Abstract
Background/Aims
More and more literature describes how to overcome challenges in the implementation of adaptive designs for trials for drug approval process. Most adaptive trials were conducted in Western Europe or USA for Phase II or Phase II/III settings; however, examples of non-oncology pivotal adaptive trials used for regulatory approval in Japan are rare. This article elaborates on our experience with designing and implementing a Phase III adaptive confirmatory trial permitting unblinded sample size re-estimation and futility analysis after a single interim analysis in Japanese patients with palmoplantar pustulosis (PPP) receiving guselkumab.
Methods
We provide insights into consideration at the design stage of an adaptive study, including design options, development duration, operational risks, and statistical methods. We also share our experience from two aspects of implementation: conducting an adaptive clinical trial in Japan and setting up a Japanese domestic independent data monitoring committee.
Results
Final analysis results of this study successfully demonstrated the effectiveness of guselkumab for the treatment of PPP. Based on the interim analysis results, it was recommended to continue the study without sample size adjustment.
Conclusion
We discuss results versus design assumptions and advantages to the conduct of the study with the adaptive design approach. Significant cost savings were gained and development time was reduced compared to the alternative option of a fixed conservative design. Several limitations of our study are also discussed.
Keywords: Clinical trial design, Guselkumab, Methodology, Palmoplantar pustulosis
1. Introduction
With recent growth in the use of adaptive designs not only in oncology but also in other areas, more and more literature describes how to overcome challenges for implementation of adaptive designs for trials for drug approval process [[1], [2], [3]]. However, as noted by Bothwell et al. [4], most Phase II or Phase II/III adaptive trials have been conducted in Western Europe or USA. Examples of pivotal adaptive trials in fields outside of oncology used for regulatory approval in Japan are rare. However, some guidelines have been released by the Japan Pharmaceuticals and Medical Devices Agency (PMDA) or Japan Pharmaceutical Manufacturers Association (JPMA) for independent data monitoring committees and/or interim analyses (IA) [5,6] and some experience with review of adaptive trials by the PMDA has been reported [7]. Sample size re-estimation (SSR), defined as “A study design using a flexible sample size adjustment or re-estimation based on interim analysis of accumulating data” represents one type of adaptive design and has been used in 8% of adaptive trials [4].
Guselkumab (CNTO 1959), a development program was initiated to obtain the indication of palmoplantar pustulosis (PPP), a skin disease which is considered distinct from psoriasis in Japan. After two randomized, double-blind, placebo-controlled trials, including a 49-patients Phase II (CNTO1959PPP2001) trial and a 159-patients Phase III trial (CNTO1959PPP3001), successfully demonstrated significant reductions in the signs and symptoms of PPP, guselkumab was approved by the Japan Ministry of Health, Labour and Welfare (MHLW) for use in patients with PPP on 21 November 2018 [8]. Results of these two clinical trials were presented by Terui T et al. [9,10]. Phase III trial of this development program was conducted using an adaptive design, permitting one unblinded SSR and futility analysis at a single IA.
In this article, we elaborate on our experience with designing and implementing a Phase III adaptive confirmatory trial for guselkumab, a biologic monoclonal antibody previously approved for treating moderate-to-severe plaque psoriasis (PSO), for the indication of PPP. We discuss design options for a Phase III study, in light of Phase II results, our choice of an adaptive design, and our experience with both implementing an adaptive clinical trial in Japan and setting up a Japanese domestic Independent Data Monitoring Committee (IDMC). We review trial results versus design assumptions and the advantages of employing an adaptive design; we also present statistical simulation results used to evaluate the proposed trial design. Overall, we believe our experience can be helpful for planning and implementing future adaptive design trials in Japan.
2. Methods
2.1. Overview of the design
Guselkumab, a biologic agent that specifically targets interleukin (IL)-23, was recently approved for the treatment of moderate-to-severe PSO in the US and European Union mainly based on data from three global trials [[11], [12], [13]], and for PSO, psoriatic arthritis (PsA), generalized pustular psoriasis (GPP) and erythrodermic psoriasis (EP) not adequately responding to conventional treatments in Japan mainly based on two local Japanese trials [14,15].
In addition to the global indication of PSO, a broader development strategy for guselkumab was pursued in Japan. This included clinical trials of the CNTO1959PPP2001 [9] and CNTO1959PPP3001 [10] trials to evaluate the therapeutic potential of guselkumab in PPP, a skin disease which is considered distinct from psoriasis [9] in Japan and may be driven by the IL-23/IL-17 pathway [10]. While how PPP is classified within the spectrum of psoriatic diseases is subject to debate in some regions of the world, PPP is recognized as a disease state distinct from PSO and pustular psoriasis in Japan [16]. The prevalence of PPP in Japan has been reported to be 0.12% as compared to 0.34% [17] for PSO in Japan, which indicates patient enrolment in PPP would be much more challenging than in psoriasis.
