Abstract
Context
Anti-double-stranded deoxyribonucleic acid antibodies (dsDNA Abs) are highly specific markers of systemic lupus erythematosus (SLE). Multiple methods are employed for their detection in routine diagnostics.
Objectives
The aim of this study was to evaluate a diagnostic approach for anti-dsDNA Abs using DNA-ELISA and Crithidia luciliae fluorescence test (CLIFT), in combination with antinuclear antibody (ANA) screening.
Methods
We enrolled 113 patients—53 with SLE, 50 with other systemic autoimmune rheumatic diseases (OSARD), and 10 with non-autoimmune clinical conditions (NAICC).
Patients’ samples were tested for anti-dsDNA Abs using an enzyme-linked immunosorbent assay (ELISA) and CLIFT, combined to ANA screening by indirect immunofluorescence assay (ANA-IIFA).
Results
The mean age of patients was 39.94 ± 15 years (ranges: 11–85 years). Overall, specimens from 77.3%, 11.7%, and 20% of patients with SLE, OSARD and NAICC respectively were ELISA-positive; and those from 54.7% to 4% of patients with SLE and OSARD, respectively, were CLIFT-positive. CLIFT positivity was significantly associated with high ELISA titers (p = 0.002) and homogeneous ANA-IIF pattern (p = 0.0002).
Conclusion
For better clinical relevance of anti-dsDNA antibodies, we suggest a combined detection strategy based on ELISA, CLIFT and ANA-IIFA, considering the clinical criteria of SLE.
Keywords: Anti-ds DNA antibodies, ELISA, CLIFT, Antinuclear antibodies, Diagnostic approach, Limited resource settings
Highlights
-
•
Anti-dsDNA Abs represent an excellent indicator of systemic lupus erythematosus (SLE) activity and valuable diagnostic biomarker.
-
•
We tested 103 autoimmune disease cases and 10 non-autoimmune condition cases for anti-dsDNA Abs using DNA and CLIFT, in combination with ANA-IIF screening.
-
•
CLIFT positivity was significantly associated with high DNA-ELISA titers (p = 0.002) and homogeneous ANA-IIF pattern (p = 0.0002).
-
•
High DNA-ELISA titers with a positive CLIFT are clinically relevant for the diagnosis of SLE, especially in the presence of a homogeneous ANA-IIF pattern.
1. Introduction
Anti-double-stranded deoxyribonucleic acid antibodies (anti-dsDNA Abs) embrace antibodies with a wide spectrum of fine molecular specificities that are produced persistently in the context of true autoimmunity or transiently in context of infections or other clinical conditions [1].
Despite controversies, anti-dsDNA Abs are a highly valuable biomarker and a pathogenic factor of systemic lupus erythematosus (SLE) [[2], [3], [4]]. They are considered as excellent indicators of disease activity, with their levels increasing concomitantly with the flare-ups of SLE, especially in lupus nephritis, and decreasing in response to treatment [2]. Taking into consideration the clinical manifestations, patients who met the EULAR/ACR 2019 criteria before the ACR 1982/1997 appear to have a higher frequency of class II or V lupus nephritis, anti-dsDNA and anti-Sm antibodies, and a higher SLAM (systemic lupus activity measure), suggesting that these criteria could be very useful in subgroups of patients with more severe disease [5].
Numerous studies have yielded insight into specific aspects of anti-dsDNA Abs, such as their genetics, immunogens and targets, as well as their diagnostic and pathogenic impact, particularly in SLE [1,6,7]. Yet, several aspects of these antibodies remain enigmatic, like the clinical significance of their presence in other autoimmune or non-autoimmune diseases. Further, there is a need for the development of diagnostic methods for their detection [8,9]. In fact, the clinical significance of anti-dsDNA Abs largely depends on the principle of the assay employed and the analytical variables of the methods used to quantify and characterize them [8].
In practice, anti-dsDNA Abs can be detected by enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA), Crithidia luciliae immunofluorescence test (CLIFT) [10], and recently by chemiluminescence technique [11,12]. It is hypothesized that each of these methods detects subgroups of anti-dsDNA with different properties and, of particular interest, different clinical associations [13]. Therefore, there is a need for standardization of anti-dsDNA Ab testing despite the availability of an international reference basis [14].
