Abstract
Hemophagocytic lymphohistiocytosis (HLH) constitutes a life-threatening inflammatory syndrome. Postmortem histological findings of bone marrow (BM) from COVID-19 patients showed histiocytosis and hemophagocytosis and supported the hypothesis that secondary HLH (sHLH) may be triggered by SARS-CoV-2 infection. However, there are a limited number of sHLH cases in which trephine has been performed in living post-COVID-19 patients. Here we present a recent case and a mini-review of sHLH diagnosed by trephine biopsy in living patients after COVID-19. An 81-year-old man with a past medical history of hypertension, diabetes, ischemic stroke, was referred to the hospital to evaluate leukocytosis, pyuria, and elevation of inflammatory markers four weeks after recovering from COVID-19. Computed tomography of the abdomen did not reveal focal signs of infection or hepatosplenomegaly. The patient received intravenous meropenem and two packed red blood cell units. Leukocytes and C-reactive protein were gradually decreased. A BM biopsy was performed and the patient was discharged on cefixime. BM smear revealed severe anemia, lymphopenia, and dysplastic morphologic findings of erythroblasts, neutrophils, and megakaryocytes. Trephine biopsy revealed hypercellular marrow dyserythropoiesis, plasmacytosis, lymphocytosis, histiocytosis, hemophagocytosis, and the absence of granulomas or carcinoma. Immunohistochemistry documented a mixed population of T lymphocytes (CD3+) and B lymphocytes (CD20+). Strong positivity for CD68 confirmed histiocytosis. CD138 κ, λ staining proved polyclonal plasmacytosis. Perl’s staining showed excess hemosiderin deposits. Based on our findings, we document sHLH in trephine BM biopsy of a living post-COVID-19 patient and persistent leukocytosis, underscoring the diagnostic value of trephine biopsy in preventing life-threatening conditions such as COVID-19.
Keywords: Hemophagocytic lymphohistiocytosis, Post COVID-19, Bone marrow biopsy, Trephine biopsy, Living patient, SARS-CoV-2
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a potentially life-threatening syndrome characterized by the dysregulated activity of cytotoxic T lymphocytes and natural killer (NK) cells (Al-Samkari and Berliner 2018). HLH was first reported in 1939 by Scott & Robb-Smith (Scott and Robb-Smith 1939), who described a child as having a neoplastic histiocytic disorder. The disorder was first recognized as familial by Farquhar & Claireaux (Farquhar and Claireaux 1952).
HLH includes two types that can be difficult to distinguish one from the other: A primary type that occurs due to genetic disorders and a secondary type (sHLH) triggered by various infections, autoimmune diseases, or malignancy (Scott and Robb-Smith 1939; Ramos-Casals et al. 2014). In primary HLH, several different genetic defects, such as mutations in PRF1 or UNC13D, can lead to an impaired NK/ T cytotoxicity (Usmani et al. 2013; Janka 2012). A number of external factors can lead to NK/T cell defection and excessive inflammation, causing sHLH, such as infections, malignancies, rheumatological diseases, or autoimmune/immunodeficiency conditions (Usmani et al. 2013; Dropulic and Cohen 2011; Han et al. 2017).
Malignancies are considered to be the most common factor in the development of sHLH in adults (approximately 45% of cases) and a secondary cause of sHLH in children (about 8% of cases) (Allen and McClain 2015). These patients may develop sHLH due to immune activation by tumor cells or due to loss of inhibitory immune function due to disease or bone marrow (BM) dysfunction caused by treatment (Campo and Berliner 2015). Viral agents are another cause of sHLH. The most common causes of sHLH-related infection include Epstein-Barr virus (EBV) and herpes simplex virus (HSV). In particular, Epstein-Bar Virus (EBV) is the most common infectious agent leading to disease through the proliferation and hyperactivity of EBV-infected T lymphocytes (Smith et al. 2014). However, other viruses, such as influenza, cytomegalovirus, adenovirus, hepatitis A, parvovirus B19, rotavirus, syncytial virus, and pathogens, such as bacteria and fungi and parasites, can cause sHLH (Ramos-Casals et al. 2014; Dropulic and Cohen 2011; Apodaca et al. 2018; Barba et al. 2015; Otrock and Eby 2015; Zhao et al. 2019; Lerolle et al. 2016; Henter et al. 2006; Esteban et al. 2017).
sHLH is characterized by activation of lymphocytes and macrophages with consequent immoderate immune response, cytokine storm, and hemophagocytosis, leading to multiorgan failure, overlapping with cytokine release syndrome and macrophage activation syndrome (MAS), the latter has clinical and laboratory features similar to primary genetic HLH (Usmani et al. 2013; Karakike and Giamarellos-Bourboulis 2019; Kumar et al. 2017; Lee et al. 2014; Schram and Berliner 2015). Patients with sHLH are often severely ill and may progress rapidly to a clinical picture resembling septic shock, with approximately 50% of cases presenting with respiratory symptoms (Lerolle et al. 2016).
Although the diagnosis of sHLH is based on clinical and laboratory criteria derived from primary HLH (HLH 2004 criteria) (Henter et al. 2007), in 2014, Fardet et al., presented a new rating for sHLH called HScore (Fardet et al. 2014). This score is based on nine parameters, including the presence of immunosuppression, fever, hepatosplenomegaly, high levels of triglycerides, ferritin, liver enzymes, and fibrinogen, as well as the development of cytopenia and hemophagocytosis in BM aspiration. The creators of the score found an optimal threshold (HScore of 169), which corresponds to a sensitivity of 93% and a specificity of 86% (Fardet et al. 2014).