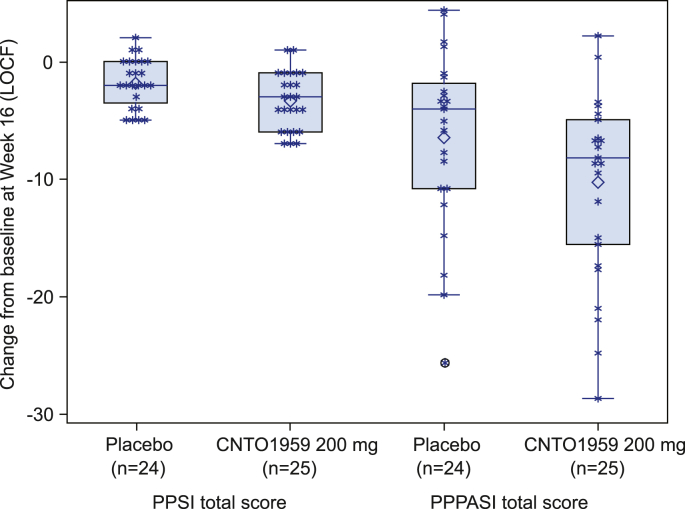
The CNTO1959PPP2001 trial was a randomized, proof-of-concept (PoC), placebo-controlled study (NCT01845987) [9] to evaluate the efficacy and safety of guselkumab 200 mg in Japanese patients with PPP. The primary efficacy endpoint was the change from baseline in the palmoplantar pustulosis severity index (PPSI) total score (ranging from 0 to 12) at week 16. Patients were randomized to receive either guselkumab 200 mg or placebo at weeks 0 and 4, 24 patients in the guselkumab 200 mg group achieved a mean 3.3 point (standard deviation (SD) 2.43) reduction in PPSI score versus a mean 1.8 point (SD 2.09) reduction for the placebo group (p = 0.03; Fig. 1). In addition, mean change from baseline in the PPP area and severity index (PPPASI) score (ranging from 0 to 72) showed a significantly greater reduction at week 16 for guselkumab (−10.2 [SD 8.07]) compared to placebo (−6.4 [SD 7.55]; difference in LS mean, −5.65; 95% CI, −9.80 to −1.50; p = 0.009) (Fig. 1).
Fig. 1.
Change in PPSI and PPPASI total score at week 16 in CNTO1959PPP2001 study.
The graph above displays boxplots overlaid by scattered plots, for the changes in PPSI and PPPASI total score at week 16 by treatment group. The display includes a box spanning the Q1-Q3 inter-quartile range, with a line drawn at the median value. The symbol of diamond (◇) is used to display the mean value. The vertical line (called whisker) issuing from the box extends to the group minimum and maximum values within the "fences" as defined as the ((Q1-1.5*IQR)-(Q3+1.5*IQR)). The "outlier" observation is displayed above or below the fences. The symbol of asterisk (*) represents the LOCF value for each subject.
2.2. Design options for the phase III trial and basis for selection
While outcomes from the CNTO1959PPP2001 study were encouraging, uncertainty surrounding primary endpoint data variability raised concerns. Because the PPPASI appeared to be more sensitive for capturing improvement in PPP than the PPSI, the PPPASI was selected as the measure for the primary endpoint in the Phase III study. However, the PPPASI had never before been used in any registrational studies prior to the CNTO1959PPP2001 trial and the degree of precision for the nuisance parameter of the SD derived from the PoC study was unclear. To address this, we quantified this uncertainty using Bayesian probabilistic methods and our estimates showed that there was greater potential for underestimation than overestimation of the SD from CNTO1959PPP2001.
In planning for the Phase III PPP program for guselkumab, a development time not exceeding 5 years (i.e. trial time ≤2 years and enrollment time ≤1 year) was felt to be desirable in order to most expeditiously address the high unmet need for PPP in Japan. Towards this, a preliminary feasibility assessment was conducted to evaluate the ability of specific investigator sites to recruit PPP patients. Results determined a maximum feasible sample size of 225 patients, allowing for a fast enrollment period for the first 150 patients, followed by a slower enrollment period for the remaining patients.
Two doses of guselkumab, 100 mg and 200 mg were selected based on clinical, and pharmacokinetic and pharmacodynamic modeling results from the CNTO1959PPP2001 study [10]. Once dosing for Phase III was determined, three options for the Phase III trial design were developed.
Option 1: a fixed design with a total sample size of 225 patients randomized to 3 arms (one placebo arm and two active treatment arms: 100 mg and 200 mg). A sample size of 75 patients per treatment group yielded a power of 91.7% for the primary endpoint at a significance level of 5% (2-sided) based on the assumptions of a relatively larger SD than that for the Phase II study (10) and an identical treatment effect (5.5) in PPPASI outcome. There was the potential for statistical power to increase to 98.5% if the assumed SD from the Phase II study decreased from 10 to 8.1.
