Introduction
Autism is a neurodevelopmental condition characterized by challenges in the area of social interactions and the presence of repetitive behaviors and restricted interests (American Psychiatric Association, 2013). Attentional processes are foundational for the development of social-communication abilities and social engagement, as a young child must notice and maintain attention to other persons in order to engage in social learning. Previous eye-tracking (ET) research in autism has primarily focused on where individuals are looking based on predefined areas-of-interest (AOIs). Very few autism studies have looked at how long the autistic individual is able to maintain sustained and focused attention to a stimulus before looking away (Hessels et al., 2016; Salley & Colombo, 2016).
Various subcategories of attention contribute to social development, including joint attention, social attention, and sustained attention (Charman, 2003; Landa et al., 2011; Parish-Morris et al., 2013; Presmanes et al., 2007; Schietecatte et al., 2012; Shi et al., 2015; Trembath et al., 2015). The majority of ET studies demonstrate differences in social attention among autistic individuals when compared to children without autism (Chawarska et al., 2012; Chawarska et al., 2013; Elsabbagh et al., 2011). Numerous ET studies have demonstrated that autistic children show distinct gaze patterns compared to children without autism when presented with socially salient stimuli (Anderson et al., 2006; Chawarska et al., 2016; Chevallier et al., 2015; Kuhn & Leekam, 2010; Werner et al., 2000). In a previous study with autistic children, we reported that children’s attention to an actress engaging in child-directed speech was associated with social-communication abilities (Murias et al., 2018). In the current study, we extend our previous work by examining whether the children’s average look duration (ALD) to a complex dynamic stimulus involving both social and nonsocial elements is correlated with a range of measures of autism symptoms and social communication skills.
There are a number of ways that look duration has been measured in young children. First, total duration of looking time reflects the total sum of all fixation lengths. Second, proportion of looking time is a measure of how long an individual spends looking in a region relative to their total looking time. For this metric, an individual with low total looking time can demonstrate high proportion looking times within certain regions if they spend most of their total time, regardless of how brief, within those regions. Next, peak look duration is the length of the single longest, non-disrupted fixation to a given AOI or to the scene as a whole. One longitudinal ET study of infants with autistic siblings (at high risk for autism) and infants without autistic siblings (at low risk for autism) studied peak look duration to faces (Hendry et al., 2018). The authors concluded that the change in peak look duration to faces between 9–15 months was negatively associated with effortful control at 36 months. However, a notable limitation of the measure peak look duration is that it only captures one distinct fixation out of many and ignores the rest, which could be problematic if valuable information is contained within the time-course of the looking behavior. Additionally, there is evidence that fixation behavior can change over the length of an experiment. For example, attentional differences between autistic and TD individuals were found to be most pronounced at later fixations when semantic-level attributes (e.g. faces) became more important (Wang et al., 2015).
ALD combines information about both the length of all looking events and the total number of looks in order to determine the average number of seconds that an individual can sustain attention to the entire visual stimulus. Looks to the screen are instances in which the individual was engaged in either a fixation or saccade event within the video frame. Gui et al. (2020) explored visual attention in autistic children and children with attention-deficit/hyperactivity disorder (ADHD) using mean peak look duration, and Del Bianco et al. (2018) used average fixation duration to explore the face-orienting response and the subsequent attentive selection in the presence of varying task instructions in autistic individuals. However, Gui and colleagues investigated fixation duration using a face AOI and Del Bianco and colleagues examined fixations to the face and body AOIs. The ALD approach, which does not utilize pre-defined areas-of-interest, need not replace AOI-based approaches but is instead a complementary method that can provide additional information regarding looking behavior. In a previous study utilizing the ALD method, Isaev et al. (2020) investigated the relative ALD difference between social and toys videos to measure attentional preferences of autistic children compared to neurotypical children. The results demonstrated that autistic and neurotypical children differed in ALD, especially when viewing the dynamic social stimuli. The present study expands on the work by Isaev et al. (2020) by utilizing eye-tracking technology to measure visual attention (i.e. gaze) automatically, whereas Isaev and colleagues used human coding of visual attention to the stimulus.
In the current study, we measured ALD in young children during an eye-tracking task while viewing complex, dynamic stimuli containing social and nonsocial elements. We calculated ALD by dividing total viewing time (i.e. the sum duration of all looks lasting at least 200 ms within a region of 1200×960 pixels) by the number of distinct periods of attention.. We also examined whether the presence of symptoms of autism and ADHD, as measured by caregiver report, were related to ALD in young autistic children. We hypothesized that children with lower levels of autism symptoms would exhibit longer ALDs toward the dynamic stimuli. We also expected levels of hyperactivity to be related to the length of looks in autistic children.
Methods
Participants
Children diagnosed with autism spectrum disorder (ASD) were part of a single site, prospective, randomized, double-blind, parallel group study of a single intravenous autologous or allogeneic, unrelated cordblood (CB) infusion in children 2–8 years (Dawson et al., 2020). 176 autistic children (140 males; mean age 65.65 months (SD ± 19.73 months)) participated in the clinical trial (ClinicalTrials.gov: NCT02176317). Legal guardians provided informed consent prior to study procedures. The study was approved by the institutional review board. Only data from the baseline visit, which were collected before infusions, were used in this analysis. Clinical diagnosis of ASD was based on Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5; American Psychiatric Association, 2013), and established by expert clinicians using Autism Diagnostic Observation Scale, Second Edition (ADOS-2; Lord et al., 1999) and Autism Diagnostic Interview, Revised (ADI-R; Rutter et al., 2005).
