Abstract
Previous studies using a murine model of coinhalation of Legionella pneumophila and Hartmannella vermiformis have shown a significantly enhanced intrapulmonary growth of L. pneumophila in comparison to inhalation of legionellae alone (J. Brieland, M. McClain, L. Heath, C. Chrisp, G. Huffnagle, M. LeGendre, M. Hurley, J. Fantone, and C. Engleberg, Infect. Immun. 64:2449–2456, 1996). In this study, we introduce an in vitro coculture model of legionellae, Mono Mac 6 cells (MM6) and Acanthamoeba castellanii, using a cell culture chamber system which separates both cell types by a microporous polycarbonate membrane impervious to bacteria, amoebae, and human cells. Whereas L. pneumophila has shown a maximal 4-log-unit multiplication within MM6, which could not be further increased by coculture with Acanthamoeba castellanii, significantly enhanced replication of L. gormanii, L. micdadei, L. steigerwaltii, L. longbeachae, and L. dumoffii was seen after coculture with amoebae. This effect was seen only with uninfected amoebae, not with Legionella-infected amoebae. The supporting effect for intracellular multiplication in MM6 could be reproduced in part by addition of a cell-free coculture supernatant obtained from a coincubation experiment with uninfected A. castellanii and Legionella-infected MM6, suggesting that amoeba-derived effector molecules are involved in this phenomenon. This coculture model allows investigations of molecular and biochemical mechanisms which are responsible for the enhancement of intracellular multiplication of legionellae in monocytic cells after interaction with amoebae.
In 1980, Rowbotham published the first report on intracellular multiplication of Legionella pneumophila within Acanthamoeba spp. and Naegleria spp. (33). Thereafter, several reports described the replication of Legionella culture isolates from clinical samples within protozoa isolated from the presumed source of infection (2, 6, 21–23, 29, 34, 36, 45). Intracellular growth within protozoa enhances the ability of L. pneumophila to infect human monocytes (18), induces phenotypic modulation (1, 4), and causes resistance to chemical disinfectants, biocides, and antibiotics (3, 5).
Inhalation of legionellae packaged in amoebae results in the induction of more-severe clinical cases of legionellosis (31, 33). This speculation was supported by a recently published mouse model of coinhalation of L. pneumophila and Hartmannella vermiformis. Coinhalation with H. vermiformis significantly enhanced the intrapulmonary growth of L. pneumophila, resulting in greater mortality than that from inhalation of legionellae alone (7). Intrapulmonary growth of mutant strains of L. pneumophila with reduced virulence for H. vermiformis but maintained virulence for monocytes was not significantly enhanced by coinhalation (8). Intrapulmonary growth of L. pneumophila was significantly greater in mice inoculated with L. pneumophila-infected H. vermiformis than in mice inoculated with an equivalent number of bacteria or coinoculated with L. pneumophila and uninfected H. vermiformis (9). The mechanism of intrapulmonary growth enhancement of legionellae by amoebae remains to be determined. We therefore established an in vitro coculture model of the Mono Mac 6 cell line (MM6), Acanthamoeba castellanii, and Legionella species with different intracellular growth rates within MM6 (30) in order to analyze the underlying molecular and biochemical mechanisms.
MATERIALS AND METHODS
Bacteria.
Legionella spp. L. gormanii ATCC 32979 (isolated from soil of a creek bank), L. longbeachae serogroup 1 ATCC 33462 (isolated from human lung), L. dumoffii ATCC 33279 (isolated from a cooling tower), L. micdadei ATCC 33218 (isolated from human blood via the yolk sac), and L. steigerwaltii ATCC 35302 (isolated from tap water) were obtained from the American Type Culture Collection. L. pneumophila serogroup 1 subtype Pontiac (isolated from a patient with severe Legionella pneumonia and passaged less than three times on buffered charcoal yeast extract agar (BCYE agar) was kindly provided by G. Ruckdeschel (University of Munich, Munich, Germany). Bacteria were grown on BCYE agar (Oxoid, Wesel, Germany) at 35°C in 3% CO2 for 3 to 5 days.
MM6.
MM6 were kindly supplied by H. W. L. Ziegler-Heitbrock (University of Munich) and were cultured as replicative nonadherent monocytes under lipopolysaccharide-free conditions in 250-ml flasks (Nunc, Roskilde, Denmark) in 25 ml of RPMI 1640 medium (Gibco, Eggenstein, Germany) supplemented with 10% fetal calf serum (FCS) (Myoclone Superplus; Gibco), 2 mM l-glutamine (Gibco), 1 mM pyruvic acid (Fluka, Buchs, Switzerland), 1% nonessential amino acids (Gibco), 9 mg of insulin (Sigma, Munich, Germany)/ml, and 1 mM oxalacetate (Sigma) (MM6 medium) at 35°C in 5% CO2 as described by Ziegler-Heitbrock et al. (47). Cells were diluted 1:3 twice a week in fresh medium.
