Abstract
Herein, we report the synthesis, characterization, and biological properties of eleven (3a-3k) novel Schiff bases. The spectral data of FT-IR, 1H NMR, 13C NMR, and LC-MS are associated with these synthesized compounds. From the FT-IR analysis, we confirmed the azomethine (-C=N-) group and from 1H NMR data, the phenolic –OH proton is appeared at range δ 13.92–14.09ppm due to hydrogen bonding. The LC-MS analysis agreed with molecular ion peaks of synthesized Schiff bases. To evaluate the antibacterial activity of newly synthesized compounds were screened against b. licheniformis, b. species, e. coli, and s. aureus. Furthermore, the antioxidant activity was investigated by two methods 2,2-diphenyl-1-picryl hydrazyl (DPPH) and hydroxyl radical scavenging methods. The (-NO2,-Cl,-Br,-I) substituted compounds have shown good antibacterial activity against tested organisms. Also, these compounds were exhibited higher antioxidant activity by given methods.
Keywords: Primary diamines, 1-hydroxy-2-acetonapthanone, 1H NMR, Mass spectrometry, Antimicrobial activity
Graphical abstract
Primary diamines; 1-hydroxy-2-acetonapthanone; 1H NMR; Mass spectrometry; Antimicrobial activity.
Specifications Table
| Subject area | Organic Chemistry |
|---|---|
| Compounds | 4- halo/nitro substituted 2,2'-((alkane-1,3-diylbis(azanylylidene))bis(ethan-1-yl-1-ylidene))bis(naphthalen-1-ol) |
| Data category | Synthesized, Spectral and Biological data. |
| Data acquisition format | FT-IR, 1H NMR, 13C NMR, Mass spectra, Elemental analysis |
| Data type | Experimental |
| Procedure | A series of substituted 4- halo/nitro substituted 2,2'-((alkane-1,3-diylbis(azanylylidene))bis(ethan-1-yl-1-ylidene))bis(naphthalen-1-ol) derivatives have been synthesized and Characterized by spectral data. Also screened for their biological potential. |
| Data accessibility | Data is with this article. |
1. Introduction
The Schiff bases synthesized by the condensation of the active carbonyl group and primary amines with azomethine (-C=N-) linkage may have numerous applications. Some Schiff bases were reported to possess antibacterial [1, 2], antioxidant [1, 2, 3], antifungal [4, 5], anti-HIV [6], antitumor [7], anti-inflammatory [8], anticancer [9], antimalarial [10, 11] antiproliferative activities [11]. Phenolic Schiff base derivatives with one or more halo groups (-Cl, -Br, –I) or nitro group in the aromatic ring may show biological activities like antibacterial [12] and antiviral [12] activities. Some isatin Schiff base derivatives were applicable for docking study [13], some Schiff bases were supposed to be adsorbed on the metal surface by its characteristic imine group [14]. Schiff bases were known to be a sort of ligands with strong coordinative ability due to the intra-hydrogen bonding [15, 16]; hence mostly all Schiff Bases can form 1:1 complexes with transition metal ions. Metal complexes of Schiff bases having O-hydroxy aromatic tetradentate Schiff bases exhibit various bioactivities like antibacterial [17, 18], antioxidant [17, 18, 19, 20], antifungal [20], DNA damage assays [20], antitumor [21], anticancer [22], antiviral [23], anti-inflammatory [24] activities. It is also used for homogeneous catalysis [25], electrocatalytic reduction [26], catalytic oxidation [27], fluorescent chemosensor for detection of Fe2+ ions [28], potentiometric sensor [29], catalytic used in heck [30] & Suzuki reaction [30], dyes [31], polymers [31], phenoxazinone synthase mimicking activity [32], and catalase mimic activity [33]. The prolongation of our research work on Schiff bases [34]; herein we report a synthesis of eleven tetradentate Schiff Bases. The structure elucidation of synthesized Schiff bases had done with spectroscopic techniques (FT-IR, 1H NMR, 13C NMR, and LC-MS). The result of this article will be useful for the upcoming researchers to gain more information about the antibacterial and antioxidant activities of Schiff bases (see Scheme 1).
Scheme 1.
Synthesis of Schiff Bases (3a-3k).
1a. R = -H, 1b. R = -Cl, 1c. R = -Br, 1d. R = -I, 1e. R = -NO2.
2a. R’= -CH2-CH2-, 2b. R’= -CH2-CH2-CH2-, 2c. R’ = -CH(C2H5)-CH2-CH2-.
3a. R = -H, R’ = -CH2-CH2-, 3b. R = -Cl, R’= -CH2-CH2-, 3c. R = -Br, R’ = -CH2-CH2-, 3d. R = -I, R’ = -CH2-CH2-, 3e. R = -NO2, R’ = -CH2-CH2-, 3f. R = -Br, R’= -CH2-CH2-CH2-, 3g. R = -I, R’ = -CH2-CH2-CH2-, 3h. R = -NO2, R’ = -CH2-CH2-CH2-, 3i. R = -Cl, R’ = -CH(C2H5)-CH2-CH2-, 3j. R = -I, R’ = -CH(C2H5)-CH2-CH2-, 3k. R = -NO2, R’ = -CH(C2H5)-CH2-CH2-.
2. Experimental
2.1. Chemical material and instrumentation
For the experimental work, the chemicals were purchased by aura, spectrochem & TCI and without further purification. In the laboratory, thin layer chromatography (TLC) took by 0.25-mm e merck gel Plates (60F-254). Synthesized compounds were dissolved in a minimum amount of acetic acid, spotted on the given TLC plate, and ran through a solution of ethyl acetate and benzene. The melting point determination of compounds was done by digital apparatus koefler banc. The elemental analysis of the synthesized compounds was carried out through perkin-elmer 240 elemental analyzer. The structure of unknown compounds was agreed upon by different spectral characterization. FT-IR spectrometer was recorded KBr pellets on a perkin-elmer 2000 at 8 cm−1 resolution in the region 4000-400 cm−1. 1H NMR spectra were carried out with bruker avance III HD 300/400 operating at 300/400 MHz using CDCl3 solution with TMS at internal standard. 13C NMR spectra obtained with bruker avance III HD 300 operating at 300 MHz using CDCl3 solution with TMS at internal standard. Mass spectra recorded on LC-MS (ESI) mass spectrometer at 70 ev.