Additional inclusion criteria included (1) stability on current medications for at least 2 months prior to the infusion, (2) participants and parents/guardians were English speaking, (3) available and qualified umbilical cord blood unit with a minimum banked total nucleated cell dose of availability of autologous umbilical cord blood unit of ≥ 2.5 × 107 cells/kg, either autologous umbilical cord blood unit or ≥4/6 HLA-matched allogeneic unrelated umbilical cord blood unit from the Carolinas Cord Blood Bank, (4) Fragile X testing performed and negative, (5) normal absolute lymphocyte count (≥1500/uL), (6) able to travel to Duke University two times (baseline and 6 months post-baseline), and parent/guardian is able to participate in interim surveys and interviews monthly, (7) and parental consent.
Exclusion Criteria.
Exclusion criteria included (1) known diagnosis of any of the following coexisting psychiatric conditions: depression, bipolar disorder, schizophrenia, obsessive compulsive disorder, Tourette syndrome, (2) has a sibling enrolled in the study, (3) records indicate that child has a known genetic syndrome such as (but not limited to) Fragile X syndrome, neurofibromatosis, Rett syndrome, tuberous sclerosis, PTEN mutation, cystic fibrosis, muscular dystrophy, (4) known pathogenic mutation or copy number variation (CNV) associated with autism (e.g., 16p11.2, 15q13.2, 2q13.3), (5) known active CNS infection, evidence of uncontrolled infection based on records or clinical assessment, or HIV positivity, (6) known metabolic disorder, known mitochondrial dysfunction, history of unstable epilepsy or uncontrolled seizure disorder, infantile spasms, Lennox Gastaut syndrome, Dravet syndrome, or other similar chronic seizure disorder, active malignancy or prior malignancy that was treated with chemotherapy, history of a primary immunodeficiency disorder, history of autoimmune cytopenias (i.e., ITP, AIHA), coexisting medical condition that would place the child at increased risk for complications of sedation or other study procedures, concurrent genetic or acquired disease or comorbidity(ies) that could require a future stem cell transplant, significant sensory (e.g., blindness, deafness, uncorrected hearing impairment) or motor (e.g., cerebral palsy) impairment, impaired renal or liver function as determined by serum creatinine >1.5mg/dL or total bilirubin >1.3mg/dL, except in patients with known Gilbert’s disease, significant hematologic abnormalities defined as: Hemoglobin <10.0 g/dL, WBC < 3,000 cells/mL, ALC <1000/uL, Platelets <150 × 10e9/uL, evidence of clinically relevant physical dysmorphology indicative of a genetic syndrome, (7) history of prior cell therapy, current or prior use of IVIG or other anti-inflammatory medications with the exception of NSAIDs, or current or prior immunosuppressive therapy.
Clinical measures
Full Scale IQ.
Cognitive/developmental ability was measured using Mullen Scales of Early Learning (Mullen, 1995) or Differential Ability Scales, Second Edition (DAS-II; Elliot, 2007) depending upon the participant’s age; children up to the age of four completed the Mullen Scales of Early Learning. For Mullen, the Early Learning Composite, and for DAS-II, the General Conceptual Ability scores were used to measure Full Scale IQ.
Clinical Global Impression– Severity Scale (CGI-S).
CGI-S is a 7-point scale that requires a clinician to rate the level of a participant’s symptoms based on a parent interview in addition to an observation of the child (Busner & Targum, 2007). There were three separate CGI-S ratings; these included social communicative functioning, restricted/repetitive interests and behaviors, and an overall score. The overall severity score was used in the present analysis.
Pervasive Developmental Disorder Behavior Inventory (PDDBI).
PDDBI was developed to assess responsiveness to intervention in autistic children (Cohen et al., 2003). PDDBI is an informant-based rating scale that is designed for children 1 year, 6 months to 12 years, 5 months. It assesses challenging behaviors as well as appropriate social, language, and learning/memory skills. PDDBI is a parent questionnaire with 188 items that takes approximately 30–45 minutes to complete.
Vineland Adaptive Behavior Scales, Third Edition (VABS) Interview Form.
VABS was administered to the parent using a semi-structured interview format. This assessment takes about 60–120 minutes to administer. VABS is a well-standardized measure of several domains of adaptive functioning including socialization, communication, daily living, and motor skills (Sparrow et al., 1984). The Socialization domain assesses play, interpersonal relationships and coping skills. The Communication domain assesses receptive, expressive and written language skills. The Daily Living Skills domain assesses personal, domestic and community living skills. The Motor Skills subdomain assesses gross and fine motor skills.
Aberrant Behavior Checklist-Community (ABC-C).
This parent-completed rating scale was used to measure challenging behaviors associated with autism, including social withdrawal (Aman & Singh, 1985). ABC-C is a validated scale that can assess drug and other treatment effects in studies of developmental delay and autism (Davis & Kollins, 2012). This parent-completed rating scale takes approximately 10 to 15 minutes to complete.
