Abstract
Objective:
We sought to define criteria associated with low lymph node metastasis risk in patients with submucosal (pT1b) gastric cancer from 3 Western and 3 Eastern countries.
Summary Background Data:
Accurate prediction of lymph node metastasis risk is essential when determining the need for gastrectomy with lymph node dissection following endoscopic resection. Under current guidelines, endoscopic resection is considered definitive treatment if submucosal invasion is only superficial, but this is not routinely assessed.
Methods:
Lymph node metastasis rates were determined for patient groups defined according to tumor pathological characteristics. Clinicopathological predictors of lymph node metastasis were determined by multivariable logistic regression and used to develop a nomogram in a randomly selected subset that was validated in the remainder. Overall survival was compared between Eastern and Western countries.
Results:
Lymph node metastasis was found in 701 of 3,166 (22.1%) Eastern and 153 of 560 (27.3%) Western patients. Independent predictors of lymph node metastasis were female gender, tumor size, distal stomach location, lymphovascular invasion, and moderate or poor differentiation. Patients fulfilling the National Comprehensive Cancer Network guideline criteria, excluding the requirement that invasion not extend beyond the superficial submucosa, had a lymph node metastasis rate of 8.9% (53 of 594). Excluding moderately differentiated tumors lowered the rate to 3.4% (10 of 296). The nomogram’s AUC was 0.690. Regardless of lymph node status, overall survival was better in Eastern patients.
Conclusions:
The lymph node metastasis rate was lowest in patients with well differentiated tumors that were ≤ 3 cm and lacked lymphovascular invasion. These criteria may be useful in decisions regarding endoscopic resection as definitive treatment for pT1b gastric cancer.
MINI-ABSTRACT
To inform decisions regarding endoscopic resection as definitive treatment for submucosal (T1b) gastric adenocarcinoma, we sought to identify pathological tumor characteristics that predict low risk of lymph node metastasis in a global cohort of 3,726 patients. The lymph node metastasis rate was lowest among patients with tumors that were well differentiated, ≤ 3 cm, and without lymphovascular invasion.
INTRODUCTION
Endoscopic resection has been increasingly adopted as treatment for early-stage gastric cancer over the last decade. Endoscopic mucosal resection (EMR) is now commonly used worldwide for well-selected gastric stage 1a (T1a) cases, while the more technically demanding endoscopic submucosal dissection (ESD) is more commonly used in Asian than in Western countries stemming from their higher incidence of early-stage gastric cancer1. The appropriate use of endoscopic resection as definitive treatment for T1b tumors rather than gastrectomy with lymph node dissection depends largely on the risk of lymph node metastasis (LNM) and remains controversial. As gastric cancer incidence and pathobiology vary between Eastern and Western countries, indications for endoscopic resection as definitive treatment in certain countries may or may not apply to other parts of the world. This makes it difficult to ascertain the true risk of LNM for individual patients and impedes treatment decision making.
The Japanese guidelines from 2020 (2nd edition) consider endoscopic resection of pT1b tumors as definitive treatment if the tumor invades the submucosa < 500 μm, is well or moderately differentiated, ≤ 3 cm in size, and lacks lymphovascular invasion, and the margins are negative2. This recommendation was based on the observation that none of the 145 Japanese patients fulfilling these criteria had LNM3. Based on the Japanese guidelines, the American National Comprehensive Cancer Network (NCCN) guideline version 3.2020 adopted similar criteria, substituting the quantitative invasion limit with the more qualitative “does not penetrate beyond the superficial submucosa,” and lowering the tumor size limit to ≤ 2 cm4. Given known differences in the epidemiology and subtypes of gastric cancer between Asian and Western patients5–14, the paucity of research on LNM risk for T1b tumors in patients from the West leaves a gap in determining the applicability of these criteria. Furthermore, the criteria regarding invasion depth are difficult to apply given that this feature is not routinely assessed in the West, and the NCCN guideline is non-quantitative and thus subject to interpretation.
To examine LNM risk in early-stage gastric cancer, we formed an international collaboration and established the Global Gastric Cancer Group (G3) Alliance, a large cohort of gastric cancer patients from large volume gastric cancer institutions in South Korea, China, Japan, the United States, and Italy, as well as the Netherlands Cancer Registry and the Dutch Gastric Cancer Trial15. This dataset enables comparison of LNM risk between global regions, which will broaden knowledge of associated differences in early-stage gastric cancer presentation, treatment, and outcomes.
The aim of this study was to determine the LNM rates for pathological T1b (pT1b) gastric cancer in patients who underwent gastrectomy among groups defined according to pathological tumor characteristics, and compare these rates, as well as other clinical features including survival, between Eastern and Western countries. We also aimed to identify clinical and pathological predictors of LNM and to construct and validate an LNM nomogram.
METHODS
Data collection
After the study protocol was designed, discussed, and approved by all G3 Alliance members, Institutional Review Board approval was obtained at Memorial Sloan Kettering Cancer Center. Each member institution has a prospectively maintained database of surgical gastric cancer patients that was queried for the purpose of this study. The Dutch cohort was derived from the Dutch Gastric Cancer Trial database and Netherlands Cancer Registry 16. The Dutch Gastric Cancer Trial randomized patients treated from 1989 to 1993 to either D1 or D2 lymph node dissection15. The NCR is a nationwide registry containing data on all patients with a cancer diagnosis since 1989 hosted by the Netherlands Comprehensive Cancer Organisation. Nearly 100% of patients with gastroesophageal cancer who underwent surgery since 2010 in the Netherlands are included in the database17.