A. castellanii.
A. castellanii ATCC 30234 was grown in 250-ml flasks (Nunc) in 25 ml of PYE broth [2% proteose peptone no. 3 (Difco, Detroit, Mich.), 0.1% yeast extract (Difco), 0.1 M glucose, 4 mM MgSO4, 0.4 M CaCl2, 0.1% sodium citrate dihydrate, 0.05 mM Fe(NH4)2(SO4)2 · 6H2O, 2.5 mM NaH2PO3, 2.5 mM K2HPO3 (Sigma), pH 6.5] at 35°C in 5% CO2 as described by Moffat and Tompkins (28) and was diluted 1:2 twice a week.
In vitro infection of MM6 and A. castellanii.
Nonadherent MM6 were harvested by centrifugation at 400 × g for 10 min. The pellet was washed twice in MM6 medium without FCS. Legionellae were harvested from BCYE agar, suspended in MM6 medium without FCS, and adjusted to an optical density at 578 nm of 0.2 (Ultrospec 2000; Pharmacia, Freiburg, Germany), corresponding to a concentration of legionellae of approximately 3 × 108 CFU/ml. For concentration, bacteria were centrifuged at 3,000 × g for 15 min. MM6 (2 × 107) were pelleted and resuspended with 2 × 109 legionellae in a volume of 1.5 ml in a well of a six-well tissue culture plate (Nunc) to provide a bacteria-to-cell ratio of 100:1 and were incubated in the presence of legionellae at 35°C in 5% CO2 for 2 h. After this period, nonphagocytized bacteria were killed by the addition of 4.5 ml of MM6 medium without FCS containing 100 μg of gentamicin/ml for 1 h at 35°C in 5% CO2. After three washes by centrifugation at 300 × g for 15 min, the cells were resuspended in coculture medium (see below) and distributed in 1-ml aliquots into the wells of a 24-well tissue culture plate (Nunc), giving a concentration of 106 infected MM6 per well. This time point was defined as time zero. Elimination of extracellular bacteria after exposure to gentamicin was verified by plating an aliquot of all washing solution onto BCYE agar.
The cells were then incubated for an additional 72 h at 35°C in 5% CO2. Every 24 h, the contents of two wells were aspirated and pelleted by centrifugation at 400 × g for 10 min. Supernatant was transfered into a sterile tube. One milliliter of sterile distilled water was added to the pellet, and final disruption of the cells was performed by aspirating the suspension through a 27-gauge needle. Supernatant and lysis fluid were pooled, and serial 10-fold dilutions were made. Then, 0.1 ml of each dilution was inoculated onto BCYE agar to determine the number of culturable legionellae after multiplication in MM6. Colonies on the agar were counted on day 5 after incubation at 35°C in 5% CO2.
The viability of MM6 and A. castellanii was determined by trypan blue exclusion at the time points indicated above.
In experiments using infected amoebae, in vitro infection of A. castellanii was performed in an identical manner except that legionellae were suspended in amoebae buffer (PYE broth without glucose) (30).
Coculture of MM6 and A. castellanii.
Infected MM6 were resuspended in a mixture of 50% MM6 medium without FCS and 50% PYE broth without glucose NaCl was added to yield a NaCl concentration of 6.5 g/liter (coculture medium), identical to that in MM6 medium. This mixture was found to support the growth of MM6 and A. castellanii as well as the above-described original cell culture media for these cells. Infected MM6 were then distributed in 1-ml aliquots into the wells of a 24-well tissue culture plate, giving a concentration of 106 infected MM6 per well. A. castellanii cells (106; uninfected or infected) were resuspended in coculture medium and were added using a Transwell insert (Costar, Bodenheim, Germany) which separated both cell types by a microporous polycarbonate membrane with a pore size of 0.1 μm, impervious to bacteria, amoebae, and MM6. The absence of legionellae from the upper chamber (uninfected A. castellanii) was determined by means of culture on BCYE agar, and the sterility of the coculture was checked by culture on Columbia blood agar (Oxoid).
The coculture was incubated for 72 h. In some experiments, 106 infected MM6 per well were coincubated for 72 h with a cell-free Transwell supernatant from a coincubation experiment of A. castellanii and MM6 infected with legionellae. Cell-free Transwell supernatant was obtained by harvesting the content of a Transwell insert (i.e., noninfected amoebae in coculture with infected MM6 and both host cells separated by a microporous membrane), with subsequent centrifugation.
Intracellular multiplication of legionellae in MM6 was determined as described in the previous section.
Multiplication within MM6 in coculture with A. castellanii or supernatant was compared with multiplication within MM6 in coculture medium. All experiments were done at least in triplicate.