Eye-Tracking Methodology
Procedures.
Participants sat approximately 65 centimeters from the screen (range 60–70) while watching a 3-minute video of a female actress engaged in child-directed speech while surrounded by four distracter toys in each corner of the screen (Chawarska et al., 2013). A research assistant stood next to participants and monitored the track status throughout the experiment; when participants moved out of the trackable range, they were re-positioned and/or encouraged to stay on task. ET was recorded using Tobii TX300 technology, which employs infrared (IR) light-emitting diodes and IR cameras to measure corneal reflections and calculate gaze location. Data were collected with a sampling rate of 120 Hz using Tobii Studio Version 3.2.2 with standardized room lighting and video volume. Before the experiment, a 5-point calibration was performed. Up to three calibration attempts were made in order to capture the 5 calibration points for each eye (10 total) with small error vectors. If no calibration points were captured, the video stimulus was still played for the child, but no eye-tracking data was collected. Offline, we deemed a calibration as valid if 2/5 points were captured for each eye (4/10 total).
Missing Data and Data Interpolation.
Eighty-eight percent, N=155 (mean FSIQ 70.60 (SD ± 21.16)), of the children provided analyzable eye-tracking data. 21 participants were excluded due to data that was deemed invalid due to behavioral non-compliance and calibration or technical errors during the eye-tracking session. For all valid eye-tracking sessions, the Tobi I-VT filter was applied to interpolate missing samples based on the velocity of directional shifts of the eye, in order to not interpret one fixation as two separate fixations. If a gap was more than 75 ms, then the fixations were considered two distinct fixation events.
Stimuli and Video Conditions.
Children were shown a video involving an Actress Dyadic Bid condition and an Actress with Toys condition, each of which lasted for approximately 41 consecutive seconds. The Actress Dyadic Bid condition consisted of four joint attention bids towards the toys with child-directed speech between bids, during which the actress made direct eye-contact with the viewer. The four joint attention bids, the instances when the actress looked at one of the four toys in the corners of the frame, was accompanied by an ‘Uh-oh’ comment from the actress. The four toys remained inactive, motionless, and silent during this condition. The Actress with Toys condition included child-directed speech, joint attention bids, as well as activated toys with movement and sound.
Eye-tracking Variables.
We calculated ALD separately for the Actress Dyadic Bid condition (Actress Dyadic Bid ALD), the Actress with Toys condition (Actress with Toys ALD), and the entire video (Total Video ALD). To measure ALD, we divided the length of total looking time in seconds by the total number of uninterrupted looks to media. The media region included the entire video frame. Samples in which a fixation or saccade occurred on the video media were deemed as part of a looking event. If gaze was not on the media for 200 ms in a row, then those samples were deemed as part of a period of visual inattention in which the participant was not looking at the video. This 200 ms threshold ensures that the look duration calculations reflect true looking events and not momentary eye-tracking artifacts caused by blinks or sudden movements.
We also measured the proportion of time spent looking at the media and at the actress’ body (Percent Time on Actress AOI) while the actress was engaged in child-directed speech, in order to compare our results to previous AOI studies. The actress region was defined as the combination of eyes, mouth, torso, and hand regions.
Analytic Strategy
We utilized multiple linear regression to predict core autism and related symptoms using eye-tracking variables. Slope coefficients, standard errors, and t-values were obtained from regression models that included covariates age and IQ. For each model, independent variables included one eye-tracking variable (the main predictor; ALD Actress with Dyadic Bid, ALD Actress with Toys, ALD Total Video, or Percent Time on Face AOI), Full Scale IQ, and age. The dependent variable was a selected caregiver-reported measure of behavior (8 clinical measures, 32 regressions total). To account for multiple testing, we calculated adjusted p-values for the main predictors using the Benjamini-Hochberg method (Benjamini& Hochberg, 1995) based on the total number of models ran. Using an alpha of 0.05, an adjusted p-value of 0.010938 was identified as the cutoff to reduce Type 1 error. Six models remained significant after correction. Assumptions of linear regression were met, including linear relationships between variables and independence of observations. Statistical analyses were performed using the lm and p.adjust functions in R Version 4.0.2.
Community Involvement.
One member of the research team is on the autism spectrum and this team member helped with the design, data collection, interpretation of data, and writing of the paper. The study received assistance by an intern who is on the autism spectrum, who is recognized in the acknowledgement section. Additionally, the Duke Center for Autism and Brain Development, where this research was conducted, has a Community Engagement Board that provides input and feedback on studies conducted at the Center. This board includes two persons on the autism spectrum.
Results
Full regression results (slope coefficients, standard errors, and t-values) are presented in Table 1.
Table 1.