In the context of systemic autoimmune rheumatic diseases, auto-Abs as detected by the indirect immunofluorescence assay (IIFA) on HEp-2 cells are recognized as important diagnostic markers. In addition, screening for antinuclear antibodies (ANA) is one of the mandatory steps in the diagnosis of SLE or any other non-specific autoimmune disease [15] Indeed, the 2019 EULAR/ACR classification criteria for SLE consider that ANA would be better employed as an obligatory entry criterion than as a classification criterion [16].
Obviously, the clinical association of positive ANA-IIFA is diverse, but some ANA IIFA patterns may have a good orientation value towards certain specificities, including anti-dsDNA Abs, and therefore should order specific tests accordingly [15]. Hence, the detection and identification of auto-Abs depend on the platform used and the performance of the mentioned tests, either alone or in combination.
The aim of this study was to assess a diagnostic approach for anti-dsDNA Abs using DNA-ELISA and CLIFT in combination with ANA-IIFA.
2. Patients and methods
A cross-sectional study was carried out on 113 adults and pediatric patients presenting various clinical conditions: SLE (n = 53), other systemic autoimmune rheumatic diseases (OSARD) including rheumatoid arthritis (RA, n = 23), Sjögren syndrome (SjS, n = 9), mixed connective tissue disease (MCTD, n = 8), systemic sclerosis (SSc, n = 5), inflammatory myositis (IM, n = 5), and non-auto-immune clinical conditions (NAICC, n = 10), including 5 cases of inflammatory neurologic diseases and 5 cases of non-lupus glomerulonephritis. The diagnosis of each case was retained on the basis of clinical criteria supported by appropriate biological and radiological investigations, extracted from electronic medical records, in consultation with physicians, and in accordance with the ethical principles of medical research involving human subjects.
Blood samples collected from the patients were centrifuged (2500 g × 15 min), and the serum was separated into three cryogenic tubes, each containing 300 μl. Samples were kept at 4 °C, if their analysis was planned within the next 48 h, or frozen at −20 °C for testing planned later. Samples were tested for anti-dsDNA Abs using ELISA (Bio-Rad Kallestad Anti-dsDNA EIA™, IgG conjugate, threshold: 15 IU/mL) combined with CLIFT (Kallstad, Bio-rad, threshold: 1:10). Screening for ANA was performed by the IIF method using a Hep-2 cell substrate (Kallstad, Bio-rad, threshold: 1:160 for patients older than 18 and 1:80 for those younger than 18).
For statistical analysis, Chi-Square test was performed using Epi Info7™ software, and the variables studied were considered significant if the p-value was less than 0.05.
3. Results
The mean age of patients was 39.94 ± 15 years (range, 11–85 years), and they were predominantly females (female to male sex ratio = 4.94).
Overall, 42.4% (n = 48), 27.4% (n = 31), and 78.8% (n = 89) of patients with selected clinical conditions were positive for DNA-ELISA, DNA-CLIFT, and ANA-IIFA anti-dsDNA Ab screening, respectively (Table 1).
Table 1.
General results of DNA-ELISA, DNA-CLIFT and ANA-IIF for selected clinical conditions.
| Testing Methods |
DNA–ELISA Positive patients |
DNA–CLIFT Positive patients |
ANA–IIFA Positive patients |
|||
|---|---|---|---|---|---|---|
| Clinical Conditions | n | (%) | n | (%) | n | (%) |
| SLE, n = 53 | 41 | (77.3) | 29 | (54.7) | 48 | (90.56) |
| RA, n = 23 | 1 | (4.3) | 0 | (0) | 10 | (43.4) |
| SjS, n = 9 | 0 | (0) | 0 | (0) | 7 | (77.7) |
| MCTD, n = 8 | 3 | (37.5) | 2 | (20) | 8 | (100) |
| SSc, n = 5 | 0 | (0) | 0 | (0) | 5 | (100) |
| IM, n = 5 | 1 | (20) | 0 | (0) | 3 | (60) |
| NAICC, n = 10 | 2 | (20) | 0 | (0) | 8 | (80) |
| Total: n = 113 | 48 | (42.4) | 31 | (27.4) | 89 | (78.8) |
SLE: systemic lupus erythematosus; RA: rheumatoid arthritis; SjS: Sjögren syndrome; MCTD: mixed connective tissue disease; SSc: systemic sclerosis; IM: inflammatory myositis; NAICC: non auto-immune clinical conditions; ELISA: enzyme-linked immunosorbent assay; CLIFT: Crithidia luciliae immunofluorescence test; ANA-IIFA: antinuclear antibody indirect immunofluorescence assay.