Patient selection
Databases were queried for patients with pT1b gastric cancer, meaning a tumor invading no deeper than the submucosal layer, and who had undergone a (sub)total gastric resection including lymphadenectomy with negative margins. Inclusion criteria were a histologically confirmed primary T1b gastric adenocarcinoma without neoadjuvant treatment. Exclusion criteria were atypical resections including wedge gastrectomy and completion gastrectomy for recurrent or remnant cancers or following endoscopic resection, gastroesophageal junction cancers, and patients with missing information for one of the following variables: type of surgical resection, pathological tumor location, or pathological lymph node status.
Data collection
The following variables were collected from each database: age at surgery, gender, race, date of surgery, type of surgery, pathological tumor location, pathological tumor stage (pT) and lymph node status (pN), number of dissected lymph nodes, number of positive lymph nodes, pathological tumor size, differentiation, histology, presence of lymphovascular invasion, date of last follow-up, and date of death, if applicable. pT and pN were determined using the 8th edition of the AJCC staging system18.
Statistical analysis
Patient demographics and clinicopathological characteristics were compared among all 6 countries and Eastern (South Korea, China, and Japan) vs. Western (the Netherlands, United States, and Italy) countries by chi-square test for categorical variables and Mann-Whitney U test for continuous variables. Predictors of LNM were identified by univariable and multivariable logistic regression analysis. All factors with a p value of ≤ 0.10 in the univariable analysis were included for multivariable analysis. To build the nomogram, each individual dataset was randomly divided into a training and validation set at a 2:1 ratio, and logistic regression was repeated to identify independent predictors of LNM in the combined training set. Patients with missing values for the variables used in the nomogram were excluded. The discriminative ability of the nomogram in the training and validation dataset was estimated by the area under the curve (AUC). Goodness of fit was evaluated by a calibration plot in the validation set by quantiles of predicted risk. Overall survival (OS) was calculated from the date of surgery until date of death or last follow-up in the survivors. Univariable and multivariable survival analysis were performed by Cox regression analysis. Survival curves were estimated by Kaplan-Meier method and p values by log-rank test. All p values were two-sided; p-values of < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS Statistics for Windows, Version 25.0 (IBM, Armonk, New York, USA), or R version 3.6 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Demographics, clinicopathological characteristics, and LNM rate
A total of 3,726 patients were included: 1,858 from South Korea, 700 from China, 608 from Japan, 278 from the Netherlands, 244 from the United States, and 38 from Italy. After grouping the individual databases into global regions, 3,166 patients formed the Eastern cohort and 560 the Western (cohorts summarized in Table 1; each country’s data reported in Supplemental Table 1). Compared to the Western cohort, the Eastern cohort was more recently treated (median 2011, IQR 2007–2015 vs. median 2010, IQR 2001–2014), younger (median 62 years, IQR 54–70 vs. 68, IQR 59–75), included fewer women (32% vs. 48%), had fewer tumors located in the distal stomach (50% vs. 55%), less often underwent distal gastrectomy (75% vs. 79%), had more lymph nodes dissected (median 36 vs. 22), and had more well differentiated tumors (17% vs. 11%). Frequencies of signet ring cell histology (17% vs. 18%) and lymphovascular invasion (19% vs. 19%) were comparable, but data regarding these variables was missing in 39% and 46% of the Western patients, respectively (Table 1). Of the total 3,726 patients, 854 (22.9%) had pathologically positive lymph nodes; 701 (22.1%) in the Eastern cohort and 153 (27.3%) in the Western (p = 0.007) (Table 1). The rate of LNM was lowest in Japan at 19.1% and highest in the Netherlands at 29.5% (Supplemental Table 1).
Table 1.
Demographics and clinicopathological characteristics. Categorical variables are presented as n (%) and continuous variables as median (interquartile range).