Regression results for eye-tracking variables, Full Scale IQ, and age in months
| Regression Results (B, SE B, t) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Measures | Average Look Duration (Actress with Dyadic Bid) | Average Look Duration (Actress with Toys) | Average Look Duration (Total Video Presentation) | Percent Time on Actress (Dyadic Bid) | ||||||||
| ET Variable | Age (months) | Full Scale IQ | ET Variable | Age (months) | Full Scale IQ | ET Variable | Age (months) | Full Scale IQ | ET Variable | Age (months) | Full Scale IQ | |
| VABS Socialization | 1.03, 0.69, 1.50 | −0.09, 0.05, −1.78 | 0.39, 0.05, 8.28*** | 1.15, 0.74, 1.55 | −0.07, 0.05, −1.56 | 0.39, 0.05, 8.62*** | 1.46, 0.61, 2.41* | −0.08, 0.05, −1.68 | 0.37, 0.05, 7.78*** | 2.97, 7.07, 0.42 | −0.08, 0.05, −1.62 | 0.40, 0.05, 8.71*** |
| VABS Communication | 0.71, 0.69, 1.04 | −0.06, 0.05, −1.23 | 0.67, 0.05, 14.31*** | 0.95, 0.74, 1.29 | −0.05, 0.05, −1.07 | 0.67, 0.05, 14.74*** | 0.68, 0.61, 1.11 | −0.05, 0.05, −1.12 | 0.67, 0.05, 13.93*** | −1.10, 7.04, −0.16 | −0.05, 0.05, −1.0 | 0.69, 0.05, 14.85*** |
| PDDBI Receptive Expressive Social Communication | 1.02, 0.37, 2.79** | −0.04, 0.03, −1.45 | 0.26, 0.02, 10.30*** | 0.82, 0.40, 2.06* | −0.03, 0.03, −1.01 | 0.27, 0.02, 10.85*** | 0.87, 0.33, 2.66** | −0.03, 0.03, −1.14 | 0.25, 0.03, 9.93*** | 4.17, 3.82, 1.09 | −0.03, 0.03, −1.27 | 0.27, 0.025, 10.81*** |
| PDDBI Social Approach Behaviors | 0.96, 0.48, 1.99* | −0.02, 0.03, −0.59 | 0.24, 0.03, −7.22*** | 0.92, 0.52, 1.76 | −0.009, 0.03, −0.27 | 0.25, 0.03, 7.62*** | 0.89, 0.43, 2.07* | −0.01, 0.03, −0.36 | 0.23, 0.03, 6.93*** | 1.18, 5.01, 0.24 | −0.01, 0.04, −0.34 | 0.26, 0.03, 7.80*** |
| PDDBI Autism Composite | −1.59, 0.68, −2.38* | −0.03, 0.05, −0.65 | −0.17, 0.05, −3.75*** | −0.93, 0.74, −1.26 | −0.05, 0.05, −1.01 | −0.19, 0.05, −4.24*** | −1.42, 0.60, −2.38* | −0.04, 0.05, −0.93 | −0.16, 0.05, −3.51*** | −1.24, 6.94, −0.18 | −0.04, 0.05, −0.89 | −0.20, 0.05, −4.43*** |
| ABC-C Social Withdrawal | −1.07, 0.39, −2.70** | −0.007, 0.03, −0.26 | −0.11, 0.03, −4.13*** | −1.17, 0.43, −2.76** | −0.02, 0.03, −0.71 | −0.12, 0.03, −4.48*** | −1.03, 0.35, −2.93** | −0.02, 0.03, −0.58 | −0.10, 0.03, −3.82 | −7.01, 4.09, −1.71 | −0.006, 0.03, −0.20 | −0.12, 0.03, −4.53*** |
| ABC-C Hyperactivity | 0.29, 0.55, 0.53 | −0.05, 0.04, −1.23 | −0.08, 0.04, −2.11* | 0.04, 0.59, 0.07 | −0.04, 0.04, −1.16 | −0.07, 0.04, −2.02* | −0.35, 0.49, −0.72 | −0.04, 0.04, −1.14 | −0.06, 0.04, −1.65 | 0.19, 5.58, 0.03 | −0.04, 0.04, −1.13 | −0.07, 0.04 −1.99* |
| CGI Severity | −0.09, 0.04, −2.0* | −0.002, 0.003, −0.83 | −0.03, 0.003, −10.68*** | −0.08, 0.05, −1.82 | −0.003, 0.003, −1.16 | −0.03, 0.003, −11.15*** | −0.11, 0.04, −2.84** | −0.003, 0.003, −1.06 | −0.03, 0.003, −10.18*** | −0.91, 0.43, −2.11* | −0.002, 0.003, −0.55 | −0.03, 0.003, −10.95*** |
Note. Average Look Duration is measured in seconds. ABC-C: Aberrant Behavior Checklist-Community. CGI: Clinical Global Impression. ET: eye-tracking. IQ: intelligence quotient. PDDBI: Pervasive Developmental Disorder Behavior Inventory. SE: standard error. VABS: Vineland-3 Interview Form.
unadjusted p < 0.05
unadjusted p < 0.01
unadjusted p < 0.001.
AOI analyses.
To draw comparisons between analyses based on look duration and a traditional area-of-interest approach, we first examined associations between time spent looking at the actress and characteristics associated with autism while controlling for age and IQ. No associations were statistically significant.
ALD analyses.
The correlation between Total Video average look duration and proportion of looking time to the video media was r=0.85.