2.2. Synthesis
2.2.1. General synthesis of schiff base compounds (3a-3k)
Schiff bases had been synthesized by mixing of warm absolute ethanolic solution of substituted 1-(1-hydroxynaphthalen-2-yl)ethan-1-one (2 mmol) with ethane-1,2-diamine (1 mmol) (3a-3e) as well as with propane-1,3-diamine (1 mmol) (3f-3h) and also with pentane-1,3-diamine (1 mmol) (3i-3k). In each reaction mixture catalytic amount of acetic acid (2–3 drops) was added and refluxed for 3–4 h (Scheme-1). The progress of the reaction mixture was monitored on TLC using pet-ether: ethyl acetate (8:2V/V) as eluent. After completion, the reaction mixture was kept overnight at room temperature. The crystalline compound was filtered, washed with cold water, and re-crystallized from absolute ethanol. The purity of the product was checked by using TLC and the physical data were tabulated in Table 1.
Table 1.
Physical properties of Schiff bases (3a-3k).
|
compound |
molecular formula | color | yield% | M.P. (oC) |
|---|---|---|---|---|
| 3a | C26H24O2N2 | yellow | 88 | 128–130 |
| 3b | C26H22O2N2Cl2 | yellow | 85 | 158–160 |
| 3c | C26H22O2N2Br2 | brown | 90 | 172–174 |
| 3d | C26H22O2N2I2 | pale yellow | 92 | 182–184 |
| 3e | C26H22O6N4 | yellow | 82 | 205–207 |
| 3f | C27H24O2N2Br2 | brown | 92 | 135–137 |
| 3g | C27H24O2N2I2 | yellow | 88 | 195–197 |
| 3h | C27H24O6N4 | green | 78 | 220–222 |
| 3i | C29H28O2N2Cl2 | yellow | 80 | 192–194 |
| 3j | C29H28O2N2 I2 | yellow | 90 | 205–207 |
| 3k | C29H28O6N4 | brown | 81 | 235–237 |
2.2.2. Procedure for antibacterial activity
The given compounds were screened for antibacterial activity against the bacteria bacillus licheniformis, bacillus species, escherichia coli, and staphylococcus aureus by using well diffusion method. The microbial suspension (100uL) having 108 CFU mL−1 of bacteria was carried out with the help of Mueller-Hinton agar (MHA) medium. The extracts were diluted by 100% dimethyl sulphoxide at the concentrations of 5 mg/mL and the given medium was melted and cooled to 48–50 °C. The solid plates were formed by the addition of standardized inoculums (1.5 × 108C FU/mL, 0.5McFarland) to the molten agar which was poured into sterile petri dishes. The well diffusion method was used to prepare product which was in the seeded agar plate; the compound activity had checked, and that was put into the well (6 mm). The plates were incubated in the incubator at 37 °C for overnight. The antibacterial zone of inhibition of the extract had determined for the bacterial species in zone sizes around each well. The compounds produced diameters of the zone of inhibition as compared to standard ciprofloxacin [35, 36].
2.2.3. Determination of minimum inhibitory concentration (MIC)
The broth dilution method is applicable for the minimum inhibitory concentration of given compounds. The concentration of compounds was prepared 8 mg/mL in the first tube containing 1mL of broth. The conduits were vortexes to make the initial standard concentration. These were serially diluted to other cannulas. Finally, 1mL compound was discarded from the last tube and prepared the dilution of 0.25, 0.50, 0.75, 1.0 mg/mL. To all these tubes, 0.1mL of the log phase culture of target microorganisms were added separately and incubated at 37 °C for 24–48 h for bacteria. After incubation, the lowest concentration of tube solution with no detectable bacterial growth was considered a minimum inhibitory concentration.
2.2.4. Antioxidant activity
2.2.4.1. Procedure for 2, 2-diphenyl-1-Picryl hydrazyl (DPPH) assay
DPPH (2, 2, diphenyl-1-picryl hydrazyl) radical scavenging assay was carried out [37] with slight modifications. The 1mL ethanolic solution had different concentrations of synthesized compounds. It was added with an equal volume of 0.1mm ethanolic solution of DPPH. The prepared solution settled for incubation at room temperature. The decreases in the concentration of DPPH were measured by noting the absorbance at 517nm. A similar test was performed with ascorbic acid, as an internal standard, instead of Schiff's base. The percentage scavenging of DPPH free radical for each concentration of test compounds had calculated the absorbance of negative control using Eq. (1).
| (1) |
2.2.4.2. Procedure for hydroxyl radical scavenging assay
Hydroxyl radical scavenging activities of given compounds were determined by using the earlier reported method [37]. The reaction cocktail included 60μL of 1mm FeCl3, 90μL of 1mm 1,10 phenanthroline, 2.4mL of 0.2M phosphate buffer (pH 7.8), 150μL of 0.17 M H2O2, and 1.5mL of various concentrations of each compound. The prepared solutions of given compounds were kept at room temperature for 5min incubation, and absorbance was measured at 560 nm with the help of spectrophotometer. The α-tocopherol was used as the reference compound for hydroxyl radical scavenging assay.
3. Result and discussion
All the synthesized Schiff bases are in different colors and they are stable in air and moisture at room temperature. They are soluble in methanol, dimethyl sulphoxide, dimethylformamide, dichloromethane, chloroform, and partially soluble in ethanol. The spectral data of FT-IR, 1H NMR, 13C NMR, and LCMS confirmed their structure.
3.1. FT-IR analysis
FT-IR spectra of given compounds performed with the KBr pellet technique, and the observed results are in Table 2. The stretching bands are observed at 3444-3403 cm−1 due to the phenolic –OH group and 1665-1622 cm−1 due to the (–C=N) stretching mode of the imine group. The IR absorption peak at 1278–1263 cm−1 shows the phenolic ν(C–O) group with the presence of the keto-amine group having (N–O⋯H) intramolecular hydrogen bonding only in the solid-state [38]. The Schiff bases have shown two C–X stretching vibrations in the range of 794–760 cm−1 which confirms that the halogen groups are presented at the para position to the aromatic –OH group [38, 39]. Even two stretching vibrations have been observed in the range 1573–1523 cm−1 and 1413–1384 cm−1 due to the –NO2 group. Table 2 and the supplementary file (Fig. No. S1 to S11) represents spectroscopic data (IR) of given Schiff bases (3a-3k) (see Table 3).