| Total (3,726) | East (3,166) | West (560) | p | |
|---|---|---|---|---|
| Age, years | 63 (54–71) | 62 (54–70) | 68 (59–75) | < 0.001 |
| Female | 1,273 (34.2) | 1,006 (31.8) | 267 (47.7) | < 0.001 |
| Race | < 0.001 | |||
| White | 302 (8.1) | 0 (0.0) | 302 (53.9) | |
| Asian | 3,210 (86.2) | 3,166 (100) | 44 (7.9) | |
| Black | 23 (0.6) | 0 | 23 (4.1) | |
| Other or unknown | 191 (5.1) | 0 | 191 (34.1) | |
| Year of surgery | 2011 (2007–2015) | 2011 (2007–2015) | 2010 (2001–2014) | < 0.001 |
| Location | 0.023 | |||
| Proximal third | 516 (13.8) | 452 (14.3) | 64 (11.4) | |
| Middle third | 1,245 (33.4) | 1,062 (33.5) | 183 (32.7) | |
| Distal third | 1,907 (51.2) | 1,597 (50.4) | 310 (55.4) | |
| Multiple/diffuse | 58 (1.6) | 55 (1.7) | 3 (0.5) | |
| Type of surgery | 0.011 | |||
| Distal | 2,818 (75.6) | 2,374 (75.0) | 444 (79.3) | |
| Total | 825 (22.1) | 726 (22.9) | 99 (17.7) | |
| Proximal | 83 | 66 (2.1) | 17 (3.0) | |
| pN positive | 854 (22.9) | 701 (22.1) | 153 (27.3) | 0.007 |
| pN status | 0.02 | |||
| 0 | 2,872 (77.1) | 2,465 (77.9) | 407 (72.7) | |
| 1 | 535 (14.4) | 440 (13.9) | 95 (17.0) | |
| 2 | 225 (6.0) | 186 (5.9) | 39 (7.0) | |
| 3a | 83 30 | 64 (2.0) | 19 (3.4) | |
| 3b | 11 (0.3) | 11 (0.3) | 0 (0.0) | |
| Dissected nodes | 34 (25–45) | 36 (27–46) | 22 (15–32) | < 0.001 |
| Positive nodes | 0 (0–0) | 0 (0–0) | 0 (0–1) | 0.008 |
| Tumor size, mm | 25 (18–40) | 25 (18–40) | 25 (15–35) | 0.002 |
| Differentiation | 0.002 | |||
| Well | 598 (16.0) | 539 (17.0) | 59 (10.5) | |
| Moderate | 1,470 (39.5) | 1,236 (39.0) | 234 (41.8) | |
| Poor | 1,454 (39.0) | 1,220 (38.5) | 234 (41.8) | |
| Unknown | 204 (5.5) | 171 (5.4) | 33 (5.9) | |
| Signet histology | 626 (16.8) | 527 (16.6) | 99 (17.7) | < 0.001 |
| Unknown | 455 (12.2) | 237 (7.5) | 218 (38.9) | |
| Lymphovascular invasion | 705 (18.9) | 600 (19.0) | 105 (18.8) | < 0.001 |
| Unknown | 299 (8.0) | 39 (1.2) | 260 (46.4) |
LNM rate according to pathological tumor characteristics
Patients were categorized according to their tumor’s differentiation grade, tumor size, and presence of lymphovascular invasion (Table 2a–b). The group fulfilling Japanese guideline criteria for considering endoscopic resection to be definitive therapy (well or moderately differentiated, size ≤ 3 cm, no lymphovascular invasion), without requirement for limited submucosal invasion depth, had an LNM rate of 12.5% (121 of 971) (Table 2a). The group fulfilling NCCN guidelines’ criteria (well or moderately differentiated, size ≤ 2 cm, no lymphovascular invasion), disregarding the invasion depth criterion, had an LNM rate of 53 of 594 (8.9%). LNM rates below 5% were observed in patients with a well differentiated tumor, without lymphovascular invasion, and size ≤ 3 cm, and even lower rates in tumors ≤ 2 cm or ≤ 1 cm: 3.4% (10/296), 3.0% (6/200) and 1.6% (1/63), respectively (Table 2b). Of the 27 patients with tumors < 1 cm, none had LNM.
Table 2.
Proportion of patients with lymph node metastasis (pN-positive) according to differentiation, tumor size, and lymphovascular invasion 31.
| a | |||||
|---|---|---|---|---|---|
| No LVI | LVI | ||||
| Differentiation | Tumor size | Total | pN-positive | Total | pN-positive |
| Well or moderate | ≤ 2 cm | 594 | 53 (8.9%) | 134 | 20 (14.9%) |
| ≤ 3 cm | 971 | 121 (12.5%) | 241 | 47 (19.5%) | |
| > 3 cm | 518 | 134 (25.9%) | 156 | 71 (45.5%) | |
| Poor | ≤ 2 cm | 408 | 58 (14.2%) | 102 | 36 (35.3%) |
| ≤ 3 cm | 675 | 119 (17.6%) | 177 | 64 (36.2%) | |
| > 3 cm | 380 | 120 (31.6%) | 80 | 41 (51.2%) | |
| b | |||||
| No LVI | LVI | ||||
| Differentiation | Tumor size | Total | pN-positive | Total | pN-positive |
| Well | ≤ 1 cm | 63 | 1 (1.6%) | 11 | 2 (18.2%) |
| ≤ 2 cm | 200 | 6 (3.0%) | 43 | 3 (7.0%) | |
| ≤ 3 cm | 296 | 10 (3.4%) | 74 | 9 (12.2%) | |
| > 3 cm | 138 | 25 (18.1%) | 55 | 19 (34.5%) | |
| Moderate | ≤ 1 cm | 106 | 12 (11.3%) | 20 | 2 (10.0%) |
| ≤ 2 cm | 394 | 47 (11.9%) | 91 | 17 (18.7%) | |
| ≤ 3 cm | 675 | 111 (16.4%) | 167 | 38 (22.8%) | |
| > 3 cm | 380 | 109 (28.7%) | 101 | 52 (51.5%) | |
a, Well or moderately vs. poorly differentiated tumors. Bolded entries indicate patients meeting criteria for definitive treatment by endoscopic resection according to Japanese (≤ 3 cm) and NCCN (≤ 2 cm) criteria. b, Well vs. moderately differentiated tumors. Italicized entries indicate patients meeting above criteria but excluding moderate differentiation.
