Abstract
Patients and clinicians considering electroconvulsive therapy (ECT) for treatment-resistant depression are faced with limited information about the likely long-term outcomes, and the individual characteristics that predict those outcomes. We aimed to identify sociodemographic and clinical predictors of acute ECT response and subsequent long-term depression severity. This prospective longitudinal study followed adult patients at a single academic ECT center. Among 114 participants, 105 completed an index ECT series and 70 were classified as acute ECT responders. Over a 2-year follow-up period, 82 subjects provided data on depression severity (Patient Health Questionnaire; PHQ-9). Better acute ECT response was predicted by less medication resistance, shorter index episode, and psychotic features (p<0.05). PHQ-9 scores during the two-year follow-up period improved from baseline at all time points (p<0.000001) but individual scores varied widely. Lower long-term PHQ-9 scores were predicted by better acute therapeutic response to ECT (p=0.004) but not by ECT adverse effects (p>0.05). Married status and greater baseline clinician-rated severity were not associated with acute ECT response but those variables did predict lower PHQ-9 scores longitudinally (p<0.001), independent of other baseline features, initial ECT response, or intensity of ongoing treatment. These findings confirm previously identified predictors of short-term ECT response and demonstrate that distinct individual characteristics predict long-term depression outcomes. An individual’s social context appears to strongly influence long-term but not short-term outcomes, suggesting a potential target for post-ECT therapeutic interventions.
Keywords: Treatment-resistant depression, predictors, social support, long-term, electroconvulsive therapy
Introduction
Treatment-resistant depression (TRD) afflicts millions of individuals worldwide, profoundly eroding quality of life and exacting an economic toll of more than $29 billion annually in the US alone (Johnston et al., 2019; Lex et al., 2019; Mrazek et al., 2014). For some patients with severe TRD, clinicians recommend electroconvulsive therapy (ECT). Although ECT has a high success rate relative to pharmacological interventions (Kellner et al., 2012; Ross et al., 2018; UK ECT Review Group, 2003), roughly one-third of TRD patients do not respond adequately to an acute ECT series (Haq et al., 2015; Lisanby, 2007). Furthermore, ECT can cause substantial temporary cognitive side effects (Kellner et al., 2012; Lisanby, 2007; UK ECT Review Group, 2003) and relapse is common within the first 6 months after an acute ECT series (Jelovac et al., 2013; Kellner et al., 2006; Sackeim et al., 2001). Little is known about post-ECT depression trajectories beyond 6 months, but existing data regarding TRD patients more generally suggest that long-term outcomes are poor (Fekadu et al., 2009a; Jelovac et al., 2013). Thus, patients and clinicians considering ECT face a lack of information about likely outcomes as they decide whether to choose an intrusive and costly intervention.
Critical for this decision-making process is the ability to predict who is likely to experience acute improvement with ECT, and who will experience lasting benefit. Previous meta-analyses have revealed several fairly consistent predictors: better acute ECT response is generally associated with shorter index episodes, less medication resistance, psychotic features, and older age at baseline (Haq et al., 2015; Heijnen et al., 2019; Heijnen et al., 2010; van Diermen et al., 2020; van Diermen et al., 2018). In addition, existing evidence suggests that mood disorders with catatonic features respond especially well to ECT (Fink and Taylor, 2003; Kellner et al., 2012; Leroy et al., 2018; Luchini et al., 2015). To our knowledge, baseline features that predict outcomes more than 6 months after ECT have not yet been systematically examined.
This study had two objectives. First, we aimed to identify baseline sociodemographic and clinical predictors of short-term response to ECT. Based on existing literature, we hypothesized that better acute response would be associated with shorter episode duration, less medication resistance, psychotic or catatonic features, and greater age at baseline. Beyond confirming those predictors, we aimed to exhaustively evaluate dozens of other baseline features in order to identify any useful but previously unrecognized predictors. Our second objective was to describe depression severity longitudinally for a cohort of TRD patients followed for two years after ECT, and to identify predictors of long-term outcomes. We expected that better acute response to ECT would be associated with better subsequent depression scores. Furthermore, we hypothesized that the individual characteristics that predict long-term improvements in depression might differ from features that predict short-term ECT response.
Material and Methods
Design and participants
Study procedures have been reported in detail elsewhere (Mickey et al., 2018) and are summarized here. This prospective, longitudinal, observational study enrolled adult patients with TRD (DSM-IV/DSM-5 major depressive disorder or bipolar disorder) who were referred to the ECT program at the University of Michigan. Eligible patients met the following criteria: ≥ 18 years old; diagnosis of a moderate-to-severe depressive episode for at least 2 months; medication resistance during the current episode [as previously described (Mickey et al., 2018)]; clinically appropriate for ECT; and able to provide informed consent. Exclusion criteria were: medically or psychiatrically unstable or requiring a higher level of care; ECT within the past year; primary psychotic disorder (e.g., schizoaffective disorder); secondary mood or psychotic disorder (e.g., due to a general medical condition or substance use); rapid cycling within the past year or manic/mixed episode within the past 6 months; dementia; delirium; or personality disorder as a current focus of treatment. The study was approved by the local institutional review board; subjects provided written informed consent. The sample described here includes sub-samples of participants described in previous reports (Lex et al., 2019; Lex et al., 2021; Mickey et al., 2018). The study consisted of four sequential phases: Baseline, Treatment, Post-Treatment, and Follow-up.
