangladesh. [DOI] [PMC free article] [PubMed]
- Adam D.C., Wu P., Wong J.Y., Lau E.H.Y., Tsang T.K., Cauchemez S., Leung G.M., Cowling B.J. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat. Med. 2020;26:1714–1719. doi: 10.1038/s41591-020-1092-0. [DOI] [PubMed] [Google Scholar]
- Ahmed S.A.K.S., Ajisola M., Azeem K., Bakibinga P., Chen Y.-F., Choudhury N.N., Fayehun O., Griffiths F., Harris B., Kibe P., Lilford R.J., Omigbodun A., Rizvi N., Sartori J., Smith S., Watson S.I., Wilson R., Yeboah G., Aujla N., Azam S.I., Diggle P.J., Gill P., Iqbal R., Kabaria C., Kisia L., Kyobutungi C., Madan J.J., Mberu B., Mohamed S.F., Nazish A., Odubanjo O., Osuh M.E., Owoaje E., Oyebode O., Porto de Albuquerque J., Rahman O., Tabani K., Taiwo O.J., Tregonning G., Uthman O.A., Yusuf R. Impact of the societal response to COVID-19 on access to healthcare for non-COVID-19 health issues in slum communities of Bangladesh, Kenya, Nigeria and Pakistan: results of pre-COVID and COVID-19 lockdown stakeholder engagements. BMJ Glob. Heal. 2020;5 doi: 10.1136/bmjgh-2020-003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleta A., Martín-Corral D., Pastore y Piontti A., Ajelli M., Litvinova M., Chinazzi M., Dean N.E., Halloran M.E., Longini Jr I.M., Merler S., Pentland A., Vespignani A., Moro E., Moreno Y. Modelling the impact of testing, contact tracing and household quarantine on second waves of COVID-19. Nat. Hum. Behav. 2020;4:964–971. doi: 10.1038/s41562-020-0931-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amewu S., Asante S., Pauw K., Thurlow J. The economic costs of COVID-19 in Sub-Saharan Africa: insights from a simulation exercise for Ghana. Eur. J. Dev. Res. 2020;32:1353–1378. doi: 10.1057/s41287-020-00332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andam, K.S., Edeh, H., Oboh, V., Pauw, K., Thurlow, J., 2020. Estimating the economic costs of COVID-19 in Nigeria, NSSP Working Paper 63. 10.2499/p15738coll2.133846. [DOI]
- Anwar S., Nasrullah M., Hosen M.J. COVID-19 and Bangladesh: challenges and how to address them. Front. Public Health. 2020;8:154. doi: 10.3389/fpubh.2020.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin O., Emon B., Cheng S., Hong L., Chamorro L.P., Saif M.T.A. Performance of fabrics for home-made masks against the spread of COVID-19 through droplets: a quantitative mechanistic study. Extrem. Mech. Lett. 2020;40 doi: 10.1016/j.eml.2020.100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard M., Bancroft E., Nesbit J., Johnson A., Holeman I., Foth J., Rogers D., Yang J., Nardella J., Olsen H., Raghavan M., Panjabi R., Alban R., Malaba S., Christiansen M., Rapp S., Schechter J., Aylward P., Rogers A., Sebisaho J., Ako C., Choudhury N., Westgate C., Mbeya J., Schwarz R., Bonds M.H., Adamjee R., Bishop J., Yembrick A., Flood D., McLaughlin M., Palazuelos D. Prioritising the role of community health workers in the COVID-19 response. BMJ Glob. Heal. 2020;5 doi: 10.1136/bmjgh-2020-002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangladesh Bureau of Statistics, 2011. Bangladesh Population and Housing Census 2011 [WWW Document]. URL 〈http://203.112.218.65:8008/Census.aspx?MenuKey=43〉.
- Bangladesh Bureau of Statistics, 2017. Preliminary Report on Household Income and Expenditure Survey 2016. https://catalog.ihsn.org/catalog/7399/related-materials.
