Abstract
Juvenile-onset open-angle glaucoma (JOAG) is a subset of primary open-angle glaucoma that is diagnosed before 40 years of age. The disease may be familial or non-familial, with proportions varying among different populations. Myocilin mutations are the most commonly associated. JOAG is characterized by high intraocular pressures (IOP), with many patients needing surgery. The mean age at diagnosis is in the 3rd decade, with a male preponderance. Myopia is a common association. The pathophysiology underlying the disease is immaturity of the conventional outflow pathways, which may or may not be observed on gonioscopy and anterior segment optical coherence tomography. The unique optic nerve head features include large discs with deep, steep cupping associated with high IOP-induced damage. Progression rates among JOAG patients are comparable to adult primary glaucomas, but as the disease affects younger patients, the projected disability from this disease is higher. Early diagnosis, prompt management, and life-long monitoring play an important role in preventing disease progression. Gene-based therapies currently under investigation offer future hope.
Keywords: juvenile onset open angle glaucoma, juvenile open angle glaucoma, early onset glaucoma, juvenile ocular hypertension, juvenile normal tension glaucoma, myocilin, MYOC, CYP1B1, JOAG, juvenile glaucoma
1. Introduction
Juvenile-onset open angle glaucoma (JOAG) has long been a subject of interest in view of its rarity and unique clinical features. It is relatively common in people of South Asian, West Asian, and African descent when compared to European Caucasians.25 Because of genetic heterogeneity, the genotype and phenotype characteristics are incompletely understood; however, if left unattended the disease is associated with considerable morbidity in the most socioeconomically productive subgroup of the population. In one study, the economic burden of eye disorders and vision loss among the United States population less than 40 years of age was estimated to be $27.5 billion per year.217 Glaucoma in this population drew a 2% share of all medical costs. Being a disease of childhood and young adults, JOAG patients require life-long close monitoring to preserve their socially productive status.
2. Prevalence
The prevalence of JOAG varies in different populations. It was found that JOAG accounted for 3.4% of all the newly diagnosed glaucomas in a tertiary care center in Nigeria,108 as compared to 3.3% in India,43 1.9% in Saudi Arabia,7 and 0.7% in Caucasians.61 The prevalence in the United States of America was estimated to be 1 in 50,000.196
JOAG is classified under the umbrella terms of both pediatric glaucoma and adult-onset primary open-angle glaucoma (POAG), and hence its proportion in various studies is different. Studies on the profile of childhood glaucomas have shown a prevalence of 15% in South India168 and 16% in Ohio.27 These studies set an upper age limit of 16–18 years, producing the higher reported prevalence. A hospital-based study in Egypt has, however, suggested that JOAG contributes to only 1% of childhood glaucomas.142 A screening program for POAG in West Africa found that 16% of diagnosed patients were between 20 and 40 years of age.192 Goldwyn and coworkers61 found that one-fourth of adolescents and young adults between the ages of 10 and 35 years referred for glaucoma had JOAG.
3. Nomenclature and classification
Primary congenital glaucoma (PCG), JOAG, and adult-onset POAG can be considered a continuous disease spectrum. Immaturity of the conventional outflow pathway is the important underlying pathology for JOAG and PCG. At present, the definition of JOAG is limited to POAG occurring within the limits defined by age, although a classification based on genetic mutations could be useful.
3.1. Nomenclature
3.1.1. Juvenile-onset open-angle glaucoma
JOAG is a type of POAG, where the onset of disease is before 40 years of age.77 Both the upper and lower age limit considered for this diagnosis has been variable, ranging from 5–35 years,46 10–30 years,195 10–35 years,124 and 10–40 years;70, 71 however, 3 years of age is generally taken as the lower limit as the eye is not expected to enlarge in response to high IOP after this age.8 To address such discrepancy in nomenclature of childhood glaucomas, the Childhood Glaucoma Research Network proposed an International Consensus Classification193 that stated that the earliest age of onset for JOAG is considered to be 4 years, and the upper limit may extend up to 30 or 40 years, after which it will be classified under adult-onset POAG. The discrepancy in age-based classification, as reflected through recruitment criteria of different studies, remains. The crucial features for diagnosis include an IOP >21 mmHg on at least 2 occasions, with open angles, supported by distinct glaucomatous optic neuropathy with or without corroborative visual field (VF) defects.
3.1.2. Juvenile ocular hypertension (JOHT)
The term ‘juvenile ocular hypertension’ (JOHT) was first coined in 1948.93 JOHT is diagnosed when the central corneal thickness (CCT)-corrected IOP is over 22 mmHg on at least 2 different occasions in the absence of glaucomatous optic neuropathy and VF defects in patients younger than 40 years.77, 124 Up to 43% of fellow eyes of unilateral JOAG can manifest JOHT.72 It is frequently associated with an IOP greater than 30 mmHg, fluctuating IOPs, and thick corneas.184 Sun proposed that many patients with high IOP detected in the juvenile age group may normalize during adolescence and need only long-term follow up, labeling it as ‘adolescence IOP fluctuation’ or ‘adolescence ocular hypertension’.184 Rousseau and Bito44 performed primate eye studies of IOPs in rhesus monkeys and proposed that JOHT could be a physiological phenomenon critically important for normal development of the globe size. JOHT is important to be identified and monitored, however, as in some families with JOAG, the younger members may present with only high IOP in the initial stages. JOHT may sometimes be incorrectly diagnosed in young children because of difficulty in obtaining IOP measurements. In such cases an examination under sedation will be useful not only to measure IOP, but also to rule out a secondary cause that could contribute to it.
3.1.3. Juvenile normal tension glaucoma (JNTG)
JNTG is clinically defined as glaucomatous damage occurring at baseline/highest IOPs ≤ 21 mmHg in individuals less than 40 years of age. Adult onset NTG is more prevalent in Asia, estimated to comprise about 52–92% of all open-angle glaucomas;37 however, only a few series of JNTG have been documented, primarily focusing upon their distinct vascular and perimetric characteristics.5, 18, 24, 116, 151, 152 Neuroimaging to rule out possible underlying causes is important. Some unique reports of JNTG include a family of autosomal recessive juvenile glaucoma with novel LTBP2 variants162 and a 27-year-old patient with chronic low cerebrospinal fluid pressures from a shunt procedure.224 A new phenotype of familial normal-tension glaucoma with features of anterior segment digenesis was reported in a two-generation pedigree,19 though the underlying cause was not evaluated.
3.2. Classification
JOAG is clinically heterogeneous, with variations in age at onset, IOP elevation, extent of goniodysgenesis, and inheritance pattern. This range of manifestations is likely related to the underlying genetic heterogeneity. Birla and coworkers25 studied the phenotypic characteristics of 414 unrelated Indian JOAG patients and performed a cluster analysis to classify them based on the age of onset, highest untreated IOP, gonioscopic findings and iris features (Table 1). The mean age of the study population was 27 ± 9 years and mean highest untreated IOP was 38.67 ± 12.5 mmHg. Of their cases, 64% had abnormal angle features, and 30% had abnormal iris pattern. They found differences not only in the baseline IOP, but also in their apparently normal looking iris and angle morphology. Figure 1 shows the different angle and iris features observed.
Table 1 –
Phenotypic classification of JOAG by Birla et al25
| Angle | ||||
|---|---|---|---|---|
| Iris crypts | Normal | Featureless angle | High iris insertion | Prominent iris process |
| Normal | Cluster 1 | Cluster 2 | Cluster 3 | |
| 28 ± 9.2 years* | 24 ± 9.6 years* | 26 ± 7.8 years* | ||
| 36 ± 11 mmHg† | 37.8 ± 12.8 mmHg† | 41.3 ± 12.7 mmHg† | ||
| Absent Prominent | Cluster 4 | |||
| 27.4 ± 9.2 years* | ||||
Mean age at onset
Mean highest intraocular pressure
Figure 1 –
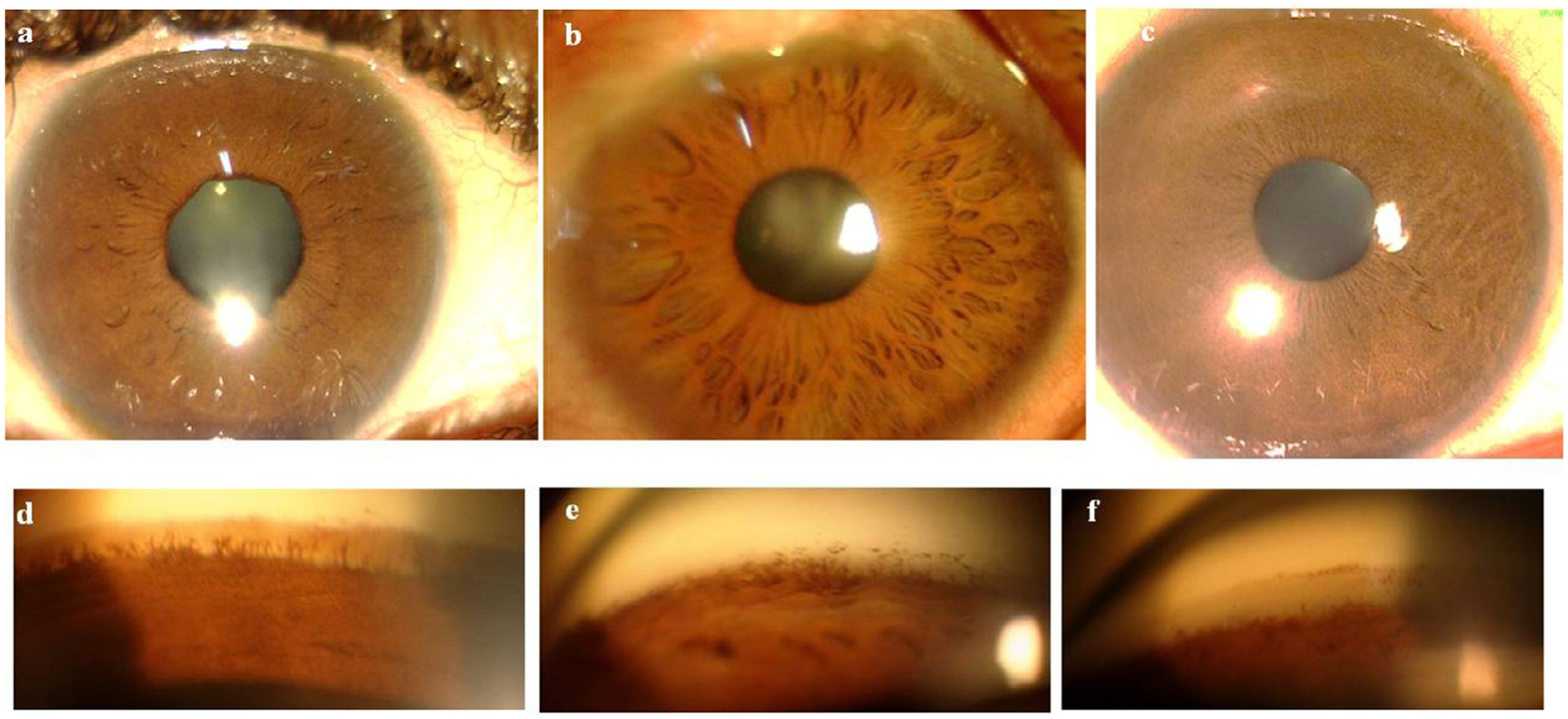
Anterior segment photographs showing a) normal iris crypts, b) prominent crypts of iris, c) lack of iris crypts. Goniophotographs showing d) prominent iris processes, e) high iris insertion, and f) a featureless angle.
From these clusters, the authors classified JOAG into 4 phenotypic groups.
Group 1: Normal angle and iris features: They presented at a later age than all other clusters and had the lower baseline untreated IOP.
Group 2: Featureless angle and normal iris pattern: They had the earliest presentation.
Group 3: Prominent iris processes or high iris insertion, with a normal iris pattern: They presented with high IOPs, and high iris insertion was associated with greater mean IOP than the presence of only prominent iris processes.
Group 4: Prominent iris crypts and high iris insertion resembling subtle forms of Axenfeld- Rieger anomaly: They had the highest baseline untreated IOP, with over half having an IOP ≥ 40 mm Hg.
Whether this classification system could be applied to all high pressure JOAG patients across different populations needs further evaluation.
4. Demographic and Clinical features
4.1. Demography
The mean age at diagnosis has varied among studies from 16 years to the 3rd decade.7, 48, 112, 142, 168 Males are more commonly affected.17, 46, 61, 112, 168 JOAG is usually bilateral,27, 72, 112 but up to 25% of patients have been reported to present with unilateral disease.72 The proportion of familial JOAG is variable across populations:30 28% among Koreans,112 30% among Indians,25 43% in Americans,61, 124 and up to 88% among Africans.48, 108 Smoking has not been found to confer any risk to disease onset or IOP levels in JOAG.202
4.2. Intraocular pressure
Mean IOP during diagnosis of JOAG is usually between 30 and 40 mmHg, significantly higher than that of JOHT.74, 84, 124 Wide diurnal fluctuations of up to 22 mm Hg have been observed. Peak IOPs could be associated with transient corneal edema, resulting in colored halos and blurred vision;153 however, one third of patients could remain totally asymptomatic.112
4.3. Gonioscopy
JOAG eyes may exhibit angle dysgenesis;199 however, this phenotypic finding may be more common in some ethnic groups than others. Urbak and coworkers198 suggested that goniodysgenesis in the form of a transparent to yellow/brown/greyish pretrabecular membrane and abnormally exposed greater arterial circle of iris may be present in some patients. In some subjects, distinct iris processes and persistent uveal meshwork were also noted. Gupta and coworkers80 studied the gonioscopic characteristics of a cohort of JOAG patients and identified 34% of them as possessing a normal looking angle, while the remaining 66% showed developmental anomalies in form of a featureless angle, a high iris insertion, or prominent iris processes. They defined these forms of angle dysgenesis as: (i) prominent iris processes, where multiple iris processes were seen extending onto the trabecular meshwork (TM) in at least 2 quadrants (180 °), (ii) high iris insertion, when the root of iris was inserted over the trabecular meshwork anterior to the ciliary body band. They may present with or without prominent iris processes (iii) a featureless angle, where there was a lack of pigmentation, and TM could not be differentiated from the scleral spur. Probably, the compact TM/persistent embryonic tissue gave a featureless/ground-glass appearance to the angle. A high iris insertion with or without prominent iris processes was the most common manifestation of angle dysgenesis in their study.25, 80
En face gonioscopy, while being an important tool to determine angle dysgenesis, may not be able to elicit the true extent of trabecular dysfunction.196 On the other hand, prominent iris processes or other features of goniodysgenesis may not necessarily indicate impaired aqueous outflow.62 In fact, the line between a normal and abnormal angle (dysgenesis) on gonioscopy is a thin one. Further, the prevalence data for gonioscopic anomalies as anatomical variants in nonglauco-matous eyes has not been analyzed.
4.4. Optic disc
In comparison to adult onset POAG, the optic discs of JOAG patients were found to be of normal size in one study,98 while another showed larger disc size.73 A relatively low frequency of disc hemorrhages and smaller beta zone parapapillary atrophy has been noted in comparison to adult-onset POAG.97, 98 Also, the size of disc hemorrhages have been observed to be smaller in JOAG as compared to NTG, contributing to lower detection rates as well.97
4.5. Myopia
Myopia is commonly associated with JOAG.61, 95, 112, 124, 134, 196 JOAG patients have been seen to have longer axial lengths and myopic refractive status when compared to adult POAG patients.107 About 42–72% of JOAG patients are myopic, compared to 59% of JOHT patients.27, 124 Lotufo and coworkers found 38.5% of JOAG eyes to have myopia of 6 diopters or more.124 An association of JOAG with keratoconus has also been described.60
5. Rare associations
The rare systemic associations of JOAG have been summarized in Table 2.