Baseline and Treatment phases
The baseline assessment incorporated the Mini International Neuropsychiatric Interview, Montgomery-Asberg Depression Rating Scale (MADRS), Hamilton Depression Rating Scale (HDRS), Global Assessment of Functioning (GAF), Clinical Global Impression Severity (CGI-S) scale, Massachusetts General Hospital (MGH) staging, Maudsley staging, modified Cumulative Illness Rating Scale, and a structured review of medical records (Endicott et al., 1976; Fava, 2003; Fekadu et al., 2009b; Guy, 1976; Montgomery and Asberg, 1979; Salvi et al., 2008; Williams et al., 1988). Questionnaires included the Patient Health Questionnaire (PHQ-9), Quick Inventory of Depressive Symptomatology (QIDS-SR), Work and Social Adjustment Scale (WSAS), Generalized Anxiety Disorder (GAD-7) scale, Adverse Childhood Experiences (ACE) scale, and World Health Organization Quality of Life (WHOQOL-BREF) scale (Felitti et al., 1998; Kroenke et al., 2001; Mundt et al., 2002; Rush et al., 1996; Spitzer et al., 2006; WHOQOL Group, 1998). ECT was administered 2–3 times per week as clinically indicated (total of 6–15 treatments, mean [SD] = 10.5 [3.0]). Brief- or ultra-brief-pulse stimuli were delivered with sedation and muscle relaxation using bitemporal electrode placement or right unilateral electrode placement at 5–6 times seizure threshold.
Post-treatment and Follow-up phases
As part of routine clinical care, nurses assessed patients at weekly intervals using the MADRS, the 17-item HDRS, and the Montreal Cognitive Assessment (MoCA). Nurses used semi-structured interviews and were trained by a psychiatrist. Nursing staff also elicited subjective reports from patients, family members, and caregivers. All these sources of information, chart review, and team consensus were incorporated into the Clinical Global Impression (CGI) Improvement scale (CGI-I), which ranges from 1 (very much improved) to 7 (very much worse), and the 4-point CGI Side Effect scale (Guy, 1976). The CGI was assessed at interdisciplinary team meetings during the post-treatment phase, defined as the 2-week period following the last treatment in the index series. Because this study was imbedded in a real-world clinical ECT service (i.e., an "effectiveness" rather than an "efficacy" study) and our overarching goal is to inform clinical care outside of research settings, we chose the CGI as the principal measure of acute ECT response. The primary short-term outcome was dichotomous ECT response (very much or much improved on the CGI-I), chosen because it represents clinically meaningful benefit that informs future decision-making. The MADRS was used as the secondary outcome measure because it is a validated continuous scale (range 0–60) that is sensitive to change (Montgomery and Asberg, 1979). During follow-up, questionnaires were administered at 6, 12, 18, and 24 months. The primary long-term outcome measure was total score on the PHQ-9, which is validated and easy to administer by mail/telephone (Kroenke et al., 2001). The intensity and type of treatment was summarized with the Antidepressant Treatment History Form - Short Form (ATHF-SF) (Sackeim et al., 2019).
Statistical analysis
We hypothesized that 5 baseline subject characteristics would predict better outcomes after ECT: shorter duration of the index episode, less medication resistance, psychotic features, catatonic features, and greater age. For each of these 5 predictors, two-tailed p < 0.05 was considered significant, and two-tailed 0.05 < p < 0.10 was considered marginal. Fifty-five additional baseline features (see Supplmental Table S1) were evaluated in exploratory analyses. Bonferroni adjustment was applied to account for multiple testing (threshold p = 0.05/55 = 0.0009). All reported p-values are two-tailed, uncorrected.
To evaluate each baseline variable as a potential predictor of the primary short-term outcome, we applied logistic regression with the sociodemographic or clinical feature as a predictor variable, and dichotomous ECT response as the outcome variable. For the secondary short-term outcome, we used linear regression with baseline MADRS score and the feature of interest as predictor variables, and post-ECT MADRS score as the outcome variable.
Long-term outcomes were analyzed with linear mixed models to accommodate missing data and repeated correlated measures over time. Longitudinal PHQ-9 total score was the outcome variable and subject intercept was the random-effects predictor. Fixed-effect predictors were baseline PHQ-9 score, time, and (in some models) ECT response. To evaluate each sociodemographic or clinical feature (Supplemental Table S1), the feature and its interaction with acute ECT response were included as fixed effects. Wald χ2 tests and corresponding p-values were calculated from fitted models. Further details of statistical analyses are provided in the Supplemental Methods.
To evaluate whether the type and intensity of treatment received during follow-up was associated with long-term outcomes, information on treatments used during each 6-month interval was collected via self-report and summarized with the ATHF-SF. For each subject, we calculated the number of psychotherapy visits, total number of medication trials, and the number of medication trials that were adequate in dose and duration (averages across time for each subject). We also recorded any reported use of ECT during follow-up as a binary variable. To examine whether each of these four treatment intensity measures was associated with longitudinal PHQ-9 scores after ECT, we constructed four linear mixed models with subject intercept as the random effect. Fixed-effect predictors were baseline PHQ-9 score, time, acute ECT response, one of the four treatment intensity measures, and the interaction of acute ECT response and treatment intensity.
Results
Baseline characteristics and short-term response to ECT
One-hundred-fourteen patients enrolled in the study and received ECT. Baseline sociodemographic and clinical data are described in Tables 1 and 2, and in the Supplemental Results.
Table 1.