Statistical analysis of data.
Based on the maximum-likelihood method, assuming a Poisson distribution, the number of bacteria in each experiment was estimated by dividing the sum of bacterial counts for different dilutions by the sum of dilution factors (32). The response variable was always the logarithm of the ratio of the bacterial counts at 72 h divided by the initial value, which is called the log multiplication within 72 h. For each species, a multifactorial analysis of variance (ANOVA) was carried out separately and included the factors (when applicable) date of experiment, coculture with A. castellanii, coculture with either uninfected or infected amoebae, supernatant from coculture of A. castellanii with MM6 infected with certain Legionella species, and NaCl supplementation and their interactions. The ANOVA models provide least-squares mean estimates of bacterial multiplication within 72 h in MM6 together with their 95% confidence limits. Post-hoc t tests for individual contrasts were adjusted (when necessary) by the method of Bonferroni. An overall significance level of 5% was adopted. Statistical analyses were performed using the statistics package JMP, version 3.2.2.
RESULTS
Viability of MM6 and A. castellanii in coculture medium.
MM6 medium without FCS and amoeba buffer without glucose were mixed in different ratios. This mixture was supplemented with NaCl to yield a NaCl concentration of 6.5 g/liter. MM6 and A. castellanii were cultured in this mixture, and cell viability was determined by trypan blue exclusion. At a ratio of 50% MM6 medium and 50% amoeba buffer, the viability of both MM6 and A. castellanii was similar to that in the original culture media. This mixture was chosen as the coculture medium for further experiments.
Multiplication of different Legionella species in MM6 and influence of coculture with A. castellanii.
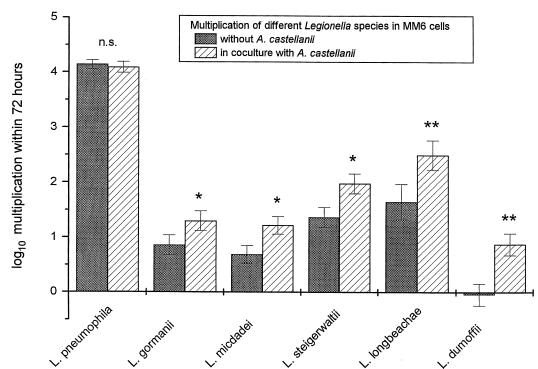
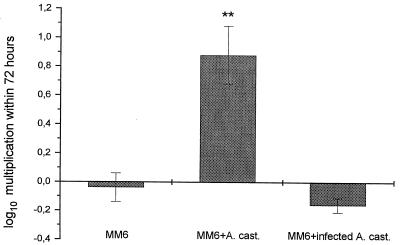
Legionellae could not multiply within MM6 medium, amoeba medium, or coculture medium without the addition of host cells (data not shown). After in vitro infection of MM6, the level of intracellular L. pneumophila increased by more than 4 orders of magnitude over a 72-h period. This maximal replication could not be further enhanced by the addition of A. castellanii. L. gormanii, L. micdadei, L. steigerwaltii, and L. longbeachae showed only moderate intracellular multiplication in MM6, which was significantly enhanced by coculture with amoebae. While L. dumoffii was not able to multiply within MM6, the addition of A. castellanii induced a 10-fold increase for this species (Fig. 1). The supporting effect of coculture with amoebae for intracellular growth of legionellae in MM6 was seen only with uninfected amoebae. A. castellanii infected with L. dumoffii was not able to enhance the intracellular growth of L. dumoffii in MM6 when used in coculture (Fig. 2).
FIG. 1.
Multiplication of different Legionella species in MM6 and influence of coculture with A. castellanii. Data are means ± 95% confidence limits from three experiments. ∗, P < 0.05; ∗∗, P < 0.01; n.s., not significant.
FIG. 2.
Multiplication of L. dumoffii in MM6 in coculture with uninfected and L. dumoffii-infected A. castellanii (A. cast.). Data are means ± 95% confidence limits from three experiments. ∗∗, P < 0.01.
Multiplication of different Legionella species in MM6 coincubated with supernatants obtained from a coculture of L. dumoffii-, L. steigerwaltii-, or L. longbeachae-infected MM6 with A. castellanii.