Table 2 –
Systemic associations with JOAG
| Disease | Systemic features | Ocular features |
|---|---|---|
| Hereditary motor and sensory neuropathy14,16,104,148,197 | Progressive weakness and atrophy of distal limb muscles. Impaired sensation in distal part of limbs. Club foot. | Congenital cataract, JOAG, JOHT, congenital glaucoma, retinitis pigmentosa, optic atrophy. |
| Nail patella syndrome119,140,166 | Dysplasia of nails, patella, elbow and iliac horns. Nephropathy. Neurological or vasomotor peripheral limb abnormalities. Epilepsy. | Ptosis, epicanthal folds, hypertelorism, sclerocornea, microcornea, keratoconus, pupillary ectopia, anisocoria, cataracts, JOAG, JOHT, OHT, POAG, NTG. |
| 22q11.2 microduplication syndrome39,45 | Mental retardation, heart and urogenital malformations, hypotonia, hearing impairment, hypothyroidism, seizures. | Downslanting palpebral fissures, hypertelorism , epicanthal folds, hyperopia, congenital cranial dysinnervation disorders, JOAG, infantile glaucoma, retinal vascular abnormalities. |
| Cutis marmorata telangiectatica congenita47,126,141,144 | Generalized red-blue reticular vascular skin pattern, port-wine stain, telangiectasia, ulcers, mental retardation. | Corneal opacity, congenital glaucoma, JOAG, peripheral retinal avascularity, neovascularisation, retinal detachment, granular appearance of macula. |
JOAG = Juvenile open angle glaucoma; JOHT = Juvenile ocular hypertension; OHT = ocular hypertension, POAG = Primary open angle glaucoma; NTG = normal tension glaucoma.
6. Differential diagnosis
Other causes of high IOP in the juvenile age group, discussed below, need to be excluded before labeling a patient as JOAG.
6.1. Pigmentary glaucoma
Pigment dispersion syndrome and pigmentary glaucoma can be distinguished by the presence of a Krukenberg spindle, Sampolesi line, excessive angle pigmentation, a very deep anterior chamber, Scheie stripe or Zentmayer ring on the lens capsule and sometimes even concave iris configuration confirmed on UBM. Patients may present with post-exercise coloured halos and/or blurred vision due to transient IOP spike.
6.2. Steroid-induced glaucoma
Steroid-induced glaucoma can be difficult to differentiate unless there is a history of steroid use, either topical or systemic. A history of seasonal/chronic red eye, itching, skin disorders, systemic diseases like asthma/nephrotic syndrome and over-the-counter medication usage may raise suspicion. Steroid-induced glaucoma is associated with very high IOPs and in many, though not all, cases IOP decreases once steroids are withdrawn, unlike untreated JOAG that progressively worsens.
6.3. Traumatic glaucoma
Traumatic glaucoma is generally unilateral and can be difficult to distinguish, especially if the history is not forthcoming. Signs of blunt trauma such as sphincter tears, iridodialysis, asymmetrically deep and irregular anterior chamber, phacodonesis, and angle recession on gonioscopy help in diagnosis.
6.4. Uveitic glaucoma
A case of uveitis with minimal telltale signs could be misdiagnosed as JOAG unless carefully looked for. Residual keratic precipitates and retrolental cells may provide a clue. Continuous steroid usage in this setting can also lead to a white eye with high IOP.
6.5. Congenital glaucoma
PCG after 3 years of age can be misdiagnosed as a JOAG, unless one carefully looks at the corneal diameters. Both conditions may have angle dysgenesis and myopia.
6.6. Other developmental glaucomas (Axenfeld-Rieger anomaly, congenital ectropion uveae)
Subtle forms of Axenfeld-Rieger anomaly may be difficult to distinguish from JOAG. Posterior embryotoxon is more common in patients with developmental glaucomas. Congenital ectropion uveae in older individuals is generally unilateral and may be misdiagnosed as JOAG unless the other eye is carefully examined. Gonioscopic anomalies in an eye with JOAG would be symmetrical, while those in congenital ectropion uveae would be more pronounced in the affected eye.
6.7. Primary angle-closure glaucoma (PACG)
Without gonioscopy, early onset PACG (or PACG of the young) may be misdiagnosed as JOAG. With gonioscopy, the high iris insertion typically observed in JOAG needs to be distinguished from the broad peripheral anterior synechiae seen in PACG.
6.8. Megalopapilla
Megalopapilla is a congenitally anomalous optic disc with surface area >2.5 mm2.115 The physiologically large disc is accompanied by a large cup, raising suspicion of juvenile glaucoma, if the patient presents at a young age; however, the neuroretinal rim, contour of blood vessels, IOP and visual fields are normal.115, 165
6.9. Optic disc cupping secondary to prematurity
There is controversy whether low birth weight is associated with larger optic disc cupping or whether large (physiologic) cupped discs are genetic.58 In a study of over 2000 children, it was shown that low birth weight, short birth length, and small head circumference at birth were associated with larger cup/disc ratio suggesting that fetal growth restriction could adversely influence optic nerve head parameters.164 Prematurity-associated periventricular leukomalacia has been shown to result in pseudoglaucomatous cupping characterized by a large horizontal cup and minimal to no displacement of nasal vessels.64, 123 Associated VF defects can result from thinning of the periventricular optic radiations and/or transsynaptic degeneration of retinal ganglion cells;64 however, the IOP tends to remain in normal range, raising suspicion of JNTG.
6.10. Chandler syndrome
Chandler syndrome, the predominantly corneal subtype of iridocorneal endothelial (ICE) syndrome, may be confused with JOAG, especially when the corneal changes are subtle. It is commonly unilateral and may progress to involve the iris at later stages. Demonstration of ICE cells on confocal microscopy can clinch the diagnosis.
7. Investigations
7.1. Structural
7.1.1. Anterior Segment
7.1.1.1. Biometry
A biometric study showed that JOAG patients had lower CCT (mean 521 ±20μ), greater axial length (mean 24.63 ±0.83mm), and deeper anterior chamber depth (mean 3.52 ±0.25mm) compared to age-matched normal subjects, likely attributed to myopia;46 however, the sample size of the study was not large. The endothelial cell counts of JOAG eyes have also been shown to be lesser.200, 225
7.1.1.2. Angle imaging
An ultrasound biomicroscopic study showed that JOAG eyes had wider angles as compared to normals,199 and another showed shorter TM height, suggesting a structural abnormality underlying the reduced outflow facility.180 The resistance to outflow in JOAG eyes could be at various levels, either pretrabecular, trabecular or at posttrabecular Schlemm canal (SC) and collector channels.199 Gupta and coworkers67 studied the angle of JOAG patients using high definition spectral domain anterior segment optical coherence tomography (OCT) and identified abnormal tissue/hyperreflective membrane covering the TM in 40% of them. Also, the SC could not be identified in 40% of the eyes (Fig. 2). Whether the inability to identify SC is a marker of primary dysgenesis or secondary to trabecular resistance and consequent poor aqueous outflow needs to be further explored.
Figure 2 –
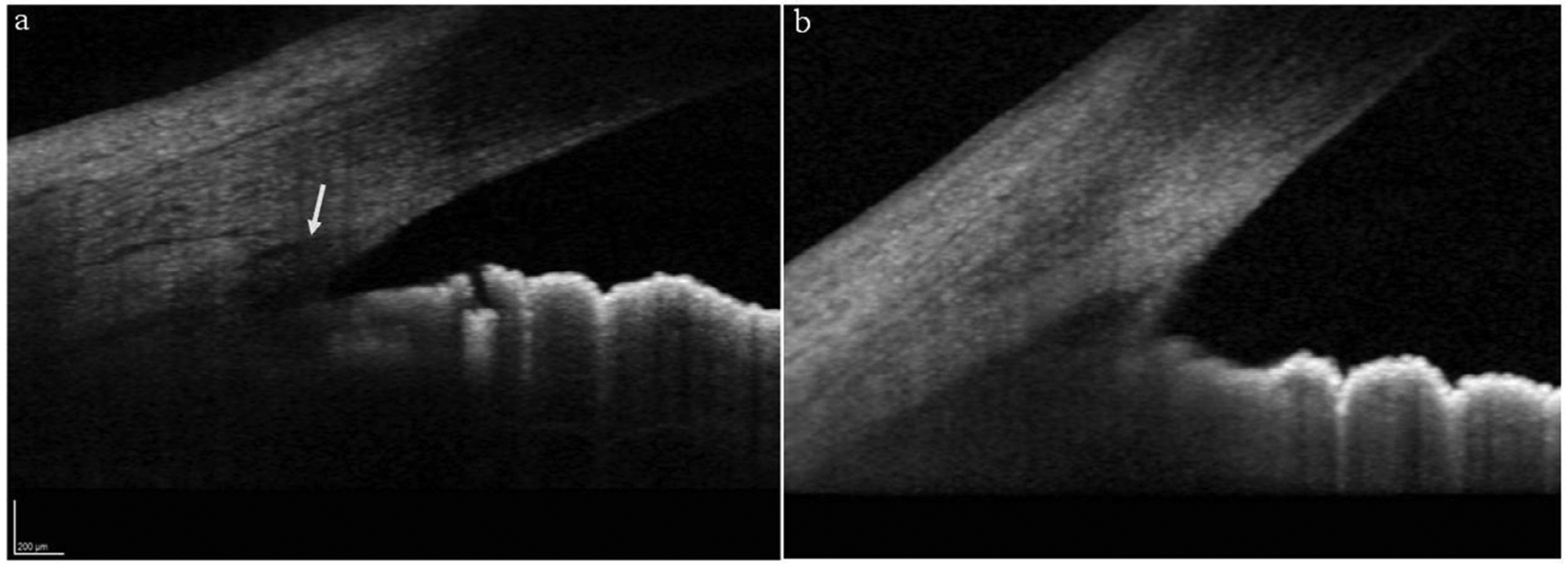
High-definition anterior segment OCT images showing a) normal appearing angle with the white arrow pointing towards the Schlemm’s canal, b) an angle of a JOAG patient showing abnormal thickness in the region of trabecular meshwork and without a visible Schlemm’s canal.
7.1.2. Posterior segment
Studying the optic disc features on scanning laser ophthalmoscopy, Gupta and coworkers73 showed that JOAG eyes had enlarged cups, with increased cup depth, cup volume, and cup to disc area ratio compared to PCG and POAG. In OCT retinal nerve fibre layer (RNFL) studies, it has been seen that JOAG eyes have thinner RNFL compared to JOHT and normal age-matched controls.143, 146
OCT angiography (OCT-A) has revealed a strong positive correlation between RNFL thickness and vascular density (VD) of disc and peripapillary region in JOAG.1 The superficial vascular plexus VD of perifoveal regions showed a trend to reduce with increase in IOP.34
In patients with JNTG, central retinal arteriolar equivalent, a measure of the diameter of retinal arterioles passing around the disc, was found to be significantly less when compared to JOAG.116 Another study5 showed narrowed arterioles in preperimetric JNTG eyes as well. This arteriolar narrowing could translate to reduced blood flow to the optic nerve head and result in glaucomatous optic neuropathy despite normal IOPs.5, 152 A subsequent study, however, aiming to characterize these vascular differences using OCT-A showed contrasting results, with JOAG eyes manifesting greater peripapillary microvascular attenuation than healthy controls or JNTG.151
7.2. Functional
Patients with JOAG usually present at moderate-advanced stages, with baseline mean deviation of <−8.5 dB.68, 72, 75, 112 The pattern of VF defects depends upon the baseline IOP at which the damage occurs. VF defects in JOAG are not very different from those of adult onset POAG; however, Ko and coworkers, and Park and coworkers found JOAG VF defects to be more diffuse and symmetric between the superior and inferior hemifields.107, 152 The authors attributed this to the longer axial length and myopic refractive status in their patients, which may confound localized glaucomatous VF defects.107 In contrast, JNTG showed vertically asymmetric VF defects, especially deeper in the superior paracentral area.152 This was proposed to be caused by localized impaired blood flow to the optic nerve.
7.3. Histopathology
The decisive pathological feature of goniodysgenesis is anomalous persistence of foetal endothelial membrane that covers the TM at the iridocorneal angle and interferes with the aqueous drainage. This membrane usually becomes fenestrated by the end of the third trimester to allow conventional aqueous outflow.160 An early histological study191 on juvenile glaucoma eyes showed thick compact tissue at the anterior chamber side of SC, representing an underdeveloped TM. An electron microscopic study59 of trabeculectomy specimens from JOAG eyes showed large amounts of extracellular material arranged in a fingerprint-like pattern, resembling basement membrane-like material, causing significant thickening of the cribriform trabecular meshwork in all cases. Characteristically, these histopathological changes may be present among JOAG patients even with normal appearing angle on en face gonioscopy,191 reinforcing the deeper layer dysgenesis that may account for glaucoma.
8. Genetic factors
Recent evidence suggests that JOAG is genetically heterogenous.76 While it was typically considered as an autosomal dominant disease with variable penetrance and expressivity,193, 211 autosomal recessive and sporadic occurrence are also common in certain ethnicities.22, 76, 102
Initial linkage analysis pointed to chromosome 1q21–31 as the locus of interest for JOAG (GLC1A), which was later refined to 1q23–25.118, 158, 196, 210–212 Further studies identified the gene encoding Myocilin (MYOC) as the JOAG causing gene in the GLC1A locus.57, 102 Apart from MYOC, CYP1B1 and less commonly LTBP2 have been associated with high-pressure JOAG. Optineurin (OPTN) and TANK-binding kinase 1 (TBK1) have been associated with JNTG. For JOAG families, genetic testing allows for identification of family members with disease-causing mutations which makes possible early diagnosis and treatment, and also obviating the need for unnecessary regular ophthalmic check-ups when negative.35, 89, 176
8.1. Myocilin (MYOC)
Myocilin was the first identified glaucoma-causing gene, and mutations are known to cause JOAG and adult onset POAG.87, 101 The distribution of MYOC mutations contributing to JOAG have varied among different ethnicities, ranging from 5.8% in Chinese cohorts87 to 12.5% in Taiwanese,222 17.4% in Iranian,22 and 34% in Brazilian cohorts.186 Such diversity also existed among familial cases, with MYOC contributing to 8% of cases among North American JOAG pedigrees,209 to 27% among Indians,77 and to 33% in a mixed-ethnicity study.170 The sample sizes widely differed among these studies, however, and not all MYOC mutations were accounted for.