Table 2.
FT-IR values of Schiff bases(3a-3k).
|
compound |
ν(O–H) cm−1 | ν(−C=N−) cm−1 | ν(C=C) cm−1 | ν(C–N–C) cm−1 | ν(C−X) cm−1 | ν(N–O) cm−1 |
|---|---|---|---|---|---|---|
| 3a | 3434 | 1629 | 1452 | 1018 | -- | -- |
| 3b | 3444 | 1640 | 1457 | 1076 | 788,761 | -- |
| 3c | 3419 | 1630 | 1440 | 1068 | 788,763 | -- |
| 3d | 3440 | 1646 | 1457 | 1070 | 794,760 | -- |
| 3e | 3413 | 1636 | 1467 | 1081 | -- | 1573, 1413 |
| 3f | 3403 | 1622 | 1450 | 1072 | 790,765 | -- |
| 3g | 3407 | 1635 | 1450 | 1076 | 790,765 | -- |
| 3h | 3417 | 1650 | 1455 | 1076 | -- | 1527, 1384 |
| 3i | 3444 | 1640 | 1452 | 1078 | 790,769 | -- |
| 3j | 3440 | 1647 | 1457 | 1018 | 786, 760 | -- |
| 3k | 3430 | 1630 | 1450 | 1027 | -- | 1523, 1388 |
Table 3.
Antibacterial activity of Schiff bases (in mm) (3a-3k).
| sample | B. Lichenifermis | Bacillus Sp. | E.coli | S.aureus |
|---|---|---|---|---|
| 3a | 08 | 07 | 07 | 08 |
| 3b | 24 | 19 | 16 | 20 |
| 3c | 17 | 20 | 11 | 21 |
| 3d | 23 | 13 | 09 | 16 |
| 3e | 09 | 16 | 18 | 20 |
| 3f | 10 | 22 | 10 | 27 |
| 3g | 16 | 12 | 11 | 13 |
| 3h | 19 | 25 | 08 | 26 |
| 3i | 25 | 23 | 19 | 28 |
| 3j | 12 | 15 | 17 | 19 |
| 3k | 26 | 24 | 10 | 18 |
| ciprofloxacin | 27 | 26 | 20 | 30 |
| DMSO | 00 | 00 | 00 | 00 |
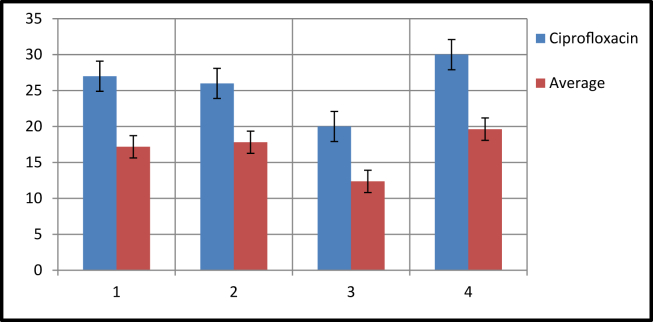
| average | 17.18 | 17.81 | 12.36 | 19.63 |
3.2. 1H NMR analysis
1H NMR spectral data of Schiff bases analyzed in CDCl3 solvent, from the data, the presence of multiplets at δ value between 6.78–8.53ppm is due to aromatic protons. The appearance of the singlet at δ 13.92–14.09ppm shows the existence of the phenolic –OH proton having (N–O⋯H) intramolecular hydrogen bonding. In compounds (3a-3h) –CH3 groups have appeared singlet at range δ 2.45–2.49ppm of 6H indicating that they are symmetrically equivalent. The compounds (3i, 3j, 3k), –CH3 groups have shown two singlets at range δ 2.44–2.47ppm, which indicates that they are symmetrically non-equivalent. The spectral data of 1H NMR is represented in the supplementary file (Fig. No.S12 to S22) of synthesized Schiff bases (3a-3k).
3.3. 13C NMR analysis
In 13C NMR spectra, the peaks are observed at the range of δ value from 105 to 140ppm is due to aromatic and olefinic carbon. The signal present at δ 175.1–175.5ppm confirms azomethine (-C=N-) group, and the phenolic C–OH carbon atom showed a sharp peak at δ 170.6–171.7ppm. The Schiff bases (3e, 3h, 3k) display signal at δ 165.1–164.5ppm due to a C–NO2 bond. Schiff bases (3b-d, 3f, 3g, 3i, 3j) have exhibited a peak at δ 132.1–135.0ppm stipulates the presence of a C–X bond and the signal present at δ 14.3–14.6ppm due to –N=C–CH3 group. The spectral data of 13C NMR is represented in the supplementary file (Fig. No. S23 to S33) of given Schiff bases (3a-3k).
3.4. LC-MS analysis
The LC-MS spectra of the Schiff bases exhibited different fragmentation patterns as expected, and results were getting to be in good agreement with their molecular formulae. –Cl and–Br containing Schiff bases gave different molecular ion peaks due to its isotopic effect. The molecular ion peak of the Schiff bases is displayed at [M + H]+ peak. Especially the aromatic nitro Schiff bases (3e, 3h, 3k) lose –NO2 radical and give molecular ion peak (M – NO2) [39]. The spectral analysis (FT-IR, 1H NMR, 13C NMR, and LC-MS) confirmed that the synthesized compounds are Schiff bases. The mass spectrometry data of synthesized Schiff bases (3a-3k) is represented in the supplementary file (Fig. No. S34 to S44).
3.5. Antibacterial activity
The experimental details and zone of inhibition of Schiff bases concerning the antibacterial activity against the bacterial strains is illustrated in table no.3. Among the eleven Schiff bases, the compound 3i has more potent against tested all four micro-organisms. The Schiff bases 3b, 3d, 3k are more effective against b. Lichenifermis and the compounds 3b, 3e, 3j show the higher activity against e. coli. Also, the compounds 3f, 3j are more potent against Bacillus sp., and compounds 3f, 3h give promising activity against s. aureus. The synthesized Schiff bases with the electron-withdrawing group such as [-Cl, -Br, -I, and -NO2] and extended carbon chain of diamines suggest more potent in antibacterial activities. The impact of electronegative groups like -Cl,-Br, and –NO2 is more effective than the –H and –I group on synthesized compounds. The antibacterial activity of these compounds is represented graphically in Figure 1.
