Abstract
Calcium absorption; Vitamin D deficiency; Lack of sunlight; Hikikomori; Vegan.
Keywords: Calcium absorption, Vitamin D deficiency, Lack of sunlight, Hikikomori, Vegan
1. Introduction
In some cases, hypocalcemia leads to tetany, arrhythmia, and fatal outcomes [1]. Calcium levels are strictly regulated by parathyroid hormone (PTH), calcitonin, and calcitriol. Humans do not endogenously produce calcium; therefore, it must be ingested and absorbed by the intestine [2]. In recent years, there has been considerable interest in vegan diets to avoid all foods from animal produce, and the percentage of vegans in Japan is said to be 1.9%. Vegan foods are considered healthy; however, a lack of food diversity and supplementation can result in nutrient deficiencies [3]. Pathological social withdrawal is referred to as “Hikikomori”, and there are more than million people in Japan who are afflicted with this condition [4]. The following diagnostic criteria for “Hikikomori” have been proposed in 2020: a) marked social isolation in one's home; b) duration of continuous social isolation of at least 6 months; c) significant functional impairment or distress associated with the social isolation [5]. We report a patient with “Hikikomori” who followed a vegan diet and experienced repeated episodes of hypocalcemia. An appropriate diagnosis is important for administering standard treatment to patients with repeated hypocalcemia.
2. Case report
A 33-year-old woman who had been withdrawing from society (Hikikomori) for more than 15 years, arrived at the emergency department (ED). At the age of 10, following a death in the family, she became unable to eat animal products. At the age of 18, she developed the habit of over-eating and vomiting. From that time onwards, she spent most of the day inside her room with the curtains closed. After that, she neither worked nor went outside. She had been previously admitted to another ED several times for hypocalcemia. Each time, she was given intravenous calcium infusion to improve her symptoms, and she went home. As she was unable to go to the hospital, her mother received the prescription for her oral medication on her behalf.; however, she often experienced symptoms of hypocalcemia.
For four days prior to her admission, she began experiencing difficulty in moving due to numbness and stiffness in her limbs. On arrival at the ED, she was underweight (153 cm, 32 kg, BMI 13.67 kg/m2), and her skin was pale (Figure 1). Trousseau's signs and Chvostek's signs were observed. Her corrected calcium levels (cCa) and ionic calcium levels (iCa) were 7.0 mg/dL and 3.0 mg/dL, respectively. Intravenous calcium was administered, and her symptoms improved by day 2 (Figure 2). Subsequently, her 1,25(OH)2-vitamin D and 25-OH vitamin D levels decreased to 8 pg/mL and <4 ng/mL (below the measurement sensitivity), respectively. Moreover, her intact PTH level increased to 394 pg/mL. There was no significant decrease in trace elements or vitamins other than vitamin D (Figure 3). Hypocalcemia was diagnosed based on inadequate calcium absorption caused by vitamin D deficiency. Therefore, she was prescribed active vitamin D and calcium supplementation. She developed rhabdomyolysis with a maximum creatine kinase (CK) level of 48333 U/L during admission, which improved with intravenous fluids without renal damage. After taking activated vitamin D, her cCa, 1,25(OH)2-vitamin D and intact PTH levels were stabilized by day 8 and her symptoms gradually improved. She was diagnosed with an eating disorder and obsessive-compulsive disorder by a psychiatrist. She exhibited significant functional impairment and was diagnosed again as having “Hikikomori” according to the diagnostic criteria proposed by Kato et al [5]. An inpatient treatment was warranted, but she refused. The patient returned home, but she self-interrupted her outpatient visits.