Recent studies show that the cytokine storm induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has significant similarities to clinical and laboratory findings of sHLH (Kim et al. 2021; Meng et al. 2021; Mehta et al. 2020; Tsatsakis et al. 2020; Stancioiu et al. 2020; Kostoff et al. 2022). SARS-CoV-2 belongs to the family of Coronaviridae and causes COVID-19 disease, which is a respiratory illness (Tsatsakis et al. 2020; Wang et al. 2020; Wu and McGoogan 2020). SARS-CoV-2 attaches to the angiotensin-converting enzyme 2 (ACE2) receptor that is highly expressed in lung epithelial cells, as well as in other tissues, and thus enters the cells (Zou et al. 2020; Stancioiu et al. 2020). Specifically, spike protein S is responsible for the attachment to the ACE2 receptor (Hoffmann et al. 2020). Protein S employs host cell transmembrane protease serine 2 (TMPRSS2) that cleavage ACE2, promoting SARS-CoV-2 fusion with the epithelial cell (Hoffmann et al. 2020). SARS-CoV-2 infection triggers an excessive immune response known as a cytokine storm in cases of severe COVID-19 Feng et al. 2020; Tsatsakis et al. 2020;. Hu et al. 2020). The cytokine storm, which is characterized by high expression of IL-6 and TNF-α, is considered to be the main cause of disease severity and death in COVID-19 (Mehta et al. 2020) and is related to high levels of circulating cytokines, severe lymphopenia, thrombosis, and massive mononuclear cell infiltration in multiple organs (Merad et al. 2020). Hirano and Murakami 2020, proposed a potential mechanism of the cytokine storm caused by the angiotensin 2 (AngII) / NF-κB pathway, which can lead to pleiotropic effects on acquired and innate immune cells, resulting in cytokine storms (Moore and June 2020). It is considered that the impaired acquired immune responses and uncontrolled inflammatory innate responses to SARS-CoV-2 may cause cytokine storms.
Although COVID-19-related cytokine storm has been associated with the development of sHLH since the onset of the pandemic (England et al. 2021), little is known about the clinical manifestations of sHLH that develop after a patient recovers from SARS-CoV-2 infection England et al. 2021; Naous et al. 2021; Naqvi and Bhutta 2021; Sangha et al. 2021; Kayaaslant al. e 2021; Kalita et al. 2021; Michaelis et al. 2021; Núñez-Torrón et al. 2021; Wiseman et al. 2021). The majority of studies on histological findings of BM in patients with COVID-19 are postmortem (Núñez-Torrón et al. 2021; Prilutskiy et al. 2020; Swoboda et al. 2021; Prieto-Pérez et al. 2020; Bryce et al. 2021). However, there are a limited number of cases in the English literature in which sHLH was documented by trephine BM biopsy in living patients with COVID-19 (Prieto-Pérez et al. 2020; Harris et al. 2021) and especially after COVID-19 (Dandu 2021). Here, we present a case of HLH based on histological and immunohistochemical findings from trephine BM biopsy in a living patient after COVID-19, with persistent leukocytosis. We also include a mini-review of BM sHLH observations in post-COVID-19 living patients.
Case report
An 81-year-old man with a medical history included hypertension, diabetes, ischemic stroke (Table 1) who was recently admitted and treated for COVID-19, was referred to a hospital physician (General Hospital of Larissa) four weeks following his recovery, due to persistent leukocytosis, pyuria, and elevation of inflammatory markers such as C-reactive protein (CRP) (Table 1). The patient had a fever (temperature > 38.4 °C). Ferritin was within normal range. Peripheral blood cell counts revealed mild anemia (hemoglobin 7.2 g/dl), increased white cell count (44 × 103/µl), and elevated CRP levels (193 mg/L) (Table 1). The liver function tests were: aspartate aminotransferase (AST) 14 IU/l, alanine aminotransferase (ALT) 13 IU/l, gamma-glutamyl transpeptidase (γGT) 18 IU/l and lactate dehydrogenase (LDH) 397 mg/dl (Table 1). The fibrinogen was 661 mg/dl (Table 1). Urinalysis showed bacteriuria. Peripheral blood smear showed hypochromic anisocytosis, dimorphic blood cell population, dysplastic neutrophils nuclei with partial lack granulation, and some neutrophils with increased toxic granulation (Table 1). The imaging investigation, which included abdominal ultrasound and chest computed tomography (CT) scan, showed a fracture of the left sciatic branch and a fracture of the fifth lumbar vertebra (Table 1), but no source of infection or hepatosplenomegaly (Table 1).
Table 1.