Option 2: the same fixed design using the same assumptions as option 1, however with a total sample size of 150 patients (50 patients per treatment group). This design yielded a power of 91.9% based on an assumed SD of 8.1 and a power of 77.7% based on an assumed SD of 10.
Option 3: an adaptive statistical design permitting one IA, with the potential to either stop the study for futility or continue the study while increasing sample size to achieve a minimum of 150 patients and an allowable maximum of 225 patients. Optimal timing for the IA was determined to be when approximately 40% of the minimum sample size of 150 patients had either completed the primary endpoint visit or ended study participation before primary endpoint visit. In doing so, the decision to stop or continue the study could be made shortly before reaching the minimum target enrollment while allowing for collection of the maximum amount of data to inform re-estimation of the sample size without potentially disrupting study enrollment. To statistically accommodate both SSR and futility analysis, use of the promising zone SSR method [18] was proposed, with a slight extension to include a non-binding futility boundary. Importantly, if the interim analysis results fall into the promising zone, the chosen adaptive method could substantially increase the power of the study. The boundary values for futility and unfavorable zones were set at 5% and 42%, respectively. Table 2 provides the operational characteristics based on the simulation results (probability of IA outcome, unconditional power and averaged sample size) of the proposed adaptive method in our trial settings.
Table 2.
Operational characteristics of the proposed adaptive method based on the simulation results.
| Simulation condition |
Interim outcome | Probability (per cent) | Proposed adaptive method |
|||
|---|---|---|---|---|---|---|
| Average Z value | Power∗1 (per cent) | ASN∗2 | ||||
| Diff. of mean | Com. SD | |||||
| 0 | 8.1 | Futility | 66.83 | – | 0.00 | 19.0 |
| Unfavorable | 20.00 | 0.474 | 0.57 | 47.0 | ||
| Promising | 9.64 | 0.783 | 0.97 | 70.4 | ||
| Favorable | 3.53 | 1.358 | 0.86 | 47.0 | ||
| Total | 100.00 | 0.658 | 2.40 | 30.5 | ||
| 5.5 | 8.1 | Futility | 5.04 | – | 0.00 | 19.0 |
| Unfavorable | 12.32 | 2.470 | 9.18 | 47.0 | ||
| Promising | 24.24 | 3.667 | 23.59 | 67.3 | ||
| Favorable | 58.40 | 3.749 | 57.16 | 47.0 | ||
| Total | 100.00 | 3.562 | 89.93 | 50.5 | ||
| Total | 100.00 | 4.491 | 98.31 | 49.4 | ||
| 0 | 10 | Futility | 66.55 | – | 0.00 | 19.0 |
| Unfavorable | 20.65 | 0.495 | 0.70 | 47.0 | ||
| Promising | 9.41 | 0.721 | 0.72 | 70.3 | ||
| Favorable | 3.39 | 1.462 | 0.91 | 47.0 | ||
| Total | 100.00 | 0.657 | 2.33 | 30.6 | ||
| 5.5 | 10 | Futility | 10.87 | – | 0.00 | 19.0 |
| Unfavorable | 19.23 | 2.132 | 11.24 | 47.0 | ||
| Promising | 27.33 | 3.119 | 24.91 | 68.0 | ||
| Favorable | 42.57 | 3.301 | 39.93 | 47.0 | ||
| Total | 100.00 | 2.993 | 76.08 | 49.7 | ||
| 0 | 12 | Futility | 66.71 | – | 0.00 | 19.0 |
| Unfavorable | 20.36 | 0.487 | 0.70 | 47.0 | ||
| Promising | 9.47 | 0.741 | 0.69 | 70.2 | ||
| Favorable | 3.46 | 1.385 | 0.88 | 47.0 | ||
| Total | 100.00 | 0.653 | 2.27 | 30.5 | ||
| 5.5 | 12 | Futility | 16.82 | – | 0.00 | 19.0 |
| Unfavorable | 22.65 | 1.878 | 10.51 | 47.0 | ||
| Promising | 27.24 | 2.716 | 21.94 | 68.6 | ||
| Favorable | 33.29 | 2.939 | 28.92 | 47.0 | ||
| Total | 100.00 | 2.577 | 61.37 | 48.2 | ||
*1: Power unconditional on interim data.
*2: Average sample size.
Futility boundary = 0.05; Unfavorable boundary = 0.42; alpha = 0.05 (2-sided); Original N per group = 47; N per group included in IA = 19; Maximum N per group = 75; number of simulations for each simulation condition = 10000.