Socio-demographic characteristics of the sample (n=114)
| Age, years, mean (SD) | 50.4 (16.0) |
| range | 19–88 |
| Female | 68 (60.0%) |
| pre-menopause | 24 (21.1%) |
| peri-menopause | 9 (7.9%) |
| post-menopause | 35 (30.7%) |
| Self-reported race, white or Caucasian | 105 (92.1%) |
| black or African American | 3 (2.6%) |
| Asian American | 3 (2.6%) |
| other or mixed | 3 (2.6%) |
| Marital status, married | 53 (46.5%) |
| single | 27 (23.7%) |
| divorced | 16 (14.0%) |
| unmarried partner | 9 (7.9%) |
| other | 1 (0.9%) |
| Living alone | 29 (24.6%) |
| Education, years, mean (SD) | 15.7 (3.0) |
| range | 9–25 |
| Employment status, full-time | 12 (10.5%) |
| retired | 20 (17.5%) |
| unemployed | 18 (15.8%) |
| student or homemaker | 13 (11.4%) |
| part-time | 7 (6.1%) |
| disability status | 44 (38.6%) |
| Annual household income a, < $25,000 | 33 (31.1%) |
| $25,000 - $49,999 | 25 (23.6%) |
| $50,000 - $74,999 | 20 (18.9%) |
| ≥ $75,000 | 28 (26.4%) |
Values represent number of subjects (%) except where indicated
8 missing values; median income in Michigan was $49,000
Table 2.
Baseline clinical features of the sample a
| DSM-IV / DSM-5 categories | |
| Primary mood disorder: major depressive disorder, recurrent | 74 (64.9%) |
| major depressive disorder, single-episode | 14 (12.3%) |
| bipolar 1 disorder | 16 (14.0%) |
| bipolar 2 disorder | 10 (8.8%) |
| Psychotic features | 14 (12.3%) |
| Melancholic features | 91 (79.8%) |
| Atypical features | 4 (3.5%) |
| Catatonic features | 6 (5.3%) |
| Severity measures | |
| CGI Severity scale, mean (SD) | 5.71 (0.70) |
| GAF scale, mean (SD) | 30.6 (9.9) |
| MADRS, mean (SD), n=104 | 34.1 (6.0) |
| 17-item HDRS, mean (SD), n=100 | 22.5 (4.7) |
| PHQ-9, mean (SD), n=91 | 20.4 (4.5) |
| QIDS-SR, mean (SD), n=89 | 18.2 (4.1) |
| WSAS, mean (SD), n=87 | 31.9 (5.5) |
| Chronicity and resistance | |
| Index episode duration, months, mean (SD) | 43.7 (80.7) |
| median (IQR), range | 18 (30), 2–500 b |
| duration ≥ 6 months | 91 (79.8%) |
| duration ≥ 1 year | 71 (62.3%) |
| duration ≥ 2 years | 48 (42.1%) |
| duration ≥ 5 years | 22 (19.3%) |
| Onset age, years, mean (SD), n=112 | 21.2 (12.7) |
| median (IQR), range | 16 (14), 4–68 |
| Lifetime number of depressive episodes (n=112), single episode | 16 (14.3%) |
| 2 | 18 (16.1%) |
| 3 | 11 (9.8%) |
| 4–10 | 25 (22.3%) |
| > 10 | 42 (37.5%) |
| MGH staging score, mean (SD), n=112 | 6.17 (3.83) |
| Maudsley staging score, mean (SD) | 8.90 (1.90) |
| Past improvement with ECT, n=113 | 31 (27.4%) |
| Other clinical features | |
| GAD-7 scale, mean (SD), n=87 | 12.0 (6.0) |
| ACE scale, mean (SD), n=86 | 2.52 (2.09) |
| median (IQR), range | 2 (3), 0–7 |
| WHOQOL-BREF, Physical health, mean (SD), n=78 | 39.6 (14.0) |
| Psychological, mean (SD) | 22.8 (12.2) |
| Social relationships, mean (SD) | 43.0 (20.9) |
| Environmental, mean (SD) | 63.7 (16.1) |
| Maternal history of mood disorder (n=101) | 44 (43.6%) |
| Paternal history of mood disorder (n=101) | 26 (25.7%) |
| Left-handed or ambidextrous | 12 (10.5%) |
| Psychiatric comorbidities (MINI Plus 5.0.0) c | |
| Any anxiety disorder, current | 79 (78.2%) |
| Generalized anxiety disorder | 53 (52.5%) |
| Agoraphobia without panic | 35 (34.7%) |
| Panic disorder with agoraphobia | 16 (15.8%) |
| Panic disorder without agoraphobia | 4 (4.0%) |
| Social phobia | 37 (36.6%) |
| Specific phobia | 9 (8.9%) |
| Obsessive-compulsive disorder | 5 (5.0%) |
| Post-traumatic stress disorder | 4 (4.0%) |
| Bulimia nervosa, current | 4 (4.0%) |
| Anorexia nervosa, current | 0 (0%) |
| Substance use disorder, past year | 15 (14.9%) |
| Alcohol | 10 (9.9%) |
| Tobacco | 6 (5.9%) |
| Other drugs | 5 (5.0%) |
| Substance use disorder, lifetime | 51 (50.5%) |
| Alcohol | 39 (38.6%) |
| Tobacco | 24 (23.8%) |
| Other drugs | 22 (21.8%) |
| Medical comorbidities (modified CIRS) d | |
| Cardiac | 11 (9.7%) |
| Hypertension | 34 (30.1%) |
| Vascular | 6 (5.4%) |
| Respiratory (mainly sleep apnea, asthma, and tobacco smoking) | 33 (31.4%) |
| Eye, ear, nose, throat, larynx | 8 (7.8%) |
| Upper gastrointestinal | 18 (16.2%) |
| Lower gastrointestinal | 5 (4.5%) |
| Hepatic | 0 (0%) |
| Renal | 3 (2.7%) |
| Other genitourinary | 13 (11.5%) |
| Musculo-skeletal-integumentary | 22 (20.8%) |
| Neurological | 22 (21.8%) |
| Endocrine-metabolic | 41 (37.6%) |
| CIRS total score e, mean (SD) | 6.4 (4.3) |
| median (IQR), range | 6 (6), 0–18 |
| Body mass index, mean (SD) | 29.6 (8.0) |
| obese (body mass index > 30) | 48 (42.1%) |
Values represent number of subjects (%) out of 114 total, except where indicated Abbreviations are defined in the text
For variables with missing values, the number of subjects with available data (n) is shown
Two participants with episode duration > 40 years were assigned a value of 500 months
complete data available from 101 subjects
data available from 101–113 subjects, depending on the disease domain
excluding Psychiatric-Behavioral scale
One-hundred-five subjects (92%) completed the acute ECT index series (Figure 1A-B). On the primary short-term outcome, 70 of the 105 completers (67%) were classified as ECT responders (CGI-I = 1 or 2). On the secondary outcome, MADRS total score decreased by 19 points (54%) on average from baseline to post-treatment (p < 10 −15, t = 17.1, df = 84, paired t-test).