Patient’s comorbidities, laboratory, imaging, and peripheral blood smear findings
| Comorbidities | Laboratory findings | Imaging findings | Peripheral blood smear | ||
|---|---|---|---|---|---|
| Hypertension | Hb | 7.2 gr/dl | Upper and lower abdomen CT |
No hepatosplenomegaly Fracture of left sciatic branch Fracture of the fifth lumbar vertebra |
Hypochromic anisocytosis |
| Diabetes | WBC | 44 × 103 /µl | Chest CT | No signs of infection | Dimorphic blood cell population |
| Ischemic stroke | PLT | 437 K/ µl | Dysplastic neutrophils nuclei with partial lack of granulation | ||
| Triglycerides | 109 mg/dl | Neutrophils with increased to toxic granulation. | |||
| AST | 14 IU/l | ||||
| ALT | 13 IU/l | ||||
| γ-GT | 18 IU/l | ||||
| LDH | 397 mg/dl | ||||
| CRP | 193 mg/l | ||||
| FIB | 661 mg/dl | ||||
| Hb, hemoglobin; WBC, white blood cells; CT, computed tomography; PLT, platelets; AST, aspartate aminotransferase; ALT, Alanine aminotransferase; γ-GT, gamma-glutamyl transpeptidase; LDH, lactate dehydrogenase; CRP, C-reactive protein; FIB, Fibrinogen. | |||||
The differential diagnosis included both a severe infection and a hematologic malignancy. Intravenous administration of meropenem and colistin was initiated due to suspected disease, and two units of packed red blood cells were given for severe anemia. Leukocytes and CRP were gradually decreased and the patient was discharged after five days. Before discharge, the leukocytes count was 9.5 × 10 3 /µl, while CRP was 66 mg/l, still not within the normal range. The patient was prescribed cefixime. Prior to discharge, BM aspiration and trephine biopsy samples were sent for evaluation.
Flow cytometry immunophenotyping was performed in BM and showed hypercellular marrow with myeloid hyperplasia, left-shifted myeloid lineage, and immature myeloid cells (Table 2). Microscopic examination of the BM smear revealed severe anemia, lymphopenia, and dysplastic morphologic findings of erythroblasts, neutrophils, and megakaryocytes (Table 2).
Table 2.
Bone marrow (BM) findings
| BM smear | Anemia | Lymphopenia | Dysplastic morphologic findings of erythroblasts, neutrophils, and megakaryocytes | |
|---|---|---|---|---|
| BM trephine | Polyclonal plasmatocytosis |
Lymphocytosis (mixed population of T- and B- lymphocytes) |
Histiocytosis with phagocytosis of red blood cells | Dysplastic changes in all three hemopoietic lineages |
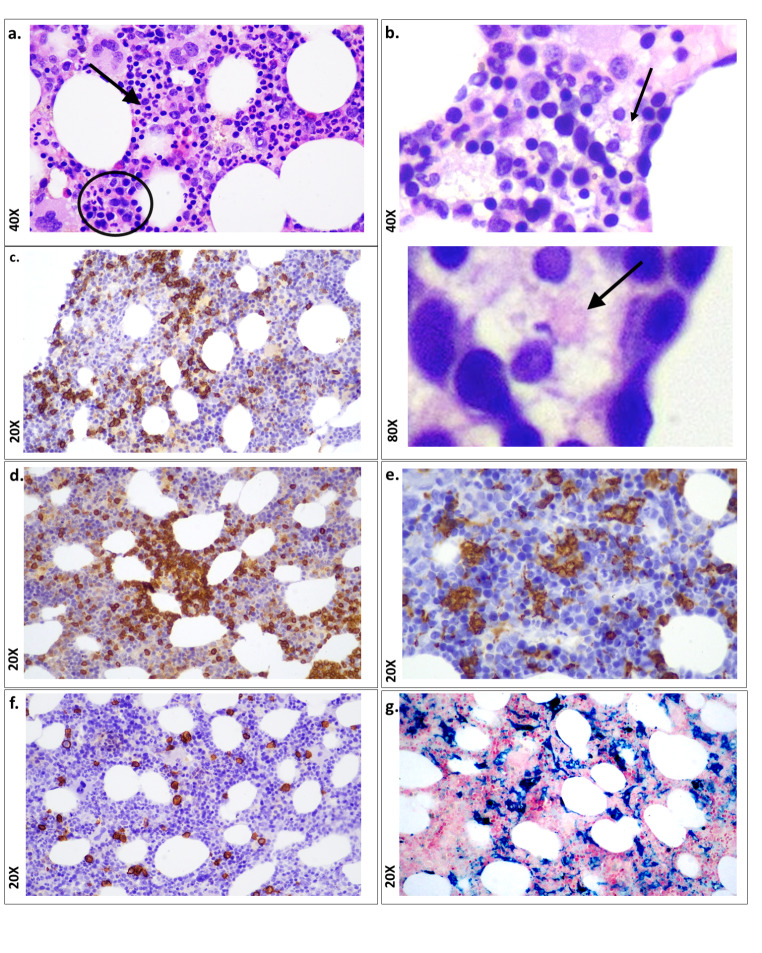
The BM biopsy was sent to the University Hospital of Larissa in the Department of Pathology. Histological examination showed hypercellular marrow with the adipocytes occupying 40% of marrow spaces and thin trabeculae indicating osteoporosis (Table 2). Hematoxylin and eosin (H&E) staining also revealed dyserythropoiesiss, plasmacytosis, lymphocytosis, histiocytosis, and absence of granulomas or carcinoma (Fig. 1, a). Another significant finding was the phagocytosis of red blood cells (Fig. 1, b). Immunohistochemistry revealed a mixed population of T lymphocytes (CD3+) (polyclonal, 1:300 DAKO, Dako Autostainer Link 48 +) (Fig. 1, c) and B lymphocytes (CD20+) (clone L26, 1:200, DAKO, Dako Autostainer Link 48 +) (Fig. 1, d). At the same time, histiocytosis was confirmed by strong positivity for CD68 (clone PG-M1, 1:100, DAKO, Dako Autostainer Link 48 +) (Fig. 1, e). CD138 (clone MI15, 1:100, DAKO, Dako Autostainer Link 48+), κ (polyclonal, 1:30000, DAKO, Dako Autostainer Link 48+), and λ (polyclonal, 1:30000, DAKO, Dako Autostainer Link 48+) stains showed that plasmacytosis was polyclonal (Fig. 1, f). In addition, Perl’s histochemical stain also showed excess hemosiderin deposits (Fig. 1, g). The histopathological diagnosis was dyshematopoiesis with excessive lymphohistiocytosis. Based on the above data, sHLH was diagnosed on trephine BM biopsy.