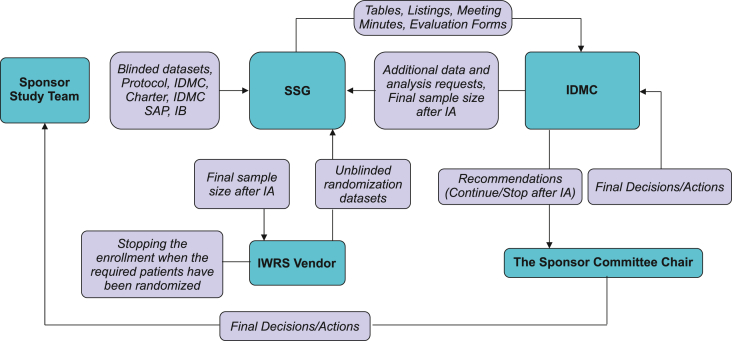
The estimated duration and pros/cons for the three options are summarized in Table 1. Note that since there would be no pause in the enrollment for option 3, the maximum duration for option 3 with continuing the study was the same as that for option 1. However, the regulatory risk of option 3 was considered to be high due to the Japan regulatory agency's position that result-based changes could weaken a study's integrity, as insinuated in Ando et al. [7]. This article also outlined the Japanese regulatory agency's perspective regarding adaptive clinical trials for new drug applications, including: 1) the advantages and disadvantages of adaptive designs (i.e., the necessity of an adaptive design), 2) the operational challenges, such as appropriate information management, and 3) the statistical procedures, such as type I error control. The major advantage of an adaptive design, and the primary rationale for choosing an unblinded SSR, was to account for the high degree of uncertainty in assumed SD. As for the second potential regulatory consideration, our simulation results showed that in any scenario, the overall type I error rates were below 3% (2-sided), across two dose groups. In addition to setting up an IDMC to address the third potential regulatory consideration of operational bias, an independent, external Statistical Support Group (SSG) was created to perform the IA and coordinate with an interactive web response system (IWRS) vendor to ensure that the appropriate number of subjects would be enrolled in the trial. Further, communication plans were put in place to ensure that any information regarding sample size adjustment (i.e., neither recommendations on sample size nor the overall magnitude of any increase in sample size) would not be revealed to study investigators. In turn, as indicated in the IDMC communication flow chart (Fig. 2), no information pertaining to sample size decisions would be shared with sponsor personnel until enrollment was completed. Ultimately, option 3 was selected, as the overall advantages of the proposed adaptive study design outweighed potential regulatory and operational risks.
Table 1.
Comparison of 3 Phase III trial design options with estimated duration.
| Option | Duration |
Pros and cons |
||||
|---|---|---|---|---|---|---|
| If stopped |
If continued |
|||||
| FPI ∼ LPI (Enrollment duration) | FPI ∼ Trial end | FPI ∼ LPI (Enrollment duration) | FPI ∼ NDA | Pros | Cons | |
| Option 1: Fixed design with a total sample size of 225 patients for 3 arms | 3 months | 9 months | 18 months | 35 months | ●Statistical power: high. Ideal statistical power (>90%) for a fixed design trial based on an assumed SD of 10. ●Trial implementation/Regulatory risk: low. |
●Enrollment risk: high. 225 patients considered to be the maximum feasible sample size, imposing the highest risk of not achieving the enrollment target. ●Development time: unfavorable. Enrollment period and trial duration almost one year longer than our desired. |
| Option 2: Fixed design with a total sample size of 150 patients for 3 arms | 3 months | 9 months | 11 months | 26 months | ●Enrollment risk: low. ●Development time: favorable. The shortest duration, fitting desired development time frame. ●Trial implementation/Regulatory risk: low. |
●Statistical power: low. Statistical power lower than 80% based on an assumed SD of 10, leading to the highest risk in trial success. |
| Option 3: Adaptive statistical design with a minimum sample size of 150 patients and a maximum sample size of 225 patients for three arms. | 3 months | 9 months | 11–18 months | 26–35 months | ●Statistical power: varies between those for Options 1 and 2. Avoids drawbacks of Options 1 and 2 with an opportunity to make mid-trial adjustments to accommodate uncertainty in the SD up to 10. ●Enrollment risk: varies between those for Options 1 and 2. ●Development time: varies between those for Options 1 and 2. |
●Trial implementation/Regulatory risk: high. More complex design compared to Options 1 and 2; lack of experience with implementing an adaptive design trial. Requires intensive upfront planning and special measures to prevent operational bias. |
FPI: First patient in; LPI: last patient in; NDA: new drug application.
Durations are rough estimates at the planning stage.
Fig. 2.
IDMC communication flow chart.
IA, interim analysis; IDMC, Independent Data Monitoring Committee; IWRS, interactive web response system; SAP, statistical analysis plan; SSG, Statistical Support Group.
2.3. Implementing an adaptive clinical trial in Japan
The Phase III CNTO1959PPP3001 trial was conducted at 40 sites in Japan, with the first subject randomized in January 2016 and the last subject randomized in December 2016. Of note, addressing the operational and logistical challenges of implementing adaptive designs [2,3] and managing those specific to trials using SSR designs [19] were not unique to this trial. Here we focus on a number of particular operational challenges that arose in our study. The predominant measure designed to prevent operational bias, creation of an IDMC, will be discussed in the next section separately.