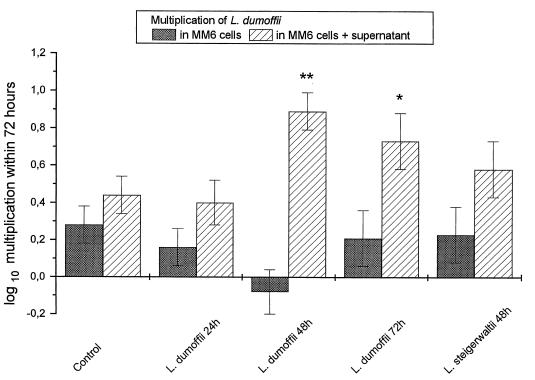
In some coculture experiments, A. castellanii was replaced by a cell-free Transwell supernatant obtained from a coincubation experiment of A. castellanii and MM6 infected with L. dumoffii, L. steigerwaltii, or L. longbeachae. Coculture supernatant of uninfected MM6 and A. castellanii served as the control. Intracellular growth of L. dumoffii in MM6 was significantly enhanced by the addition of supernatants, which were obtained after 48 and 72 h of coculture of L. dumoffii-infected MM6 and A. castellanii. The addition of supernatant which was obtained after 48 h of coculture of L. steigerwaltii-infected MM6 and A. castellanii also had a potentiating, although not significantly so, effect on the intracellular growth of L. dumoffii (Fig. 3).
FIG. 3.
Multiplication of L. dumoffii in MM6 coincubated with supernatant obtained from a coculture of L. dumoffii- or L. steigerwaltii-infected MM6 with uninfected A. castellanii. Control, coculture supernatant from uninfected MM6 and A. castellanii. Data are means ± 95% confidence limits from three experiments. ∗, P < 0.05; ∗∗, P < 0.01.
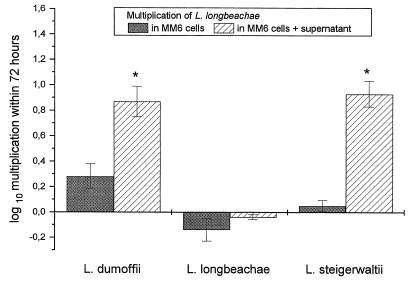
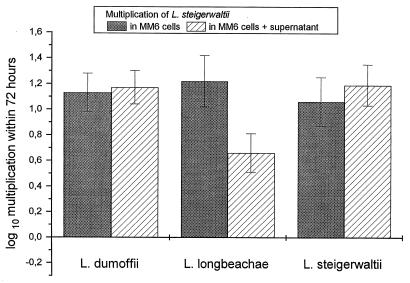
Intracellular multiplication of L. longbeachae was also significantly enhanced by supernatants from cocultures of L. steigerwaltii- and L. dumoffii-infected MM6 and A. castellanii (Fig. 4). Replication of L. steigerwaltii in MM6 could not be influenced by the addition of supernatant (Fig. 5).
FIG. 4.
Multiplication of L. longbeachae in MM6 coincubated with supernatant obtained from a coculture of L. dumoffii-, L. longbeachae-, or L. steigerwaltii-infected MM6 with uninfected A. castellanii. Data are means ± 95% confidence limits from three experiments. ∗, P < 0.05.
FIG. 5.
Multiplication of L. steigerwaltii in MM6 coincubated with supernatant obtained from a coculture of L. dumoffii-, L. longbeachae-, or L. steigerwaltii-infected MM6 with uninfected A. castellanii. Data are means ± 95% confidence limits from three experiments.
Influence of NaCl content of coculture medium on intracellular replication of L. dumoffii in MM6.
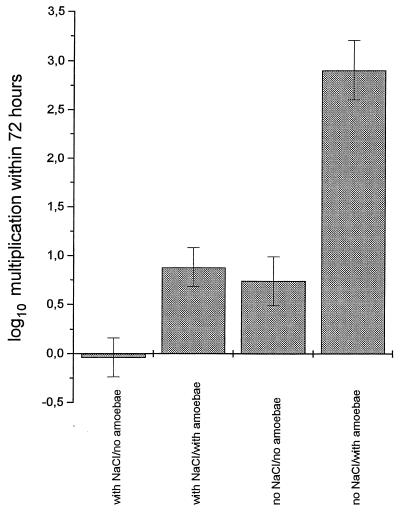
The NaCl content of coculture medium had a considerable influence on the intracellular multiplication of legionellae in MM6, as shown, e.g., for L. dumoffii. Coculture medium without supplementation of NaCl significantly enhanced the intracellular multiplication of L. dumoffii in the absence as well as in the presence of A. castellanii. The supporting effect of coculture with amoebae was more pronounced in coculture medium without supplementation of NaCl (Fig. 6).
FIG. 6.
Influence of NaCl content of coculture medium on intracellular replication of L. dumoffii in MM6. Data are means ± 95% confidence limits from three experiments.
DISCUSSION
Recently, Brieland et al. investigated the effect of inhaled H. vermiformis on the pathogenesis of Legionnaires' disease in a murine model (7, 9). They found that intratracheal coinoculation of L. pneumophila and amoebae as well as inoculation of L. pneumophila-infected amoebae significantly enhanced the intrapulmonary growth of L. pneumophila in A/J mice. The mechanism by which intrapulmonary H. vermiformis potentiates the replication of L. pneumophila in the rodent lung and the relevance of these findings for human infections remained unclear. Amoeba-induced inhibition of proinflammatory cytokine production could be excluded, since coinhalation as well as inhalation of L. pneumophila-infected H. vermiformis induced significantly enhanced levels of gamma interferon and tumor necrosis factor alpha in A/J mice, levels similar to those induced during replicative lung infections induced by L. pneumophila alone.