Fig. 1.
Histological and immunohistochemical (IHC) findings in BM trephine biopsy from a living post-COVID-19 patient. (a) Histology shows trilineage haemopoiesis with dyserythropoiesis (in circle) and dysgranulopoiesis (arrow). A few histiocytes with cytoplasmic debris are also observed; X40; (H&E: hematoxylin and eosin staining). (b) Phagocytosis of red blood cells is shown (arrow) X40 and 80X; (H&E staining). (c) CD20 positive B lymphocytes are demonstrated (immunohistochemical staining; X20); (d) CD3 positive T lymphocytes are displayed (immunohistochemical staining; X20). (e) CD68 positive histiocytes are displayed (immunohistochemical staining; X40). (f) CD138 positive plasma cells are shown (immunohistochemical staining; X20). (g) Perl’s histochemical staining shows the excess of iron in the background (blue reaction; X20)
Discussion
HLH can lead to a cytokine storm that may lead to multiple organ failure and mortality, ranging from 20.4 to 88% depending on the condition and the underlying disease, including malignancies, infections, and autoimmune disorders (Hayden et al. 2016). According to Meng et al., (Meng et al. 2021) and Yang et al., (Yang et al. 2021), COVID-19-associated sHLH is responsible for increased mortality. Thus, accurate diagnosis of sHLH can be vital for the early treatment of life-threatening conditions such as COVID-19. Here, we present a case of sHLH diagnosed by trephine BM biopsy in a living patient with resolved COVID-19.
The diagnosis of sHLH has been previously described in COVID-19 (Núñez-Torrón et al. 2021; Harris et al. 2021; Swoboda et al. 2021; Meazza Prina et al. 2021; Bryce et al. 2021; Prilutskiy et al. 2020; Debliquis et al. 2020; Prieto-Pérez et al. 2020; Tholin et al. 2020; Labro et al. 2020; Lima et al. 2020) and especially in patients after COVID-19 (Michaelis et al. 2021; Naqvi and Bhutta 2021; Sangha et al. 2021; Dandu 2021; Naous et al. 2021; Kalita et al. 2021; Kayaaslan et al. 2021; Wiseman et al. 2021) (Table 3). Nalbandian et al., defined post-acute COVID-19 syndrome as persistent symptoms and/or delayed or long-term complications of SARS-CoV-2 infection beyond four weeks after the onset of symptoms (Nalbandian et al. 2021). Post-COVID-19 syndrome has been attributed to excessive cytokine release, such as IL-6 (Tanaka et al. 2018). However, clinical, laboratory, and morphological findings appear controversial in patients recovering from COVID-19 (Bhattacharjee et al. 2020). Table 3 shows current and previous cases of sHLH described after recovery from COVID-19, based on BM findings in living patients (Naous et al. 2021; Naqvi and Bhutta 2021; Sangha et al. 2021; Kayaaslan et al. 2021; Kalita et al. 2021; Michaelis et al. 2021; Núñez-Torrón et al. 2021; Wiseman et al. 2021; Swoboda et al. 2021; Debliquis et al. 2020; Prieto-Pérez et al. 2020; Dandu 2021; Harris et al. 2021). Kayaaslan et al., showed that some patients may develop hemophagocytosis in the late COVID-19 period, even when recovering (Kayaaslan et al. 2021). Kayaaslan et al., suggested that post-COVID-19-associated sHLH was associated with significant mortality and that BM aspiration/biopsy should be performed in patients with suspected HLH, especially in patients on the margins of diagnosis (Kayaaslan et al. 2021). Wiseman et al., also described a case of sHLH developed in post-acute COVID-19 syndrome, based on the findings of hemophagocytosis from BM aspirates (Wiseman et al. 2021). In particular, Wiseman D et al., showed a delayed immune response against SARS-CoV-2. They suggested that BM aspiration and/or biopsy should be carried out promptly to confirm the diagnosis and start appropriate treatment for HLH in patients who have recovered from COVID-19. Kalita et al., described two cases of an adult and a toddler after COVID-19 (Kalita et al. 2021). Notably, they showed that the toddler had a high HScore and hemophagocytosis in BM aspiration, similar to the adult patient.
Table 3.
