Abstract
We previously reported that the norepinephrine transporter inhibitor, atomoxetine, improved standing blood pressure and lightheadedness in patients with neurogenic orthostatic hypotension (nOH). The purpose of the present study was to determine the predictors of the pressor response to atomoxetine. Patients with nOH who participated in the clinical trials (NCT00223691, NCT1316666) were included in this retrospective analysis. All subjects underwent autonomic function testing, plasma norepinephrine, systolic, diastolic blood pressure (SBP/DBP) and symptoms assessments, while seated and standing, before and 60 minutes after a single dose of atomoxetine 18 mg. A sub-set of 25 patients underwent iodine-123-labeled metaiodobenzylguanidine (MIBG) scanning to estimate the degree of cardiac sympathetic denervation. A total of 99 subjects with nOH (67±9 years old, 40 women) participated in the study, 35 with multiple system atrophy, 52 with pure autonomic failure and 12 with Parkinson’s Disease. The average orthostatic decrease in their SBP/DBP was −52±26/−22±15 mm Hg. Supine plasma norepinephrine levels predicted the standing SBP (adjusted R2 was 0.12, F (3,80) =4.66, p=0.007) and DBP (adjusted R2 was 0.18, F (3, 80) =7.04, p=0.001) in response to atomoxetine. The increase in SBP after atomoxetine was associated with the decrease in nOH-related symptoms (R2=0.14, F (1,44) =8.16 p=0.007). In conclusion, plasma norepinephrine was modestly associated with the pressor response to atomoxetine in patients with nOH. Additionally, the improvement in nOH-related symptoms was associated with the increase in the pressor response to atomoxetine.
Keywords: orthostatic hypotension, norepinephrine transporter inhibitors, atomoxetine, biomarker, norepinephrine
Graphical Abstract
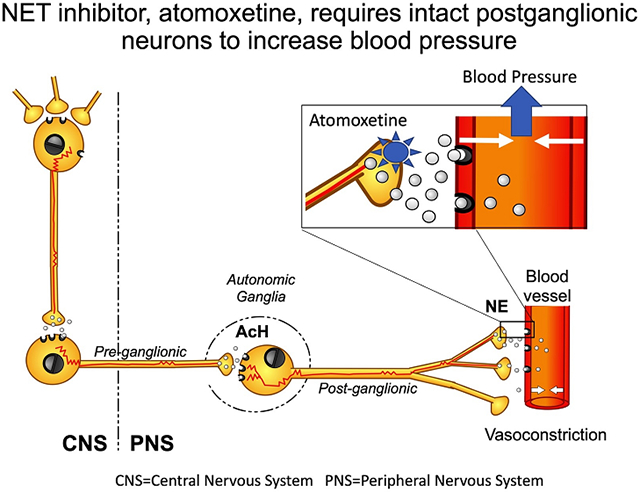
Introduction
Autonomic failure is a distinctive feature of the synucleinopathies, a group of neurodegenerative disorders caused by the abnormal accumulation of misfolded α-synuclein in the central and peripheral nervous systems.1 The presence and nature of the autonomic and neurological abnormalities in the different phenotypes depend on the distribution of the pathological α-synuclein deposits. In patients with multiple system atrophy (MSA), α-synuclein predominantly accumulates in glial cytoplasmic inclusions in the spinal cord, brainstem, basal ganglia and cerebellum, causing neurodegeneration and impairment of central autonomic pathways; however, the peripheral sympathetic fibers are mostly spared.2 In contrast, in patients with the other two synucleinopathies, pure autonomic failure (PAF) and Parkinson disease(PD), α-synuclein predominantly accumulates in peripheral post-ganglionic sympathetic neurons, resulting in denervation of the vasculature.3 Plasma norepinephrine (NE) levels while resting supine, a biomarker of the integrity of sympathetic post-ganglionic neurons, are frequently normal in MSA but low in PAF and PD.4 Despite the different pathophysiology, all synucleinopathies can manifest with neurogenic orthostatic hypotension (nOH) because of blunted baroreflex-mediated sympathetic activation and impaired vasoconstriction. However, early on in the disease process, the distinction between the synucleinopathies can be difficult to detect.5,6 In these patients, nOH results in cerebral hypoperfusion7 with lightheadedness, dizziness, syncope, and falls8 all being common symptoms, which contribute to morbidity, disability, and death in this population.9
We previously showed that atomoxetine, a norepinephrine transporter (NET) blocker that increases the availability of synaptic NE by hindering its reuptake,10(Graphical Abstract) induces an increase in blood pressure, particularly in patients with MSA. In a proof-of-concept study,11 an acute dose of 18 mg of atomoxetine increased seated systolic blood pressure (SBP) by ~50 mm Hg compared with placebo in MSA patients. In these patients, atomoxetine increased plasma NE levels by 26%, suggesting a mechanistic link between the neurohumoral response and its hemodynamic effect. In a subsequent study, atomoxetine had a greater preferential pressor response upon standing than midodrine and only atomoxetine brought about a significant reduction in clinical symptoms (lightheadedness and dizziness) compared with placebo.12
The purpose of the present study was to determine the predictors of the pressor response to atomoxetine in patients with nOH. We hypothesized that higher plasma NE levels would predict a more pronounced pressor response to atomoxetine.