Three possible explanations for the potentiating effect of coinhalation for Legionella infection were discussed: modification of the host response to L. pneumophila infection by amoebae, function of amoebae as implanted host cells, and enhanced virulence of amoeba-associated bacteria (7).
In this study, we introduce a coculture model of legionellae, MM6, and A. castellanii that enables molecular and biochemical investigations of interactions between amoebae, bacteria, and human monocytes as the typical host cells in human Legionella infections. Legionella species which show different human prevalences and different degrees of multiplication within MM6 were investigated. The first observed replication in MM6 and A. castellanii of the Legionella species investigated in this model has been described recently (30).
We could show that the number of cells of L. pneumophila, the most common cause of Legionnaires' disease, increased by more than 4 orders of magnitude over a 72-h period in MM6. This maximal multiplication within MM6 could not be further enhanced by coculture with A. castellanii (Fig. 1). This result is in contrast to those obtained with the A/J mouse model, where coinhalation of L. pneumophila and uninfected H. vermiformis resulted in an increase of intrapulmonary L. pneumophila in mice (7). Use of different amoebal hosts, different multiplicities of infection, and interference with other host cells and their mediators in the mouse model could possibly be the reasons for the contrasting results between the animal model and the in vitro model. In our coculture model, maximal intracellular multiplication of L. pneumophila within MM6 seems to be independent from support by amoebae.
Less-common causes of legionellosis such as L. micdadei, L. gormanii, L. longbeachae, and L. dumoffii or environmental species such as L. steigerwaltii showed moderate or deficient intracellular multiplication in MM6, which was significantly enhanced by coculture with amoebae (Fig. 1). We showed in a previous investigation that L. dumoffii is the only one of these species which is able to grow efficiently within A. castellanii (30), a phenomenon which can be explained by the specialized adaptation of certain Legionella species to certain protozoa (19, 20, 24, 28, 35, 37, 39, 40). In the mouse model, only strains of L. pneumophila which were able to replicate within H. vermiformis showed maximal intrapulmonary growth when coinoculated with amoebae, whereas the growth of mutants with reduced virulence for H. vermiformis was not potentiated by coinhalation (8). In the in vitro coculture model of MM6 and A. castellanii, significant enhancement of intracellular multiplication in MM6 could also be achieved by coculture of MM6 and amoebae for species which are not able to multiply in A. castellanii.
When L. dumoffii was used for infection, the potentiating effect of coculture with amoebae for intracellular growth of legionellae in MM6 was seen only with uninfected amoebae, whereas A. castellanii infected with L. dumoffii was not able to enhance the intracellular growth of L. dumoffii in MM6 when used in coculture (Fig. 2). In the mouse model of coinhalation, intrapulmonary growth of L. pneumophila was significantly greater in mice inoculated with L. pneumophila-infected H. vermiformis than in mice inoculated with an equivalent number of bacteria or coinoculated with L. pneumophila and uninfected H. vermiformis (9). The difference between this animal model and our results could be due to the different Legionella species used for the investigations or due to the separation of the cell populations in the in vitro model, whereas the mouse model allowed close contact between lung macrophages and Legionella-infected amoebae.
The supporting effect of coculture on the replication of L. dumoffii in MM6 could be reproduced by the addition of supernatants, which were obtained after 48 and 72 h of coculture of L. dumoffii-infected MM6 and A. castellanii (Fig. 3). Intracellular multiplication of L. longbeachae was also significantly enhanced by supernatants from cocultures of L. steigerwaltii- and L. dumoffii-infected MM6 and A. castellanii (Fig. 4). Replication of L. steigerwaltii in MM6 could not be influenced by the addition of supernatant (Fig. 5). These data suggest (i) that despite enhanced intracellular multiplication in MM6 during coculture with A. castellanii (Fig. 1), supernatants from coculture of A. castellanii and L. longbeachae-infected MM6 were not able to stimulate intracellular replication (Fig. 4 and 5) and (ii) that only certain Legionella species are susceptible to the potentiating effect of coculture supernatant since intracellular growth of L. steigerwaltii could not be influenced by the addition of supernatants (Fig. 5). The cause for these differences remains to be investigated.