Predicting LNM
Factors significantly and independently associated with LNM included female gender (OR 1.36, 95% CI 1.13–1.64, p = 0.001), increasing tumor size (OR 1.03, 95% CI 1.02–1.03, p < 0.001), tumors located in the distal stomach (OR 1 .78, 95% CI 1.34–2.38, p < 0.001), lymphovascular invasion (OR 2.44, 95% CI 1.99–2.99, p < 0.001), and moderate or poor differentiation (OR 2.70, 95% CI 1.99–3.67, p < 0.001 and OR 3.07, 95% CI 2.26–4.18, p < 0.001, respectively) (Table 3). After multivariable analysis, East vs. West was not associated with a different LNM rate.
Table 3.
Univariable and multivariable logistic regression analysis for lymph node metastasis
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| East vs. West | 0.76 | 0.62–0.93 | 0.007 | 0.89 | 0.64–1.23 | 0.471 |
| Age, years | 1.01 | 1.00–1.01 | 0.124 | |||
| Female vs. male | 1.35 | 1.15–1.58 | < 0.001 | 1.36 | 1.13–1.64 | 0.001 |
| Year of surgery | 1.01 | 0.99–1.02 | 0.428 | |||
| Tumor size, mm | 1.02 | 1.02–1.03 | < 0.001 | 1.03 | 1.02–1.03 | < 0.001 |
| Location | ||||||
| Proximal | 1 | 1 | ||||
| Middle | 1.41 | 1.08–1.85 | 0.012 | 1.28 | 0.94–1.74 | 0.112 |
| Distal | 1.74 | 1.35–2.24 | < 0.001 | 1.78 | 1.34–2.38 | < 0.001 |
| Multiple | 1.19 | 0.59–2.38 | 0.63 | 1.48 | 0.70–3.11 | 0.301 |
| Number of dissected nodes | 1.00 | 1.00–1.01 | 0.102 | |||
| Signet histology | 1.07 | 0.87–1.31 | 0.529 | |||
| Lymphovascular invasion | 2.19 | 1.83–2.62 | < 0.001 | 2.44 | 1.99–2.99 | < 0.001 |
| Differentiation | ||||||
| Well | 1 | 1 | ||||
| Moderate | 2.45 | 1.85–3.23 | < 0.001 | 2.70 | 1.99–3.67 | < 0.001 |
| Poor | 2.83 | 2.14–3.73 | < 0.001 | 3.07 | 2.26–4.18 | < 0.001 |
To develop the nomogram, each country’s cohort was then divided into training and validation sets (n = 2,492 and 1,234, respectively). There were no significant differences in demographics or clinicopathological characteristics between training and validation sets (Supplemental Table 2). In the training set, the same factors were associated with LNM as in the total population (Supplemental Table 3); these were incorporated into the nomogram predicting LNM (Fig. 1a). The nomogram’s area under the curve was 0.700 (95% CI 0.675–0.726) in the training set and 0.690 (0.653–0.728) in the validation set (Fig. 1b). The calibration plot shows that the nomogram’s predicted risk is less than the observed risk in the low-risk and high-risk groups, and greater than the observed risk in the middle-risk groups (Supplemental Fig. 1).
Figure 1.
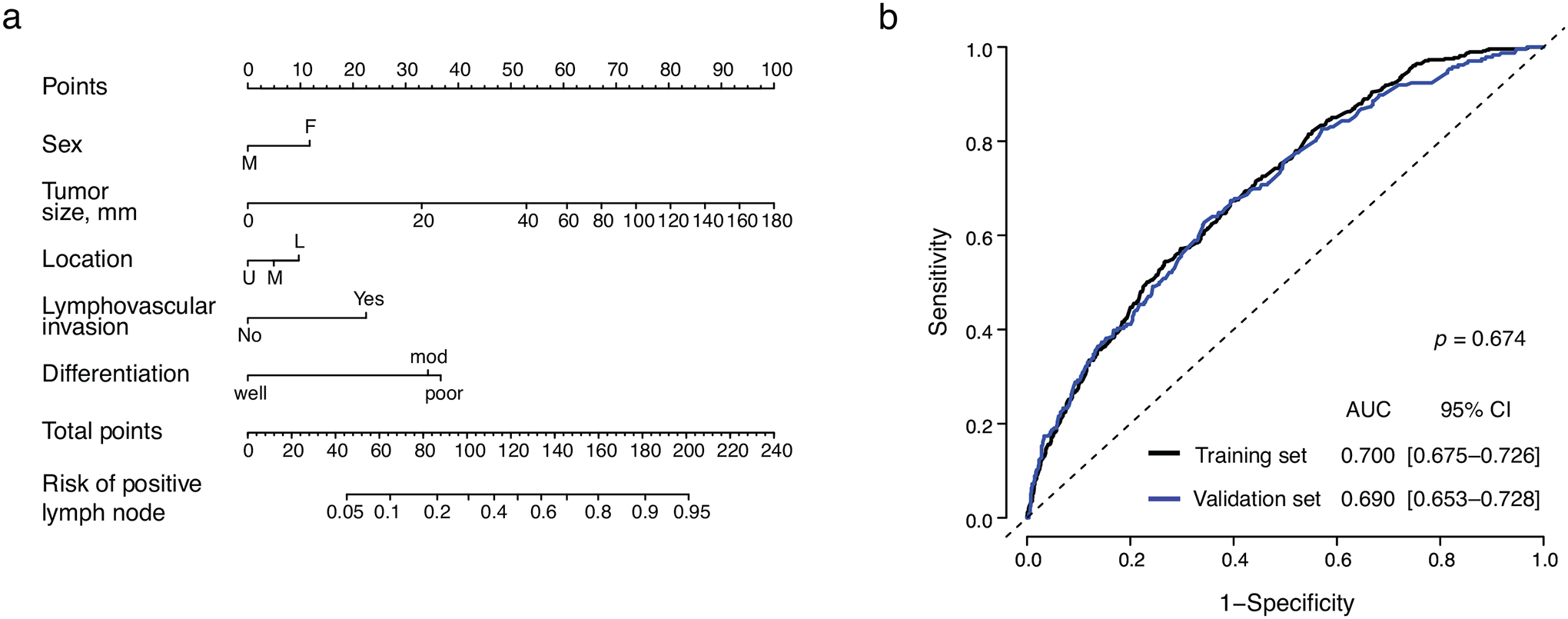
Nomogram for lymph node metastasis in pT1b gastric cancer patients. (a) Scoring and interpretation. (b) ROC curve in the training set (black line) and validation set (blue line).