To test this hypothesis, we integrated the data from two clinical trials (clinicaltrials.gov NCT00223691) that tested the acute effect of 18 mg of atomoxetine on upright blood pressure and pre-syncopal symptoms and a prospective, 3-year longitudinal study (clinicaltrials.gov NCT1316666).
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study subjects
All subjects enrolled in the randomized clinical trial (clinicaltrials.gov NCT00223691), and the prospective longitudinal study (clinicaltrials.gov NCT1316666) were included in this study. Our sample size consisted of 99 patients with nOH. OH was defined as a decrease in systolic blood pressure (SBP) of at least 20 mm Hg or diastolic blood pressure (DBP) of at least 10 mm Hg within 3 minutes of standing or 60° head-up tilt. 13 The diagnosis of nOH was ascertained following current guidelines.14 All subjects underwent autonomic function tests that showed impairment of the baroreflex, with absence of a pressor response during late phase II and phase IV of the Valsalva maneuver (VM). Patients were excluded if they had autonomic failure secondary to any of the following conditions: diabetes mellitus, amyloidosis, paraneoplastic autonomic neuropathy such as autoimmune autonomic neuropathy, or ganglionopathy. Subjects with known intolerance to atomoxetine, use of monoamine oxidase inhibitors and closed-angle glaucoma were also excluded.
The diagnosis of probable MSA was made according to current consensus criteria.15
These studies were conducted in two national referral centers for autonomic disorders (Vanderbilt Autonomic Dysfunction Center and NYU Langone Medical Center Dysautonomia Center). In addition, the Vanderbilt and NYU Institutional Review Boards approved these studies. Finally, all subjects provided informed consents and all testing procedures were standardized between these two institutions
Acute medication trial
All studies were conducted in the morning, in a post-void state, at least 2.5 hours after breakfast to avoid the post-prandial hemodynamic effects. Patients were given atomoxetine (18 mg, Eli Lilly Pharmaceuticals, Indianapolis, IN). Atomoxetine is a selective norepinephrine transporter inhibitor that increases the availability of NE in the synaptic cleft (Graphical abstract).
Studies were conducted with the patients seated on a chair, with their feet on the floor. At baseline, SBP, DBP and heart rate (HR) were measured every 2 minutes for 6 minutes. If the patient had a seated SBP>150 mm Hg, the study was stopped to prevent worsening hypertension. Orthostatic vital signs were obtained at 1, 3, 5 and 10 minutes or until tolerated. Moreover, patients were asked to rate their OH-related symptoms using the Orthostatic Hypotension Questionnaire (OHQ).16 Atomoxetine was then administered, and the SBP, DBP, and HR were assessed after 60 minutes. We repeated the orthostatic vital signs assessment and symptom evaluation at the end of this period, as described previously.11 Blood pressure and HR were measured using an automated brachial sphygmomanometer (Dinamap, GE Medical Systems Information Technologies, Milwaukee, WI). Finally, the data were digitally transferred into a custom-designed database (Access, Microsoft Corporation, Bellevue, WA).