The NaCl content of coculture medium had a considerable influence on the intracellular multiplication of legionellae in MM6 as shown, e.g., for L. dumoffii. Coculture medium without supplementation of NaCl significantly enhanced the intracellular multiplication of L. dumoffii in the absence as well as in the presence of A. castellanii. The supporting effect of coculture with amoebae was more pronounced in coculture medium without supplementation of NaCl (Fig. 6). The same effects could be shown for L. longbeachae, whereas intracellular multiplication of L. pneumophila was not influenced by osmolarity (data not shown). It is well established that NaCl is inhibitory for virulent and exponential-phase, but not for avirulent and postexponential, strains of L. pneumophila (16, 17, 41), but we could exclude the possibility that L. dumoffii or L. longbeachae multiplied in low-NaCl coculture medium in the absence of host cells (data not shown). Therefore, a low NaCl concentration in coculture medium obviously influenced the host cells. Supplementation of the coculture medium with raffinose, KCl, or Na2SO4 to establish an osmolarity identical to those of MM6 medium and coculture medium with NaCl, respectively, resulted in the complete abrogation of enhanced intracellular replication of both Legionella species in coculture medium (data not shown). Thus, decreased extracellular osmolarity rather than reduced sodium or reduced chloride accounted for the stimulation of replication. A decrease of extracellular osmolarity leads to osmotic cell swelling, which modifies a variety of cellular functions, such as transport, metabolism, cell proliferation, and cell death (for a review, see reference 27). Most notably, it is well established that cell swelling results in alkalinization whereas cell shrinkage results in acidification of endosomal pH (10–15, 42–44). Previous investigations have shown that, due to inhibition of the fusion of the phagosome and lysosome, the phagosome of L. pneumophila does not become acidified (25, 26, 38) and therefore enables the intracellular multiplication of bacteria. It remains to be determined whether or not the non-L. pneumophila species used in this investigation are unable to avoid acidification of their phagosomes in isotonic extracellular medium but benefit from the alkalinization of phagosomal pH by osmotic swelling of the host cell during incubation in hyposmolar coculture medium.
In summary, by this study we introduce a new in vitro coculture model of legionellae, monocytes, and A. castellanii. By using non-L. pneumophila species, this model allows investigations of mechanisms which are responsible for the enhancement of intracellular multiplication of legionellae in monocytic cells after interaction with amoebae. Since the stimulating effect for intracellular replication of legionellae in monocytes could be reproduced in part by the addition of supernatants obtained from previous coincubation experiments, secreted amoebal substances responsible for the phenomenon should be investigated.
We could also show that the reduced osmolarity of the cell culture medium induced intracellular multiplication of L. dumoffii and L. longbeachae in MM6 due to the swelling of the host cells. Studies are in progress to characterize possible underlying cellular mechanisms, such as modulation of phagosomal pH, activity of a cell volume-regulated kinase (46), and cell volume-regulated transport systems including ion channels (27) in swollen MM6 infected with legionellae. A possible link between the effects of coculture with amoebae and cell swelling remains to be investigated. Moreover, further studies are necessary to clarify the cause of the differences between L. pneumophila and non-L. pneumophila species in terms of susceptibility to interaction with amoebae and the effect of hyposmolarity.
REFERENCES
- 1.Abu-Kwaik Y, Eisenstein B I, Engleberg N C. Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infect Immun. 1993;61:1320–1329. doi: 10.1128/iai.61.4.1320-1329.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbaree J M, Fields B S, Feeley J C, Gorman G W, Martin W T. Isolation of protozoa from water associated with a legionellosis outbreak and demonstration of intracellular multiplication of Legionella pneumophila. Appl Environ Microbiol. 1986;51:422–424. doi: 10.1128/aem.51.2.422-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker J, Brown M R, Collier P J, Farrell I, Gilbert P. Relationship between Legionella pneumophila and Acanthamoeba polyphaga: physiological status and susceptibility to chemical inactivation. Appl Environ Microbiol. 1992;58:2420–2425. doi: 10.1128/aem.58.8.2420-2425.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker J, Lambert P A, Brown M R W. Influence of intra-amoebic and other growth conditions on the surface properties of Legionella pneumophila. Infect Immun. 1995;61:3503–3510. doi: 10.1128/iai.61.8.3503-3510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker J, Scaife H, Brown R W. Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrob Agents Chemother. 1995;39:2684–2688. doi: 10.1128/aac.39.12.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breiman R F, Fields B S, Sanden G N, Volmer L J, Meier A, Spika J S. Association of shower use with Legionnaires' disease. JAMA. 1990;263:2924–2926. [PubMed] [Google Scholar]