Overall survival
After a median follow-up of 60.7 (IQR 30–100) months, 5-year OS was 92.0% among Eastern and 76.5% among Western patients (p < 0.001) (Fig. 2a). After adjustment for age, gender, race, number of dissected lymph nodes, lymphovascular invasion, and differentiation, OS remained significantly better in Eastern patients (HR 0.54, 95% CI 0.41–0.71, p < 0.001) (Supplemental Table 4). Among pathological lymph node (pN)-negative patients, 5-year OS was 93.2% in the East and 80.4% in the West (p < 0.001) (Fig. 2b) and significantly better in Eastern patients after adjustment (HR 0.52, 95% CI 0.37–0.72, p < 0.001) (Supplemental Table 4). In pN-positive patients, 5-year OS was 87.7% in the East and 65.5% in the West (p < 0.001) (Fig. 2c), and significantly better in Eastern patients after adjustment (HR 0.56, 95% CI 0.35–0.91, p = 0.020) (Supplemental Table 4).
Figure 2.
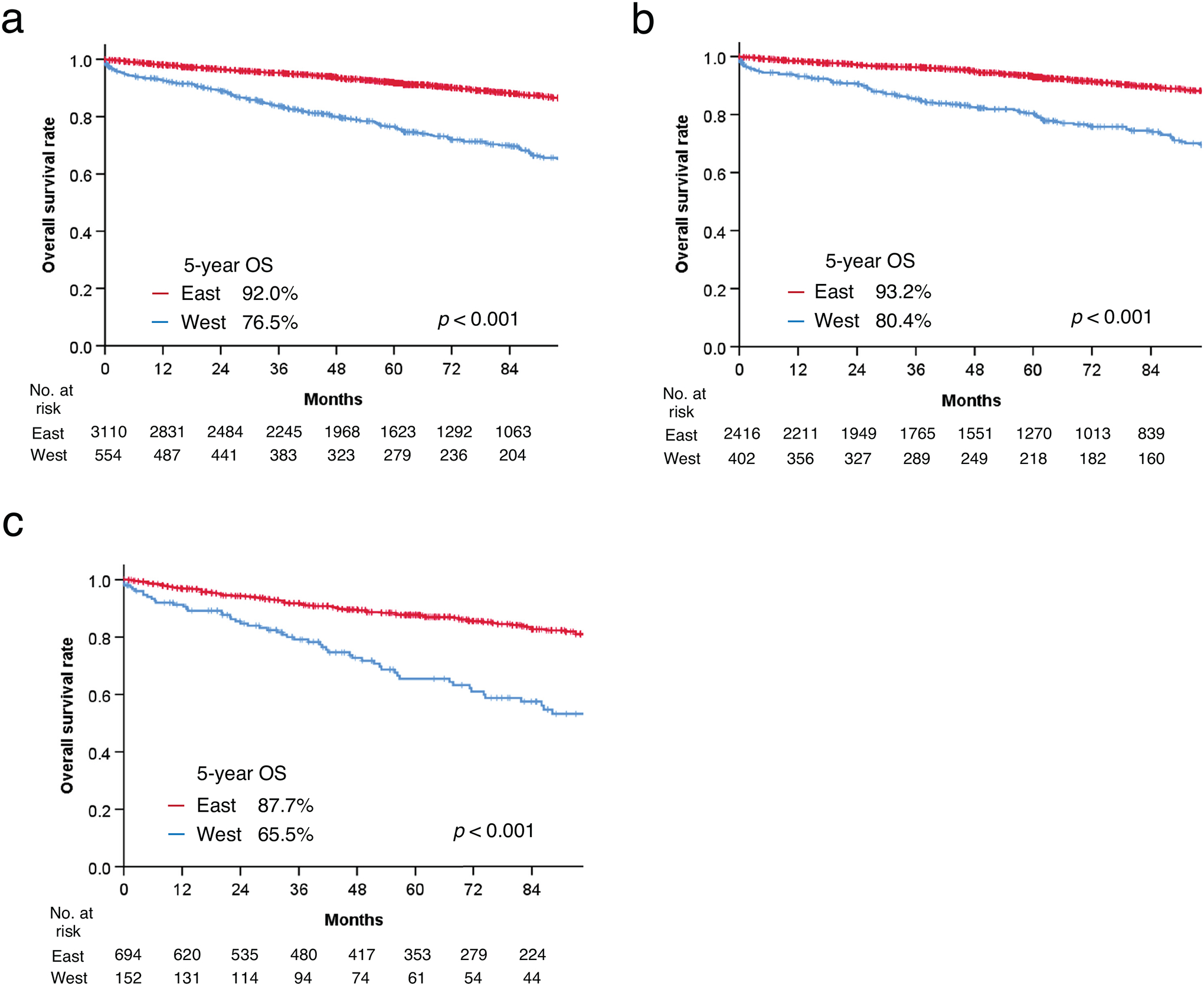
Comparison of overall survival. (a) Eastern vs. Western countries; (b) Eastern vs. Western countries in pN-negative patients only; (c) Eastern vs. Western countries in pN-positive patients.