The proportion of ELISA-positive patients with SLE was more than that of CLIFT-positive ones (77.3% [n = 41] vs 54.7% [n = 29]). Among the 50 patients with OSARD, three patients with MCTD, one with RA, and one with IM were ELISA-positive. However, only two of the patients with MCTD were CLIFT-positive. Among the 10 patients with NAICC, only 2 were ELISA-positive (Table 1).
The DNA-ELISA test was positive for 48 patients (42.4%), among which 34 patients had titers higher than 50 IU/mL, 16 patients had titers varying between 30 and 50 IU/mL, whereas 32 patients had equivocal titers (15–30 IU/mL), and 31 patients were negative.
ANA were positive for 78.7% (n = 89) of patients, distributed as follows: 48 patients (90.5%) with SLE, 10 (43.4%) with RA, 8 (100%) with MCTD, 7 (77.7%) with SjS, 5 (100%) with SSc, 3 (60%) with IM, and 8 (80%) with NAICC (Table 1).
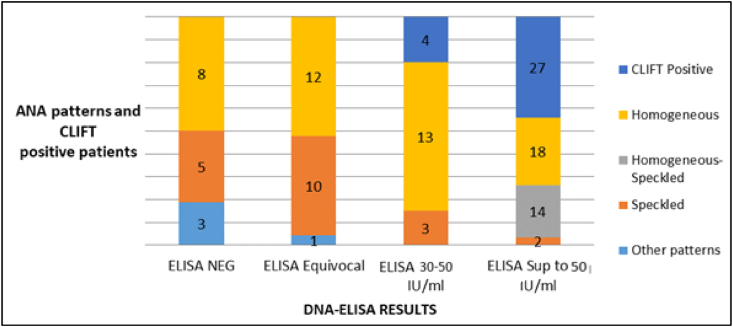
Among the 50 patients positive for DNA-ELISA, 31 (62%) were also positive for CLIFT, and these patient were significantly associated with high titers of DNA-ELISA (>50 IU/mL; p = 0.002); the titers of four patients varied between 30 and 50 IU/mL. None of the negative or equivocal DNA-ELISA patients were positive for CLIFT (Fig. 1).
Fig. 1.
Distribution of ELISA-DNA titers according to ANA-IIFA patterns and CLIFT results.
ANA: antinuclear antibodies; CLIFT: Crithidia luciliae immunofluorescence test; ELISA: enzyme-linked immunosorbent assay; NEG: negative; Sup: superior.
The most prevalent fluorescence pattern was homogeneous, observed in 57.3% of ANA-IIF positive patients (n = 51), followed by speckled pattern in 22.5% (n = 20), mixed homogeneous-speckled in 15.7% (n = 14), and nucleolar (n = 2), nucleolar-speckled (n = 1), and nucleolar-homogeneous (n = 1) in 4.5% (n = 4) (Table 2).
Table 2.
Distribution of ANA-IIFA patterns according to DNA-ELISA results and titers.
| DNA-ELISA Results ANA-IF Patterns | ELISA-Negative | ELISA-Equivocal | ELISA-Positive 30–50 IU/mL |
ELISA-Positive >50 IU/mL |
Total |
|---|---|---|---|---|---|
| Homogeneous (Ho) | 8 | 12 | 13 | 18 | 51 |
| Speckled (Sp) | 5 | 10 | 3 | 2 | 20 |
| Mixed Ho-Sp | 0 | 0 | 0 | 14 | 14 |
| Other Patterns | 3 | 1 | 0 | 0 | 4 |
| ANA-Negative | 15 | 9 | 0 | 0 | 24 |
| CLIFT | 0 | 0 | 4 | 27 | 31 |
| Total | 31 | 32 | 16 | 34 | 113 |
DNA: deoxyribonucleic acid; ELISA: enzyme-linked immunosorbent assay; CLIFT: Crithidia luciliae immunofluorescence test; ANA-IIFA: antinuclear antibody immunofluorescence assay.