When considering the entire video presentation, shorter Total Video ALD was associated with increased global autism symptoms (CGI Severity; B=−0.11, p=0.005, SE B=0.04, t=−2.84), reduced communication skills (PDDBI Receptive/Expressive Social Communication Abilities; B=0.87, p=0.009, SE B=0.33, t=2.66), and increased social withdrawal (ABC-C Lethargy/Social Withdrawal; B=−1.03, p=0.004, SE B=0.35, t=−2.93).
When considering ALD during specific segments of the ET stimulus video, shorter ALD during the Actress Dyadic Bid condition was associated with reduced communication skills (PDDBI Receptive/Expressive Social Communication Abilities; B=1.02, p=0.006, SE B=0.37, t=2.79) and increased social withdrawal (ABC-C Lethargy/Social Withdrawal; B=−1.07, p=0.008, SE B=0.39, t=−2.70). Shorter ALD during the Actress with Toys condition was associated with increased social withdrawal (ABC-C Lethargy/Social Withdrawal; B=−1.17, p=0.007, SE B=0.43, t=−2.76). No significant results emerged for the remaining parent report measures, including hyperactivity.
Discussion
In a relatively large sample of young autistic children, we examined the associations between a child’s ALD to a dynamic video containing both social and nonsocial elements and several measures of autism symptoms and social communication skills. We found that children with a better ability to maintain focus on the stimulus, as reflected in longer look durations, had fewer autism symptoms, higher social communication abilities, and reduced social withdrawal. The dynamic stimulus shown in this study incorporated both social (e.g. direct gaze and communication directed towards the child) and highly reinforcing nonsocial elements (e.g. interesting toys), mimicking a common, real-world setting in which a child is likely to view an environment with multiple inputs that compete for the child’s attention. This is especially relevant for autistic children who often have difficulty responding to multiple sensory inputs at once (Bruinsma et al., 2004; Mundy & Acra, 2006). The current findings demonstrate that ALD during the entire video and during social segments containing child-directed communication (Actress Dyadic Bid condition), were more consistently associated with children’s symptoms and social skills than ALD during other conditions which did not involve direct social bids. Additionally, ALD measures were correlated with children’s autism symptoms and social abilities, as compared to traditional measures of proportion looking time within social AOIs, which were not correlated with any clinical measures after accounting for age and IQ. The ability to look at a stimulus for longer periods, measured via ALD, may reflect advanced self-regulatory skills and lead to enhanced engagement with and comprehension of social information.
It is possible that longer durations of attention represent reduced flexibility in gaze behavior. The tendency to fixate for long periods has been studied in autistic children, referred to as “sticky attention” (Sacrey et al., 2014). Sticky attention occurs when an individual has difficulty disengaging from a salient stimulus once they have initiated a fixation. Additionally, sluggish cognitive tempo has been observed in autistic children and children with ADHD (Becker et al., 2016; McFayden et al., 2020), a characteristic that is associated with lethargy, lower memory retrieval, lower levels of alertness, and slower processing speed. Children with sluggish cognitive tempo might get visually stuck on the stimulus, but not because they are actually engaged or attentive. Alternatively, longer fixations to complex stimuli might provide opportunities for social learning. Our results support this interpretation; children with more advanced social and communication skills were more likely to demonstrate longer look durations.
ADHD, which is characterized by inattention and hyperactivity, is another condition in which children demonstrate reduced sustained attention. Distractibility/hyperactivity and poor focused attention have been linked previously (Hsieh et al., 2016). Given that 30–50% of autistic persons also display ADHD symptoms (Davis & Kollins, 2012), and an estimated 30–80% of autistic individuals have a clinical diagnosis of comorbid ADHD (Leitner, 2014; Van Der Meet et al., 2012), it is important to consider the overlap between autism and ADHD in the context of sustained attention abilities. Interestingly, in our study, autistic children whose caregivers reported higher levels of hyperactivity symptoms were not more likely to show differences in their look duration, suggesting that differences in look duration are associated with autism rather than co-occurring ADHD symptoms. Future research that includes autistic children who have diagnosed co-morbid ADHD is needed to fully understand the contribution of ADHD symptoms to shorter look duration in autistic children.
Tsang et al. (2018) utilized ET to count the number of fixations and saccades towards social AOIs in autistic children, autistic children with co-occurring ADHD, and children without autism. While the total number of fixation counts was lowest for autistic children with co-occurring ADHD, autistic children had the fewest saccades between AOIs. Other studies have reported that individuals with ADHD demonstrate greater difficulties in sustaining attention than autistic individuals (Davis & Kollins, 2012; Johnson et al., 2007). It is possible that autistic children have reduced sustained attention to social stimuli, while autism children with co-occurring ADHD have lower levels of sustained attention generally. Furthermore, Gui et al. (2020) studied the mean peak look duration (the peak look was averaged across trials from various stimuli types) and found that longer mean peak look durations to the face in infancy were related to increased mid-childhood ADHD traits and familial/genetic liability for ADHD, but not autism. Taken together, these studies suggest that autism and ADHD have distinct attention profiles, despite the high comorbidity rate between the two disorders. Future studies that include children without autism or ADHD and children with ADHD are needed to understand the overlap between the two disorders.