DISCUSSION
We found that the lymph node metastasis rate in pT1b gastric cancer is 23%, and significantly lower in those treated in Eastern countries (South Korea, China, and Japan) than in Western countries (the Netherlands, United States, and Italy). The LNM rates among patients fulfilling current guideline criteria for considering endoscopic resection as definitive treatment for pT1b gastric cancer, without regard for invasion depth, were relatively high, at 8.9% for NCCN and 12.5% for the Japanese guidelines. One of the independent predictors for LNM was moderate differentiation; excluding patients with moderately differentiated tumors lowered the LNM rate to 3.0% for NCCN and 3.4% for Japanese criteria. These findings may be used to inform decision making regarding endoscopic resection as definitive treatment in pT1b gastric cancer.
The overall LNM rate we report is similar to that in a meta-analysis by Kwee et al., which found a median risk of 19%19. However, of the 40 studies included in that analysis, 39 were in Asian populations and most in Japan. Their finding is thus in agreement with our LNM rate of 22% in Eastern countries and 19% in Japan. The only Western study included in the meta-analysis had the highest LNM rate at 33% (8 of 24)20. Among the 6 other studies of LNM rates in Western T1b gastric cancer patients 21–26, one reported a substantially lower LNM rate of 9%, but that study included only 15% of patients with ≥ 15 lymph nodes removed21; the other 5 found LNM rates between 25–32%22–26, concordant with our LNM rate of 27% in Western countries. Although Western patients had fewer lymph nodes resected (median 22 vs. 36), the number of nodes resected was not associated with the presence of LNM. We believe the recommendation to examine ≥ 15 lymph nodes for accurate staging remains adequate 4, 27.
LNM rates in patients fulfilling most criteria for endoscopic resection as definitive treatment in pT1b tumors were relatively high, at 12.5% for the Japanese guidelines and 8.9% for NCCN. Invasion depth was not considered because most pathologists in Western countries do not assess this feature. The former rate is higher than that in a recent meta-analysis of Asian studies, in which the LNM rate in patients fulfilling all of the Japanese criteria, including maximum invasion depth, was 8 of 315 (2.5%)28. Thus, it appears that excluding this criterion leads to the inclusion of patients who are more likely to harbor LNM. Even considering disadvantages of a surgical resection regarding comorbidity, complications, and quality of life, a predicted LNM risk of 8.9% may be unacceptable; in cases where invasion depth is not assessed, more stringent criteria regarding available information may be necessary. However, the LNM risk threshold is an individual decision for each patient.
Importantly, we found that excluding moderately differentiated tumors from the criteria for definitive treatment substantially decreased LNM risk. Moderate differentiation was seen in a greater proportion of patients compared with well differentiation in most countries: 29% vs. 30% of tumors in Japan, 34% vs. 11% in Italy, 40% vs. 16% in Korea, 42% vs. 13% in the US, 43% vs. 8% in the Netherlands, and 47% vs. 10% in China (Supplemental Table 1). While the distinction between well and moderate differentiation is generally considered equivocal and subjective, even in this international cohort, the greater LNM risk in moderately than well differentiated tumors was consistent across countries (Supplemental Table 5). This distinct LNM risk has not previously been reported, as other studies have grouped well and moderately differentiated tumors together into “differentiated”3, 19, as did Japanese and NCCN guidelines2, 4. The better understanding of factors associated with LNM in pT1b gastric cancer provided here can inform decisions about optimal management of patients after endoscopic resection, in the context of other patient-related variables and preferences.
Our nomogram appears to underestimate LNM risk in patients fulfilling almost all guideline criteria. The reasons for this suboptimal predictive ability (AUC of 0.69 in the validation set) are unclear, as the meta-analysis from Kwee et al. found the same variables to be significantly associated with LNM 19. However, the association between distal stomach tumors and LNM was unexpected. A potential reason is that patients with tumors in the distal stomach have more nodes retrieved and thus a higher chance of finding LNM; however, this was not observed, as the median number of lymph nodes resected in upper, middle, and distal stomach tumors was similar. Another unexpected finding was the lower prevalence of distal stomach tumors in the East vs. the West (50% vs. 55%), likely resulting from the low prevalence of tumors in this location in Japan (30%) (Supplemental Table 1). This might be explained by Japan’s national gastric cancer screening program, as screened patients more often have cancer in the middle stomach29, as confirmed here (49% vs. 20–33% in other countries; Supplemental Table 1).