Figure 1.
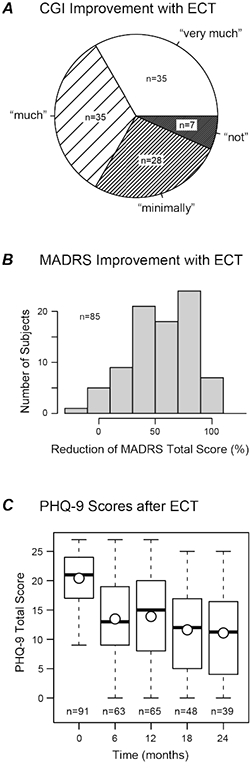
Depression outcomes following ECT. (A) Primary short-term outcome: acute response to ECT measured with the Clinical Global Impression – Improvement (CGI-I) scale. (B) Secondary short-term outcome: acute response shown as percentage decrease in MADRS total score from baseline to post-ECT. (C) Long-term outcome: Patient Health Questionnaire (PHQ-9) score at baseline (time = 0) and at four time points during the Follow-Up phase. Boxplots show quartiles, mean values (circles), and most extreme individual values (whiskers). Number of observations at each time point is shown (n).
Long-term outcomes after ECT
Follow-up data were available from 82 of 114 participants (72%). One died from chronic medical problems at 11 months; another died of suicide at 14 months. The 82 subjects did not differ from those lost to follow-up with respect to baseline sociodemographic variables, clinical features, or acute ECT response (all p > 0.05), suggesting that participants with follow-up data were representative of the broader sample.
PHQ-9 total scores varied widely, spanning the full range of this scale (0–27). On average, scores improved relative to baseline (mean change = −6.4 to −8.7 across time points; all p < 10−6, paired t-tests; Figure 1C). Scores decreased slightly between 6 and 24 months (β = −1.58 points per year, p = 0.014, linear mixed model). The proportion of subjects with a decrease of at least 5 points (the minimal clinically important difference (Lowe et al., 2004)) ranged 51–63% across time points. The proportion of subjects with PHQ-9 scores < 10 (mild or better) varied from 27% to 44%.
Baseline predictors of short-term ECT response
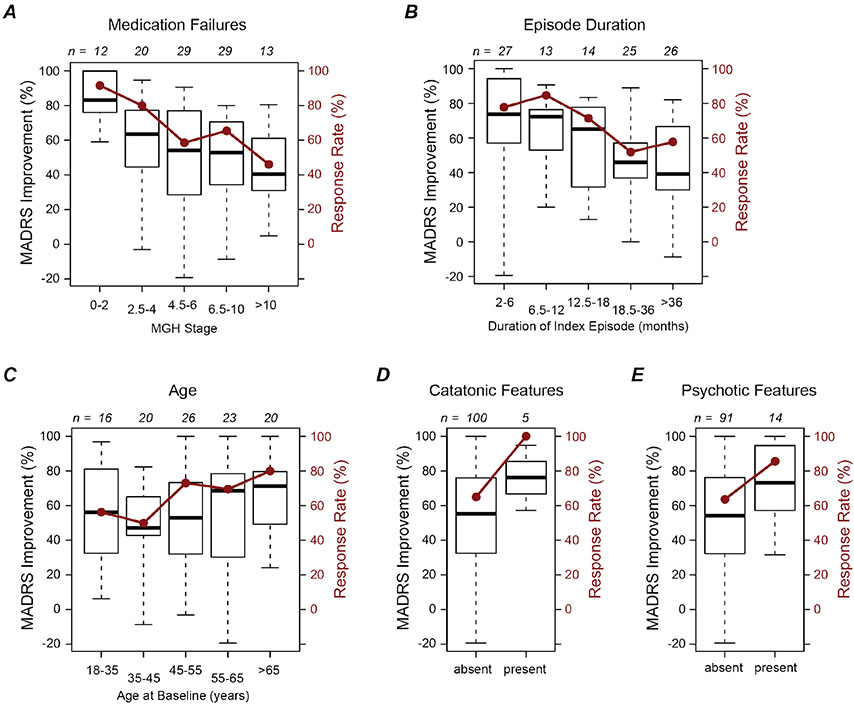
As shown in Figure 2 and Supplemental Table S1, ECT response was more likely among participants with lower MGH stages, i.e., less medication resistance (Hedges’ g = −0.51; p = 0.021, logistic regression). At marginal levels of significance, ECT response was more common among those with shorter episode duration (g = −0.41; p = 0.059) and greater age (g = 0.36; p = 0.085). Subjects with catatonic features were more likely to respond to ECT (5/5 versus 65/100), as were patients with psychotic features (12/14 versus 58/91, odds ratio = 3.4), but neither association reached significance (p = 0.99 and 0.12, respectively, logistic regressions).
Figure 2.
Predictors of acute response to electroconvulsive therapy (ECT). Each panel shows improvement in Montgomery-Asberg Depression Rating Scale (MADRS) from baseline (boxplots, left axis) and rate of categorical response on the Clinical Global Impression - Improvement scale (red filled circles, right axis). Boxplots represent quartiles and most extreme values. The number of subjects in each group (n) is shown above each plot. (A-E) ECT response stratified according to each of five different baseline features.