The present study provides the first evidence that ALD is related to level of autism symptoms in young autistic children. One limitation of the current study is that when children diverted their gaze from the screen, they were prompted to look back using pointing or verbal prompts when necessary. Sustained attention is optimally measured in a context in which participants attend spontaneously without prompts. A second limitation of the current study is that children were also participating in a clinical trial that involved specific inclusion and exclusion criteria relevant to that trial. As such, the results of this study may not be generalizable to the broader community of autistic children. Similarly, the clinical trial involved a predetermined set of clinical measures related to the attentional constructs being studied.
In conclusion, the ability to sustain attention to a complex, social stimulus may reflect advanced self-regulatory skills in young autistic children. Maintenance of focused attention may promote the development of social-communication skills by allowing the child to more easily process the dynamic flow of dyadic, human interaction in the environment.
Figure 1.
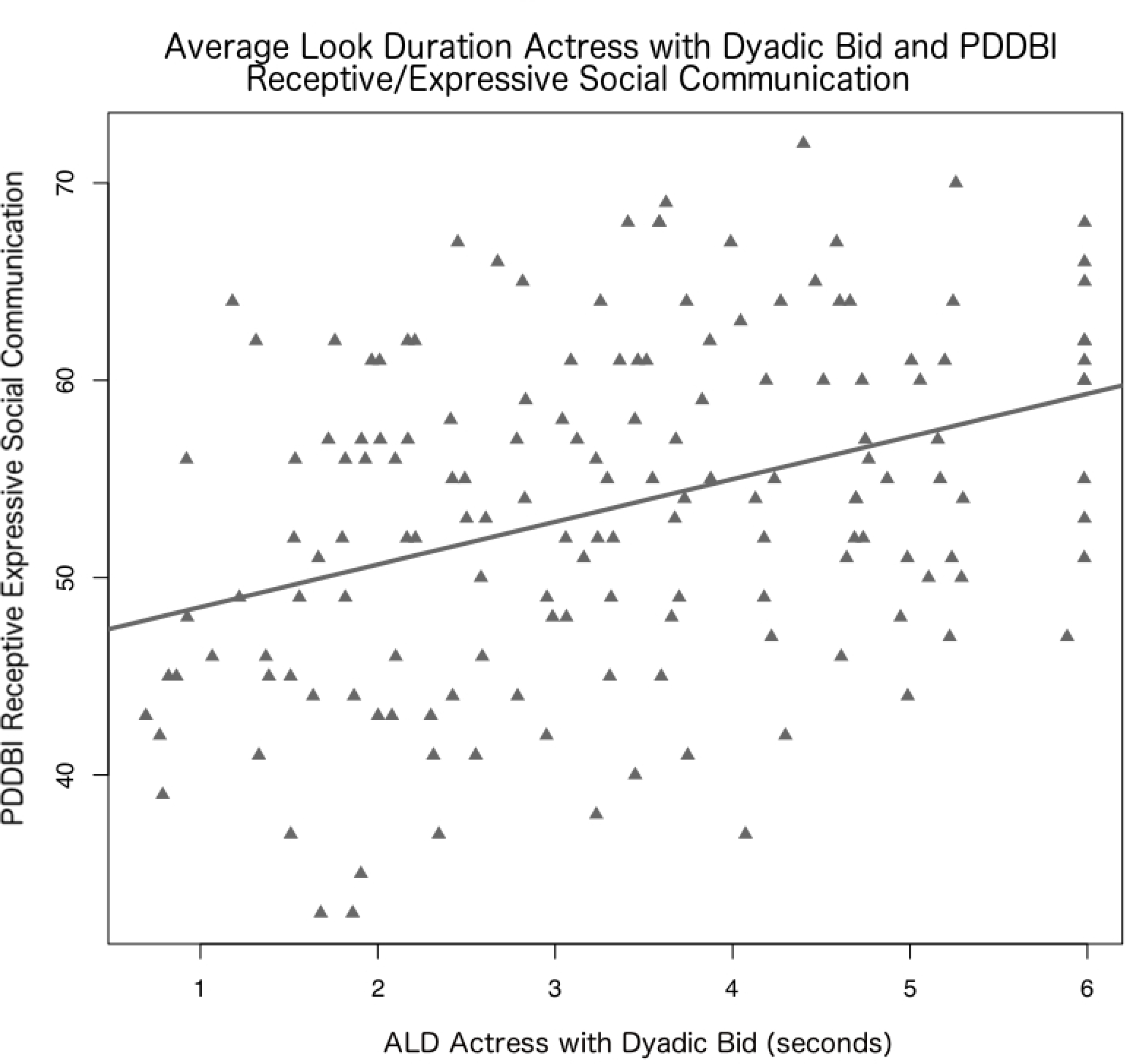
Scatterplot of Average Look Duration (ALD), the mean fixation length, during the Actress with Dyadic Bid condition (X-axis) and communication abilities measured by Pervasive Developmental Disorder Behavior Index (PDDBI) Receptive Expressive Social Communication T-Score (y-axis). The line of best fit was generated using linear regression.
Acknowledgements:
We are grateful for the children and their families who participated in this study, and the staff members who helped conduct the clinical trial. We also thank Raghav Swaminathan for his assistance with study procedures during data collection.