Figure 1.
Antibacterial activity of Schiff bases (3a-3k) against gram positive and gram negative error bars represents the standard deviation of triplicate measurements.
The minimum inhibitory concentration was done at 0.25, 0.50, 0.75, and 1.0 mg/mL; the observed results are presented in Table 4. The Schiff base 3i has a good inhibition character at minimum concentration against referred microorganisms. It indicates the -Cl group and extended carbon chain of diamine which gives more potent biological activity. The Schiff bases 3b, 3d, 3k have shown good inhibition against b. licheniformis (gram-positive), and compounds 3f, 3h, 3k show high potent against b. species (gram-positive). The compounds 3e, 3j against e. coli (gram-negative) strain, and compounds 3f, 3h against s. aureus (gram-positive) organisms have conveyed good inhibition at a minimum concentration (0.25 mg/mL).
Table 4.
MIC of Schiff bases (In Mg/mL) (3a-3k).
| sample | bacterial pathogens |
|||
|---|---|---|---|---|
| B.Lichenifermis Mg/mL |
Bacillus sp. Mg/mL |
E.coli. Mg/mL |
S.aureus Mg/mL |
|
| 3a | 0.638 | 0.792 | 0.792 | 0.653 |
| 3b | 0.172 | 0.261 | 0.267 | 0.256 |
| 3c | 0.267 | 0.272 | 0.272 | 0.291 |
| 3d | 0.229 | 0.642 | 0.690 | 0.667 |
| 3e | 0.531 | 0.267 | 0.192 | 0.269 |
| 3f | 0.639 | 0.218 | 0.609 | 0.235 |
| 3g | 0.351 | 0.739 | 0.752 | 0.761 |
| 3h | 0.279 | 0.162 | 0.713 | 0.169 |
| 3i | 0.149 | 0.140 | 0.149 | 0.138 |
| 3j | 0.632 | 0.275 | 0.172 | 0.329 |
| 3k | 0.153 | 0.158 | 0.571 | 0.269 |
| ciprofloxacin | 0.107 | 0.107 | 0.097 | 0.112 |
| average | 0.367273 | 0.356909 | 0.470818 | 0.367 |
3.6. Antioxidant activity
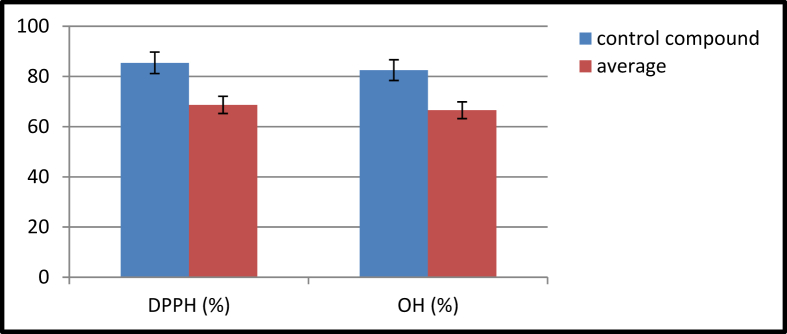
The antioxidant activity is one of the prime activities for the Schiff bases by using DPPH and hydroxyl radical scavenging assay. Ascorbic acid is used as a control compound for the DPPH method whereas α-tocopherol is used for the hydroxy method; the observed results are illustrated in Table 5. The compounds (3b, 3g, 3h, 3k) containing an electron-withdrawing group at the para position to phenolic –OH group were shown more potent with antioxidant activity by both methods. The results of both techniques reveal that the electron-withdrawing substitution with an extended carbon chain of diamines perform alternating antioxidant activity. The obtained data of compounds is mean graphically in Figure 2.
Table 5.
Antioxidant activity of Schiff bases (3a-3k).
| sample | DPPH (%) | OH (%) |
|---|---|---|
| 3a | 55.89 ± 0.55 | 62.93 ± 0.25 |
| 3b | 75.19 ± 0.15 | 72.50 ± 0.15 |
| 3c | 72.99 ± 0.15 | 56.12 ± 0.51 |
| 3d | 59.91 ± 0.89 | 47.80 ± 0.76 |
| 3e | 62.89 ± 0.55 | 61.93 ± 0.15 |
| 3f | 52.24 ± 0.03 | 57.17 ± 0.53 |
| 3g | 78.56 ± 0.45 | 73.28 ± 0.17 |
| 3h | 78.28 ± 0.36 | 77.77 ± 0.91 |
| 3i | 67.99 ± 0.51 | 78.25 ± 0.1 |
| 3j | 72.29 ± 0.45 | 70.55 ± 0.78 |
| 3k | 79.28 ± 0.36 | 73.77 ± 0.31 |
| ascorbic acid | 85.42 ± 0.78 | -- |
| α- tocopherol | -- | 82.50 ± 0.84 |
| average | 68.68 | 66.55 |
Figure 2.
Antioxidant activity of Schiff bases (3a-3k) error bars represents the standard deviation of triplicate measurements.
3.7. Spectral data
[E]-2,2'-((ethane-1,2-diylbis(azanylylidene))bis(ethan-1-yl-1-ylidene))bis(naphthalen-1-ol) (3a)
Yield – 349.53 mg, 88%, Color -Yellow, M.P. 128–130 °C.
FT-IR (KBr, cm−1): 3434(νOH),1629(νC = N),1452(νC = C-), 1018 (νC-N-C).
1H NMR (400MHz,CDCl3):δ 14.02 (s,2H,Ar-OH),8.48–6.82 (m,12H,Ar-H),4.05 (s,4H,-CH2), 2.49(s,6H-CH3).
13C NMR (300MHz,CDCl3):δ 175.2(−C=N−),171.6(C–O),137.2–108.8(Ar-C-),35.2(−N-CH2-), 14.5(-N=C-CH3).
ESIMS (m/z): 397.40.
Anal. Cal. For C26H24O2N2: C 78.78, H 6.60, N 7.07; found C 78.60, H 6.82, N 7.72.
[E]-2,2'-((ethane-1,2-diylbis(azanylylidene))bis(ethan-1-yl-1-ylidene))bis(4-chloronaphthalen-1-ol) (3b)
Yield – 395.34 mg, 85%, Color -Yellow, M.P. 158–160 °C.