High DNA-ELISA titers were associated mainly with homogeneous and/or mixed homogeneous-speckled patterns (34 cases). The 30–50 IU/mL ELISA-positive titers were associated with homogeneous (16 patients) and speckled patterns (3 cases). The equivocal or negative DNA-ELISA results were associated with various patterns, including homogeneous, speckled, nucleolar, homogeneous-nucleolar, and nucleolar-speckled.
The positive cases in CLIFT (62% of cases) corresponded to homogeneous, mixed homogeneous-speckled, and speckled ANA-IIF patterns in 15, 14 and 2 cases respectively. A positive result for CLIFT was significantly associated to a greater extent with homogeneous staining than with speckled staining (p = 0.0002). In lines with the ANA-IIF pattern and fluorescence intensity, the probability of detecting anti-dsDNA Abs was proportional to the titer intensity of homogeneous staining (Table 3).
Table 3.
Probability of detecting anti-dsDNA Abs using the ANA-IF pattern and fluorescence intensity (Results are given in percentage).
| ANA-IF titers | 1:160 | 1:320 | 1:640 | 1:1280 | Irrespective of IF Intensity |
|---|---|---|---|---|---|
| Anti-dsDNA | |||||
| Homogeneous (Ho) | 0 | 3.2 | 19.3 | 29.0 | 31.4 |
| Speckled (Sp) | 0 | 0 | 3.2 | 0 | 0.5 |
| Mixed Ho-Sp | 0 | 0 | 19.3 | 25.8 | 1.0 |
| Other Patterns | 0 | 0 | 0 | 0 | 0 |
| Irrespective of ANA pattern | 0 | 0.1 | 14.6 | 19.1 | 34.8 |
This was estimated to be 52.5% when the homogeneous IF intensity was greater than or equal to 1:320 and to be 45.1% with a mixed homogeneously speckled IF staining with ANA titers greater than or equal to 1:640. The probability of detecting anti-dsDNA Abs was high with homogeneous patterns irrespective of the ANA-IIF intensity, and was proportionally associated with ANA-IIF titers irrespective of ANA-IIF patterns. Similarly, the probability was high with mixed homogeneous-speckled IIF staining (Table 3).
4. Discussion
The immunology laboratory plays an important role in diagnosing and monitoring SLE as well as other connective tissue diseases [17]. The detection of anti-dsDNA Abs is generally conducted with respect to the best adequacy between biological results and clinical relevance.
Using a sequential approach, this study established that DNA-ELISA could be used to detect and quantify anti-dsDNA Abs with moderately high sensitivity (77.3% of SLE patients), but with a low specificity (42.4% of all clinical conditions). DNA-CLIFT corroborated the positive results of DNA-ELISA in only 54.7% of SLE patients and 27.4% of all patients.
ELISA is a useful and widely used laboratory test to screen for anti-dsDNA Abs [13,18] because of its high sensitivity, quantitative results, and simplicity, as well as relatively low cost compared to other assays [19,20], while CLIFT provides higher clinical specificity than ELISA [10]. Therefore, many studies recommend confirming positive ELISA results using CLIFT or rarely Farr test, thus ensuring improved specificity [17,21].
Most commercial ELISA systems have great advantages in routine laboratory testing but may detect not specific anti-dsDNA Abs, which results in false positives in non-SLE patients [[22], [23]]. In our study, two well-established anti-dsDNA Abs were observed in non-SLE patients, which is consistent with published data suggesting the possible association of anti-dsDNA Abs with clinical conditions other than SLE.