Funding Source:
NICHD P50HD093074 and the Marcus Foundation
Conflict of Interests and Disclosures: Dr. Dawson is on the Scientific Advisory Boards of Janssen Research and Development, Akili, Inc, LabCorp, Inc, Roche Pharmaceutical Company, and Tris Pharma, and is a consultant to Apple, Gerson Lehrman Group, Guidepoint, Inc, Axial Ventures, Teva Pharmaceutical, and is CEO of DASIO, LLC. Dr. Dawson has received book royalties from Guilford Press, Oxford University Press, Springer Nature Press. In addition, Dr. Dawson has the following patent applications: 1802952, 1802942, 15141391, and 16493754. Drs. Dawson, Sapiro, and Carpenter helped develop technology that has been licensed and Drs. Dawson, Sapiro, and Carpenter, and Duke University have benefited financially. Dr. Howard reports personal fees from Roche. Dr. Tenenbaum reports consulting fees from MapLight Therapeutics.
References
- Aman MG, Singh NN, Stewart AW, & Field CJ (1985). The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. American journal of mental deficiency. [PubMed] [Google Scholar]
- American Psychiatric Association & American Psychiatric Association. DSM-5 Task Force (2013). Diagnostic and statistical manual of mental disorders: DSM-5 (5th ed.). Washington, D.C.: American Psychiatric Association. [Google Scholar]
- Anderson CJ, Colombo J, & Jill Shaddy D (2006). Visual scanning and pupillary responses in young children with autism spectrum disorder. Journal of Clinical and Experimental Neuropsychology, 28(7), 1238–1256. [DOI] [PubMed] [Google Scholar]
- Becker SP, Ciesielski HA, Rood JE, Froehlich TE, Garner AA, Tamm L, & Epstein JN (2016). Uncovering a clinical portrait of sluggish cognitive tempo within an evaluation for attention-deficit/hyperactivity disorder: A case study. Clinical Child Psychology and Psychiatry, 21(1), 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinsma Y, Koegel RL, & Koegel LK (2004). Joint attention and children with autism: A review of the literature. Mental retardation and developmental disabilities research reviews, 10(3), 169–175. [DOI] [PubMed] [Google Scholar]
- Busner J, & Targum SD (2007). The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont), 4(7), 28. [PMC free article] [PubMed] [Google Scholar]
- Charman T (2003). Why is joint attention a pivotal skill in autism? Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 358(1430), 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Macari S, & Shic F (2012). Context modulates attention to social scenes in toddlers with autism. Journal of Child Psychology and Psychiatry, 53(8), 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Macari S, & Shic F (2013). Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biological psychiatry, 74(3), 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Ye S, Shic F, & Chen L (2016). Multilevel differences in spontaneous social attention in toddlers with autism spectrum disorder. Child development, 87(2), 543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Parish‐Morris J, McVey A, Rump KM, Sasson NJ, Herrington JD, & Schultz RT (2015). Measuring social attention and motivation in autism spectrum disorder using eye‐tracking: Stimulus type matters. Autism Research, 8(5), 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IL, Schmidt-Lackner S, Romanczyk R, & Sudhalter V (2003). The PDD Behavior Inventory: a rating scale for assessing response to intervention in children with pervasive developmental disorder. Journal of autism and developmental disorders, 33(1), 31–45. [DOI] [PubMed] [Google Scholar]
- Davis NO & Kollins SH (2012). Treatment for co-occurring attention deficit/hyperactivity disorder and autism spectrum disorder. Neurotherapeutics, 9(3), 518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Sun JM, Baker J, Carpenter K, Compton S, Deaver M, … & Kurtzberg J (2020). A phase II randomized clinical trial of the safety and efficacy of intravenous umbilical cord blood infusion for treatment of children with autism spectrum disorder. The Journal of pediatrics, 222, 164–173. [DOI] [PubMed] [Google Scholar]
- Del Bianco T, Mazzoni N, Bentenuto A, & Venuti P (2018). An investigation of attention to faces and eyes: Looking time is task-dependent in autism spectrum disorder. Frontiers in psychology, 9, 2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot CD (2007). Differential Ability Scales-Second Edition (2nd ed.). San Antonio, TX. Harcourt Assessment, Inc. [Google Scholar]
- Elsabbagh M, Holmboe K, Gliga T, Mercure E, Hudry K, Charman T, … & BASIS Team. (2011). Social and attention factors during infancy and the later emergence of autism characteristics. Brain Research (Vol. 189, pp. 195–207). [DOI] [PubMed] [Google Scholar]
- Gui A, Mason L, Gliga T, Hendry A, Ali JB, Pasco G, … & Meaburn E (2020). Look duration at the face as a developmental endophenotype: elucidating pathways to Autism and ADHD. Development and Psychopathology, 1–20. [DOI] [PubMed] [Google Scholar]
- Hendry A, Jones EJ, Bedford R, Gliga T, Charman T, Johnson MH, & BASIS Team. (2018). Developmental change in look durations predicts later effortful control in toddlers at familial risk for ASD. Journal of neurodevelopmental disorders, 10(1), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessels RS, Kemner C, van den Boomen C, & Hooge IT (2016). The area-of-interest problem in eye tracking research: A noise-robust solution for face and sparse stimuli. Behavior research methods, 48(4), 1694–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S, Wu M, & Tang CH (2016). Inhibiting prepotent responses in the elderly: distraction and disinhibition. Cognitive, Affective, & Behavioral Neuroscience, 16(1), 124–134. [DOI] [PubMed] [Google Scholar]