FT-IR(KBr,cm−1):3444(νOH),1640(νC = N),1457(νC = C-),1076(νC−N−C),788(νC-Cl),761(νC-Cl) cm−1;
1H NMR (300MHz,CDCl3):δ 13.93 (s,2H,Ar-OH),8.50–6.79 (m,10H,Ar-H),4.09 (s,4H,-CH2), 2.45 (s, 6H, -CH3).
13C NMR (300MHz,CDCl3):δ 175.3(−C=N−),171.7(C−O),137.1–108.2(Ar-C-),132.6(-C-Cl), 35.1(−N-CH2-),14.5(-N=C-CH3).
ESIMS(m/z): 465.70.
Anal. Cal. For C26H22O2N2Cl2: C 67.24, H 4.74, N 6.03; found C 67.03, H 4.93, N 6.65.
[E]-2,2'-((ethane-1,2-diylbis(azanylylidene))bis(ethan-1-yl-1-ylidene))bis(4-bromonaphthalen-1-ol) (3c)
Yield – 496.80 mg, 90%, Color -Brown, M.P. 172–174 °C.
FT-IR(KBr,cm−1): 3419(νOH),1630(νC = N),1440(νC = C-),1068(νC−N−C),788(νC-Br),763(νC-Br) cm−1.
1H NMR (400MHz,CDCl3):δ 14.02 (s,2H,Ar-OH),8.50–6.90 (m,10H,Ar-H),4.04 (s,4H,-CH2) 2.48 (s,6H,-CH3).
13C NMR (300MHz,CDCl3):δ 175.2(−C=N−),171.7(C−O),137.1–108.2(Ar-C-),132.4(-C-Br),35.1(−N-CH2-),14.4(-N=C-CH3).
ESIMS(m/z): 556.10.
Anal. Cal. For C26H22O2N2Br2: C 56.31, H 3.09, N 5.05; found C 56.11, H 3.31, N 5.78.
[E]-2,2'-((ethane-1,2-diylbis(azanylylidene))bis(ethan-1-yl-1-ylidene))bis(4-iodonaphthalen-1-ol) (3d)
Yield– 597.06 mg, 92%, Color–Pale Yellow, M.P. 182–184 °C.
FT-IR(KBr, cm−1): 3440(νOH),1646(νC = N),1457(νC = C-),1070(νC−N−C),794(νC-I),760(νC-I) cm−1.
1H NMR (300MHz,CDCl3):δ 13.92 (s,2H,Ar-OH),8.50–6.79 (m,10H,Ar-H),4.09 (s,4H,-CH2), 2.46 (s,6H,-CH3).
13C NMR (300MHz,CDCl3):δ 175.2(−C=N−),171.7(C−O),137.1–108.1(Ar-C-),132.4(-C-I), 35.0(−N-CH2-),14.3(-N=C-CH3).
ESIMS(m/z): 649.80.
Anal. Cal. For C26H22O2N2I2: C 48.14, H 3.39, N 4.42; found C 48.03, H 3.69, N 4.64.
[E]-2,2'-((ethane-1,2-diylbis(azanylylidene))bis(ethan-1-yl-1-ylidene))bis(4-nitronaphthalen-1-ol) (3e)
Yield – 399.47 mg, 82%, Color-Yellow, M.P. 205–207 °C.
FT-IR(KBr,cm−1): 3413(νOH),1636(νC = N),1467(νC = C-),1573(νC-NO2),1413(νC-NO2),1081(νC−N−C) cm−1.
1H NMR (400MHz,CDCl3):δ 14.02 (s,2H,Ar-OH),8.47–6.87 (m,10H,Ar-H),4.04 (s,4H,-CH2),2.48 (s,6H,-CH3).
13C NMR (300MHz,CDCl3):δ 175.2(−C=N−),171.7(C−O),137.1–108.2(Ar-C-),133.2(-C-NO2), 35.1(−N-CH2-),14.4(-N=C-CH3).
ESIMS(m/z): 487.70.
Anal. Cal. For C26H22O6N4: C 64.19, H 4.52, N 11.52; found C 64.06, H 4.74, N 11.86.
[E]-2,2'-((propane-1,3-diylbis(azanylylidene))bis(ethan-1-yl-1-ylidene))bis(4-bromonaphthalen-1-ol) (3f)
Yield – 520.73 mg, 92%, Color- Brown, M.P.135-137 °C.
FT-IR(KBr,cm−1): 3403(νOH),1622(νC = N),1450(νC = C-),1076(νC−N−C),790(νC-Br),765(νC-Br) cm−1.
1H NMR (400MHz,CDCl3):δ 14.02 (s,2H,Ar-OH),8.45–7.26 (m,10H,Ar-H),3.81 (t,4H,J = 4.5Hz,-CH2),2.47 (s,6H,-CH3),2.32 (m,2H,J = 4.5Hz,-CH2).
13C NMR (300MHz,CDCl3):δ 175.2(−C=N−),171.7(C−O),137.1–108.2(Ar-C-),132.4(-C-Br), 42.1(−N-CH2-),29.2(HC-C-CH2-),14.4(-N=C-CH3).
ESIMS(m/z): 566.40.
Anal. Cal. For C27H24O2N2Br2: C 57.04, H 4.25, N 4.92; found C 56.84, H 4.51, N 5.09.
[E]-2,2'-((propane-1,3-diylbis(azanylylidene))bis(ethan-1-yl-1-ylidene))bis(4-iodonaphthalen-1-ol) (3g)
Yield – 583.44 mg, 88%, Color - Yellow, M.P.195-197 °C.
FT-IR(KBr,cm−1): 3407(νOH),1635(νC = N),1450(νC = C-),1076(νC−N−C),790(νC-I),765(νC-I) cm−1.
1H NMR (400MHz,CDCl3): δ 14.02 (s,2H,Ar-OH),8.45–7.26 (m,10H,Ar-H),3.81 (t,4H,J = 4.5Hz,-CH2),2.47 (s,6H,-CH3),2.32 (m,2H,J = 4.5Hz,-CH2).
13C NMR (300MHz,CDCl3): δ 175.1(−C=N−),171.6(C−O),137.1–108.2(Ar-C-),132.1(-C-I),42.1(−N-CH2-),29.2(HC-C-CH2-),14.4(-N=C-CH3).
ESIMS(m/z): 662.92.