On the secondary acute outcome measure, MADRS total score, more improvement was found among subjects with lower MGH stages (β = 0.79 points per staging point, p = 0.001, linear regression) and shorter episode duration (β = 1.5 points per doubling of duration, p = 0.003). MADRS scores improved more among subjects with psychotic features at baseline (β = −6.8 points relative to non-psychotic, p = 0.046) and a similar trend was found for catatonia (β = −8.7 points relative to non-catatonic, p = 0.099). Age did not predict MADRS improvement (β = −0.48 points per decade, p = 0.45).
Exploratory analyses of a wide range of other potential predictors showed that no baseline features survived a conservative threshold of p = 0.0009 to account for multiple testing (Supplemental Results and Supplemental Table S1).
Acute ECT response predicts long-term outcome
As expected, acute ECT responders reported lower PHQ-9 depression scores during the subsequent 2-year follow-up period (β = −4.5 points relative to non-responders, p = 0.004, linear mixed model; Figure 3A). Long-term PHQ-9 outcomes did not differ significantly between full responders and partial responders (CGI-I = 1 versus 2; p = 0.42), or between those with minimal response versus no response (CGI-I = 3 versus 4; p = 0.80). The proportion of responders with subsequent PHQ-9 < 10 (mild or better) ranged 38%–58% across assessments; among non-responders, the proportion was 6–21%. PHQ-9 scores were not significantly associated with CGI Side Effect score or MoCA score post-ECT (p > 0.05, linear mixed models), suggesting that long-term outcomes were influenced by therapeutic effects but not by adverse effects of ECT.
Figure 3.
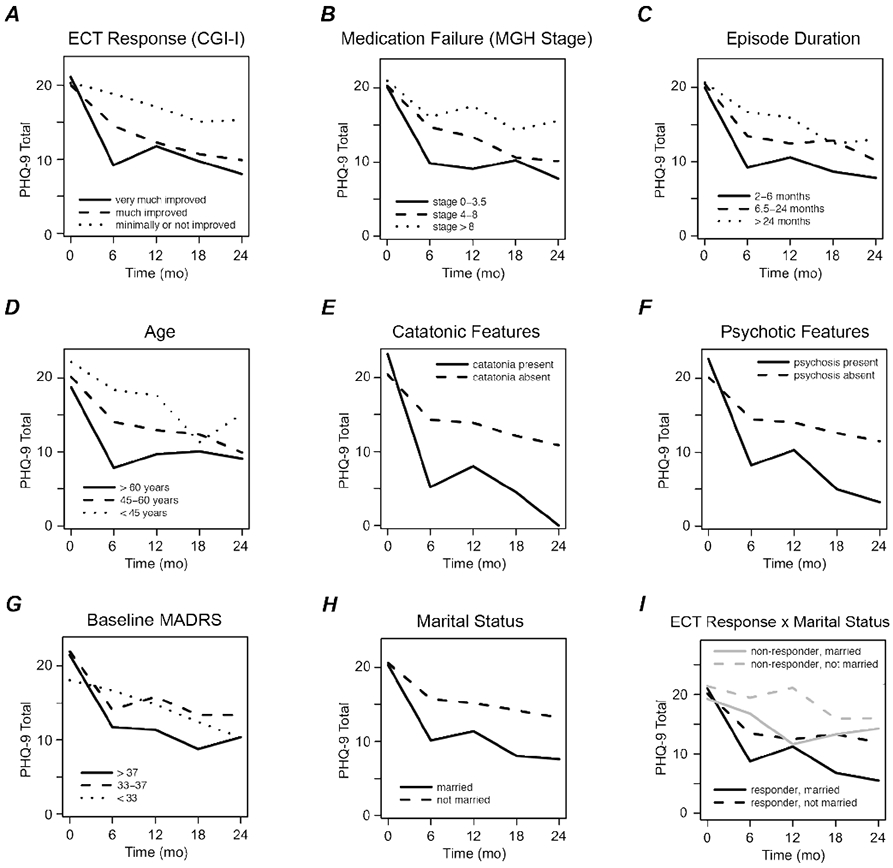
Predictors of long-term depression severity after electroconvulsive therapy (ECT). Each panel illustrates mean Patient Health Questionnaire (PHQ-9) scores at baseline before ECT (time = 0) and at four time points during the Follow-Up phase. (A) PHQ-9 outcomes stratified by acute response to ECT. Subjects rated much or very much improved are classified as ECT responders. (B-H) PHQ-9 outcomes stratified by each of seven different baseline characteristics. (I) PHQ-9 outcomes stratified by both acute response and marital status.
Baseline predictors of long-term outcome
We next tested baseline variables (Supplemental Table S1) as predictors of longitudinal PHQ-9 scores while disregarding acute ECT response (Figure 3B-F). Better long-term PHQ-9 scores were found for participants with shorter episode duration (β = 0.85 points per doubling of duration, p = 0.044, linear mixed model), psychotic features (β = −5.4 points relative to non-psychotic, p = 0.021), and catatonic features at baseline (β = −9.8 points relative to non-catatonic, p = 0.021). PHQ-9 scores also tended to improve more in older subjects (β = −1.0 points per decade, p = 0.067) but were not significantly associated with MGH stage (β = 0.27 points per staging point, p = 0.21). Thus, some but not all baseline variables that predicted acute ECT improvement also predicted long-term outcomes. Multivariate analyses suggested that psychotic features and catatonic features were independent predictors (see Supplemental Results).