Actually, ELISA may exhibit positive results of anti-dsDNA Abs in other autoimmune diseases [24], [25], [26]], as well as in other clinical conditions such as chronic liver diseases, Epstein-Barr, hepatitis B virus and bacterial infections, and even in cancers and blood donors [27]. It is also important to rule out other potential causes of false anti-ds DNA Abs, especially drugs like hydralazine, tumor necrosis factor inhibitors, interferon treatment, and sulfasalazine, to ensure accurate diagnosis of SLE [28,29].
Therefore, these shortcomings underline the need of considering other useful and efficient methods for more accuracy of anti-dsDNA Ab results [20,28].
ELISA detects anti-dsDNA Abs of low avidity and uncertain clinical relevance [13], whereas Farr assays detect those of high avidity, which are more related to SLE pathogenesis, particularly in nephritis [13,30]. Accordingly, an important cause of discrepancies between results obtained with different methods might be related to the avidity of the Abs. In fact, ELISA assays detect Abs of both low and high avidity, while Farr and CLIFT assays predominantly detect those of high avidity [13,30].
However, Farr assay requires a special set-up for radioactive labeling of DNA and is also time-consuming, because of which its use has been declining in recent years, to be definitively abandoned in favor of other alternatives, such as that using intercalating fluorescent dsDNA dye, without the radioactive material and organic solvents [31]. Similarly, CLIFT may be considered as an alternative confirmatory test and its relevance seems increasingly defendable. Indeed, despite its low sensitivity, globally ranging from 30% to 60% [19,25,32,33], but owing to its high specificity, CLIFT is considered useful in the diagnosis of SLE and to distinguish it from other diseases [17,31,34].
We assessed the possibility of detecting anti-dsDNA Abs in ANA-positive patients using immunofluorescence. The ANA pattern may be indicative of the type of autoimmune reactivities responsible for IF staining [35]. In fact, homogeneous IF pattern is regularly correlated to anti-dsDNA Abs, and speckled staining to anti-ENA specificities, especially ribonucleoproteins [36,37]. However, the identification of more antinuclear specificities may certainly increase the positive predictive value of positive ANA for diagnosing SLE or other connective tissue diseases, such as Sjögren syndrome and polymyositis [38].
According to the guidelines of the International Consensus on ANA Patterns (ICAP) initiative which previously established the clinical relevance of distinct HEp-2 IIFA patterns, a homogeneous pattern is generally found in patients with SLE, chronic autoimmune hepatitis or juvenile idiopathic arthritis, and if SLE is clinically suspected, it is recommended to perform a follow-up test for anti-dsDNA Abs [15]. In our study, anti-dsDNA Abs were significantly associated with homogeneous ANA staining, with the probability increasing proportionally with the ANA fluorescence intensity. However, two of the speckled ANA-IF patterns were also positive in CLIFT among the 29 patients of the homogeneous and mixed homogeneous-speckled ANA patterns (p = 0.002). The association of anti-dsDNA Abs with a speckled IIF pattern has already been described. Therefore, the rare occurrence of anti-dsDNA Abs at clinically significant ANA titers associated with such a pattern may mask the diagnosis of SLE [39].
The existence of anti-dsDNA Abs in the absence of positive ANA results is thought to be a rare phenomenon that is attributed to laboratory errors [40]. Nevertheless, low titers of ANA are not necessarily insignificant, as they may also contain anti-dsDNA auto-Abs [35].
Finally, as limitations, the results of this cross-sectional study may have been biased by the relatively small sample size and the possible imprecision of the diagnosis of both SLE and other clinical conditions. Therefore, for more relevance, these results must be validated on a larger sample size, including well-documented cases of SLE, its differential diagnoses or even non-autoimmune clinical conditions.
5. Conclusion
The results of our study allow us to suggest a diagnostic strategy based on the detection and quantification of anti-dsDNA Abs by ELISA, followed if positive by CLIFT and ANA-IIF screening. High titers of anti-DNA Abs on ELISA associated with positive CLIFT are almost relevant, especially when associated with a homogeneous ANA-IIF pattern, taking into consideration the clinical criteria of SLE.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Informed consent
Not applicable.
Author's contribution
Brahim ADMOU: Study conceptualization and manuscript writing.
Fatima-ezzohra EDDEHBI: Data collection and references’ management.