The hemodynamic parameters and symptom questionnaires were evaluated at baseline and 60 minutes after drug administration, consistent with the time for active metabolites of atomoxetine to reach their peak plasma levels.10
Plasma Catecholamines
Supine and upright plasma samples were collected through an intravenous catheter placed >30 minutes before sampling. Catecholamines (norepinephrine, epinephrine) were quantified using high-performance liquid chromatography (HPLC) with electrochemical detection as has been previously described by our group.17
I-123-metaiodobenzylguanidine– (MIBG–SPECT)
To assess the functional integrity of cardiac postganglionic sympathetic neurons, we obtained an iodine-123-labeled metaiodobenzylguanidine (MIBG) scan in 25 patients with an early (<5 years) history of orthostatic hypotension without any motor symptoms. This imaging technique has been reported to have high sensitivity and specificity in distinguishing between MSA and Lewy body disorders (PD and PAF).18-20
Statistics
Differences in baseline demographic and clinical variables among groups were assessed based on descriptive statistics such as means and standard deviations for continuous variables.
For the primary analyses, the independent variables were the standing SBP and DBP after atomoxetine administration. The dependent variables were the supine plasma NE levels and the MIBG heart to mediastinum ratio (H/M). We used linear regression to assess the association between continuous variables. Furthermore, post-treatment standing SBP, DBP, and HR were adjusted for age and gender and analyses were performed using SPSS for Windows (version 26.0; SPSS Inc., Chicago, IL, USA).
Results
Subjects
Of the 99 subjects with nOH who participated in the study, 35 fulfilled the criteria for MSA, 52 for PAF, and 12 for PD. The subjects’ characteristics are shown in Table 1. The average orthostatic decrease was −52±26/−22±15 mm Hg (SBP/DBP). All patients had impaired cardiovascular reflexes with absence of late phase II and phase IV of the VM; the VM heart rate ratio was markedly reduced indicating impaired parasympathetic responses 1.10±0.16 (the normative values of the VM ratio are above 1.2). Thus, autonomic testing indicated severe sympathetic and parasympathetic impairment.
Table 1.
Patient Characteristics
| Parameters | MSA (N=35) |
PAF/PD (N=64) |
All patients (N=99) |
|---|---|---|---|
| Age, years | 61±7.5 | 70±8.3 | 67±9.0 |
| BMI, kg/m2 | 26±4.1 | 26±3.50 | 26±3.7 |
| Sex, female/male | 17/18 | 23/41 | 40/59 |
| Supine SBP, mm Hg | 139±24.6 | 132±24.2 | 135±24.4 |
| Supine DBP, mm Hg | 84±16.2 | 77±12.0 | 80±13.9 |
| Supine, HR, bpm | 71±11.9 | 69±10.8 | 70±11.2 |
| Upright SBP, mm Hg | 90±19.3 | 81±15.9 | 85±17.8 |
| Upright DBP, mm Hg | 59±10.8 | 53±10.9 | 56±11.2 |
| Upright HR, bpm | 85±13.7 | 82±13.4 | 83±13.4 |
| OHSA, question 1 | 4.0±3.5 | 4.4±3.6 | 4.3±3.5 |
| Supine Norepinephrine, pg/mL | 212±126.4 | 109±92.8 | 150±125.4 |
| Supine Epinephrine, pg/mL | 23±16.0 | 22±37.0 | 22±30.9 |
| Upright Norepinephrine, pg/mL | 355±235.6 | 204±192.0 | 254±218.0 |
| Upright Epinephrine, pg/mL | 40±26.0 | 31±36.2 | 34±33.2 |
OHSA, orthostatic hypotension symptom assessment; MSA, multiple system atrophy; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate, BMI, body mass index.
Acute medication trial
The baseline BP was 106±23/63±11 mm Hg, and 60 minutes after the administration of atomoxetine18 mg increased to 135±33/79±16 mm Hg (p<0.001), (Figure 1 A,B). Similarly, these patients increased their standing BP, after 3-min from 83±22/55±16 mm Hg at baseline, to 106±31/67±18 mm Hg (p<0.001) 60-min after drug administration (Figure 1 C,D). What is more, no adverse events were noted during the medication trial with the acute dose of 18 mg of atomoxetine.
Figure 1.