- Isaev DY, Major S, Murias M, Carpenter KL, Carlson D, Sapiro G, & Dawson G (2020). Relative Average Look Duration and its Association with Neurophysiological Activity in Young Children with Autism Spectrum Disorder. Scientific Reports, 10(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Robertson IH, Kelly SP, Silk TJ, Barry E, Dáibhis A, … & Gill M (2007). Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neuropsychologia, 45(10), 2234–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn G, Kourkoulou A, & Leekam SR (2010). How magic changes our expectations about autism. Psychological Science, 21(10), 1487–1493. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, O’Neill AH, & Stuart EA (2011). Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: A randomized controlled trial. Journal of Child Psychology and Psychiatry, 52(1), 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner Y (2014). The co-occurrence of autism and attention deficit hyperactivity disorder in children–what do we know? Frontiers in human neuroscience, 8, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop SL (1999). ADOS. Autism diagnostic observation schedule manual. Los Angeles, CA: WPS. [Google Scholar]
- McFayden T, Jarrett MA, White SW, Scarpa A, Dahiya A, & Ollendick TH (2020). Sluggish cognitive tempo in autism spectrum disorder, ADHD, and their comorbidity: Implications for impairment. Journal of Clinical Child & Adolescent Psychology, 1–8. [DOI] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning (pp. 58–64). Circle Pines, MN: AGS. [Google Scholar]
- Mundy P, & Acra CF (2006). Joint attention, social engagement, and the development of social competence. The development of social engagement: Neurobiological perspectives, 81–117. [Google Scholar]
- Murias M, Major S, Davlantis K, Franz L, Harris A, Rardin B, … & Dawson G (2018). Validation of eye‐tracking measures of social attention as a potential biomarker for autism clinical trials. Autism Research, 11(1), 166–174. [DOI] [PubMed] [Google Scholar]
- Parish-Morris J, Chevallier C, Tonge N, Letzen J, Pandey J, & Schultz RT (2013). Visual attention to dynamic faces and objects is linked to face processing skills: a combined study of children with autism and controls. Frontiers in psychology, 4, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presmanes AG, Walden TA, Stone WL, & Yoder PJ (2007). Effects of different attentional cues on responding to joint attention in younger siblings of children with autism spectrum disorders. Journal of autism and developmental disorders, 37(1), 133–144. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C, Faggioli R (2005). Autism Diagnostic Interview-Revised (ADI-R). Torrance, CA: Western Psychological Services. [Google Scholar]
- Sacrey LAR, Armstrong VL, Bryson SE, & Zwaigenbaum L (2014). Impairments to visual disengagement in autism spectrum disorder: a review of experimental studies from infancy to adulthood. Neuroscience & Biobehavioral Reviews, 47, 559–577. [DOI] [PubMed] [Google Scholar]
- Salley B, & Colombo J (2016). Conceptualizing social attention in developmental research. Social Development, 25(4), 687–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schietecatte I, Roeyers H, & Warreyn P (2012). Exploring the nature of joint attention impairments in young children with autism spectrum disorder: Associated social and cognitive skills. Journal of Autism and Developmental Disorders, 42(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Shi L, Zhou Y, Ou J, Gong J, Wang S, Cui X, … & Luo X (2015). Different visual preference patterns in response to simple and complex dynamic social stimuli in preschool-aged children with autism spectrum disorders. PloS one, 10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S, Balla D, Cicchetti D (1984). Vineland Adaptive Behavior Scales: Interview Edition. Circile Pines, MN: American Guidance Service. [Google Scholar]
- Trembath D, Vivanti G, Iacono T, & Dissanayake C (2015). Accurate or assumed: Visual learning in children with ASD. Journal of Autism and Developmental Disorders, 45(10), 3276–3287. [DOI] [PubMed] [Google Scholar]
- Tsang V, & Chu PCK (2018). Comparing Eye-tracking Data of Children with High-functioning ASD, Comorbid ADHD, and of a Control Watching Social Videos. JoVE (Journal of Visualized Experiments), (142), e58694. [DOI] [PubMed] [Google Scholar]
- Van Der Meer JM, Oerlemans AM, Van Steijn DJ, Lappenschaar MG, De Sonneville LM, Buitelaar JK, & Rommelse NN (2012). Are autism spectrum disorder and attention-deficit/hyperactivity disorder different manifestations of one overarching disorder? Cognitive and symptom evidence from a clinical and population-based sample. Journal of the American Academy of Child & Adolescent Psychiatry, 51(11), 1160–1172. [DOI] [PubMed] [Google Scholar]
- Wang S, Jiang M, Duchesne XM, Laugeson EA, Kennedy DP, Adolphs R, & Zhao Q (2015). Atypical visual saliency in autism spectrum disorder quantified through model-based eye tracking. Neuron, 88(3), 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E, Dawson G, Osterling J, & Dinno J (2000). Recognition of autism spectrum disorder before 1 year of age. Journal of Autism and Developmental Disorders, 30, 157–162. [DOI] [PubMed] [Google Scholar]