The lifetime risk of developing open-angle glaucoma in MYOC mutation carriers is estimated to be 60–100%.76 The gene was initially cloned as a steroid-response protein from cultured TM cells, named ‘trabecular meshwork-induced glucocorticoid response (TIGR) protein’.9, 96 Subsequently, the gene was isolated and localized to cilium connecting the inner and outer segments of photoreceptor cells, and therefore named the ‘myocilin’.209 Myocilin is an extracellular protein widely expressed in normal tissues, with high expression in the trabecular meshwork.188, 205 MYOC mutations cause intracellular protein aggregation resulting in reduced amounts of extracellular protein. Aggregated mutant protein can cause apoptosis.205 A histochemical study of a JOAG case caused by MYOC p.Tyr437His identified abundant accumulated mutant protein within endoplasmic reticulum (ER) of TM cells that caused ER stress and cell loss.82
Over 250 MYOC mutations have been reported, among which 37.7% are deemed pathogenic.205 At least 93% of disease-causing mutations are located in the 3rd exon that encodes the olfactomedin-homology domain.28, 57, 111, 145, 196, 205 The majority of pathogenic MYOC mutations are missense alleles (84%); however, the most common MYOC mutation (in both JOAG and POAG) is a nonsense mutation (p.Gln368stop) that also causes protein aggregation.205 MYOC mutations can cause both JOAG and adult onset POAG, however, most of the mutations cause JOAG,88, 133, 196, 205, 214, 222 and usually familial disease.77, 170, 209 p.Pro370Leu is a frequent and severe JOAG causing MYOC mutation.76 Selected MYOC mutations have been summarised in Table 3. Many MYOC mutations have only been found in one or few families, such as p.Asn57Asp,170 p.Cys245Tyr,54 p.Pro274Arg,129 p.Ile345Met,186 p.Thr377Arg,207 p.Asp384His,87 p.Glu385Lys,41 p.Tyr453MetfsTer11,186 and p.Ile499Ser.170 Several common MYOC polymorphisms have also been identified including, p.Glu340Glu, p.Arg76Lys and p.Gly122Gly.77, 181 A combination of the p.Asn480Lys mutation and IVS2 730+35 G>A polymorphism has also been proposed to increase the susceptibility to JOAG.139 A Brazilian study supported a founder effect of MYOC p.Cys433Arg variant in JOAG, predicting its age to be of 43–59 generations.130
Table 3 –
Selected JOAG-causing mutations.
| Chromosome | Locus | Gene | Mutations |
|---|---|---|---|
| 1q23–25 | GLC1A | MYOC | p.Gln48His77,178 |
| p.Gly252Arg26,170 | |||
| p.Gln337Arg77,182 | |||
| p.Gly367Arg77,90,135,136,220 | |||
| p.Gln368stop10,186 | |||
| p.Pro370Leu35,87,135,136,170,181,186,208,209 | |||
| p.Thr377Met10,156 | |||
| p.Lys423Glu29,186 | |||
| p.Val426Phe121,128 | |||
| p.Cys433Arg130,155,186 | |||
| p.Tyr437His10,82,209 | |||
| p.Ile477Asn10,159 | |||
| 2p21 | GLC3A | CYP1B1 | p.Gly61Glu2,20 |
| p.Glu229Lys4,78 | |||
| p.Arg368His4,78,100,201 | |||
| p.Arg390His87,183 | |||
| 14q24 | GLC3D | LTBP2 | p.Pro432Leu173 |
| p.Pro989Arg162 | |||
| p.Asn1745Lys162 |
While MYOC mutations are commonly associated with autosomal dominant JOAG, de novo mutations have also been reported.111, 175 A study among JOAG patients (both familial and sporadic forms) found that MYOC mutations were much more common in familial (27%) as compared to sporadic JOAG cases (2%) and concluded that genetic screening for MYOC among the sporadic group may not be entirely fruitful.77
8.2. Cytochrome P450 Family 1 Subfamily B Member 1 (CYP1B1)
The CYP1B1 gene has been implicated in PCG, JOAG and adult onset POAG.132, 137, 185 The prevalence of CYP1B1 mutations causing JOAG have also varied across the world, ranging from 2% among Brazilian cases186 to 11.7% in Indian,4 17.4% in Iranian,22 and nearly 86% among Saudi Arabian cases.2
It has been hypothesized that CYP1B1 and MYOC genes may act through a common biochemical pathway, with the former acting as a modifier for the latter’s expression.201 Svidnicki and coworkers186 performed gene sequencing in Brazilian JOAG patients and identified both MYOC and CYP1B1 variants. The MYOC damaging variants were identified in 34%, but CYP1B1 damaging variants were found only in 2%. While the role of CYP1B1 in glaucoma is not entirely understood, animal models have shown that CYP1B1 deficiency leads to abnormalities in the TM.186 Acharya and coworkers4 suggested that monoallelic CYP1B1 mutations may lead to JOAG, while biallelic mutations cause PCG. Digenic inheritance of JOAG was also identified, where both MYOC and CYP1B1 mutations led to JOAG in a family, while only MYOC mutation manifested as POAG.201
Gupta and coworkers78 studied the frequency of CYP1B1 p.Glu229Lys and p.Arg368His mutations among 120 families of sporadic JOAG and found that only 7.5% and 5.8% of them harboured the mutations respectively. Interestingly, Abu-Amero and coworkers2 identified p.Gly61Glu mutations in 86% of JOAG patients of Saudi Arabian origin. Bashir and coworkers20 reported a Pakistani family with coexisting recessively inherited JOAG and PCG from the same mutation (Gly61Glu).
Selected CYP1B1 mutations identified in JOAG cases are summarised in Table 3. The less frequent mutations reported are p.Pro52Leu,186 p.Trp57Cys,4 p.Thr404SerfsTer30,186 p.Ile471Ser,87 and p.Arg523Thr.4
8.3. Latent transforming growth factor-beta-binding protein 2 (LTBP2)
An autosomal recessive form of JOAG associated with two novel variants of LTBP2 was identified by Saeedi and coworkers,162 although LTBP2 mutations have been classically associated with Weil-Marchesani syndrome. A case of digenic inheritance of MYOC and LTBP2 in a family with autosomal dominant JOAG is also reported.173
8.4. Optineurin (OPTN) and TANK-binding kinase 1 (TBK1)
The evidence for a role of OPTN (optic neuropathy–inducing protein) in JOAG has been conflicting. OPTN is expressed in trabecular meshwork, nonpigmented ciliary epithelium, retina, and in other nonocular tissues such as brain.216 The role of OPTN as an autophagy receptor has been linked with glaucoma pathogenesis. OPTN-mediated autophagy is promoted by TBK1.83 Both have been associated with familial NTG. Rare mutations and variants of OPTN such as p.Leu41Leu, p.His486Arg, p.Arg329Gly and p.Leu494Trp have been identified in some JOAG patients in the Canadian, Chinese, and Korean population,87, 150, 216 but these variants have uncertain pathogenicity and these results have not been validated in functional studies.203, 221 Two large families of autosomal dominant TBK1 associated JNTG have also been reported.24, 56
8.5. Others
Other loci for JOAG candidate genes have been proposed at 5q, 9q22, 15q22-q24 and 20p12 by genome-wide scan maps.149, 204, 213 Mutations in several other genes that have an association with JOAG are:
The CPAMD8 gene (complement component 3- and pregnancy zone protein-like alpha-2-macroglobulin 360 domain-containing protein 8) was associated with 4 members of 2 families with apparent JOAG, with one of the families also having Stickler syndrome.171 Most patients with CPAMD8 variants in this study had cataract, iris hypoplasia and retinal detachments and hence may not be the classical primary JOAG.
Mutations in LMX1B (LIM homeobox transcription factor 1-beta) gene have been shown to increase the susceptibility of glaucoma in patients with nail patella syndrome.140, 194
Hereditary motor and sensory neuropathy has been associated with congenital and juvenile glaucomas,14, 104, 197 with some reports suggesting myotubularin-related protein (MTMR) genes to play a role.16, 148
22q11.2 microduplication syndrome has also been associated with infantile and juvenile glaucomas, likely attributed to mutations in CLDN-5 (claudin-5) gene.39, 45
Pediatric glaucoma, congenital more often than JOAG, has been diagnosed in many patients with cutis marmorata telangiectatica congenita (CMTC).47, 144 Generalised CMTC disease and CMTC lesions on the face posed higher risk of glaucoma.31 Their genetic relations are yet to be known in detail.
Variants of EFEMP1 (epidermal growth factor containing fibulin-like extracellular matrix protein 1) have been associated with severe JOAG in three Philippine families, with 76% of the affected blind in at least one eye.38
Fernández-Martínez and coworkers reported heterozygous variants in RPGRIP1 (retinitis pigmentosa GTPase regulator-interacting protein 1) to be associated with JOAG.55
A heterozygous mutation in oligoadenylate synthetase 3 (OAS3) gene was reported in a Chinese JOAG family.218
The role of glutathione S-transferase (GSTM1, GSTT1) polymorphisms in JOAG susceptibility has been studied but significant associations were not detected.127
9. Genotype-phenotype
Understanding the relationship between disease-causing mutations and clinical phenotype can help define a surveillance and treatment plan for affected patients; however, for many JOAG-related mutations clear genotype-phenotype correlations are not defined, partly because of variable expressivity and, in some cases, reduced penetrance. Additionally it is possible that environmental factors can also modify disease phenotypes.220
Depending on the site and nature of the MYOC mutation, most JOAG patients have higher IOP and a more severe disease course compared to adult onset POAG.190 MYOC mutation carriers usually respond less well to medical and laser therapies, warranting early surgical interventions.57, 84, 90, 138, 220 The penetrance also varies; for example, the penetrance at 30 years of age in pedigrees carrying MYOC p.Pro370Leu was up to 100%, while for p.Asn450Tyr, p.Gly367Arg it was 50%, and 25% for p.Gln368stop mutation (unpublished data).35, 90, 227
Disease secondary to MYOC Pro370Leu mutations exhibit earlier onset and a more aggressive phenotype, with high IOP refractory to medical management.32, 208, 227, 228 MYOC p.Gly246Arg, p.Tyr437His and p.Asn450Tyr also have been shown to manifest early with severe disease.15, 207 On the other end of the spectrum are p.Gln368stop and p.Cys433Arg mutations associated with milder disease that manifested at a later age with lower IOP.10, 214, 227 Phenotypic features of other MYOC mutations appear to be intermediate between these extremes.121, 207 Table 4 summarizes the mean age at diagnosis and maximum IOPs recorded for selected JOAG causing MYOC mutations.
Table 4 –
| Amino acid change | Nucleotide change | Weighted Mean age at diagnosis (years) | Weighted Mean Maximum recorded IOP (mmHg) |
|---|---|---|---|
| p.Gly246Arg | NM_000261.1:c.736G>A | 21.1 ± 6.2 | 31.8 ± 6.1 |
| p.Gly252Arg | NM_000261.1:c.754G>A | 35 ± 7.3 | 31.7 ± 6.5 |
| p.Gly364Val | NM_000261.1:c.1091G >T | 34 | 36 |
| p.Gly367Arg | NM_000261.1:c.1099G >A | 26.4 ± 6.3 | 36.3 ± 8.1 |
| p.Gln368Ter | NM_000261.1:c.1102C >T | 37.8±5.4 | 21.1 ± 2.4 |
| p.Pro370Leu | NM_000261.1:c.1109C >T | 13.3 ± 2.4 | 30.9 ± 3 |
| p.Lys423Glu | NM_000261.1:c.1267A>G | 28.8 ± 8.1 | 31.3 ± 3 |
| p.Val426Phe | NM_000261.1:c.1276G >T | 18.6 ± 1.4 | 30.6 ± 0.8 |
| p.Cys433Arg | NM_000261.1:c.1297T>C | 22.8 ± 8.9 | 20±6.3 |
| p.Tyr437His | NM_000261.1:c.1309T>C | 21.7 ± 1.2 | 44.3±1 |
| p.Asn450Tyr | NM_000261.1:c.1348A>T | 25.7 ± 4.3 | 48.3 ± 12.1 |
| p.Ile477Asn | NM_000261.2:C.1430T>A | 20.7 ± 1.3 | 39.6 ± 1.9 |
Phenotypic variability associated with MYOC mutations is also population dependent. Yao and coworkers220 reported a recurrent missense p.Gly367Arg MYOC mutation transmitted as autosomal dominant JOAG in 5 generations of a Chinese family. This genotype was characterized by early age at onset, incomplete penetrance, high IOP with rapid deterioration after 30 years of age, and no significant gender disparity. On the other hand, Iliev and coworkers90 studied the same mutation in a large Swiss pedigree and summarized the phenotype as a late-onset JOAG.
Gupta and coworkers81 showed that familial JOAG patients presented at least 4 years earlier than those without a family history of glaucoma and had better disease outcomes, likely due to their awareness and early presentation. A negative family history was associated with ten-fold higher likelihood of presenting with a severe glaucomatous VF defect; however, another larger study by the same authors comparing the phenotype of familial versus non-familial patients found no significant differences between age at diagnosis, gender distribution, baseline IOP, goniodysgenesis, and high myopia.74
There are limited studies examining the genotype-phenotype correlations of other JOAG contributing genes. JOAG patients with CYP1B1 mutations are in general younger and manifested a more severe glaucoma when compared to those without them.177 The phenotype of TBK1 duplications was characterized by severe, early-onset optic neuropathy with vision loss and a normal IOP.83
10. Gene-based therapy
Gene-based therapies have promise to be effective for JOAG caused by MYOC mutations. Trimethylamine N-oxide (TMAO), a natural osmolyte, facilitated folding and secretion of mutant MYOC and rescued cells from apoptosis by alleviating ER stress.94 Another investigational drug, sodium 4-phenylbutyrate, a molecular chaperone that relieves misfolded protein response in urea cycle disorders, has also been shown to relieve ER stress and lowered IOP in transgenic MYOC mouse.111, 214 Since MYOC associated JOAG results in a continuous disease process, it is difficult to administer these drugs over the entire lifetime of the patient. Genome editing using CRISPR/Cas9 system has shown promise by reducing expression of mutant MYOC and ER stress, that resulted in lowered IOP and prevented glaucoma development in a mouse model.92
11. Management
11.1. Medical
Juvenile glaucoma is often considered difficult to control medically;6 however, those with not so high IOP and milder disease severity can be controlled on medical therapy. Gupta and coworkers75 showed that up to 50% of patients could achieve optimal IOP control on medical therapy over 5 years; however, in their series VF worsening was more evident in medically treated rather than surgically treated patients.
While all classes of glaucoma agents can be used in older JOAG patients, alpha agonists are generally avoided in children less than 10 years of age. Latanoprost shows a good ocular hypotensive effect in JOAG eyes as compared to other pediatric glaucomas.3, 51, 52, 125 Dorzolamide, in association or in fixed combination with timolol, has been shown to improve retinal blood flow in JOAG patients.40 Miotics though effective, maybe poorly tolerated in JOAG due to ciliary spasm and induced myopia; however, pilocarpine gel forms and Ocusert cause lesser side effects and may be tried in older patients.189
Pregnant and nursing JOAG patients pose a therapeutic challenge.23 Brimonidine is the only drug that is considered possibly safe in pregnancy (FDA category-B); however it is best to avoid it closer to delivery in view of the possible central nervous depression to the newborn. While all other drugs fall into category-C, apraclonidine and pilocarpine can be prescribed in all three trimesters based on the limited data available. Beta-blockers may be used in the first two trimesters, while prostaglandins can be used in third and possibly second trimester as well, with a risk of early labor. Latanoprostene bunod is to be avoided in all trimesters.
The use of oral acetazolamide (FDA category-C) in pregnancy has remained controversial. While animal studies showed teratogenic insults, its usage in pregnant women to treat idiopathic intracranial hypertension did not show a higher incidence of congenital malformations.53, 85, 114 The dosage varied from 250 mg to 2 g per day and was used in all trimesters.53, 114
11.2. Laser trabeculoplasty
Early studies on trabeculoplasty in JOAG eyes using Argon laser showed poor response, and even worsening in a few eyes with pressure spikes reaching up to 35–70 mmHg.86, 131, 215 Melamed and coworkers131 proposed trabeculopuncture as a modality to be tried before invasive glaucoma surgeries were planned for JOAG. In this technique, Q-switched YAG laser energy was delivered to create holes in the TM disrupting it, to penetrate into the Schlemm canal, under topical anesthesia. Blood reflux from SC and retro displacement of the iris adjacent to treatment site could be seen. They compared two techniques of trabeculopuncture–the confluent (2 clock hours) versus focal (4 punctures in 4 quadrants),–and concluded that the former treatment was superior, simulating trabeculotomy. The energy levels required for 1-clock hour of trabeculotomy was 156 mJ and for one focal trabeculopuncture was 42 mJ. This treatment is now rarely used.