2.3.1. Management of accrual information
To prevent unblinding of SSR trials, attention must be paid to not reveal critical information, such as adjusted final sample size, to subjects and the investigators that could be used for back-calculation of the observed treatment effect at the IA [19]. However, in our study, once the total number of randomized patients exceeded the minimum sample size (50 per group), parties authorized to access total accrual information would have known whether the sample size was adjusted or not, which, in turn, could have introduced evaluation bias on the part of investigators. To address this challenge, a clear action plan, mainly consisting of limiting the number of sponsor personnel with access to accrual information and requiring each to participate in a thorough e-learning exercise and sign an agreement to not leak total accrual information to investigators. Unlike other ongoing company-sponsored studies, enrollment status was not reported in any internal trial systems database that could be accessed by sponsor personnel. Only sponsor personnel deemed necessary were authorized to access total accrual information, including study team members, site monitors and operation management board members. Moreover, necessary sponsor personnel was required to sign an agreement before being granted access to ensure that knowledge of the total numbers of screened, randomized, and dosed patients would not be conveyed to investigators, site staff, and study patients. In addition, completing an e-learning exercise was mandatory for all study sponsor personnel, regardless of their involvement in the trial, for purposes of improving awareness of adaptive designs and avoiding incidental information leaks. The e-learning exercise included an overview of adaptive designs and the general SSR algorithm, as well as the requirement of not disclosing confidential information to investigators (e.g., the total number of enrolled patients) as such inferable information could cause subtle changes in study conduct by investigators and potential operational bias.
2.3.2. Optimizing the enrollment speed
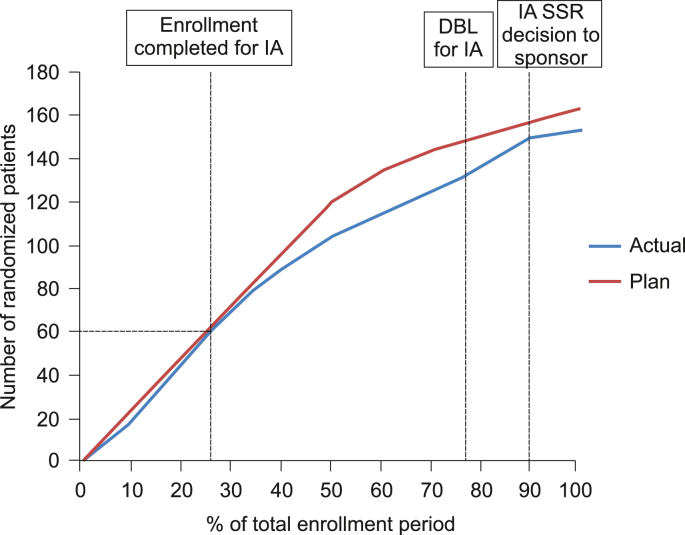
The conduct of this trial was further complicated by efforts to optimize speed of enrollment to enable conducting the IA without disrupting study enrollment overall. Optimally, study enrollment would be rapid for the initial 60 patients (i.e., the number of patients required for the IA), followed by slower but stable enrollment to allow for IA preparations (e.g., data cleaning, report generation, scheduling of the IDMC IA meeting). The planned vs. actual enrollment curves are displayed in Fig. 3. The targeted optimal enrollment curve was achieved, as the first 60 patients (40% of the minimum sample size) were enrolled in approximately the initial quartile of the overall enrollment period, while the remaining 90 patients (60% of the minimum sample size) were enrolled in roughly the latter three quarters of the enrollment period. It is noteworthy that speed of enrollment was controlled by periodically adding patients to those sites with the potential to increase enrollment, instead of opening more sites, in an effort to avoid any sudden changes in the enrollment plan leading to operational biases as described above.
Fig. 3.
Planned vs. actual enrolment curve.
DBL, database lock; IA, interim analysis; SSG, Statistical Support Group.
2.3.3. Conducting the interim analysis
The IA was based on the first 60 randomized subjects. After the IA review meeting, the IDMC recommended continuing the trial without a final sample size adjustment (i.e. 50 patients per group) based on an interim conditional power that fell within the favorable zone (i.e. ≥90%). Subsequently, IWRS immediately put a cap on the number of randomized patients required for the final sample size. All sponsor personnel, including the study team, sponsor committee and medical monitor, were not informed of whether the final sample size was adjusted or not, until when study team was notified by the IWRS that the cap for randomized patients had been reached and that enrolment was completed.
2.4. Setting up a Japanese domestic independent data monitoring committee
This trial included an unblinded SSR, which was crucial for determining whether final sample size adjustment would be triggered. For unblinded SSR designs, an IDMC is essential for maintaining the blind and represents the most effective safeguard against operational bias. Given that Japanese dermatologists consider PPP a distinct entity [20] and have considerable expertise in PPP, a Japanese domestic IDMC was established to monitor data on an ongoing basis to ensure the continuing safety of the enrolled subjects. In addition to its traditional role of safety monitoring, the IDMC was also charged with reviewing the results from the unblinded IA and making critical recommendations for continuing the study, including whether to stop the study for futility, continuing the study without modifications, or continuing the study with increased sample size. General considerations for establishing and operating IDMCs have been provided in regulatory guidance documents (EMA, 2005; FDA, 2006; PMDA, 2010). Publications by Chow et al. [21], Sanchez-Kam et al. [22] and Turnbull [23] also describe IDMC-related challenges that are specific to adaptive designs.