Lahcen EL MOUMOU: Laboratory investigation.
Saad EL MOJADILI: Software and statistical analysis.
Abdelmouïne SALAMI: Laboratory investigation and editing.
Imane BRAHIM: Data curation and review.
Raja HAZIME: Validation and supervision.
Declaration of competing interest
The authors have no conflict of interest to declare.
References
- 1.Rekvig O.P. Anti-dsDNA antibodies as a classification criterion and a diagnostic marker for systemic lupus erythematosus: critical remarks. Clin. Exp. Immunol. 2015;179(1):5–10. doi: 10.1111/cei.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manson J.J., Ma A., Rogers P., Mason L.J., Berden J.H., van der Vlag J., et al. Relationship between anti-dsDNA, anti-nucleosome and anti-alpha-actinin antibodies and markers of renal disease in patients with lupus nephritis: a prospective longitudinal study. Arthritis Res. Therap. 2009;11(5):1–9. doi: 10.1186/ar2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rekvig O.P. Autoimmunity and SLE: factual and semantic evidence-based critical analyses of definitions, etiology, and pathogenesis. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.569234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rekvig O.P. The dsDNA, Anti-dsDNA antibody, and lupus nephritis: what we agree on, what must be done, and what the best strategy forward could be. Front. Immunol. 2019;10:1104. doi: 10.3389/fimmu.2019.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ugarte-Gil M.F., Pons-Estel G.J., Harvey G.B., Vilá L.M., Griffin R., Alarcón G.S. Arthritis Care & Research; 2020. Applying the 2019 EULAR/ACR Lupus Criteria to Patients from the LUMINA Cohort. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan M.R., Wang C., Marion T.N. Anti-DNA autoantibodies initiate experimental lupus nephritis by binding directly to the glomerular basement membrane in mice. Kidney Int. 2012;82(2):184–192. doi: 10.1038/ki.2011.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seredkina N., Van Der Vlag J., Berden J., Mortensen E., Rekvig O.P. Lupus nephritis: enigmas, conflicting models and an emerging concept. Mol. Med. 2013;19(1):161–169. doi: 10.2119/molmed.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghirardello A., Villalta D., Morozzi G., Afeltra A., Galeazzi M., Gerli R., et al. Evaluation of current methods for the measurement of serum anti–double-stranded DNA antibodies. Ann. New York Acad. Sci. 2007;1109(1):401–406. doi: 10.1196/annals.1398.045. [DOI] [PubMed] [Google Scholar]
- 9.Isenberg D.A., Manson J.J., Ehrenstein M.R., Rahman A. 2007. Fifty Years of Anti-ds DNA Antibodies: Are We Approaching Journey's End? [DOI] [PubMed] [Google Scholar]
- 10.Lakos G., Gonzalez M., Flaherty D., Bentow C., Ibarra C., Stimson D., et al. Detection of anti-dsDNA antibodies by computer-aided automated immunofluorescence analysis. J. Immunol. Methods. 2016;433:17–22. doi: 10.1016/j.jim.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Bentow C., Lakos G., Martis P., Wahl E., Garcia M., Viñas O., et al. International multi-center evaluation of a novel chemiluminescence assay for the detection of anti-dsDNA antibodies. Lupus. 2016;25(8):864–872. doi: 10.1177/0961203316640917. [DOI] [PubMed] [Google Scholar]