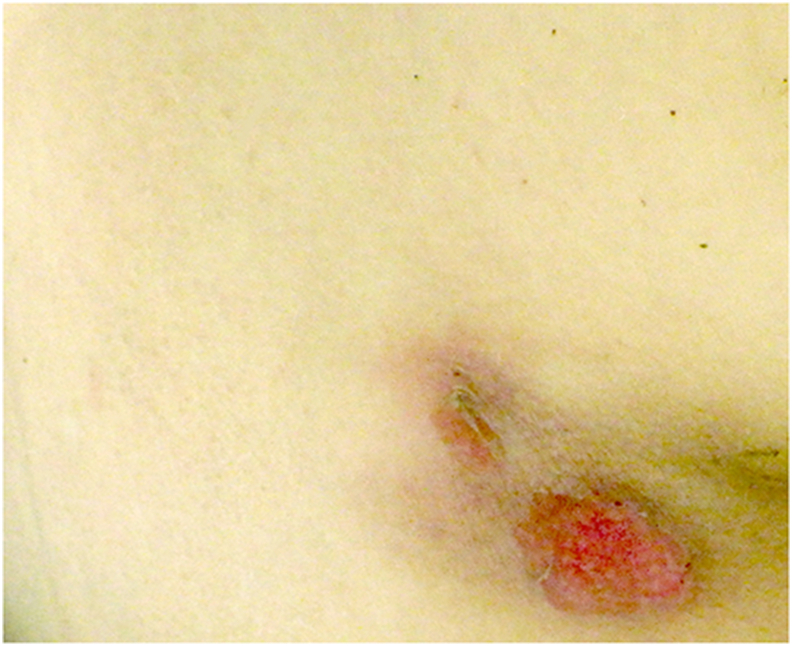
Figure 1.
An image of the pressure ulcer on the patient's back near the buttocks. The patient's skin was pale.
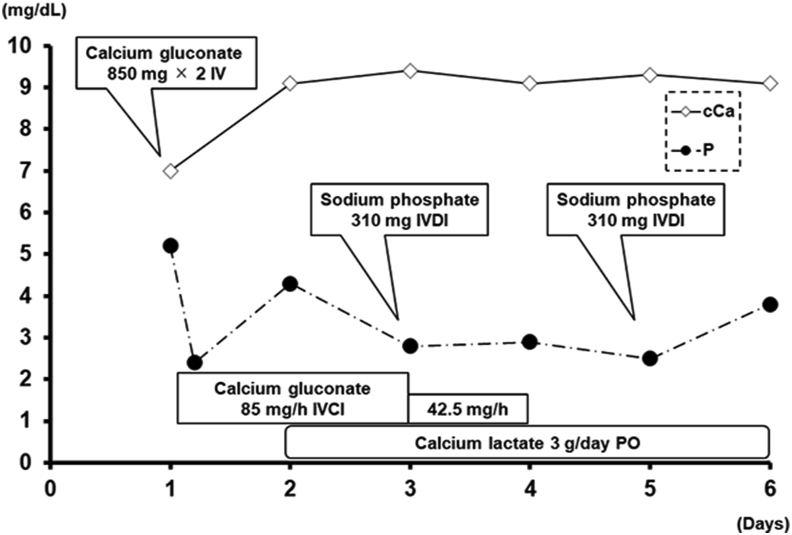
Figure 2.
Changes in calcium and phosphorus levels after hospitalization. Intravenous calcium correction was necessary until Day 4. As needed, phosphorus was administered intravenously. IV: Intravenous injection; IVDI: Intravenous drip infusion; IVCI: Intravenous continuous infusion; PO: Per OS.
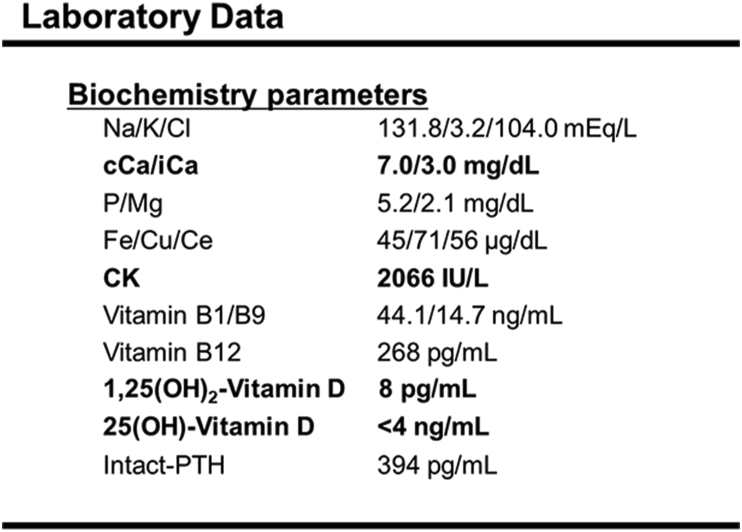
Figure 3.