- Bodas M., Peleg K. Self-isolation compliance in the COVID-19 era influenced by compensation: findings from a recent survey In Israel. Health Aff. 2020;39:936–941. doi: 10.1377/hlthaff.2020.00382. [DOI] [PubMed] [Google Scholar]
- Byrne A.W., McEvoy D., Collins Á.B., Hunt K., Casey M., Barber A., Butler F., Griffin J., Lane E.A., McAloon C., O’Brien K., Wall P., Walsh K.A., More S.J. Inferred duration of infectious period of SARS-CoV-2: rapid scoping review and analysis of available evidence for asymptomatic and symptomatic COVID-19 cases. BMJ Open. 2020;10(8):e039856. doi: 10.1136/bmjopen-2020-039856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cele S., Gazy I., Jackson L., Hwa S.-H., Tegally H., Lustig G., Giandhari J., Pillay S., Wilkinson E., Naidoo Y., Karim F., Ganga Y., Khan K., Bernstein M., Balazs A.B., Gosnell B.I., Hanekom W., Moosa M.-Y.S., Lessells R.J., de Oliveira T., Sigal A. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature. 2021;593(7875):142–146. doi: 10.1038/s41586-021-03471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R., Luhar S., Khan N., Choudhury S.R., Matin I., Franco O.H. Long-term strategies to control COVID-19 in low and middle-income countries: an options overview of community-based, non-pharmacological interventions. Eur. J. Epidemiol. 2020;35:743–748. doi: 10.1007/s10654-020-00660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A., Jit M., Warren-Gash C., Guthrie B., Wang H.H., Mercer S.W., Sanderson C., McKee M., Troeger C., Ong K.I., Checchi F., Perel P., Joseph S., Gibbs H.P., Banerjee A., Eggo R., CMMID COVID-19 working group How many are at increased risk of severe COVID-19 disease? Rapid global, regional and national estimates for 2020. medRxiv. 2020 doi: 10.1101/2020.04.18.20064774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus COVID-19 Dashboard, 2020 [WWW Document], 2020. URL 〈http://103.247.238.81/webportal/pages/covid19.php〉 (accessed 11.19.20).
- Cowley, L.A., Afrad, M.H., Rahman, S.I.A., Mahfuz-Al-Mamun, M., Chin, T., Mahmud, A.S., Rahman, M.Z., Billah, M.M., Khan, M.H., Sultana, S., Khondaker, T., Baker, S., Banik, N., Alam, A.N., Mannor, K., Banu, S., Chowdhury, A., Flora, M.S., Thomson, N.R., Buckee, C.O., Qadri, F., Shirin, T., 2021. Genomic and mobility data reveal mass population movement as a driver of SARS-CoV-2 dissemination and diversity in Bangladesh. medRxiv 2021.01.05.21249196. 10.1101/2021.01.05.21249196. [DOI]
- Davies A., Thompson K.-A., Giri K., Kafatos G., Walker J., Bennett A. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med. Public Health Prep. 2013;7:413–418. doi: 10.1017/dmp.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.G., Klepac P., Liu Y., Prem K., Jit M., Eggo R.M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- Davies N.G., Kucharski A.J., Eggo R.M., Gimma A., Edmunds W.J., Jombart T., O’Reilly K., Endo A., Hellewell J., Nightingale E.S., Quilty B.J., Jarvis C.I., Russell T.W., Klepac P., Bosse N.I., Funk S., Abbott S., Medley G.F., Gibbs H., Pearson C.A.B., Flasche S., Jit M., Clifford S., Prem K., Diamond C., Emery J., Deol A.K., Procter S.R., van Zandvoort K., Sun Y.F., Munday J.D., Rosello A., Auzenbergs M., Knight G., Houben R.M.G.J., Liu Y. Effects of non-pharmaceutical interventions on COVID-19 cases, deaths, and demand for hospital services in the UK: a modelling study. Lancet Public Heal. 2020;5:e375–e385. doi: 10.1016/S2468-2667(20)30133-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control, 2020. Download today’s data on the geographic distribution of COVID-19 cases worldwide.
- Ferguson, N.M., Laydon, D., Nedjati-Gilani, G., Imai, N., Ainslie, K., Baguelin, M., Bhatia, S., Boonyasiri, A., Cucunubá, Z., Cuomo-Dannenburg, G., Dighe, A., Dorigatti, I., Fu, H., Gaythorpe, K., Green, W., Hamlet, A., Hinsley, W., Okell, L.C., van Elsland, S., Thompson, H., Verity, R., Volz, E., Wang, H., Wang, Y., Walker, P.G., Walters, C., Winskill, P., Whittaker, C., Donnelly, C.A., Riley, S., Ghani, A.C., 2020. Report 9: Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand. 10.25561/77482. [DOI]
- Flaxman S., Mishra S., Gandy A., Unwin H.J.T., Mellan T.A., Coupland H., Whittaker C., Zhu H., Berah T., Eaton J.W., Monod M., Ghani A.C., Donnelly C.A., Riley S., Vollmer M.A.C., Ferguson N.M., Okell L.C., Bhatt S. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584:257–261. doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- Google, 2021. COVID-19 Community Mobility Reports [WWW Document]. URL 〈https://www.google.com/covid19/mobility/〉 (accessed 8.10.21).