Selective laser trabeculoplasty (SLT) has gained popularity in treatment of open-angle glaucomas in view of its simplicity, safety, and repeatability. A case report174 on the use of SLT in a treatment naïve JOAG patient showed 40% IOP drop at one month. Another study122 compared the outcomes of SLT in JOAG and JOHT against adult onset POAG and found comparable results. Over 70% of the young patients in this study attained success at 12 months, with mean IOP reduction of 30%. High pretreatment IOP was identified as a predictor for SLT success. Gupta and coworkers,71 in a longitudinal 12-month follow up, evaluated SLT as secondary therapy for medically-uncontrolled JOAG patients and found that 43% of eyes showed >20% reduction in IOP. Absence of angle dysgenesis on gonioscopy was a favorable prognostic factor. Hence, SLT may not be a contraindication in JOAG and could be tried before resorting to surgery. This could also be a viable option in young pregnant women with JOAG.157
11.3. Surgical
Many patients of JOAG may require surgical intervention, especially those who are non-compliant or refractory to medical/laser therapy.84, 196, 210 It has been shown that 40–70% of JOAG require filtering surgeries.75, 154, 210 Glaucoma surgeries for JOAG can be broadly classified into micro- (or minimally) invasive glaucoma surgery (MIGS) that are performed via an ab interno microincision with minimal tissue trauma163 or invasive surgeries that require external dissection of the conjunctiva and sclera.
11.3.1. Micro-(or minimally)invasive glaucoma surgery
11.3.1.1. Goniotomy
Goniotomy showed 53% complete success and an additional 23.5% qualified success in JOAG eyes at an average 9 years follow-up.223 Being less invasive with fewer complication rates, it improved the aqueous outflow via the conventional drainage system and spared the conjunctiva for future surgeries if needed. Therefore, it was proposed as the initial procedure of choice in JOAG by some authors,223 but has not been universally agreed upon due to lack of extensive studies.
Goniotomy with Kahook dual blade has an advantage over conventional incisional goniotomy in that the TM tissue can be excised, and thus prevents fibrosis of the TM by adhesion of the incised leaflets. Khouri and coworkers showed >30% drop in IOP up to 18-months, using the Kahook dual blade in a 14-year-old JOAG patient.103
11.3.1.2. Ab interno trabeculotomy
Ab interno trabeculotomy can be performed as microcatheter/suture aided Gonioscopy assisted transluminal trabeculotomy (GATT) or by using a microhook. Grover and coworkers65 reported 100% clinical success for microcatheter (iTrack, Ellex, Menlo Park, California, USA) aided GATT at mean 20 months in a series of JOAG eyes. Hyphema was the only reported complication, and open trabecular shelves (>180 °) could be seen in all operated eyes at the last follow-up. A subsequent study206 using the same device showed complete success of only 58% and qualified success of 81% at 18 months in JOAG patients. Another similar device (Trab360™, Sight Sciences, Menlo Park CA, USA) showed 83% success at mean 16 months follow-up, but only six JOAG eyes were studied.12 Ab interno trabeculotomy using prolene suture cannulation is a similar cost-effective alternative that has also been attempted in a few JOAG eyes.21
Microhook (Inami & Co. Ltd, Tokyo, Japan) trabeculotomy has been performed in a few JOAG eyes, but a series91 showed severe hypotony with annular ciliochoroidal detachment in two eyes, attributed to excessive uveoscleral outflow or development of cyclodialysis cleft. The authors proposed that young age and JOAG can be important predictors to risk of ciliochoroidal detachment after microhook ab interno trabeculotomy.
11.3.1.3. Ab interno trabeculectomy
Using a trabectome (NeoMedix Corporation, Tustin, CA) to perform ab interno trabeculectomy, a case of JOAG showed IOP and VF stabilization without need for topical glaucoma medications for up to 4.5 years.147 A subsequent larger case series, however, showed survival rate of 73% at 12 months post-op, with no significant drop in the number of medications.13
11.3.1.4. XEN implantation
Usage of the XEN implant has been reported in two JOAG cases; one with intraoperative mitomycin C (MMC) showed better result,106 while the other in a pregnant woman required several needlings with 5-fluorouracil post-partum to combat early bleb encystment.226 A few JOAG cases have been included as a part of large-scale XEN studies, but their individual outcomes are unavailable.167, 169
11.3.2. Invasive surgeries
11.3.2.1. Ab-externo Trabeculotomy
Trabeculotomy has shown high success rates in JOAG eyes in the long term.63, 105 With the advent of the illuminated micro catheter, the procedure of trabeculotomy became more predictable and safe. Complete 360-degree Schlemm canal cannulation was possible in 69–100% of JOAG eyes using this technique.42, 161 Two studies showed encouraging results with 90–100% success at mean 15–43 months follow-up,42, 161 while another study showed only 53% success at 36 months follow-up, with no significant drop in the number of topical glaucoma medicines.120 Transient hyphema developed in up to 20% of eyes.42 Another variation was to combine 180 ° trabeculotomy with sinusotomy (microtrephination of scleral flap over the Schlemm canal); however, it did not show any added benefit in JOAG eyes.110
11.3.2.2. Nonpenetrating glaucoma surgeries
To obviate the complications of penetrating surgeries, nonpenetrating surgeries were tried. Viscocanalostomy showed 80% overall success at the end of 3 years, though there were trabeculo-Descemet-membrane micro- and macroperforation in 10%.179 Nonperforating sclerectomy with intrascleral sodium hyaluronate implant has also been tried in JOAG eyes.109
11.3.2.3. Trabeculectomy
The success of trabeculectomy in JOAG has been variable, ranging from 50–90% at 3 years.154, 195, 219 The complete success rates have been reported to be 80% in 5 years154 and 52% at 10 years.172
Though the results of trabeculectomy may appear encouraging, they also carry a higher risk of complications. Early bleb failure necessitating needling developed in 20% of cases treated with MMC,49 and younger age at onset has been identified as a risk factor for failure.154 On the other hand, hypotony also occurred in 20–25% with the usage of MMC in JOAG eyes.49, 195 A case of hypotony and subsequent maculopathy 14 years after trabeculectomy was reported in a JOAG patient with high myopia. This was hypothesized to be the result of progressive scleral thinning and loss of rigidity with age, leading to biomechanical weakening and collapse of the scleral wall.99 Chen and coworkers36 proposed that prophylactic sclerotomy could be combined with standard trabeculectomy in high risk JOAG cases to reduce the chances of massive choroidal effusion or suprachoroidal hemorrhage.
11.3.2.4. Glaucoma drainage devices (GDD)
In an attempt to obtain better long-term IOP control and safety profiles, GDD have been tried in JOAG. At 1 year, Ahmed and Baerveldt drainage implants have shown 90.7% success in JOAG eyes, with postoperative complication rates of 9.3%.113 A long-term study of Molteno implants in juvenile glaucomas showed 83% success at mean 12 years; however, the post-operative complication rate was high in this study, with 53% of cases requiring one or more surgical interventions later.6
12. Long-term outcomes and prognosis
There are only a few studies looking at progression rates among JOAG patients. Gupta and coworkers75 showed that JOAG patients from India exhibited 4.7% structural progression and 7.1% functional progression over 5 years. On the other hand, Kwun and coworkers112 showed 28% functional progression in Korean patients over a 7-year period. A deterioration of −0.9% per year on the VF index (VFI) was reported among treated JOAG patients.68 A subsequent study11 divided functional progression as fast (progression rate <−1 to −2 dB/yr) and catastrophic (progression rate <−2 dB/yr), and identified 3.1% and 1.5% of JOAG eyes to fall within these groups respectively. Though the progression is similar to other primary glaucomas, their younger age portends that a larger proportion could end up with visual impairement.11 Higher IOP at last hospital visit and long-term IOP fluctuations have been identified as significant risk factors for progression.68, 112 Another recent study66 found higher degrees of myopia among JOAG patients to be a significant risk factor for progression. The glaucoma progressors also showed significant progression of myopia in this study.
Among untreated preperimetric JOAG patients from Korea, Bak and coworkers reported 43% overall progression at 5 years, 39% by structural criteria and 5% by functional criteria.18 Older age at presentation, baseline lamina pore visibility, baseline temporal raphe sign, and greater pattern standard deviation at baseline were identified as risk factors.
Among JOAG patients, reversal of structural and functional parameters has also been observed. With surgical control of IOP, reversal of cupping occurred in up to 17% of JOAG cases.75, 117, 187 This was proposed to be dependent upon the degree of IOP reduction, age at presentation, compliance of lamina cribrosa and composition of tissues supporting the retinal ganglion cells;117 however, the changes in RNFL have not been consistent. While a report noted supportive increase in RNFL thickness and reversal of VF defect,117 another case showed decrease in RNFL, as well as macular ganglion cell complex thickness, over a 3-year period despite a reversal of optic disc cupping after lowering of IOP.187 It has also been suggested that, though cupping may show reversal after surgical lowering of IOP, this may not reflect a truly healthy rim, but just proliferative glial tissue.50
13. Visual disability and quality of life
Visual loss occurring from glaucoma results in substantial decrease in patient utility values and quality of life.61 The patient’s socioeconomic status (SES) also influences the disease outcome, clinical management, and medication expenses.33 Lower SES and poor health literacy among JOAG patients, who are generally not targeted in glaucoma screening programs, have been associated with severe disease at presentation.81
About two-thirds of Nigerian JOAG patients had a visual acuity of 6/18 or worse in their better eye at the time of presentation.108 Among Indian JOAG patients presenting to a tertiary care facility, 15% were bilaterally blind, and an additional 21% were unilaterally blind by World Health Organization criteria.70 In Cameroon, the prevalence of bilateral blindness among JOAG patients was 33% at presentation. The affected eye was blind in 50% of patients with unilateral glaucoma. School eye health programs and community-based case detection could aid in early detection, reducing morbidity arising from this disease.108 Gupta and coworkers68 showed that the projected risk of bilateral blindness was 10% over a JOAG patient’s lifetime even under treatment. This risk could be dependent upon ethnicity. Quality of life of glaucoma patients, as of others, is directly related to the degree of visual acuity loss in the better eye.79 In a study, Gupta and coworkers69 found JOAG patients to have a better quality of life compared to adult-onset POAG with the same level of visual disability, primarily because the younger patients had better social support systems and fewer co-morbidities.
14. Conclusion
JOAG is a relatively uncommon subset of the primary glaucomas that has an important impact on the quality of life of young adults who form a productive part of the family and society. The prevalence and inheritance patterns are widely variable in different populations. There may be many genetic causative loci, and only a few have been discovered and understood. Further research on genetic etiology of JOAG will likely identify new and interesting causative genes that may allow a better classification of the disease. Surgical treatment is needed for many JOAG patients, and although MIGS procedures are being tried, longer term studies are needed to establish their efficacy in this subgroup of patients. Early identification of those at risk and treatment at an early stage remain key to avoid irreversible visual disability.
15. Method of literature search
Literature search was performed in PubMed Medline using the following keywords: juvenile open angle glaucoma, JOAG, juvenile ocular hypertension, juvenile normal tension glaucoma, MYOC glaucoma, childhood glaucoma surgery and pediatric glaucoma surgery. Articles published up to December 2020 were reviewed and suitable ones between 1990 and 2020 were mainly included. A few selected articles published before 1990 have been included for historical purposes. Reference lists of included articles were also reviewed to identify additional relevant papers. Articles in English were mainly considered. If non-English articles possessed an English abstract, they were also reviewed. Case reports were included only if they contributed to new information about the disease.
15.1. Limitation
A uniform diagnostic criterion for JOAG was lacking in many of the studies, and sometimes, adolescent secondary glaucomas were included under the umbrella term ‘juvenile glaucoma’. This discrepancy made comparison of data difficult between the studies, and generalized the outcomes in some.
Disclosures
The authors report no proprietary or commercial interest in any product or company mentioned or concept discussed in this article. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
H Selvan, S Gupta, V Gupta: Nothing to disclose
J L Wiggs: Partially supported by NIH NEI EY031820 to study the genetic etiologies of childhood glaucoma. Consultant for Allergan, Editas, Maze, RegenXbio and has received research support from Aerpio.