- 12.Infantino M., Meacci F., Bentow C., Martis P., Benucci M., Afeltra A., et al. Clinical comparison of QUANTA Flash dsDNA chemiluminescent immunoassay with four current assays for the detection of anti-dsDNA autoantibodies. J. Immunol. Res. 2015;2015 doi: 10.1155/2015/902821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egner W. The use of laboratory tests in the diagnosis of SLE. Journal of clinical pathology. 2000;53(6):424–432. doi: 10.1136/jcp.53.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Launay D., Schmidt J., Lepers S., Mirault T., Lambert M., Kyndt X., et al. Comparison of the Farr radioimmunoassay, 3 commercial enzyme immunoassays and Crithidia luciliae immunofluorescence test for diagnosis and activity assessment of systemic lupus erythematosus. Clin. Chim. Acta. 2010;411(13-14):959–964. doi: 10.1016/j.cca.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Damoiseaux J., Andrade L.E.C., Carballo O.G., Conrad K., Francescantonio P.L.C., Fritzler M.J., et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: the International Consensus on ANA patterns (ICAP) perspective. Ann. Rheumat. Dis. 2019;78(7):879–889. doi: 10.1136/annrheumdis-2018-214436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aringer M., Costenbader K., Daikh D., Brinks R., Mosca M., Ramsey-Goldman R., et al. 2019 European League against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71(9):1400–1412. doi: 10.1002/art.40930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinico R.A., Radice A. The clinical immunology laboratory in diagnosis and monitoring of systemic lupus erythematosus and connective tissue diseases. G Ital Nefrol. 2005;22(Suppl 33):S21–S26. [PubMed] [Google Scholar]
- 18.Wichainun R., Kasitanon N., Wangkaew S., Hongsongkiat S., Sukitawut W., Louthrenoo W. Sensitivity and specificity of ANA and anti-dsDNA in the diagnosis of systemic lupus erythematosus: a comparison using control sera obtained from healthy individuals and patients with multiple medical problems. Asian Pacific J. Aller. Immunol. 2013;31(4):292. doi: 10.12932/AP0272.31.4.2013. [DOI] [PubMed] [Google Scholar]
- 19.Janyapoon K., Jivakanont P., Choosang K., Surbrsing R., Charoenying V., Baithong S. Comparative study of anti-double stranded DNA detection by ELISA and Crithidia luciliae immunofluorescence. Southeast Asian J. Tropic. Med. Pub. Health. 2003;34(3):646–650. [PubMed] [Google Scholar]
- 20.Boguszewska K., Szewczuk M., Urbaniak S., Karwowski B.T. Immunoassays in DNA damage and instability detection. Cell. Mol. Life Sci. 2019;76(23):4689–4704. doi: 10.1007/s00018-019-03239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agmon-Levin N., Damoiseaux J., Kallenberg C., Sack U., Witte T., Herold M., et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann. Rheumat. Dis. 2014;73(1):17–23. doi: 10.1136/annrheumdis-2013-203863. [DOI] [PubMed] [Google Scholar]
- 22.Wigand R., Gottschalk R., Falkenbach A., Matthias T., Kaltwasser J.P., Hoelzer D. Detection of dsDNA antibodies in diagnosis of systemic lupus erythematosus–comparative studies of diagnostic effectiveness of 3 ELISA methods with different antigens and a Crithidia luciliae immunofluorescence test. Zeitschrift fur Rheumatologie. 1997;56(2):53–62. doi: 10.1007/s003930050020. [DOI] [PubMed] [Google Scholar]
- 23.Werle E., Blazek M., Fiehn W. The clinical significance of measuring different anti-dsDNA antibodies by using the Farr assay, an enzyme immunoassay and a Crithidia luciliae immunofluorescence test. Lupus. 1992;1(6):369–377. doi: 10.1177/096120339200100606. [DOI] [PubMed] [Google Scholar]
- 24.Derksen R., Bast E., Strooisma T., Jacobs J.W.G. A comparison between the Farr radioimmunoassay and a new automated fluorescence immunoassay for the detection of antibodies against double stranded DNA in serum. Ann. Rheumat. Dis. 2002;61(12):1099–1102. doi: 10.1136/ard.61.12.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haugbro K., Nossent J.C., Winkler T., Figenschau Y., Rekvig O.P. Anti-dsDNA antibodies and disease classification in antinuclear antibody positive patients: the role of analytical diversity. Ann. Rheumat. Dis. 2004;63(4):386–394. doi: 10.1136/ard.2003.016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riboldi P., Gerosa M., Moroni G., Radice A., Allegri F., Sinico A., et al. Anti-DNA antibodies: a diagnostic and prognostic tool for systemic lupus erythematosus? Autoimmunity. 2005;38(1):39–45. doi: 10.1080/08916930400022616. [DOI] [PubMed] [Google Scholar]