Exploratory analyses identified additional baseline features that, while not associated with acute ECT response, did predict long-term PHQ-9 scores. Better scores were found among participants who were married (β = −5.7 points relative to non-married, p = 0.00007, linear mixed model; Figure 3H), those who reported stronger social support (WHOQOL-BREF social domain, p = 0.02), and those who lived with other people (p = 0.04). Greater longitudinal improvements in PHQ-9 scores were also predicted by more severe clinician-rated illness at baseline – specifically, MADRS (β = −0.46 points per MADRS point, p = 0.0004; Figure 3G), CGI-S (p = 0.001), and GAF (p = 0.009). After applying a conservative threshold of p = 0.0009 to account for multiple testing, marital status and baseline MADRS score survived as significant predictors. Long-term outcomes did not differ for bipolar versus unipolar subjects (p = 0.70) or for other baseline characteristics in Supplemental Table S1.
Analyses that controlled for initial ECT response indicated that the influences of episode duration and psychotic features on long-term PHQ-9 scores were at least partially mediated through acute response to ECT. In contrast, married status and baseline MADRS continued to predict long-term PHQ-9 scores when acute ECT response was included in the model, indicating that the long-term influence of these predictors was independent of the initial therapeutic effects of ECT (Figure 3I). Similarly, married status and baseline MADRS predicted long-term PHQ-9 scores independent of age, episode duration, psychotic features, or catatonic features. See the Supplemental Results for details.
Long-term outcomes and intensity of naturalistic treatment
We evaluated whether naturalistic treatment reported during the follow-up period was associated with long-term PHQ-9 scores. Data on treatments received during the 2-year follow-up phase were available from 66 of the 82 subjects (80%). Participants reported 3.0 ± 1.5 (mean ± SD) medication interventions and 13 ± 18 psychotherapy visits within each 6-month window. Twenty-six percent reported additional ECT between the 6-month and 24-month time points. Longitudinal PHQ-9 scores were not associated with the number of medication interventions, number of psychotherapy visits, or use of ECT (p > 0.10, linear mixed models). These results suggested that long-term outcomes were not confounded by intensity of treatment during follow-up.
Discussion
The present study aimed to comprehensively evaluate dozens of baseline sociodemographic and clinical features as potential predictors of short- and long-term outcomes following ECT. Our findings largely support prior reports that less medication resistance, shorter episode duration, psychotic features, and catatonic features predict better acute post-ECT outcomes. We also confirmed the hypothesis that subjects who responded acutely to ECT would experience better long-term depression outcomes. Short- and long-term outcomes were predicted by distinct baseline features. Married status and greater clinician-rated severity at baseline were not associated with acute ECT response but did predict lower depression scores longitudinally. Overall, these findings suggest that individual characteristics associated with long-term mood stability differ from those that predict acute relief following ECT.
The associations of shorter index episode and less medication resistance with better acute response to ECT are consistent with previous meta-analyses of these variables (Haq et al., 2015; Heijnen et al., 2010). For example, our estimated effect size of −0.41 for episode duration falls well within the 95%-confidence interval estimated meta-analytically (Haq et al., 2015). Because episode duration and medication failures were strongly correlated in our sample, disentangling their distinct influences on acute ECT response is challenging; studies with larger samples will likely be needed. In the meantime, we can conclude that greater chronicity or resistance portends less favorable outcomes with ECT.
Individuals in our sample with psychotic or catatonic features were more likely to respond to ECT, but the small proportion of subjects with these features (12% and 5%, respectively) resulted in wide confidence intervals with limited power to detect effects. The underrepresentation of these features is likely due to the difficulties enrolling extremely ill patients (see Supplemental Results). Nonetheless, our finding that 86% of psychotic subjects responded is consistent with recent studies showing that psychotic features are a strong predictor of acute ECT response, especially among older patients and those with less medication resistance (van Diermen et al., 2020; van Diermen et al., 2018). Similarly, our finding that all ECT completers with catatonic features were classified as responders adds to previous evidence that mood disorders with catatonic features respond particularly well to ECT (Fink and Taylor, 2003; Kellner et al., 2012; Leroy et al., 2018; Luchini et al., 2015) and concurs with recent reports that patients with greater psychomotor disturbance respond better to ECT acutely (Heijnen et al., 2019; van Diermen et al., 2020; van Diermen et al., 2019).
We found that patients experience widely divergent long-term depression scores after ECT (Figure 1C). For roughly a third of participants (27–44% across assessments), depression was well-managed (PHQ-9 scores < 10), and a majority of subjects experienced a decrease of at least 5 points from baseline (estimated as the minimal clinically important difference (Lowe et al., 2004)). On the other hand, more than half continued to live with moderate or severe depression (PHQ-9 ≥ 10), highlighting the long-term challenges faced by patients with treatment-resistant mood disorders.
What explains this wide variability in long-term outcomes? We evaluated ongoing psychotherapy, pharmacotherapy, and ECT as potential explanatory variables and found no significant associations between intensity of treatment and depression scores. Initial therapeutic response to ECT did account for some of the variation in subsequent outcomes (Figure 3A) while global ECT side effects did not. These observations are consistent with the clinical experience that the antidepressant effects of ECT appear to be durable for many patients, while the side effects tend to be transient. Long-term PHQ-9 total scores below 10 were common for acute ECT responders (38–58%) and less common for non-responders (6–21%), demonstrating that the initial response to ECT is important but not deterministic for long-term prognosis.