2.4.1. Selection of independent data monitoring committee members
Our IDMC consisted of two Japanese dermatologists (a professor at a national university as chairperson, and a chief physician of a general hospital), and a statistician (an associate professor at a national university). Although all regulatory guidelines mentioned above emphasize that the “expertise” and “experience in clinical trials and in serving on other DMCs” are key factors to consider in selecting IDMC members, it was extremely difficult to find experienced IDMC members in Japan given the overall limited experience of Japanese stakeholders on DMCs/DSMBs [24]. In turn, participation in the IDMC for this study represented the first such experience for each of the 3 committee members selected. Consequently, our study will help increase the pool of experienced IDMC members in Japan.
2.4.2. Statistical supporting group
An SSG independent of the sponsor was set up to support the IDMC, as recommended by PMDA IDMC guidelines [5]. The SSG for our study was composed of personnel from a Japanese contract research organization company. The responsibilities of the SSG were further divided into two roles: an SSG Statistician and Programmer and an IDMC bureau. The responsibilities of the SSG Statistician and Programmer included reviewing the IDMC Statistical Analysis Plan (SAP), preparing programs to generate IDMC reports, and ascertaining the algorithm and decision rules regarding sample size re-estimation documented in the IDMC SAP. The IDMC bureau served as a liaison between IDMC members and the Sponsor Committee, and was responsible for distributing the data package to the IDMC, transmitting recommendations to the Sponsor Committee, and receiving the final sample size determination (after sample size re-estimation) from the IDMC and relaying this information directly to the IWRS vendor. The IDMC bureau also had other administrative responsibilities, such as scheduling and arranging telecommunications for meetings as well as recording IDMC meeting minutes.
This study will be conducted in accordance with the ethical principles of the Declaration of Helsinki and per the International Council for Harmonization and Good Clinical Practice guidelines. Informed consent forms will be reviewed and approved by all the appropriate Ethics Committees prior to enrolment of the patients into the study.
3. Discussion
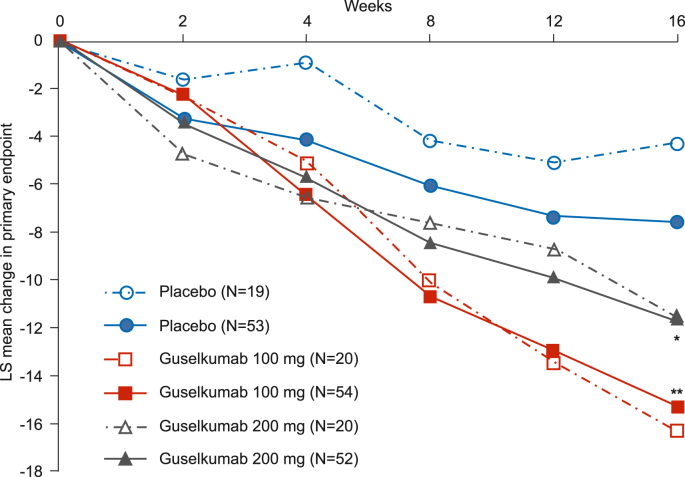
Our chosen adaptive design in this confirmatory Phase III study for guselkumab in the treatment of PPP enabled an unblinded SSR and futility assessment based on an IA. Fig. 4 shows results for the primary endpoint analysis based on IA data that were available to the IDMC. Based on an interim conditional power that fell within the favorable zone (i.e. ≥90%), the IDMC recommended continuing the trial without final sample size adjustment (i.e. maintaining target enrollment of approximately 50 patients per group). After the study had been completed and the full analysis was performed, it was determined that primary objectives were met (Fig. 4). The rationale for choosing an unblinded SSR option was based on the estimated high probability of the SD in this Phase III trial being greater than that of the Phase II trial. In turn, results of the Phase III trial yielded an SD of 9.9 for the primary endpoint for the guselkumab group vs a SD of 8.1 in the Phase II trial; consequently, this outcome supports our decision to choose this adaptive study design. Thus, we think that it is important to quantitatively evaluate the uncertainty of nuisance parameters and to then account for these in the design of trials at the next phase of development. It was also found that both the IA and final efficacy results were somehow similar to those presented in our Phase II trial, which implied a smaller initial sample size (<50 patients per group) may have been able to address the efficacy objectives. The initial sample size for this Phase III trial was decided as such to obtain an evaluable 1-year safety population. All of these data/findings are believed to be useful for the sponsor when considering the design and patient enrollment strategy for the future trials in the PPP area.
Fig. 4.
Interim and final study results.