Anal. Cal. For C27H24O2N2I2: C 48.94, H 3.39, N 4.32; found C 48.72, H 3.68, N 4.56.
[E]-2,2'-((propane-1,3-diylbis(azanylylidene))bis(ethan-1-yl-1-ylidene))bis(4-nitronaphthalen-1-ol) (3h)
Yield – 436.01 mg, 87%, Color - Green, M.P.220–222 °C.
FT-IR(KBr,): 3417(νOH),1650(νC=N),1455(νC=C-),1527(νC-NO2),1384(νC-NO2),1076(νC−N−C) cm−1.
1H NMR (400MHz,CDCl3):δ 14.02 (s,2H,Ar-OH,),8.50–6.82 (m,10H,Ar-H),3.82 (t,4H,J=4.5Hz,-CH2),2.47 (s,6H,-CH3),2.32 (m,2H,J=4.5Hz,-CH2).
13C NMR (300MHz,CDCl3):δ 175.5(−C=N−),171.9(C–O),137.1–108.4(Ar-C-),133.6(-C-NO2),42.1(−N-CH2-),29.3(HC-C-CH2-),14.6(-N=C-CH3).
ESIMS(m/z): 500.52.
Anal. Cal. For C27H24O6N4: C 64.08, H 4.08, N 11.02; found C 63.83, H 4.32, N 11.53.
[E]-2,2’-((pentane-1,3-diylbis(azanylylidene))bis(ethan-1-yl-1-ylidene))bis(4-chloronaphthalen-1-ol) (3i)
Yield- 406.52 mg, 80%, Color- yellow, M.P. 192–194 °C.
FT-IR(KBr,cm−1): 3444(νOH),1640(νC = N),1452(νC=C-),1078(νC−N−C),790(νC-Cl),769(νC-Cl) cm−1.
1H NMR (400MHz,CDCl3): δ 14.03 (s,2H,Ar-OH),8.53–6.80 (m,10H,Ar-H),4.11 (t,2H,J=4.5Hz,-CH2),3.70 (m,1H,J=4.6Hz,-CH),2.47 (s,3H,-CH3),2.45 (s,3H,-CH3),2.25 (q,2H,J=4.3Hz,-CH2),1.86 (m,2H,J=4.1Hz,-CH2),1.03 (t,3H,J=4.1Hz,-CH3).
13C NMR (300MHz,CDCl3):δ 175.2(-C=N-),170.7(C-O),137.1–105.9(Ar-C-),135.0(-C-Cl),54.1(-N-C-H),41.7(-N-CH2-),34.6(HC-C-CH2-),29.4(H3C-C-CH-),14.4(-N=C-CH3),10.2(-C-CH2-CH-);
ESIMS(m/z): 508.51.
Anal. Cal. For C29H28O2N2Cl2: C 68.77, H 5.53, N 5.53; found C 68.60, H 5.81, N 5.72.
[E]-2,2’-((pentane-1,3-diylbis(azanylylidene))bis(ethan-1-yl-1-ylidene))bis(4-iodonaphthalen-1-ol) (3j)
Yield – 621.92 mg, 90%, Color - yellow, M.P. 205–207 °C.
FT-IR(KBr,cm−1): 3440(νOH),1647(νC=N),1457(νC=C-),1088(νC−N–C),786(νC-I),760(νC-I)cm−1.
1H NMR (300 MHz,CDCl3): δ 14.09 (s,2H,Ar-OH),8.50–6.79 (m,10H,Ar-H),4.09 (t,2H,J=4.5Hz,-CH2),3.65 (m,1H,J=4.6Hz,-CH),2.46 (s,3H,-CH3),2.35 (s,3H,-CH3),2.24 (q,2H,J=4.3Hz,-CH2),1.84 (m,2H,J=4.1Hz,-CH2),1.04 (t,3H,J=4.1Hz,-CH3).
13C NMR (300MHz,CDCl3):δ 175.2(-C=N-),170.6(-C-O),137.0–105.8(Ar-C-),134.9(-C-I),54.1(-N-C-H),41.7(-N-CH2-),34.6(HC-C-CH2-),29.4(H3C-C-CH-),14.4(-N=C-CH3),10.2(-C-CH2-CH-).
ESIMS(m/z): 691.20.
Anal. Cal. For C29H28O2N2I2: C 50.43, H 4.05, N 4.05; found C 50.20, H 4.27, N 4.35.
[E]-2,2’-((pentane-1,3-diylbis(azanylylidene))bis(ethan-1-yl-1-ylidene))bis(4-nitronaphthalen-1-ol) (3k)
Yield -428.65 mg, 81%, Color - Brown, M.P. 235–237 °C.
FT-IR (KBr, cm−1): 3430(νOH),1665(νC=N),1450(νC=C-),1523(νC-NO2),1388(νC-NO2), 1027(νC-N-C) cm−1.
1H NMR (400MHz,CDCl3):δ 14.01 (s,2H,Ar-OH),8.50–6.78 (m,10H,Ar-H),4.12 (t,2H,J=4.5Hz,-CH2),3.66 (m,1H,J=4.6Hz,-CH),2.44 (s,3H,-CH3),2.33 (s,3H,-CH3),2.35 (q,2H,J=4.3Hz,-CH2),1.90 (m,2H,J=4.1Hz,-CH2),1.07 (t,3H,J=4.1Hz,-CH3).
13C NMR (300MHz,CDCl3):δ 175.5(-C=N-),170.9(C-O),137.4–106.0(Ar-C-),136.2(-C-NO2),54.3(-N-C-H),41.7(-N-CH2-),34.7(HC-C-CH2-),29.5(H3C-C-CH-),14.5(-N=C-CH3),10.2(-C-CH2-CH-).
ESIMS (m/z): 528.70.
Anal. Cal. For C27H24O6N4 C 65.90, H 5.30, N 10.60; found C 65.72, H 5.68, N 10.96.