Biochemistry analysis results on admission: Both cCa and iCa levels were decreased. No decrease in trace elements was observed. CK was elevated from the time of admission. Vitamin D deficiency was present, but Vitamin B levels were within the normal limits.
Written consent was obtained from the patient's family and patient's personal information was anonymized with great care. The patient provided informed consent for the publication of her case history.
3. Discussion
The presence of 1,25(OH)2-vitamin D enhances intestinal absorption of calcium and phosphorus; therefore, inadequate vitamin D levels reduce gastrointestinal calcium absorption by up to 50% [6]. Vitamin D is provided by two pathways, namely, biosynthesis in the skin using the energy of ultraviolet radiation, and ingestion from food, although the former pathway accounts for a larger proportion. Hypocalcemia can occur in people with a low exposure to ultraviolet light, especially in those with a diet that is deficient in vitamins [7]. In this case, tetany was suspected to be induced by hypocalcemia, because her symptoms improved after her calcium levels were elevated with intravenous calcium administration. Before admission, hypocalcemia was thought to be caused only by inadequate calcium intake, as she was a vegan who had several prior visits to the ED. However, our data revealed that her repeated hypocalcemia was due to vitamin D deficiency, mainly caused by a lack of sunlight exposure.
Vegan diets can provide vitamins and minerals if proper supplements are consumed. Therefore, vegans do not usually experience nutritional deficiencies. In our case, there were no deficiencies in trace elements or other vitamins. Good sources of calcium can be obtained by increasing the intake of calcium-rich foods from plant sources; however, as no diet can provide adequate amounts of active vitamin D, exposure to sunlight is essential [8]. In a Japanese cohort-study, vitamin D deficiency was significantly associated with the female sex, examined month, current smoking status, lack of regular walking, higher intact PTH, and poor daily vitamin D intake [9]. Koda reported an eating disorder and vitamin D deficiency in a patient, which was caused by a combination of inadequate nutrition intake and lack of sunlight [10], as seen in our case. Miyakoshi reported that “Hikikomori” causes calcium and vitamin D deficiency due to a lack of sunlight exposure [11]. On a sunny day in Japan, the skin can produce adequate vitamin D in only 10 min [12]. Therefore, lack of sunlight, which causes vitamin D deficiency, is thought to occur only in severe “Hikikomori” who spend most of their day inside the house. Vitamin D deficiency is defined as a 25(OH)-vitamin D level of less than 12 ng/mL [6]. The patient's 25(OH)-vitamin D level had decreased to <4 ng/mL, which was below measurement sensitivity. In the human brain, vitamin D receptors are expressed, and a vitamin D deficiency fosters the development of several psychiatric diseases including depression, bipolar disorder, and schizophrenia [13].
It is possible that “Hikikomori” occurs with a variety of psychiatric disorders as a contributor to psychopathology [5]. This patient was also diagnosed with an eating disorder and obsessive-compulsive disorder. Recently, the disease concept of orthorexia nervosa (ON) has also emerged, described as a pathological obsession with healthy eating. Weight loss is not considered the desired result in people with ON, because there is an intense preoccupation about consuming healthy food instead. It has been suggested that veganism may be a risk factor for ON [14]. Although we do not know the direct causal relationship between veganism and “Hikikomori”, it has been suggested that vegans might have a risk of psychiatric disorders in this way and may be associated with “Hikikomori” as well. Patients with obsessive-compulsive disorder should be treated with cognitive-behavioral therapy, a selective serotonin reuptake inhibitor (SSRI) medication, or both. In this case, the patient refused to be hospitalized; therefore, her obsessive-compulsive disorder could not be addressed, resulting in self-interruption of treatment. In the future, the patient might be at a risk of being transported with arrhythmia or other fatal disease, so it might have been better to continue hospitalization and treatment.