- 7.Brieland J, McClain M, Heath L, Chrisp C, Huffnagle G, LeGendre M, Hurley M, Fantone J, Engleberg C. Coinoculation with Hartmannella vermiformis enhances replicative Legionella pneumophila infection in a murine model of Legionnaires' disease. Infect Immun. 1996;64:2449–2456. doi: 10.1128/iai.64.7.2449-2456.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brieland J, McClain M, Legendre M, Engleberg C. Intrapulmonary Hartmannella vermiformis: a potential niche for Legionella pneumophila replication in a murine model of legionellosis. Infect Immun. 1997;65:4892–4896. doi: 10.1128/iai.65.11.4892-4896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brieland J K, Fantone J C, Remick D G, Legendre M, McClain M, Engleberg C. The role of Legionella pneumophila-infected Hartmannella vermiformis as an infectious particle in a murine model of Legionnaires' disease. Infect Immun. 1997;65:5330–5333. doi: 10.1128/iai.65.12.5330-5333.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busch G, Guenther E, Hewig B, Zrenner E, Lang F. Effect of cell swelling, NH4Cl and glutamate on acridine orange fluorescence in retinal ganglion cells. Cell Physiol Biochem. 1997;6:185–194. [Google Scholar]
- 11.Busch G L, Schreiber R, Dartsch P C, Völkl H, vom Dahl S, Häussinger D, Lang F. Involvement of microtubules in the link between cell volume and pH of acidic cellular compartments in rat and human hepatocytes. Proc Natl Acad Sci USA. 1994;91:9165–9169. doi: 10.1073/pnas.91.19.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busch G L, Wiesinger H, Gulbins E, Wagner H J, Hamprecht B, Lang F. Effect of astroglial cell swelling on pH of acidic intracellular compartments. Biochim Biophys Acta. 1996;1285:212–218. doi: 10.1016/s0005-2736(96)00163-0. [DOI] [PubMed] [Google Scholar]
- 13.Busch G L, Lang H J, Lang F. Studies on the mechanism of swelling-induced lysosomal alkalinization in vascular smooth muscle cells. Pflügers Arch. 1996;431:690–696. doi: 10.1007/BF02253831. [DOI] [PubMed] [Google Scholar]
- 14.Busch G L, Völkl H, Haller T, Ritter M, Siemen D, Moest J, Koch F, Lang F. Vesicular pH is sensitive to changes in cell volume. Cell Physiol Biochem. 1997;7:25–34. [Google Scholar]
- 15.Busch G L, Kaba N K, Bukara M, Lang F. Osmotic cell swelling alkalinizes acidic cellular compartments of pancreatic islet and RINm5F cells. Pancreas. 1997;15:420–423. doi: 10.1097/00006676-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Byrne B, Swanson M. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catrenich C E, Johnson W. Characterization of the selective inhibition of growth of virulent Legionella pneumophila by supplemented Mueller-Hinton medium. Infect Immun. 1989;57:1862–1864. doi: 10.1128/iai.57.6.1862-1864.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cirillo J D, Falkow S, Tompkins L S. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect Immun. 1994;62:3254–3261. doi: 10.1128/iai.62.8.3254-3261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fields B S, Shotts E B, Jr, Feeley J C, Gorman G W, Martin W T. Proliferation of Legionella pneumophila as an intracellular parasite of the ciliated protozoan Tetrahymena pyriformis. Appl Environ Microbiol. 1984;47:467–471. doi: 10.1128/aem.47.3.467-471.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fields B S, Barbaree J M, Shotts E B, Jr, Feeley J C, Morrill W E, Sanden G N, Dykstra M J. Comparison of guinea pig and protozoan models for determining virulence of Legionella species. Infect Immun. 1986;53:553–559. doi: 10.1128/iai.53.3.553-559.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fields B S, Sanden G N, Barbaree J M, Morrill W E, Wadowsky R M, White E H, Feeley J C. Intracellular multiplication of Legionella pneumophila in amoebae isolated from hospital hot water tanks. Curr Microbiol. 1989;16:131–137. [Google Scholar]
- 22.Fields B S, Nerad T A, Sawyer T K, King C H, Barbaree J M, Martin W T, Morrill W E, Sanden G N. Characterization of an axenic strain of Hartmannella vermiformis obtained from an investigation of nosocomial legionellosis. J Protozool. 1990;37:581–583. doi: 10.1111/j.1550-7408.1990.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 23.Henke M, Seidel K M. Association between Legionella pneumophila and amoebae in water. Isr J Med Sci. 1986;22:690–695. [PubMed] [Google Scholar]
