Abstract
This study aimed to investigate the genetic polymorphisms of miR-146a SNPs (rs2910164, rs57095329, and rs2431697) in systemic lupus erythematosus (SLE) patients and their association with clinical manifestations. The implication of SNPs on miR-146a expression level was also evaluated. SLE patients (113) and healthy controls (104) were registered in this study. The miR-146a SNPs were genotyped by polymerase chain reaction/restriction fragment length polymorphism (PCR-RFLP). Quantitative real-time PCR was used to measure the miR-146a expression in peripheral blood mononuclear cells (PBMCs). Our results showed that the genotype frequency of miR-146a SNPs didn't deviate significantly from the Hardy–Weinberg equilibrium (HWE). The AG genotype and G allele of miR-146a (rs57095329 A/G) might be considered a risk factor for the disease (OR = 2.27; CI: 0.78–6.57 and OR: 2.35; CI: 0.79–6.92 for AG genotype and G allele, respectively). Although, no statistical significance in the distribution of miR-146a SNPs (rs2910164, rs57095329, and rs2431697) was found, indicating the lack of association between the three SNPs and SLE susceptibility. Significantly, the higher frequency of the AA genotype of miR-146a (rs57095329) was associated with pancytopenia (P < 0.05), while the CT genotype of miR-146a (rs2431697) was associated (P < 0.05) with the antiphospholipid syndrome (APS). SLE patients had significantly higher levels of miR-146a compared to controls (P < 0.05). Elevation of miR-146a was independent of any SNP genotypes. In conclusion, this pilot study shows no association between miR-146a SNPs in our population group and susceptibility to lupus. Studies concerning other miRNAs in larger sample sizes are essential for a better understanding of their role in susceptibility to SLE disease.
Keywords: SLE, miR-146a, SNPs, RFLP, PCR
Highlights
-
•
This study is concerned with genetic polymorphisms of miR-146a in Lupus patients.
-
•
The AG genotype and G allele of miR-146a (rs57095329 A/G) might be considered a risk factor.
-
•
No significance in the distribution of miR-146a SNPs (rs2910164, rs57095329, and rs2431697).
-
•
miR-146a level was significantly elevated in SLE irrespective to any SNP genotypes.
-
•
AA genotype of rs57095329 was associated with pancytopenia while the CT genotype of rs2431697 was associated with the APS.
1. Introduction
Systemic lupus erythematosus (SLE) is a multi-systemic autoimmune disease (AID) prototype. Antibodies against self-antigens, such as anti-dsDNA and antiphospholipid (aPL) antibodies, immune complex accumulation in tissues, and excessive complement activation are all hallmarks of SLE. The production of such autoantibodies affects multiple organ systems, including the skin, joints, lungs, kidneys, heart, blood vessels, and the central nervous system. Also, the raised levels of aPL antibodies are commonly associated with the antiphospholipid syndrome (APS) [1,2]. In SLE, Interestingly, the severity of some organ manifestations with consequences for morbidity and mortality varies among populations. SLE influences human health and quality of life [[3], [4], [5]]. The overall incidence and prevalence of SLE have increased from 1.4 to 21.9% and from 7.4 to 159.4 cases per 100,000 people, respectively [[5], [6], [7], [8]]. The disease incidence is higher in females than in males, with ten times (the ratio is 9:1) in reproductive age [9]. The manifestations of SLE and its prevalence differ between different geographical and ethnic populations [5,9,10]. The genetic factor represents the most potent SLE risk factor [9]. Genome-wide association studies (GWASs) have confirmed genetic associations of over a hundred loci (including different variants of protein-coding genes) with SLE risks that achieve genome-wide significance [11]. Genetic risk factors for SLE may encompass single-nucleotide polymorphisms (SNPs) [4,11,12].
MicroRNAs (miRNAs) are a class of endogenous non-coding RNAs that play essential roles in regulating gene expression. The miRNA expression profiles of SLE patients prove that miRNAs are relevant to the pathogenesis of SLE [[13], [14], [15]]. Functions of abnormally expressed miRNAs have elucidated the mechanisms involved in SLE development. One of the numerous miRNAs contributing to different aspects of the abnormal immune responses in SLE is miR-146a [16]. It is located on chromosome 5q33.3. It is a member of short oligonucleotides (∼22 nucleotides), non-coding RNA regulatory molecules, which at the post-transcriptional level can adversely regulate the expression of its target gene [[17], [18], [19]]. Taganov et al. [20) initially described it in the human acute monocytic leukemia (AML) cell line. It was unregulated in response to IL-1β, TNF-α, and lipopolysaccharide (LPS). TNF receptor-associated factor 6 (TRAF6) and IL-1 receptor-associated kinase1 (IRAK1) are regulated by miR-146a. Hence, it plays as a negative feedback regulator of the Toll-like receptor (TLR) signaling, suggesting that the increased miR-146a might inhibit the effect of these pro-inflammatory molecules as a negative feedback mechanism [20]. Also, miR-146a has been associated with regulating diverse immune functions, including T-cell selection, B cell maturation, and development of regulatory T cells (Tregs), indicating the regulatory role of miR-146ais central in immunological tolerance [21,22]. Abnormal expression of miR-146a has been associated with SLE [16,23]. The common rs2910164C/G SNP identified by Hu et al. (2008) is located at the pre-miRNA regions of miR-146a. The rs57095329 A/G SNP presents in the miR-146a promoter region [23]. Also, the rs24311697C/T SNP is located in an intergenic region on chromosome 5q33.3 with 24.23 kb downstream of the pituitary tumor–transforming 1 (PTTG1) gene and 15.3 kb upstream of miR-146a [17,19,24]. These three SNPs of miR-146a (rs2910164, rs57095329, and rs2341697) were involved in regulating miR-146a expression and maturation or mRNA recognition of miRNA-146a and causing pathogenic regulation of its target gene expression [[24], [25], [26]].
Several studies showed a significant association between these three SNPs and SLE in different populations [23,25,27]; although; limited researches have been performed on Egyptian SLE patients [[28], [29], [30]], none of them examined their effect on miR-146a expression. Therefore, the present case/control study aimed to evaluate whether the polymorphisms in miR-146a (rs2910164, rs57095329, and rs2431697) affect its mature level, conferring SLE susceptibility in the Egyptian population.
2. Subjects and methods
2.1. Patients and controls
This study enrolled one hundred thirteen SLE patients and 104 controls from the Rheumatology and Rehabilitation Department, El-Kasr El-Ainy Hospital, Cairo University, Egypt. SLE patients were diagnosed under the classification criteria of the Systemic Lupus International Collaborating Clinics (SLICC) 2012 [31]. At the time of data collection, routine laboratory and immunological investigations for each patient were assessed. Our institutional ethics committee approved the study, and all patients obtained informed consent. As SLE is a female-based disease, our patient group consists of 103 females and 10 males. The assessment of disease activity [32] in SLE patients was performed using the SLE Disease Activity Index (SLEDAI), and it was 9.28 ± 0.73. The patients were excluded if they had a viral infection, received a blood transfusion within the last three months or AIDs (except for SLE), had cancer, or pregnant women.