Marital status and social support emerged as individual characteristics that influenced long-term but not short-term outcomes. This finding was unanticipated but it is consistent with evidence that greater social support is associated with lower levels of depression in the general population (Gariepy et al., 2016), among people with physical disabilities (Tough et al., 2017), among older adults (Laird et al., 2019; Schwarzbach et al., 2014), and among those with a depression diagnosis (Wang et al., 2018). Nonetheless, the magnitude of the effects of marital status and social support that we observed were striking. For example, similar magnitudes of long-term benefit were found for ECT responders versus non-responders and for married versus unmarried people (Figure 3A and 3H) and the influences of these two predictors were additive (Figure 3I). The mechanisms through which married status and social relationships might reduce depression are unclear. Social supports might act indirectly by facilitating access to care or improving adherence, but this explanation appears unlikely for our cohort since intensity of treatment was not associated with PHQ-9 scores. Alternatively, social relationships and social support may directly benefit emotional well-being and, furthermore, they may act indirectly as a “stress buffer” whereby the onset of a major stressor activates the individual’s social support network, which enhances the individual’s ability to cope or adjust (Thoits, 2011). By identifying the specific mechanisms through which social supports enhance outcomes of TRD, it may be possible to develop adjunctive interventions that target those mechanisms for patients who lack a robust social network. Beyond social context, clinician-rated severity at baseline (MADRS) did not predict acute ECT response but did predict long-term depression outcomes. Further studies are warranted to replicate and explicate these findings.
Limitations of this study include the sample size, which likely limited our ability to detect weak-to-moderate effects of baseline predictors on outcomes. Missing follow-up data could have altered findings through attrition bias, although our analyses suggested that at baseline those who were included resembled those lost to follow-up. Although this study used validated depression outcome measures, it did not evaluate other short- or long-term outcomes such as anxiety. The persistence of anxiety disorders and symptoms should be addressed in future studies. Personality disorders were not rigorously assessed in this study. While extreme personality disorders were likely excluded from our sample, it remains unclear whether outcomes were impacted by less severe personality disorders or traits. Our sample from a single US academic medical center, largely of European ancestry, may not be representative of TRD patients in other settings or parts of the world. Future longitudinal studies are needed to confirm these findings and evaluate how generalizable they are. The observational design and lack of a control group in this study do not permit causal inferences. Strengths of this study include the relatively long follow-up period and the extensive phenotyping of sociodemographic and clinical features.
In conclusion, this study provides new evidence that features that predict long-term depression outcomes differ from features that predict short-term outcomes. Future studies should develop robust predictive models and targeted interventions in order to durably enhance the well-being of people afflicted by TRD.
Supplementary Material
Acknowledgments
Funded by National Institute of Mental Health (K23MH092648), Michigan Institute for Clinical & Health Research (National Center for Advancing Translational Sciences, 2UL1TR000433), Taubman Medical Research Institute, and University of Michigan Depression Center
The funders of this work played no role in the design, conduct, or reporting of the study.
We thank Adam Sitzmann and Clara Grayhack for assistance with data collection, and the patients and staff of the University of Michigan ECT Program for their enthusiastic support of this study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BJM has received research support from LivaNova and Novartis for work unrelated to this manuscript. All authors report no financial or other conflict of interest relevant to this work.
Previous presentation: A portion of this work was presented in abstract form at the 3rd International Brain Stimulation Conference, Vancouver, Canada: Mickey et al. (2019) “Social and clinical variables that influence longitudinal depression outcomes after brain stimulation,” Brain Stimulation 12(2):497.
References
- Endicott J, Spitzer RL, Fleiss JL, Cohen J, 1976. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 33(6), 766–771. [DOI] [PubMed] [Google Scholar]
- Fava M, 2003. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 53(8), 649–659. [DOI] [PubMed] [Google Scholar]
- Fekadu A, Wooderson SC, Markopoulo K, Donaldson C, Papadopoulos A, Cleare AJ, 2009a. What happens to patients with treatment-resistant depression? A systematic review of medium to long term outcome studies. J Affect Disord 116(1-2), 4–11. [DOI] [PubMed] [Google Scholar]
- Fekadu A, Wooderson SC, Markopoulou K, Cleare AJ, 2009b. The Maudsley Staging Method for treatment-resistant depression: prediction of longer-term outcome and persistence of symptoms. J Clin Psychiatry 70(7), 952–957. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS, 1998. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- Fink M, Taylor MA, 2003. Catatonia: A Clinician's Guide to Diagnosis and Treatment. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Gariepy G, Honkaniemi H, Quesnel-Vallee A, 2016. Social support and protection from depression: systematic review of current findings in Western countries. Br J Psychiatry 209(4), 284–293. [DOI] [PubMed] [Google Scholar]
- Guy W, 1976. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare, Rockville, MD. [Google Scholar]
- Haq AU, Sitzmann AF, Goldman ML, Maixner DF, Mickey BJ, 2015. Response of depression to electroconvulsive therapy: a meta-analysis of clinical predictors. J Clin Psychiatry 76(10), 1374–1384. [DOI] [PubMed] [Google Scholar]
- Heijnen W, Kamperman AM, Tjokrodipo LD, Hoogendijk WJG, van den Broek WW, Birkenhager TK, 2019. Influence of age on ECT efficacy in depression and the mediating role of psychomotor retardation and psychotic features. J Psychiatr Res 109, 41–47. [DOI] [PubMed] [Google Scholar]
- Heijnen WT, Birkenhager TK, Wierdsma AI, van den Broek WW, 2010. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol 30(5), 616–619. [DOI] [PubMed] [Google Scholar]