- Gupta M., Wahl B., Adhikari B., Bar-Zeev N., Bhandari S., Coria A., Erchick D.J., Gupta N., Hariyani S., Kagucia E.W., Killewo J., Limaye R.J., McCollum E.D., Pandey R., Pomat W.S., Rao K.D., Santosham M., Sauer M., Wanyenze R.K., Peters D.H. The need for COVID-19 research in low- and middle-income countries. Glob. Health Res. Policy. 2020;5:33. doi: 10.1186/s41256-020-00159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin S.J., McIvor C., Whyatt G., Adams A., Harvey O., McLean L., Walshaw C., Kemp S., Corrado J., Singh R., Collins T., O’Connor R.J., Sivan M. Postdischarge symptoms and rehabilitation needs in survivors of COVID‐19 infection: a cross‐sectional evaluation. J. Med. Virol. 2020;93(2):1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- Hodgins S., Saad A. Will the higher-income country blueprint for COVID-19 work in low- and lower middle-income countries? Glob. Heal. Sci. Pract. 2020;8:136–143. doi: 10.9745/GHSP-D-20-00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homaira, N., Islam, M.S., Hassan, Z., Haider, N., Satter, S.M., 2020. Contact tracing for covid-19 in low- and middle-income countries. BMJ Opin.
- Hong L., Lin A., He Z.-B., Zhao H.-H., Zhang J.-G., Zhang C., Ying L.-J., Ge Z.-M., Zhang X., Han Q.-Y., Chen Q.-Y., Ye Y.-H., Zhu J.-S., Chen H.-X., Yan W.-H. Mask wearing in pre-symptomatic patients prevents SARS-CoV-2 transmission: an epidemiological analysis. Travel Med. Infect. Dis. 2020;36 doi: 10.1016/j.tmaid.2020.101803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, J., Huang, A., Li, Z., Tufekci, Z., Zdimal, V., van der Westhuizen, H.-M., von Delft, A., Price, A., Fridman, L., Tang, L.-H., Tang, V., Watson, G.L., Bax, C.E., Shaikh, R., Questier, F., Hernandez, D., Chu, L.F., Ramirez, C.M., Rimoin, A.W., 2020. Face masks against COVID-19: an evidence review. Preprints. 10.20944/preprints202004.0203.v3. [DOI] [PMC free article] [PubMed]
- Hu Zhiliang, Song C., Xu C., Jin G., Chen Y., Xu X., Ma H., Chen W., Lin Y., Zheng Y., Wang J., Hu Zhibin, Yi Y., Shen H. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci. China Life Sci. 2020;63:706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- icddrb, 2020. The IEDCR and partners share insights on the prevalence, seroprevalence and genomic epidemiology of COVID-19 in Dhaka city [WWW Document]. URL 〈https://www.icddrb.org/quick-links/press-releases?id=97&task=view〉 (accessed 9.27.21).
- icddrb, 2021. Higher COVID-19 seropositivity observed among residents in Dhaka and Chattogram [WWW Document]. URL 〈https://www.icddrb.org/news-and-events/news?id=878〉 (accessed 9.27.21).
- Iwasaki A. What reinfections mean for COVID-19. Lancet Infect. Dis. 2021;21:3–5. doi: 10.1016/S1473-3099(20)30783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasundara P., Peariasamy K.M., Law K.B., Abd Rahim K.N.K., Lee S.W., Ghazali I.M.M., Abayawardana M., Le L.V., Khalaf R.K.S., Razali K., Le X., Chong Z.L., McBryde E.S., Meehan M.T., Caldwell J.M., Ragonnet R., Trauer J.M. Sustaining effective COVID-19 control in Malaysia through large-scale vaccination. Epidemics. 2021;37 doi: 10.1016/j.epidem.2021.100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling M.J., Hill E.M., Gorsich E.E., Penman B., Guyver-Fletcher G., Holmes A., Leng T., McKimm H., Tamborrino M., Dyson L., Tildesley M.J. Predictions of COVID-19 dynamics in the UK: short-term forecasting and analysis of potential exit strategies. PLOS Comput. Biol. 2021;17 doi: 10.1371/journal.pcbi.1008619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr C.C., Stuart R.M., Mistry D., Abeysuriya R.G., Rosenfeld K., Hart G.R., Núñez R.C., Cohen J.A., Selvaraj P., Hagedorn B., George L., Jastrzębski M., Izzo A.S., Fowler G., Palmer A., Delport D., Scott N., Kelly S.L., Bennette C.S., Wagner B.G., Chang S.T., Oron A.P., Wenger E.A., Panovska-Griffiths J., Famulare M., Klein D.J. Covasim: an agent-based model of COVID-19 dynamics and interventions. PLOS Comput. Biol. 2021;17 doi: 10.1371/journal.pcbi.1009149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krispin, R., Byrnes, J., 2021. coronavirus: The 2019 Novel Coronavirus COVID-19 Dataset. R package version 0.3.21.