The healthy volunteers were 89 females and 15 males. They all had no evidence of a family history of SLE or chronic inflammatory disease, including asthma, obesity, food allergy, chronic urticarial, inflammatory bowel disease, etc.
2.2. Genomic DNA extraction
We obtained 5 ml EDTA-treated venous blood for genomic DNA extraction from each participant. According to the manufacturer's instructions, genomic DNA was extracted using GentraPuregene Blood Kit (Qiagen Company, Hilden, Germany). The DNA purity and concentration were spectrophotometrically measured by NanoDrop™ 2000/2000c (Thermo Fisher Scientific, Waltham, MA, USA). The integrity of extracted DNA was assessed by 1% agarose gel electrophoresis. The extracted pure DNA is stored at −20 °C for further experiments.
2.3. miR-146a genotyping
Gene polymorphism of miR-146a was analyzed by polymerase chain reaction-restriction fragment length polymorphism (PCR–RFLP) [33]. In Table 1, the primer sequences and restriction enzymes for three SNPs of miR-146a (rs2910164, rs57095329, and rs2431697) were summarized [19,34,35]. The PCR mixture was performed in one tube with a total reaction volume of 12.5 μl containing MyTaq™ Dream Mastermix (2 × ) (Fermentas, Thermo Fisher Scientific Inc.), 10 pmol of each primer, and 150 ng of template DNA. The PCR was done in a PCR thermal cycler (Biomatra, Germany). The length of the PCR product was visualized by 2% agarose gel electrophoresis and was estimated by comparing with 50bp and 100bp DNA Ladder (Fermentas, Thermo Fisher Scientific Inc.). The restricted PCR product was verified in 4% agarose gel electrophoresis estimated by comparing with 50 bp DNA Ladder (Fermentas, Thermo Fisher Scientific Inc.) for the three SNPs rs2910164 (Fig. 1), rs57095329 (Fig. 2), and rs2431697 (Fig. 3), respectively. As a quality measurement, 10% of all samples for each SNP had been analyzed twice.
Table 1.
Primers, PCR and restriction conditions of miR-146a SNPs [rs2910164, rs2431697 and rs57095329].
| miR-146a SNP |
Primers |
PCR conditions |
Restriction enzyme |
Length of expected bands |
||
|---|---|---|---|---|---|---|
| Step | Temperature/Time | Cycles | ||||
| rs2910164 C/G |
Forward: 5′-CATGGGTTGTGTCAGTGTCAGAGCT-3′ Reverse: 5′-TGCCTTCTGTCTCCAGTCTTCCAA-3′ |
Pre-heating | 95 °C [5 min] | 1 cycle | SacI [at 37 °C/30 min] |
CC: 122/25 bp CG: 147/122/25 bp GG: 147 bp |
| [PCR–RFLP] | Denaturation | 95 °C [45 s] | 30 cycles | |||
| Annealing | 61 °C [45 s] | |||||
| Extension | 72 °C [45 s] | |||||
| Final extension | 72 °C [7 min] | 1 cycle | ||||
| rs57095329 A/G |
Forward: 5′-GGGGCTGCGGAGAGTACCG-3′ Reverse: 5′-GGACCCTCTTGCAGCACGTGTC-'3 |
Pre-heating | 95 °C [5 min] | 1 cycle | MSpI [at 37 °C/1 h] |
AA: 160 bp AG: 160/143/17 bp GG: 143/17 |
| [PCR–RFLP] | Denaturation | 95 °C [45 s] | 30 cycles | |||
| Annealing | 61 °C [45 s] | |||||
| Extension | 72 °C [45 s] | |||||
| Final extension | 72 °C [7 min] | 1 cycle | ||||
| rs2431697 C/T |
Forward: 5′-AGAGGGGGTGAAAGAAGGAA-3′ Reverse: 5′-TTCTCAGTGCCAATGTGAGG-3′ |
Pre-heating | 95 °C [5 min] | 1 cycle | TaqI [at 65 °C/30 min] |
CC: 260 bp CT: 260/199/61 bp TT: 199/61 bp |
| [PCR–RFLP] | Denaturation | 95 °C [45 s] | 30 cycles | |||
| Annealing | 57 °C [45 s] | |||||
| Extension | 72 °C [45 s] | |||||
| Final extension | 72 °C [7 min] | 1 cycle | ||||
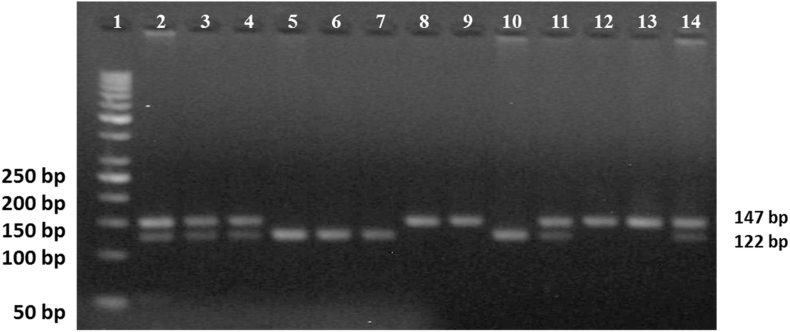
Fig. 1.
miR-146a (2910164C/G) was detected by PCR-RFLP. PCR product after digestion with SacI enzyme. Lane (1) 50 bp DNA ladder. Sample lanes (2, 3, 4, 11, and 14): CG heterozygous genotype. Sample lanes (5, 6,7, and 10): wild homozygous CC genotype. Sample lanes (8, 9, 12, and 13): mutant homozygous GG genotype. All experiments were performed on 104 controls and 113 SLE patients.
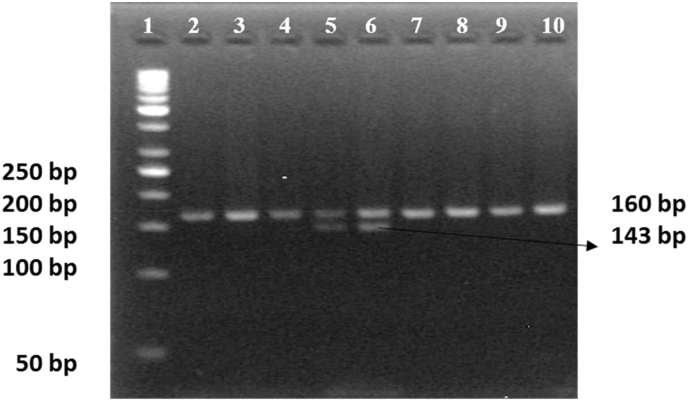
Fig. 2.
miR-146a (rs57095329 A/G) was detected by PCR-RFLP. PCR product after digestion with MSpI enzyme. Lane (1) 50 bp DNA ladder. Sample lanes (5 and 6): CG heterozygous genotype. Sample lanes (2, 3,4, and 7 to 10): AA wild genotype. All experiments were performed on 104 controls and 113 SLE patients.