Graph represents interim (open symbols, dashed lines) and final (solid symbols and lines) primary endpoint analysis results for placebo guselkumab 100 mg and guselkumab 200 mg groups. *p = 0.017, **p = 0.001 vs. placebo (final analysis).
Although results based on the IA did not lead to increasing the final sample size or stopping the study for futility, the chosen adaptive design combining a futility analysis and unblinded SSR had several potential advantages. Should the futility analysis have determined that the study be stopped, further exposure of patients to a non-efficacious drug could have been avoided and the study would have been shortened by around one and a half years. Based on retrospective assessments, we determined that use of our adaptive design reduced the duration of the trial by about one year and lead to cost savings of about 2.9 million USD compared to a traditional fixed design. Furthermore, failure to enroll the targeted number of patients is a significant concern for clinical trials conducted in Japan. However, the flexibility of an unblinded SSR approach to minimize the required sample size may improve the efficiency of patient allocation, especially for relatively uncommon diseases like PPP.
Beyond time and cost savings, the design and implementation of this adaptive trial enhanced interactions among both internal and external key stakeholders. Many of the challenges associated with trial design and execution that emerged were addressed by cooperative teamwork between multiple disciplinary partners. It should be emphasized that exposing a broad scope of cross-functional partners, including data managers and statisticians, to trial operations was much more of a priority for this trial compared to fixed design trials typically conducted by the sponsor. For instance, a trial statistician provided substantial input into the source data verification plan to ensure increased but focused monitoring of data relative to the IA. Moreover, this study created an opportunity for Japanese stakeholders to gain experience as IDMC participants and provide their professional input making critical the IA recommendations based on the IA and monitoring patient safety. We believe that initiatives such as those that we describe here with our trial are important for expanding the pool of experienced Japanese IDMC participants. Nonetheless, there are limitations pertaining to our case study. For example, other SSR approaches, such as use of Bayesian SSR methodology, were not evaluated at the planning stage and the chosen promising zone design did not surpass other approaches in overall power. Some issues were particular to our case study, such as challenges to enrollment due to low prevalence of the targeted disease (PPP).
4. Conclusion
We believe our case study is of interest because it illustrates a successful example of designing and implementing an adaptive design trial, incorporating appropriate operational measures to preserve trial integrity, to obtain pharmaceutical regulatory approval in Japan. The challenges discussed above, such as the need for a more comprehensive information management plan, and the solutions adopted to address these challenges, may be helpful for future adaptive trials both in Japan and elsewhere in the world.
Authors’ contributions
Richuan Zheng served as a trial statistician of the trial and drafted the manuscript. Yoichi M. Ito served as the statistician member for the IDMC. Richuan Zheng, Soyoku Nobeyama, Motonari Yunoki and Kazuki Minoda participated in the planning and operation of the trial. All authors reviewed and made the critical revisions to the manuscript and approved the final manuscript.
Funding
This study was funded by Janssen Pharmaceutical K.K., Tokyo, Japan. The author(s) received no financial support for the research, authorship and/or publication of this article.
Trial registration
ClinicalTrials.gov identifier: NCT02641730 (https://clinicaltrials.gov/ct2/show/NCT02641730)
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Janssen Pharmaceutical K.K. sponsored this study with trial registration number NCT02641730. We thank Kenji Kabashima who chaired the IDMC and Yujiro Takae who also served as a member of the IDMC of this study. We also thank reviewers in Janssen Pharmaceutical K.K. and Janssen Research & Development for their valuable comments, and in particular Vladimir Dragalin for his skillful and tireless support of this study and Kentaro Matsuura (ex-employee of Janssen Pharmaceutical K.K.) for his statistical simulation work. Hirose Keiichiro (ex-employee of Janssen Pharmaceutical K.K.) provided key statistical advice for the study design and this manuscript. The viewpoints expressed herein are based on authors' work and do not necessarily represent the viewpoint of Janssen Pharmaceutical K.K. We also thank Jyothi Ramanathan (Ph.D.), Nigar Malik (B.Pharm) and Sangita Patil (Ph.D., CMPP) of SIRO Clinpharm Pvt. Ltd (funded by Janssen Pharmaceutical K.K) provided medical writing and editorial support.
References
- 1.Krams M., Sharma S., Dragalin V., et al. Adaptive approaches in clinical drug development: opportunities and challenges in design and implementation. Pharm. Med. (Hamps.) 2009;23(3):139–148. [Google Scholar]
- 2.Quinlan J., Gaydos B., Maca J., et al. Barriers and opportunities for implementation of adaptive designs in pharmaceutical product development. Clin. Trials. 2010;7(2):167–173. doi: 10.1177/1740774510361542. [DOI] [PubMed] [Google Scholar]
- 3.Chow S.C., Corey R. Benefits, challenges and obstacles of adaptive clinical trial designs. Orphanet J. Rare Dis. 2011;6(1):79–89. doi: 10.1186/1750-1172-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bothwell L.E., Avorn J., Khan N.F., et al. Adaptive design clinical trials: a review of the literature and ClinicalTrials.gov. BMJ Open. 2018;8(2) doi: 10.1136/bmjopen-2017-018320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Japan Pharmaceuticals and Medical Devices Agency (PMDA): Guideline on Data Monitoring Committee https://www.pmda.go.jp/files/000157932.pdf (April 4, 2013). Accessed 9 June 2019.