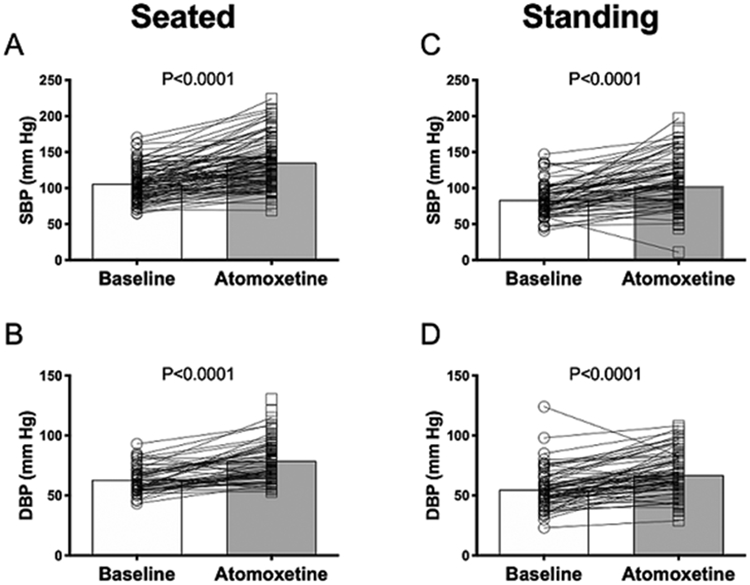
Changes in systolic and diastolic blood pressure 60 minutes after administration of 18 mg of atomoxetine during seated (A & B) and standing (C & D) positions.
Predictors of the pressor response of NET inhibition
We assessed the relationship between baseline supine plasma NE levels and standing SBP/DBP with atomoxetine. Higher supine plasma NE levels at baseline were modestly associated with a higher standing SBP (unadjusted R2 was 0.07, P=0.01) and DBP (unadjusted R2 was 0.11, P=0.002) (Figure 2 A,B). This association seem to be driven by two MSA subjects with a supine plasma NE >500 pg/dl. If these subjects are removed from analyses, the association between plasma NE levels and standing SBP (P=0.212) or DBP (0.120) was no longer significant. However, these patients were not on medication that could affect their NE measurements, and based on the underlying disease (MSA), NE levels were expected to be normal or elevated. Thus, we had no reason to believe that these patients are outliers. Nevertheless, this analysis indicates that the supine NE alone is not a reliable predictor of the pressor response to atomoxetine.
Figure 2.
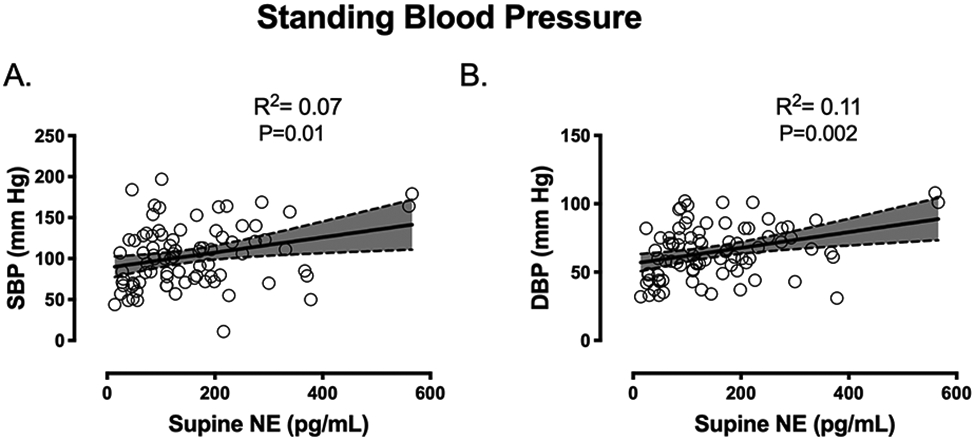
Association between supine norepinephrine (NE) levels and standing systolic blood pressure (SBP, panel A) and diastolic blood pressure (DBP, panel B) after atomoxetine.
The age and gender-adjusted R2 was 0.12 (SE, 35.15), F (3,80) =4.66, p=0.007 which indicates that the supine NE levels were modestly associated with a 12% of the variability in standing SBP after atomoxetine. Similarly, the age and gender-adjusted R2 was 0.18 (SE,17.5), F (3, 80) =7.04, p=0.002 (Figure 2 A,B) indicating that the supine NE levels were modestly associated with a 18% of the variability in standing DBP after atomoxetine.