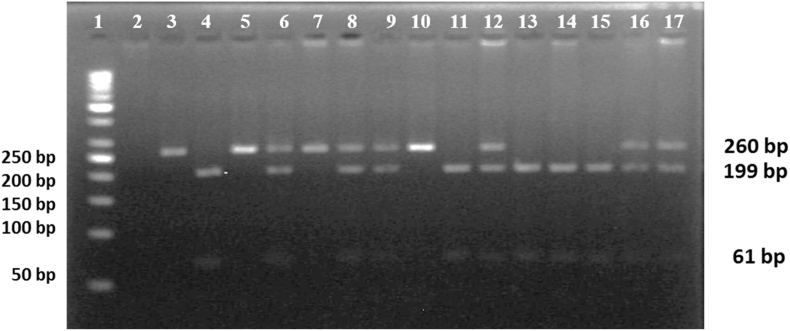
Fig. 3.
miR-146a (rs2431697C/T) was detected by PCR-RFLP. PCR product after digestion with TaqI enzyme. Lane (1) 50 bp DNA ladder. Sample lanes (6, 8, 9, 12, 16, and 17): CT heterozygous genotype. Sample lanes (3, 5, 7, and 10): wild homozygous CC genotype. Sample lanes (4, 11, and 13 to 15): mutant homozygous TT genotype. All experiments were performed on 104 controls and 113 SLE patients.
2.4. RNA extraction and quantification of miR-146a expression level
The 5 ml EDTA-treated venous blood for each patient and control were used to get peripheral blood mononuclear cells (PBMCs)as previously described [36]. According to the manufacturer's protocol, the extraction of total human RNA was done from freshly prepared PBMCs by using TRIzol (Life Technologies Ltd. UK). NanoDrop™ 2000/2000c spectrophotometer [Thermo Fisher Scientific, Waltham, MA, USA] was used to measure the concentration and purity of RNA. The RNA integrity was verified by 1%agarose gel electrophoresis. A total of 100 ng of each RNA sample was used for quantitative real-time RT-PCR (qRT-PCR) using AriaMx Real-Time PCR System (Agilent Technologies). According to the miScript PCR System manufacturer's directions, the reverse-transcribed cDNA was done using the miScript II RT kit (Qiagen, Valencia, CA, United States), which uses total RNA that contains miRNA as the starting material for cDNA synthesis (catalog numbers 218061). qRT-PCR for detecting Hs_miR-146a was carried out in 25 μL PCR reactions using miScript Primer Assays (Qiagen) and miScript SYBR Green PCR Kit (catalog numbers 218073) according to the protocol guidelines. The PCR cycles were as follows: 95 °C for 15 min, followed by 40 cycles of 95 °C for 15s, 55 °C for the 30s, and 70 °C for 30s. The post-amplification melting curve program was run at 95 °C for the 30s, 65 °C for 30s, followed by 95 °C at 30s. The U6B small nuclear RNA (RNU6B) expression was used as an endogenous control for data normalization. The cycle threshold (Ct) values variations between the tested miRNA and reference genes were computed to define the relative expression levels, calculated using the 2−ΔΔCt method with data analysis center-Qiagen (https://www.qiagen.com/us/shop/genes-andpathways/data-analysis-center-overview-page).
2.5. Statistical analysis
Statistical analyses were performed by SPSS [Statistical Package for the Social Science, IBM Corporation, USA, version 19). Comparisons between controls and SLE patients were made using an independent t-test, while Comparisons among multiple groups were performed by one-way analysis of variance (ANOVA). The Tukey test was used as a post-doc test. Clinical data were statistically described as mean ± standard deviation (±SD), frequencies when appropriate. Chi-square tests examined the allele frequency and genotype distribution differences between different groups. Odds ratios [95% confidence interval (CI)] were calculated to measure the relative risks. The online tool SNPstats was used to perform haplotype reconstruction from population genotype data, Linkage Disequilibrium (LD) parameters (D/and r2) (https://www.snpstats.net/start.htm.). Each polymorphism was examined in the control population to confirm that the distribution of the genotypes approved Hardy–Weinberg expectations (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl). All values were two-tailed, and p < 0.05 was considered to be statistically significant.
3. Results
3.1. Subject's characteristics
In this investigation, we analyzed the SNPs of rs2910164C/G, rs57095329 A/G, and rs2431697C/T in 113 SLE patients and 104 healthy controls. The mean age of patients and controls was 32.60 ± 9.50 and 27.31 ± 9.70, respectively. The clinical and demographic features and laboratory data of cases and controls are presented in Table 2, Table 3. Among SLE patients, 83% had mucocutaneous manifestation, 73% had a malar rash, 75% had ANA, 67% had anti-ds DNA, 56% had arthritis, 51% had oral ulcers, 47% had renal disorders, and 40% had serositis.
Table 2.
Biochemical characteristics of SLE patients and healthy controls.
|
Characteristics |
Control group [N = 104] | Patient group [N = 113] | P |
|---|---|---|---|
| ESR [mm/hr] | 6.19 ± 1.97 | 52.94 ± 32.84 | P<0.001 |
| Hemoglobin [g/dl] | 13.31 ± 1.33 | 11.92 ± 4.68 | P<0.01 |
| WBC [109/L] | 8.17 ± 1.29 | 7.89 ± 3.61 | NS |
| Platelets [x1000/μl] | 290.43 ± 56.90 | 235.25 ± 129.84 | P<0.001 |
| Creatinine [mg/dl] | 0.85 ± 0.21 | 0.89 ± 0.73 | NS |
| AST [IU/L] | 23.77 ± 5.21 | 23.38 ± 15.18 | NS |
| ALT [IU/L] | 20.81 ± 5.02 | 21.41 ± 15.74 | NS |
All data are presented as mean ± SD.
Erythrocytes sedimentation rate [ESR]; Alanine aminotransferase [ALT], Aspartate aminotransferase [AST], While bold cell [WBC].
Table 3.