- 24.Holden E P, Winkler H H, Wood D O, Leinbach E D. Intracellular growth of Legionella pneumophila within Acanthamoeba castellanii Neff. Infect Immun. 1984;45:18–24. doi: 10.1128/iai.45.1.18-24.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horwitz M A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz M A, Maxfield F R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984;99:1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang F, Busch G L, Volkl H. The diversity of volume regulatory mechanisms. Cell Physiol Biochem. 1998;8:1–45. doi: 10.1159/000016269. [DOI] [PubMed] [Google Scholar]
- 28.Moffat J F, Tompkins L S. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect Immun. 1992;60:296–301. doi: 10.1128/iai.60.1.296-301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nahapetian K, Challemel O, Beurtin D, Dubrou S, Gounon P, Squinazi F. The intracellular multiplication of Legionella pneumophila in protozoa from hospital plumbing systems. Res Microbiol. 1991;142:677–685. doi: 10.1016/0923-2508(91)90081-k. [DOI] [PubMed] [Google Scholar]
- 30.Neumeister B, Schöniger S, Faigle M, Eichner M, Dietz K. Multiplication of different Legionella species in Mono Mac 6 cells and in Acanthamoeba castellani. Appl Environ Microbiol. 1997;63:1219–1224. doi: 10.1128/aem.63.4.1219-1224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Brien S J, Bhopal R S. Legionnaires' disease: the infective dose paradox. Lancet. 1993;342:5–6. doi: 10.1016/0140-6736(93)91877-o. [DOI] [PubMed] [Google Scholar]
- 32.Roberts E A, Coote G G. The estimation of concentration of viruses and bacteria from dilution counts. Biometrics. 1965;21:600–615. [Google Scholar]
- 33.Rowbotham T J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol. 1980;33:1179–1183. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowbotham T J. Isolation of Legionella pneumophila from clinical specimens via amoebae, and the interaction of those and other isolates with amoebae. J Clin Pathol. 1983;36:978–986. doi: 10.1136/jcp.36.9.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowbotham T J. Current views on the relationships between amoebae, legionellae and man. Isr J Med Sci. 1986;22:678–689. [PubMed] [Google Scholar]
- 36.Sanden G N, Morrill W E, Fields B S, Breiman R F, Barbaree J M. Incubation of water samples containing amoebae improves detection of legionellae by the culture method. Appl Environ Microbiol. 1992;58:2001–2004. doi: 10.1128/aem.58.6.2001-2004.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith-Somerville H E, Huryn V B, Walker C, Winters A L. Survival of Legionella pneumophila in the cold water ciliate Tetrahymena vorax. Appl Environ Microbiol. 1991;57:2742–2749. doi: 10.1128/aem.57.9.2742-2749.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swanson M S, Isberg R R. Identification of Legionella pneumophila mutants that have aberrant intracellular fates. Infect Immun. 1996;64:2585–2594. doi: 10.1128/iai.64.7.2585-2594.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tyndall R L, Domingue E L. Cocultivation of Legionella pneumophila and free-living amoebae. Appl Environ Microbiol. 1982;44:954–959. doi: 10.1128/aem.44.4.954-959.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandenesch F, Surgot M, Bornstein N, Paucod J C, Marmet D, Isoard P, Fleurette J. Relationship between free amoebae and Legionella: studies in vitro and in vivo. Int J Med Microbiol. 1990;272:265–275. doi: 10.1016/s0934-8840(11)80027-7. [DOI] [PubMed] [Google Scholar]
- 41.Vogel J P, Roy C, Isberg R R. Use of salt to isolate Legionella pneumophila mutants unable to replicate in macrophages. Ann N Y Acad Sci. 1996;797:271–272. doi: 10.1111/j.1749-6632.1996.tb52975.x. [DOI] [PubMed] [Google Scholar]
- 42.Völkl H, Friedrich F, Häussinger D, Lang F. Effect of cell volume on acridine orange fluorescence in hepatocytes. Biochem J. 1993;295:11–14. doi: 10.1042/bj2950011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Völkl H, Rehwald W, Waitz W, Häussinger D, Lang F. Acridine orange fluorescence in renal proximal tubules: effects of NH3/NH4+ and cell volume. Cell Physiol Biochem. 1993;3:28–33. [Google Scholar]
- 44.Völkl H, Busch G L, Häussinger D, Lang F. Alkalinization of acidic cellular compartments following cell swelling. FEBS Lett. 1994;338:27–30. doi: 10.1016/0014-5793(94)80110-x. [DOI] [PubMed] [Google Scholar]
- 45.Wadowsky R M, Butler L J, Cook M K, Verma S M, Paul M A, Fields B S, Keleti G, Sykora J L, Yee R B. Growth-supporting activity for Legionella pneumophila in tap water cultures and implication of hartmannellid amoebae as growth factors. Appl Environ Microbiol. 1988;54:2677–2682. doi: 10.1128/aem.54.11.2677-2682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waldegger S, Barth P, Raber G, Lang F. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci USA. 1997;94:4440–4445. doi: 10.1073/pnas.94.9.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziegler-Heitbrock H W L, Thiel E, Fütterer A, Herzof V, Wirtz A, Riethmüller G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer. 1988;41:456–461. doi: 10.1002/ijc.2910410324. [DOI] [PubMed] [Google Scholar]