- 27.Žigon P., Lakota K., Čučnik S., Švec T., Ambrožič A., Sodin-Šemrl S., et al. Comparison and evaluation of different methodologies and tests for detection of antidsdna antibodies on 889 Slovenian patients' and blood donors' sera. Croatian Med. J. 2011;52(6):694–702. doi: 10.3325/cmj.2011.52.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barzilai O., Ram M., Shoenfeld Y. Viral infection can induce the production of autoantibodies. Curr. Opin. Rheumatol. 2007;19(6):636–643. doi: 10.1097/BOR.0b013e3282f0ad25. [DOI] [PubMed] [Google Scholar]
- 29.Sladkova V., Mareš J., Lubenova B., Hluštík P., Kanovskỳ P. Drug-induced systemic lupus erythematosus in interferon beta-1b therapy. Neuroendocrinol. Lett. 2011;32(1) [PubMed] [Google Scholar]
- 30.Hamann D.S.R. second ed. ed. Elsevier; Burlington (MA: 2007. dsDNA Autoantibodies; p. 872. [Google Scholar]
- 31.Lakota K., Švec T., Kveder T., Sodin-Šemrl S., Žigon P., Ambrožič A., et al. Autoantibodies against dsDNA measured with nonradioactive Farr assay—an alternative for routine laboratories. Clin. Rheumatol. 2019;38(2):353–359. doi: 10.1007/s10067-018-4271-3. [DOI] [PubMed] [Google Scholar]
- 32.Antico A., Platzgummer S., Bassetti D., Bizzaro N., Tozzoli R., Villalta D., et al. Diagnosing systemic lupus erythematosus: new-generation immunoassays for measurement of anti-dsDNA antibodies are an effective alternative to the Farr technique and the Crithidia luciliae immunofluorescence test. Lupus. 2010;19(8):906–912. doi: 10.1177/0961203310362995. [DOI] [PubMed] [Google Scholar]
- 33.Chiaro T.R., Davis K.W., Wilson A., Suh-Lailam B., Tebo A.E. Significant differences in the analytic concordance between anti-dsDNA IgG antibody assays for the diagnosis of systemic lupus erythematosus—implications for inter-laboratory testing. Clin. Chim. Acta. 2011;412(11-12):1076–1080. doi: 10.1016/j.cca.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 34.Gerlach S., Affeldt K., Pototzki L., Kraus.e C., Voigt J., Fraune J., et al. Automated evaluation of Crithidia luciliae based indirect immunofluorescence tests: a novel application of the EUROPattern-Suite technology. J. Immunol. Res. 2015;2015 doi: 10.1155/2015/742402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peene I., Meheus L., Veys E.M., De Keyser F. Detection and identification of antinuclear antibodies (ANA) in a large and consecutive cohort of serum samples referred for ANA testing. Ann. Rheumat. Dis. 2001;60(12):1131–1136. doi: 10.1136/ard.60.12.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sebastian W., Roy A., Kini U., Mullick S. Correlation of antinuclear antibody immunofluorescence patterns with immune profile using line immunoassay in the Indian scenario. Indian J. Pathol. Microbiol. 2010;53(3):427. doi: 10.4103/0377-4929.68262. [DOI] [PubMed] [Google Scholar]
- 37.Sharmin S., Ahmed S., Saleh A.A., Rahman F., Choudhury M.R., Hassan M.M. Association of immunofluorescence pattern of antinuclear antibody with specific autoantibodies in the Bangladeshi population. Bangladesh Med. Res. Council Bull. 2014;40(2):74–78. doi: 10.3329/bmrcb.v40i2.25225. [DOI] [PubMed] [Google Scholar]
- 38.Slater C.A., Davis R.B., Shmerling R.H. Antinuclear antibody testing: a study of clinical utility. Arch. Int. Med. 1996;156(13):1421–1425. [PubMed] [Google Scholar]
- 39.Almogren A. Anti-double stranded antibody. Saudi Med. J. 2010;31(1):32–36. [PubMed] [Google Scholar]
- 40.Petri M., Orbai A.-M., Alarcón G.S., Gordon C., Merrill J.T., Fortin P.R., et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheumatism. 2012;64(8):2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]