- 6.Japan pharmaceutical Manufacturers association (JPMA): guindance on operating data monitoring committee and conducting interim analysis. June 2013. http://www.jpma.or.jp/medicine/shinyaku/tiken/allotment/pdf/monitoring.pdf
- 7.Ando Y., Hirakawa A., Uyama Y. Adaptive clinical trials for new drug applications in Japan. Eur. Neuropsychopharmacol. 2011;21(2):175–179. doi: 10.1016/j.euroneuro.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Janssen Pharmaceutical K.K. press release: https://www.janssen.com/japan/press-release/20171208. Accessed 9 June 2019.
- 9.Terui T., Kobayashi S., Okubo Y., et al. Efficacy and safety of guselkumab, an anti–interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA dermatol. 2018;154(3):309–316. doi: 10.1001/jamadermatol.2017.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terui T., Kobayashi S., Okubo Y., et al. Efficacy and safety of guselkumab in Japanese patients with palmoplantar pustulosis: a phase 3 randomized clinical trial. JAMA Dermatol. 2019;155(10):1153–1161. doi: 10.1001/jamadermatol.2019.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blauvelt A., Papp K.A., Griffiths C.E., et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo-and active comparator–controlled VOYAGE 1 trial. J. Am. Acad. Dermatol. 2017;76(3):405–417. doi: 10.1016/j.jaad.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 12.Reich K., Armstrong A.W., Foley P., et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo-and active comparator–controlled VOYAGE 2 trial. J. Am. Acad. Dermatol. 2017;76(3):418–431. doi: 10.1016/j.jaad.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 13.Ferris L.K., Ott E., Jiang J., et al. Efficacy and safety of guselkumab, administered with a novel patient-controlled injector (One-Press),for moderate-to-severe psoriasis: results from the phase 3 ORION study. J. Dermatol. Treat. 2019:1–8. doi: 10.1080/09546634.2019.1587145. [DOI] [PubMed] [Google Scholar]
- 14.Ohtsuki M., Kubo H., Morishima H., et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J. Dermatol. 2018;45(9):1053–1062. doi: 10.1111/1346-8138.14504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sano S., Kubo H., Morishima H., et al. Guselkumab, a human interleukin‐23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52‐week, phase 3, multicenter, open‐label study. J. Dermatol. 2018;45(5):529–539. doi: 10.1111/1346-8138.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Waal A.C., van de Kerkhof P.C. Pustulosis palmoplantaris is a disease distinct from psoriasis. J. Dermatol. Treat. 2011;22(2):102–105. doi: 10.3109/09546631003636817. [DOI] [PubMed] [Google Scholar]
- 17.Kubota K., Kamijima Y., Sato T., et al. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open. 2015;5(1) doi: 10.1136/bmjopen-2014-006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta C.R., Pocock S.J. Adaptive increase in sample size when interim results are promising: a practical guide with examples. Stat. Med. 2011;30(28):3267–3284. doi: 10.1002/sim.4102. [DOI] [PubMed] [Google Scholar]
- 19.Pritchett Y.L., Menon S., Marchenko O., et al. Sample size re-estimation designs in confirmatory clinical trials – current state, statistical considerations, and practical guidance. Stat. Biopharm. Res. 2015;7(4):309–321. [Google Scholar]
- 20.Yamamoto T. Clinical characteristics of Japanese patients with palmoplantar pustulosis. Clin. Drug Invest. 2019;39(3):241–252. doi: 10.1007/s40261-018-00745-6. [DOI] [PubMed] [Google Scholar]
- 21.Chow S.C., Corey R., Lin M. On the independence of data monitoring committee in adaptive design clinical trials. J. Biopharm. Stat. 2012;22(4):853–867. doi: 10.1080/10543406.2012.676536. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Kam M., Gallo P., Loewy J., et al. A practical guide to data monitoring committees in adaptive trials. Ther. Innov. Regul. Sci. 2014;48(3):316–326. doi: 10.1177/2168479013509805. [DOI] [PubMed] [Google Scholar]
- 23.Turnbull B.W. Adaptive designs from a data safety monitoring board perspective: some controversies and some case studies. Clin. Trials. 2017;14:462–469. doi: 10.1177/1740774516689261. [DOI] [PubMed] [Google Scholar]
- 24.Japan Pharmaceutical Manufacturers Association (JPMA) Data Safety Monitoring Board (DSMB) Training. 4 April 2014. http://www.jpma.or.jp/medicine/shinyaku/tiken/symposium/pdf/20140404/20140404_01.pdf 9 June 2019.