Laboratory and clinical characteristics of SLE patients.
| Parameters | M ± SE | Parameters | No [%] | Parameters | No [%] |
|---|---|---|---|---|---|
| Disease duration [months] | 7.21 ± 0.53 | Malar rash | 82 [73%] | Nonscarring Alopecia | 34 [30%] |
| Female/Male | 103/10 | Photosensitivity | 60 [54%] | Thrombocytopenia | 27 [24%] |
| Total SLEDAI | 9.28 ± 0.73 | Oral Ulcers | 57 [51%] | Hemolytic anemia | 17 [15%] |
| Serum albumin | 3.32 ± .077 | Arthritis | 63 [56%] | Leucopenia | 34 [30%] |
| C3 titter [mg/dl] | 65.10 ± 6.60 | Serositis | 45 [40%] | Neutropenia | 18 [16%] |
| C4 titter [mg/dl] | 32.98 ± 4.46 | Renal disorders | 53 [47%] | Lymphopenia | 35 [31%] |
| Neuropsychiatric disorders | 14 [12%] | ||||
| Treatment | No [%] | Pancytopenia | 26 [23%] | ||
| Corticosteroid | 91 [81%] | Anti-nuclear antibodies | 85 [75%] | ||
| Hydroxychloroquine | 95 [84%] | Anti-dsDNA antibodies | 76 [67%] | ||
| Cyclophosphamide | 54 [48%] | Anti-phospholipid syndrome [APS] | 22 [19%] | ||
| Azathioprine | 71 [63%] | Mucocutaneous manifestation | 93 [83%] | ||
| Biologic | 5 [4%] |
3.2. Genetic polymorphism analysis
The frequency of genotypes for miR-146a SNPs did not deviate significantly from the Hardy–Weinberg equilibrium (HWE). The genotype frequencies of the three SNPs in SLE patients and controls with miR-146a (rs2910164 C/G, rs57095329 A/G, and rs2431697 C/T) genotypes and allele frequencies are shown in Table 4. Analysis of miR-146a (rs2910164C/G) SNP revealed that the CG genotype and G allele were the most frequent genotype/allele in both groups, while the CC genotype and C allele were the least frequent ones. There was no statistically significant difference in the distribution of all genotypes or alleles in miR-146a (rs2910164C/G) SNP between the two studied groups.
Table 4.
The genotype and allele frequencies of miR-146a gene polymorphisms in SLE patients and controls.
| Polymorphisms | Control [N = 104] N [%] |
SLE [N = 113] N [%] |
OR [95% CI] | P |
|---|---|---|---|---|
| miR-146a [rs2910164C/G] | ||||
|
Alleles C G |
80 [38.5%] 128 [61.5%] |
99 [43.8%] 127 [56.2%] |
1.24 [0.84–1.83] 0.80 [0.54–1.17] |
NS NS |
|
Genotypes CC |
18 [17.3%] | 24 [21.2%] | 1.28 [0.65–2.54] | NS |
| CG | 44 [42.3%] | 51 [45.2%] | 1.12 [0.65–1.92] | NS |
| GG | 42 [40.4%] | 38 [33.6%] | 0.75 [0.43–1.30] | NS |
| miR-146a [rs57095329 A/G] | ||||
|
Alleles A G |
203 [97.6%] 5 [2.4%] |
212 [94.6%] 12 [5.4%] |
0.43 [0.15–1.25] 2.27[0.78–6.57] |
NS NS |
|
Genotype AA |
99 [95.2%] | 101 [89.4%] | 0.42 [0.14–1.25] | NS |
| AG | 5 [4.8%] | 12 [10.6%] | 2.35 [0.79–6.92] | NS |
| GG | 0 [0] | 0 [0] | – | NS |
| miR-146a [rs2431697 C/T] | ||||
|
Alleles C T |
83 [39.9%] 125 [60.1%] |
97 [42.9%] 129 [57.1%] |
1.13 [0.77–1.66] 0.88 [0.60–1.29] |
NS NS |
|
Genotype CC |
14 [13.4%] | 20 [17.7%] | 1.38 [0.66–2.90] | NS |
| CT | 55 [52.9%] | 57 [50.4%] | 0.91 [0.53–1.55] | NS |
| TT | 35 [33.7%] | 36 [31.9%] | 0.92 [0.52–1.63] | NS |
Genotyping of miR-146a (rs57095329 A/G) SNP showed that the AA genotype and A allele were more frequent. In contrast, the AG genotype and G allele are less frequent genotypes/alleles in patients and controls and might be considered a risk factor for the disease (OR = 2.27; CI: 0.78–6.57 and OR:2.35; CI: 0.79–6.92 for AG genotype and G allele, respectively). A total loss of GG genotype in both groups was noticeable. miR-146a (rs57095329 A/G) SNP did not show any statistical significance in distributing all genotypes or alleles between both groups. Concerning miR-146a (rs2431697C/T) SNP, the CT genotype is the most frequent than the CC and TT genotypes, while the T allele was more frequent than the C allele in both groups. Analysis of miR-146a (rs2431697C/T) SNP demonstrated no statistical significance in the distribution of all genotypes or alleles between two groups.
As shown in Supplementary Table 1, no association was found between rs2910164C/G SNP and clinical manifestations of SLE. SLE patients with pancytopenia have a slight significant association with rs57095329 AA genotype SNP, while rs2431697 CT genotype was significantly associated with APS manifestation.
3.3. Haplotype analysis
The possible six haplotype frequencies are shown in Table 5. GAT is the major frequent haplotype that accounted for 34.5% and 30.9% of all haplotypes in both the cases and controls. The CGT haplotype was observed in SLE patients with total disappearance in the control group from the haplotype analysis.
Table 5.
Haplotype frequencies of miR-146a gene polymorphism in SLE patients and controls.
|
Haplotype |
Control [N = 104] [%] |
SLE [N = 113] [%] |
OR [95% CI] | P |
|---|---|---|---|---|
| GAT | 34.5 | 30.9 | 1.00 | NS |
| GAC | 24.7 | 23.4 | 1.08 [0.56–2.07] | NS |
| CAT | 23.3 | 20.9 | 1.03 [0.58–1.85] | NS |
| CAC | 15.1 | 19.5 | 1.34 [0.75–2.40] | NS |
| GGT | 2.4 | 2.4 | 1.19 [0.31–4.57] | NS |
| CGT | 0 | 2.9 | 117381136.37 [117381135.10–117381137.65]] | P<0.001 |
| CGC | 0 | 0 | – | – |
3.4. Expression of miR-146a
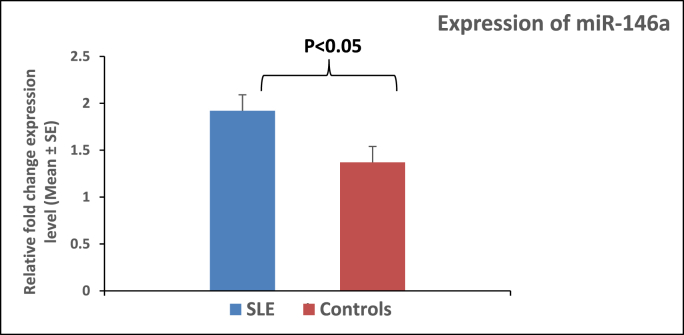
Using RT-qPCR analysis, miR-146a expression levels in PBMCs of SLE patients and healthy controls were determined. Fold change in the miR-146a expression was significantly elevated (P < 0.05) in SLE patients (1.92 ± 0.17) compared with those in healthy controls (1.37 ± 0.17) (Fig. 4).