Bone marrow (BM) findings and HScore in post-COVID-19/COVID-19 patients, mini literature review
| Post COVID-19 patients | Author | Number of deceased patients |
Number of alive patients | HScore / *HScore range |
BM aspirate | BM biopsy | BM autopsy | |
|---|---|---|---|---|---|---|---|---|
| Current case | 0 | 1 | 92 | Hemophagocytosis | Dyshematopoiesis, histiocytosis hemophagocytosis |
N/A | ||
| Michaelis S, et al. | 0 | 1 | N/A | Hemophagocytosis, megakaryocytic hypocellularity | N/A | N/A | ||
| Naqvi WA, et al. | 0 | 1 | N/A | Hemophagocytosis | N/A | N/A | ||
| Sangha G, et al. | 0 | 1 | 191 | Hemophagocytosis, hypocellular marrow | N/A | N/A | ||
| Dandu H, et al. | 0 | 13 | *138–198 | Hemophagocytosis, histiocytic hyperplasia | Hemophagocytosis, histiocytic hyperplasia | N/A | ||
| Naous E, et al. | 0 | a1 | 205 | Hemophagocytosis | N/A | N/A | ||
| Kalita P, et al. | 0 | 2 | *213–239 | Hemophagocytosis | N/A | N/A | ||
| Kayaaslan BU, et al. | 0 | 1 | 209 | Hemophagocytosis | N/A | N/A | ||
| Wiseman D, et al. | 0 | 1 | 197 | Hemophagocytosis | N/A | N/A | ||
| Dewaele K, et al. | 0 | 1 | N/A | Hemophagocytosis and erythroid hyperplasia | N/A | N/A | ||
| COVID-19 patients | Harris CK, et al. | a20 | 0 | *35–269 | N/A | N/A | Hemophagocytic histiocytosis | |
| 0 | 2 | b19 | N/A |
cLeft-shifted myelopoiesis but no apparent hemophagocytosis; dFew hemophagocytic histiocytes |
N/A | |||
| Swoboda J, et al. | 15 | 0 | 19–191 | N/A | N/A | Hemophagocytosis | ||
| Meazza Prina M, et al. | 0 | 1 | 269 | No aspects of hemophagocytosis | No aspects of hemophagocytosis | N/A | ||
| Núñez-Torrón C, et al. | 16 | 0 | *54–304 | N/A | N/A | Hemophagocytosis | ||
| Bryce C, et al. | e100 | 0 | N/A | N/A | N/A |
Hemophagocytosis (8/11) Left shift granulopoiesis (4/11) Decreased erythroid precursors (1/11) Decreased megakaryocytes (1/11) |
||
| Prieto-Pérez L, et al. | f33 | 0 | N/A | N/A | N/A | Hypercellular marrow, ghemophagocytosis | ||
| 0 | 3 | N/A | N/A | Hypercellular marrow, hemophagocytosis | N/A | |||
| Debliquis A, et al. | 0 | 3 | 35, 84, 207 | Hemophagocytosis | N/A | N/A | ||
| Tholin B, et al. | 0 | 1 | *221–230 | Hemophagocytosis | N/A | N/A | ||
| Labro G, et al. | 0 | h6 | *35–207 | Hemophagocytosis | N/A | N/A | ||
| Prilutskiy A, et al. | i4 | 0 | *96–217 | N/A | N/A | Left-shifted myeloid hyperplasia, histiocytic hyperplasia, NO hemophagocytosis | ||
| Lima R, et al. | 0 | j1 | N/A | Increased number of activated macrophages with prominent hemophagocytosis of hematopoietic elements | Increased macrophage activity | N/A | ||
aBM sample in 19 out of 20 patients; bHScore evaluated in 1 out of 2 alive patients; cThe patient with Hscore 19; dThe patient with no Hscore; eBM autopsy on 11 out of 100 patients; fBM autopsy on 17 out of 33 patients; gHemophagocytosis in 16/17 BM autopsies; hNumber of patients were alive at the time of BM examination (4 of them died during hospitalization); iBM autopsy on 2 out of 4 patients; jThe patient was alive at the time of BM examination but died during hospitalization; N/A: not applicable
Although, several studies have linked high HScore levels to increased mortality in patients with COVID-19 (Mehta et al. 2020; Bordbar et al. 2021), in most cases there is a lack of COVID-19-associated sHLH criteria, such as high fever, cytopenia, and signs of abnormalities through CT scan imaging analysis. However, morphological findings such as BM aspirate may support the diagnosis of sHLH (Tholin et al. 2020). It is worth mentioning that, Michaelis et al., showed hemophagocytosis in BM aspirations of a post-COVID-19 female patient, five weeks after her initial diagnosis of SARS-CoV-2 infection, although no clinical or laboratory findings were found for sHLH (Michaelis et al. 2021) (Table 3). Although our clinical and laboratory data resulted in low HScore, BM smear and biopsy supported sHLH in a living patient after COVID-19.
There are a limited number of cases in the English literature in which trephine biopsy was performed in living patients with COVID-19 (Harris et al. 2021; Prieto-Pérez et al. 2020), and especially after COVID-19 (Dandu et al. 2021) (Table 3). Dandu et al., reported the cases of thirteen COVID-19 patients with severe respiratory distress even after the infection had resolved (Dandu et al. 2021). Only five of them had HScore over 169, but all had hemophagocytosis in BM biopsies. Prieto-Pérez et al., examined bone marrow biopsies from three living post-COVID-19 patients who met the criteria for sHLH diagnosis. Bone marrow examination in these living patients confirmed sHLH with severe erythrophagocytosis (Prieto-Pérez et al. 2020) (Table 3). In a recent study by Harris CK et al., BM was examined in 20 autopsies and in two living patients with COVID-19, with HScore ranging from 35 to 269 (Harris et al. 2021) (Table 3). The BM of the two living patients showed maturation of trilineage hematopoiesis, including a few hemophagocytic histiocytes. In our study, BM biopsy histology showed dyshematopoiesis with excessive lymphohistiocytosis, and hemophagocytosis (Fig. 1). Also, immunohistochemistry revealed T-lymphocytes and B lymphocytes, indicated by the presence of CD3 + and CD20 + cells, respectively, polyclonal plasmacytes, as suggested by the presence of CD138 + cells and polytypic for kappa and lambda light chains, and histiocytes, as confirmed by the presence of CD68 + cells (Fig. 1). All these findings suggest persistent BM inflammation one month after the resolution of COVID-19 and are suggestive of a sHLH.