Moreover, no differences between MIBG heart to mediastinum (H/M) ratio and standing SBP after atomoxetine were identified, (Figure 3). As expected, the majority of our patients with MSA had H/M ratio higher than 1.6 indicating preserved cardiac sympathetic innervation, consistent with previous studies. 21
Figure 3.
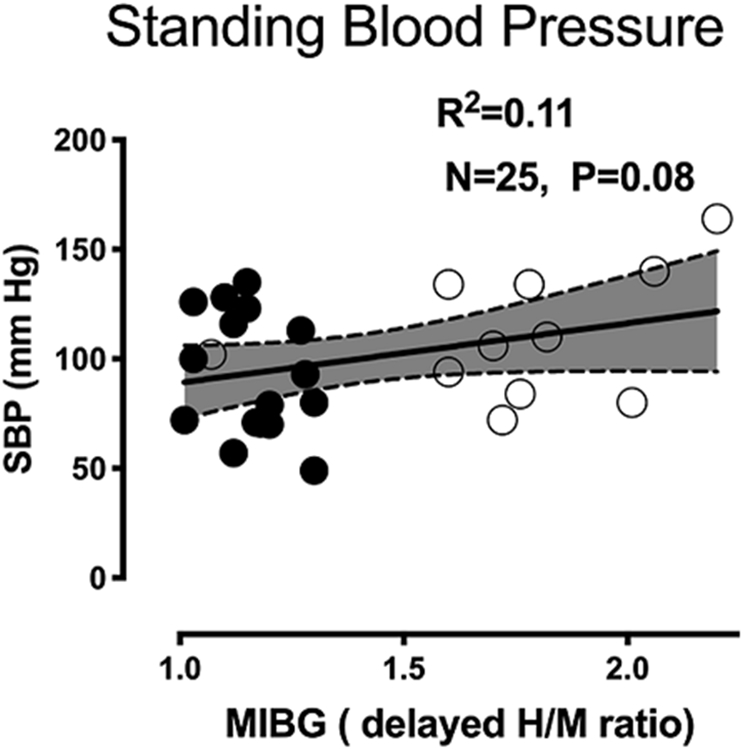
Association between standing systolic blood pressure (SBP) after atomoxetine and Iodine-123-labeled metaiodobenzylguanidine (MIBG) heart to mediastinum ratio. Close circles denoted non-MSA patients, open circles are MSA patients. Four MSA patient had H/M ratio less than 1.6.
Sub-analyses: predictors of the improvement in pre-syncopal symptoms.
We performed a sub-analysis in patients with nOH who had clinically significant symptoms as defined by the OHSA question 1 above 4 points (N=44). The average improvement in pre-syncopal symptoms as measured by a decrease in question 1 of the OHSA survey (ΔOHSA Q1) was −3.0±3.42. The improvement in ΔOHSA Q1 was associated with an increase in standing SBP after atomoxetine (R2=0.18, p=0.004) (Figure 4). The linear regression equation is ΔOHSA Q1 =−0.04(SBP)+0.79, indicating that to reduce 1 point in OHSA question 1 (clinically meaningful improvement), the standing SBP needs to increase by ~5 mm Hg after atomoxetine.
Figure 4.
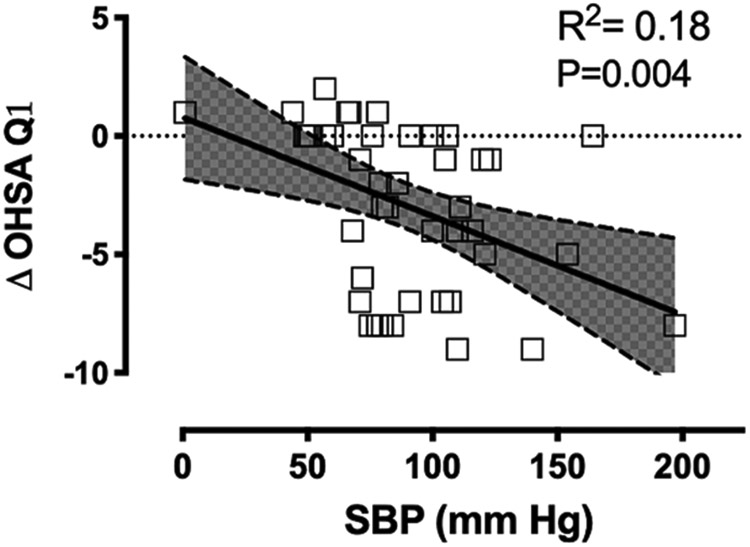
Association between the decrease in orthostatic-related symptoms (ΔOHSA Q1) and standing systolic blood pressure (SBP) after atomoxetine.