- Liang W., Guan W., Li C., Li Y., Liang H., Zhao Y., Liu X., Sang L., Chen R., Tang C., Wang T., Wang W., He Q., Chen Zi-sheng, Wong S.-S., Zanin M., Liu Jun, Xu X., Huang J., Li J., Ou L., Cheng B., Xiong S., Xie Z., Ni Z., Hu Yu, Liu L., Shan H., Lei C., Peng Y., Wei L., Liu Y., Hu Ya-hua, Peng P., Wang J., Liu Ji-yang, Chen Zhong, Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Cheng L., Ye F., Li S., Zheng J., Zhang N., Zhong N., He J. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicentre) and outside Hubei (non-epicentre): a nationwide analysis of China. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00562-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton N., Kobayashi T., Yang Y., Hayashi K., Akhmetzhanov A., Jung S., Yuan B., Kinoshita R., Nishiura H. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J. Clin. Med. 2020;9:538. doi: 10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Funk S., Flasche S., Centre for Mathematical Modelling of Infectious Diseases nCoV Working Group The contribution of pre-symptomatic infection to the transmission dynamics of COVID-2019. Wellcome Open Res. 2020;5:58. doi: 10.12688/wellcomeopenres.15788.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Sherlock P., Ebrahim S., Geffen L., McKee M. Bearing the brunt of covid-19: older people in low and middle income countries. BMJ. 2020;368 doi: 10.1136/bmj.m1052. [DOI] [PubMed] [Google Scholar]
- Madewell Z.J., Yang Y., Longini I.M., Halloran M.E., Dean N.E. Household transmission of SARS-CoV-2. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBryde E.S., Meehan M.T., Adegboye O.A., Adekunle A.I., Caldwell J.M., Pak A., Rojas D.P., Williams B.M., Trauer J.M. Role of modelling in COVID-19 policy development. Paediatr. Respir. Rev. 2020;35:57–60. doi: 10.1016/j.prrv.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas D., Bruel T., Grzelak L., Guivel-Benhassine F., Staropoli I., Porrot F., Planchais C., Buchrieser J., Rajah M.M., Bishop E., Albert M., Donati F., Prot M., Behillil S., Enouf V., Maquart M., Smati-Lafarge M., Varon E., Schortgen F., Yahyaoui L., Gonzalez M., De Sèze J., Péré H., Veyer D., Sève A., Simon-Lorière E., Fafi-Kremer S., Stefic K., Mouquet H., Hocqueloux L., van der Werf S., Prazuck T., Schwartz O. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat. Med. 2021;27(5):917–924. doi: 10.1038/s41591-021-01318-5. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2021. R: A language and environment for statistical computing.
- Rahaman K.R., Mahmud S., Mallick B. Challenges of testing COVID-19 cases in Bangladesh. Int. J. Environ. Res. Public Health. 2020;17:6439. doi: 10.3390/ijerph17186439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, H.Z., Das, N., Matin, I., Mohammad Abdul Wazed, S.A., Jahan, N., Zillur, U., 2020. Livelihoods, coping, and support during COVID-19 crisis, Power and Participation Research Centre (PPRC) & BRAC Institute for Governance and Development (BIGD).
- Rees E.M., Nightingale E.S., Jafari Y., Waterlow N.R., Clifford S., B. Pearson C.A., Group C.W., Jombart T., Procter S.R., Knight G.M. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18:270. doi: 10.1186/s12916-020-01726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidpath D.D., Allotey P., O’Brien-Malone A., Diamond M.R. Re: Tackling covid-19: are the costs worth the benefits? COVID in (country) context. BMJ. 2020 doi: 10.1136/bmj.m1496. [DOI] [Google Scholar]
- Saha, Senjuti, Tanmoy, A.M., Sium, S.M. Al, Tanni, A.A., Goswami, S., Rahman, H., Saha, Samir, Hooda, Y., 2021a. Detection of the B.1.1.7 and B.1.351 SARS-CoV-2 variants in Bangladesh [WWW Document]. URL 〈https://virological.org/t/detection-of-the-b-1–1-7-and-b-1–351-sars-cov-2-variants-in-bangladesh/668〉 (accessed 4.6.21).