The clinical features of COVID-19 can vary from asymptomatic or mild symptoms to severe pneumonia (Mizumoto et al. 2020; Yang et al. 2020). Laboratory findings, such as highly elevated CRP and hyperferritinemia, are critical parameters for the diagnosis of sHLH and are often elevated even after COVID-19 resolution (Colafrancesco et al. 2014; Rosário et al. 2013; Sharif et al. 2018). However, the laboratory findings in our case did not show signs of hyperferritinemia with documented high CRP levels (193 mg/L), which remained at non-normal levels after the patient recovered from COVID-19 (66 mg/L), supporting the diagnosis of post-COVID-19 sHLH. In addition, coagulopathy has been described in up to 60% of cases with sHLH (Valade et al. 2015). When coagulopathy and abnormal liver are evident in SaRS-CoV-2 infected patients, it may indicate that a subgroup of them has sHLH (Wu et al. 2020). Elevated fibrinogen levels (FIB) are often reported in coagulopathies and are therefore an additional diagnostic criterion for HLH (Yin et al. 2020; Jordan et al. 2019). The laboratory findings of our patient showed elevated levels of fibrinogen (661 mg/dl) that can reflect sHLH. In general, the presence of comorbidities, such as hypertension or diabetes, may result in severe complications in COVID-19 patients (Du et al. 2020). Our patient’s comorbidities included hypertension and diabetes (Table 2), further supporting the development of sHLH due to SARS-CoV-2 infection.
Here, we present a rare case of sHLH, documented in a trephine BM biopsy in a living patient after SARS-CoV-2 infection, consistent with previous postmortem diagnosis of sHLH in patients with COVID-19. Although limitations in this study may include non-evidence of hepatosplenomegaly or ferritin measurements resulting in low HScore, our histological and immunohistochemical findings from trephine BM biopsy strongly supported the diagnosis of post-COVID-19 sHLH. Based on our findings, we document sHLH in a trephine BM biopsy from a living patient with resolved COVID-19 and persistent leukocytosis, underscoring the diagnostic value of trephine biopsy in preventing life-threatening conditions such as COVID-19.
Acknowledgements
Not applicable.
Authors’ contributions
MI, KZ, and DPV, were involved in conceiving and designing the study. KZ, VT, MDD, GK, KK, GK, and MI contributed to patient data collection. MI, KZ, VT and DPV confirmed the authenticity of all the raw data. DPV, SGD, KZ, VT, and MI contributed to the interpretation of data. KZ, DPV, VT, SGD and MI are involved in the preparation of the original draft. DPV, SGD, and MI, critically revised the manuscript. All authors have read and approved the final version of the manuscript.
Funding
No funding was received.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The hospital to which the patient was admitted is a teaching hospital, and all patients signed a written consent for participation and publication of their associated data.
Patient consent for publication
The patient provided oral and written approval for participation and publication of the information presented in the current study. All identifying information was removed.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Samkari H, Berliner N. Hemophagocytic Lymphohistiocytosis (Review) Annu Rev Pathol. 2018;13:27–49. doi: 10.1146/annurev-pathol-020117-043625. [DOI] [PubMed] [Google Scholar]
- Allen CE, McClain KL (2015) Pathophysiology and epidemiology of hemophagocytic lymphohistiocytosis (Review). Hematol Am Soc Hematol Educ Program 2015:177 – 82. 10.1016/j.hoc.2015.06.009 [DOI] [PubMed]
- Dropulic LK, Cohen JI. Severe viral infections and primary immunodeficiencies (Review) Clin Infect Dis. 2011;73(9):e2705–e2712. doi: 10.1093/cid/cir610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca E, et al. Prognostic factors and outcomes in adults with secondary hemophagocytic lymphohistiocytosis: a single-center experience. Clin Lymphoma Myeloma Leuk. 2018;18(10):e373–e380. doi: 10.1016/j.clml.2018.06.014. [DOI] [PubMed] [Google Scholar]
- Barba T, et al. Hemophagocytic lymphohistiocytosis in Intensive Care Unit: a 71-case strobe-compliant retrospective study. Med (Baltim) 2015;94(51):e2318. doi: 10.1097/MD.0000000000002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo M, Berliner N. Hemophagocytic lymphohistiocytosis in adults (Review) Hematol Oncol Clin North Am. 2015;29(5):915–925. doi: 10.1016/j.hoc.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S, Banerjee M, Pal R. COVID-19 Associated Hemophagocytic Lymphohistiocytosis and Coagulopathy: Targeting the Duumvirate (Review) Indian Pediatr. 2020;57(9):827–833. doi: 10.1007/s13312-020-1962-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordbar M, et al. Assessment of the HScore as a predictor of disease outcome in patients with COVID-19. BMC Pulm Med. 2021;21(1):338. doi: 10.1186/s12890-021-01706-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce C, et al. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol. 2021;34(8):1456–1467. doi: 10.1038/s41379-021-00793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colafrancesco S, et al. sCD163 in AOSD: a biomarker for macrophage activation related to hyperferritinemia. Immunol Res. 2014;60(2–3):177–183. doi: 10.1007/s12026-014-8563-7. [DOI] [PubMed] [Google Scholar]