REFERENCES
- 1.Abdelrahman AM, Eltanamly RM, Elsanabary Z, Hassan LM. Optical coherence tomography angiography in juvenile open angle glaucoma: correlation between structure and perfusion. Int Ophthalmol. 2021;41:883–9. doi: 10.1007/s10792-020-01643-7. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Amero KK, Morales J, Aljasim LA, Edward DP. CYP1B1 mutations are a major contributor to juvenile-onset open angle glaucoma in saudi arabia. Ophthalmic Genet. 2015;36:184–7. doi: 10.3109/13816810.2013.841961. [DOI] [PubMed] [Google Scholar]
- 3.Black AC, Jones S, Yanovitch TL, Enyedi LB, Stinnett SS, Freedman SF. Latanoprost in pediatric glaucoma–pediatric exposure over a decade. J AAPOS. 2009;13:558–62. doi: 10.1016/j.jaapos.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Acharya M, Mookherjee S, Bhattacharjee A, Bandyopadhyay AK, Daulat Thakur SK, Bhaduri G, Sen A, Ray K. Primary role of CYP1B1 in Indian juvenile-onset POAG patients. Mol Vis. 2006;12:399–404. [PubMed] [Google Scholar]
- 5.Adiarti R, Ekantini R, Agni AN, Wong TY, Sasongko MB. Retinal arteriolar narrowing in young adults with glaucomatous optic disc. J Glaucoma. 2018;27:699–702. doi: 10.1097/IJG.0000000000000997. [DOI] [PubMed] [Google Scholar]
- 6.Ah-Chan JJ, Molteno ACB, Bevin TH, Herbison P. Otago glaucoma surgery outcome study: follow-up of young patients who underwent molteno implant surgery. Ophthalmology. 2005;112:2137–42. doi: 10.1016/j.ophtha.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Al Obeidan SA, Dewedar A, Osman EA, Mousa A. The profile of glaucoma in a tertiary ophthalmic university center in riyadh, saudi arabia. Saudi J Ophthalmol. 2011;25:373–9. doi: 10.1016/j.sjopt.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allingham RR, Damji KF, Freedman SF, Moroi SE, Rhee DJ, Shields MB. Shields textbook of glaucoma. 6th Ed. Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 9.Alward WL. The genetics of open-angle glaucoma: the story of GLC1A and myocilin. Eye Lond Engl. 2000;14:429–36. doi: 10.1038/eye.2000.127. [DOI] [PubMed] [Google Scholar]
- 10.Alward WL, Fingert JH, Coote MA, Johnson AT, Lerner SF, Junqua D, Durcan FJ, McCartney PJ, Mackey DA, Sheffield VC, Stone EM. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A). N Engl J Med. 1998;338:1022–7. [DOI] [PubMed] [Google Scholar]
- 11.Anderson AJ, Chaurasia AK, Sharma A, Gupta A, Gupta S, Khanna A, Gupta V. Comparison of Rates of Fast and Catastrophic Visual Field Loss in Three Glaucoma Subtypes. Invest Ophthalmol Vis Sci. 2019;60:161–7. [DOI] [PubMed] [Google Scholar]
- 12.Areaux RG, Grajewski AL, Balasubramaniam S, Brandt JD, Jun A, Edmunds B, Shyne MT, Bitrian E. Trabeculotomy Ab Interno With the Trab360 Device for Childhood Glaucomas. Am J Ophthalmol. 2020;209:178–86. [DOI] [PubMed] [Google Scholar]
- 13.Arora S, Maeda M, Francis B, Maeda M, Sit AJ, Mosaed S, Nazarali S, Damji KF. Efficacy and safety of ab interno trabeculectomy in juvenile open-angle glaucoma. Can J Ophthalmol. 2018;53:482–6. [DOI] [PubMed] [Google Scholar]
- 14.Arruda WO, Comerlato EA, Scola RH, Silvado CE, Werneck LC. Hereditary motor and sensory neuropathy with congenital glaucoma. Report on a family. Arq Neuropsiquiatr. 1999;57:190–4. doi: . [DOI] [PubMed] [Google Scholar]
- 15.Avisar I, Lusky M, Robinson A, Shohat M, Dubois S, Raymond V, Gaton DD. The novel Y371D myocilin mutation causes an aggressive form of juvenile open-angle glaucoma in a Caucasian family from the Middle-East. Mol Vis. 2009;15:1945–50. [PMC free article] [PubMed] [Google Scholar]
- 16.Azzedine H, Bolino A, Taïeb T, Birouk N, Di Duca M, Bouhouche A, Benamou S, Mrabet A, Hammadouche T, Chkili T, Gouider R, Ravazzolo R, Brice A, Laporte J, LeGuern E. Mutations in MTMR13, a new pseudophosphatase homologue of MTMR2 and Sbf1, in two families with an autosomal recessive demyelinating form of Charcot-Marie-Tooth disease associated with early-onset glaucoma. Am J Hum Genet. 2003;72:1141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bachman JA. Juvenile onset primary open-angle glaucoma: three case studies and review. J Am Optom Assoc. 1998;69:785–95. [PubMed] [Google Scholar]
- 18.Bak E, Kim YW, Ha A, Kim YK, Park KH, Jeoung JW. Preperimetric open angle glaucoma with young age of onset: natural clinical course and risk factors for progression. Am J Ophthalmol. 2020;216:121–31. doi: 10.1016/j.ajo.2020.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Barkana Y, Shoshany N, Almer Z, Pras E. Familial juvenile normal-tension glaucoma with anterior segment dysgenesis: a clinical report of a new phenotype. J Glaucoma. 2013;22:510–14. doi: 10.1097/IJG.0b013e318255dbcf. [DOI] [PubMed] [Google Scholar]
- 20.Bashir R, Tahir H, Yousaf K, Naz S, Naz S. Homozygous p.G61E mutation in a consanguineous Pakistani family with co-existence of juvenile-onset open angle glaucoma and primary congenital glaucoma. Gene. 2015;570:295–8. doi: 10.1016/j.gene.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Baumgarten S, Kürten D, Lohmann T, Schellhase H, Plange N, Walter P, Fuest M. Outcomes of 360 ° suture trabeculotomy after unsuccessful canaloplasty. Graefes Arch Clin Exp Ophthalmol. 2020;258:387–93. [DOI] [PubMed] [Google Scholar]
- 22.Bayat B, Yazdani S, Alavi A, Chiani M, Chitsazian F, Tusi BK, Suri F, Narooie-Nejhad M, Sanati MH, Elahi E. Contributions of MYOC and CYP1B1 mutations to JOAG. Mol Vis. 2008;14:508–17. [PMC free article] [PubMed] [Google Scholar]
- 23.Belkin A, Chen T, DeOliveria AR, Johnson SM, Ramulu PY, Buys YM. American Glaucoma Society and the Canadian Glaucoma Society. A Practical Guide to the Pregnant and Breastfeeding Patient with Glaucoma. Ophthalmol Glaucoma. 2020;3:79–89. [DOI] [PubMed] [Google Scholar]
- 24.Bennett SR, Alward WL, Folberg R. An autosomal dominant form of low-tension glaucoma. Am J Ophthalmol. 1989;108:238–44. doi: 10.1016/0002-9394(89)90112-8. [DOI] [PubMed] [Google Scholar]
- 25.Birla S, Gupta D, Somarajan BI, Gupta S, Chaurasia AK, Kishan A, Gupta V. Classifying juvenile onset primary open angle glaucoma using cluster analysis. Br J Ophthalmol. 2020;104:827–35. [DOI] [PubMed] [Google Scholar]
- 26.Booth AP, Anwar R, Chen H, Churchill AJ, Jay J, Polansky J, Nguyen T, Markham AF. Genetic screening in a large family with juvenile onset primary open angle glaucoma. Br J Ophthalmol. 2000;84:722–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouhenni RA, Ricker I, Hertle RW. Prevalence and clinical characteristics of childhood glaucoma at a tertiary care children’s Hospital. J Glaucoma. 2019;28:655–9. doi: 10.1097/IJG.0000000000001259. [DOI] [PubMed] [Google Scholar]
- 28.Braghini CA, Neshich IAP, Neshich G, Soardi FC, de Mello MP, Costa VP, de Vasconcellos JPC, de Melo MB. New mutation in the myocilin gene segregates with juvenile-onset open-angle glaucoma in a Brazilian family. Gene. 2013;523:50–7. [DOI] [PubMed] [Google Scholar]
- 29.Bruttini M, Longo I, Frezzotti P, Ciappetta R, Randazzo A, Orzalesi N, Fumagalli E, Caporossi A, Frezzotti R, Renieri A. Mutations in the myocilin gene in families with primary open-angle glaucoma and juvenile open-angle glaucoma. Arch Ophthalmol Chic Ill 1960. 2003;121:1034–8. [DOI] [PubMed] [Google Scholar]
- 30.Budde WM, Jonas JB. Family history of glaucoma in the primary and secondary open-angle glaucomas. Graefes Arch Clin Exp Ophthalmol. 1999;237:554–7. doi: 10.1007/s004170050278. [DOI] [PubMed] [Google Scholar]
- 31.Bui TNPT, Corap A, Bygum A. Cutis marmorata telangiectatica congenita: a literature review. Orphanet J Rare Dis. 2019;14. doi: 10.1186/s13023-019-1229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campos-Mollo E, Sánchez-Sánchez F, López-Garrido MP, López-Sánchez E, López-Martínez F, Escribano J. MYOC gene mutations in Spanish patients with autosomal dominant primary open-angle glaucoma: a founder effect in southeast Spain. Mol Vis. 2007;13:1666–73. [PubMed] [Google Scholar]
- 33.Chakravarti T The association of socioeconomic status with severity of glaucoma and the impacts of both factors on the costs of glaucoma medications: a cross-sectional study in West Bengal, India. J Ocul Pharmacol Ther. 2018;34:442–51. doi: 10.1089/jop.2017.0135. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Wang X, Hu X, Sun X. The evaluation of juvenile ocular hypertension by optical coherence tomography angiography. BMC Ophthalmol. 2020;20:423. doi: 10.1186/s12886-020-01641-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Jiang D, Wan B, Yu L, Sun X. Presymptomatic genetic diagnosis for consulters from a large Chinese family with juvenile open angle glaucoma. Mol Vis. 2006;12:360–6. [PubMed] [Google Scholar]
- 36.Chen YH, Wen W, Wu N, Ling ZH, Chen JY, Chen Q, Sun XH. [The effect of trabeculectomy combined with prophylactic sclerotomy as a treatment of late stage juvenile open angle glaucoma and primary congenital glaucoma patients: a primary observational study]. Zhonghua Yan Ke Za Zhi Chin J Ophthalmol. 2019;55:347–54. [DOI] [PubMed] [Google Scholar]
- 37.Cho H-K, Kee C Population-based glaucoma prevalence studies in Asians. Surv Ophthalmol. 2014;59:434–47. doi: 10.1016/j.survophthal.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Collantes E, Delfin M, Fan BJ, Tooregosa J, Siguan-Bell C, Florcruz N, Martinez J, Masna-Hidalgo B, Guzman V, Anotado-Flores J, Levina F, Hernandez S, Collantes A, Sibulo M, Rong S, Wiggs J. EFEMP1 rare variants cause juvenile-onset open angle glaucoma in families from the Philippines. Hum Mutat. 2021. Authorea preprint. [Google Scholar]
- 39.Cordovez JA, Capasso J, Lingao MD, Sadagopan KA, Spaeth GL, Wasserman BN, Levin AV. Ocular manifestations of 22q11.2 microduplication. Ophthalmology. 2014;121:392–8. [DOI] [PubMed] [Google Scholar]
- 40.Costagliola C, Campa C, Parmeggiani F, Incorvaia C, Perri P, D’Angelo S, Lamberti G, Sebastiani A. Effect of 2% dorzolamide on retinal blood flow: a study on juvenile primary open-angle glaucoma patients already receiving 0.5% timolol. Br J Clin Pharmacol. 2007;63:376–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Criscione J, Ji W, Jeffries L, McGrath JM, Soloway S, Pusztai L, Lakhani S. Identification of a novel MYOC variant in a Hispanic family with early-onset primary open-angle glaucoma with elevated intraocular pressure. Cold Spring Harb Mol Case Stud. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dao JB, Sarkisian SR, Freedman SF. Illuminated microcatheter-facilitated 360-degree trabeculotomy for refractory aphakic and juvenile open-angle glaucoma. J Glaucoma. 2014;23:449–54. doi: 10.1097/IJG.0b013e31829484df. [DOI] [PubMed] [Google Scholar]
- 43.Das J, Bhomaj S, Chaudhuri Z, Sharma P, Negi A, Dasgupta A. Profile of glaucoma in a major eye hospital in North India. Indian J Ophthalmol. 2001;49:25. [PubMed] [Google Scholar]
- 44.De Rousseau CJ, Bito LZ. Intraocular pressure of rhesus monkeys (Macaca mulatta). II.Juvenile ocular hypertension and its apparent relationship to ocular growth. Exp Eye Res. 1981;32:407–17. doi: 10.1016/s0014-4835(81)80020-6. [DOI] [PubMed] [Google Scholar]
- 45.Di Matteo F, Bettin P, Ferrari G, Fiori M, Ciampi C, Manfredini E, Rabiolo A, Bandello F. 22q11.2 microduplication syndrome and juvenile glaucoma. Ophthalmic Genet. 2018;39:532–8. [DOI] [PubMed] [Google Scholar]
- 46.Elgin U, Şen E, Uzel M, Yılmazbaş P. Comparison of refractive status and anterior segment parameters of juvenile open-angle glaucoma and normal subjects. Turk J Ophthalmol. 2018;48:295–8. doi: 10.4274/tjo.68915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elitt MS, Tamburro JE, Moran RT, Traboulsi E. Cutis marmorata telangiectatica congenita: a focus on its diagnosis, ophthalmic anomalies, and possible etiologic factors. Ophthalmic Genet. 2020;41:101–7. doi: 10.1080/13816810.2020.1744018. [DOI] [PubMed] [Google Scholar]
- 48.Ellong A, Ebana Mvogo C, Nyouma Moune E, Bella-Hiag A. [Juvenile glaucoma in Cameroon]. Bull Soc Belge Ophtalmol. 2007:69–77. [PubMed] [Google Scholar]
- 49.El-Sayyad F, El-Saied HMA, Abdelhakim MASE. Trabeculectomy with ologen versus mitomycin c in juvenile open-angle glaucoma: a 1-year study. Ophthalmic Res. 2017;57:230–8. doi: 10.1159/000456719. [DOI] [PubMed] [Google Scholar]
- 50.Ely AL, El-Dairi MA, Freedman SF. Cupping reversal in pediatric glaucoma–evaluation of the retinal nerve fiber layer and visual field. Am J Ophthalmol. 2014;158:905–15. doi: 10.1016/j.ajo.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 51.Enyedi LB, Freedman SF. Latanoprost for the treatment of pediatric glaucoma. Surv Ophthalmol. 2002;47(Suppl 1):S129–32. doi: 10.1016/s0039-6257(02)00303-x. [DOI] [PubMed] [Google Scholar]
- 52.Enyedi LB, Freedman SF, Buckley EG. The effectiveness of latanoprost for the treatment of pediatric glaucoma. J AAPOS. 1999;3:33–9. doi: 10.1016/s1091-8531(99)70092-3. [DOI] [PubMed] [Google Scholar]
- 53.Falardeau J, Lobb BM, Golden S, Maxfield SD, Tanne E. The use of acetazolamide during pregnancy in intracranial hypertension patients. J Neuro-Ophthalmol. 2013;33:9–12. doi: 10.1097/WNO.0b013e3182594001. [DOI] [PubMed] [Google Scholar]
- 54.Fan BJ, Leung DYL, Wang DY, Gobeil S, Raymond V, Tam POS, Lam DSC, Pang CP. Novel myocilin mutation in a Chinese family with juvenile-onset open-angle glaucoma. Arch Ophthalmol Chic Ill. 2006;124:102–6 1960. [DOI] [PubMed] [Google Scholar]
- 55.Fernández-Martínez L, Letteboer S, Mardin CY, Weisschuh N, Gramer E, Weber BH, Rautenstrauss B, Ferreira PA, Kruse FE, Reis A, Roepman R, Pasutto F. Evidence for RPGRIP1 gene as risk factor for primary open angle glaucoma. Eur J Hum Genet. 2011;19:445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fingert JH, Robin AL, Stone JL, Roos BR, Davis LK, Scheetz TE, Bennett SR, Wassink TH, Kwon YH, Alward WLM, Mullins RF, Sheffield VC, Stone EM. Copy number variations on chromosome 12q14 in patients with normal tension glaucoma. Hum Mol Genet. 2011;20:2482–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fingert JH, Stone EM, Sheffield VC, Alward WLM. Myocilin glaucoma. Surv Ophthalmol. 2002;47:547–61. doi: 10.1016/s0039-6257(02)00353-3. [DOI] [PubMed] [Google Scholar]