Discussion
The main findings of this study are: first, supine plasma norepinephrine levels in patients with nOH were modestly associated with the standing SBP and DBP after the administration of atomoxetine 18 mg and second, the improvement in nOH-related symptoms was directly associated with an increase in BP after atomoxetine.
Two previously published clinical trials showed that acute doses of the NET inhibitor, atomoxetine, improved standing blood pressure and pre-syncopal symptoms in patients with nOH caused by autonomic failure.11, 12 A new clinical trial (clinicaltrials.gov NCT02784535) that is testing the chronic effect of atomoxetine on pre-syncopal symptoms in nOH is underway. In this context, we hypothesized that plasma NE levels may be useful to identify nOH patients that are more likely to benefit from NET therapy. Nevertheless, our results showed that plasma NE is not a good predictor of response. What’s more, when we removed the subjects with the highest NE levels, the association was null; this was in part due to a significant variability in the blood pressure response in patients with normal or low NE levels. Therefore, we postulate that the pressor response to atomoxetine does not depend only on the level of endogenous norepinephrine but also on the different degrees of post-synaptic alpha-1 adrenergic hypersensitivity in the nOH patient.
Still, our findings provide some information about the mode of action of the NET inhibitors. The NET is responsible for ~90% reuptake of the NE released in the neurovascular junction, and is the primary mechanism by which the physiological effects of NE are terminated.22 By blocking its reuptake, atomoxetine increases NE concentrations (Graphical Abstract), prolonging its effect on the post-synaptic α1-adrenergic receptor to induce vasoconstriction. To elicit a pressor response in patients with autonomic failure, atomoxetine needs at least some residual preservation of endogenous NE release. Our findings that higher supine plasma NE levels were modestly associated with high standing blood pressure after atomoxetine administration also supports this hypothesis. The reverse relationship was observed after administration of droxidopa, a synthetic norepinephrine precursor which, contrary to atomoxetine, increases blood pressure predominantly in patients with peripheral sympathetic denervation, as shown by their low norepinephrine levels.23
Although, cardiac MIBG does not automatically entail vascular innervation, it measured spared cardiac post-ganglionic sympathetic innervation and these neurons are required for atomoxetine to elicit a pressor effect. Yet, we did not observe a significant higher standing blood pressure after atomoxetine administration in patients with preserved cardiac sympathetic innervation. Based on these data, we conclude that predicting the pressor response to atomoxetine is more complex than expected, neither plasma NE nor cardiac sympathetic preservation predict the patients who are most likely to benefit from NET therapy.
In the management of patients with nOH, the most important clinical outcome is the reduction in nOH-related symptoms. The changes in seated and standing blood pressure after receiving pressor agents are surrogate biomarkers for this clinical outcome. We evaluated whether the changes in blood pressure in response to atomoxetine predict the changes in nOH-related symptoms. For this analysis, we only included symptomatic nOH patients with an OHSA question Q1 score of 4 or more. We found a significant association between the increase in the seated systolic blood pressure with atomoxetine and the reduction in nOH-related symptoms. To reduce 1-point the OHSA Q1 score, the seated SBP needed to increase by ~ 19 mm Hg, which is a modest pressor response for this patient population, considering that the average increase for the entire group was 29±17 mm Hg. In addition to the direct peripheral pressor effects of atomoxetine, we do not rule out the possibility that its effect on CNS might contribute to the improvement in symptoms. Atomoxetine crosses the blood-brain barrier, and recent data have shown that it increases cerebral blood flow in rats,24 and healthy subjects25 in areas of the brain that are rich in the NET. As a result, the increase in endogenous NE in the brain and the associated increase in dopamine levels might also contribute to this beneficial effect.