Fig. 4.
Comparison of miR-146a expression in PBMCs from controls and SLE groups. The expression profile of miR146a was measured by real-time PCR. Each sample was duplicated, and all samples were presented as relative fold change. Each column represents the average normalized miRNA level from 104 controls and 113 SLE patients. The relative level of miRNA over U6 (internal standard) was calculated as 2−ΔΔCT, where ΔΔCT = ΔCT patient group (miRNA146a–U6) – ΔCT control group (miRNA146a–U6). Error bars represent the standard error of each group's mean calculated relative fold change. P-value was performed by T-test statistical analysis.
3.5. Differential expression of miR-146a in patients and controls according to polymorphisms
miR-146a was significantly elevated in all SLE patients in different genotypes than their control counterparts. In miR-146a rs2910164 C/G, the maximum production of miR-146a was observed in SLE patients harboring CG and GG genotypes compared with CC genotype, indicating the possible association of the G allele with an elevation of miR-146a (Table 6).
Table 6.
miR-146aexpression in different genotypes in SLE patients and controls.
|
miR-146a SNPs |
Controls [M±SE] [N = 104] |
Patients [M±SE] [N = 113] |
P |
|---|---|---|---|
| miR-146a [rs2910164C/G] | |||
| CC [18, 24] | 0.70 ± 0.43 | 1.51 ± 0.43 | P<0.001 |
| CG [44, 51] | 1.03 ± 0.22 | 2.11 ± 0.33 | P<0.001 |
| GG [38, 42] | 1.59 ± 0.39 | 2.13 ± 0.27 | P<0.001 |
| miR-146a [rs57095329 A/G] | |||
| AA [99, 101] | 1.17 ± 0.20 | 2.00 ± 0.23 | P<0.001 |
| AG [5, 12] | 2.00 ± 1.34 | 1.78 ± 0.32 | NS |
| GG [0, 0] | – | – | – |
| miR-146a [rs2431697 C/T] | |||
| CC [14, 20] | 1.39 ± 0.39 | 2.16 ± 0.59 | P<0.001 |
| CT [51, 55] | 1.02 ± 0.25 | 1.93 ± 0.24 | P<0.001 |
| TT [35, 36] | 1.37 ± 0.44 | 1.92 ± 0.27 | P<0.001 |
4. Discussion
The pathogenesis of SLE is multifactorial, involving genetic, epigenetic, environmental, and immunological factors. The balance of immune response can be enlightened by the need of the immune system to avoid uncontrolled and overreacted immune responses, which potentially lead to substantial self-damage [5]. miRNAs play a crucial role in regulating the immune response's ‘fine-tuning’ manner [37]. The importance of this regulation is demonstrated by the severe impairment of the development and/or function of immune cells when miRNA expression is experimentally altered [23]. The miR-146a is one of the critical regulators of innate and adaptive immune responses. Many studies proved that dysregulated miR-146a could contribute to the pathogenesis of autoimmune diseases, chronic inflammation, and malignancies [38].
Although several studies attempted to evaluate its relationship with SLE development [23,26,27], minimal studies correlated miR-146a polymorphisms with SLE pathogenesis in Egyptians [[28], [29], [30]]. No single research analyzed the 3 SNPs (rs2710164, rs57095329, and rs2341697) together with miR-146a expression. Therefore, the present study was assessed to investigate the polymorphisms of the miR-146a gene (rs2710164, rs57095329, and rs2431697) in SLE patients compared to controls and their effect on expression level implication on clinical manifestations.
There was no association between rs2910164C/G SNP in the present study either with disease risk or SLE clinical manifestations. In the same line with our data, several studies on Asian [39], Chinese [25], Mexican [40], and Sweden [23] populations documented that no variations were monitored in the distribution of rs2910164 SNP between SLE patients and healthy controls. Also, many meta-analysis studies reported the absence of an association between this SNP and SLE risk [12,27,41,42]. Contradictory, Alemán-Ávila et al. showed significant variations in genotypes distribution of rs2910164 SNP between Mexican SLE patients and controls [4]. Ambivalent to the current study, Wu et al. observed that Chinese patients with lupus nephritis [LN] increased the frequency of the C allele against patients without nephritis [43]. Also, they documented that the C allele and genotype carriers were associated with LN risk while the G allele worked as a protective factor against increasing the disease complication [43]. Inconsistent with our study, Labib et al. [28] found significant differences in alleles and genotypes of rs2910164 SNP between Egyptian SLE patients and controls. They also found a significant association between this SNP and clinical manifestations such as arthritis and nephritis.
The current study showed an unpredictable lack of significant association between rs57095329, rs24311697 SNPs, and SLE patients compared to their control counterparts for the other two SNPs. However, the frequency of the G allele and AG genotype of rs57095329 SNP were insignificantly elevated in patients against controls; they could be disease risk factors. AA genotype of rs57095329 SNP was significantly associated with pancytopenia, while APS was associated with CT genotype of rs24311697 SNP.
Similarly, Luo et al. [25] recruited SLE patients and controls from different places in Chinese and found no association between rs57095329 and SLE risk in Chinese patients from Hong Kong. Another study on Chinese [44] and European [23] populations didn't find any association between SLE disease and different genotypes of rs57095329 SNP. In the same line as our study, Fouda et al. and Mohammed et al. showed no significant association between rs57095329 and SLE in the Egyptian population [29,30]. In contrast, Luo et al. [25] documented a significant association between rs57095329 and SLE risk in Chinese recruited from mainland China, Taiwan, and Bangkok. Contradictory to our data, meta-analysis studies [26,27,41,42] reported a significant variation in the distribution of alleles and genotypes of rs57095329 SNP between SLE patients and controls.
In contrast to the present study, the rs2431697 T allele and TT were significantly associated with SLE risk in the Chinese population [19,25,45]. Lee et al. reported that the rs2431697 was associated with SLE risk in the Korean population [46]. From previous studies, a robust significant association between the miR-146a rs2431697 SNP was detected in both Asian and European populations [26,27]. In disagreement, Fouda et al. [29] discovered that the T allele and TT genotype of rs2431697 SNP were significantly elevated in SLE than in controls. Further, this rs2431697 SNP was associated with all SLE clinical manifestations. Hence, they documented that the miR-146a rs2431697 T allele could be a potential risk factor contributing to SLE susceptibility, development of LN, and disease activity in the Egyptian population.