- 58.Fledelius H Optic disc cupping and prematurity. Large cups as a possible low birth weight sequel. Acta Ophthalmol (Copenh). 1978;56:563–73. doi: 10.1111/j.1755-3768.1978.tb01369.x. [DOI] [PubMed] [Google Scholar]
- 59.Furuyoshi N, Furuyoshi M, Futa R, Gottanka J. Lütjen-Drecoll E. Ultrastructural changes in the trabecular meshwork of juvenile glaucoma. Ophthalmologica. 1997;21:140–6. doi: 10.1159/000310781. [DOI] [PubMed] [Google Scholar]
- 60.Goel S, Ganger A, Gupta V. Bilateral juvenile onset primary open-angle glaucoma among keratoconus patients. J Glaucoma. 2015;24:e25–7. doi: 10.1097/IJG.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 61.Goldwyn R, Waltman SR, Becker B. Primary open-angle glaucoma in adolescents and young adults. Arch Ophthalmol Chic Ill 1960. 1970;84:579–82. [DOI] [PubMed] [Google Scholar]
- 62.Graff C, Urbak SF, Jerndal T, Wadelius C. Confirmation of linkage to 1q21–31 in a Danish autosomal dominant juvenile-onset glaucoma family and evidence of genetic heterogeneity. Hum Genet. 1995;96:285–9. doi: 10.1007/BF00210408. [DOI] [PubMed] [Google Scholar]
- 63.Groh MJ, Behrens A, Händel A, Küchle M. [Mid- and long-term results after trabeculectomy in patients with juvenile and late-juvenile open-angle glaucoma]. Klin Monatsbl Augenheilkd. 2000;217:71–6. doi: 10.1055/s-2000-10387. [DOI] [PubMed] [Google Scholar]
- 64.Groth SL, Donahue SP, Reddy A, Sarma A, Wushensky C. Periventricular leukomalacia in patients with pseudo-glaucomatous cupping. Am J Ophthalmol. 2020;211:31–41. doi: 10.1016/j.ajo.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 65.Grover DS, Smith O, Fellman RL, Godfrey DG, Butler MR, Montes de Oca I, Feuer WJ. Gonioscopy assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy for the treatment of primary congenital glaucoma and juvenile open angle glaucoma. Br J Ophthalmol. 2015;99:1092–6. [DOI] [PubMed] [Google Scholar]
- 66.Gupta S, Singh A, Mahalingam K, Selvan H, Gupta P, Pandey S, Somarajan BI, Gupta V. Myopia and glaucoma progression among patients with juvenile onset open angle glaucoma: A retrospective follow up study. Ophthalmic Physiol Opt. 2021;41:475–85. [DOI] [PubMed] [Google Scholar]
- 67.Gupta V, Chaurasia AK, Gupta S, Gorimanipalli B, Sharma A, Gupta A. In vivo analysis of angle dysgenesis in primary congenital, juvenile, and adult-onset open angle glaucoma. Invest Ophthalmol Vis Sci. 2017;58:6000–5. doi: 10.1167/iovs.17-22695. [DOI] [PubMed] [Google Scholar]
- 68.Gupta V, Devi KS, Kumar S, Pandey RM, Sihota R, Sharma A, Gupta S. Risk of perimetric blindness among juvenile glaucoma patients. Ophthalmic Physiol Opt. 2015;35:206–11. [DOI] [PubMed] [Google Scholar]
- 69.Gupta V, Dutta P, Ov M, Kapoor KS, Sihota R, Kumar G. Effect of glaucoma on the quality of life of young patients. Invest Ophthalmol Vis Sci. 2011;52:8433–7. doi: 10.1167/iovs.11-7551. [DOI] [PubMed] [Google Scholar]
- 70.Gupta V, Ganesan VL, Kumar S, Chaurasia AK, Malhotra S, Gupta S. Visual disability among juvenile open-angle glaucoma patients. J Glaucoma. 2018;27:e87–9. doi: 10.1097/IJG.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 71.Gupta V, Ghosh S, Sujeeth M, Chaudhary S, Gupta S, Chaurasia AK, Sihota R, Gupta A, Kapoor KS. Selective laser trabeculoplasty for primary open-angle glaucoma patients younger than 40 years. Can J Ophthalmol. 2018;53:81–5. [DOI] [PubMed] [Google Scholar]
- 72.Gupta V, Gupta S, Dhawan M, Sharma A, Kapoor KS. Sihota R. Extent of asymmetry and unilaterality among juvenile onset primary open angle glaucoma patients. Clin Experiment Ophthalmol. 2011;39:633–8. doi: 10.1111/j.1442-9071.2011.02522.x. [DOI] [PubMed] [Google Scholar]
- 73.Gupta V, James MK, Singh A, Kumar S, Gupta S, Sharma A, Sihota R, Kennedy DJ. Differences in Optic Disc Characteristics of Primary Congenital Glaucoma, Juvenile, and Adult Onset Open Angle Glaucoma Patients. J Glaucoma. 2016;25:239–43. [DOI] [PubMed] [Google Scholar]
- 74.Gupta V, Markan A, Somarajan BI, Sihota R, Gupta A, Gupta S, Sharma A. Phenotypic differences between familial versus non-familial Juvenile onset open angle glaucoma patients. Ophthalmic Genet. 2018;39:63–7. [DOI] [PubMed] [Google Scholar]
- 75.Gupta V, Ov M, Rao A, Sharma A, Sihota R. Long-term structural and functional outcomes of therapy in juvenile-onset primary open-angle glaucoma: a five-year follow-up. Ophthalmologica. 2012;228:19–25. doi: 10.1159/000334033. [DOI] [PubMed] [Google Scholar]
- 76.Gupta V, Somarajan B i, Gupta S, Chaurasia A k, Kumar S, Dutta P, Gupta V, Sharma A, Tayo B o, Nischal K. The inheritance of juvenile onset primary open angle glaucoma. Clin Genet. 2017;92:134–42. [DOI] [PubMed] [Google Scholar]
- 77.Gupta V, Somarajan BI, Gupta S, Walia GK, Singh A, Sofi R, Chaudhary RS, Sharma A. The mutational spectrum of Myocilin gene among familial versus sporadic cases of Juvenile onset open angle glaucoma. Eye Lond Engl. 2021;35:400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gupta V, Somarajan BI, Walia GK, Kaur J, Kumar S, Gupta S, Chaurasia AK, Gupta D, Kaushik A, Mehta A, Gupta V, Sharma A. Role of CYP1B1, p.E229K and p.R368H mutations among 120 families with sporadic juvenile onset open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2018;256:355–62. [DOI] [PubMed] [Google Scholar]
- 79.Gupta V, Srinivasan G, Mei SS, Gazzard G, Sihota R, Kapoor KS. Utility values among glaucoma patients: an impact on the quality of life. Br J Ophthalmol. 2005;89:1241–4. doi: 10.1136/bjo.2005.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gupta V, Srivastava RM, Rao A, Mittal M, Fingert J. Clinical correlates to the goniodysgensis among juvenile-onset primary open-angle glaucoma patients. Graefes Arch Clin Exp Ophthalmol. 2013;251:1571–6. doi: 10.1007/s00417-013-2262-2. [DOI] [PubMed] [Google Scholar]
- 81.Gupta V, Srivastava RM, Sihota R, Kaur J, Kumar S, Singh D. Determinants of severity at presentation among young patients with early onset glaucoma. Indian J Ophthalmol. 2013;61:546–51. doi: 10.4103/0301-4738.121064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van der Heide CJ, Alward WLM, Flamme-Wiese M, Riker M, Syed NA, Anderson MG, Carter K, Kuehn MH, Stone EM, Mullins RF, Fingert JH. Histochemical Analysis of Glaucoma Caused by a Myocilin Mutation in a Human Donor Eye. Ophthalmol Glaucoma. 2018;1:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van der Heide CJ, Miller MA, Fingert JH. Chapter 7 - Early-onset glaucoma.Gao XR, editor. Genetics and Genomics of Eye Disease. Academic Press; 2020. p. 95–116. doi: 10.1016/B978-0-12-816222-4.00007-1. [DOI] [Google Scholar]
- 84.Hewitt AW, Mackey DA, Craig JE. Myocilin allele-specific glaucoma phenotype database. Hum Mutat. 2008;29:207–11. doi: 10.1002/humu.20634. [DOI] [PubMed] [Google Scholar]
- 85.Hoffmann J, Mollan SP, Paemeleire K, Lampl C, Jensen RH, Sinclair AJ. European headache federation guideline on idiopathic intracranial hypertension. J Headache Pain. 2018;19. doi: 10.1186/s10194-018-0919-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horns DJ, Bellows AR, Hutchinson BT, Allen RC. Argon laser trabeculoplasty for open angle glaucoma: a retrospective study of 380 eyes. Trans Ophthalmol Soc U K. 1983;103:288–96. [PubMed] [Google Scholar]
- 87.Huang C, Xie L, Wu Z, Cao Y, Zheng Y, Pang C-P, Zhang M. Detection of mutations in MYOC, OPTN, NTF4, WDR36 and CYP1B1 in Chinese juvenile onset open-angle glaucoma using exome sequencing. Sci Rep. 2018;8:4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang X, Li M, Guo X, Li S, Xiao X, Jia X, Liu X, Zhang Q. Mutation analysis of seven known glaucoma-associated genes in Chinese patients with glaucoma. Invest Ophthalmol Vis Sci. 2014;55:3594–602. [DOI] [PubMed] [Google Scholar]
- 89.Hulsman CAA, De Jong PTVM, Lettink M, Van Duijn CM, Hofman A, Bergen AAB. Myocilin mutations in a population-based sample of cases with open-angle glaucoma: the Rotterdam Study. Graefes Arch Clin Exp Ophthalmol. 2002;240:468–74. doi: 10.1007/s00417-002-0455-1. [DOI] [PubMed] [Google Scholar]
- 90.Iliev ME, Bodmer S, Gallati S, Lanz R, Sturmer J, Katsoulis K, Wolf S, Trittibach P, Sarra GM. Glaucoma phenotype in a large Swiss pedigree with the myocilin Gly367Arg mutation. Eye Lond Engl. 2008;22:880–8. [DOI] [PubMed] [Google Scholar]
- 91.Ishida A, Mochiji M, Manabe K, Matsuoka Y, Tanito M. Persistent hypotony and annular ciliochoroidal detachment after microhook ab interno trabeculotomy. J Glaucoma. 2020;29:807–12. doi: 10.1097/IJG.0000000000001560. [DOI] [PubMed] [Google Scholar]
- 92.Jain A, Zode G, Kasetti RB, Ran FA, Yan W, Sharma TP, Bugge K, Searby CC, Fingert JH, Zhang F, Clark AF, Sheffield VC. CRISPR-Cas9-based treatment of myocilin-associated glaucoma. Proc Natl Acad Sci U S A. 2017;114:11199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jean-Gallois null[Two cases of juvenile ocular hypertension; verification and comparison tonometers; total peripheral visual field]. Bull Soc Ophtalmol Fr. 1948;27:13–19. [PubMed] [Google Scholar]
- 94.Jia L-Y, Gong B, Pang C-P, Huang Y, Lam DS-C, Wang N, Yam GH-F. Correction of the disease phenotype of myocilin-causing glaucoma by a natural osmolyte. Invest Ophthalmol Vis Sci. 2009;50:3743–9. [DOI] [PubMed] [Google Scholar]
- 95.Johnson AT, Richards JE, Boehnke M, Stringham HM, Herman SB, Wong DJ, Lichter PR. Clinical phenotype of juvenile-onset primary open-angle glaucoma linked to chromosome 1q. Ophthalmology. 1996;103:808–14. [DOI] [PubMed] [Google Scholar]
- 96.Johnson DH. Myocilin and glaucoma: a TIGR by the tail? Arch Ophthalmol Chic Ill 1960. 2000;118:974–8. [PubMed] [Google Scholar]
- 97.Jonas JB, Budde WM. Optic nerve head appearance in juvenile-onset chronic high-pressure glaucoma and normal-pressure glaucoma. Ophthalmology. 2000;107:704–11. doi: 10.1016/s0161-6420(99)00172-4. [DOI] [PubMed] [Google Scholar]
- 98.Jonas JB, Gründler A. Optic disc morphology in juvenile primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 1996;234:750–4. doi: 10.1007/BF00189356. [DOI] [PubMed] [Google Scholar]
- 99.Kao S-T, Lee S-H, Chen Y-C. Late-onset hypotony maculopathy after trabeculectomy in a highly myopic patient with juvenile open-angle glaucoma. J Glaucoma. 2017;26:e137–41. doi: 10.1097/IJG.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaur A, Vanita V, Singh J. Screening of CYP1B1 Arg368His as predominant mutation in North Indian primary open angle glaucoma and juvenile onset glaucoma patients. Mol Biol Res Commun. 2018;7:181–6. doi: 10.22099/mbrc.2018.30630.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kennan AM, Mansergh FC, Fingert JH, Clark T, Ayuso C, Kenna PF, Humphries P, Farrar GJ. A novel Asp380Ala mutation in the GLC1A/myocilin gene in a family with juvenile onset primary open angle glaucoma. J Med Genet. 1998;35:957–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khan AO. Genetics of primary glaucoma. Curr Opin Ophthalmol. 2011;22:347–55. doi: 10.1097/ICU.0b013e32834922d2. [DOI] [PubMed] [Google Scholar]
- 103.Khouri AS, Zhu Y, Sadek H. Ab interno trabeculectomy with the dual blade in juvenile open-angle glaucoma. Eur J Ophthalmol. 2021;31:NP43–5. doi: 10.1177/1120672119892440. [DOI] [PubMed] [Google Scholar]
- 104.Kiwaki T, Umehara F, Takashima H, Nakagawa M, Kamimura K, Kashio N, Sakamoto Y, Unoki K, Nobuhara Y, Michizono K, Watanabe O, Arimura H, Osame M. Hereditary motor and sensory neuropathy with myelin folding and juvenile onset glaucoma. Neurology. 2000;55:392–7. [DOI] [PubMed] [Google Scholar]
- 105.Kjer B, Kessing SV. Trabeculotomy in juvenile primary open-angle glaucoma. Ophthalmic Surg. 1993;24:663–8. [PubMed] [Google Scholar]
- 106.Klug E, Solá-Del Valle D. Bilateral XEN gel stent implantation in juvenile-onset open-angle glaucoma. Case Rep Ophthalmol. 2020;11:336–41. doi: 10.1159/000508391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ko Y-C, Liu CJ-L, Chou JC-K, Chen M-R, Hsu W-M, Liu J-H. Comparisons of risk factors and visual field changes between juvenile-onset and late-onset primary open-angle glaucoma. Ophthalmologica. 2002;216:27–32. doi: 10.1159/000048293. [DOI] [PubMed] [Google Scholar]
- 108.Komolafe O, Olawoye O, Fafowora O, Ashaye A, Baiyeroju AM. Demographic and clinical profile of patients with juvenile onset open angle glaucoma in southwestern Nigeria. Niger J Clin Pract. 2011;14:395–9. doi: 10.4103/1119-3077.91742. [DOI] [PubMed] [Google Scholar]
- 109.Krzywicki S, Szała E. [Non-perforating deep sclerectomy ab externo with intrascleral implant in juvenile glaucoma]. Klin Oczna. 2002;104:222–5. [PubMed] [Google Scholar]
- 110.Kubota T, Takada Y, Inomata H. Surgical outcomes of trabeculotomy combined with sinusotomy for juvenile glaucoma. Jpn J Ophthalmol. 2001;45:499–502. doi: 10.1016/s0021-5155(01)00375-6. [DOI] [PubMed] [Google Scholar]
- 111.Kuchtey J, Chowdhury UR, Uptegraft CC, Fautsch MP, Kuchtey RW. A de novo MYOC mutation detected in juvenile open angle glaucoma causes non-secretion of associated with reduced myocilin protein in aqueous humor. Eur J Med Genet. 2013;56:292–6. doi: 10.1016/j.ejmg.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kwun Y, Lee EJ, Han JC, Kee C. Clinical characteristics of Juvenile-onset open angle glaucoma. Korean J Ophthalmol. 2016;30:127–33. doi: 10.3341/kjo.2016.30.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Le PH, Nguyen M, Humphrey K-A, Klifto MR. Ahmed and baerveldt drainage implants in the treatment of juvenile open angle glaucoma. J Glaucoma. 2021;30:276–80. doi: 10.1097/IJG.0000000000001732. [DOI] [PubMed] [Google Scholar]
- 114.Lee AG, Pless M, Falardeau J, Capozzoli T, Wall M, Kardon RH. The use of acetazolamide in idiopathic intracranial hypertension during pregnancy. Am J Ophthalmol. 2005;139:855–9. doi: 10.1016/j.ajo.2004.12.091. [DOI] [PubMed] [Google Scholar]
- 115.Lee HS, Park SW, Heo H. Megalopapilla in children: a spectral domain optical coherence tomography analysis. Acta Ophthalmol (Copenh). 2015;93:e301–5. doi: 10.1111/aos.12545. [DOI] [PubMed] [Google Scholar]
- 116.Lee JY, Yoo C, Park J, Kim YY. Retinal vessel diameter in young patients with open-angle glaucoma: comparison between high-tension and normal-tension glaucoma. Acta Ophthalmol (Copenh). 2012;90:e570–1. doi: 10.1111/j.1755-3768.2011.02371.x. [DOI] [PubMed] [Google Scholar]