- Dandu H, et al. Hemophagocytic histiocytosis in severe SARS-CoV-2 infection: A bone marrow study. Int J Lab Hematol. 2021;43(6):1291–1301. doi: 10.1111/ijlh.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debliquis A, et al. Haemophagocytosis in bone marrow aspirates in patients with COVID-19. Br J Haematol. 2020;190(2):e70–e73. doi: 10.1111/bjh.16860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020;201(11):1372–1379. doi: 10.1164/rccm.202003-0543oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England JT, et al. Weathering the COVID-19 storm: lessons from hematologic cytokine syndromes (Review) Blood Rev. 2021;45:100707. doi: 10.1016/j.blre.2020.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban YM, et al. An overview of hemophagocytic lymphohistiocytosis (Review) Pediatr Ann. 2017;46(8):e309–e313. doi: 10.3928/19382359-20170717-01. [DOI] [PubMed] [Google Scholar]
- Fardet L, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613–2620. doi: 10.1002/art.38690. [DOI] [PubMed] [Google Scholar]
- Farquhar JW, Claireaux AE. Familial haemophagocytic reticulosis. Arch Dis Child. 1952;27(136):519–525. doi: 10.1136/adc.27.136.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, et al. COVID-19 with Different Severity: A Multi-center Study of Clinical Features. Am J Respir Crit Care Med. 2020;201(11):1380–1388. doi: 10.1164/rccm.202002-0445oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han XC, et al. Cytokine profiles as novel diagnostic markers of Epstein-Barr virus–associated hemophagocytic lymphohistiocytosis in children. J Crit Care. 2017;39:72–77. doi: 10.1016/j.jcrc.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Harris CK, et al. Bone Marrow and Peripheral Blood Findings in Patients Infected by SARS-CoV-2. Am J Clin Pathol. 2021;155(5):627–637. doi: 10.1093/ajcp/aqaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden A, et al. Hemophagocytic syndromes (HPSs) including hemophagocytic lymphohistiocytosis (HLH) in adults: a systematic scoping review. (Review) Blood Rev. 2016;30(6):411–420. doi: 10.1016/j.blre.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Henter JI, et al. Cytotoxic therapy for severe avian influenza A (H5N1) infection (Review) Lancet. 2006;367(9513):870–873. doi: 10.1016/S0140-6736(06)68232-9. [DOI] [PubMed] [Google Scholar]
- Henter JI, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. (Review) Annu Rev Med. 2012;63:233–246. doi: 10.1146/annurev-med-041610-134208. [DOI] [PubMed] [Google Scholar]
- Hirano T, Murakami M. COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity. 2020;52(5):731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, et al. The cytokine storm and COVID-19. J Med Virol. 2021;93(1):250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MB, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: recommendations from the North American Consortium for Histiocytosis (NACHO) (Review) Pediatr Blood Cancer. 2019;66(11):e27929. doi: 10.1002/pbc.27929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalita P, et al. Secondary Hemophagocytic Lymphohistiocytosis in Post-COVID-19 Patients: A Report of Two Cases. Cureus. 2021;13(8):e17328. doi: 10.7759/cureus.17328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakike E, Giamarellos-Bourboulis EJ. Macrophage Activation-Like Syndrome: A Distinct Entity Leading to Early Death in Sepsis (Review) Front Immunol. 2019;10:55. doi: 10.3389/fimmu.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaaslan BU, et al. A case of Hemophagocytic lymphohistiocytosis induced by COVID-19, and review of all cases reported in the literature. (Review) J Infect Dev Ctries. 2021;15(11):1607–1614. doi: 10.3855/jidc.14829. [DOI] [PubMed] [Google Scholar]
- Kim JS et al (2021) Immunopathogenesis and treatment of cytokine storm in Covid-19 (Review). 11(1):316–329. Theranostics 10.7150/thno.49713 [DOI] [PMC free article] [PubMed]
- Kostoff RN, et al. Contributing factors common to COVID19 and gastrointestinal cancer. Oncol Rep. 2022;47(1):16. doi: 10.3892/or.2021.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B, et al. A personalized diagnostic and treatment approach for macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in adults. J Clin Immunol. 2017;37(7):638–643. doi: 10.1007/s10875-017-0439-x. [DOI] [PubMed] [Google Scholar]
- Labro G, et al. Macrophage Activation in COVID-19 Patients in Intensive Care Unit. Med Cases. 2020;11(7):211–214. doi: 10.14740/jmc3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerolle N, et al. Diversity and combinations of infectious agents in 38 adults with an infection-triggered reactive haemophagocytic syndrome: a multicenter study. Clin Microbiol Infect. 2016;22(3):268. doi: 10.1016/j.cmi.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Lima R, et al. Hemophagocytic syndrome and COVID-19. Respir Med Case Rep. 2020;31:101162. doi: 10.1016/j.rmcr.2020.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meazza Prina M, et al. Hemophagocytic syndrome secondary to SARS-Cov-2 infection: a case report. BMC Infect Dis. 2021;21(1):811. doi: 10.1186/s12879-021-06532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng M, et al. Risk factors for secondary hemophagocytic lymphohistiocytosis in severe coronavirus disease 2019 adult patients. BMC Infect Dis. 2021;21(1):398. doi: 10.1186/s12879-021-06094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S, et al. Concomitant immune thrombocytopenia and bone marrow hemophagocytosis in a patient with SARS-CoV-2. Clin Chem Lab Med. 2021;59(9):e358–e361. doi: 10.1515/cclm-2021-0169. [DOI] [PubMed] [Google Scholar]