In parallel, the alleles and genotypes of rs2431697 SNP were identical between SLE patients and controls in European [47] and Chinese [25] populations. Pons-Estel et al. [1] showed that SLE patients with APS have reduced survival; they are at increased risk of renal and neuropsychiatric disorders. In parallel to the Pons-Estel et al. study, our SLE patients harboring CT genotype might be at risk due to association with APS [1]. Therefore, the management of APS in SLE patients should be regarded as a priority and should be carefully balanced against this risk factor. In contrast, the rs2431697 was associated with overall SLE risk in the European population [17,23,48,49].
The results of miR-146a polymorphisms with the risk of SLE were inconsistent because ethnicity is the primary source of genetic heterogeneity [27]. Luo et al. [25] found that one Chinese population who lives in Hong Kong, Taiwan, and Beijing has a different spectrum of SLE risk alleles.
Our study showed a significantly increased level of miR-146a in PBMCs of SLE patients compared to controls. Our research agrees that the miR-146a level was overexpressed in SLE patients compared to controls in Europeans [50] and Egyptian [51] populations. In contrast, the miR-146a level was decreased as compared to healthy controls in Egyptian [52], European [23], and Chinese [25,53,54] populations. The inconsistent and controversial observations on the miR-146a expression levels in SLE may be attributed to the differences in the pathogenic pathways of the disease as well as the stage at which the samples have been taken during disease development and genetic variations [23].
The present study showed that miR-146a expression was exalted in SLE patients with different genotypes of rs2910164C/G SNP against controls. The most significant expression of miR-146a was observed with CG and GG genotypes. In the same line, Alemán-Ávila et al. showed that miR-146a expression was significantly increased with CG and GG genotypes. The C allele affects the processing and maturation of miR-146a, decreasing the generation of the mature form compared with the G allele [4]. Thus, the presence of the G allele might be associated with increased miR-146a expression in SLE patients, which could be a protective factor for the disease [4]. In agreement, rs2910164 SNP affects miR-146a expression in LN patients where the carrier harboring CC genotype had low miR-146a expression than CG and GG. Also, they documented that the minor G allele might be a protective factor from LN [43].
On the contrary, Luo et al. [25] showed no association between rs2910164SNP and miR-146a expression. On the other hand, miR-146a was significantly increased in SLE patients with AA genotype compared to controls. Similarly, rs57095329 SNP effects on miR-146a expression where AA genotype carriers had higher miR-146a expression than the other genotype carriers [25].
Finally, our study showed that miR-146a expression was elevated in SLE patients with CC genotype of rs2431697 SNP while it was reduced with CT and TT genotypes against their control counterparts. Likewise, rs2431697 SNP was associated with miR-146a expression in the Chinese and Sweden population [19,23]. Therefore, the presence of the T allele caused a reduction in miR-146a expression. Contrary to the present study, Luo et al. [25] documented no significant connection between the genotypes of rs2431697andmiR-146a expression level.
5. Conclusion
In conclusion, the current findings of our pilot study pointed to the elevation in the frequency of AG genotypes and G allele of rs57095329 A/G in SLE patients, which may suggest their role in the susceptibility of lupus in Egyptians. No significant effect of the other SNPs on disease susceptibility was observed in our data. One of the study's limitations was selecting subjects from one governorate of Egypt, and this province represents a small part of the country. Larger scale studies considering more SNPs in miR-146a and another miRNA with relative importance in SLE are still required to evaluate further the role of gene polymorphisms and their interaction with susceptibility to SLE. This might help in treatment services management.
Funding source
This study was supported by research funds from the Science and Technological Development Fund (STDF), Academy of Scientific Research and Technology (Grant No: 15123).
Author contributions
This work was carried out in collaboration between all authors: Talaat RM: designed the research, contributed new reagents/analytic tools; Bassyouni IH: Provided patients and revised the clinical part; El-Akhras BA and Ali YBM: performed the experiment; Ali YBM and Talaat RM: analyzed the data, El-Akhras BA, and Talaat RM: write the first version of the manuscript; El-Masry SA and El-Sayed IH: revised the manuscript. All authors approved the final version of the manuscript.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ncrna.2022.05.001.
Contributor Information
Basima A. El-Akhras, Email: basima.elakhras@gebri.usc.edu.eg.
Yasser B.M. Ali, Email: yasser.ali@gebri.usc.edu.eg.
Samir A. El-Masry, Email: samir.elmasry@gebri.usc.edu.eg.
Iman H. Bassyouni, Email: iman.bassyouni@kasralainy.edu.eg.
Ibrahim H. El-Sayed, Email: Ibrahimelsayed@sci.kfs.edu.eg.
Roba M. Talaat, Email: roba.talaat@gebri.usc.edu.eg, robamtalaat@gmail.com.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Pons-Estel G.J., Andreoli L., Scanzi F., Cervera R., Tincani A. The antiphospholipid syndrome in patients with systemic lupus erythematosus. J. Autoimmun. 2017;76:10–20. doi: 10.1016/j.jaut.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Abdelal I., Gaballah N., Kamel B., Kamel L. Frequency of antiphospholipid antibodies in systemic lupus erythematosus patients and their clinical associations. ZUMJ. 2022;28:130–136. doi: 10.21608/zumj.2020.20187.1632. [DOI] [Google Scholar]
- 3.Liu Z., Davidson A. Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat. Med. 2012;18:871–882. doi: 10.1038/nm.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alemán-Ávila I., Jiménez-Morales M., Beltrán-Ramírez O., Barbosa-Cobos R.E., Jiménez-Morales S., Sánchez-Muñoz F., Valencia-Pacheco G., Amezcua-Guerra L.M., Juárez-Vicuña Y., Razo-Blanco Hernández D.M., et al. Functional polymorphisms in pre-miR146a and pre-miR499 are associated with systemic lupus erythematosus but not with rheumatoid arthritis or Graves' disease in Mexican patients. Oncotarget. 2017;8:91876–91886. doi: 10.18632/oncotarget.19621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernández Cruz B., Alonso F., CalvoAlén J., Pego-Reigosa J.M., López-Longo F.J., Galindo-Izquierdo M., Olivé A., Tomero E., Horcada L., Uriarte E., et al. Differences in clinical manifestations and increased severity of systemic lupus erythematosus between two groups of Hispanics: European Caucasians versus Latin American mestizos (data from the RELESSER registry) Lupus. 2020;29:27–36. doi: 10.1177/0961203319889667. [DOI] [PubMed] [Google Scholar]