4. Conclusion
The current study describes the synthesis of new eleven Schiff bases having an electronegative group (-Cl, -Br, –I, and –NO2) located at para position to the phenolic –OH group. They are confirmed by using instrumental techniques viz. FT-IR, 1H NMR, 13C NMR, and LCMS analysis, also evaluated biologically by antibacterial and antioxidant activities. From the FT-IR analysis, we confirm the azomethine (-C=N-) group, and the 1H NMR peaks of the phenolic –OH proton are shown at δ 13.92–14.09ppm due to hydrogen bonding. The LC-MS analysis agrees with molecular ion peaks of synthesized Schiff bases. The screening data for antibacterial activity shows that electronegative-substituted Schiff bases are more potent against the tested gram-positive and gram-negative bacteria. Among these Schiff bases, compound 3i shows the best Antibacterial activity against all tested pathogens. From this, it is concluded that the influence of the –Cl group and extended carbon chain of diamines on synthesized compounds is more effective than other substituent's. The comparative determination of the total antioxidant capacity also shows the highest value for the electronegative Schiff bases with an extended carbon chain of diamines by DPPH and the hydroxyl radical scavenging method.
Declarations
Author contribution statement
Bhagwat Vhanale: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Digambar Kadam: Analyzed and interpreted the data.
Avinash Shinde: Conceived and designed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The authors do not have permission to share data.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Ejidike I.P., Ajibade P.A. Synthesis and in vitro anticancer, antibacterial, and antioxidant studies of unsymmetrical Schiff base derivatives of 4-[(1E)-N-(2-aminoethyl)ethanimidoyl]benzene-1,3-diol. Res. Chem. Intermed. 2016;42:6543–6555. [Google Scholar]
- 2.Cinarli A., Gürbüz D., Tavman A., Birteksöz A.S. Spectral characterization and antimicrobial activity of some schiff bases derived from 4-chloro-2-aminophenol and various salicylaldehyde derivatives. Chin. J. Chem. 2012;30:449–459. [Google Scholar]
- 3.Beena D. Kumar, Rawat D.S. Synthesis and antioxidant activity of thymol and carvacrol based Schiff bases. Bioorg. Med. Chem. Lett. 2013;23:641–645. doi: 10.1016/j.bmcl.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Da Silva C.M., Da Silva D.L., Martins C.V.B., De Resende M.A., Dias E.S., Magalhaes T.F.F., Rodrigues L.P., Sabino A.A., Alves R.B., Fatima A.D. Synthesis of aryl aldimines and their activity against fungi of clinical interest. Chem. Biol. Drug Des. 2011;78:810–815. doi: 10.1111/j.1747-0285.2011.01185.x. [DOI] [PubMed] [Google Scholar]
- 5.Gürbüz D., Cinarli A., Tavman A., Birteksöz A.S. Spectral characterization and antimicrobial activity of some schiff bases derived from 4-Methyl-2-aminophenol. Chin. J. Chem. 2012;30:970–978. [Google Scholar]
- 6.Sriram D., Yogeeswari P., Myneedu N.S., Saraswat V. Abacavir prodrugs: microwave-assisted synthesis and their evaluation of anti-HIV activities. Bioorg. Med. Chem. Lett. 2006;16:2127–2129. doi: 10.1016/j.bmcl.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 7.Liang C., Xia J., Lei D., Li X., Yao Q., Gao J. Synthesis in vitro and in vivo antitumor activity of symmetrical bis-Schiff base derivatives of isatin. Eur. J. Med. Chem. 2014;74:742–750. doi: 10.1016/j.ejmech.2013.04.040. [DOI] [PubMed] [Google Scholar]
- 8.Noureen A., Saleem S., Farima T., Siddiqi H.M., Mirza B. Synthesis, characterization, biological evaluation and QSAR of some Schiff base esters: promising new antitumor, antioxidant and anti-inflammatory agents. Pak. J. Pharm. Sci. 2013;26:113–123. [PubMed] [Google Scholar]
- 9.Chattopadhyay B., Basu S., Chakraborty P., Choudhuri S.K., Mukherjee A.K. Synthesis, spectroscopic characterization, X-ray powder structure analysis, DFT study and in vitro anticancer activity of N-(2-methoxyphenyl)-3-methoxysalicylaldimine. J. Mol. Struct. 2009;932:90–96. [Google Scholar]
- 10.Matar S.A., Talib W.H., Mustafa M.S., Mubarak M.S., AlDamen M.A. Synthesis, characterization, and antimicrobial activity of Schiff bases derived from benzaldehydes and 3,3’-diaminodipropylamine. Arab. J. Chem. 2015;8:850–857. [Google Scholar]
- 11.Jarrahpour A., Shirvani P., Sharghi H., Aberi M., Sinou V., Latour C., Brunel J.M. Synthesis of novel mono- and bis-Schiff bases of morpholine derivatives and the investigation of their antimalarial and antiproliferative activities. Med. Chem. Res. 2015;24:4105–4112. [Google Scholar]
- 12.Da Silva C.M., Da Silva D.L., Modolo L.V., Alves R.B., Resende M.D., Martins C.V.B., Fatima A. Schiff bases: a short review of their antimicrobial activities. J. Adv. Res. 2011;2:1–8. [Google Scholar]
- 13.Azizian J., Mohammadi M.K., Firuzi O., Razzaghi-Asl N., Miri R. Synthesis, biological activity and docking study of some new isatin Schiff base derivatives. Med. Chem. Res. 2012;21:3730–3740. [Google Scholar]
- 14.Kumar B., Kuntail J., Verma D.K., Rastogi R.B., Sinha I. Mechanism of triboactivity of Schiff bases: experimental and molecular dynamics simulations studies. J. Mol. Liq. 2019;289 [Google Scholar]
- 15.Szady-Chełmieniecka A., Kołodziej B., Morawiak M., Kamieński B., Schilf W. Spectroscopic studies of the intramolecular hydrogen bonding in o-hydroxy Schiff bases, derived from diaminomaleonitrile, and their deprotonation reaction products. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018;189:330–341. doi: 10.1016/j.saa.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 16.Naskar S., Naskar S., Butcher R.J., Chattopadhyay S.K. Synthesis, X-ray crystal structures and spectroscopic properties of two Ni(II) complexes of pyridoxal Schiff’s bases with diamines: importance of steric factor in stabilization of water helices in the lattices of metal complex. Inorg. Chim. Acta. 2010;363:404–411. [Google Scholar]