- Mizumoto K, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the diamond Princess cruise SHIP, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10):2000180. doi: 10.2807/1560-7917.es.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- Nalbandian A et al (2021) Post-acute COVID-19 syndrome (Review). Nat Med 27(4): 601–615, 2021. 27(4):601–615. 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed]
- Naous E, et al. Hemophagocytic lymphohistiocytosis, a new cause of death during ‘post-acute COVID-19 syndrome?‘ A case report. J Hematop. 2021;14(3):229–233. doi: 10.1007/s12308-021-00452-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi WA, Bhutta MJ, Cureus (2021) 10.7759/cureus.19292
- Núñez-Torrón C et al (2021) Secondary haemophagocytic lymphohistiocytosis in COVID-19: correlation of the autopsy findings of bone marrow haemophagocytosis with HScore. 10.1136/jclinpath-2020-207337. J Clin Pathol jclinpath-2020-207337 [DOI] [PubMed]
- Otrock ZK, Eby CS. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 2015;90(3):220–224. doi: 10.1002/ajh.23911. [DOI] [PubMed] [Google Scholar]
- Prieto-Pérez L, et al. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection. Mod Pathol. 2020;33(11):2139–2146. doi: 10.1038/s41379-020-0613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prilutskiy A, et al. SARS-CoV-2 Infection-Associated Hemophagocytic Lymphohistiocytosis. Am J Clin Pathol. 2020;154(4):466–474. doi: 10.1093/ajcp/aqaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Casals M, et al. Adult haemophagocytic syndrome (Review) Lancet. 2014;383(9927):1503–1516. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- Rosário C, et al. The hyperferritinemic syndrome: macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome (Review) BMC Med. 2013;11:185. doi: 10.1186/1741-7015-11-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha G, et al. Limited utility of the HScore in detecting secondary haemophagocytic lymphohistiocytosis in COVID-19. Br J Haematol. 2021;194(4):686–688. doi: 10.1111/bjh.17533. [DOI] [PubMed] [Google Scholar]
- Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient (Review) Blood. 2015;125(19):2908–2914. doi: 10.1182/blood-2015-01-551622. [DOI] [PubMed] [Google Scholar]
- Scott RB, Robb-Smith AHT. Histiocytic medullary reticulosis. Lancet. 1939;234:P194–198. doi: 10.1016/S0140-6736(00)61951-7. [DOI] [Google Scholar]
- Sharif K, et al. Eppur Si Muove: ferritin is essential in modulating inflammation. Clin Exp Immunol. 2018;191(2):149–150. doi: 10.1111/cei.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MC, et al. The ambiguous boundary between EBV-related hemophagocytic lymphohistiocytosis and systemic EBV-driven T cell lymphoproliferative disorder (Review) Int J Clin Exp Pathol. 2014;7(9):5738–5749. [PMC free article] [PubMed] [Google Scholar]
- Stancioiu F, et al. A dissection of SARSCoV2 with clinical implications (Review) Int J Mol Med. 2020;46(2):489–508. doi: 10.3892/ijmm.2020.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoboda J, et al. Bone marrow haemophagocytosis indicates severe infection with severe acute respiratory syndrome coronavirus 2. Histopathology. 2021;78(5):727–737. doi: 10.1111/his.14281. [DOI] [PubMed] [Google Scholar]
- Tanaka T, et al. Interleukin (IL-6) immunotherapy (Review) Cold Spring Harb Perspect Biol. 2018;10(8):a028456. doi: 10.1101/cshperspect.a028456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsakis A, et al. SARS-CoV-2 pathophysiology and its clinical implications: An integrative overview of the pharmacotherapeutic management of COVID-19. Food Chem Toxicol. 2020;146:111769. doi: 10.1016/j.fct.2020.111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholin B, et al. Hemophagocytic lymphohistiocytosis in a patient with COVID-19 treated with tocilizumab: a case report. J Med Case Reports. 2020;14(1):187. doi: 10.1186/s13256-020-02503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usmani GN, et al. Advances in understanding the pathogenesis of HLH. Br J Haematol. 2013;161(5):609–622. doi: 10.1111/bjh.12293. [DOI] [PubMed] [Google Scholar]
- Valade S, et al. Coagulation disorders and bleedings in critically ill patients with hemophagocytic lymphohistiocytosis. Med (Baltim) 2015;94(40):e1692. doi: 10.1097/md.0000000000001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, et al. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front Cell Infect Microbiol. 2020;10:587269. doi: 10.3389/fcimb.2020.587269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman D. Haemophagocytic lymphohistiocytosis in an adult with postacute COVID-19 syndrome. BMJ Case Rep. 2021;14(9):e245031. doi: 10.1136/bcr-2021-245031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Wu C, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, et al. Infection-associated Hemophagocytic Syndrome in Critically Ill Patients with COVID-19. Curr Med Sci. 2021;41(1):39–45. doi: 10.1007/s11596-021-2315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G, et al. The prognostic role of plasma fibrinogen in adult secondary hemophagocytic lymphohistiocytosis. Orphanet J Rare Dis. 2020;15(1):332. doi: 10.1186/s13023-020-01622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, et al. Risk factors of early death in adult patients with secondary hemophagocytic lymphohistiocytosis: a single-institution study of 171 Chinese patients. Hematology. 2019;24(1):606–612. doi: 10.1080/16078454.2019.1660458. [DOI] [PubMed] [Google Scholar]
- Zou X, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.