- Jelovac A, Kolshus E, McLoughlin DM, 2013. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology 38(12), 2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston KM, Powell LC, Anderson IM, Szabo S, Cline S, 2019. The burden of treatment-resistant depression: A systematic review of the economic and quality of life literature. J Affect Disord 242, 195–210. [DOI] [PubMed] [Google Scholar]
- Kellner CH, Greenberg RM, Murrough JW, Bryson EO, Briggs MC, Pasculli RM, 2012. ECT in Treatment-Resistant Depression. Am J Psychiatry 169(12), 1238–1244. [DOI] [PubMed] [Google Scholar]
- Kellner CH, Knapp RG, Petrides G, Rummans TA, Husain MM, Rasmussen K, Mueller M, Bernstein HJ, O'Connor K, Smith G, Biggs M, Bailine SH, Malur C, Yim E, McClintock S, Sampson S, Fink M, 2006. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE). Arch Gen Psychiatry 63(12), 1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, 2001. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird KT, Krause B, Funes C, Lavretsky H, 2019. Psychobiological factors of resilience and depression in late life. Transl Psychiatry 9(1), 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy A, Naudet F, Vaiva G, Francis A, Thomas P, Amad A, 2018. Is electroconvulsive therapy an evidence-based treatment for catatonia? A systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 268(7), 675–687. [DOI] [PubMed] [Google Scholar]
- Lex H, Ginsburg Y, Sitzmann AF, Grayhack C, Maixner DF, Mickey BJ, 2019. Quality of life across domains among individuals with treatment-resistant depression. J Affect Disord 243, 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex H, Nevers SW, Jensen EL, Ginsburg Y, Maixner DF, Mickey BJ, 2021. Long-term quality of life in treatment-resistant depression after electroconvulsive therapy. J Affect Disord 291, 135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanby SH, 2007. Electroconvulsive therapy for depression. N Engl J Med 357(19), 1939–1945. [DOI] [PubMed] [Google Scholar]
- Lowe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K, 2004. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care 42(12), 1194–1201. [DOI] [PubMed] [Google Scholar]
- Luchini F, Medda P, Mariani MG, Mauri M, Toni C, Perugi G, 2015. Electroconvulsive therapy in catatonic patients: Efficacy and predictors of response. World J Psychiatry 5(2), 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickey BJ, Ginsburg Y, Sitzmann AF, Grayhack C, Sen S, Kirschbaum C, Maixner DF, Abelson JL, 2018. Cortisol trajectory, melancholia, and response to electroconvulsive therapy. J Psychiatr Res 103, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M, 1979. A new depression scale designed to be sensitive to change. Br J Psychiatry 134, 382–389. [DOI] [PubMed] [Google Scholar]
- Mrazek DA, Hornberger JC, Altar CA, Degtiar I, 2014. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatr Serv 65(8), 977–987. [DOI] [PubMed] [Google Scholar]
- Mundt JC, Marks IM, Shear MK, Greist JH, 2002. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry 180, 461–464. [DOI] [PubMed] [Google Scholar]
- Ross EL, Zivin K, Maixner DF, 2018. Cost-effectiveness of Electroconvulsive Therapy vs Pharmacotherapy/Psychotherapy for Treatment-Resistant Depression in the United States. JAMA Psychiatry 75(7), 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH, 1996. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med 26(3), 477–486. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Aaronson ST, Bunker MT, Conway CR, Demitrack MA, George MS, Prudic J, Thase ME, Rush AJ, 2019. The assessment of resistance to antidepressant treatment: Rationale for the Antidepressant Treatment History Form: Short Form (ATHF-SF). J Psychiatr Res 113, 125–136. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Haskett RF, Mulsant BH, Thase ME, Mann JJ, Pettinati HM, Greenberg RM, Crowe RR, Cooper TB, Prudic J, 2001. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA 285(10), 1299–1307. [DOI] [PubMed] [Google Scholar]
- Salvi F, Miller MD, Grilli A, Giorgi R, Towers AL, Morichi V, Spazzafumo L, Mancinelli L, Espinosa E, Rappelli A, Dessi-Fulgheri P, 2008. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J Am Geriatr Soc 56(10), 1926–1931. [DOI] [PubMed] [Google Scholar]
- Schwarzbach M, Luppa M, Forstmeier S, Konig HH, Riedel-Heller SG, 2014. Social relations and depression in late life-a systematic review. Int J Geriatr Psychiatry 29(1), 1–21. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Lowe B, 2006. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 166(10), 1092–1097. [DOI] [PubMed] [Google Scholar]
- Thoits PA, 2011. Mechanisms linking social ties and support to physical and mental health. J Health Soc Behav 52(2), 145–161. [DOI] [PubMed] [Google Scholar]
- Tough H, Siegrist J, Fekete C, 2017. Social relationships, mental health and wellbeing in physical disability: a systematic review. BMC Public Health 17(1), 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK ECT Review Group, 2003. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet 361(9360), 799–808. [DOI] [PubMed] [Google Scholar]
- van Diermen L, Poljac E, Van der Mast R, Plasmans K, Van den Ameele S, Heijnen W, Birkenhager T, Schrijvers D, Kamperman A, 2020. Toward Targeted ECT: The Interdependence of Predictors of Treatment Response in Depression Further Explained. J Clin Psychiatry 82(1). [DOI] [PubMed] [Google Scholar]
- van Diermen L, van den Ameele S, Kamperman AM, Sabbe BCG, Vermeulen T, Schrijvers D, Birkenhager TK, 2018. Prediction of electroconvulsive therapy response and remission in major depression: meta-analysis. Br J Psychiatry 212(2), 71–80. [DOI] [PubMed] [Google Scholar]
- van Diermen L, Vanmarcke S, Walther S, Moens H, Veltman E, Fransen E, Sabbe B, van der Mast R, Birkenhager T, Schrijvers D, 2019. Can psychomotor disturbance predict ect outcome in depression? J Psychiatr Res 117, 122–128. [DOI] [PubMed] [Google Scholar]
- Wang J, Mann F, Lloyd-Evans B, Ma R, Johnson S, 2018. Associations between loneliness and perceived social support and outcomes of mental health problems: a systematic review. BMC Psychiatry 18(1), 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHOQOL Group, 1998. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med 28(3), 551–558. [DOI] [PubMed] [Google Scholar]
- Williams JBW, Link MJ, Rosenthal NE, Terman M, 1988. Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders Version (SIGH-SAD). New York State Psychiatric Institute, New York. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.