Perspective
In this large retrospective cohort of patients with nOH, higher plasma NE levels were modestly associated with the pressor response to atomoxetine; thus, considering the significant variability in this association, we concluded that plasma NE alone may not be a reliable predictor to atomoxetine’s pressor effect, particularly in patients with low NE levels.
Table 2.
Predictors of the pressor response to atomoxetine
| Predictor: SBP w/atomoxetine | R | B | SEM | 95%CI for P | P value |
|---|---|---|---|---|---|
| Age, years | 0.83 | 0.53 | 0.43 | (−3.19, 1.37) | 0.219 |
| Gender (1=M; 0=F) | 0.22 | 18.32 | 7.86 | (2.69, 33.96) | 0.022 |
| Supine NE | 0.28 | 0.97 | 0.035 | (0.03, 33.96) | 0.007 |
| Predictor: DBP w/atomoxetine | |||||
| Age, years | −0.06 | −0.05 | 0.212 | (−0.47, 0.37) | 0.813 |
| Gender (1=M; 0=F) | 0.31 | 12.31 | 3.913 | (4.52,20.098) | 0.002 |
| Supine NE | 0.33 | 0.058 | 0.017 | 0.023, 0.92) | 0.001 |
B, unstandardized; SME, coefficient standard error
Novelty and Significance:
1). What is New?
We identified a biomarker that modestly predicted the pressor response to atomoxetine, a norepinephrine transporter inhibitor, and novel pathway for the treatment of neurogenic orthostatic hypotension.
2). What is Relevant?
The treatment of neurogenic orthostatic hypotension is challenging because of significant heterogenicity in the response to pressor agents. Our study provide evidence that plasma norepinephrine levels were modestly associated with the pressor response to atomoxetine, a norepinephrine transporter inhibitor. This class of drugs are currently being developed for the treatment of this condition.
3). Summary
We determined the predictors of the pressor response to atomoxetine in a large cohort of patients with neurogenic orthostatic hypotension. Plasma norepinephrine levels obtained in supine position were modestly associated with the pressor response to atomoxetine. Predicting the pressor response to atomoxetine is challenging; residual sympathetic activity coupled with different degrees of post-synaptic alpha-1 adrenergic hypersensitivity need to be considered.
Sources of Funding
C.A.S. was supported by FDA grant R01 FD04778-02. The study was partially funded by the Vanderbilt CTSA grant ULTR000445 from NCATS/NIH.
Conflict of Interest/Disclosures
C.A.S. has received research grant from Doris Duke Foundation. I.B., C.A.S. and H.K. received grant support from Office of Orphan Products Development. Food and Drug Administration, Grant #FD-R-04778-01-A3. C.A.S. has received speaker honorarium from Lundbeck Pharmaceuticals. I.B., C.A.S. and H.K. received consulting honoraria from Lundbeck and Theravance Biopharma. C.A.S is member of the Board for the American Autonomic Society.
JAP has received consulting honoraria from Lundbeck and is the principal investigator of trials funded by Theravance Biopharma.