Overall survival was independently and significantly better in Eastern patients compared with Western, regardless of lymph node status. The survival rates of patients in each Eastern country were more similar to each other than the survival from Western countries, calling for investigation into the reasons for differences in outcomes of patients with early gastric cancer between global regions. The significant age difference between East and West (median 62 vs. 68 years) could cause residual confounding, though our analysis adjusted for this factor. As the clinicopathological characteristics we analyzed were similar between the Eastern and Western cohort, such studies should focus on cancer biology, environmental factors, other patient characteristics, or treatment differences.
Because this study was based on data from the postoperative pathologic report, the findings can only be used to inform decision making after endoscopic resection, not indications for endoscopic resection. Determining initial indications for minimally invasive treatment would require assessment of lymphovascular invasion on biopsy specimens, which is challenging. Such guidelines are further complicated by the fact that clinical assessment of tumor size and invasion depth by endoscopic ultrasound or even newer technology like narrow-band imaging is inexact.
This retrospective multi-institutional cohort study had several limitations. Although data were collected from representative high-volume centers and well-organized databases, practice, type of operation, and pathological evaluations were not standardized, and imaging and surgical video were not centrally reviewed. In addition, the lack of assessment of the degree of submucosal tumor invasion in Western countries limited our ability to precisely compare outcomes with studies from the East. Invasion depth data could have improved our prediction model and the predictive accuracy of our nomogram, and we would likely have found a lower LNM rate in patients with tumors with limited submucosal invasion. To enhance the reliability of predictions regarding outcomes of endoscopic treatment of T1b gastric cancer in the West, we recommend standardized pathological assessment of submucosal invasion depth.
A well-designed international prospective study could confirm our results. In addition, comparison of survival outcomes between early gastric cancer patients with and without additional surgery following endoscopic resection could validate the usefulness of our nomogram. Furthermore, our nomogram predicting LNM can be applied for gastric cancer patients following endoscopic resection, because this nomogram was created in patients with pathologically confirmed T1b disease and the nomogram includes lymphovascular invasion status, which is unavailable before resection. Although clinical tumor depth data would be difficult to collect in an international multi-institutional study, accurate pre-treatment data would allow creation of a nomogram to predict LNM among early gastric cancer patients to inform the decision of whether to perform an endoscopic dissection. Combining our nomogram (i.e., post-endoscopic resection) with g b b such a pre-endoscopic resection nomogram would enable more patient-specific treatment for early gastric cancer patients, which might lead better oncological outcomes, quality of life, and cost-effectiveness. The strength of our study is the large study population, including multiple countries from the East and West, and the inclusion of representative high-volume centers.
Conclusions
In patients with pT1b gastric cancer, those treated in Eastern countries (South Korea, China, and Japan) were significantly less likely to harbor LNM than those in Western countries (the Netherlands, United States, and Italy). The LNM rates among patients fulfilling current guideline criteria for considering endoscopic resection as definitive treatment for pT1b gastric cancer, without regard for invasion depth, had relatively high LNM rates, but excluding patients with moderately differentiated tumors lowered the LNM rate from 8.9% to 3.0% for NCCN and from 12.5% to 3.4% for Japanese criteria. These findings may be used to inform decision making regarding endoscopic resection as definitive treatment in pT1b gastric cancer.
Supplementary Material
Supplemental Figure 1. Calibration curve comparing the nomogram’s predicted risk vs. the observed risk for lymph node metastasis.
Supplemental Table 1. Demographics and clinicopathological characteristics by country.
Supplemental Table 2. Demographics and clinicopathological characteristics of the training and validation sets used to develop and validate the nomogram.
Supplemental Table 3. Univariable and multivariable logistic regression analysis of factors associated with lymph node metastasis in the training set.
Supplemental Table 4. Univariable and multivariable Cox regression analysis of factors associated with overall survival.
Supplemental Table 5. Association of tumor differentiation and lymph node metastasis within each country.
ACKNOWLEDGEMENTS
The authors thank Murray F. Brennan, MD, for his intellectual contribution to the manuscript and Jessica Moore, MS, for editing.
Funding:
This study was supported in part by an NCI Cancer Center Support Grant (P30 CA008748) to the institution for core resources.