4. Conclusion
Taking active vitamin D and calcium supplementation should be considered to prevent repeated hypocalcemia in patients with “Hikikomori” following a vegan diet, who are thought to have low calcium intake and sunlight exposure. Psychiatric intervention is essential, but treatment can be difficult.
Declarations
Author contribution statement
All authors listed have significantly contributed to the investigation, development and writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
References
- 1.Martha J.W., Wibowo A., Pranata R. Hypocalcemia is associated with severe COVID-19: a systematic review and meta-analysis. Diabetes Metabol. Syndr. 2021;15:337–342. doi: 10.1016/j.dsx.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drake T.M., Gupta V. Treasure Island (FL) StatPearls Publishing; 2022. Calcium. [Google Scholar]
- 3.Weikert C., Trefflich I., Menzel J., Obeid R., Longree A., Dierkes J., et al. Vitamin and mineral status in a vegan diet. Dtsch. Arztebl. Int. 2020;117:575–582. doi: 10.3238/arztebl.2020.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watts J. Public health experts concerned about “Hikikomori”. Lancet. 2002;359:1131. doi: 10.1016/s0140-6736(02)08186-2. [DOI] [PubMed] [Google Scholar]
- 5.Kato T.A., Kanba S., Teo A.R. Defining pathological social withdrawal: proposed diagnostic criteria for hikikomori. World Psychiatr. 2020;19:116–117. doi: 10.1002/wps.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 7.Cooper M.S., Gittoes N.J. Diagnosis and management of hypocalcaemia. BMJ. 2008;336:1298–1302. doi: 10.1136/bmj.39582.589433.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baroni L., Goggi S., Battaglino R., Berveglieri M., Fasan I., Filippin D., et al. Vegan nutrition for mothers and children: practical tools for healthcare providers. Nutrients. 2019;11:5. doi: 10.3390/nu11010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshimura N., Muraki S., Oka H., Morita M., Yamada H., Tanaka S., et al. Profiles of vitamin D insufficiency and deficiency in Japanese men and women: association with biological, environmental, and nutritional factors and coexisting disorders: the ROAD study. Osteoporos. 2013;24:2775–2787. doi: 10.1007/s00198-013-2372-z. [DOI] [PubMed] [Google Scholar]
- 10.Koda R., Miyazaki S., Iino N., Sato Y., Hirano K., Sunami E., et al. Vitamin D deficiency-induced osteomalacia in a patient with anorexia nervosa. Intern. Med. 2020;60:1731–1736. doi: 10.2169/internalmedicine.5911-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyakoshi T., Satoh M., Nomura F., Hashimoto T., Aizawa T. A case of hypocalcaemia due to vitamin D deficiency in ‘Hikikomori’ syndrome. Eur. J. Case. Rep. Intern. Med. 2017;4 doi: 10.12890/2017_000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyauchi M., Hirai C., Nakajima H. The solar exposure time required for vitamin D3 synthesis in the human body estimated by numerical simulation and observation in Japan. J. Nutr. Sci. Vitaminol. 2013;59:257–263. doi: 10.3177/jnsv.59.257. [DOI] [PubMed] [Google Scholar]
- 13.Lang F., Ma K., Leibrock C.B. 1,25(OH) 2 D 3 in brain function and neuropsychiatric disease. Neurosignals. 2019;27:40–49. doi: 10.33594/000000182. [DOI] [PubMed] [Google Scholar]
- 14.McComb S.E., Mills J.S. Orthorexia nervosa: a review of psychosocial risk factors. Appetite. 2019;140:50–75. doi: 10.1016/j.appet.2019.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.