- 117.Leung CKS, Woo J, Tsang MK, Tse KK. Structural and functional recovery in juvenile open angle glaucoma after trabeculectomy. Eye Lond Engl. 2006;20:132–4. doi: 10.1038/sj.eye.6701819. [DOI] [PubMed] [Google Scholar]
- 118.Lichter PR, Richards JE, Boehnke M, Othman M, Cameron BD, Stringham HM, Downs CA, Lewis SB, Boyd BF. Juvenile glaucoma linked to GLCIA in a Panamanian family. Trans Am Ophthalmol Soc. 1996;94:335–46 discussion 347–351. [PMC free article] [PubMed] [Google Scholar]
- 119.Lichter PR, Richards JE, Downs CA, Stringham HM, Boehnke M, Farley FA. Cosegregation of open-angle glaucoma and the nail-patella syndrome. Am J Ophthalmol. 1997;124:506–15. doi: 10.1016/s0002-9394(14)70866-9. [DOI] [PubMed] [Google Scholar]
- 120.Lim ME, Dao JB, Freedman SF. 360-degree trabeculotomy for medically refractory glaucoma following cataract surgery and juvenile open-angle glaucoma. Am J Ophthalmol. 2017;175:1–7. doi: 10.1016/j.ajo.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 121.Lim P, Lichter PR, Higashi M, Downs CA, Richards JE. Septuagenarian’s phenotype leads to ascertainment of familial MYOC gene mutation. J Glaucoma. 2003;12:98–103. doi: 10.1097/00061198-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 122.Liu D, Chen D, Tan Q, Xia X, Jiang H, Jiang J. Outcome of selective laser trabeculoplasty in young patients with primary open-angle glaucoma and ocular hypertension. J Ophthalmol. 2020;2020:5742832. doi: 10.1155/2020/5742832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Longmuir SQ, Brodsky MC. Postnatal optic disc cupping in a non-glaucomatous infant with periventricular leukomalacia: nosologic implications. Br J Ophthalmol. 2012;96:606–7. doi: 10.1136/bjophthalmol-2011-301086. [DOI] [PubMed] [Google Scholar]
- 124.Lotufo D, Ritch R, Szmyd L, Burris JE. Juvenile glaucoma, race, and refraction. JAMA. 1989;261:249–52. doi: 10.1001/jama.1989.03420020103038. [DOI] [PubMed] [Google Scholar]
- 125.Maeda-Chubachi T, Chi-Burris K, Simons B, Brémond-Gignac D, Freedman S, Khaw PT, Wirostko B, Yan E. A6111137 Study Group. Impact of age, diagnosis, and history of glaucoma surgery on outcomes in pediatric patients treated with latanoprost. J Glaucoma. 2013;22:614–19. [DOI] [PubMed] [Google Scholar]
- 126.Makita LS, Muniz BC, Medina FMC. Ophthalmologic alterations in cutis marmorata telangiectatica congenita: a series of cases. Arq Bras Oftalmol. 2020;83:239–41. doi: 10.5935/0004-2749.20200064. [DOI] [PubMed] [Google Scholar]
- 127.Malik MA, Gupta V, Shukla S, Kaur J. Glutathione S-transferase (GSTM1, GSTT1) polymorphisms and JOAG susceptibility: A case control study and meta-analysis in glaucoma. Gene. 2017;628:246–52. doi: 10.1016/j.gene.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 128.Mansergh FC, Kenna PF, Ayuso C, Kiang AS, Humphries P, Farrar GJ. Novel mutations in the TIGR gene in early and late onset open angle glaucoma. Hum Mutat. 1998;11:244–51 3 <244::AID-HUMU10>3.0.CO;2-Z. doi: 10.1002/(SICI)1098-1004(1998)11. [DOI] [PubMed] [Google Scholar]
- 129.Markandaya M, Ramesh TK, Selvaraju V, Dorairaj SK, Prakash R, Shetty J, Kumar A. Genetic analysis of an Indian family with members affected with juvenile-onset primary open-angle glaucoma. Ophthalmic Genet. 2004;25:11–23. [DOI] [PubMed] [Google Scholar]
- 130.Marques AM, Ananina G, Costa VP, de Vasconcellos JPC, de Melo MB. Estimating the age of the p.Cys433Arg variant in the MYOC gene in patients with primary open-angle glaucoma. PloS One. 2018;13:e0207409. doi: 10.1371/journal.pone.0207409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Melamed S, Latina MA, Epstein DL. Neodymium:YAG laser trabeculopuncture in juvenile open-angle glaucoma. Ophthalmology. 1987;94:163–70. doi: 10.1016/s0161-6420(87)33481-5. [DOI] [PubMed] [Google Scholar]
- 132.Melki R, Colomb E, Lefort N, Brézin AP, Garchon H-J. CYP1B1 mutations in French patients with early-onset primary open-angle glaucoma. J Med Genet. 2004;41:647–51. doi: 10.1136/jmg.2004.020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Menaa F, Braghini CA, Vasconcellos JPCD, Menaa B, Costa VP, Figueiredo ESD, Melo MBD. Keeping an Eye on Myocilin: A Complex Molecule Associated with Primary Open-Angle Glaucoma Susceptibility. Molecules. 2011;16:5402–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Merritt JC, Reid LA, Smith R, Harris DF. Diurnal intraocular pressure in juvenile open-angle glaucoma. Ann Ophthalmol. 1979;11:253–60. [PubMed] [Google Scholar]
- 135.Michels-Rautenstrauss K, Mardin C, Wakili N, Jünemann AM, Villalobos L, Mejia C, Soley GC, Azofeifa J, Ozbey S, Naumann GOH, Reis A, Rautenstrauss B. Novel mutations in the MYOC/GLC1A gene in a large group of glaucoma patients. Hum Mutat. 2002;20:479–80. [DOI] [PubMed] [Google Scholar]
- 136.Michels-Rautenstrauss KG, Mardin CY, Budde WM, Liehr T, Polansky J, Nguyen T, Timmerman V, Van Broeckhoven C, Naumann GO, Pfeiffer RA, Rautenstrauss BW. Juvenile open angle glaucoma: fine mapping of the TIGR gene to 1q24.3-q25.2 and mutation analysis. Hum Genet. 1998;102:103–6. [DOI] [PubMed] [Google Scholar]
- 137.Millá E, Mañé B, Duch S, Hernan I, Borràs E, Planas E, Dias M de S, Carballo M, Gamundi MJ. Survey of familial glaucoma shows a high incidence of cytochrome P450, family 1, subfamily B, polypeptide 1 (CYP1B1) mutations in non-consanguineous congenital forms in a Spanish population. Mol Vis. 2013;19:1707–22. [PMC free article] [PubMed] [Google Scholar]
- 138.Miller MA, Fingert JH, Bettis DI. Genetics and genetic testing for glaucoma. Curr Opin Ophthalmol. 2017;28:133–8. doi: 10.1097/ICU.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 139.Mimivati Z, Nurliza K, Marini M, Liza-Sharmini A. Identification of MYOC gene mutation and polymorphism in a large Malay family with juvenile-onset open angle glaucoma. Mol Vis. 2014;20:714–23. [PMC free article] [PubMed] [Google Scholar]
- 140.Mimiwati Z, Mackey DA, Craig JE, MacKinnon JR, Rait JL, Liebelt JE, Ayala-Lugo R, Vollrath D, Richards JE. Nail-patella syndrome and its association with glaucoma: a review of eight families. Br J Ophthalmol. 2006;90:1505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Miranda I, Alonso MJ, Jimenez M, Tomas-Barberan S, Ferro M, Ruiz R. Cutis marmorata telangiectatica congenita and glaucoma. Ophthalmic Paediatr Genet. 1990;11:129–32. doi: 10.3109/13816819009012958. [DOI] [PubMed] [Google Scholar]
- 142.Mokbel TH, El Hefney EM, Hagras SM, ALNagdy AA, Badawi AE, Kasem MA, El Shaer SM. Childhood glaucoma profile in Dakahelia, Egypt: a retrospective study. Int J Ophthalmol. 2018;11:674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mrugacz M, Bakunowicz-Lazarczyk A. Optical coherence tomography measurement of the retinal nerve fiber layer in normal and juvenile glaucomatous eyes. Ophthalmologica. 2005;219:80–5. doi: 10.1159/000083265. [DOI] [PubMed] [Google Scholar]
- 144.Murphy CC, Khong CH, Ward WJ, Morgan WH. Late-onset pediatric glaucoma associated with cutis marmorata telangiectatica congenita managed with Molteno implant surgery: Case report and review of the literature. JAAPOS. 2007;11:519–21. doi: 10.1016/j.jaapos.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 145.Myocilin.com, Myocilin allele-specific phenotype database | Home. Last accessed February 6, 2021. Available at: http://www.myocilin.com/
- 146.Nadeau S, Gire J, Coste R, Cornand E, Denis D. [Papillary retinal nerve fiber layer thickness measurement using optical coherence tomography in children with ocular hypertension and juvenile glaucoma]. J Fr Ophtalmol. 2010;33:249–57. doi: 10.1016/j.jfo.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 147.Nazarali S, Murphy P, Damji KF. Case of Ab Interno Trabeculectomy in Juvenile Open-angle Glaucoma with 5-year Follow-up. Can J Ophthalmol. 2018;53:e39–41. doi: 10.1016/j.jcjo.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 148.NEGRÃO L, ALMENDRA L, RIBEIRO, MATOS A, GERALDO A, PINTO-BASTO J. Charcot-Marie-Tooth 4B2 caused by a novel mutation in the MTMR13/SBF2 gene in two related portuguese families. Acta Myol. 2014;33:144–8. [PMC free article] [PubMed] [Google Scholar]
- 149.Pang CP, Fan BJ, Canlas O, Wang DY, Dubois S, Tam POS, Lam DSC, Raymond V, Ritch R. A genome-wide scan maps a novel juvenile-onset primary open angle glaucoma locus to chromosome 5q. Mol Vis. 2006;12:85–92. [PubMed] [Google Scholar]
- 150.Park J, Kim M, Park CK, Chae H, Lee S, Kim Y, Jang W, Chi HY, Park H-YL, Park SH. Molecular analysis of myocilin and optineurin genes in Korean primary glaucoma patients. Mol Med Rep. 2016;14:2439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Park J-H, Yoo C, Kim YY. Peripapillary vessel density in young patients with open-angle glaucoma: comparison between high-tension and normal-tension glaucoma. Sci Rep. 2019;9. doi: 10.1038/s41598-019-55707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Park J-H, Yoo C, Park J, Kim YY. Visual field defects in young patients with open-angle glaucoma: comparison between high-tension and normal-tension glaucoma. J Glaucoma. 2017;26:541–7. doi: 10.1097/IJG.0000000000000667. [DOI] [PubMed] [Google Scholar]
- 153.Park SC, Kee C. Large diurnal variation of intraocular pressure despite maximal medical treatment in juvenile open angle glaucoma. J Glaucoma. 2007;16:164–8. doi: 10.1097/01.ijg.0000212278.03595.39. [DOI] [PubMed] [Google Scholar]
- 154.Pathania D, Senthil S, Rao HL, Mandal AK, Garudadari CS. Outcomes of trabeculectomy in juvenile open angle glaucoma. Indian J Ophthalmol. 2014;62:224–8. doi: 10.4103/0301-4738.101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Povoa CA, Malta RFS, Rezende M de M, de Melo KFS, Giannella-Neto D. Correlation between genotype and phenotype in primary open angle glaucoma of Brazilian families with mutations in exon 3 of the TIGR/MYOC gene. Arq Bras Oftalmol. 2006;69:289–97. doi: 10.1590/s0004-27492006000300002. [DOI] [PubMed] [Google Scholar]
- 156.Puska P, Lemmelä S, Kristo P, Sankila E-M, Järvelä I. Penetrance and phenotype of the Thr377Met Myocilin mutation in a large finnish family with juvenile- and adult-onset primary open-angle glaucoma. Ophthalmic Genet. 2005;26:17–23. doi: 10.1080/13816810590918208. [DOI] [PubMed] [Google Scholar]
- 157.Razeghinejad MR, Masoumpour M, Eghbal MH, Myers JS, Moster MR. Glaucoma Surgery in Pregnancy: A Case Series and Literature Review. Iran J Med Sci. 2016;41:437–45. [PMC free article] [PubMed] [Google Scholar]
- 158.Richards JE, Lichter PR, Boehnke M, Uro JL, Torrez D, Wong D, Johnson AT. Mapping of a gene for autosomal dominant juvenile-onset open-angle glaucoma to chromosome Iq. Am J Hum Genet. 1994;54:62–70. [PMC free article] [PubMed] [Google Scholar]
- 159.Richards JE, Ritch R, Lichter PR, Rozsa FW, Stringham HM, Caronia RM, Johnson D, Abundo GP, Willcockson J, Downs CA, Thompson DA, Musarella MA, Gupta N, Othman MI, Torrez DM, Herman SB, Wong DJ, Higashi M, Boehnke M. Novel trabecular meshwork inducible glucocorticoid response mutation in an eight-generation juvenile-onset primary open-angle glaucoma pedigree. Ophthalmology. 1998;105:1698–707. [DOI] [PubMed] [Google Scholar]
- 160.Ritch R, Shields MB, Krupin T. The Glaucomas. Vol 1 & 2. Mosby; 1989. [Google Scholar]
- 161.Rojas C, Bohnsack BL. Rate of Complete Catheterization of Schlemm’s Canal and Trabeculotomy Success in Primary and Secondary Childhood Glaucomas. Am J Ophthalmol. 2020;212:69–78. doi: 10.1016/j.ajo.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 162.Saeedi O, Yousaf S, Tsai J, Palmer K, Riazuddin S, Ahmed ZM. Delineation of Novel Compound Heterozygous Variants in LTBP2 Associated with Juvenile Open Angle Glaucoma. Genes. 2018;9. doi: 10.3390/genes9110527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Saheb H, Ahmed IIK. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23:96–104. doi: 10.1097/ICU.0b013e32834ff1e7. [DOI] [PubMed] [Google Scholar]
- 164.Samarawickrama C, Huynh SC, Liew G, Burlutsky G, Mitchell P. Birth weight and optic nerve head parameters. Ophthalmology. 2009;116:1112–18. doi: 10.1016/j.ophtha.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 165.Sampaolesi R, Sampaolesi JR. Large optic nerve heads: megalopapilla or megalodiscs. Int Ophthalmol. 2001;23:251–7. doi: 10.1023/a. [DOI] [PubMed] [Google Scholar]
- 166.Sawamura H, Aihara M, Araie M. Juvenile onset of ocular hypertension associated with de novo nail-patellar syndrome. J Glaucoma. 2014;23:e122–5. doi: 10.1097/IJG.0b013e3182953b08. [DOI] [PubMed] [Google Scholar]
- 167.Schlenker MB, Gulamhusein H, Conrad-Hengerer I, Somers A, Lenzhofer M, Stalmans I, Reitsamer H, Hengerer FH, Ahmed IIK. Efficacy, Safety, and Risk Factors for Failure of Standalone Ab Interno Gelatin Microstent Implantation versus Standalone Trabeculectomy. Ophthalmology. 2017;124:1579–88. [DOI] [PubMed] [Google Scholar]
- 168.Senthil S, Badakere S, Ganesh J, Krishnamurthy R, Dikshit S, Choudhari N, Garudadri C, Mandal AK. Profile of childhood glaucoma at a tertiary center in South India. Indian J Ophthalmol. 2019;67:358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sheybani A, Dick HB, Ahmed IIK. Early clinical results of a novel ab interno gel stent for the surgical treatment of open-angle glaucoma. J Glaucoma. 2016;25:e691–6. doi: 10.1097/IJG.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 170.Shimizu S, Lichter PR, Johnson AT, Zhou Z, Higashi M, Gottfredsdottir M, Othman M, Moroi SE, Rozsa FW, Schertzer RM, Clarke MS, Schwartz AL, Downs CA, Vollrath D, Richards JE. Age-dependent prevalence of mutations at the GLC1A locus in primary open-angle glaucoma. Am J Ophthalmol. 2000;130:165–77. [DOI] [PubMed] [Google Scholar]
- 171.Siggs OM, Souzeau E, Taranath DA, Dubowsky A, Chappell A, Zhou T, Javadiyan S, Nicholl J, Kearns LS, Staffieri SE, Narita A, Smith JEH, Pater J, Hewitt AW, Ruddle JB, Elder JE, Mackey DA, Burdon KP, Craig JE. Biallelic CPAMD8 Variants Are a Frequent Cause of Childhood and Juvenile Open-Angle Glaucoma. Ophthalmology. 2020;127:758–66. doi: 10.1016/j.ophtha.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 172.Sihota R, Shakrawal J, Sidhu T, Sharma AK, Dada T, Pandey V. Does TRABECULECTOMY meet the 10–10–10 challenge in PACG, POAG, JOAG and Secondary glaucomas? Int Ophthalmol. 2020;40:1233–43. doi: 10.1007/s10792-020-01289-5. [DOI] [PubMed] [Google Scholar]