- 17.Al Zoubi W., Al-Hamdani A.A.S., Kaseem M. Synthesis and antioxidant activities of Schiff bases and their complexes: a review. Appl. Organomet. Chem. 2016;30:810–817. [Google Scholar]
- 18.Hasi Q.M., Fan Y., Yao X.Q., Hu D.C., Liu J.C. Synthesis, characterization, antioxidant and antimicrobial activities of a bidentate Schiff base ligand and its metal complexes. Polyhedron. 2016;109:75–80. [Google Scholar]
- 19.Rouco L., Liberato A., Fernandez-Trujillo M.J., Manez A., Basallote M.G., Alvarino R., Alfonso A., Botana L.M., Maneiro M. Salen-manganese complexes for controlling ROS damage: neuroprotective effects, antioxidant activity and kinetic studies. J. Inorg. Biochem. 2020;203 doi: 10.1016/j.jinorgbio.2019.110918. [DOI] [PubMed] [Google Scholar]
- 20.Ramesh G., Daravath S., Swathi M., Sumalatha V., Shankar D.S. Shivaraj, Investigation on Co(II), Ni(II), Cu(II) and Zn(II) complexes derived from quadridentate salen-type Schiffbase: structural characterization, DNA interactions, antioxidant proficiency and biological evaluation. Chem. Data Collect. 2020 000. [Google Scholar]
- 21.Tzubery A., Tshuva E.Y. Trans titanium(IV) complexes of salen ligands exhibit high antitumor activity. Inorg. Chem. 2011;50:7946–7948. doi: 10.1021/ic201296h. [DOI] [PubMed] [Google Scholar]
- 22.Majumdar D., Philip J.E., Das S., Kundu B.K., Saini R.V., Chandan G., Bankura K., Mishra D. Experimental and theoretical corroboration of antimicrobial and anticancer activities of two pseudohalides induced structurally diverse Cd (II)-Salen complexes. J. Mol. Struct. 2020;1225 [Google Scholar]
- 23.Li L., et al. Design, synthesis, and biological activities of aromatic gossypol schiff base derivatives. J. Agric. Food Chem. 2014;62:11080–11088. doi: 10.1021/jf504411g. [DOI] [PubMed] [Google Scholar]
- 24.Pontiki E., Hadjipavlou-Litina D., Chaviara A.T. J. Evaluation of anti-inflammatory and antioxidant activities of Copper (II) Schiff mono-base and Copper(II) Schiff base coordination compounds of dien with heterocyclic aldehydes and 2-amino-5-methyl-thiazole. Enzyme Inhib. Med. Chem. 2008;23:1011–1017. doi: 10.1080/14756360701841251. [DOI] [PubMed] [Google Scholar]
- 25.Li Z., Wu S., Ding H., Zheng D., Hu J., Wang X., Huo Q., Guan J., Kan Q. Immobilized Cu(II) and Co(II) salen complexes on graphene oxide and their catalytic activity for aerobic epoxidation of styrene. New J. Chem. 2013;37:1561–1568. [Google Scholar]
- 26.Ourari A., Ouennoughi Y., Aggoun D., Mubarak M.S., Pasciak E.M., Peters D.G. Synthesis, characterization, and electrochemical study of a new tetradentate nickel(II)-Schiff base complex derived from ethylenediamine and 5′-(N-methyl-N-phenylaminomethyl)-2′-hydroxyacetophenone. Polyhedron. 2014;67:59–64. [Google Scholar]
- 27.Salavati-Niasari M., Bazarganipour M. Synthesis, characterization and catalytic oxidation properties of multi-wall carbon nanotubes with a covalently attached copper(II) salen complex. Appl. Surf. Sci. 2009;255:7610–7617. [Google Scholar]
- 28.Finelli A., Chabert V., rault N.H., Crochet A., Kim C., Fromm K.M. Sequential multiple-target sensor: in3+, Fe2+, and Fe3+ discrimination by an anthracene-based probe. Inorg. Chem. 2019;58:13796–13806. doi: 10.1021/acs.inorgchem.9b01478. [DOI] [PubMed] [Google Scholar]
- 29.Xu L., Yang Y., Wang Y., Gao J. Chiral salen Mn(III) complex-based enantioselective potentiometric sensor for l-mandelic acid. Anal. Chim. Acta. 2009;653:217–221. doi: 10.1016/j.aca.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Sadeghzadeh S.M. Ruthenium salen complex immobilized on FeNi3 magnetic nanoparticles: the efficient, green and reusable nanocatalyst for heck and Suzuki coupling reactions. Catal. Lett. 2016;146:2555–2565. [Google Scholar]
- 31.Pepels M.P.F., Bouyahyi M., Heise A., Duchateau R. Kinetic investigation on the catalytic ring-opening (Co)polymerization of (Macro)Lactones using aluminum salen catalysts. Macromolecules. 2013;46:4324–4334. [Google Scholar]
- 32.Roy S., Dutta T., Drew M.G.B., Chattopadhyay S. Phenoxazinone synthase mimicking activity of a dinuclear copper(II) complex with a half salen type Schiff base ligand. Polyhedron. 2020;178 [Google Scholar]
- 33.Erxleben A. Transition metal salen complexes in bioinorganic and medicinal chemistry. Inorg. Chim. Acta. 2018;472:40–57. [Google Scholar]
- 34.Vhanale B.T., Deshmukh N.J., Shinde A.T. Synthesis, characterization, spectroscopic studies and biological evaluation of Schiff bases derived from 1–hydroxy-2-acetonapthanone. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chinnamanayakar R., Ezhilarasi M.R., Prabha B., Kulandhaivel M. In vitro antimicrobial activity and in silico activity of 1-thiocarbamoyl substituted pyrazole derivatives. Asian J. Chem. 2018;30:783–789. [Google Scholar]
- 36.Omeke P.O., Obi J.O., Orabueze N.A.I., Ike A.C. Antibacterial activity of leaf extract of Chromolaena odorata and the effect of its combination with some conventional antibiotics on Pseudomonas aeruginosa isolated from wounds. J. Appl. Biol. Biotechnol. 2019;7:36–40. [Google Scholar]
- 37.Zhou K., Yu L. Effects of extraction solvent on wheat bran antioxidant activity estimation. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2004;37:717–721. [Google Scholar]
- 38.Ünver H. Synthesis and spectroscopic studies in some new Schiff bases. Spectrosc. Lett. 2001;34:783–791. [Google Scholar]
- 39.Salman S.R., Kamounah F.S. Mass spectral study of tautomerism in some 1-hydroxy-2-naphthaldehyde Schiff bases. Spectrosc. Lett. 2002;35:327–335. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.