REFERENCES
- 1.Gotoda T Endoscopic resection of early gastric cancer. Gastric Cancer 2007; 10(1):1–11. [DOI] [PubMed] [Google Scholar]
- 2.Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc 2020. [DOI] [PubMed] [Google Scholar]
- 3.Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3(4):219–225. [DOI] [PubMed] [Google Scholar]
- 4.Ajani JA, D’Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016; 14(10):1286–1312. [DOI] [PubMed] [Google Scholar]
- 5.Li P, Huang CM, Zheng CH, et al. Comparison of gastric cancer survival after R0 resection in the US and China. J Surg Oncol 2018; 118(6):975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shim JH, Song KY, Jeon HM, et al. Is gastric cancer different in Korea and the United States? Impact of tumor location on prognosis. Ann Surg Oncol 2014; 21(7):2332–9. [DOI] [PubMed] [Google Scholar]
- 7.Strong VE, Song KY, Park CH, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg 2010; 251(4):640–6. [DOI] [PubMed] [Google Scholar]
- 8.Strong VE, Wu AW, Selby LV, et al. Differences in gastric cancer survival between the U.S. and China. J Surg Oncol 2015; 112(1):31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strong VE, Russo A, Yoon SS, et al. Comparison of Young Patients with Gastric Cancer in the United States and China. Ann Surg Oncol 2017; 24(13):3964–3971. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi Y, Yoshikawa T, Tsuburaya A, et al. Is gastric carcinoma different between Japan and the United States? Cancer 2000; 89(11):2237–46. [PubMed] [Google Scholar]
- 11.Lu J, Yoon C, Xu B, et al. Long-Term Survival after Minimally Invasive Versus Open Gastrectomy for Gastric Adenocarcinoma: A Propensity Score-Matched Analysis of Patients in the United States and China. Ann Surg Oncol 2020; 27(3):802–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schumacher SE, Shim BY, Corso G, et al. Somatic copy number alterations in gastric adenocarcinomas among Asian and Western patients. PLoS One 2017; 12(4):e0176045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YW, Joo J, Yoon HM, et al. Different survival outcomes after curative R0-resection for Eastern Asian and European gastric cancer: Results from a propensity score matched analysis comparing a Korean and a German specialized center. Medicine (Baltimore) 2016; 95(28):e4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada T, Yoshikawa T, Taguri M, et al. The survival difference between gastric cancer patients from the UK and Japan remains after weighted propensity score analysis considering all background factors. Gastric Cancer 2016; 19(2):479–489. [DOI] [PubMed] [Google Scholar]
- 15.Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999; 340(12):908–14. [DOI] [PubMed] [Google Scholar]
- 16.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005; 138(1):8–13. [DOI] [PubMed] [Google Scholar]
- 17.Busweiler LA, Wijnhoven BP, van Berge Henegouwen MI, et al. Early outcomes from the Dutch Upper Gastrointestinal Cancer Audit. Br J Surg 2016; 103(13):1855–1863. [DOI] [PubMed] [Google Scholar]
- 18.MB Amin SE, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed.: New York: Springer, 2017. [Google Scholar]
- 19.Kwee RM, Kwee TC. Predicting lymph node status in early gastric cancer. Gastric Cancer 2008; 11(3):134–48. [DOI] [PubMed] [Google Scholar]
- 20.Jatzko G, Lisborg PH, Klimpfinger M. Extended lymphadenectomy against early gastric cancer. Jpn J Clin Oncol 1992; 22(1):26–9. [PubMed] [Google Scholar]
- 21.Borie F, Millat B, Fingerhut A, et al. Lymphatic involvement in early gastric cancer: prevalence and prognosis in France. Arch Surg 2000; 135(10):1218–23. [DOI] [PubMed] [Google Scholar]
- 22.Folli S, Morgagni P, Roviello F, et al. Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC). Jpn J Clin Oncol 2001; 31(10):495–9. [DOI] [PubMed] [Google Scholar]
- 23.Holscher AH, Drebber U, Monig SP, et al. Early gastric cancer: lymph node metastasis starts with deep mucosal infiltration. Ann Surg 2009; 250(5):791–7. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad R, Setia N, Schmidt BH, et al. Predictors of Lymph Node Metastasis in Western Early Gastric Cancer. J Gastrointest Surg 2016; 20(3):531–8. [DOI] [PubMed] [Google Scholar]
- 25.Tran TB, Worhunsky DJ, Squires MH, et al. Clinicopathologic score predicting lymph node metastasis in T1 gastric cancer. Surgery 2018; 163(4):889–893. [DOI] [PubMed] [Google Scholar]
- 26.Bausys R, Bausys A, Vysniauskaite I, et al. Risk factors for lymph node metastasis in early gastric cancer patients: Report from Eastern Europe country- Lithuania. BMC Surg 2017; 17(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karpeh MS, Leon L, Klimstra D, et al. Lymph node staging in gastric cancer: is location more important than Number? An analysis of 1,038 patients. Ann Surg 2000; 232(3):362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdelfatah MM, Barakat M, Lee H, et al. The incidence of lymph node metastasis in early gastric cancer according to the expanded criteria in comparison with the absolute criteria of the Japanese Gastric Cancer Association: a systematic review of the literature and meta-analysis. Gastrointest Endosc 2018; 87(2):338–347. [DOI] [PubMed] [Google Scholar]
- 29.Klevebro F, Lindblad M, Johansson J, et al. Outcome of neoadjuvant therapies for cancer of the oesophagus or gastro-oesophageal junction based on a national data registry. Br J Surg 2016; 103(13):1864–1873. [DOI] [PubMed] [Google Scholar]
- 30.National Comprehensive Cancer Network. Gastric Cancer (Version 5.2021). https://www.nccn.org › physician_gls › pdf › gastric. Accessed May 2021. [DOI] [PubMed]
- 31.Wang K, Johnson A, Ali SM, et al. Comprehensive Genomic Profiling of Advanced Esophageal Squamous Cell Carcinomas and Esophageal Adenocarcinomas Reveals Similarities and Differences. Oncologist 2015; 20(10):1132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Calibration curve comparing the nomogram’s predicted risk vs. the observed risk for lymph node metastasis.
Supplemental Table 1. Demographics and clinicopathological characteristics by country.
Supplemental Table 2. Demographics and clinicopathological characteristics of the training and validation sets used to develop and validate the nomogram.
Supplemental Table 3. Univariable and multivariable logistic regression analysis of factors associated with lymph node metastasis in the training set.
Supplemental Table 4. Univariable and multivariable Cox regression analysis of factors associated with overall survival.
Supplemental Table 5. Association of tumor differentiation and lymph node metastasis within each country.