- 6.Ortega L.M., Schultz D.R., Lenz O., Pardo V., Contreras G.N. Review: lupus nephritis: pathologic features, epidemiology and a guide to therapeutic decisions. Lupus. 2010;19:557–574. doi: 10.1177/0961203309358187. [DOI] [PubMed] [Google Scholar]
- 7.Wu S., Wang J., Li F. Dysregulation of miRNA-146a contributes to the development of lupus nephritis via targeting of TRAF6. Per. Med. 2017;14:131–139. doi: 10.2217/pme-2016-0065. [DOI] [PubMed] [Google Scholar]
- 8.Stojan G., Petri M. Epidemiology of systemic lupus erythematosus: an update. Curr. Opin. Rheumatol. 2018;30:144–150. doi: 10.1097/BOR.0000000000000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Husakova M. MicroRNAs in the key events of systemic lupus erythematosus pathogenesis, Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 2016;160:327–342. doi: 10.5507/bp.2016.004. [DOI] [PubMed] [Google Scholar]
- 10.Pons-Estel G.J., Alarcón G.S., Scofield L., Reinlib L., Cooper G.S. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin. Arthritis Rheum. 2010;39:257–268. doi: 10.1016/j.semarthrit.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid S., Alexsson A., Frodlund M., Morris D., Sandling J.K., Bolin K., Svenungsson E., Jönsen A., Bengtsson C., Gunnarsson I., et al. High genetic risk score is associated with early disease onset, damage accrual and decreased survival in systemic lupus erythematosus. Ann. Rheum. Dis. 2020;79:363–369. doi: 10.1136/annrheumdis-2019-216227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y.H., Bae S.C. The miR-146a polymorphism and susceptibility to systemic lupus erythematosus and rheumatoid arthritis: a meta-analysis. Z. Rheumatol. 2015;74:153–156. doi: 10.1007/s00393-014-1509-6. [DOI] [PubMed] [Google Scholar]
- 13.Qu B., Shen N. miRNAs in the pathogenesis of systemic lupus erythematosus. Int. J. Mol. Sci. 2015;16:9557–9572. doi: 10.3390/ijms16059557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X., Peng J., Yi C. The epitranscriptome of small non-coding RNAs. Non-coding RNA Res. 2021;6:167–173. doi: 10.1016/j.ncrna.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong S., Liu C., Yin Z., Wu L., Qu B., Shen N. MicroRNAs in systemic lupus erythematosus: a perspective on the path from biological discoveries to clinical practice. Curr. Rheumatol. Rep. 2020;22:17. doi: 10.1007/s11926-020-00895-7. [DOI] [PubMed] [Google Scholar]
- 17.Harley J.B., Alarcon-Riquelme M.E., Criswell L.A., Jacob C.O., Kimberly R.P., Moser K.L., Tsao B.P., Vyse T.J., Langefeld C.D., Nath S.K., et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat. Genet. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui Y., Sheng Y., Zhang X. Genetic susceptibility to SLE: recent progress from GWAS. J. Autoimmun. 2013;41:25–33. doi: 10.1016/j.jaut.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Tang Z.M., Wang P., Chang P.P., Hasahya T., Xing H., Wang J., Hu L. Association between rs2431697 T allele on 5q33.3 and systemic lupus erythematosus: case-control study and meta-analysis. Clin. Rheumatol. 2015;34:1893–1902. doi: 10.1007/s10067-015-3045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taganov K.D., Boldin M.P., Chang K.J., Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. U.S.A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pauley K.M., Cha S., Chan E.K. MicroRNA in autoimmunity and autoimmune diseases. J. Autoimmun. 2009;32:189–194. doi: 10.1016/j.jaut.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsitsiou E., Lindsay M.A. microRNAs and the immune response. Curr. Opin. Pharmacol. 2009;9:514–520. doi: 10.1016/j.coph.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George G.P., Gangwar R., Mandal R.K., Sankhwar S.N., Mittal R.D. Genetic variation in microRNA genes and prostate cancer risk in North Indian population. Mol. Biol. Rep. 2011;38:1609–1615. doi: 10.1007/s11033-010-0270-4. [DOI] [PubMed] [Google Scholar]
- 24.Lofgren S.E., Frostegard J., Truedsson L., Pons-Estel B.A., D'Alfonso S., Witte T., Lauwerys B.R., Endreffy E., Kovács L., Vasconcelos C., et al. Genetic association of miRNA-146a with systemic lupus erythematosus in Europeans through decreased expression of the gene. Gene Immun. 2012;13:268–274. doi: 10.1038/gene.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo X., Yang W., Ye D.Q., Cui H., Zhang Y., Hirankarn N., Qian X., Tang Y., Lau Y.L., de Vries N., et al. A functional variant in microRNA-146a promoter modulates its expression and confers disease risk for systemic lupus erythematosus. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park R., Lee W.J., Ji J.D. Association between the three functional miR-146a single-nucleotide polymorphisms, rs2910164, rs57095329, and rs2431697, and autoimmune disease susceptibility: a meta-analysis. Autoimmunity. 2016;49:451–458. doi: 10.3109/08916934.2016.1171854. [DOI] [PubMed] [Google Scholar]
- 27.Ji J.D., Cha E.S., Lee W.J. Association of miR-146a polymorphisms with systemic lupus erythematosus: a meta-analysis. Lupus. 2014;23:1023–1030. doi: 10.1177/0961203314534512. [DOI] [PubMed] [Google Scholar]
- 28.Labib D.A., Shaker O.G., ElRefai R.M., Ghoniem S.A., Elmazny A. Association between miRNA-146a and polymorphisms of its target gene, IRAK1, regarding susceptibility to and clinical features of systemic lupus erythematous and multiple sclerosis. Lab. Med. 2019;50:34–41. doi: 10.1093/labmed/lmy033. [DOI] [PubMed] [Google Scholar]
- 29.Fouda M.E., Nour E Din D.M., Mahgoub M.Y., Elashkar A.E., Abdel Halim W.A. Genetic variants of microRNA-146a gene: an indicator of systemic lupus erythematosus susceptibility, lupus nephritis, and disease activity. Mol. Biol. Rep. 2020;47:7459–7466. doi: 10.1007/s11033-020-05802-y. [DOI] [PubMed] [Google Scholar]
- 30.Mohammed S.R., Shaker O.G., Mohammed A.A., Fouad N.A., Hussein H.A., Ahmed N.A. Impact of miR-155 (rs767649 A>T) and miR-146a (rs57095329 A>G) polymorphisms in System Lupus Erythematosus susceptibility in an Egyptian cohort. Eur. Rev. Med. Pharmacol. Sci. 2021;25:1425–1435. doi: 10.26355/eurrev_202102_24850. [DOI] [PubMed] [Google Scholar]