- Saha Senjuti, Tanmoy A.M., Tanni A.A., Goswami S., Sium S.M.Al, Saha Sudipta, Islam S., Hooda Y., Malaker A.R., Anik A.M., Haq M.S., Jabin T., Hossain M.M., Tabassum N., Rahman H., Hossain M.J., Islam M.S., Saha S.K. New waves, new variants, old inequity: a continuing COVID-19 crisis. BMJ Glob. Health. 2021;6:1–5. doi: 10.1136/bmjgh-2021-007031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020;26:1470–1477. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetaert K., Petzoldt T., Setzer R.W. Solving differential equations in R: Package deSolve. J. Stat. Softw. 2010;33:1–25. [Google Scholar]
- Stutt R.O.J.H., Retkute R., Bradley M., Gilligan C.A., Colvin J. A modelling framework to assess the likely effectiveness of facemasks in combination with ‘lock-down’ in managing the COVID-19 pandemic. Proc. R. Soc. A Math. Phys. Eng. Sci. 2020;476 doi: 10.1098/rspa.2020.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thunström L., Newbold S.C., Finnoff D., Ashworth M., Shogren J.F. The benefits and costs of using social distancing to flatten the curve for COVID-19. J. Benefit-Cost. Anal. 2020;11:179–195. doi: 10.1017/bca.2020.12. [DOI] [Google Scholar]
- Torres-Rueda S., Sweeney S., Bozzani F., Vassall A. The health sector cost of different policy responses to COVID-19 in low- and middle- income countries. medRxiv. 2020 doi: 10.1101/2020.08.23.20180299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truelove S., Abrahim O., Altare C., Lauer S.A., Grantz K.H., Azman A.S., Spiegel P. The potential impact of COVID-19 in refugee camps in Bangladesh and beyond: a modeling study. PLOS Med. 2020;17 doi: 10.1371/journal.pmed.1003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division, 2019a. Database on Household Size and Composition 2019 [WWW Document]. URL 〈https://www.un.org/development/desa/pd/data/household-size-and-composition〉 (accessed 1.11.21).
- United Nations, Department of Economic and Social Affairs, Population Division, 2019b. World Population Prospects 2019 [WWW Document]. URL 〈https://population.un.org/wpp/Download/Standard/Population/〉 (accessed 9.17.20).
- Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., Cuomo-Dannenburg G., Thompson H., Walker P.G.T., Fu H., Dighe A., Griffin J.T., Baguelin M., Bhatia S., Boonyasiri A., Cori A., Cucunubá Z., FitzJohn R., Gaythorpe K., Green W., Hamlet A., Hinsley W., Laydon D., Nedjati-Gilani G., Riley S., van Elsland S., Volz E., Wang H., Wang Y., Xi X., Donnelly C.A., Ghani A.C., Ferguson N.M. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect. Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespignani A., Tian H., Dye C., Lloyd-Smith J.O., Eggo R.M., Shrestha M., Scarpino S.V., Gutierrez B., Kraemer M.U.G., Wu J., Leung K., Leung G.M. Modelling COVID-19. Nat. Rev. Phys. 2020;2:279–281. doi: 10.1038/s42254-020-0178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Tian H., Zhang L., Zhang M., Guo D., Wu W., Zhang X., Kan G.L., Jia L., Huo D., Liu B., Wang X., Sun Y., Wang Q., Yang P., MacIntyre C.R. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Glob. Health. 2020;5 doi: 10.1136/bmjgh-2020-002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation (WHO), 2020a. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19), The WHO-China Joint Mission on Coronavirus Disease 2019.
- World Health Organisation (WHO), 2020b. Hospital beds (per 10 000 population) [WWW Document]. URL 〈https://www.who.int/data/gho/data/indicators/indicator-details/GHO/hospital-beds-(per-10–000-population)〉 (accessed 9.17.20).
- Yi C., Aihong W., Bo Y., Keqin D., Haibo W., Jianmei W., Hongho S., Sia W., Guozhang X. Epidemiological characteristics of infection among close contacts of new coronavirus pneumonia in Ningbo City. Chin. J. Endemiol. 2020;41 doi: 10.3760/cma.j.cn112338-20200304-00251. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
All data and code can be accessed via out Github repository (https://github.com/boydorr/BGD_Covid-19/ tree/main/ BGD_NPI_model). The interactive app is available at http://boydorr.gla.ac.uk/BGD_Covid-19/CEEDS/.