References
- 1.Jordan J, Shibao C and Biaggioni I. Multiple system atrophy: using clinical pharmacology to reveal pathophysiology. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2015;25:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burn DJ and Jaros E. Multiple system atrophy: cellular and molecular pathology. MolPathol. 2001;54:419–426. [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufmann H, Hague K and Perl D. Accumulation of alpha-synuclein in autonomic nerves in pure autonomic failure. Neurology. 2001;56:980–981. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein DS, Polinsky RJ, Garty M, Robertson D, Brown RT, Biaggioni I, Stull R and Kopin IJ. Patterns of plasma levels of catechols in neurogenic orthostatic hypotension. Ann Neurol. 1989;26:558–563. [DOI] [PubMed] [Google Scholar]
- 5.Anonymous. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann H, Norcliffe-Kaufmann L and Palma JA. Baroreflex Dysfunction. The New England journal of medicine. 2020;382:163–178. [DOI] [PubMed] [Google Scholar]
- 7.Novak V, Novak P, Spies JM and Low PA. Autoregulation of cerebral blood flow in orthostatic hypotension. Stroke; a journal of cerebral circulation. 1998;29:104–111. [DOI] [PubMed] [Google Scholar]
- 8.Low PA, Opfer-Gehrking TL, McPhee BR, Fealey RD, Benarroch EE, Willner CL, Suarez GA, Proper CJ, Felten JA, Huck CA and et al. Prospective evaluation of clinical characteristics of orthostatic hypotension. Mayo Clin Proc. 1995;70:617–622. [DOI] [PubMed] [Google Scholar]
- 9.Shibao C, Lipsitz LA and Biaggioni I. ASH position paper: evaluation and treatment of orthostatic hypotension. Journal of clinical hypertension (Greenwich, Conn). 2013;15:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauer JM, Ring BJ and Witcher JW. Clinical pharmacokinetics of atomoxetine. ClinPharmacokinet. 2005;44:571–590. [DOI] [PubMed] [Google Scholar]
- 11.Shibao C, Raj SR, Gamboa A, Diedrich A, Choi L, Black BK, Robertson D and Biaggioni I. Norepinephrine transporter blockade with atomoxetine induces hypertension in patients with impaired autonomic function. Hypertension. 2007;50:47–53. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez CE, Okamoto LE, Arnold AC, Gamboa A, Diedrich A, Choi L, Raj SR, Robertson D, Biaggioni I and Shibao CA. Efficacy of atomoxetine versus midodrine for the treatment of orthostatic hypotension in autonomic failure. Hypertension. 2014;64:1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, Isaacson S, Karabin B, Kuritzky L, Lew M, Low P, Mehdirad A, Raj SR, Vernino S and Kaufmann H. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz IJ, Schondorf R, Stewart JM and van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. AutonNeurosci. 2011;161:46–48. [DOI] [PubMed] [Google Scholar]
- 15.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K and Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K and Freeman R. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. ClinAutonRes. 2012;22:79–90. [DOI] [PubMed] [Google Scholar]
- 17.He HB, Deegan RJ, Wood M and Wood AJJ. Optimization of high-performance liquid chromatographic assay for catecholamines. Journal of Chromatography. 1992;574:213–218. [PubMed] [Google Scholar]
- 18.Braune S, Reinhardt M, Schnitzer R, Riedel A and Lucking CH. Cardiac uptake of [123I]MIBG separates Parkinson's disease from multiple system atrophy. Neurology. 1999;53:1020–1025. [DOI] [PubMed] [Google Scholar]
- 19.Mitsui J, Saito Y, Momose T, Shimizu J, Arai N, Shibahara J, Ugawa Y, Kanazawa I, Tsuji S and Murayama S. Pathology of the sympathetic nervous system corresponding to the decreased cardiac uptake in 123I-metaiodobenzylguanidine (MIBG) scintigraphy in a patient with Parkinson’s disease. JNeurolSci. 2006;243:101–104. [DOI] [PubMed] [Google Scholar]
- 20.Orimo S, Kanazawa T, Nakamura A, Uchihara T, Mori F, Kakita A, Wakabayashi K and Takahashi H. Degeneration of cardiac sympathetic nerve can occur in multiple system atrophy. Acta Neuropathol. 2007;113:81–86. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein DS, Holmes C, Cannon RO, Eisenhofer G and Kopin IJ. Sympathetic cardioneuropathy in dysautonomias. New England Journal of Medicine. 1997;336:696–702. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J. Norepinephrine transporter inhibitors and their therapeutic potential. Drugs Future. 2004;29:1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palma JA, Norcliffe-Kaufmann L, Martinez J and Kaufmann H. Supine plasma NE predicts the pressor response to droxidopa in neurogenic orthostatic hypotension. Neurology. 2018;91:e1539–e1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Easton N, Marshall F, Fone K and Marsden C. Atomoxetine produces changes in cortico-basal thalamic loop circuits: assessed by phMRI BOLD contrast. Neuropharmacology. 2007;52:812–826. [DOI] [PubMed] [Google Scholar]
- 25.Marquand AF, O'Daly OG, De Simoni S, Alsop DC, Maguire RP, Williams SC, Zelaya FO and Mehta MA. Dissociable effects of methylphenidate, atomoxetine and placebo on regional cerebral blood flow in healthy volunteers at rest: a multi-class pattern recognition approach. Neuroimage. 2012;60:1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]