- 31.Petri M., Orbai A.M., Alarcón G.S., Gordon C., Merrill J.T., Fortin P.R., Bruce I.N., Isenberg D., Wallace D.J., Nived O., et al. Derivation and validation of the systemic lupus international collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bombardier C., Gladman D.D., Urowitz M.B., Caron D., Chang C.H. Derivation of the SLEDAI. A disease activity index for lupus patients. The committee on prognosis studies in SLE. Arthritis Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 33.Haliassos A., Chomel J.C., Tesson L., Baudis M., Kruh J., Kaplan J.C., Kitzis A. Modification of enzymatically amplified DNA for the detection of point mutations. Nucleic Acids Res. 1989;17:3606. doi: 10.1093/nar/17.9.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei L., Zhou Q., Hou S., Bai L., Liu Y., Qi J., Xiang Q., Zhou Y., Kijlstra A., Yang P. MicroRNA-146a and Ets-1 gene polymorphisms are associated with pediatric uveitis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yadegari Z.S., Akrami H., Hosseini S.V., Erfani N. miR-146a gene polymorphism and susceptibility to gastric cancer. Br. J. Biomed. Sci. 2016;73:201–203. doi: 10.1080/09674845.2016.1233790. [DOI] [PubMed] [Google Scholar]
- 36.AboElAtta A.S., Ali Y.B.M., Bassyouni I.H., Talaat R.M. Upregulation of miR-221/222 expression in rheumatoid arthritis (RA) patients: correlation with disease activity. Clin. Exp. Med. 2019;19:47–53. doi: 10.1007/s10238-018-0524-3. [DOI] [PubMed] [Google Scholar]
- 37.Fujii Y.R. Quantum language of MicroRNA: application for new cancer therapeutic targets. Methods Mol. Biol. 2018;1733:145–157. doi: 10.1007/978-1-4939-7601-0_12. [DOI] [PubMed] [Google Scholar]
- 38.Ahmadi K., Soleimani A., Motlagh S.S., Ahmadi S.B., Almasian M., Kiani A.A. Polymorphisms of pre-miR-499 rs3746444 T/C andPre-miR-146a rs2910164 C/G in the autoimmune diseases of rheumatoid arthritis and systemic lupus erythematosus in the west of Iran. Iran. J. Public Health. 2020;49:782–790. [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J., Yang B., Ying B., Li D., Shi Y., Song X., Cai B., Huang Z., Wu Y., Wang L. Association of pre-microRNAs genetic variants with susceptibility in systemic lupus erythematosus. Mol. Biol. Rep. 2011;38:1463–1468. doi: 10.1007/s11033-010-0252-6. [DOI] [PubMed] [Google Scholar]
- 40.Jiménez-Morales S., Gamboa-Becerra R., Baca V., Del Río-Navarro B.E., López-Ley D.Y., Velázquez-Cruz R., Saldaña-Alvarez Y., Salas-Martínez G., Orozco L. miR‐146a polymorphism is associated with asthma but not with systemic lupus erythematosus and juvenile rheumatoid arthritis in Mexican patients. Tissue Antigens. 2012;80:317–321. doi: 10.1111/j.1399-0039.2012.01929.x. [DOI] [PubMed] [Google Scholar]
- 41.Fu L., Jin L., Yan L., Shi J., Wang H., Zhou B., Wu X. Comprehensive review of genetic association studies and meta-analysis on miRNA polymorphisms and rheumatoid arthritis and systemic lupus erythematosus susceptibility. Hum. Immunol. 2016;77:1–6. doi: 10.1016/j.humimm.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Liu F., Liang Y., Zhao Y., Chen L., Wang X., Zhang C. Meta-analysis of association of microRNAs genetic variants with susceptibility to rheumatoid arthritis and systemic lupus erythematosus. Medicine (Baltim.) 2021;100 doi: 10.1097/MD.0000000000025689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu S., Wang J., Li F. Dysregulation of miRNA-146a contributes to the development of lupus nephritis via targeting of TRAF6. Pers. Med. 2017;14:131–139. doi: 10.2217/pme-2016-0065. [DOI] [PubMed] [Google Scholar]
- 44.Leng R., Wang W., Cen H., Zhou M., Feng C., Zhu Y., Yang X., Yang M., Zhai Y., Li B., et al. Gene-gene and gene-sex epistatic interactions of MiR146a, IRF5, IKZF1, ETS1, and IL21 in systemic lupus erythematosus. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheng Y., Xu J., Wu Y., Zuo X., Gao J., Lin Y., Zhu Z., Wen L., Yang L., Liu L., et al. Association analyses confirm five susceptibility loci for systemic lupus erythematosus in the Han Chinese population. Arthritis Res. Ther. 2015;17:85. doi: 10.1186/s13075-015-0602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee H.S., Kim T., Bang S.Y., Na Y.J., Kim I., Kim K., Kim J.H., Chung Y.J., Shin H.D., Kang Y.M., et al. Ethnic specificity of lupus-associated loci identified in a genome-wide association study in Korean women, Ann. Rheum. Dis. 2014;73:1240–1245. doi: 10.1136/annrheumdis-2012-202675. [DOI] [PubMed] [Google Scholar]
- 47.Chung S.A., Taylor K.E., Graham R.R., Nititham J., Lee A.T., Ortmann W.A., Jacob C.O., Alarcón-Riquelme M.E., Tsao B.P., Harley J.B., et al. Differential genetic associations for systemic lupus erythematosus based on anti-dsDNA autoantibody production. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orozco G., Eyre S., Hinks A., Bowes J., Morgan A.W., Wilson A.G., Wordsworth P., Steer S., Hocking L. UKRAG consortium et al., study of the common genetic background for rheumatoid arthritis and systemic lupus erythematosus. Ann. Rheum. Dis. 2011;70:463–468. doi: 10.1136/ard.2010.137174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramos P.S., Criswell L.A., Moser K.L., Comeau M.E., Williams A.H., Pajewski N.M., Chung S.A., Graham R.R., Zidovetzki R., Kelly J.A., et al. A comprehensive analysis of shared loci between systemic lupus erythematosus (SLE) and sixteen autoimmune diseases reveals limited genetic overlap. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1002406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shumnalieva R., Kachakova D., Shoumnalieva-Ivanova V., Miteva P., Kaneva R., Monov S. Whole peripheral blood miR-146a and miR-155 expression levels in Systemic lupus erythematosus patients. Acta. Rheumatol. Port. 2018;43:217–225. [PubMed] [Google Scholar]
- 51.Labib D.A., Koptan D., Ghoniem S., Salah S.H., ElShazly R., ElRefai R.M. Dysregulation of microRNA146a-5p expression in systemic lupus erythematosus females: diagnostic potential and association with ocular manifestations. Egypt. Rheumatologist. 2020;42:117–121. [Google Scholar]
- 52.Hashad D.I., Abdelmagid M.H., Elsherif S.H. microRNA146a expression in lupus patients with and without renal complications. J. Clin. Lab. Anal. 2012;26:35–40. doi: 10.1002/jcla.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hai-yan W., Yang L., Mei-hong C., Hui Z. Expression of MicroRNA146a in peripheral blood mononuclear cells in patients with systemic lupus erythematosus. Zhongguo Yi XueKeXue Yuan XueBao. 2011;33:185–188. doi: 10.3881/j.issn.1000-503X.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Tang Y., Luo X., Cui H., Ni X., Yuan M., Guo Y., Huang X., Zhou H., de Vries N., Tak P.P., et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60:1065–1075. doi: 10.1002/art.24436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.