- 173.Somarajan BI, Gupta S, Mahalingam K, Azmira K, Gupta V. Digenic inheritance in juvenile open-angle glaucoma. J Pediatr Genet. 2021. Published online ahead of print February 2. doi: 10.1055/s-0040-1722213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Song J, Song A, Palmares T, Song M. Selective laser trabeculoplasty success in pediatric patients with glaucoma: two case reports. J Med Case Reports. 2013;7:198. doi: 10.1186/1752-1947-7-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Souzeau E, Burdon KP, Ridge B, Dubowsky A, Ruddle JB, Craig JE. A novel de novo Myocilin variant in a patient with sporadic juvenile open angle glaucoma. BMC Med Genet. 2016;17. doi: 10.1186/s12881-016-0291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Souzeau E, Glading J, Ridge B, Wechsler D, Chehade M, Dubowsky A, Burdon KP, Craig JE. Predictive genetic testing in minors for Myocilin juvenile onset open angle glaucoma. Clin Genet. 2015;88:584–8. [DOI] [PubMed] [Google Scholar]
- 177.Souzeau E, Hayes M, Zhou T, Siggs OM, Ridge B, Awadalla MS, Smith JEH, Ruddle JB, Elder JE, Mackey DA, Hewitt AW, Healey PR, Goldberg I, Morgan WH, Landers J, Dubowsky A, Burdon KP, Craig JE. Occurrence of CYP1B1 Mutations in Juvenile Open-Angle Glaucoma With Advanced Visual Field Loss. JAMA Ophthalmol. 2015;133:826–33. [DOI] [PubMed] [Google Scholar]
- 178.Sripriya S, Uthra S, Sangeetha R, George RJ, Hemamalini A, Paul PG, Amali J, Vijaya L, Kumaramanickavel G. Low frequency of myocilin mutations in Indian primary open-angle glaucoma patients. Clin Genet. 2004;65:333–7. [DOI] [PubMed] [Google Scholar]
- 179.Stangos AN, Whatham AR, Sunaric-Megevand G. Primary viscocanalostomy for juvenile open-angle glaucoma. Am J Ophthalmol. 2005;140:490–6. doi: 10.1016/j.ajo.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 180.Stegman Z, Sokol J, Liebmann JM, Cohen H, Tello C, Ritch R. Reduced trabecular meshwork height in juvenile primary open-angle glaucoma. Arch Ophthalmol Chic Ill 1960. 1996;114:660–3. doi: 10.1001/archopht.1996.01100130652003. [DOI] [PubMed] [Google Scholar]
- 181.Stoilova D, Child A, Brice G, Desai T, Barsoum-Homsy M, Ozdemir N, Chevrette L, Adam MF, Garchon HJ, Pitts Crick R, Sarfarazi M. Novel TIGR/MYOC mutations in families with juvenile onset primary open angle glaucoma. J Med Genet. 1998;35:989–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stoilova D, Child A, Brice G, Crick RP, Fleck BW, Sarfarazi M. Identification of a new “TIGR” mutation in a family with juvenile-onset primary open angle glaucoma. Ophthalmic Genet. 1997;18:109–18. doi: 10.3109/13816819709057124. [DOI] [PubMed] [Google Scholar]
- 183.Su C-C, Liu Y-F, Li S-Y, Yang J-J, Yen Y-C. Mutations in the CYP1B1 gene may contribute to juvenile-onset open-angle glaucoma. Eye. 2012;26(10):1369–77. doi: 10.1038/eye.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sun X [Management of juvenile ocular hypertension]. Zhonghua Yan Ke Za Zhi Chin J Ophthalmol. 2012;48:481–4. [PubMed] [Google Scholar]
- 185.Suri F, Kalhor R, Zargar SJ, Nilforooshan N, Yazdani S, Nezari H, Paylakhi SH, Narooie-Nejhad M, Bayat B, Sedaghati T, Ahmadian A, Elahi E. Screening of common CYP1B1 mutations in Iranian POAG patients using a microarray-based PrASE protocol. Mol Vis. 2008;14:2349–56. [PMC free article] [PubMed] [Google Scholar]
- 186.Svidnicki PV, Braghini CA, Costa VP, Schimiti RB, de Vasconcellos JPC, de Melo MB. Occurrence of MYOC and CYP1B1 variants in juvenile open angle glaucoma Brazilian patients. Ophthalmic Genet. 2018;39:717–24. doi: 10.1080/13816810.2018.1546405. [DOI] [PubMed] [Google Scholar]
- 187.Takagi S, Tomita G. Changes in the thickness of the macular ganglion cell complex and retinal nerve fiber layer over time after surgery in a case of juvenile glaucoma. Am J Ophthalmol Case Rep. 2016;2:41–3. doi: 10.1016/j.ajoc.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Takahashi H, Noda S, Mashima Y, Kubota R, Ohtake Y, Tanino T, Kudoh J, Minoshima S, Oguchi Y, Shimizu N. The myocilin (MYOC) gene expression in the human trabecular meshwork. Curr Eye Res. 2000;20:81–4. [PubMed] [Google Scholar]
- 189.Talbot AW, Russell-Eggitt I. Pharmaceutical management of the childhood glaucomas. Expert Opin Pharmacother. 2000;1:697–711. doi: 10.1517/14656566.1.4.697. [DOI] [PubMed] [Google Scholar]
- 190.Tamm ER, Russell P. The role of myocilin/TIGR in glaucoma: results of the Glaucoma Research Foundation catalyst meeting in Berkeley, California, March 2000. J Glaucoma. 2001;10:329–39. doi: 10.1097/00061198-200108000-00014. [DOI] [PubMed] [Google Scholar]
- 191.Tawara A, Inomata H. Developmental immaturity of the trabecular meshwork in juvenile glaucoma. Am J Ophthalmol. 1984;98:82–97. doi: 10.1016/0002-9394(84)90193-4. [DOI] [PubMed] [Google Scholar]
- 192.Tchabi S, Doutétien C, Amoussouga A, Babagbéto M, Lawani R, Déguénon J, Bassabi SK. [Intraocular pressure in the Benin: screening for primary open-angle glaucoma]. J Fr Ophtalmol. 2005;28:623–6. [DOI] [PubMed] [Google Scholar]
- 193.Thau A, Lloyd M, Freedman S, Beck A, Grajewski A, Levin AV. New classification system for pediatric glaucoma: implications for clinical care and a research registry. Curr Opin Ophthalmol. 2018;29:385–94. doi: 10.1097/ICU.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 194.Tolman NG, Balasubramanian R, Macalinao DG, Kearney AL, MacNicoll KH, Montgomery CL, de Vries WN, Jackson IJ, Cross SH, Kizhatil K, Nair KS, John SWM. Genetic background modifies vulnerability to glaucoma-related phenotypes in Lmx1b mutant mice. Dis Model Mech. 2021;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tsai J-C, Chang H-W, Kao C-N, Lai I-C, Teng M-C. Trabeculectomy with mitomycin C versus trabeculectomy alone for juvenile primary open-angle glaucoma. Ophthalmoogica. 2003;217:24–30. doi: 10.1159/000068250. [DOI] [PubMed] [Google Scholar]
- 196.Turalba AV, Chen TC. Clinical and genetic characteristics of primary juvenile-onset open-angle glaucoma (JOAG). Semin Ophthalmol. 2008;23:19–25. doi: 10.1080/08820530701745199. [DOI] [PubMed] [Google Scholar]
- 197.Unoki K, Sakamoto Y, Ohba N, Kiwaki T, Umehara F, Isashiki Y, Nakagawa M, Osame M. Hereditary motor and sensory neuropathy associated with juvenile glaucoma. Arch Ophthalmol Chic Ill. 2001;119:1547–50 1960. [PubMed] [Google Scholar]
- 198.Urbak SF. Clinical phenotype of juvenile open-angle glaucoma linked to chromosome 1q in a Danish family. Acta Ophthalmol Scand. 1999;77:568–74. doi: 10.1034/j.1600-0420.1999.770518.x. [DOI] [PubMed] [Google Scholar]
- 199.Urbak SF. Ultrasound biomicroscopical study of the irido-corneal angle in dominant juvenile open-angle glaucoma, in POAG, and in normal eyes. Acta Ophthalmol Scand. 1999;77:160–4. doi: 10.1034/j.1600-0420.1999.770209.x. [DOI] [PubMed] [Google Scholar]
- 200.Urban B, Bakunowicz-Łazarczyk A, Michalczuk M, Krętowska M. Evaluation of corneal endothelium in adolescents with juvenile glaucoma. J Ophthalmol. 2015;2015:895428. doi: 10.1155/2015/895428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vincent AL, Billingsley G, Buys Y, Levin AV, Priston M, Trope G, Williams-Lyn D, Héon E. Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. Am J Hum Genet. 2002;70:448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang D, Huang Y, Huang C, Wu P, Lin J, Zheng Y, Peng Y, Liang Y, Chen J-H, Zhang M. Association analysis of cigarette smoking with onset of primary open-angle glaucoma and glaucoma-related biometric parameters. BMC Ophthalmol. 2012;12:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang DY, Fan BJ, Canlas O, Tam POS, Ritch R, Lam DSC, Fan DSP, Pang CP. Absence of myocilin and optineurin mutations in a large Philippine family with juvenile onset primary open angle glaucoma. Mol Vis. 2004;10:851–6. [PubMed] [Google Scholar]
- 204.Wang DY, Fan BJ, Chua JKH, Tam POS, Leung CKS, Lam DSC, Pang CP. A genome-wide scan maps a novel juvenile-onset primary open-angle glaucoma locus to 15q. Invest Ophthalmol Vis Sci. 2006;47:5315–21. [DOI] [PubMed] [Google Scholar]
- 205.Wang H, Li M, Zhang Z, Xue H, Chen X, Ji Y. Physiological function of myocilin and its role in the pathogenesis of glaucoma in the trabecular meshwork (Review). Int J Mol Med. 2019;43:671–81. doi: 10.3892/ijmm.2018.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang Y, Wang H, Han Y, Shi Y, Xin C, Yin P, Li M, Cao K, Wang N. Outcomes of gonioscopy-assisted transluminal trabeculotomy in juvenile-onset primary open-angle glaucoma. Eye Lond Engl; 2020. December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Waryah AM, Narsani AK, Sheikh SA, Shaikh H, Shahani MY. The novel heterozygous Thr377Arg MYOC mutation causes severe Juvenile Open Angle Glaucoma in a large Pakistani family. Gene. 2013;528:356–9. doi: 10.1016/j.gene.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 208.Wei Y-T, Li Y-Q, Bai Y-J, Wang M, Chen J-H, Ge J, Zhuo Y-H. Pro370Leu myocilin mutation in a Chinese pedigree with juvenile-onset open angle glaucoma. Mol Vis. 2011;17:1449–56. [PMC free article] [PubMed] [Google Scholar]
- 209.Wiggs JL, Allingham RR, Vollrath D, Jones KH, De La Paz M, Kern J, Patterson K, Babb VL, Del Bono EA, Broomer BW, Pericak-Vance MA, Haines JL. Prevalence of mutations in TIGR/Myocilin in patients with adult and juvenile primary open-angle glaucoma. Am J Hum Genet. 1998;63:1549–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wiggs JL, Bono EAD, Schuman JS, Hutchinson BT, Walton DS. Clinical Features of Five Pedigrees Genetically Linked to the Juvenile Glaucoma Locus on Chromosome 1q21-q31. Ophthalmology. 1995;102:1782–9. doi: 10.1016/S0161-6420(95)30793-2. [DOI] [PubMed] [Google Scholar]
- 211.Wiggs JL, Damji KF, Haines JL, Pericak-Vance MA, Allingham RR. The distinction between juvenile and adult-onset primary open-angle glaucoma. Am J Hum Genet. 1996;58:243–4. [PMC free article] [PubMed] [Google Scholar]
- 212.Wiggs JL, Haines JL, Paglinauan C, Fine A, Sporn C, Lou D. Genetic linkage of autosomal dominant juvenile glaucoma to 1q21-q31 in three affected pedigrees. Genomics. 1994;21:299–303. doi: 10.1006/geno.1994.1269. [DOI] [PubMed] [Google Scholar]
- 213.Wiggs JL, Lynch S, Ynagi G, Maselli M, Auguste J, Del Bono EA, Olson LM, Haines JL. A Genomewide Scan Identifies Novel Early-Onset Primary Open-Angle Glaucoma Loci on 9q22 and 20p12. Am J Hum Genet. 2004;74:1314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wiggs JL, Pasquale LR. Genetics of glaucoma. Hum Mol Genet. 2017;26:R21–7. doi: 10.1093/hmg/ddx184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wilensky JT, Weinreb RN. Early and late failures of argon laser trabeculoplasty. Arch Ophthalmol Chic Ill 1960. 1983;101:895–7. doi: 10.1001/archopht.1983.01040010895007. [DOI] [PubMed] [Google Scholar]
- 216.Willoughby CE, Chan LLY, Herd S, Billingsley G, Noordeh N, Levin AV, Buys Y, Trope G, Sarfarazi M, Héon E. Defining the pathogenicity of optineurin in juvenile open-angle glaucoma. Invest Ophthalmol Vis Sci. 2004;45:3122–30. [DOI] [PubMed] [Google Scholar]
- 217.Wittenborn JS, Zhang X, Feagan CW, Crouse WL, Shrestha S, Kemper AR, Hoerger TJ, Saaddine JB. The Economic Burden of Vision Loss and Eye Disorders among the United States Population Younger than 40 Years. Ophthalmology. 2013;120:1728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xiao X, Huang C, Cao Y, Chen S, Xu Y, Chen H, Pang C, Zhang M. Exome Sequencing Reveals a Heterozygous OAS3 Mutation in a Chinese Family With Juvenile-Onset Open-Angle Glaucoma. Invest Ophthalmol Vis Sci. 2019;60:4277–84. [DOI] [PubMed] [Google Scholar]
- 219.Yalvac IS, Nurözler A, Kahraman C, Kasim R, Duman S. The results of trabeculectomy with and without mitomycin C in young patients. Ophthalmoogica. 1998;212:399–403. doi: 10.1159/000027375. [DOI] [PubMed] [Google Scholar]
- 220.Yao Y-H, Wang Y-Q, Fang W-F, Zhang L, Yang J-H, Zhu Y-H. A recurrent G367R mutation in MYOC associated with juvenile open angle glaucoma in a large Chinese family. Int J Ophthalmol. 2018;11:369–74. doi: 10.18240/ijo.2018.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yen Y-C, Yang J-J, Chou M-C, Li S-Y. Absence of optineurin (OPTN) gene mutations in Taiwanese patients with juvenile-onset open-angle glaucoma. Mol Vis. 2008;14:487–94. [PMC free article] [PubMed] [Google Scholar]
- 222.Yen Y-C, Yang J-J, Chou M-C, Li S-Y. Identification of mutations in the myocilin (MYOC) gene in Taiwanese patients with juvenile-onset open-angle glaucoma. Mol Vis. 2007;13:1627–34. [PubMed] [Google Scholar]
- 223.Yeung HH, Walton DS. Goniotomy for juvenile open-angle glaucoma. J Glaucoma. 2010;19:1–4. doi: 10.1097/IJG.0b013e3181a2fa31. [DOI] [PubMed] [Google Scholar]
- 224.Yusuf IH, Ratnarajan G, Kerr RS, Salmon JF. Juvenile-onset normal tension glaucoma from chronic, recurrent low cerebrospinal fluid pressure. J Glaucoma. 2016;25:e738–40. doi: 10.1097/IJG.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 225.Zarnowski T, Lekawa A, Dyduch A, Turek R, Zagórski Z. [Corneal endothelial density in glaucoma patients]. Klin Oczna. 2005;107:448–51. [PubMed] [Google Scholar]
- 226.Zehavi-Dorin T, Heinecke E, Nadkarni S, Green C, Chen C, Kong YXG. Bilateral consecutive Xen gel stent surgery during pregnancy for uncontrolled early-onset primary open angle glaucoma. Am J Ophthalmol Case Rep. 2019;15. doi: 10.1016/j.ajoc.2019.100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhao X, Yang C, Tong Y, Zhang X, Xu L, Li Y. Identification a novel MYOC gene mutation in a Chinese family with juvenile-onset open angle glaucoma. Mol Vis. 2010;16:1728–35. [PMC free article] [PubMed] [Google Scholar]
- 228.Zhuo Y-H, Wei Y-T, Bai Y-J, Duan S, Lin M-K, Saragovi HU, Ge J. Pro370Leu MYOC gene mutation in a large Chinese family with juvenile-onset open angle glaucoma: correlation between genotype and phenotype. Mol Vis. 2008;14:1533–9. [PMC free article] [PubMed] [Google Scholar]


